Abstract
Background
Reduced reward learning might contribute to the onset and maintenance of major depressive disorder (MDD). In particular, the inability to utilize rewards to guide behavior is hypothesized to be associated with anhedonia, a core feature and potential trait marker of MDD. Few studies have investigated whether reduced reward learning normalizes with treatment and/or reward learning predicts clinical outcome. Our goal was to test that MDD is characterized by reduced reward learning, especially in the presence of anhedonic symptoms, and to investigate the relationship between reward learning and MDD diagnosis after 8 weeks of treatment.
Methods
Seventy-nine inpatients and 63 healthy controls performed a probabilistic reward task yielding an objective measure of participants’ ability to modulate behaviour as a function of reward. We compared reward responsiveness between depressed patients and controls, as well as high vs. low anhedonic MDD patients. Further, we evaluated whether reward learning deficits predicted persistence of MDD after 8 weeks of treatment.
Results
Relative to controls, MDD patients showed reduced reward learning. Moreover, patients with high anhedonia showed diminished reward learning compared to patients with low anhedonia. Reduced reward learning at study entry increased the odds of a persisting diagnosis of MDD after 8 weeks of treatment (OR: 7.84).
Conclusions
Our findings indicate that depressed patients, especially those with anhedonic features, are characterized by an impaired ability to modulate behaviour as a function of reward. Moreover, reduced reward learning increased the odds for the diagnosis of MDD to persist after 8 weeks of treatment.
Keywords: Reward Learning, Anhedonia, Depression, Outcome
1. Introduction
Anhedonia is a core feature and potential trait marker of major depressive disorder (MDD) (1,2). The presence of anhedonic symptoms has been found to predict poor treatment outcome in MDD (3). However, the precise mechanisms underlying anhedonia in MDD remains poorly understood. Clinical and neurobiological studies over the last 10 years suggest that anhedonia is not a monolithic phenomenon, but can be parsed in (A) a reduction in experienced pleasure (liking reward), (B) a dysfunction in the approach-related system subserving motivated behaviour (wanting reward), and/or (C) disrupted reward learning (4, 5). In particular, evidence suggests that anhedonia in MDD is associated with an inability to respond to positive reinforcers, leading to abnormal reward-based decision-making and impairment in goal-directed behaviour (6, 7). For instance, depressed patients have shown a reduced ability to integrate prior reinforcements and modulate behaviour accordingly (8, 9) and several studies have described altered patterns of reinforcement-related decision making in depressed patients compared to controls (10,11). These behavioral abnormalities have been complemented by reports of dysfunctional activation in MDD within mesocorticolimbic regions critically implicated in reward processing (12–16).
In spite of compelling evidence indicating that MDD is characterized by reduced reward responsiveness, several important questions remain largely unanswered. First, it is unclear whether such dysfunctions are generally present in MDD or might be characteristic of patients reporting anhedonic symptoms in their daily life. Second, few studies have investigated whether blunted reward learning persists after treatment of MDD. Finally, little is known about whether blunted reward learning predicts a persisting MDD diagnosis despite treatments.
The goal of the current study was to address these gaps in the literature. Accordingly, we aimed to assess reward learning in a relatively large sample of treatment-seeking patients with MDD and investigate the association between anhedonia and reward learning within an inpatient depressed sample. In addition, we investigated the relationship between reward learning and clinical outcome after 8 weeks of treatment. To pursue these goals, we used a laboratory-based probabilistic reward task that objectively measures participants’ ability to modulate behaviour as a function of reward (17). We hypothesize that, relative to controls, MDD inpatients would show reduced reward learning toward a more frequently rewarded stimulus, and patients with high anhedonic symptoms would show such dysfunction compared to less anhedonically depressed subjects. Further, we expected that reduced reward learning would predict a persisting diagnosis of MDD after 8 weeks of treatment.
2. Methods and Materials
2.1 Participants
Eighty-three patients meeting DSM-IV criteria for MDD were included. All patients were hospitalized at the University Psychiatric Center of the University of Leuven and were evaluated within their first week of admission. Participants with bipolar disorder, substance-related disorders or any other unstable medical condition were excluded. Almost all patients already started antidepressant treatment before admission. During follow up, treatment was not standardized and patients were treated with psychopharmacology and/or psychotherapy, as clinically appropriate. Sixty-eight healthy volunteers were included. Controls were matched by age and gender. Exclusion criteria for controls were: any current or past psychiatric disorder; past mood disorder; or any other current unstable medical condition including thyroid problems. After 8 weeks, a follow-up session with clinical assessments and re-administration of the reward task took place for the depressed group (controls were tested only at baseline). Participants signed an informed consent and the study was approved by the local ethics committee.
