Abstract
Previous studies examining age-group differences in working memory load-related neural activity have yielded mixed results. When present, age-group differences in working memory capacity are frequently proposed to underlie these neural effects. However, direct relationships between working memory capacity and working memory load-related activity have only been observed in younger adults. These relationships remain untested in healthy aging. Therefore, the present study examined patterns of working memory load-related activity in 22 younger and 20 older adults and assessed the contribution of working memory capacity to these load-related effects. Participants performed a partial-trial delayed response item recognition task during functional magnetic resonance imaging. In this task, participants encoded either 2 or 6 letters, maintained them during a delay, and then indicated whether a probe was present in the memory set. Behavioral results revealed faster and more accurate responses to load 2 versus 6, with age-group differences in this load condition effect for the accuracy measure. Neuroimaging results revealed one region (medial superior frontal gyrus) that showed age-group differences in load-related activity during the retrieval period, with less (greater) neural activity for the low versus high load condition in younger (older) adults. Furthermore, for older adults, load-related activity did not vary as a function of working memory capacity. Thus, working memory-related activity varies with healthy aging, but these patterns are not due solely to working memory capacity. Neurocognitive aging theories that feature capacity will need to account for these results.
Keywords: working memory, capacity, functional magnetic resonance imaging (fMRI), healthy aging
1. Introduction
Working memory has been defined as the temporary storage and manipulation of information (Baddeley, 1986). These fundamental operations allow for on-line processing, which is necessary for many higher-level cognitive functions including reasoning, planning, and problem solving. In healthy aging, working memory performance declines, especially for tasks that place demands on processing operations (e.g., storing information for extended delays, manipulating or transforming information, increasing the amount of information to be remembered) (Craik & Jennings, 1992).
Individual and age-related differences in working memory performance may be attributed to differences in working memory capacity. Working memory capacity refers to the amount of information that can be stored and processed in working memory. In turn, these capacity limits impose constraints on information processing. For healthy younger adults, the capacity of working memory is thought to be approximately four items or “chunks” of items (Cowan, 2001; Luck & Vogel, 1997), with some estimates as high as seven plus or minus two items (Miller, 1956). For older adults, however, declines in working memory capacity limits are observed (e.g., Bopp & Verhaeghen, 2005).
Behavioral research has identified working memory capacity as one key factor that might account for a substantial portion of the variability in performance across many cognitive functions including reasoning (Kyllonen & Christal, 1990) and general intelligence (Conway, Kane, & Engle, 2003; Engle, Tuholski, Laughlin, & Conway, 1999). More recently, researchers have sought to determine whether working memory capacity also accounts for variability in task-related neural activity. Whereas capacity has been directly related to working memory-related activity in younger adults (Prabhakaran et al., 2011; Todd & Marois, 2004, 2005; Xu & Chun, 2006), these relationships remain untested in healthy aging. Therefore, the present study aimed to examine working memory load-related activity in younger and older adults and to assess the contribution of working memory capacity to these age-related neural effects.
1.1 Benefits of the Delayed Response Item Recognition Task
Neuroimaging studies have revealed that, in general, working memory engages a distributed frontal-parietal network. Across a variety of paradigms, working memory-related neural activity has been observed in lateral prefrontal regions including dorsolateral prefrontal cortex (DLPFC) and Broca’s area, medial prefrontal regions such as premotor and supplementary motor areas, and posterior parietal cortex (Cabeza & Nyberg, 2000; Smith & Jonides, 1997).
The neural substrates of working memory component processes can be further isolated using the delayed response item recognition task (Sternberg, 1966). In this task, participants encode a set of items, maintain them during a delay, and then indicate whether or not a single probe matches an item from the memory set. Thus, an appealing feature of this task includes the ability to separately assess neural activity attributed to cognitive processes occurring during each of the three task periods (encoding, maintenance, and retrieval). Demands on working memory can also be manipulated by varying the number of items in the memory set (i.e., the working memory load). A generation of research has established that this load manipulation yields monotonic increases in reaction time and decreases in accuracy as working memory load increases (for a review see Sternberg, 1975).
1.2 Working Memory-Related Activity in Younger Adults
In young adults, neuroimaging studies that used delayed response item recognition tasks observed greater activity during higher versus lower working memory load conditions (Cairo, Liddle, Woodward, & Ngan, 2004; Cappell, Gmeindl, & Reuter-Lorenz, 2010; Carp, Gmeindl, & Reuter-Lorenz, 2010; Eldreth et al., 2006; Motes & Rypma, 2010; Rypma, Berger, & D'Esposito, 2002; Rypma, Berger, Genova, Rebbechi, & D'Esposito, 2005; Rypma & D'Esposito, 1999; Zarahn, Rakitin, Abela, Flynn, & Stern, 2005). These working memory load-related effects were reported primarily in prefrontal brain regions (e.g., dorsolateral and ventrolateral prefrontal cortex; superior, middle, and inferior frontal gyri) during all task periods. Additional load-related effects have also been observed in parietal, occipital, cerebellar, and subcortical regions during encoding and retrieval periods. Thus, they are thought to reflect strategic engagement of executive processes (possibly in the service of chunking operations) when there are large amounts of information to be remembered (Rypma & Gabrieli, 2001).
1.2.1 Patterns of working memory load-related activity
A handful of studies have systematically examined working memory load-related effects in younger adults and identified three distinct patterns that are thought to reflect the effect of working memory capacity on working memory-related neural activity (Callicott et al., 1999; Linden et al., 2003). In one “capacity independent” pattern, increases in working memory-related neural activity plateau early (i.e., at very low working memory loads) and remain constant across working memory load conditions. Thus, this pattern might reflect changes in task-related neural activity that are independent of working memory capacity. In contrast, the other two patterns, “capacity unconstrained” and “capacity dependent”, are frequently cited as evidence that working memory capacity modulates task-related neural activity in younger adults. The capacity-unconstrained pattern refers to increases in working memory-related neural activity as a function of working memory load. Similarly, the capacity-dependent pattern refers to working memory-related neural activity that initially increases with working memory load until it reaches a peak after which it decreases with additional increases in working memory load. The peak of this inverted U-shape pattern has been proposed to be a neural marker of working memory capacity (Callicott, et al., 1999; Nagel et al., 2011; Todd & Marois, 2005). Thus, in the same way that the working memory load manipulation is associated with monotonic changes in behavioral performance, it is also associated with linear increases in working memory-related activity in both the capacity-unconstrained and capacity-dependent patterns, at least at sub-capacity loads (i.e., prior to the peak in the capacity-dependent pattern).
1.2.2 Working memory capacity-related activity
Direct support for the notion that working memory capacity moderates working memory-related neural activity in younger adults comes from electrophysiological (Vogel & Machizawa, 2004) and functional neuroimaging studies (Prabhakaran, et al., 2011; Todd & Marois, 2004, 2005; Xu & Chun, 2006) that assessed relationships between these measures. Across studies, higher working memory capacity was associated with a more negative load-related slow wave or greater working memory load-related activity (e.g., difference between high versus low load) in frontal (e.g., DLPFC), parietal (e.g., intraparietal sulcus) and occipital (e.g., lateral occipital cortex, intraoccipital sulcus) regions. These capacity-related effects were observed during all task periods, with most studies reporting them during the maintenance period. Thus, they may reflect the allocation of attention to the memory set during encoding (e.g., Yantis & Serences, 2003) and/or storage of the representations in memory during maintenance (Smith & Jonides, 1997).
1.3 Working Memory-Related Activity in Older Adults
In contrast to the previously cited literature on working memory load-related and capacity-related effects in younger adults, it remains unknown whether working memory capacity modulates working memory-related neural activity in healthy aging. Whereas studies in young adults have primarily reported increases in working memory-related neural activity as a function of working memory load (i.e., capacity-unconstrained and capacity-dependent patterns), neuroimaging studies using delayed response item recognition tasks in aging have reported negative (Carp, et al., 2010), capacity-unconstrained (Cappell, et al., 2010; Rypma, et al., 2005; Schneider-Garces et al., 2010, absolute data), and capacity-dependent (Cappell, et al., 2010) patterns in older adults. The negative pattern, in which working memory-related neural activity decreases as a function of working memory load, was observed primarily in frontal (e.g., DLPFC; inferior, middle and superior frontal gyrus; anterior cingulate) and parietal (e.g., precuneus) regions during encoding and maintenance task periods (Carp, et al., 2010). The capacity-unconstrained and capacity-dependent patterns, on the other hand, were observed mostly in frontal regions (e.g., DLPFC, inferior frontal gyrus, motor cortex, anterior cingulate) during maintenance (Cappell, et al., 2010; Rypma, et al., 2005; Schneider-Garces, et al., 2010). Thus, across studies, there has been substantial variability in the direction of significant effects and the brain regions and task periods in which they occur.
