Abstract
Background
PTK6 is an intracellular tyrosine kinase that is distantly related to SRC family kinases. PTK6 is nuclear in normal prostate epithelia, but nuclear localization is lost in prostate tumors. Increased expression of PTK6 is detected in human prostate cancer, especially at metastatic stages, and in other types of cancers, including breast, colon, head and neck cancers, and serous carcinoma of ovary.
Materials and Methods
Potential novel substrates of PTK6 identified by mass spectrometry were validated in vitro. The significance of PTK6 induced phosphorylation of these substrates was addressed using human prostate cell lines by knockdown of endogenous PTK6, or overexpression of targeted PTK6 to different intracellular compartments.
Results
We identified AKT, p130CAS and FAK as novel PTK6 substrates and demonstrated their roles in promoting cell proliferation, migration, and resistance to anoikis. In prostate cancer cells, active PTK6 is primarily associated with membrane compartments, although the majority of total PTK6 is localized within the cytoplasm. Ectopic expression of membrane-targeted PTK6 transforms immortalized fibroblasts. Knockdown of endogenous cytoplasmic PTK6 in PC3 prostate cancer cells impairs proliferation, migration, and anoikis resistance. However, reintroduction of PTK6 into the nucleus significantly decreases cell proliferation, suggesting context specific functions for nuclear PTK6.
Conclusions
In human prostate cancer, elevated PTK6 expression, translocation of PTK6 from the nucleus to the cytoplasm, and its activation at the plasma membrane, contribute to increased phosphorylation and activation of its substrates such as AKT, p130CAS and FAK, thereby promoting prostate cancer progression.
Keywords: PTK6, BRK, FAK, p130CAS, AKT, ERK5
INTRODUCTION
Prostate cancer is the most common form of cancer, other than skin cancer, in American men. About one out of six men will be diagnosed with prostate cancer during his lifetime. Although prostate cancer has a relatively low mortality rate, it remains the second leading cause of cancer-related deaths in American men [1]. The major cause of death is metastases resulting from lymphatic, blood, or contiguous local spread. Unfortunately, we still lack effective means to treat metastatic prostate cancer, and the use of tyrosine kinase inhibitors is being explored as a treatment option [2, 3].
Roles for non-receptor tyrosine kinases in prostate cancer have been previously reviewed [4]. SRC, FAK, JAK1/2, and ETK play indispensable roles in different aspects of prostate cancer including proliferation, migration, apoptosis and metastasis [4]. During the last few years we have made substantial progress in understanding functions of the intracellular tyrosine kinase Protein Tyrosine kinase 6 (PTK6) and its potential contributions to prostate cancer, and these new findings are reviewed here [5–9].
PTK6 belongs to the PTK6 family of intracellular non-receptor tyrosine kinases, which includes Fyn-Related Kinase (FRK, also known as RAK, BSK, Iyk, and Gtk) and SRC-Related kinase lacking C-terminal regulatory tyrosine and N-terminal Myristoylation Sites (SRMS). These proteins are structurally similar to the SRC-family kinases, consisting of Src-Homology-3 (SH3) and SH2 domains followed by a tyrosine kinase catalytic domain. However, they lack an SH4 domain, which facilitates lipid modification and membrane association, and they exhibit flexibility in intracellular localization (reviewed in [10]). PTK6 family members share a highly conserved gene structure that is distinct from other intracellular tyrosine kinase families, including the SRC family [11, 12]. Human protein tyrosine kinase 6 (PTK6) was identified in cultured human melanocytes [13] and breast tumor cells [14], and has been commonly referred to as BReast tumor Kinase (BRK). Its mouse orthologue was cloned from normal small intestinal epithelial cell RNA in a screen for factors that regulate epithelial cell turnover, and was thus given the name SRC-related intestinal kinase (Sik) [15, 16].
Different roles for PTK6 in normal tissues and cancer
In normal tissues, expression of PTK6 is highest in the non-dividing, differentiated epithelial cells of the gastrointestinal tract [16, 17]. PTK6 is also detected in other differentiated epithelial cells including those of the prostate [18], oral cavity [19] and skin [16, 20]. A variety of studies suggest that PTK6 negatively regulates proliferation and promotes the differentiation of epithelial cells. In the cultured human keratinocyte cell line HaCaT and embryonic mouse keratinocyte cell line EMK, addition of calcium promotes differentiation, which is accompanied by increased PTK6 expression and activation, and elevated levels of the epidermal differentiation marker keratin-10 in HaCaT cells and filaggrin in EMK cells [20, 21]. Studies in a Ptk6-deficient mouse model demonstrated roles for PTK6 in promoting cell cycle exit and differentiation in the normal intestinal epithelium in vivo. Increased growth of small intestinal villi was accompanied by an expanded zone of proliferation and delayed enterocyte differentiation in Ptk6−/− mice [22].
PTK6 also plays important roles in regulating the survival of normal cells in response to a variety of apoptotic stimuli. PTK6 sensitized immortalized nontransformed Rat1A cells to apoptosis induced by serum deprivation and UV irradiation [23]. Further in-vivo studies revealed that PTK6 expression is induced in small intestinal crypt epithelial cells by γ-radiation, where it appears to promote DNA damage-induced apoptosis by inhibiting prosurvival signaling including AKT and ERK1/2 [24]. In the colon, induction of PTK6 in crypt base epithelial cells following administration of the carcinogen azoxymethane was also positively correlated with apoptosis [25].
Although PTK6 is not expressed in the normal human mammary gland, it is aberrantly expressed in a high percentage of breast tumors [26, 27]. Elevated expression of PTK6 has also been detected in other types of cancer including colon [17], prostate [8], head and neck cancer [28], serous carcinoma of ovary [29], lung [30] and thyroid cancer [31]. Recently however, expression of PTK6 transcripts were found to be downregulated in esophageal squamous carcinomas as a consequence of epigenetic modification, and PTK6 appears to have tumor suppressor activities in this type of cancer [32].