2.2 Task and procedures
2.2.1 Clinical assessment
The Structured Clinical Interview for DSM-IV-TR (SCID-I) (18) was used to ascertain whether patients met DSM-IV criteria for MDD. The (17-item) Hamilton Rating Scale for Depression (HDRS) (19) was used to assess depression severity. Further, all participants completed the Snaith Hamilton Pleasure Scale (SHAPS) (20). The SHAPS is a 14-item questionnaire designed to measure hedonic tone or its absence, anhedonia. Higher scores indicate higher anhedonic symptoms (21).
2.2.2 Reward task
We used a computerized reward learning task rooted in signal detection theory that yields an objective measurement of participant’s ability to modulate behaviour as a function of rewards (6). The task was previously shown to have adequate test-retest reliability (17), as well as convergent and predictive validity. Specifically, among non-clinical samples, reward learning in this task correlated negatively with self-reported anhedonic symptoms and predicted these symptoms 30–40 days later (17, 22). In addition, the task was found to objectively measure reward responsiveness in healthy volunteers (6, 17), as well as MDD (6, 23) and bipolar disorder patients (24). The task is described extensively in Pizzagalli et al. (17).
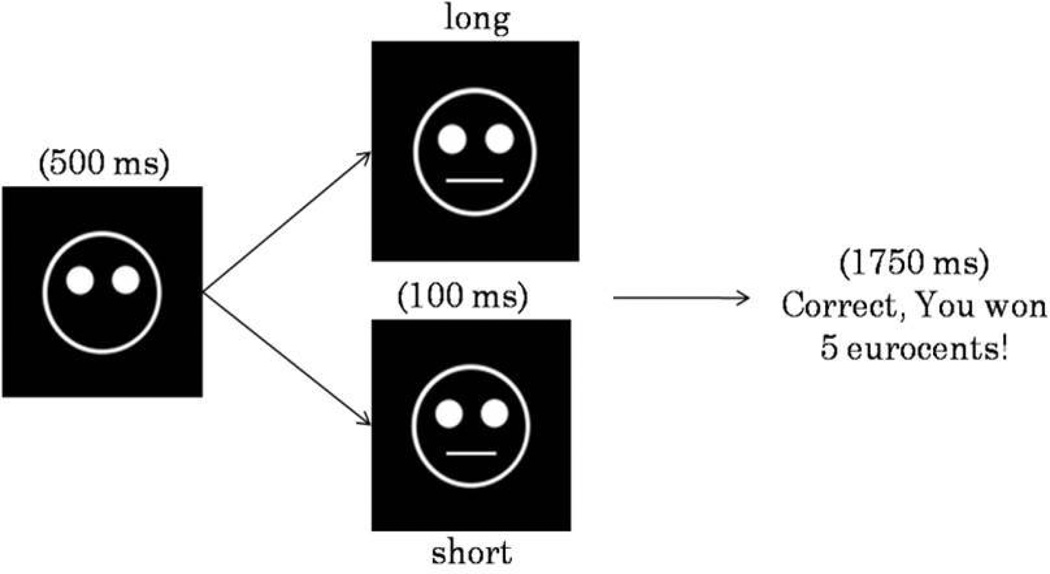
Briefly, the task was presented on a 17” flat screen using E-prime software (version 1.2; Psychology Software Tools Inc., Pittsburgh, Pennsylvania). The task lasted approximately 25 minutes and included 300 trials, divided in 3 blocks of 100 trials, separated by two short breaks (30 sec). Each trial started with a fixation point, shown for 500 msec in the middle of the screen, which was replaced with a mouthless cartoon face. After 500 msec, a short (11.5 mm) or long (13 mm) mouth appeared for 100 msec (Figure 1). Participants were instructed to make a key response to identify which type of mouth had been presented.
Figure 1.
Schematic representation of the probabilistic reward task. In each trial, participants identified (via key press) whether a short or long month stimulus had been presented in the mouthless face on the screen. In approximately 40% of the trials, a positive reinforcement (monetary reward) was presented.
