Abstract
Polymorphisms in noncoding regions of the vasopressin 1a receptor gene (Avpr1a) are associated with a variety of socioemotional characteristics in humans, chimpanzees, and voles, and may impact behavior through site-specific variation in gene expression. The socially monogamous prairie vole offers a unique opportunity to study such neurobiological control of individual differences in complex behavior. Vasopressin 1a receptor (V1aR) signaling is necessary for the formation of the pair bond in males, and prairie voles exhibit greater V1aR binding in the reward-processing ventral pallidum than do asocial voles of the same genus. Diversity in social behavior within prairie voles has been correlated to natural variation in neuropeptide receptor expression in specific brain regions. Here we use RNA interference to examine the causal relationship between intraspecific variation in V1aR and behavioral outcomes, by approximating the degree of naturalistic variation in V1aR expression. Juvenile male prairie voles were injected with viral vectors expressing shRNA sequences targeting Avpr1a mRNA into the ventral pallidum. Down-regulation of pallidal V1aR density resulted in a significant impairment in the preference for a mated female partner and a reduction in anxiety-like behavior in adulthood. No effect on alloparenting was detected. These data demonstrate that within-species naturalistic-like variation in V1aR expression has a profound effect on individual differences in social attachment and emotionality. RNA interference may prove a useful technique to unite the fields of behavioral ecology and neurogenetics to perform ethologically relevant studies of the control of individual variation and offer insight into the evolutionary mechanisms leading to behavioral diversity.
Keywords: vasopressin, Microtus ochrogaster, ventral pallidum, pair bond, RNAi, evolution
Introduction
Central vasopressin 1a receptors (V1aR) regulate a variety of socioemotional behaviors including conspecific recognition and memory (Landgraf et al., 1995; Bielsky et al., 2004), territoriality (Ferris et al., 1984; Albers, 2012), aggression (Winslow et al., 1993; Gobrogge et al., 2009), paternal care (Wang et al., 1994), and anxiety-related behaviors (Wigger et al., 2004). Prairie voles (Microtus ochrogaster) provide an excellent opportunity to study the neural mechanisms underlying complex social behavior as they form life-long socially monogamous relationships with their mates. In male prairie voles, V1aR activation is necessary and sufficient for pair bonding and other behaviors associated with monogamy (Winslow and Insel, 1993; Wang et al., 1994; Liu et al., 2001; Lim and Young, 2004; Donaldson et al., 2010).
Comparative studies with closely related, asocial Microtus species have suggested that variation in V1aR distribution in the brain is associated with diversity in social behavioral phenotypes (Insel et al., 1994; Young et al., 1997). Non-monogamous vole species have lower densities of V1aR in the ventral pallidum (VP) than do prairie voles and infusion of a V1aR antagonist into the VP of male prairie voles prevents mating-induced partner preferences, a laboratory index of pair bonding (Lim and Young, 2004). In addition, over-expressing the V1aR gene (Avpr1a) in the VP of male meadow voles confers the ability to develop partner preferences in this promiscuous species (Lim et al., 2004).
Variation in length and composition of a microsatellite upstream of the Avpr1a gene between monogamous and promiscuous vole species and has been hypothesized to impact gene expression and behavior (Young et al., 1999; Hammock and Young, 2004; Young and Hammock, 2007). Similar allelic variation in microsatellites upstream of the AVPR1A gene in both humans and chimpanzees has been linked to relationship quality (Walum et al., 2008), personality traits (Ebstein, 2006; Meyer-Lindenberg et al., 2009; Ebstein et al., 2012; Hopkins et al., 2012), and autism spectrum disorders (Kim et al., 2002; Wassink et al., 2004; Yirmiya et al., 2006). Together, these observations suggest that natural variation in neural expression patterns, not protein structure, significantly contributes to diversity in sociobehavioral traits.
Significant natural variation in mating and parenting strategies also exist within prairie voles, with some males taking on a life of “wandering” from mate to mate while others become “residents” and faithfully defend their partner (Getz and Carter, 1993; Roberts et al., 1998). Individual variation in V1aR expression has been correlated with behavioral variation and Avpr1a microsatellite composition in males (Phelps and Young, 2003; Hammock et al., 2005; Hammock and Young, 2005; Ophir et al., 2008). However, the exact contributions of the microsatellite to both inter and intra-specific regional V1aR expression and behavior remains controversial (Hammock et al., 2005; Hammock and Young, 2005; Fink et al., 2006; Young and Hammock, 2007; Ophir et al., 2008). While previous studies demonstrated that ectopically expressing V1aR alters social behaviors (Young et al., 1999; Pitkow et al., 2001; Lim et al., 2004; Gobrogge et al., 2009), there has been no direct, causal demonstration that endogenous variation in Avpr1a expression is behaviorally relevant. Prairie voles typically display an approximately 30–40% difference in ventral pallidal V1aR density between upper and lower quartiles in both laboratory and wild-caught populations (Barrett and Young, unpublished observations; Hammock et al., 2005, Phelps and Young, 2003). Here, we use RNA interference (RNAi) to manipulate endogenous Avpr1a expression and examined social behavior in male prairie voles to determine whether a naturalistic degree of variation in V1aR expression causally generates behavioral diversity within a species.
Materials and Methods
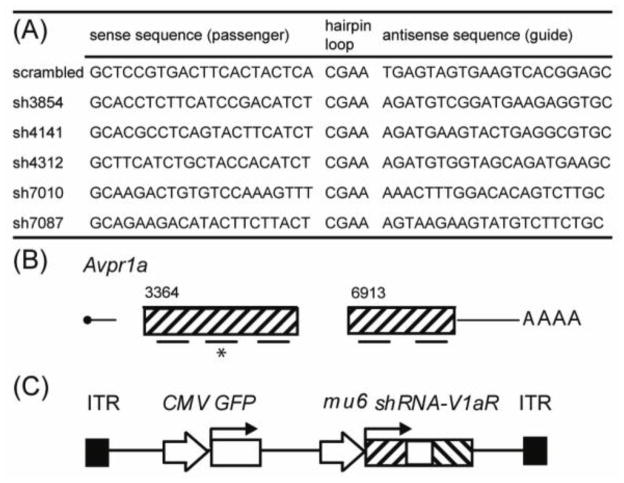
Development of short hairpin RNA sequences
Short hairpin RNA sequences (shRNAs) targeting the prairie vole Avpr1a coding sequence were designed using Invitrogen’s BLOCK-iT™ RNAi Designer software (Invitrogen, Carlsbad, CA). To minimize off-target effects, a BLAST search against other Microtus sequences was performed to verify specificity. We subsequently confirmed using a BLAST search of the recently available M. ochrogaster genome (taxid:79684) that the shRNA sequences do not target any gene other than the Avpr1a. Three sequences targeting exon 1 and two targeting exon 2 were chosen based on the efficacy predicted by the algorithm (Figure 1A, B). One scrambled control sequence, composed of the same nucleotide makeup as sh4141 but in a different order, was also designed and does not target any known mammalian gene sequence. Numbers in the shRNA descriptors indicate the distance from the beginning of the M. ochrogaster Avpr1a locus as numerated in the Genbank entry Accession number AF069304.2. Sequences were cloned into a pENTR™/U6 vectors, with polymerase III-dependent U6 driven expression, sequenced to identify clones with proper insertion, and tested for knockdown efficacy in vitro before in vivo use. Similar shRNA technology has been used before to successfully knockdown target gene expression in other rodents (Musatov et al., 2006; Tiscornia et al., 2006; Garza et al., 2008).
Figure 1. Design of short hairpin RNA sequences.
Candidate shRNA sequences consisted of a 21nt sense sequence followed by a 4nt hairpin loop and a 21nt antisense guide strand that is complementary to the target Avpr1a sequence (A). Three shRNA sequences against exon 1 and two against exon 2 of the prairie vole Avpr1a gene were designed (B) and ultimately inserted into an adenoassociated viral vector driving shRNA expression with murine U6 and co-expressing GFP under control of a uniquitous CMV promoter (C). The asterisk indicates sh4141, the sequence used to generate virus for behavioral testing.