Several studies have demonstrated oncogenic roles for PTK6 using established cancer cell lines and animal models. PTK6 promotes breast cancer cell proliferation through phosphorylating and activating its substrates STAT3 [33] and STAT5b [34], and this process may be facilitated by STAP2 [35, 36], a scaffold protein that is also a PTK6 substrate. Interestingly, the suppressor of cytokine signaling 3 (SOCS3) has been identified as an inhibitor of PTK6 [37]. PTK6 phosphorylates Paxillin and p190RhoGAP-A to promote EGF-dependent cell migration and invasion [38, 39]. PTK6 was identified from an siRNA screen as a critical regulator of IGF-1 mediated anchorage-independent survival of breast and ovarian tumor cells [40]. In addition, positive crosstalk between PTK6 and membrane receptors such as EGFR, HER2/Neu, or MET has also been reported (reviewed in [10, 41, 42]) [43]. Recently, PTK6 was shown to sustain EGFR signaling by directly phosphorylating EGFR and inhibiting its downregulation [44].
In a WAP-driven PTK6 transgenic FVB/N mouse model, delayed involution of the mammary gland might be caused by activation of a p38 MAPK pro-survival signaling pathway, and aged mice developed infrequent tumors with reduced latency compared with wild type mice [27]. In the AOM/DSS (azoxymethane/dextran sodium sulfate) murine colon cancer model, disruption of Ptk6 impaired colon tumorigenesis, probably due to significantly reduced STAT3 activation [25].
While most available data suggest that PTK6 overexpression in cancer is oncogenic, some studies have correlated PTK6 expression with increased survival [45, 46], and tumor suppression [32]. This suggests that PTK6 could have more complex roles in cancer, which may be related to tumor heterogeneity and its intracellular localization and access to specific substrates. The “pro”-oncogenic role of PTK6 in most tumors and the “anti”-tumorigenic role of PTK6 in normal cells might be achieved by activation of distinct signaling pathways due to cell/tissue type, PTK6 expression levels, alterations in intracellular location, and different environmental stimuli. Oncogenic functions of PTK6 are enhanced when the protein is targeted to the plasma membrane in HEK-293 cells [47]. Our group first proposed that intracellular localization of PTK6 will have an impact on its cellular functions [18, 48], and discovered that nuclear-targeted PTK6 negatively regulates, whereas membrane-targeted active PTK6 enhances endogenous β-catenin/TCF transcriptional activity in SW620 colon cancer cells [49].
Aberrant expression, localization and activation of PTK6 in human prostate cancer
To understand the role of PTK6 in prostate cancer, we analyzed the NCBI human genome microarray dataset GDS2545 that contains 171 samples [50]. PTK6 mRNA levels were significantly higher in prostate tumor samples, especially in metastatic prostate tumor samples compared with normal prostate tissue and normal tissue adjacent to the tumor, indicating an oncogenic role for PTK6 in prostate tumorigenesis and metastasis [8].
PTK6 is primarily localized within the nuclei of normal human prostate epithelial cells, but it is localized to the cytoplasm and at the membrane in poorly differentiated prostate tumors [18]. In the established human prostate cancer cell lines PC3 (Androgen Receptor/AR negative) and LNCaP (AR positive), the majority of PTK6 is localized within the cytoplasm, although nuclear PTK6 can also be detected by immunostaining in the more differentiated LNCaP cancer cell line [8, 18]. Prostate cancer cells provide a suitable system to investigate the biological significance of PTK6 translocation.
Interestingly, although only a small fraction of total PTK6 was localized in the membrane compartment in PC3 cells, membrane-associated PTK6 was highly phosphorylated at tyrosine residue 342, which is a marker for its kinase activation. In contrast, the more abundant pool of cytoplasmic PTK6 was not phosphorylated at this tyrosine residue. Targeting exogenous PTK6 with a mutation of its inhibitory tyrosine residue 447 (Y-F) to membrane compartments by addition of a palmitoylation/myristoylation consensus sequence (Palm) at the amino-terminus largely increases the active pool of PTK6 in PC3 cells [8]. In addition, compared with untargeted PTK6-YF, Palm-PTK6-YF (membrane-targeted) showed substantially higher activity in SYF cells (Src−/−, Yes−/−, Fyn−/− mouse embryonic fibroblasts) [8, 9]. These data indicate that membrane localization of PTK6 is critical for its activation, and support the hypothesis that translocation of PTK6 from nucleus to cytoplasm/membrane in prostate cancer could promote its activation and access to different substrates. Understanding the regulation of this relocalization and activation of PTK6 could shed light on novel mechanisms that drive prostate tumorigenesis and metastasis.
Membrane-associated active PTK6 promotes prostate cancer cell migration by phosphorylating p130CAS and activating ERK5
To further understand PTK6 signaling mechanisms and identify new substrates, proteins whose phosphorylation was increased upon ectopic expression of active PTK6 in human cells were identified using liquid chromatography coupled with tandem mass spectrometry [9]. Along with the previously identified PTK6 substrates Sam68, Paxillin and PSF, we identified several novel candidates, including p130 CRK-associated substrate (p130CAS) [8] and focal adhesion kinase (FAK) [9], and further demonstrated that PTK6 directly phosphorylates them in vitro. p130CAS is a scaffolding protein that includes a domain containing 15 repeats of a YXXP motif that can be targeted by SRC family kinases [51]. Tandem mass spectrometry revealed 11 tyrosine residues within the substrate domain that can be targeted by PTK6 in vitro [8]. FAK is also a multidomain protein that can be phosphorylated by SRC family kinases at several tyrosine residues including 576/577, 861 and 925. Phosphorylation of tyrosine residues 576 and 577 is crucial in achieving maximum kinase activity, while phosphorylated tyrosine residue 925 is believed to be a high affinity Grb2 binding site (reviewed in [52]). Tandem mass spectrometry analyses showed that PTK6 phosphorylates FAK at tyrosine residue 861 in vitro, although the biological significance of the phosphorylation on this residue is not clear [9].