1.4 The Present Study
The present study was designed to clarify the previously reported mixed findings by examining age-group differences in working memory load-related activity and to assess the contribution of working memory capacity to these neural effects. Younger and older adults completed a partial-trial delayed response item recognition task with a working memory load manipulation (2 versus 6 letters) during fMRI scanning. The partial-trial manipulation was designed to isolate neural activity (i.e., blood oxygen level-dependent, BOLD) from each task period because the neural response from one task period is not independent from activity during previous, adjacent task periods (Ollinger, Shulman, & Corbetta, 2001). This approach of controlling for correlations among task periods improved our ability to detect age-group differences in working memory load-related activity across the three task periods, providing us with some leverage to potentially adjudicate between previous studies that have yielded mixed results using delayed response item recognition tasks.
Consistent with previous behavioral findings, we predicted that older adults would show working memory performance decrements compared to younger adults (i.e., slower and less accurate performance, and larger load-related effects) and show lower working memory capacity relative to younger adults. Consistent with patterns reported in the previously cited neuroimaging literature, we further predicted increases in working memory-related neural activity as a function of increasing working memory load for younger adults (i.e., capacity-unconstrained and capacity-dependent patterns). For older adults, similar capacity-unconstrained and capacity-dependent patterns were expected if working memory-related neural activity was modulated by working memory capacity, whereas the capacity-independent and negative patterns would indicate that the measures are independent.
Importantly, the present study also directly examined the contribution of working memory capacity to working memory load-related activity by assessing relationships between these measures in both age groups. Working memory capacity was measured using WAIS-III Digit Span backward performance and Cowan’s K. We were specifically interested in the role of working memory capacity in regions showing age group differences in working memory load-related activity (i.e., Age Group × Load Condition interactions) as well as regions showing significant working memory load-related activity (i.e., main effects of Load Condition). Whereas previous studies have observed significant capacity-related increases in working memory load-related activity in younger adults (Prabhakaran, et al., 2011; Todd & Marois, 2004, 2005; Xu & Chun, 2006), this crucial test of the working memory capacity-activity relationship has not been conducted in older adults. Thus, these results will inform current theories of neurocognitive aging in which capacity plays a central role.
2. Method
2.1 Participants
Twenty-two younger (21.8 ± 2.5 years, 10 female) and 20 healthy older (65.3 ± 5.3 years, 12 female) adults were recruited through the University of Texas at Dallas and from local advertising online and in newspapers. Neuropsychological characterizations for each group are presented in Table 1. All individuals gave informed consent, and were provided either payment or course credit for their participation. The University of Texas at Dallas Institutional Review Board approved the experimental procedures.
Table 1.
Neuropsychological test results
| Neuropsychological test | Cognitive Function | Younger Adults | Older Adults | t |
|---|---|---|---|---|
| MoCA | Screen for dementia | 27.5 ± 2.7 | 27.2 ± 2.4 | −0.4 |
| WAIS-III Digit Span forward | Working memory | 10.4 ± 2.3 | 10.5 ± 1.5 | 0.2 |
| WAIS-III Digit Span backward | Working memory | 7.3 ± 1.9 | 6.7 ± 1.6 | −1.1 |
| WAIS-III Digit Symbol coding | Processing speed | 83.0 ±10.5 | 66.3 ±14.0 | −4.3** |
| Trail Making Test A | Processing speed | 19.5 ± 4.7 | 29.6 ± 9.8 | 4.2** |
| Trail Making Test B | Executive function | 43.8 ±10.6 | 81.5 ±49.7 | 3.4* |
| WASI Vocabulary | Vocabulary | 61.6 ± 8.4 | 68.1 ± 6.1 | 2.8* |
Notes. All scores are given as mean ± SD, with neuropsychological test scores based on raw data. Independent sample t tests show group effects
p < .05,
p < .001.
One younger and one older adult did not complete the neuropsychological test battery. MoCA = Montreal Cognitive Assessment; WASI = Wechsler Abbreviated Scale of Intelligence; and WAIS-III = Wechsler Adult Intelligence Scale, 3rd edition.
Participants were screened for conditions that would affect their ability to complete the computer-based task (e.g., uncorrected vision, arthritis, and back problems that would make it difficult to see items presented on the computer screen or comfortably push the response buttons), prevent them from being able to enter the MRI scanner (e.g., being pregnant, having ferrous metal implants, having difficulty lying in the supine position for 30 minutes, and being claustrophobic), or impair their cognitive functioning (e.g., stroke, dementia, diabetes, and uncontrolled depression or hypertension).
2.2 Working Memory Tasks
Prior to MRI scanning, participants completed the WAIS-III backward digit span subtest (Wechsler, 1997), an index of working memory capacity, as part of a brief neuropsychological test battery (see Table 1).
During MRI scanning, participants completed a delayed response item recognition working memory task (Sternberg, 1966) with a partial-trial modification (Motes & Rypma, 2010). On each trial, the screen displayed a yellow box in the upper part of a black screen, a cyan box in the lower part of the screen, and a fixation cross in the center. During the encoding period, six letters were presented in the yellow box. Working memory load was manipulated by instructing participants to remember a set of either two or six letters that appeared in yellow brackets. During the maintenance period, the letters disappeared and participants were instructed to hold the memory set in mind. In the retrieval period, a new set of six letters were presented in the cyan box, and participants indicated whether or not a single probe letter within cyan brackets was part of the memory set using a right or left button press, respectively.
Letter stimuli (target sets, probe, and foils) were randomly selected from the following set: B, F, G, H, J, L, M, N, Q, R, S, W, and X. The positions of the letters were randomly determined on each trial, except that target sets for load condition 2 were always adjacent to each other during encoding. For both load conditions, foils appearing during the retrieval period were randomly selected from the letters that were not used during the encoding period. This approach was designed to minimize the potential influence of inhibiting task-irrelevant distractors (e.g., letters presented during the encoding phase of load condition 2 that were not part of the memory set)(Lavie, 2005), though they have previously been shown to have no significant effect on neuroimaging results obtained using a similar working memory task (Rypma, Prabhakaran, Desmond, & Gabrieli, 2001).
The partial-trial modification involved “full trials” consisting of all three task periods (as in the traditional delayed response item recognition task) in addition to three types of “partial trials”. Full trials included encoding (2 s), maintenance (8 s), and retrieval (2 s) periods. Partial trials included subsets of only the encoding (encode), encoding and maintenance (encode-maintain), or encoding and retrieval (encode-respond) periods of a given trial. The fixation cross, which appeared green during all task periods, would turn red during rest periods that followed every trial. When it turned red after the encode and encode-maintain partial trials, this would cue participants to “stop trying to remember the letters”.
Participants completed three runs that contained 12 full trials and 4 of each partial trial type, presented in a fixed random order, with an equal number of each load condition (2 or 6 letters) per trial type. Rest periods (4, 6, 8, 10, and 12 s) were randomly selected without replacement (n = 12, 6, 3, 2, and 1 s per run, respectively). See Motes and Rypma (2010) for additional details.
2.3 Imaging Data Acquisition
Images were acquired using a Philips Achieva 3.0 Tesla MRI system at the University of Texas Southwestern Advanced Imaging Research Center. An imaging technician positioned participants in the supine position in the scanner with an 8-element, SENSE, receive-only head coil. A mirror mounted on the head coil allowed them to view the computer screen during scanning. Fitted padding was used to minimize head movements.
Functional imaging sequences for each run of the working memory task used the following parameters: scan time = 6:18 minutes, TR = 2000 ms, TE = 30 ms, 70° flip angle, FOV = 220 × 144 × 220 mm, matrix size = 64 × 64, spatial resolution = 3.5 × 4 × 3.5 mm, 36 axial interleaved slices/volume, and 180 volumes/run.
A single high resolution T1-weighted MPRAGE image was acquired with the following parameters: scan time = 3:57 minutes, TR = 8.1 ms, TE = 3.7 ms, 12° flip angle, FOV = 256 × 160 × 204 mm, matrix size = 256 × 204, spatial resolution = 1 mm3, and 160 sagittal slices.
2.4 Imaging Data Preprocessing
Functional data were pre-processed using FMRI Expert Analysis Tool (FEAT) Version 5.98 (http://www.fmrib.ox.ac.uk/fsl/feat5/index.html). After removing the first two volumes, data for each run were slice-time corrected for interleaved acquisition, spatially smoothed (5 mm FWHM Gaussian Kernel), high-pass filtered (100 s), and corrected for motion using MCFLIRT (Jenkinson, Bannister, Brady, & Smith, 2002). Each functional run was also registered first to the participant's own MPRAGE using a transformation with six degrees of freedom, and then normalized to the MNI152 standard space template using a transformation with 12 degrees of freedom. For all analyses, Z statistics were thresholded using clusters determined by Z > 2.7 and a cluster significance threshold of P < 0.05, with false discovery rate (FDR) procedures to correct for multiple comparisons.