Cell culture testing of shRNA sequences
To identify the most effective shRNA sequence, an in vitro reporter assay tested the ability of 5 shRNA plasmids to knockdown a prairie vole Avpr1a-GFP fusion protein in comparison to a scrambled control sequence. As specific antibodies against the V1aR are not available, the amount of GFP immunoreactivity relative to scrambled transfected controls was used to assess knockdown of the V1aR. The day before transfection, HEK 293T cells were plated with Dulbecco Modified Eagle Medium (DMEM, Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum and incubated at 37°C in 6-well plates (5ml media and 2E6 cells per well). Once at 70–80% confluence, 3ml media was removed and cells were cotransfected with 6μg of RNAi entry vector, 2μg of a target AVPR1A-GFP fusion protein vector, and 10μl Lipofectamine-2000 Invitrogen transfection reagent brought up to 500μl with Opti-MEM (Invitrogen, Carlsbad, CA). After 4–6hr, 2.5ml DMEM was added back. Knockdown was assessed 48hr after co-transfection in three experiments, each with duplicate wells. Half of each sample was saved for analysis by either western blot or quantitative real-time PCR.
Western blot analysis
Cells were washed with cold phosphate-buffered saline (PBS) and homogenized in Ripa Lysis Buffer (sc-24948, Santa Cruz Biotechnology). After determining protein concentration by BCA assay (Pierce, Rockford, IL), 5mg of denatured protein extract was loaded into NuPAGE 4–12% Bis-Tris gel (Invitrogen, Carlsbad, CA). Proteins were separated by gel electrophoresis and transferred to a nitrocellulose membrane. The blot was blocked with 2% milk and incubated in 1:1000 rabbit anti-GFP (A6455, Invitrogen, Carlsbad, CA) for 80min. Primary antibody reactivity was detected by incubation 1:10,000 anti-rabbit fractionate and detected using Pierce SuperSignal West Pico Chemiluminescence followed by 20s film exposure. Blots were stripped for 15 minutes with Pierce ReStore Plus stripping solution and incubated in primary antibody specific for GAPDH (10R-G1099, Fitzgerald, Acton, MA) for 80 minutes at a dilution of 1:5000. Primary antibody reactivity was detected by incubation in secondary antibody and detected using Pierce SuperSignal West Pico Chemiluminescence followed by 15s film exposure. Images were taken with Alpha Innotech imager, and optical density measuremets were analyzed using NIH Image J Software. In vitro knockdown efficiencies were determined relative to the scrambled control.
Quantitative real time PCR (qRT PCR)
RT PCR was performed on transfected cells using the POWER SYBR Green Cells-to CT Kit according the manufacturer’s protocol (Invitrogen, Carlsbad, CA). Briefly, cells were washed with cold PBS, counted, and mixed with cell lysis buffer. Genomic DNA was degraded by treatment with DNAse. Complementary DNA (cDNA) was synthesized by reverse transcription under the following cycling conditions: 60 min at 37°C; 5 min at 95°C; hold at 4°C. Quantitative PCR was performed with 4ul cDNA, 10mM forward and reverse primers, and 12.5ul Power SYBR Green PCR master mix on an AB 7500 (Applied Biosystems, Carlsbad, CA) under the following cycling conditions: 10 min at 95°C, 40 cycles of 15s at 95°C and 1min at 60°C. Both avpr1a (FWD 5-ACTGAGCACGCCTCAGTACTTCAT-3, RVS 5-TGAAGCCATAGCAGGTACCCAAGA-3) and human GAPDH control (FWD 5-AGCCTCAAGATCATCAGCAATGCC-3 and RVS 5-TGTGGTCATGAGTCCTTCCACGAT-3) primer sets were used. Relative quantification to cDNA from scrambled transfected cells using the ΔΔCT method was used to compare expression across samples. The reference gene GAPDH was used as an internal control for each individual, and samples from three replicate experiments were run in duplicate. Fold changes in expression were calculated as 2(−ΔΔCT).
Animal handling and care
Animals were laboratory-bred male prairie voles, derived from a field-caught stock originating from Illinois. The colony is systematically outbred to maintain genetic diversity. Animals were maintained on a 14:10 h light:dark cycle with lights on at 07:00 at 22°C with access to food (Purina high-fiber rabbit chow) and water ad libitum. Breeder housing consisted of large ventilated cages (34 × 30 × 19 cm) lined with bedding (bed-o-cob, Maumee, OH, USA). At 21 days of age, male subjects were injected with either scrambled (n=13) or shRNA-Avpr1a (n=14) virus, and weaned into same-sex same-treatment pairs or trios in smaller (30 × 18 × 19 cm) cages. No animals were exposed to subsequent litters in their natal group. Subjects remained undisturbed until adulthood, at which point they underwent testing for partner preference, alloparental behavior, and anxiety-like behavior in the elevated plus maze (EPM), with a two-week interval between each test (PND60-100). Stimulus animals were estrogen-primed sexually-experienced ovariectomized adult female voles, and served as either “partner” or “stranger” in the partner preference test (see below). All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Emory University Institutional Animal Care and Use Committee.
Viral production
Sequences verified to knockdown in vitro were made into adeno-associated viral (AAV) vectors as previously described (Ross et al., 2009). The constructs consisted of a cytomegalovirus (CMV) promoter driving GFP expression, followed by a high-output murine U6 Pol III element driving expression of the shRNA sequence. The AAV vectors used in this study are of the AAVrh10 serotype, which is highly neurotropic and exhibits particularly strong gene transfer into brain neurons. AAV vector stock titters were 1013 infectious particles per ml as determined by real-time PCR. To verify their knockdown ability in vivo, 1μl of either AAV-sh4141 and AAV-sh7087 were injected unilaterally into the mediodorsal thalamus (MD Thal; coordinates −0.14 anteroposterior (A/P), −0.11 mediolateral (M/L), −0.35 dorsoventral (D/V)) in 3 animals each with methods as described below. Brains were harvested at least 2 months after injection.
Viral vector injection
In order to knockdown V1aR expression long term, males were injected at the time of weaning with shRNA-pvAvpr1a and expression lasted until adulthood. Juvenile male prairie voles were bilaterally injected into the ventral pallidum with 1μl of sh4141, the AAV virus found to most efficiently produce V1aR knockdown in vitro and in vivo, from here on simply referred to as shRNA-pvAvpr1a (n=14) or the scrambled AAV control (n=13). AAV infusions were performed under isoflurane anesthesia in a Kopf stereotax fitted with an Ultra Micro Pump II (World Precision Instruments, Sarasota, FL) apparatus and a 26 gauge 5ml Hamilton syringe. Ventral pallidum coordinates were modified from (Lim et al., 2004) for juveniles and verified using dye injections. Coordinates relative to bregma in the anterior-posterior and medial-lateral planes and the top of the skill at the injection site in the dorsal-ventral plane were +0.14 A/P, ±0.08 M/L, and −0.54 D/V. Virus was infused at a rate of 3nl/s, and the needle was left in place for 3 min after the injection to prevent capillary action. The incision was closed with absorbable sutures (Vicryl 5-0 Ethicon, Piscataway, NJ). Animals were group housed until adulthood when they underwent behavioral testing. Subjects were paired with a female for a total of 3d for partner preference testing. As males display mating-induced aggression, animals were singly housed for the remainder of behavioral testing. After all of the behavioral studies, brains were harvested and analyzed for V1aR knockdown using autoradiography and injection site accuracy using GFP fluorescence. Preliminary studies indicated that expression was stable after 14d.