Both p130CAS and FAK are concentrated at focal adhesions [53, 54]. Following integrin clustering, FAK phosphorylates p130CAS at its C-terminal Y664DYVHL motif and then SRC phosphorylates p130CAS at several tyrosine residues within its substrate domain, which provide binding sites for the adaptor protein CRK, leading to the activation of the small GTPase RAC that is able to induce membrane ruffling, cytoskeleton remodeling and cell migration [55, 56]. Expression of membrane-targeted active PTK6 in PC3 cells induced membrane ruffling and formation of specific structures called peripheral adhesion complexes, which have been observed in active SRC expressing KM12C colon cancer cells [8, 57]. Both the kinase activity and membrane localization of PTK6 are necessary for the formation of peripheral adhesion complexes. Tyrosine phosphorylation of p130CAS and FAK are both induced and enriched in these structures [8]. Interestingly, the formation of peripheral adhesion complexes induced by PTK6 is dependent on p130CAS but not FAK, since knockdown of p130CAS impaired their formation, but knockdown of FAK did not. It is possible that PTK6, unlike SRC, does not rely on FAK to initiate the phosphorylation at the YDYVHL motif of p130CAS [8].
We also demonstrated that ERK5 but not ERK1/2 is enriched in peripheral adhesion complexes induced by membrane-targeted active PTK6. Knockdown of p130CAS impaired ERK5 activation in response to serum stimulation, indicating ERK5 is activated downstream of p130CAS [8]. p130CAS may serve as a scaffold protein that provides multiple phosphorylated tyrosine residues as binding sites for downstream interacting partners, to convey the Palm-PTK6-YF induced oncogenic signaling. It has been reported that PTK6 forms complexes with ERK5 in various human cells [58], but whether p130CAS and ERK5 are in the same complex is not known. As with other focal adhesion-like structures such as invadopodia and podosome, formation of peripheral adhesion complexes is accompanied by increased cell migration. This is dependent on p130CAS and ERK5, as knockdown of either protein impaired the formation of peripheral adhesion complexes and cell migration [8].
Activation of PTK6 at the membrane protects cells from anoikis through activation of FAK and AKT survival signaling
Membrane-targeted active PTK6 is able to transform murine embryonic fibroblasts, even in the absence of the SRC family kinases Src, Yes and Fyn. One of the most striking features of Palm-PTK6-YF transformed SYF cells is their ability to overcome anoikis and maintain proliferation under suspended growth conditions. Palm-PTK6-YF mediated FAK phosphorylation and the subsequent activation of AKT survival signaling contribute to this process [9].
In the absence of FAK, Palm-PTK6-YF was still able to protect Fak−/− MEFs from anoikis, indicating it has the ability to activate survival signaling independent of FAK. However, stable co-expression of FAK and Palm-PTK6-YF in Fak −/− MEFs synergistically activated AKT survival signaling and protected cells against anoikis. These data demonstrated that, while not essential, FAK is involved in PTK6 meditated anoikis resistance. Interestingly, Palm-PTK6-YF failed to protect Akt1/2−/− MEFs from anoikis, suggesting AKT is a critical downstream player that mediates survival signaling induced by PTK6 or FAK activation [9].
PTK6 directly phosphorylates AKT at tyrosine residues and promotes its activation
AKT is a direct substrate of PTK6. Tandem mass spectrometry analysis revealed that tyrosine residues 215 and 326 of AKT can be phosphorylated by PTK6. Further point mutation studies demonstrated that tyrosine residues 315 and 326 of AKT are the primary targets of PTK6, residues that can also be phosphorylated by SRC [7, 59]. Importantly, PTK6 induced the tyrosine phosphorylation of AKT in SYF cells, which lack endogenous Src, Yes and Fyn. In the presence of exogenous PTK6, SYF cells have increased levels of AKT activation (marked by phosphorylation of Threonine 308 and Serine 473) in response to physiological levels of EGF, which is accompanied by increased AKT tyrosine phosphorylation. SYF cells expressing active PTK6 but not kinase-dead PTK6 showed increased cell proliferation [7].
Knockdown of endogenous PTK6 impairs tumorigenicity of prostate cancer cells
PTK6 is primarily localized within the cytoplasm in the highly tumorigenic PC3 prostate cancer cell line [8]. Stable knockdown of PTK6 in PC3 cells using two different shRNA constructs impaired cell proliferation and colony formation [5]. PTK6 also plays an important role in cell migration, as transient knockdown of PTK6 using siRNAs largely decreased cell migration, which was accompanied by decreased p130CAS phosphorylation and ERK5 activation [8]. Moreover, PTK6 is primarily responsible for the anoikis resistance of metastatic prostate cancer PC3 cells, which are able to maintain an ~80% survival rate under suspended growth conditions for 8 days [9]. Knockdown of PTK6 in PC3 cells induced apoptosis under suspended growth conditions, which was accompanied by reduced FAK and AKT activation [9]. Interestingly, while PTK6 and SRC share several common substrates, knockdown of SRC in PC3 cells had only a small impact on the anoikis resistance of PC3 cells, and knockdown of both SRC and PTK6 did not have a synergistic effect, indicating PTK6 is the key player in protecting prostate cancer cells from anoikis [9]. These data demonstrate the oncogenic role of PTK6 in various aspects of tumorigenicity, including growth, survival and cell migration, and suggest that targeting PTK6 might be beneficial in treating human prostate cancer.
Introducing PTK6 into the nucleus of PC3 cells negatively regulates growth
Knockdown of PTK6 in PC3 cells led to growth inhibition, supporting a growth-promoting role for endogenous cytoplasmic PTK6 in this prostate cancer cell line. However, reintroduction of ectopic PTK6 into the PC3 cell nucleus by addition of a SV40 nuclear localization signal to the amino-terminus of PTK6 also led to growth inhibition that was dependent upon PTK6 activity [5]. These data suggest that nuclear PTK6 is important for maintaining proper growth regulation in the normal prostate.