2.4.1 Contrasts of interest
For each functional run, a lower-level analysis separately modeled each combination of task period (encoding, maintenance, retrieval) and load condition (2, 6) using six explanatory variables. Note that this model included task period and load condition information from both full and partial trials. Thus, across the three runs, the 36 full trials and 12 of each partial trial type yielded 36 estimates of the encoding period, 24 estimates of the maintenance period, and 24 estimates of the retrieval period per load condition. The six modeled components were convolved with a gamma-variate hemodynamic response function (standard deviation = 3 s, mean lag = 6 s). Six contrasts (versus rest) of these main effects (e.g., encode 2, encode 6, etc.) generated parameter estimates for use in the higher-level analyses. Use of parameter estimates, independent of their associated error terms, permitted between-group comparisons robust to known age differences in BOLD signal variability (Rypma & D'Esposito, 2001).
A second-level analysis separately modeled the data for each individual so as to capture their unique variance across runs. For each of the six contrasts from the lower-level analysis, one explanatory variable was created for each individual, and one contrast was used to combine these data.
Finally, age-group differences in working memory load-related neural activity (as measured by parameter estimates) were assessed with Age Group (younger, older) × Load Condition (2, 6) mixed design ANOVAs that were conducted separately for each task period. In these models, explanatory variables coded for the main effect of Load Condition and the Age Group × Load Condition interaction. Additional explanatory variables of no interest were created for each participant to control for within-subject variance across Load Condition. Analyses were restricted to task-positive networks by using prethreshold masks of regions showing positive task-related activity (i.e., Z > 0) in each combination of age group and load condition. The Age Group × Load Condition interaction was further masked by positive activation (i.e., Z > 0) from the Load Condition contrasts (i.e., Load 2 > 6 and 6 > 2). For significant clusters from the Age Group × Load Condition interaction, maximum percent signal change values were extracted from each participant’s data from the second-level analysis using Featqueary.
Because mixed design ANOVA models in FSL do not accurately account for between-group variance, the main effect of Age Group, independent of Load Condition, was assessed with unpaired t-tests that were conducted separately for each task period. For these analyses, an additional second-level analysis was used to separately model the data for each individual across both Load Conditions and runs within each task period. Contrast masks restricted the Age Group analyses (i.e., Younger > Older, Older > Younger) to regions showing positive task-related activity (i.e., Z > 0) in each age group.
3. Results
3.1 Behavioral Results
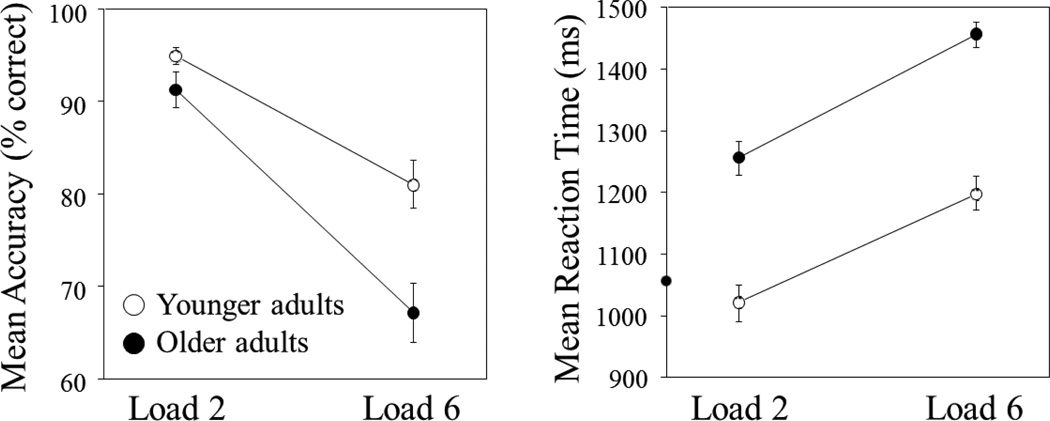
To assess age-group differences in working memory performance, we conducted Age Group (younger, older) × Load Condition (2, 6) mixed design ANOVAs separately for mean accuracy and mean reaction time on correct trials, with Age Group as a between-subject variable and Load Condition as a within-subject variable. These data are presented in Figure 1 and Table 2.
Figure 1.
Behavioral results are presented separately for mean percent correct and mean reaction time as a function of working memory load for each age group. Responses were significantly slower and less accurate for older versus younger adults (main effects of Age Group) and for load 6 versus 2 (main effects of Load Condition), with a significant Age Group by Load Condition interaction for the accuracy measure.
Table 2.
Behavioral results
| Younger Adults | Older Adults | |
|---|---|---|
| Mean Accuracy (% correct) | 88.0 ± 11.6 | 79.2 ± 16.9 |
| Load 2 | 95.0 ± 4.4 | 91.2 ± 8.4 |
| Load 6 | 81.0 ± 12.3 | 67.1 ± 14.4 |
| Mean Reaction Time (ms) | 1109.8 ± 159.7 | 1356.4 ± 146.9 |
| Load 2 | 1021.1 ± 168.9 | 1256.5 ± 124.5 |
| Load 6 | 1198.6 ± 128.1 | 1456.3 ± 88.2 |
Notes. Behavioral results (M ± SD) are presented for mean accuracy (% correct) and mean reaction times on correct trials (ms) separately for each age group and load condition.
3.1.1 Accuracy
There was a significant main effect of Age Group, F(1, 40) = 9.7, p < .01, with more accurate responses in younger versus older adults. There were also significant effects of Load Condition, F(1, 40) = 134.1, p < .001, and Load Condition × Age Group, F(1, 40) = 9.4, p < .01. As expected, responses were more accurate in Load 2 relative to Load 6, and the magnitude of this Load Condition effect (Load 2 minus Load 6) was larger in the older versus younger group (24.1 versus 14.0 %).
3.1.2 Reaction time
There was a significant main effect of Age Group, F(1, 40) = 49.1, p < .001, with significantly faster responses in younger versus older adults. In addition, a significant Load Condition effect, F(1, 40) = 194.7, p < .001, revealed that, as expected, participants responded faster in Load 2 than in Load 6.
3.2 Functional Imaging Results: Group-Level Load Effects
3.2.1 Working memory load effects
To assess age-group differences in working memory load-related activity, Age Group (younger, older) × Load Condition (2, 6) ANOVAs were conducted separately for neural activity during each task period (encoding, maintenance, retrieval).
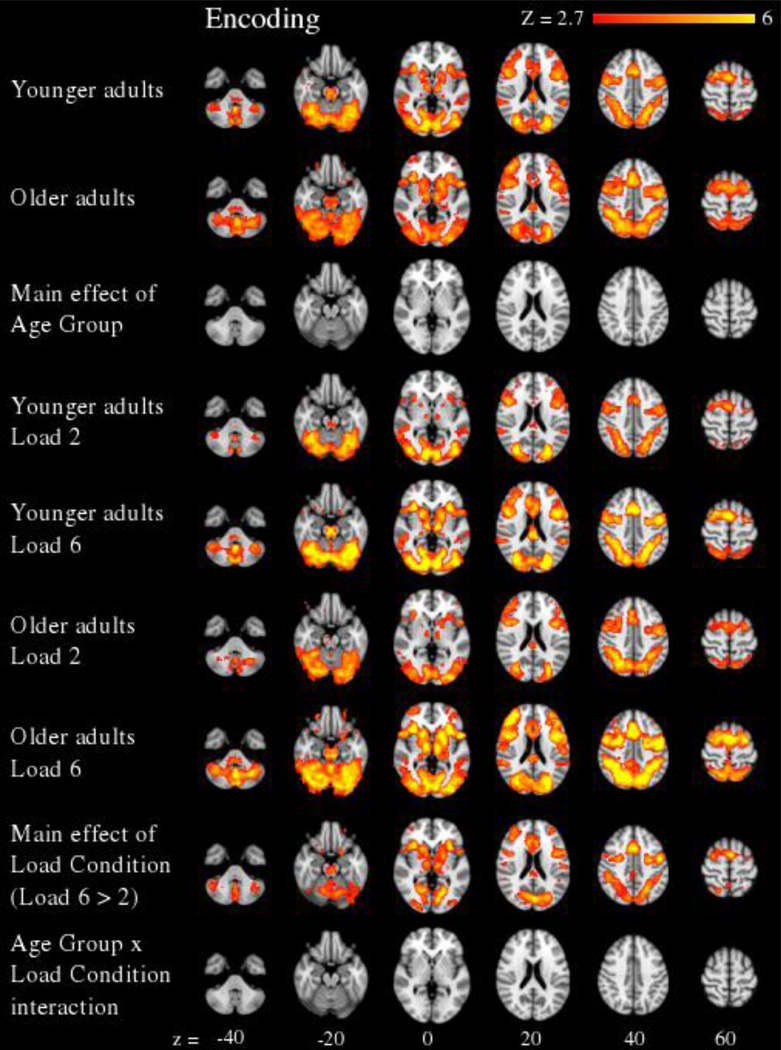
For the encoding period (see Figure 2 and Supplementary Table 1), a significant main effect of Load Condition revealed greater activity in Load 6 relative to Load 2 in bilateral middle frontal gyrus (which encompassed the DLPFC), left superior temporal gyrus, left cerebellum, and a large cluster encompassing bilateral frontal, parietal, occipital, and thalamic regions (largest maxima in left occipital cortex). There were no significant Age Group × Load Condition interactions during encoding.