In each block, both stimuli were shown an equal number of times, and a monetary reward feedback was given to approximately 40 correct answers. To induce a response bias, an asymmetrical reinforcer schedule was used, such as correct responses for one mouth (referred to as the ‘rich stimulus’) were rewarded three times more frequently (30 vs. 10) than correct responses of the other mouth (referred to as the ‘lean stimulus’). The reinforcement allocation and key presses were randomized across subjects. Coupled with the small difference between mouth sizes and the brief stimulus exposure time (100 ms), the asymmetric reinforcement schedule reliably induces a response bias among healthy participants (25). Before the task, participants received verbal and written instructions. It was emphasized that the main goal of the task was to win as much money as possible and the total amount of accumulated money (approximately 5 Euro) would be handed out in cash at the end of the experiment. Participants were informed that not all correct responses would result in a monetary reward. However, it was emphasized that more correct identifications would result in more earnings. Due to the unequal frequency of reward feedback, participants with high reward responsiveness were expected to develop a response bias in favor of the rich stimulus. Subjects with low reward responsiveness were expected to develop a smaller or no bias. Participants were informed about the task contingencies and fully debriefed only at the end of the study.
2.3 Data collection and reduction
Prior to analyses, outlier responses were identified using a two-step procedure (17): first trials with reaction time (RT) shorter than 150 ms or longer than 1500 ms were excluded; second, for each participant, trials with mean ± 3 SD were excluded (after applying a log transformation to normalize RT distribution). Moreover, participants with more than 30 outlier trials were excluded from the analyses.
Task performance was assessed by analyzing two main variables: (1) discriminability (DIS), which is an index of participants’ ability to perceptually distinguish between the two stimuli and is used as a proxy of task difficulty, and (2) response bias (RB), which reflects participants’ preference for the stimulus paired with more frequent rewards. RB towards the rich stimulus was used as a measure for reward learning (see formulas) and was our main behavioural variable of interest. To enable RB and DIS calculation in cases with zero in one cell of the formula, 0.5 was added to each cell in the matrix (6).
2.4 Statistical analysis
Analyses were performed with SAS version 9.2. χ2–tests and unpaired t-tests were run to examine group differences in sociodemographic and clinical variables. Several sets of analyses were performed to test study hypotheses. First, to assess overall group differences in the reward task, separate mixed ANOVAs with Group (MDD, controls) and Block (1, 2, 3) as factors were performed for RB and DIS. Second, to test the hypothesis that high anhedonic MDD patients would specifically show blunted reward learning, mixed ANOVAs with MDD Subgroup (low vs. high Anhedonic MDD and Block as factors were run on RB and DIS. MDD subgroups were defined by using a median split of SHAPS score; patients with SHAPS scores >7 were defined as “high anhedonic” and those with SHAPS ≤ 7 as “low anhedonic”. This categorical approach was supplemented by complementary analyses in which anhedonic symptoms were considered as continuum. Thus, Pearson correlations and hierarchical regression analyses were computed to evaluate the relation between SHAPS scores and RB. To directly assess overall reward learning ability, a difference score (∆response bias) between RB over time was calculated (∆RB3-1= RBBlock3 – RBBlock1). Third, to evaluate whether treatment normalized reward learning dysfunction, mixed ANOVAs on RB and DIS were performed comparing controls’ baseline data and patients’ follow-up data (after 8 weeks of treatment). Finally, a logistic regression analysis was performed to test whether baseline reward learning (∆RB3-1) predicted a persisting diagnosis of MDD after 8 weeks of treatment, while controlling for depression severity at baseline. Throughout the ANOVAs, significant effects were followed up with one-way ANOVA entering Block (1–3) as repeated measure for each group separately as well as post-hoc Tukey-Kramer tests. The Greenhouse-Geisser correction was used when appropriate.
3. Results
3.1 Demographic and Clinical data
Eighty-three MDD patients and 68 control subjects were included. We excluded 4 MDD and 5 control subjects due to task non-compliance, leaving 79 patients and 63 control subjects for the analyses. Forty-four patients had high anhedonic symptoms (SHAPS > 7) and 35 patients had low anhedonic symptoms (SHAPS ≤ 7). None of the controls had high anhedonic symptoms. Nineteen patients dropped out before the assessment at 8 weeks, so 60 patients completed the follow-up screening after 8 weeks. Sociodemographic and clinical information of the final sample are listed in Tables 1–3.