Behavioral testing
Partner preference test
After 1.5–2 months of viral expression, adult males (range 64–78 days of age) were paired with age-matched, sexually-experienced stimulus females in a clean cage. Females were ovariectomized and induced into estrus with 2μg estradiol benzoate (Sigma-Aldrich BP958) daily for 3 days prior to pairing as previously described (Modi and Young, 2011). The cohabitation period was video-recorded for 3 hr to verify mating. Four animals that did not mate were excluded from the analysis (three from the control group and one from the shRNA-pvAvpr1a group). Partner preference was performed 24hr after pairing using a paradigm previously described (Williams et al., 1994). After 24hr of cohabitation, the male subjects were put in the center of a 3-chambered arena with “partner” females tethered to one end and novel “stranger” females tethered to the other. “Stranger” females underwent the same sociosexual experience as the “partners.” The experimental animal was allowed to freely move throughout the arena. Time spent in immobile social contact (huddling) with each female during this 3hr session is scored with an automated system (SocialScan 2.0, Clever Sys Inc., Reston, VA, USA) as previously described (Ahern et al., 2009). After testing, males were placed back in the cohabitation cages alone, and females were returned to the colony.
Alloparental behavior
Because exposure to pups has been shown to alter subsequent social behavior (Stone et al., 2010), alloparental care was assessed after partner preference testing. Two weeks after partner preference testing, males (age range 75–95d) were tested for their willingness to care for novel, genetically unrelated stimulus pups. Subject animals were habituated to the testing room for at least 1 hr. They were then placed in a large clean cage (37 × 31 × 19cm) lined with bedding (bed-o-cob) and allowed to acclimate for 10min. The test began when 2 novel pups (PND2-5) were placed in the opposite corner. For 10min the latency, frequency, and duration of behaviors including pup licking, carrying, and crouching over pups were scored live by an observer blind to the experimental condition (Stopwatch+, Center for Behavioral Neuroscience, GA; http://www.cbn-atl.org/research/stopwatch.shtml). Testing immediately stopped if pups were attacked. A latency of 600s was assigned if subjects did not approach the pups for the duration of the test. Males were categorized as “alloparental” if they spent a total of at least 5s licking and 30s crouching over the pups without attacking (Lonstein and De Vries, 1999; Olazábal and Young, 2005).
Elevated Pluz Maze
At least two weeks after alloparental testing, males (age range 100–109) were tested for anxiety-like behavior in an EPM as previously described (Ahern and Young, 2009). Males had been isolated for at least 4 wks prior to EPM testing. Animals were habituated in a room adjacent to the behavioral testing room for at least 1 hr prior to testing, which occurred between 09:00 and 12:00 h. The apparatus was raised 80 cm above the floor, and consisted of 2 closed arms (50 × 6 × 15.5 cm), 2 open arms (50 × 6 × 0.6 cm), and a center platform (6 × 6 cm). The subjects were placed in the center, and behavior was scored from above using an automated scoring system (SocialScan 2.0, Clever Sys Inc., Reston, VA, USA) for 5 min. The arena was cleaned with 5% EtOH between animals. Arms were further divided into distal and proximal subsections, as willingness to enter the distal portion of the open arm may be a more potent measure of anxiety (Wright et al., 1992; Fernandes and File, 1996; Garcia-Cairasco et al., 1998). Raw measurements included duration, frequency, and distance in each subsection (open, distal open, closed, distal closed, center) and total distance moved in the entire arena. Measures relevant to anxiety and exploration were then calculated as follows: percent entries in the distal open arms [entries into distal open arms/(entries into distal open arms + entries into distal closed arms) × 100], percent duration in the distal open arm [time in distal open arms/(time in distal open arms + time in distal closed arms) × 100]. The same calculations were performed for percent time and entries into the entire open arms.
Brain harvest and processing
At the conclusion of behavioral testing, subject males were euthanized with CO2 inhalation in their home cage. Brains were rapidly removed from the skull, frozen in methylbutane chilled with dry ice, and stored at −80°C until sectioning. The brains were sectioned through the ventral pallidum in 4 series at 20μM onto super-frost plus slides (Fisher Scientific, 12-550-15), and maintained at −80°C until assayed.
GFP visualization
One series of slides was coverslipped with Krystalon (EMD Chemicals) to visualize GFP expression at the injection site. Subjects were excluded if GFP expression was not observed in the ventral pallidum. Four shRNA and one scrambled-injected animals were excluded from the autoradiographic and behavioral analyses due to injection misses. One shRNA-injected animal was only successfully targeted unilaterally, but was kept in the analysis. This left samples sizes of n=9 s shRNA-pvAvpr1a and n=9 scrambled for brain and behavioral analyses.
Receptor autoradiography
Receptor binding for V1aR to determine whether group differences in expression was achieved, and for oxytocin receptor (OTR) as a control, was localized by autoradiography as described previously (Young et al., 1997; Ahern et al., 2009; Ross et al., 2009)(Young 1997, Ross 2009, Ahern 2009). Slides were removed from −80°C storage, air dried, dipped in 0.1% paraformaldehyde-PBS (pH 7.4), and washed twice in 50mM Tris buffer (pH 7.4) to remove endogenous OT and AVP. Sections were then incubated for 1 hr in tracer buffer (pH 7.4) containing 0.05nM of either 125I- linier V1a antagonist for V1aR (NEX310050UC Perkin Elmer) or 125I-OVTA for OTR (NEX 254050UC PerkinElmer). Slides were washed four times in 4°C 50mM Tris-MgCl (pH 7.4), followed by a 30min room temperature stirring rinse to remove unbound radioligand. After a dip in dH2O and air drying, slides were exposed to BioMaxMRfilm (Kodak, Rochester, NY) for 72 hr for quantification and a subsequent 8d for higher resolution images. For quantification, 125I autoradiographic standards (ARI 0133A, American Radiolabeled Chemicals) were included in the cassette.
Autoradiograms were quantified as previously described (Phelps and Young 2003, Lim et al., 2005, Ahern 2009). Films were digitized (MTI CCD72 camera) and quantified using AIS software version 6.0 (Imaging Research Inc., Ontario, Canada). Optical density measures of V1aR binding were taken for each brain region bilaterally and averaged across 6–12 sections encompassing the entire ventral pallidum (VP), and 3–4 sections for the olfactory bulb (OB), lateral septum (LS), and S1 region of the cortex by an experimenter blind to the experimental treatment. A total of 9 animals from each group were analyzed for V1aR expression. To assess the specificity of knockdown, OTR binding the in nucleus accumbens (NAcc) shell and core was quantified across 6–12 sections. Tissue was damaged from one shRNA-injected male, thus leaving 8 shRNA and 9 scrambled subjects for OTR analysis. For initial in vivo testing of the virus, 5–10 bilateral sections in the MD Thal were measured for V1aR binding. Optical densities were converted to decompositions per minute (DPM)/mg tissue equivalents using 125I microscale standards on each film. Specific binding was calculated by subtracting non-specific background binding in the S1 region of the cortex from total binding in each region. This region was chosen over the corpus callosum because it contains neuronal cell bodies rather than just axon tracks.
Perfusion and immunohistochemistry
An additional 5 adult animals were injected bilaterally into the ventral pallidum with 1μl of either shRNA-pvAvpr1a or scrambled virus for post-processing with immunohistochemistry for GFP to assess viral spread and neuronal health. At least 2 months after injection, an approximately equivalent length of viral expression in the experimental subjects, animals were sacrificed by isoflurane. Animals were perfused transcardially with 4% paraformaldehyde. Brains were then removed, stored in 4°C sucrose, and sectioned coronally at 40μM on a freezing microtome. Free-floating sections were stored in cryoprotectant in a 24 well tissue culture plate until assayed for GFP expression. Every other section (every 80μM) was run in one assay. Sections were rinsed thoroughly in PBS (pH 7.4) to remove cryoprotectant, pre-blocked in PBS containing 0.3% Triton X-100 and 5% normal goat serum (NGS), and incubated for 44 hr at 4°C with 1:2000 chicken polyclonal anti-GFP (ab13970, Abcam, Cambridge, MA) in PBST with 1%NGS. Tissue was then rinsed with PBST, and incubated in 1:2000 Alexa fluor anti-chicken secondary antibody (A-11039, Invitrogen, Carlsbad, CA) in PBST for 3 hr at 4°C. After washing in PBST followed by PBS, sections were mounted onto superfrost-plus slides, air dried, and coverslipped using vectashield-mounting medium (Vector Labs, Burlingame, CA).