Figure 2.
Neural effects during the encoding period of the working memory task are presented across 6 axial slices (cluster corrected at Z > 2.7, p < 0.05, and FDR corrected for multiple comparisons). There we no significant effects of Age Group or Age Group × Load Condition. However, a main effect of Load Condition revealed greater activity at higher versus lower working memory loads.
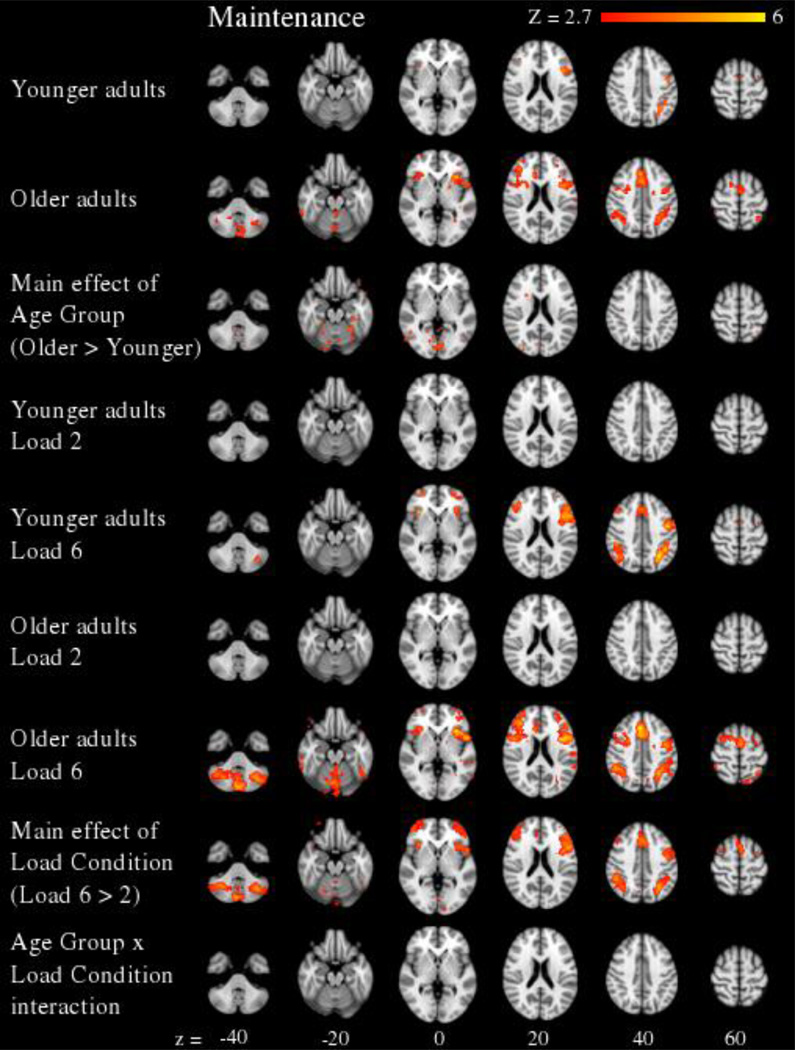
For the maintenance period (see Figure 3 and Supplementary Table 2), a significant main effect of Load Condition revealed greater activity in Load 6 relative to Load 2 in frontal (left frontal pole, left superior frontal gyrus, bilateral middle frontal gyrus, and bilateral insula) and parietal (left superior parietal lobe, right inferior parietal lobe) cortex, and bilateral cerebellum. There were no significant Age Group × Load Condition interactions during the maintenance period.
Figure 3.
Neural effects during the maintenance period of the working memory task are presented across 6 axial slices (cluster corrected at Z > 2.7, p < 0.05, and FDR corrected for multiple comparisons). Significant effects of Age Group and Load Condition revealed greater activity in older versus younger adults and at higher versus lower working memory loads, respectively. The Age Group × Load Condition interaction was not significant.
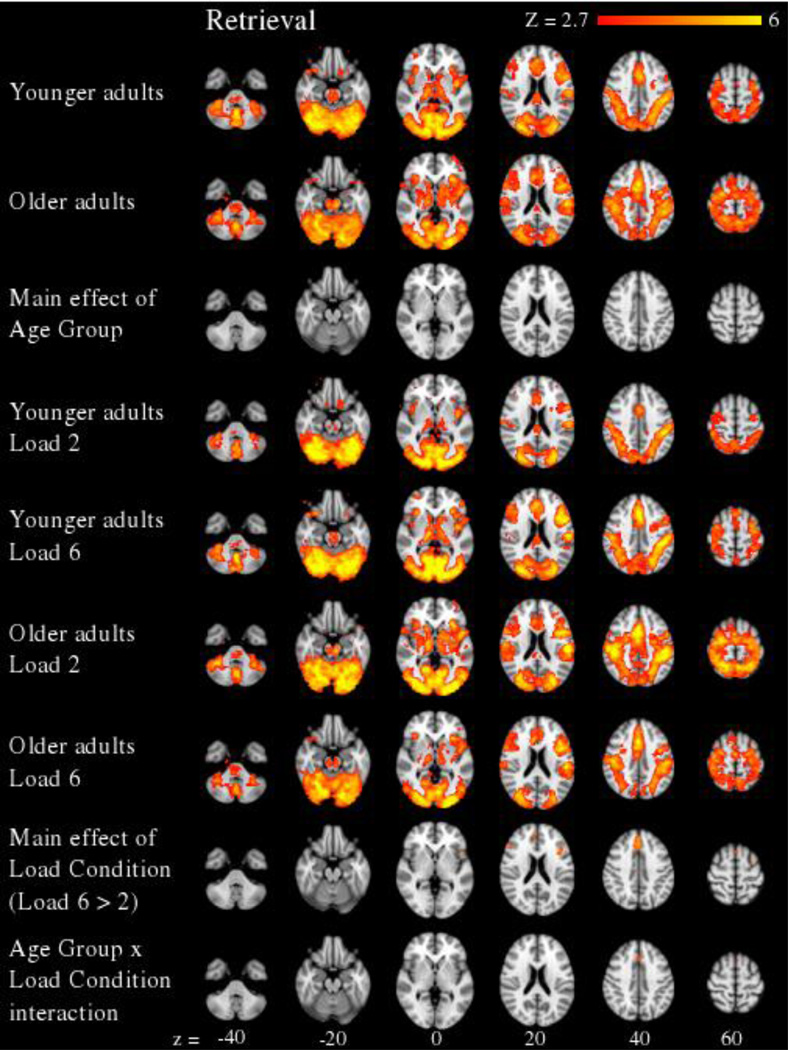
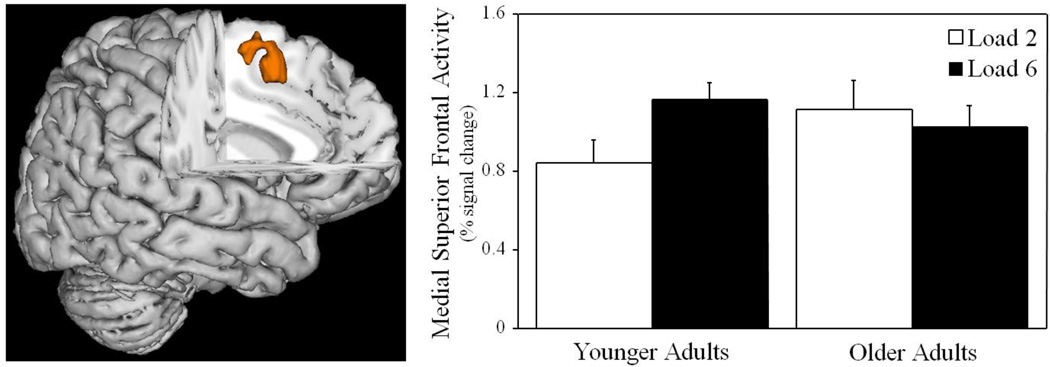
For the retrieval period (see Figure 4 and Supplementary Table 3), a significant main effect of Load Condition revealed greater activity in Load 6 relative to Load 2 in medial superior frontal gyrus and left middle frontal gyrus. Importantly, the retrieval period also revealed a significant Age Group × Load Condition interaction in medial superior frontal gyrus. In this region, within-group t-tests revealed significantly greater activity during Load 6 versus Load 2 for younger adults, t(21) = 2.7, p < .02, but non-significantly greater activity during Load 2 relative to Load 6 for older adults, p > .46. These data are presented in Figure 5.