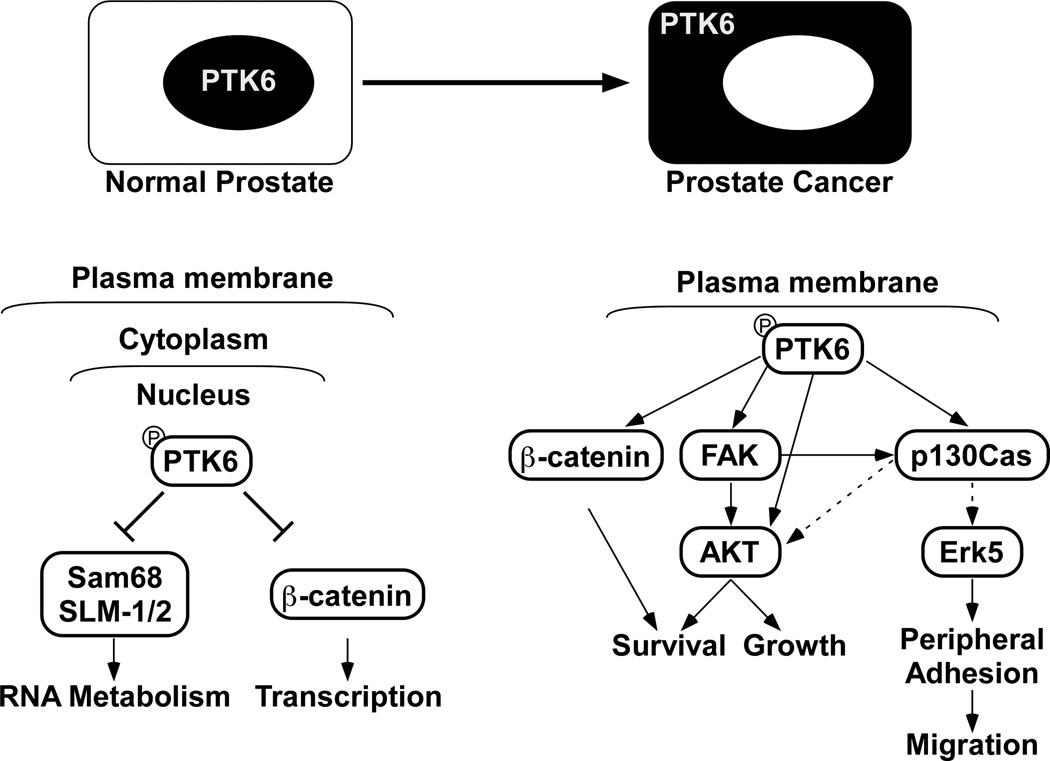
Different functions for PTK6 within the nucleus and at the membrane could be explained by its access to unique sets of substrates and interacting proteins in the different compartments. Sam68, a known PTK6 substrate [60], is a multifunctional KH-domain containing protein with several proline-rich motifs that has context-specific RNA-binding and adaptor protein functions [61, 62]. It is largely nuclear in normal prostate and prostate tumors [18], and is phosphorylated on tyrosine residues by nuclear-targeted PTK6 [5]. Sam68 has been implicated in development of prostate cancer. Expression of Sam68 was increased in 35% of prostate cancer samples examined and knockdown of Sam68 led to reduced proliferation of cultured prostate cancer cells [63]. Nuclear PTK6 in the normal prostate may inhibit growth by increasing tyrosine phosphorylation of Sam68 and related RNA-binding proteins, leading to inhibition of their RNA binding activities and affecting different aspects of RNA metabolism, including RNA stability, translation and transport [5, 60]. Following its relocalization to the cytoplasm/membrane in prostate cancer, PTK6 would no longer have access to nuclear Sam68 (Figure 1).
Fig. 1. Distinct functions for PTK6 in the nucleus and at the plasma membrane.
In normal prostate epithelial cells, total and active PTK6 is enriched in the nucleus where it has access to specific nuclear substrates and interacting proteins. These include the RNA binding proteins Sam68 [60] and SLM1 and SLM2 [48], as well as β-catenin [49]. PTK6 inhibits the RNA binding abilities of Sam68 [60] and the transcriptional activities of β-catenin [49]. Relocalization of PTK6 from nucleus to cytoplasm in prostate cancer facilitates its activation at the membrane. Active PTK6 can then phosphorylate and activate its cytoplasmic and membrane substrates including p130CAS [8], FAK [9], AKT [7], and β-catenin [49] to promote cancer cell proliferation, survival and migration.
PTK6 can also interact with and phosphorylate β-catenin on multiple tyrosine residues [49]. β-catenin, which has distinct membrane and nuclear functions, regulates both cell adhesion and transcription [64]. It is a key component of the WNT signaling pathway that is involved in promoting prostate cancer progression [65]. Nuclear-targeted PTK6 was shown to inhibit β-catenin/TCF regulated transcription in colon cancer cells [49]. In contrast, expression of membrane-targeted active PTK6 led to increased β-catenin/TCF regulated transcription [49]. At the membrane, PTK6 can phosphorylate β-catenin on tyrosine residue 142, inhibiting its membrane and promoting its nuclear functions [49] (Figure 1). Differential regulation of β-catenin would provide another mechanism by which nuclear PTK6 could inhibit, while membrane associated PTK6 could promote prostate epithelial cell proliferation, and requires further investigation.
Potential mechanisms underlying PTK6 translocation: a role for the alternative PTK6 transcript ALT-PTK6?
PTK6 lacks both membrane and nuclear targeting signals, but it is largely nuclear in normal prostate epithelium and cytoplasmic/membrane associated in prostate cancer [18]. A particularly interesting question that still needs to be addressed is, how does PTK6 translocate from the nucleus to the cytoplasm during the development/progression of prostate cancer? We determined that translocation is not due to aberrant nuclear export mediated by Crm-1/exportin-1, because inhibiting Crm-1/exportin-1 using leptomycin B did not result in nuclear accumulation of PTK6 in PC3 cells. Moreover, overexpression of Sam68, a nuclear binding partner of PTK6, was not sufficient to bring PTK6 into the nucleus [5].