Statistical analyses
Data are presented as mean ± SEM, unless otherwise noted. The normality of behavioral data was tested with a one-sample Kolmogorov-Smirnov test. In the partner preference test, time spent huddling with the partner and stranger animals was analyzed with repeated measures (RM) ANOVA with viral treatment as a between subjects factor and stimulus animal as a within-subjects repeated measure. Planned post-hoc paired t-tests were performed on huddling time with partner versus stranger if a significant interaction effect was detected. A Fisher exact test was used to compare the number of animals that formed a partner preference in each treatment group, with preference defined as spending twice as much time in social contact with the partner over the stranger animal. In addition to group differences, behavioral parameters in the partner preference test after 24hr of cohabitation were correlated with V1aR expression using linear regression. In the alloparental test, one-way ANOVAs were performed after excluding one scrambled injected animal that attacked the pups. A Fisher exact test was used to compare the proportion of animals defined as alloparental between groups. One-way ANOVAs were used to compare behavior in the EPM between groups, and linear regression was used to correlate behavior with V1aR expression. To determine whether group differences in V1aR expression was achieved, independent t-tests were used to compare mean V1aR in the VP, LS, and OB. Similarly, OTR expression in the NAcc core and shell was analyzed with independent t-tests. Statistics were performed with SPSS Statistics 17.0 with significance set at p<0.05 and two-tailed tests unless otherwise noted.
Results
In vitro and in vivo efficacy
The 5 candidate shRNA sequences were tested for knockdown of a target V1aR-GFP fusion protein by examination of immunofluorescence, qRT-PCR, and western blot. V1aR mRNA was suppressed 83–93% and protein levels were reduced 71–88% as compared to scrambled transfected controls (Fig 2A–C). Neurotropic adeno-associated viral vectors were constructed from the shRNA sequences that consistently and efficiently knocked down expression in vitro (sh4141 and sh7087) and tested for knockdown ability in vivo. When injected unilaterally into the MD Thal, AAV-sh4141 and AAV-sh7087 were found to knockdown 41% and 32% of V1aR expression in comparison to the non-injected site (Fig 2D). Thus, we proceeded with AAV-sh4141 in the behavioral experiments.
Figure 2. Characterization of shRNA knockdown in vitro and in vivo.
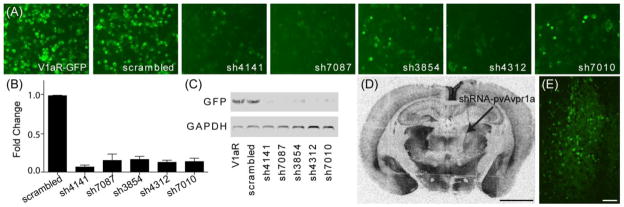
ShRNA plasmids targeting different regions of the prairie vole Avpr1a gene, but not a scrambled control, knocked-down expression of a V1aR-GFP fusion protein as assessed by GFP visualization in HEK 293T cells (A), quantitative RT PCR (B), and western blot (C; V1aR-GFP, 74kDa, GAPDH, 37kDa) Unilateral injection of shRNA(sh4141)-pvAvpr1a into the mediodorsal thalamus resulted in 41% average knockdown as compared to contralateral expression (D, scale bar 1mm). Viral injection sites were verified with GFP visualization (E, scale bar 50μm).
Partner Preference testing results
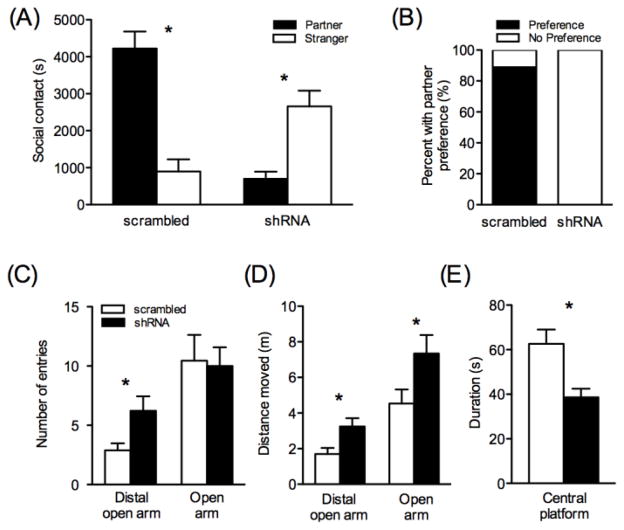
Males injected with shRNA-pvAvpr1a showed significant impairments in partner preference after 24hr of cohabitation with a sexually receptive female (Figure 3A–B). RM-ANOVA revealed that there was a significant main effect of virus (F(1,16)=6.36, p=0.023) between groups, indicating the scrambled males spent more overall time in social contact than did the shRNA males. A significant interaction between virus and time spent with either stimulus animal was detected (F(1,16)= 48.84, p<0.001). There was no main effect of stimulus animal across both groups (F(1,16)=3.25, p=0.09). Planned posthoc pairwise t-tests revealed significantly more time spent huddling with that partner over the stranger in the scrambled group (p=0.001), but significantly more time spent with the stranger over the partner in the shRNA group (p=0.002; Figure 3A). Additionally, significantly more scrambled animals formed a partner preference (p=0.0004, Fisher’s test, Figure 3B). As a measure of activity, distance travelled in the center arena was compared between groups and there was no difference (p=0.336), suggesting differences in testing were not due to overall changes in locomotion. Finally, to compare the degree of V1aR knockdown to partner preference behavior, VP V1aR densities were compared to time spent huddling with the partner or stranger during the test. Across both treatments, V1aR densities significantly predicted time spent with the stranger (R=−0.523, R2=0.273, p=0.026), with high expressing animals spending less time in stranger contact, however no correlation was detected between V1aR and partner contact.
Figure 3. Partner preference and elevated-plus maze behavior in shRNA-pvAvpr1a and scrambled injected male prairie voles.
After 24hr of mated cohabitation with a female, scrambled injected males spent significantly more time in social contact with the partner than the stranger, whereas shRNA injected males spent significantly more time with the stranger (A). The percent of animals forming a partner preference, defined as spending more than twice as much time with the partner than the stranger, differed significantly between groups, with no shRNA injected males forming a preference after 24hr (B). shRNA injected males displayed decreased measures of anxiety-like behaviors, with a higher frequency of distal, but not entire, open arm entries (C), a greater distance moved in the distal and entire open arms (D), and less time spent in the central platform (E). Asterisks indicates p-values <0.05.
Alloparental behavior
One scrambled injected animal was removed from the analyses of mean duration, frequency and latency of paternal care because of attacking the pups. No significant group differences in any paternal behavior measured were observed (p>0.05, one-way ANOVA). Likewise, there was no significant difference between scrambled (n=6/9) and shRNA (n=9/9) in the number of animals that displayed alloparental care (p=1.00, Fisher’s test).
Elevated plus maze
No difference in the time spent in the entire open (F(1,16)=0.116, p=0.737, one-way ANOVA) or closed (F(1,16)=0.895, p=0.358, one-way ANOVA) arms was detected between shRNA and scrambled males. Groups also did not differ in total number of entries into the entire open (F(1,16)=0.027, p=0.871, one-way ANOVA, Figure 3C) or closed (F(1,16)=0.636, p=0.437, one-way ANOVA). However, the shRNA males entered the distal portion of the open arm significantly more than did the scrambled injected males (F(1,16)=6.04, p=0.026, one-way ANOVA, Figure 3C), indicative of decreased anxiety. Similarly, the shRNA group moved a greater distance in both the distal open arm (F(1,16)=7.25, p=0.016, one-way ANOVA, Figure 3D) and the entire open arm (F(1,16)=4.70, p=0.046, one-way ANOVA, Figure 3D). Time spent in the central platform also differed between groups, with scrambled males spending significantly more time in this region (F(1,16)=10.17, p=0.006, one-way ANOVA, Figure 3E). No difference in overall activity in the EPM as measured by total distance moved was detected (F(1,16)=3.44, p=0.082, one-way ANOVA). Duration in the central platform correlated both with V1aR expression in the VP (R=0.495, R2=0.245, p=0.037) and with time spent huddling with the partner after 24hr cohabitation (R=0.512, R2=0.262, p=0.030) across both treatment groups. Only within the shRNA injected group, percent entries into the open arm correlated with pallidal V1aR (R=−0.667, R2=0.444, p=0.045).