Figure 4.
Neural effects during the retrieval period of the working memory task are presented across 6 axial slices (cluster corrected at Z > 2.7, p < 0.05, and FDR corrected for multiple comparisons). The main effect of Age Group was not significant. However, a main effect of Load Condition revealed greater activity at higher versus lower working memory loads. Importantly, the main effect of Load Condition was qualified by a significant Age Group × Load Condition interaction. Whereas younger adults showed greater activity to Load 6 versus Load 2, older adults showed greater activity to Load 2 versus Load 6 in medial superior frontal gyrus.
Figure 5.
Medial superior frontal gyrus (shown on the left) revealed a significant interaction between Age Group and Load Condition during the retrieval period of the working memory task (cluster corrected at Z > 2.7, p < 0.05, and FDR corrected for multiple comparisons). Bar graphs (shown on the right) indicate that younger adults exhibited significantly greater activity at higher versus lower working memory loads (p < .01), whereas older adults showed similar or slightly greater activity at lower versus higher working memory loads (p > .23).
3.2.2 Age group effects
Age-Group differences in working memory-related activity, independent of Load Condition, were also assessed using between-group t-tests for each task period. For the maintenance period (see Supplementary Table 2), significantly greater activity was found in older adults relative to younger adults in occipital cortex (medial and right lateral regions) and left cerebellum. There were no significant main effects of Age Group during encoding or retrieval. Because age group differences were not significant in the retrieval period, it is unlikely that mean age group differences in working memory-related activity contributed to the significant age group differences observed in working memory load-related activity in the Age Group × Load Condition interaction.
3.3 Functional Imaging Results: Individual-Level Capacity Effects
The effect of working memory capacity on working memory load-related activity was assessed in additional analyses that used working memory capacity as measured by WAIS-III Digit Span backward performance (i.e., number correct) and Cowan’s K. Cowan’s K was calculated based on performance in Load Condition 6 according to the following equation: K = (hit rate + correct rejection rate - 1) * N, where N = 6 (Cowan, 2001). One younger adult and one older adult were excluded from analyses using WAIS-III Digit Span because their data were not available.
These two measures were selected because they are among the most commonly used indices of working memory capacity, thus allowing for direct comparisons between our results and those from related studies. Furthermore, these measures provide unique but complementary assessments of working memory capacity in that WAIS-III Digit Span backward permits calculation of an index of capacity that is independent from the delayed response item recognition task, whereas Cowan’s K is derived from performance on the same task used to measure working memory-related neural activity. Both capacity measures were predicted to reveal similar patterns of results.
3.3.1 Correlations with age group differences in load effects
We posited that increases in working memory-related activity as a function of working memory load (e.g., capacity-unconstrained and capacity-dependent patterns) would be expected if working memory-related neural activity was modulated by capacity, whereas decreased or unchanging load-related activity (e.g., negative and capacity-independent patterns) may indicate that the measures are independent. As reported in section 3.2.1, the Age Group × Load Condition interaction during the retrieval period revealed a medial superior frontal region in which younger adults showed significant increases in working memory load-related activity (i.e., greater activity during Load 6 versus Load 2) whereas older adults showed non-significant decreases in load-related activity. Therefore, we first tested our prediction by examining whether the working memory load-related increase seen in younger adults varied as a function of working memory capacity, whereas the load-related decrease observed in older adults did not.
Individual measures of working memory capacity (WAIS-III Digit Span backward number correct and Cowan’s K) were separately correlated with load-related activity (% signal change difference between Load 6 and Load 2) from the cluster showing a significant Age Group × Load Condition interaction during the retrieval period. The pairwise comparisons were conducted separately for each age group. We applied a Bonferroni adjusted alpha level of 0.025 to account for multiple comparisons across capacity measures.
For younger adults, a positive correlation between load-related activity in the medial superior frontal gyrus and capacity attained significance for WAIS-III Digit Span backward performance, r = 0.56, p < .01, but not Cowan’s K, r = 0.27, p > .23. For older adults, no correlations with either capacity measure were significant, ps > .84. These findings are consistent with the notion that increases in working memory load-related activity are mediated by working memory capacity (as in younger adults), whereas decreases in load-related activity are not (as in older adults).
3.3.2 Correlations with load effects across age groups
Relationships between working memory load-related activity and working memory capacity identified in the previous section may be driven by the patterns of load-related activity (i.e., positive capacity-unconstrained and capacity-dependent versus negative and capacity-independent patterns) or by age group (i.e., younger versus older adults). To dissociate these possibilities, we further tested our prediction by examining whether increases in working memory load-related activity observed in both age groups varied as a function of working memory capacity.
Individual measures of working memory capacity (WAIS-III Digit Span backward number correct and Cowan’s K) were separately correlated with load-related activity (% signal change difference between Load 6 and Load 2) from the clusters showing significant main effects of Load Condition during each task period. The pairwise comparisons were conducted separately for each age group and follow-up regression analyses tested whether significant working memory activity-capacity relationships differed between age groups. We applied Bonferroni adjusted alpha levels of 0.005, 0.0025, and 0.0125 for the encoding, maintenance, and retrieval periods, respectively, to account for multiple comparisons across the number of significant clusters and capacity measures per age group and task period.
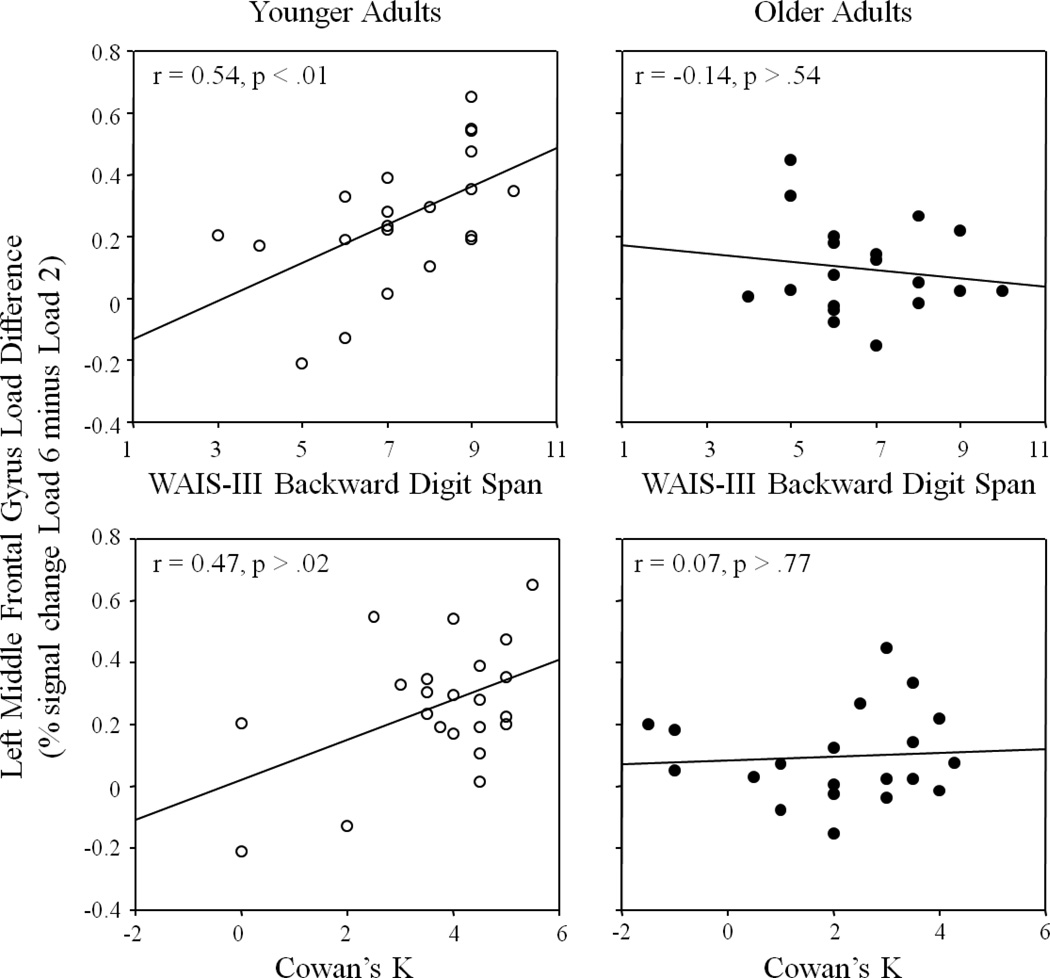
Correlations between working memory load-related activity and capacity did not attain significance for either age group in the encoding, ps > .01, and maintenance, ps > .02, periods. For the retrieval period, however, younger adults showed a significant positive correlation between load-related activity in the left middle frontal gyrus and capacity as measured by WAIS-III Digit Span backward performance, r = 0.54, p < .008. For older adults, correlations between load-related activity from either cluster or capacity measure did not approach significance, ps > .41. Data for the left middle frontal gyrus are presented in Figure 6.