Interestingly, expression of ALT-PTK6, which is encoded by an alternatively spliced PTK6 transcript, is able to affect the intracellular localization of exogenous PTK6 in HEK-293 cells. ALT-PTK6 contains the intact SH3 domain of PTK6 and could compete with PTK6 for binding of specific cytoplasmic substrates and binding partners. In cotransfection studies, increased ectopic expression of ALT-PTK6 led to increased nuclear localization of full length active PTK6 and inhibition of β-catenin/TCF transcription. ALT-PTK6 may compete with full length PTK6 for binding to a cytoplasmic retention factor that has yet to be identified, allowing PTK6 to reenter the nucleus and inhibit β-catenin-induced transcription [6]. ALT-PTK6 expression is decreased in human prostate cancer samples, which may enhance the cytoplasmic retention and oncogenic signaling of PTK6 [6].
CONCLUSIONS
Functions of PTK6 are highly context dependent and distinct in normal tissues and cancer. Increased growth, impaired enterocyte differentiation [22], and impaired DNA-damage induced apoptosis [24] led to the hypothesis that PTK6 might act as a tumor suppressor in the intestine. Surprisingly, Ptk6 null mice were resistant to AOM/DSS induced colon tumorigenesis refuting this notion [25]. However, recent studies suggest that PTK6 functions as a tumor suppressor in esophageal squamous cell cancers [32]. In contrast, oncogenic roles for PTK6, particularly in breast cancers (reviewed in [10, 41]) are supported by a large body of data. Identified inhibitors of PTK6 that may have therapeutic potential under the appropriate conditions include the BRAF inhibitor PLX4032 [66], Geldanamycin, an Hsp90 inhibitor [67], SOCS3 [37], and a series of substituted imidazo[1,2-a]pyrazin-8-amines [68].
The prostate provides a unique model where the importance of PTK6 expression levels and intracellular localization can be addressed. Increased expression, altered intracellular localization and activation at the plasma membrane are characteristics of PTK6 in prostate cancer. These characteristics may promote its access to distinct cytoplasmic and membrane associated substrates and interacting proteins including AKT [7], β-catenin [49], p130CAS [8], FAK [9], and members of the Sam68 family of RNA-binding proteins [48, 60]. PTK6-mediated phosphorylation and and/or association with these proteins could lead to the activation of oncogenic signaling pathways that are involved in regulating cell growth, survival and migration, therefore promoting prostate cancer progression. The relocalization of PTK6 and its activation in different cellular compartments could serve as a marker for cancer staging and prognosis. Recently, we demonstrated that targeting PTK6 enhances the response of colon cancer cells to chemotherapeutic agents [69]. Studies in prostate cancer cells suggest that targeting PTK6 in prostate cancer may also have significant therapeutic benefits.
ACKNOWLEDGEMENTS
A.L.T. is supported by NIH grant DK44525, DOD grant PC110752 and pilot funding from the University of Illinois Cancer Center. We thank Patrick Brauer (University of Toronto), Zebin Wang, Maoyu Peng, Priya Mathur, and Ben Hitchinson (University of Illinois at Chicago) for their helpful comments and suggestions.
REFERENCES
- 1.ACS. Cancer Facts & Figures 2012. Atlanta: American Cancer Society; 2012. [Google Scholar]
- 2.Saad F. Src as a therapeutic target in men with prostate cancer and bone metastases. BJU international. 2009;103:434–440. doi: 10.1111/j.1464-410X.2008.08249.x. [DOI] [PubMed] [Google Scholar]
- 3.Edwards J. Src kinase inhibitors: an emerging therapeutic treatment option for prostate cancer. Expert Opin Investig Drugs. 2010;19:605–614. doi: 10.1517/13543781003789388. [DOI] [PubMed] [Google Scholar]
- 4.Chang YM, Kung HJ, Evans CP. Nonreceptor tyrosine kinases in prostate cancer. Neoplasia. 2007;9:90–100. doi: 10.1593/neo.06694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brauer PM, Zheng Y, Wang L, Tyner AL. Cytoplasmic retention of protein tyrosine kinase 6 promotes growth of prostate tumor cells. Cell Cycle. 2010;9:4190–4199. doi: 10.4161/cc.9.20.13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brauer PM, Zheng Y, Evans MD, Dominguez-Brauer C, Peehl DM, Tyner AL. The Alternative Splice Variant of Protein Tyrosine Kinase 6 Negatively Regulates Growth and Enhances PTK6-Mediated Inhibition of beta-Catenin. PLoS ONE. 2011;6:e14789. doi: 10.1371/journal.pone.0014789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng Y, Peng M, Wang Z, Asara JM, Tyner AL. Protein tyrosine kinase 6 directly phosphorylates AKT and promotes AKT activation in response to epidermal growth factor. Molecular and Cellular Biology. 2010;30:4280–4292. doi: 10.1128/MCB.00024-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng Y, Asara JM, Tyner AL. Protein-tyrosine Kinase 6 Promotes Peripheral Adhesion Complex Formation and Cell Migration by Phosphorylating p130 CRK-associated Substrate. The Journal of Biological Chemistry. 2012;287:148–158. doi: 10.1074/jbc.M111.298117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng Y, Gierut J, Wang Z, Miao J, Asara JM, Tyner AL. Protein Tyrosine Kinase 6 Protects Cells from Anoikis by Directly Phosphorylating Focal Adhesion Kinase and Activating AKT. Oncogene. 2012 doi: 10.1038/onc.2012.427. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brauer PM, Tyner AL. Building a better understanding of the intracellular tyrosine kinase PTK6 - BRK by BRK. Biochimica et biophysica acta. 2010;1806:66–73. doi: 10.1016/j.bbcan.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee H, Kim M, Lee KH, Kang KN, Lee ST. Exon-intron structure of the human PTK6 gene demonstrates that PTK6 constitutes a distinct family of non-receptor tyrosine kinase. Mol Cells. 1998;8:401–407. [PubMed] [Google Scholar]