V1aR, OTR, and GFP expression
Autoradiography was performed to assess the degree of knockdown in the ventral pallidum. Figure 4 shows representative images of V1aR in the ventral pallidum (A, B), and OTR binding in the NAcc (E, F) between shRNA and scrambled males. Injection site was verified by visualization of GFP expression, and a total of 5 animals were excluded from all analyses (1 scrambled, 4 shRNA). Injection of the shRNA-pvAvpr1a vector led to a 30% reduction in V1aR expression in the ventral pallidum as compared to scrambled controls (p=0.008, t-test, Figure 4D). V1aR binding in the LS (p=0.13) or OB (p=0.477) and OTR binding in the nucleus accumbens (NAcc) core (p=0.28) or shell (p=0.26) was not significantly different between groups (t-tests). Additional animals were injected with either virus to visualize viral spread with immunohistochemistry on perfused tissue. Representative images of GFP expression as visualized by immunohistochemistry shows clear neuronal processes and health of cells, confirming that neurons were successfully targeted and alive (Figure 4G, H). Thus, the observed knockdown of V1aR expression was not due to neuronal death.
Figure 4. Analysis of V1aR knockdown, OTR and GFP expression in shRNA-pvAvpr1a and scrambled injected male prairie voles.
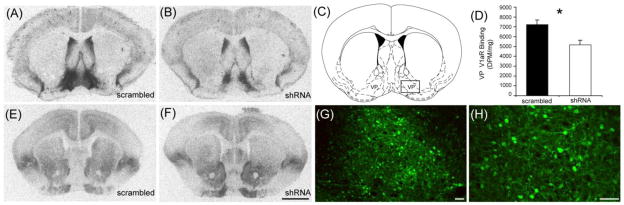
Representative V1aR autoradiograms display a reduction in binding in shRNA-injected animals as compared to control, scramble injected animals (A, B). The square surrounding the ventral pallidum (VP) represents the location of injection sites (reprinted from Paxinos and Watson, 1998) (C). V1aR expression was reduced by 30% in shRNA-injected subjects as compared to controls (D). OTR expression in the nucleus accumbens was unchanged, as seen in representative autoradiograms (E, F). GFP immunoreactivity at the injection site displays typical viral spread (10X, G) and clear expression in neuronal cell bodies and processes (20X, H). Scale bars are 1mm in F and 50μm in G, H. Asterisk indicates p-value <0.05.
Discussion
These results support the hypothesis that variation in Avrp1a expression directly contributes to natural variation in pair bonding and anxiety-related behaviors and are consistent with previous work demonstrating the role of V1aR in promoting partner preferences (Donaldson et al., 2010) and increasing anxiety (Pitkow et al., 2001). We achieved approximately 30% knockdown of V1aR expression in the ventral pallidum of male prairie voles, comparable to the degree of natural variation seen in this brain region (Phelps and Young, 2003; Hammock et al., 2005; Ophir et al., 2008). This chronic, but only partial, down-regulation of expression profoundly impaired partner preference formation after one day of cohabitation, with shRNA males displaying significantly more social contact with a novel stranger female. V1aR density in the VP correlated with time huddling with the stranger during testing, indicating that the degree of intraspecific variation in expression is behaviorally relevant. However, there was no difference in alloparental responsiveness between virally injected males. Vasopressin-mediated pair bond formation and paternal care may be controlled by disparate neural circuitry, as enhancing VP V1aR density in meadow voles also promotes partner preference but not alloparenting (Lim et al., 2004). In the EPM, shRNA males displayed a reduction in anxiety-like behaviors. Those with low V1aR expression entered and explored distal ends of the open arms more willingly, and spent less time in the central platform. Duration in the central platform has previously been associated with anxiety, approach/avoidance conflict and reduced decision making, and is reduced after treatment with anxiolytics (Cruz et al., 1994; Rodgers and Johnson, 1995; Ohl et al., 2001; Olazabal and Young, 2005). Thus, natural variation in Avpr1a expression contributes to variation in both stress and sociality.
Pair bonding is believed to arise from neuropeptide-mediated coding of socially-relevant cues of a partner (e.g. olfactory signatures) converging with dopaminergic and opioid mediated reward and reinforcement pathways activated during mating or social interaction (Young and Wang, 2004; Burkett and Young, 2012). This convergence essentially leads to a conditioned partner preference. Blockade of ventral pallidal V1aR inhibits the formation of a pair bond (Lim and Young, 2004) and over-expression accelerates partner preference formation in male voles (Pitkow et al., 2001; Lim et al., 2004). Thus, V1aR in the VP is thought to strengthen the neural connections encoding social cues and reward, and this process appears directly relate to V1aR density in the VP. A naturalistic-degree of V1aR knockdown in this region significantly impaired male partner preference formation, possibly by reducing the strength of the connection between the neuropeptide’s and reward circuitries. Whether a long-term reduction in V1aR expression has consequences on the expression or function of other receptor systems remains to be explored, although we did not detect a difference in OTR NAcc expression. Interestingly, in kidney cell lines, knockdown of V1aR expression resulted in an impairment of aldosterone function by reducing transport of the mineralcorticoid receptor (Izumi et al., 2011; Hori et al., 2012). Thus, the use of RNAi may help reveal such downstream molecular effects of V1aR in the brain.
In addition to linking to social reward circuitry, the V1aR may also impact social attachment through its regulation of anxiety. Evolutionarily, it has been hypothesized that demanding environments with sparse resources were one of the driving forces behind prairie vole mating systems; and indeed activation of the stress axis promotes male pair bonding (Getz, 1978; DeVries et al., 1996). Infusion of V1aR antisense oligonucleotides or antagonists into the LS or complete knockout of the V1aR reduce anxiety-like behaviors, whereas V1aR over-expression in the LS or VP increases these behaviors (Landgraf et al., 1995; Liebsch et al., 1996; Pitkow et al., 2001; Bielsky et al., 2005). We observed reduced anxiety-like behavior in the males with partial V1aR knockdown. Time spent in the central platform correlated with partner huddling in the partner preference test, suggesting a possible interaction between the V1aR in linking social cues with the reward system, and in regulating emotionality. Diversity in VP V1aR, both between and within species, may be a means to behaviorally adapt to evolutionary pressures.
Although even a 30% reduction in pallidal V1aR expression has behavioral relevance, V1aR densities in certain other socioemotional brain regions can vary even more dramatically between prairie voles, with up to two-fold differences in expression between individuals (Phelps and Young, 2003). Variation in V1aR density in the laterodorsal thalamus, posterior cingulate, LS, and medial amygdala has also been correlated with social behavior in prairie voles (Hammock et al., 2005; Hammock and Young, 2005). V1aR binding in the laterodorsal thalamus and posterior cingulate predicts pair bonding measures in the field and may be important in space use and territoriality in wild populations (Ophir et al., 2008). Within-species variation in the LS of prairie voles may be behaviorally relevant, as males with high levels V1aR exhibit enhanced social motivation to investigate females (Ophir et al., 2009). Examination of region-specific expression both in laboratory and naturalistic contexts can offer insight to the neural mechanisms controlling individual variation in behavior.