Figure 6.
Working memory load-related activity in the left middle frontal gyrus (significant cluster from the Load Condition effect during retrieval), measured as the difference in activity between Load 6 and Load 2, is plotted as a function of working memory capacity, measured by WAIS-III Digit Span backward (top) and Cowan’s K (bottom), separately for younger (left) and older (right) adults. Higher working memory capacity was associated with significantly greater load-related activity in younger adults, whereas load-related activity did not vary as a function of working memory capacity for older adults.
To test whether age group significantly moderated the working memory activity-capacity relationship in this left middle frontal cluster, regression coefficients for younger and older adults were compared using follow-up regression analysis (see Clogg, Petkova, & Haritou, 1995). WAIS-III Digit Span backward performance was the predictor variable and load-related activity from the left middle frontal gyrus was the outcome variable. This test revealed significant age-group differences in the working memory activity-capacity regression coefficients, z = 3.4, p < .001. These results indicate that the effect of working memory capacity on working memory load-related activity differed significantly between the age groups.
Taken together, working memory load-related activity from left middle frontal (significant cluster from the main effect of Load Condition) and medial superior frontal (significant cluster from Age Group × Load Condition interaction) regions during the retrieval period was related to working memory capacity in younger adults, but not older adults. Thus, importantly, working memory capacity did not mediate patterns of either increased or decreased load-related activity in older adults.
3.3.3 Correlations with age group effects
Individual measures of working memory capacity (WAIS-III Digit Span backward number correct and Cowan’s K) were separately correlated with age-related activity (% signal change) from the clusters showing significant main effects of Age Group during the maintenance period. The pairwise comparisons were conducted separately for each age group. We applied a Bonferroni adjusted alpha level of 0.008 to account for multiple comparisons across the number of significant clusters and capacity measures per age group. Results revealed that neither capacity measure was related to activity in these regions in younger, ps > .35, or older, ps > .01, adults. Thus, working memory capacity does not mediate age-elated differences in working memory-related activity.
3.3.4 Behavioral analysis
Age-group differences in working memory capacity were assessed with unpaired t-tests conducted separately for each capacity measure. For Cowan’s K, older adults (2.1 ± 1.7) had significantly lower capacity relative to younger adults (3.7 ± 1.5), t(40) = −3.3, p < .01. For WAIS-III Digit Span backward, older adults (6.7 ± 1.6) also showed decreased working memory capacity relative to younger adults (7.3 ± 1.9), but the group difference was not significant (p > .28). These results suggest that, for both age groups, the current Load 2 was within participants’ working memory capacity (according to both capacity measures), whereas Load 6 was either above (according to Cowan’s K) or below (according to WAIS-III Digit Span backward) their capacity.
Relationships between working memory capacity (WAIS-III Digit Span backward number correct and Cowan’s K) and working memory performance were examined separately for working memory load-related accuracy (difference between Load 2 and Load 6) and reaction time on correct trials (difference between Load 6 and Load 2). The pairwise comparisons were conducted separately for each age group. We applied a Bonferroni adjusted alpha level of 0.0125 to account for multiple comparisons across capacity and behavioral measures.
As expected, results revealed significant working memory performance-capacity relationships in both age groups. Working memory load-related accuracy was significantly correlated with Cowan’s K in younger, r = −.93, p < .001, and older, r = −.82, p < .001, adults. Load-related accuracy was also significantly correlated with WAIS-III Digit Span backward in younger adults, r = −.59, p < .01, but not older adults, p > .50. However, follow-up regression analysis comparing the regression coefficients for younger and older adults revealed no significant age-group differences in these working memory performance-capacity regression coefficients, z = −0.74, p > .22. Working memory load-related reaction time was not significantly correlated with either capacity measure in either age group, ps > .05. Thus, the previously reported age-group differences in working memory performance cannot be attributed to differences in working memory capacity.
4. Discussion
The present study examined age-group differences in working memory load-related activity and assessed the contribution of working memory capacity to these neural effects separately for healthy younger and older adults. Behavioral data revealed that, as expected (Anders, Fozard, & Lillyquist, 1972; Sternberg, 1966), older adults were slower and less accurate on the working memory task relative to younger adults, and they had larger working memory load effects (i.e., the difference in performance to Load 2 versus Load 6). Older adults also had significantly lower working memory capacity (as measured by Cowan’s K) relative to younger adults. Neuroimaging data revealed two main results. First, there were significant age-group differences in working memory load-related activity during the retrieval period. In this region, working memory-related activity increased significantly as a function of working memory load in younger adults, but decreased (or remained unchanged) with increasing working memory load in older adults. Second, relationships between working memory capacity and working memory load-related activity were significant for younger, but not for older, adults. Taken together, the neuroimaging findings do not support the view that working memory capacity contributes to working memory-related neural activity in aging.
4.1 Age Group Differences in Load-Related Activity
When collapsed across age group, working memory-related activity increased as a function of working memory load. Consistent with previous reports, task-positive load-related increases in activity were observed in frontal (superior and middle frontal gyrus, motor and supplementary motor cortices), parietal (superior and inferior regions), occipital and cerebellar cortices (Cabeza & Nyberg, 2000; Smith & Jonides, 1997). Specifically, these load-related increases were found in the left middle frontal gyrus across all three task periods, supporting the notion that the DLPFC is engaged in executive processes (e.g., chunking operations, cognitive control, and sustained attention) when there are large amounts of information to be remembered (Knight, Staines, Swick, & Chao, 1999; Rypma & Gabrieli, 2001).
For the retrieval period, however, significant age-group differences in working memory load-related activity were also observed. In medial superior frontal gyrus, working memory-related activity increased significantly as a function of working memory load in younger adults, and decreased (or remained unchanged) as a function of working memory load in older adults. This pattern of age-group differences has been reported in previous studies that also examined age-group differences in working memory load-related activity using delayed response item recognition tasks (Cappell, et al., 2010; Carp, et al., 2010). Their effects, however, were observed in frontal (e.g., right DLPFC; bilateral inferior, middle and superior frontal gyrus; right anterior cingulate; medial orbitofrontal cortex) and parietal (e.g., left precuneus) regions during encoding and maintenance task periods. Thus, across studies, there remains considerable variability in both the brain regions and task periods within which the patterns of age-group differences in working memory load-related activity occur.
Methodological differences may account for this between-study variability. For example, the partial-trial methodology used in the current study allowed us to isolate neural activity to the distinct but adjacent task periods within a full trial. Unique estimates of neural activity attributed to each task period were calculated using separate regressors that collapsed across full and partial trials (Motes & Rypma, 2010). This method minimized the influence of activity during one task period (e.g., encoding) upon other task periods (e.g., maintenance or retrieval). The two previous studies reduced such colinearity by varying the duration of the maintenance phase and inter-trial intervals (see Cappell, et al., 2010; Carp, et al., 2010). Thus, differences in the extent to which multicolinear influences were controlled using these various methods could account for differences between the current results and those observed previously, especially for differences in the task periods during which neural effects were observed.