- 12.Serfas MS, Tyner AL. Brk, Srm, Frk and Src42A form a distinct family of intracellular Src-like tyrosine kinases. Oncol Res. 2003;13:409–419. doi: 10.3727/096504003108748438. [DOI] [PubMed] [Google Scholar]
- 13.Lee ST, Strunk KM, Spritz RA. A survey of protein tyrosine kinase mRNAs expressed in normal human melanocytes. Oncogene. 1993;8:3403–3410. [PubMed] [Google Scholar]
- 14.Mitchell PJ, Barker KT, Martindale JE, Kamalati T, Lowe PN, Page MJ, Gusterson BA, Crompton MR. Cloning and characterisation of cDNAs encoding a novel non-receptor tyrosine kinase, brk, expressed in human breast tumours. Oncogene. 1994;9:2383–2390. [PubMed] [Google Scholar]
- 15.Siyanova EY, Serfas MS, Mazo IA, Tyner AL. Tyrosine kinase gene expression in the mouse small intestine. Oncogene. 1994;9:2053–2057. [PubMed] [Google Scholar]
- 16.Vasioukhin V, Serfas MS, Siyanova EY, Polonskaia M, Costigan VJ, Liu B, Thomason A, Tyner AL. A novel intracellular epithelial cell tyrosine kinase is expressed in the skin and gastrointestinal tract. Oncogene. 1995;10:349–357. [PubMed] [Google Scholar]
- 17.Llor X, Serfas MS, Bie W, Vasioukhin V, Polonskaia M, Derry J, Abbott CM, Tyner AL. BRK/Sik expression in the gastrointestinal tract and in colon tumors. Clin Cancer Res. 1999;5:1767–1777. [PubMed] [Google Scholar]
- 18.Derry JJ, Prins GS, Ray V, Tyner AL. Altered localization and activity of the intracellular tyrosine kinase BRK/Sik in prostate tumor cells. Oncogene. 2003;22:4212–4220. doi: 10.1038/sj.onc.1206465. [DOI] [PubMed] [Google Scholar]
- 19.Petro BJ, Tan RC, Tyner AL, Lingen MW, Watanabe K. Differential expression of the non-receptor tyrosine kinase BRK in oral squamous cell carcinoma and normal oral epithelium. Oral Oncol. 2004;40:1040–1047. doi: 10.1016/j.oraloncology.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Vasioukhin V, Tyner AL. A role for the epithelial-cell-specific tyrosine kinase Sik during keratinocyte differentiation. Proc Natl Acad Sci U S A. 1997;94:14477–14482. doi: 10.1073/pnas.94.26.14477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang TC, Jee SH, Tsai TF, Huang YL, Tsai WL, Chen RH. Role of breast tumour kinase in the in vitro differentiation of HaCaT cells. Br J Dermatol. 2005;153:282–289. doi: 10.1111/j.1365-2133.2005.06604.x. [DOI] [PubMed] [Google Scholar]
- 22.Haegebarth A, Bie W, Yang R, Crawford SE, Vasioukhin V, Fuchs E, Tyner AL. Protein tyrosine kinase 6 negatively regulates growth and promotes enterocyte differentiation in the small intestine. Molecular and Cellular Biology. 2006;26:4949–4957. doi: 10.1128/MCB.01901-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haegebarth A, Nunez R, Tyner AL. The Intracellular Tyrosine Kinase Brk Sensitizes Non-Transformed Cells to Inducers of Apoptosis. Cell Cycle. 2005;4:1239–1246. doi: 10.4161/cc.4.9.1965. [DOI] [PubMed] [Google Scholar]
- 24.Haegebarth A, Perekatt AO, Bie W, Gierut JJ, Tyner AL. Induction of protein tyrosine kinase 6 in mouse intestinal crypt epithelial cells promotes DNA damage-induced apoptosis. Gastroenterology. 2009;137:945–954. doi: 10.1053/j.gastro.2009.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gierut J, Zheng Y, Bie W, Carroll RE, Ball-Kell S, Haegebarth A, Tyner AL. Disruption of the Mouse Protein Tyrosine Kinase 6 Gene Prevents STAT3 Activation and Confers Resistance to Azoxymethane. Gastroenterology. 2011;141:1371–1380. e2. doi: 10.1053/j.gastro.2011.06.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barker KT, Jackson LE, Crompton MR. BRK tyrosine kinase expression in a high proportion of human breast carcinomas. Oncogene. 1997;15:799–805. doi: 10.1038/sj.onc.1201241. [DOI] [PubMed] [Google Scholar]
- 27.Lofgren KA, Ostrander JH, Housa D, Hubbard GK, Locatelli A, Bliss RL, Schwertfeger KL, Lange CA. Mammary gland specific expression of Brk/PTK6 promotes delayed involution and tumor formation associated with activation of p38 MAPK. Breast cancer research : BCR. 2011;13:R89. doi: 10.1186/bcr2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin HS, Berry GJ, Fee WE, Jr, Terris DJ, Sun Z. Identification of tyrosine kinases overexpressed in head and neck cancer. Arch Otolaryngol Head Neck Surg. 2004;130:311–316. doi: 10.1001/archotol.130.3.311. [DOI] [PubMed] [Google Scholar]
- 29.Schmandt RE, Bennett M, Clifford S, Thornton A, Jiang F, Broaddus RR, Sun CC, Lu KH, Sood AK, Gershenson DM. The BRK Tyrosine Kinase is Expressed in High-Grade Serous Carcinoma of the Ovary. Cancer Biol Ther. 2006;5:1136–1141. doi: 10.4161/cbt.5.9.2953. [DOI] [PubMed] [Google Scholar]
- 30.Fan C, Zhao Y, Liu D, Zhang X, Wang E. Detection of Brk expression in non-small cell lung cancer: clinicopathological relevance. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2011;32:873–880. doi: 10.1007/s13277-011-0188-z. [DOI] [PubMed] [Google Scholar]