RNAi may be a particularly useful tool to study the causal effects of diversity in receptor expression in an ethologically significant context. Antisense oligonucleotides have been used to temporarily knockdown neural V1aR expression previously (Landgraf et al., 1995; Bosch and Neumann, 2008; Kelly et al., 2011). However, to our knowledge, this is the first report to use viral vector mediated RNAi to manipulate endogenous gene expression in the brain in a nontraditional mammalian species. RNAi has several advantages to other approaches used to investigate the role of neuropeptide receptors in regulating behavior. Viral vector mediated over expression, which has been used in voles (Pitkow et al., 2001; Lim et al., 2004), mice (Bielsky et al., 2005) and rat (Landgraf et al., 2003), drives expression of transgenes indiscriminately and ectopically in the infused region, making circuits not involved in the natural regulation of the behavior sensitive to the peptide. Additionally, commonly used pharmacological manipulations are transient and use agents that often diffuse far beyond the site of infusion and bind to related receptor subtypes (Manning et al., 2008), and are optimally designed for studying acute effects, rather than long-term investigations into the role of social neuropeptides. Conventional knockout mouse approaches often involve developmental compensation (Landgraf, 2006). RNAi utilizes an endogenous regulatory pathway to selectively degrade double stranded RNA and results in a reduction, not absolute loss, of the targeted transcript. Consequently, in our study, the reduction in V1aR expression was limited to the region near the injection site, was within the range of what is naturally observed, and only affected cells that endogenously express the AVPR1A.
RNAi also has limitations in terms of potential off-target effects (Grimm et al., 2006; Manjunath et al., 2009). Indeed, there is a possibility that the shRNA sequences used here could target other genes, which could in turn impact behavior. It is important to note that there was no impact on the expression of OTR, which shares high homology with V1aR, in the adjacent NAcc, suggesting that the knockdown was specific to the Avpr1a. Furthermore, a BLAST search of the available M. ochrogaster genome did not reveal any other sequences within the genome matching our shRNA.
Viral spread may have reached surrounding regions including the NAcc and LS as has been reported before (Pitkow et al., 2001), but only significant knockdown in the VP was achieved, and regions without V1aR were not affected. Thus, a viral vector mediated shRNA approach provides an ideal means to examine the impact of natural variation in neuropeptide receptor on behavioral diversity in an ethologically relevant manner, and subjects can be examined over multiple behavioral tests.
Social and mating systems in Microtus are evolutionarily labile and thus must evolve rapidly under pressure of natural selection forces. Even within the prairie vole species, there is substantial variation across geographic locations, environmental conditions, and within shared enclosures (Thomas and Birney, 1979; Roberts et al., 1998). It has been proposed that the polymorphic microsatellite in the 5′ flanking region of the prairie vole Avpr1a, due to inherent instability of the repetitive DNA, may generate diversity in Avpr1a gene expression and therefore behavior. Microsatellite length has been associated with V1aR expression in the ventral pallidum, with long allele males displaying approximately 20% higher levels of binding (Ophir et al., 2008). However, the same report did not find a link between microsatellite length and reproductive success or mating tactics in field enclosures. Although there are conflicting reports on the specific relationship between microsatellite lengths, site-specific V1aR expression, and laboratory and field behavior (Hammock et al., 2005; Hammock and Young, 2005; Ophir et al., 2008; Solomon et al., 2009; Mabry et al., 2011), these discrepancies may be in part be due to microsatellite length being an imperfect indicator of the functional polymorphism, which may be better assessed by analyzing microsatellite sequence. Further, not all studies control for parental genotype and the possibility of epigenetic effects on V1aR expression, as early postnatal experiences can impact adult V1aR (Francis et al., 2002; Bales et al., 2007; Lukas et al., 2010). Our results suggest that if the microsatellite does influence expression in the ventral pallidum, it represents a genomic mechanism for generating diversity in social behaviors. Whether a causal down-regulation of V1aR expression impairs field measures of monogamy warrants further investigation.
Microsatellite sequences in the human AVPR1a 5′ flanking region have been associated with variation in human social behaviors. Specifically the (CT)4-TT-(CT)8-(GT)24 complex repeat (RS3) has been related to autism spectrum disorder, increased amygdala activation, and lower relationship quality in males (Kim et al., 2002; Walum et al., 2008; Meyer-Lindenberg et al., 2009). Interestingly, chimpanzees (Pan troglodytes) are polymorphic for a 5′ deletion flanking the AVPR1A gene that encompasses RS3 (Donaldson et al., 2008), and individuals homozygous for the deletion display sex differences in dominance and conscientiousness not present in heterozygous animals (Hopkins et al., 2012). Thus, microsatellite evidence from humans, chimpanzees, and voles alike suggest that polymorphisms in noncoding regions may mediate rapid evolution in social behavior.
These results indicate that the degree of V1aR expression in the ventral pallidum is a means to control individual differences in socioemotional behaviors, and this diversity may maintain adaptability of prairie vole social structures to changing socioecological conditions. The prairie vole mating system was once purely a focus in field ecology, but has since proven invaluable in the study of the neurogenetic control of sociality. Whether this degree of variation has relevance in field measures of sociality remains to be explored. The advancements in our ability to manipulate the genome of this nontraditional, behaviorally rich species with innovative technologies, including viral vector mediated shRNA and transgenesis (Donaldson et al., 2009; Keebaugh et al., 2012), will advance our ability to explore the mechanistic underpinnings of social behavior in an ecologically relevant context.
Highlights.
RNAi viral vectors were used to recapitulate natural variation in V1aR.
Reducing pallidal V1aR impairs pair bond formation and reduces anxiety.
Variation in V1aR in the ventral pallidum contributes to socio-emotional diversity.
Acknowledgments
The authors would like to thank Kiyoshi Inoue, Zoe Donaldson, James Burkett, and Sara Freeman for training CEB, Pravina Fernandez for laboratory assistance, Lorra Matthews for maintenance of the animal colony, and two anonymous reviewers for comments. This work was supported by NIH grants MH056897 and MH064692 to LJY and an NSF Predoctoral Fellowship to CEB. Additional support was provided by the National Center for Research Resources P51RR165 to YNPRC, which is currently supported by the Office of Research Infrastructure Programs/OD P51OD11132.