4.1.1 Theoretical implications of group-level patterns of load-related activity
Despite variability in the spatial (brain regions) and temporal (task periods) properties of age-group differences in working memory load-related activity, the direction of these patterns were consistent across the current study and two previous studies. That is, working memory load-related activity increased as a function of working memory load in younger adults (i.e., capacity-unconstrained pattern) and primarily decreased or remained unchanged with increasing working memory load in older adults (i.e., negative or capacity-independent patterns). In the neurocognitive aging literature, this pattern of positive working memory load-related activity in younger adults but negative load-related activity in older adults has been interpreted to reflect compensation models such as compensation related utilization of neural circuits (CRUNCH) (Reuter-Lorenz & Cappell, 2008) (see Carp, et al., 2010; Nagel, et al., 2011). According to CRUNCH, older adults have reduced cognitive capacity relative to younger adults. Thus, whereas younger adults are able to successfully increase neural recruitment in response to task demands, older adults must compensate for their diminished cognitive capacity by recruiting more neural resources when task demands are low, resulting in increased neural activity during low working memory load conditions. These compensatory responses fail, however, when task demands are high (i.e., above their cognitive capacity), resulting in decreased neural activity during high working memory load conditions.
Importantly, this pattern of age-group differences in neural activity as a function of working memory load (i.e., positive working memory load-related activity in younger adults but negative load-related activity in older adults) is also consistent with other interpretations. Efficiency theories, for example, posit that when fundamental processes are performed quickly, neural processing can be minimized and cognitive performance maximized (Vernon, 1983). Previous studies have shown decreased neural activity in better performing (i.e., faster and/or more accurate) younger adults relative to their worse performing counterparts (Eldreth, et al., 2006; Rypma et al., 2006; Rypma & D'Esposito, 1999). Increased neural activity, on the other hand, has been observed in older adults relative to younger adults and in better performing older adults relative to their slower and less accurate counterparts (Persson et al., 2004; Reuter-Lorenz et al., 2000; Rypma, et al., 2005; Rypma & D'Esposito, 2000; Rypma, Eldreth, & Rebbechi, 2007), consistent with age-related reductions in neural efficiency (Rypma, et al., 2006; Rypma & Prabhakaran, 2009). Because performance is typically better at lower versus higher working memory loads, the neural efficiency hypothesis predicts reduced neural activity at lower versus higher working memory loads in younger adults, but greater neural activity at lower versus higher loads in older adults.
Taken together, the pattern of age-group differences in working memory load-related activity, which was observed here and reported previously, is consistent with predictions of both neural efficiency and CRUNCH models. Therefore, the group-level working memory load-related results alone are not sufficient to adjudicate between one neurocognitive aging theory and another. In order to dissociate these theories, direct relationships between the proposed underlying mechanism (e.g., working memory capacity or neural efficiency) and the pattern of age-group differences in working memory load-related activity should be demonstrated.
4.2 Age Group Differences in Capacity-Related Activity
Toward this end, the current study further assessed the contribution of working memory capacity to working memory load-related activity by conducting additional analyses that directly related these measures within each age group. Importantly, no previous study has examined associations between working memory capacity and working memory load-related activity in older adults as was done here (cf. Schneider-Garces, et al., 2010). Our results revealed that higher working memory capacity was associated with larger Load Condition effects in medial superior and left middle frontal regions in younger adults, though these relationships were not significant for older adults. Furthermore, there was a significant age-group difference in the relationship between working memory capacity and working memory load-related activity in younger and older adults, indicating that age group moderated the working memory capacity-activity relationship. Taken together, these findings are consistent with previous reports in younger adults (Prabhakaran, et al., 2011; Todd & Marois, 2004, 2005; Xu & Chun, 2006). However, they indicated that working memory capacity alone cannot account for working memory load-related activity in older adults. Thus, these findings are not consistent with predictions of theories that posit capacity limits as a central mediator of neural activity.
In contrast to the current findings, one previous study reported that age-group differences in working memory load-related activity were accounted for by individual differences in working memory capacity (also measured by Cowan’s K) in younger and older adults (Schneider-Garces, et al., 2010). Schneider-Garces et al. examined working memory-related activity across five load conditions in younger and older adults, and found that age group differences in load-related activity were no longer significant after adjusting for each participant’s capacity. According to their results, the magnitude of working memory load-related increases in activity should be significantly related to working memory capacity in both younger and older adults. In the current study, however, these relationships were only significant for younger adults, even in regions that showed an overall increase in activity as a function of load (i.e., clusters from the main effect of Load Condition). These divergent findings might be due, at least in part, to use of event-related (current study) versus block (Schneider-Garces, et al., 2010) designs. Use of block design methodology in the latter study required that estimates of working memory load-related effects were collapsed across entire trials. This could distort the spatial (brain regions) and temporal (task periods) patterns of age-group differences in working memory load-related activity relative to the current study, which estimated these neural effects separately for each task period. Future research will be necessary to determine whether working memory capacity differentially modulates the various patterns of working memory-related activity (e.g., capacity-unconstrained versus capacity-dependent) and whether or not these effects vary in aging.
4.3 Potential Limitations
The present study employed an extreme-conditions design. One concern is that this approach might have limited our ability to detect an inverted U-shaped capacity-dependent pattern when supra-capacity loads are included. Previous aging studies have used either three load conditions that approached or were above participants’ working memory capacity limits (4, 5, and 7 letters; Cappell, et al., 2010; Carp, et al., 2010) or five load conditions that spanned the same capacity range as in the current study (load conditions 2 through 6; Schneider-Garces, et al., 2010). Across these and other studies (e.g., Rypma, et al., 2005), primarily linear or nearlinear changes in working memory-related neural activity were observed as a function of working memory load, especially for sub-capacity loads (i.e., prior to the peak in the capacity-dependent pattern) (cf. Cappell, et al., 2010). Thus, the extreme load condition design would not have influenced our ability to identify monotonic changes associated with the positive capacity-unconstrained, negative, and capacity-independent patterns that were observed here. Furthermore, results of the current study revealed that both load condition 2 (according to both capacity measures) and load condition 6 (according to WAIS-III Digit Span backward) were below participants’ working memory capacity. Accordingly, the extreme-condition design would have been sensitive to linear increases associated with the capacity-dependent pattern at these sub-capacity loads. To test these claims, research currently underway in our laboratory is designed to compare extreme-conditions designs to parametric designs that include a range of load conditions across the three task periods using the partial-trial manipulation.
Finally, whereas working memory-related activation reveals task-positive networks, task-related deactivation is observed in default mode or resting state networks (Fox et al., 2005; Raichle et al., 2001). Because the current study was among the first to examine the effect of aging and capacity on working memory load-related activity, we focused on task-positive activation by restricting our analyses to positive working memory-related activity (see Contrast of Interest section of the Methods). However, the magnitude of task-positive activation and default mode deactivation is known to vary with both aging and task demands (Persson, Lustig, Nelson, & Reuter-Lorenz, 2007). Correlated activity within these networks has also been shown to interact (Biswal, Eldreth, Motes, & Rypma, 2010). Thus, the interplay of task-positive and default mode activity, and their relationships to working memory capacity, should be addressed in future studies.
4.4 Conclusions
In summary, the present study identified significant age-group differences in working memory load-related activity. During the retrieval period, a medial superior frontal region showed positive working memory load-related activity in younger adults but negative load-related activity in older adults. These patterns were consistent with current neurocognitive aging theories related to age-group differences in working memory capacity and neural efficiency. Follow-up analyses directly tested relationships between working memory capacity and working memory load-related activity. These analyses were also conducted in regions that showed positive working memory load-related activity across age groups. Results revealed that working memory capacity accounted for working-memory load-related activity in younger but not older adults suggesting that it plays a minimal role as a mediator of age-related differences in working memory-related neural activity. Future research will certainly be required to understand the cognitive (e.g., capacity) and neural mechanisms (e.g., neural efficiency) that underlie age-related differences in working memory.
Supplementary Material
Highlights.