- 31.Cho NL, Lin CI, Du J, Whang EE, Ito H, Moore FD, Jr, Ruan DT. Global tyrosine kinome profiling of human thyroid tumors identifies Src as a promising target for invasive cancers. Biochemical and biophysical research communications. 2012;421:508–513. doi: 10.1016/j.bbrc.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 32.Ma S, Bao JY, Kwan PS, Chan YP, Tong CM, Fu L, Zhang N, Tong AH, Qin YR, Tsao SW, Chan KW, Lok S, Guan XY. Identification of PTK6, via RNA sequencing analysis, as a suppressor of esophageal squamous cell carcinoma. Gastroenterology. 2012;143:675–686. e1–e12. doi: 10.1053/j.gastro.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Liu L, Gao Y, Qiu H, Miller WT, Poli V, Reich NC. Identification of STAT3 as a specific substrate of breast tumor kinase. Oncogene. 2006;25:4904–4912. doi: 10.1038/sj.onc.1209501. [DOI] [PubMed] [Google Scholar]
- 34.Weaver AM, Silva CM. Signal transducer and activator of transcription 5b: a new target of breast tumor kinase/protein tyrosine kinase 6. Breast Cancer Res. 2007;9:R79. doi: 10.1186/bcr1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ikeda O, Miyasaka Y, Sekine Y, Mizushima A, Muromoto R, Nanbo A, Yoshimura A, Matsuda T. STAP-2 is phosphorylated at tyrosine-250 by Brk and modulates Brk-mediated STAT3 activation. Biochem Biophys Res Commun. 2009;384:71–75. doi: 10.1016/j.bbrc.2009.04.076. [DOI] [PubMed] [Google Scholar]
- 36.Ikeda O, Sekine Y, Mizushima A, Nakasuji M, Miyasaka Y, Yamamoto C, Muromoto R, Nanbo A, Oritani K, Yoshimura A, Matsuda T. Interactions of STAP-2 with Brk and STAT3 participate in cell growth of human breast cancer cells. J Biol Chem. 2010;285:38093–38103. doi: 10.1074/jbc.M110.162388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao Y, Cimica V, Reich NC. Suppressor of cytokine signaling 3 inhibits breast tumor kinase activation of STAT3. The Journal of Biological Chemistry. 2012;287:20904–20912. doi: 10.1074/jbc.M111.334144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen HY, Shen CH, Tsai YT, Lin FC, Huang YP, Chen RH. Brk activates rac1 and promotes cell migration and invasion by phosphorylating paxillin. Mol Cell Biol. 2004;24:10558–10572. doi: 10.1128/MCB.24.24.10558-10572.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen CH, Chen HY, Lin MS, Li FY, Chang CC, Kuo ML, Settleman J, Chen RH. Breast tumor kinase phosphorylates p190RhoGAP to regulate rho and ras and promote breast carcinoma growth, migration, and invasion. Cancer Res. 2008;68:7779–7787. doi: 10.1158/0008-5472.CAN-08-0997. [DOI] [PubMed] [Google Scholar]
- 40.Irie HY, Shrestha Y, Selfors LM, Frye F, Iida N, Wang Z, Zou L, Yao J, Lu Y, Epstein CB, Natesan S, Richardson AL, Polyak K, Mills GB, et al. PTK6 regulates IGF-1-induced anchorage-independent survival. PLoS ONE. 2010;5:e11729. doi: 10.1371/journal.pone.0011729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ostrander JH, Daniel AR, Lange CA. Brk/PTK6 signaling in normal and cancer cell models. Current opinion in pharmacology. 2010;10:662–669. doi: 10.1016/j.coph.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Locatelli A, Lofgren KA, Daniel AR, Castro NE, Lange CA. Mechanisms of HGF/Met signaling to Brk and Sam68 in breast cancer progression. Horm Cancer. 2012;3:14–25. doi: 10.1007/s12672-011-0097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ludyga N, Anastasov N, Gonzalez-Vasconcellos I, Ram M, Hofler H, Aubele M. Impact of protein tyrosine kinase 6 (PTK6) on human epidermal growth factor receptor (HER) signalling in breast cancer. Mol Biosyst. 2011;7:1603–1612. doi: 10.1039/c0mb00286k. [DOI] [PubMed] [Google Scholar]
- 44.Li X, Lu Y, Liang K, Hsu JM, Albarracin C, Mills GB, Hung MC, Fan Z. Brk/PTK6 sustains activated EGFR signaling through inhibiting EGFR degradation and transactivating EGFR. Oncogene. 2012;31:4372–4383. doi: 10.1038/onc.2011.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aubele M, Auer G, Walch AK, Munro A, Atkinson MJ, Braselmann H, Fornander T, Bartlett JM. PTK (protein tyrosine kinase)-6 and HER2 and 4, but not HER1 and 3 predict long-term survival in breast carcinomas. Br J Cancer. 2007;96:801–807. doi: 10.1038/sj.bjc.6603613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xie T, G DA, Lamb JR, Martin E, Wang K, Tejpar S, Delorenzi M, Bosman FT, Roth AD, Yan P, Bougel S, Di Narzo AF, Popovici V, Budinska E, et al. A comprehensive characterization of genome-wide copy number aberrations in colorectal cancer reveals novel oncogenes and patterns of alterations. PLoS ONE. 2012;7:e42001. doi: 10.1371/journal.pone.0042001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim HI, Lee ST. Oncogenic Functions of PTK6 Are Enhanced by Its Targeting to Plasma Membrane But Abolished by Its Targeting to Nucleus. Journal of biochemistry. 2009;146:133–139. doi: 10.1093/jb/mvp050. [DOI] [PubMed] [Google Scholar]
- 48.Haegebarth A, Heap D, Bie W, Derry JJ, Richard S, Tyner AL. The nuclear tyrosine kinase BRK/Sik phosphorylates and inhibits the RNA-binding activities of the Sam68-like mammalian proteins SLM-1 and SLM-2. J Biol Chem. 2004;279:54398–54404. doi: 10.1074/jbc.M409579200. [DOI] [PubMed] [Google Scholar]