Footnotes
Conflict of interest
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahern TH, Modi ME, Burkett JP, Young LJ. Evaluation of two automated metrics for analyzing partner preference tests. J Neurosci Methods. 2009;182:180–188. doi: 10.1016/j.jneumeth.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern TH, Young LJ. The impact of early life family structure on adult social attachment, alloparental behavior, and the neuropeptide systems regulating affiliative behaviors in the monogamous prairie vole (Microtus ochrogaster) Front Behav Neurosci. 2009;3:17. doi: 10.3389/neuro.08.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers HE. The regulation of social recognition, social communication and aggression: vasopressin in the social behavior neural network. Horm Behav. 2012;61:283–292. doi: 10.1016/j.yhbeh.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Bales KL, Plotsky PM, Young LJ, Lim MM, Grotte N, Ferrer E, Carter CS. Neonatal oxytocin manipulations have long-lasting, sexually dimorphic effects on vasopressin receptors. Neuroscience. 2007;144:38–45. doi: 10.1016/j.neuroscience.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47:503–513. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29:483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, I, Neumann D. Brain vasopressin is an important regulator of maternal behavior independent of dams’ trait anxiety. Proc Natl Acad Sci U S A. 2008;105:17139–17144. doi: 10.1073/pnas.0807412105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett JP, Young LJ. The behavioral, anatomical and pharmacological parallels between social attachment, love and addiction. Psychopharmacology (Berl) 2012 doi: 10.1007/s00213-012-2794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz AP, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav. 1994;49:171–176. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- DeVries AC, DeVries MB, Taymans SE, Carter CS. The effects of stress on social preferences are sexually dimorphic in prairie voles. Proc Natl Acad Sci U S A. 1996;93:11980–11984. doi: 10.1073/pnas.93.21.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, Kondrashov FA, Putnam A, Bai Y, Stoinski TL, Hammock EA, Young LJ. Evolution of a behavior-linked microsatellite-containing element in the 5′ flanking region of the primate AVPR1A gene. BMC Evol Biol. 2008;8:180. doi: 10.1186/1471-2148-8-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, Spiegel L, Young LJ. Central vasopressin V1a receptor activation is independently necessary for both partner preference formation and expression in socially monogamous male prairie voles. Behav Neurosci. 2010;124:159–163. doi: 10.1037/a0018094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, Yang SH, Chan AW, Young LJ. Production of germline transgenic prairie voles (Microtus ochrogaster) using lentiviral vectors. Biol Reprod. 2009;81:1189–1895. doi: 10.1095/biolreprod.109.077529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebstein RP. The molecular genetic architecture of human personality: beyond self-report questionnaires. Mol Psychiatry. 2006;11:427–445. doi: 10.1038/sj.mp.4001814. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Knafo A, Mankuta D, Chew SH, Lai PS. The contributions of oxytocin and vasopressin pathway genes to human behavior. Horm Behav. 2012;61:359–379. doi: 10.1016/j.yhbeh.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Fernandes C, File SE. The influence of open arm ledges and maze experience in the elevated plus-maze. Pharmacol Biochem Behav. 1996;54:31–40. doi: 10.1016/0091-3057(95)02171-x. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Albers HE, Wesolowski SM, Goldman BD, Luman SE. Vasopressin injected into the hypothalamus triggers a stereotypic behavior in golden hamsters. Science. 1984;224:521–523. doi: 10.1126/science.6538700. [DOI] [PubMed] [Google Scholar]
- Fink S, Excoffier L, Heckel G. Mammalian monogamy is not controlled by a single gene. Proc Natl Acad Sci U S A. 2006;103:10956–10960. doi: 10.1073/pnas.0602380103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis DD, Young LJ, Meaney MJ, Insel TR. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: gender differences. J Neuroendocrinol. 2002;14:349–353. doi: 10.1046/j.0007-1331.2002.00776.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Cairasco N, Oliveira JA, Wakamatsu H, Bueno ST, Guimaraes FS. Reduced exploratory activity of audiogenic seizures susceptible Wistar rats. Physiol Behav. 1998;64:671–674. doi: 10.1016/s0031-9384(98)00129-2. [DOI] [PubMed] [Google Scholar]
- Garza JC, Kim CS, Liu J, Zhang W, Lu XY. Adeno-associated virus-mediated knockdown of melanocortin-4 receptor in the paraventricular nucleus of the hypothalamus promotes high-fat diet-induced hyperphagia and obesity. J Endocrinol. 2008;197:471–482. doi: 10.1677/JOE-08-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz LL. Speculation on social structure and population cycles of microtine rodents. The Biologist. 1978;60:134–147. [Google Scholar]
- Getz LL, Carter CS. Social organization of the prairie vole (Microtus ochrogaster) J Mammal. 1993;74:44–58. [Google Scholar]
- Gobrogge KL, Liu Y, Young LJ, Wang Z. Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. Proc Natl Acad Sci U S A. 2009;106:19144–19149. doi: 10.1073/pnas.0908620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D, Streetz KL, Jopling CL, Storm TA, Pandey K, Davis CR, Marion P, Salazar F, Kay MA. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature. 2006;441:537–541. doi: 10.1038/nature04791. [DOI] [PubMed] [Google Scholar]
- Hammock EA, Lim MM, Nair HP, Young LJ. Association of vasopressin 1a receptor levels with a regulatory microsatellite and behavior. Genes Brain Behav. 2005;4:289–301. doi: 10.1111/j.1601-183X.2005.00119.x. [DOI] [PubMed] [Google Scholar]
- Hammock EA, Young LJ. Functional microsatellite polymorphism associated with divergent social structure in vole species. Mol Biol Evol. 2004;21:1057–1063. doi: 10.1093/molbev/msh104. [DOI] [PubMed] [Google Scholar]
- Hammock EA, Young LJ. Microsatellite instability generates diversity in brain and sociobehavioral traits. Science. 2005;308:1630–1634. doi: 10.1126/science.1111427. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Donaldson ZR, Young LJ. A polymorphic indel containing the RS3 microsatellite in the 5′ flanking region of the vasopressin V1a receptor gene is associated with chimpanzee (Pan troglodytes) personality. Genes Brain Behav. 2012;11:552–558. doi: 10.1111/j.1601-183X.2012.00799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori K, Nagai T, Izumi Y, Kimura M, Hasuike Y, Nakayama Y, Nanami M, Tokuyama M, Otaki Y, Kuragano T, Kohda Y, Obinata M, Kawahara K, Tanoue A, Tomita K, Nakanishi T, Nonoguchi H. Vasopressin V1a receptor is required for nucleocytoplasmic transport of mineralocorticoid receptor. Am J Physiol Renal Physiol. 2012;303:F1080–1088. doi: 10.1152/ajprenal.00052.2012. [DOI] [PubMed] [Google Scholar]
- Insel TR, Wang ZX, Ferris CF. Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. J Neurosci. 1994;14:5381–5392. doi: 10.1523/JNEUROSCI.14-09-05381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Hori K, Nakayama Y, Kimura M, Hasuike Y, Nanami M, Kohda Y, Otaki Y, Kuragano T, Obinata M, Kawahara K, Tanoue A, Tomita K, Nakanishi T, Nonoguchi H. Aldosterone requires vasopressin V1a receptors on intercalated cells to mediate acid-base homeostasis. J Am Soc Nephrol. 2011;22:673–680. doi: 10.1681/ASN.2010050468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keebaugh AC, Modi ME, Barrett CE, Jin C, Young LJ. Identification of variables contributing to superovulation efficiency for production of transgenic prairie voles (Microtus ochrogaster) Reprod Biol Endocrinol. 2012;10:54. doi: 10.1186/1477-7827-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Kingsbury MA, Hoffbuhr K, Schrock SE, Waxman B, Kabelik D, Thompson RR, Goodson JL. Vasotocin neurons and septal V1a-like receptors potently modulate songbird flocking and responses to novelty. Horm Behav. 2011;60:12–21. doi: 10.1016/j.yhbeh.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Young LJ, Gonen D, Veenstra-VanderWeele J, Courchesne R, Courchesne E, Lord C, Leventhal BL, Cook EH, Jr, Insel TR. Transmission disequilibrium testing of arginine vasopressin receptor 1A (AVPR1A) polymorphisms in autism. Mol Psychiatry. 2002;7:503–507. doi: 10.1038/sj.mp.4001125. [DOI] [PubMed] [Google Scholar]
- Landgraf R. The involvement of the vasopressin system in stress-related disorders. CNS Neurol Disord Drug Targets. 2006;5:167–179. doi: 10.2174/187152706776359664. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Frank E, Aldag JM, Neumann ID, Sharer CA, Ren X, Terwilliger EF, Niwa M, Wigger A, Young LJ. Viral vector-mediated gene transfer of the vole V1a vasopressin receptor in the rat septum: improved social discrimination and active social behaviour. Eur J Neurosci. 2003;18:403–411. doi: 10.1046/j.1460-9568.2003.02750.x. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Gerstberger R, Montkowski A, Probst JC, Wotjak CT, Holsboer F, Engelmann M. V1 vasopressin receptor antisense oligodeoxynucleotide into septum reduces vasopressin binding, social discrimination abilities, and anxiety-related behavior in rats. J Neurosci. 1995;15:4250–4258. doi: 10.1523/JNEUROSCI.15-06-04250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebsch G, Wotjak CT, Landgraf R, Engelmann M. Septal vasopressin modulates anxiety-related behaviour in rats. Neurosci Lett. 1996;217:101–104. [PubMed] [Google Scholar]
- Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF, Young LJ. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429:754–757. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience. 2004;125:35–45. doi: 10.1016/j.neuroscience.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Liu Y, Curtis JT, Wang Z. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster) Behav Neurosci. 2001;115:910–919. doi: 10.1037//0735-7044.115.4.910. [DOI] [PubMed] [Google Scholar]
- Lukas M, Bredewold R, Neumann ID, Veenema AH. Maternal separation interferes with developmental changes in brain vasopressin and oxytocin receptor binding in male rats. Neuropharmacology. 2010;58:78–87. doi: 10.1016/j.neuropharm.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Mabry KE, Streatfeild CA, Keane B, Solomon NG. avpr1a length polymorphism is not associated with either social or genetic monogamy in free-living prairie voles. Anim Behav. 2011;81:11–18. doi: 10.1016/j.anbehav.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjunath N, Wu H, Subramanya S, Shankar P. Lentiviral delivery of short hairpin RNAs. Adv Drug Deliv Rev. 2009;61:732–745. doi: 10.1016/j.addr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning M, Stoev S, Chini B, Durroux T, Mouillac B, Guillon G. Peptide and non-peptide agonists and antagonists for the vasopressin and oxytocin V1a, V1b, V2 and OT receptors: research tools and potential therapeutic agents. Prog Brain Res. 2008;170:473–512. doi: 10.1016/S0079-6123(08)00437-8. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kolachana B, Gold B, Olsh A, Nicodemus KK, Mattay V, Dean M, Weinberger DR. Genetic variants in AVPR1A linked to autism predict amygdala activation and personality traits in healthy humans. Mol Psychiatry. 2009;14:968–975. doi: 10.1038/mp.2008.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi ME, Young LJ. D-cycloserine facilitates socially reinforced learning in an animal model relevant to autism spectrum disorders. Biol Psychiatry. 2011;70:298–304. doi: 10.1016/j.biopsych.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. RNAi-mediated silencing of estrogen receptor {alpha} in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proc Natl Acad Sci U S A. 2006;103:10456–10460. doi: 10.1073/pnas.0603045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohl F, Toschi N, Wigger A, Henniger MS, Landgraf R. Dimensions of emotionality in a rat model of innate anxiety. Behav Neurosci. 2001;115:429–436. [PubMed] [Google Scholar]
- Olazabal DE, Young LJ. Variability in “spontaneous” maternal behavior is associated with anxiety-like behavior and affiliation in naive juvenile and adult female prairie voles (Microtus ochrogaster) Dev Psychobiol. 2005;47:166–178. doi: 10.1002/dev.20077. [DOI] [PubMed] [Google Scholar]
- Ophir AG, Campbell P, Hanna K, Phelps SM. Field tests of cis-regulatory variation at the prairie vole avpr1a locus: association with V1aR abundance but not sexual or social fidelity. Horm Behav. 2008;54:694–702. doi: 10.1016/j.yhbeh.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Ophir AG, Wolff JO, Phelps SM. Variation in neural V1aR predicts sexual fidelity and space use among male prairie voles in semi-natural settings. Proc Natl Acad Sci U S A. 2008;105:1249–1254. doi: 10.1073/pnas.0709116105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir AG, Zheng DJ, Eans S, Phelps SM. Social investigation in a memory task relates to natural variation in septal expression of oxytocin receptor and vasopressin receptor 1a in prairie voles (Microtus ochrogaster) Behav Neurosci. 2009;123:979–991. doi: 10.1037/a0016663. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. San Diego: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- Phelps SM, Young LJ. Extraordinary diversity in vasopressin (V1a) receptor distributions among wild prairie voles (Microtus ochrogaster): patterns of variation and covariation. J Comp Neurol. 2003;466:564–576. doi: 10.1002/cne.10902. [DOI] [PubMed] [Google Scholar]
- Pitkow LJ, Sharer CA, Ren X, Insel TR, Terwilliger EF, Young LJ. Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. J Neurosci. 2001;21:7392–7396. doi: 10.1523/JNEUROSCI.21-18-07392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RL, Miller AK, Taymans SE, Carter CS. Role of social and endocrine factors in alloparental behavior of prairie voles (Microtus ochrogaster) Can J Zool. 1998;76:1862–1868. [Google Scholar]
- Roberts RL, Williams JR, Wang AK, Carter CS. Cooperative breeding and monogamy in prairie voles: influence of the sire and geographical variation. Anim Behav. 1998;55:1131–1140. doi: 10.1006/anbe.1997.0659. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Johnson NJ. Factor analysis of spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety. Pharmacol Biochem Behav. 1995;52:297–303. doi: 10.1016/0091-3057(95)00138-m. [DOI] [PubMed] [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J Neurosci. 2009;29:1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon NG, Richmond AR, Harding PA, Fries A, Jacquemin S, Schaefer RL, Lucia KE, Keane B. Polymorphism at the avpr1a locus in male prairie voles correlated with genetic but not social monogamy in field populations. Mol Ecol. 2009;18:4680–4695. doi: 10.1111/j.1365-294X.2009.04361.x. [DOI] [PubMed] [Google Scholar]
- Stone AI, Mathieu D, Griffin L, Bales KL. Alloparenting experience affects future parental behavior and reproductive success in prairie voles (Microtus ochrogaster) Behav Processes. 2010;83:8–15. doi: 10.1016/j.beproc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JA, Birney EC. Parental Care and Mating System of the Prairie Vole, Microtus ochrogaster(Behav. Ecol Sociobiol. 1979;5:171–186. [Google Scholar]
- Tiscornia G, Singer O, Verma IM. Design and cloning of lentiviral vectors expressing small interfering RNAs. Nat Protoc. 2006;1:234–240. doi: 10.1038/nprot.2006.36. [DOI] [PubMed] [Google Scholar]
- Walum H, Westberg L, Henningsson S, Neiderhiser JM, Reiss D, Igl W, Ganiban JM, Spotts EL, Pedersen NL, Eriksson E, Lichtenstein P. Genetic variation in the vasopressin receptor 1a gene (AVPR1A) associates with pair-bonding behavior in humans. Proc Natl Acad Sci U S A. 2008;105:14153–14156. doi: 10.1073/pnas.0803081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Ferris CF, De Vries GJ. Role of septal vasopressin innervation in paternal behavior in prairie voles (Microtus ochrogaster) Proc Natl Acad Sci U S A. 1994;91:400–404. doi: 10.1073/pnas.91.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassink TH, Piven J, Vieland VJ, Pietila J, Goedken RJ, Folstein SE, Sheffield VC. Examination of AVPR1a as an autism susceptibility gene. Mol Psychiatry. 2004;9:968–972. doi: 10.1038/sj.mp.4001503. [DOI] [PubMed] [Google Scholar]
- Wigger A, Sanchez MM, Mathys KC, Ebner K, Frank E, Liu D, Kresse A, Neumann ID, Holsboer F, Plotsky PM, Landgraf R. Alterations in central neuropeptide expression, release, and receptor binding in rats bred for high anxiety: critical role of vasopressin. Neuropsychopharmacology. 2004;29:1–14. doi: 10.1038/sj.npp.1300290. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. Effects of central vasopressin administration to infant rats. Eur J Pharmacol. 1993;233:101–107. doi: 10.1016/0014-2999(93)90354-k. [DOI] [PubMed] [Google Scholar]
- Wright IK, Heaton M, Upton N, Marsden CA. Comparison of acute and chronic treatment of various serotonergic agents with those of diazepam and idazoxan in the rat elevated X-maze. Psychopharmacology (Berl) 1992;107:405–414. doi: 10.1007/BF02245168. [DOI] [PubMed] [Google Scholar]
- Yirmiya N, Rosenberg C, Levi S, Salomon S, Shulman C, Nemanov L, Dina C, Ebstein RP. Association between the arginine vasopressin 1a receptor (AVPR1a) gene and autism in a family-based study: mediation by socialization skills. Mol Psychiatry. 2006;11:488–494. doi: 10.1038/sj.mp.4001812. [DOI] [PubMed] [Google Scholar]
- Young LJ, Hammock EA. On switches and knobs, microsatellites and monogamy. Trends Genet. 2007;23:209–212. doi: 10.1016/j.tig.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Young LJ, Nilsen R, Waymire KG, MacGregor GR, Insel TR. Increased affiliative response to vasopressin in mice expressing the V1a receptor from a monogamous vole. Nature. 1999;400:766–768. doi: 10.1038/23475. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Young LJ, Winslow JT, Nilsen R, Insel TR. Species differences in V1a receptor gene expression in monogamous and nonmonogamous voles: behavioral consequences. Behav Neurosci. 1997;111:599–605. doi: 10.1037//0735-7044.111.3.599. [DOI] [PubMed] [Google Scholar]