-
▪
Age group differences in working memory load-related activity were examined
-
▪
A partial-trial manipulation isolated neural activity from each task period
-
▪
Activity increased (decreased) as a function of load in younger (older) adults
-
▪
The contribution of working memory capacity to load effects was also assessed
-
▪
For older adults, load-related activity did not vary with working memory capacity
Acknowledgements
The authors thank M. Amanda Earl Colby and Meghana Karnik-Henry for their help with data collection and Michael A. Motes for his contribution to data analysis. This research was supported by NIA/NIH grants F32 AG038299 and R01 AG029523.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anders TR, Fozard JL, Lillyquist TD. Effects of age upon retrieval from short-term memory. Dev Psychol. 1972;6:214–217. [Google Scholar]
- Baddeley A. Working Memory. Oxford: Oxford University Press; 1986. [Google Scholar]
- Biswal BB, Eldreth DA, Motes MA, Rypma B. Task-dependent individual differences in prefrontal connectivity. Cereb Cortex. 2010;20:2188–2197. doi: 10.1093/cercor/bhp284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bopp KL, Verhaeghen P. Aging and verbal memory span: a meta-analysis. J Gerontol B Psychol Sci Soc Sci. 2005;60:P223–P233. doi: 10.1093/geronb/60.5.p223. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Cairo TA, Liddle PF, Woodward TS, Ngan ET. The influence of working memory load on phase specific patterns of cortical activity. Brain Res Cogn Brain Res. 2004;21:377–387. doi: 10.1016/j.cogbrainres.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, et al. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cerel Cortex. 1999;9:20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- Cappell KA, Gmeindl L, Reuter-Lorenz PA. Age differences in prefontal recruitment during verbal working memory maintenance depend on memory load. Cortex. 2010;46:462–473. doi: 10.1016/j.cortex.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp J, Gmeindl L, Reuter-Lorenz PA. Age differences in the neural representation of working memory revealed by multi-voxel pattern analysis. Front Hum Neurosci. 2010;4:217. doi: 10.3389/fnhum.2010.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clogg CC, Petkova E, Haritou A. Statistical methods for comparing regression coefficients between models. Am J Sociol. 1995;100:1261–1293. [Google Scholar]
- Conway AR, Kane MJ, Engle RW. Working memory capacity and its relation to general intelligence. Trends Cogn Sci. 2003;7:547–552. doi: 10.1016/j.tics.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Beh Brain Sci. 2001;24:87–114. doi: 10.1017/s0140525x01003922. discussion 114-185. [DOI] [PubMed] [Google Scholar]
- Craik FI, Jennings JM. Human memory. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. 1st ed. Hillsdale, NJ: Lawrence Erlbaum Assoc; 1992. pp. 51–110. [Google Scholar]
- Eldreth DA, Patterson MD, Porcelli AJ, Biswal BB, Rebbechi D, Rypma B. Evidence for multiple manipulation processes in prefrontal cortex. Brain Res. 2006;1123:145–156. doi: 10.1016/j.brainres.2006.07.129. [DOI] [PubMed] [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, Conway AR. Working memory, short-term memory, and general fluid intelligence: a latent-variable approach. J Exp Psychol Gen. 1999;128:309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Knight RT, Staines WR, Swick D, Chao LL. Prefrontal cortex regulates inhibition and excitation in distributed neural networks. Acta Psychol (Amst) 1999;101:159–178. doi: 10.1016/s0001-6918(99)00004-9. [DOI] [PubMed] [Google Scholar]
- Kyllonen PC, Christal RE. Reasoning ability is (little more than) working-memory capacity?! Intelligence. 1990;14:389–433. [Google Scholar]
- Lavie N. Distracted and confused?: selective attention under load. Trends Cogn Sci. 2005;9:75–82. doi: 10.1016/j.tics.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Linden DE, Bittner RA, Muckli L, Waltz JA, Kriegeskorte N, Goebel R, et al. Cortical capacity constraints for visual working memory: dissociation of fMRI load effects in a fronto-parietal network. Neuroimage. 2003;20:1518–1530. doi: 10.1016/j.neuroimage.2003.07.021. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature. 1997;390:279–281. doi: 10.1038/36846. [DOI] [PubMed] [Google Scholar]
- Miller GA. The magical number seven plus or minus two: some limits on our capacity for processing information. Psychol Rev. 1956;63:81–97. [PubMed] [Google Scholar]
- Motes MA, Rypma B. Working memory component processes: Isolating BOLD signal changes. Neuroimage. 2010;49:1933–1941. doi: 10.1016/j.neuroimage.2009.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel IE, Preuschhof C, Li SC, Nyberg L, Backman L, Lindenberger U, et al. Load modulation of BOLD response and connectivity predicts working memory performance in younger and older adults. J Cogn Neurosci. 2011;23:2030–2045. doi: 10.1162/jocn.2010.21560. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Shulman GL, Corbetta M. Separating processes within a trial in event-related functional MRI. Neuroimage. 2001;13:210–217. doi: 10.1006/nimg.2000.0710. [DOI] [PubMed] [Google Scholar]
- Persson J, Lustig C, Nelson JK, Reuter-Lorenz PA. Age differences in deactivation: a link to cognitive control? J Cogn Neurosci. 2007;19:1021–1032. doi: 10.1162/jocn.2007.19.6.1021. [DOI] [PubMed] [Google Scholar]
- Persson J, Sylvester CY, Nelson JK, Welsh KM, Jonides J, Reuter-Lorenz PA. Selection requirements during verb generation: differential recruitment in older and younger adults. Neuroimage. 2004;23:1382–1390. doi: 10.1016/j.neuroimage.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Prabhakaran V, Rypma B, Narayanan NS, Meier TB, Austin BP, Nair VA, et al. Capacity-speed relationships in prefrontal cortex. PLoS One. 2011;6:e27504. doi: 10.1371/journal.pone.0027504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Curr Dir Psychol Sci. 2008;17:177–182. [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, et al. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Rypma B, Berger JS, D'Esposito M. The influence of working-memory demand and subject performance on prefrontal cortical activity. J Cogn Neurosci. 2002;14:721–731. doi: 10.1162/08989290260138627. [DOI] [PubMed] [Google Scholar]
- Rypma B, Berger JS, Genova HM, Rebbechi D, D'Esposito M. Dissociating age-related changes in cognitive strategy and neural efficiency using event-related fMRI. Cortex. 2005;41:582–594. doi: 10.1016/s0010-9452(08)70198-9. [DOI] [PubMed] [Google Scholar]
- Rypma B, Berger JS, Prabhakaran V, Bly BM, Kimberg DY, Biswal BB, et al. Neural correlates of cognitive efficiency. Neuroimage. 2006;33:969–979. doi: 10.1016/j.neuroimage.2006.05.065. [DOI] [PubMed] [Google Scholar]
- Rypma B, D'Esposito M. The roles of prefrontal brain regions in components of working memory: effects of memory load and individual differences. Proc Natl Acad Sci U S A. 1999;96:6558–6563. doi: 10.1073/pnas.96.11.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, D'Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nat Neurosci. 2000;3:509–515. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- Rypma B, D'Esposito M. Age-related changes in brain-behaviour relationships: Evidence from event-related functional MRI studies. Eur J Cogn Psychol. 2001;13:235–256. [Google Scholar]
- Rypma B, Eldreth DA, Rebbechi D. Age-related differences in activation-performance relations in delayed-response tasks: a multiple component analysis. Cortex. 2007;43:65–76. doi: 10.1016/s0010-9452(08)70446-5. [DOI] [PubMed] [Google Scholar]
- Rypma B, Gabrieli JD. Functional neuroimaging of short-term memory: The neural mechanisms of mental storage. Beh Brain Sci. 2001;24:143–144. [Google Scholar]
- Rypma B, Prabhakaran V. When less is more and when more is more: The mediating roles of capacity and speed in brain-behavior efficiency. Intelligence. 2009;37:207–222. doi: 10.1016/j.intell.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V, Desmond JE, Gabrieli JD. Age differences in prefrontal cortical activity in working memory. Psychol Aging. 2001;16:371–384. doi: 10.1037//0882-7974.16.3.371. [DOI] [PubMed] [Google Scholar]
- Schneider-Garces NJ, Gordon BA, Brumback-Peltz CR, Shin E, Lee Y, Sutton BP, et al. Span, CRUNCH, and beyond: working memory capacity and the aging brain. J Cogn Neurosci. 2010;22:655–669. doi: 10.1162/jocn.2009.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Working memory: a view from neuroimaging. Cogn Psychol. 1997;33:5–42. doi: 10.1006/cogp.1997.0658. [DOI] [PubMed] [Google Scholar]
- Sternberg S. High speed scanning in human memory. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Sternberg S. Memory scanning: New findings and current controversies. Q J Exp Psychol. 1975;27:1–32. [Google Scholar]
- Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428:751–754. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- Todd JJ, Marois R. Posterior parietal cortex activity predicts individual differences in visual short-term memory capacity. Cogn Affect Beh Neurosci. 2005;5:144–155. doi: 10.3758/cabn.5.2.144. [DOI] [PubMed] [Google Scholar]
- Vernon PA. Speed of information processing and general intelligence. Intelligence. 1983;7:53–70. [Google Scholar]
- Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428:748–751. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale. 3rd ed. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Xu Y, Chun MM. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature. 2006;440:91–95. doi: 10.1038/nature04262. [DOI] [PubMed] [Google Scholar]
- Yantis S, Serences JT. Cortical mechanisms of space-based and object-based attentional control. Curr Opin Neurobiol. 2003;13:187–193. doi: 10.1016/s0959-4388(03)00033-3. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Rakitin B, Abela D, Flynn J, Stern Y. Positive evidence against human hippocampal involvement in working memory maintenance of familiar stimuli. Cereb Cortex. 2005;15:303–316. doi: 10.1093/cercor/bhh132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.