- 49.Palka-Hamblin HL, Gierut JJ, Bie W, Brauer PM, Zheng Y, Asara JM, Tyner AL. Identification of beta-catenin as a target of the intracellular tyrosine kinase PTK6. J Cell Sci. 2010;123:236–245. doi: 10.1242/jcs.053264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu YP, Landsittel D, Jing L, Nelson J, Ren B, Liu L, McDonald C, Thomas R, Dhir R, Finkelstein S, Michalopoulos G, Becich M, Luo JH. Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J Clin Oncol. 2004;22:2790–2799. doi: 10.1200/JCO.2004.05.158. [DOI] [PubMed] [Google Scholar]
- 51.Cabodi S, Tinnirello A, Di Stefano P, Bisaro B, Ambrosino E, Castellano I, Sapino A, Arisio R, Cavallo F, Forni G, Glukhova M, Silengo L, Altruda F, Turco E, et al. p130Cas as a new regulator of mammary epithelial cell proliferation, survival, and HER2-neu oncogene-dependent breast tumorigenesis. Cancer Res. 2006;66:4672–4680. doi: 10.1158/0008-5472.CAN-05-2909. [DOI] [PubMed] [Google Scholar]
- 52.Schlaepfer DD, Mitra SK. Multiple connections link FAK to cell motility and invasion. Curr Opin Genet Dev. 2004;14:92–101. doi: 10.1016/j.gde.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 53.Petch LA, Bockholt SM, Bouton A, Parsons JT, Burridge K. Adhesion-induced tyrosine phosphorylation of the p130 src substrate. J Cell Sci. 1995;108(Pt 4):1371–1379. doi: 10.1242/jcs.108.4.1371. [DOI] [PubMed] [Google Scholar]
- 54.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 55.Tachibana K, Urano T, Fujita H, Ohashi Y, Kamiguchi K, Iwata S, Hirai H, Morimoto C. Tyrosine phosphorylation of Crk-associated substrates by focal adhesion kinase. A putative mechanism for the integrin-mediated tyrosine phosphorylation of Crk-associated substrates. J Biol Chem. 1997;272:29083–29090. doi: 10.1074/jbc.272.46.29083. [DOI] [PubMed] [Google Scholar]
- 56.Sakai R, Iwamatsu A, Hirano N, Ogawa S, Tanaka T, Mano H, Yazaki Y, Hirai H. A novel signaling molecule, p130, forms stable complexes in vivo with v-Crk and v-Src in a tyrosine phosphorylation-dependent manner. Embo J. 1994;13:3748–3756. doi: 10.1002/j.1460-2075.1994.tb06684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Avizienyte E, Wyke AW, Jones RJ, McLean GW, Westhoff MA, Brunton VG, Frame MC. Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signalling. Nature cell biology. 2002;4:632–638. doi: 10.1038/ncb829. [DOI] [PubMed] [Google Scholar]
- 58.Castro NE, Lange CA. Breast tumor kinase and extracellular-signal-regulated kinase 5 mediate Met receptor signaling to cell migration in breast cancer cells. Breast Cancer Res. 2010;12:R60. doi: 10.1186/bcr2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen R, Kim O, Yang J, Sato K, Eisenmann KM, McCarthy J, Chen H, Qiu Y. Regulation of Akt/PKB activation by tyrosine phosphorylation. J Biol Chem. 2001;276:31858–31862. doi: 10.1074/jbc.C100271200. [DOI] [PubMed] [Google Scholar]
- 60.Derry JJ, Richard S, Valderrama Carvajal H, Ye X, Vasioukhin V, Cochrane AW, Chen T, Tyner AL. Sik (BRK) phosphorylates Sam68 in the nucleus and negatively regulates its RNA binding ability. Mol Cell Biol. 2000;20:6114–6126. doi: 10.1128/mcb.20.16.6114-6126.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bielli P, Busa R, Paronetto MP, Sette C. The RNA binding protein Sam68 is a multifunctional player in human cancer. Endocrine-related cancer. 2011;18:R91–R102. doi: 10.1530/ERC-11-0041. [DOI] [PubMed] [Google Scholar]
- 62.Richard S. Reaching for the stars: Linking RNA binding proteins to diseases. Advances in experimental medicine and biology. 2010;693:142–157. [PubMed] [Google Scholar]
- 63.Busa R, Paronetto MP, Farini D, Pierantozzi E, Botti F, Angelini DF, Attisani F, Vespasiani G, Sette C. The RNA-binding protein Sam68 contributes to proliferation and survival of human prostate cancer cells. Oncogene. 2007;26:4372–4382. doi: 10.1038/sj.onc.1210224. [DOI] [PubMed] [Google Scholar]
- 64.Bienz M. beta-Catenin: a pivot between cell adhesion and Wnt signalling. Curr.Biol. 2005;15:R64–R67. doi: 10.1016/j.cub.2004.12.058. [DOI] [PubMed] [Google Scholar]
- 65.Kypta RM, Waxman J. Wnt/beta-catenin signalling in prostate cancer. Nat Rev Urol. 2012 doi: 10.1038/nrurol.2012.116. In press. [DOI] [PubMed] [Google Scholar]
- 66.Sala E, Mologni L, Truffa S, Gaetano C, Bollag GE, Gambacorti-Passerini C. BRAF silencing by short hairpin RNA or chemical blockade by PLX4032 leads to different responses in melanoma and thyroid carcinoma cells. Molecular cancer research : MCR. 2008;6:751–759. doi: 10.1158/1541-7786.MCR-07-2001. [DOI] [PubMed] [Google Scholar]
- 67.Kang SA, Cho HS, Yoon JB, Chung IK, Lee ST. Hsp90 rescues PTK6 from proteasomal degradation in breast cancer cells. The Biochemical journal. 2012;447:313–320. doi: 10.1042/BJ20120803. [DOI] [PubMed] [Google Scholar]
- 68.Zeng H, Belanger DB, Curran PJ, Shipps GW, Jr, Miao H, Bracken JB, Arshad Siddiqui M, Malkowski M, Wang Y. Discovery of novel imidazo[1,2-a]pyrazin-8-amines as Brk/PTK6 inhibitors. Bioorg Med Chem Lett. 2011;21:5870–5875. doi: 10.1016/j.bmcl.2011.07.101. [DOI] [PubMed] [Google Scholar]
- 69.Gierut JJ, Mathur PS, Bie W, Han J, Tyner AL. Targeting Protein Tyrosine Kinase 6 Enhances Apoptosis of Colon Cancer Cells Following DNA Damage. Molecular cancer therapeutics. 2012;11:2311–2320. doi: 10.1158/1535-7163.MCT-12-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]