Table 1.
Demographic and clinical features of control group (n=63) and depressed group at baseline (n=79).
| Control | MDD | Statistics | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p | ||
| Age | 44.5 | 11.6 | 45.0 | 11.9 | t=0.3 | >0.5 |
| Gender (female/male) | 38/25 | - | 48/31 | - | χ2<0.01 | >0.5 |
| Anxiety disorder (%)a | - | - | 49.4 | - | - | - |
| Age of onset | - | - | 36.0 | 13.0 | - | - |
| Number of episodesb | - | - | 2.5 | 1.9 | - | - |
| Medication (YES/NO)c | - | - | (76/3) | - | - | - |
| HDRS | - | - | 16.9 | 4.9 | - | - |
| SHAPS | 0.4 | 0.9 | 7.3 | 3.6 | t=14.8 | <0.01 |
Anxiety disorder= SCID diagnosis of panic disorder and/or agoraphobia (n = 33), social phobia (n = 5) and/or OCD (n = 1).
Number of episodes= average (34.5% first episode; 32.2% second episode; 33.3% third or higher episode).
Medication: number of patients taking psychopharmacology yes/no (40.5% SSRI; 34.2% SNRI; 5.3% others (e.g., tricyclic, mirtazepine, bupropion)).
HDRS: Hamilton Depression Rating Scale; SHAPS: Snaith Hamilton Pleasure Scale.
Table 3.
Demographic and clinical features of MDD subgroups [Low anhedonic patients (n= 34) and high anhedonic patients (n= 44)] at baseline.
| Low anhedoniaa | High anhedoniab | Statistics | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p | ||
| Age | 47.6 | 11.9 | 43.0 | 11.6 | t= 1.7 | 0.1 |
| Gender (female/male) | 17/17 | - | 30/14 | - | χ2=2.3 | 0.1 |
| Anxiety disorder (%)c | 48.6 | - | 50.0 | - | χ2=0.02 | >0.5 |
| Age of onset | 36.8 | 14.0 | 35.0 | 12.2 | t=0.61 | >0.5 |
| Number of episodesd | 2.3 | 1.9 | 2.6 | 2.0 | t=0.64 | >0.5 |
| Medication (YES/NO)e | 33/1 | 42/2 | χ2=0.01 | >0.5 | ||
| HDRS | 14.7 | 4.5 | 18.6 | 4.5 | t=3.8 | 0.01 |
| SHAPS | 4.0 | 2.4 | 9.8 | 1.9 | t=12.0 | <0.01 |
Low anhedonia=SHAPS score ≤ 7;
High anhedonia=SHAPS > 7.
Anxiety disorder= SCID diagnosis of panic disorder and/or agoraphobia, social phobia and/or OCD.
Number of episodes= average (Low anhedonia = 35.9% first; 38.5.7% second; 25.6% third or more. High anhedonia = 33.3% first; 26.7% second; 40.0% third or more).
Medication: number of patients taking psychopharmacology yes/no (Low anhedonia=35.0% SSRI; 40.0 % SNRI; 25.0 % others. High anhedonia=37.8% SSRI; 35.6 % SNRI; 22.2 % others).
HDRS: Hamilton Depression Rating Scale; SHAPS: Snaith Hamilton Pleasure Scale.
As assessed by the SCID-I, 49.37% suffered from a comorbid anxiety disorder (33 patients with panic disorder and/or agoraphobia, 8 with social phobia and 1 with OCD). Patients with comorbid anxiety did not differ from those without anxiety with regards to age, gender ratio, HDRS scores and SHAPS scores (all ps> 0.07). Furthermore, ANOVA analyses revealed no significant differences in RB and DIS between MDD patients with vs. without anxiety comorbidity (all Fs < 0.36, all ps> 0.35). Age of onset and number of previous MDD episodes were not significant predictors of the overall response bias at baseline or after 8 weeks of treatment. Similarly, HDRS scores did not correlate with overall response bias at baseline. Moreover, ANOVA analysis showed no significant differences in task performance (both in RB and DIS) between MDD patients on SSRI, SNRI or another psychopharmacological treatment at baseline. Among the MDD sample, baseline HDRS and SHAPS scores were significantly correlated (Pearson r = 0.49, p< 0.0001).
3.2 Task performance
3.2.1 Between Group (MDD, control) comparisons
Response Bias
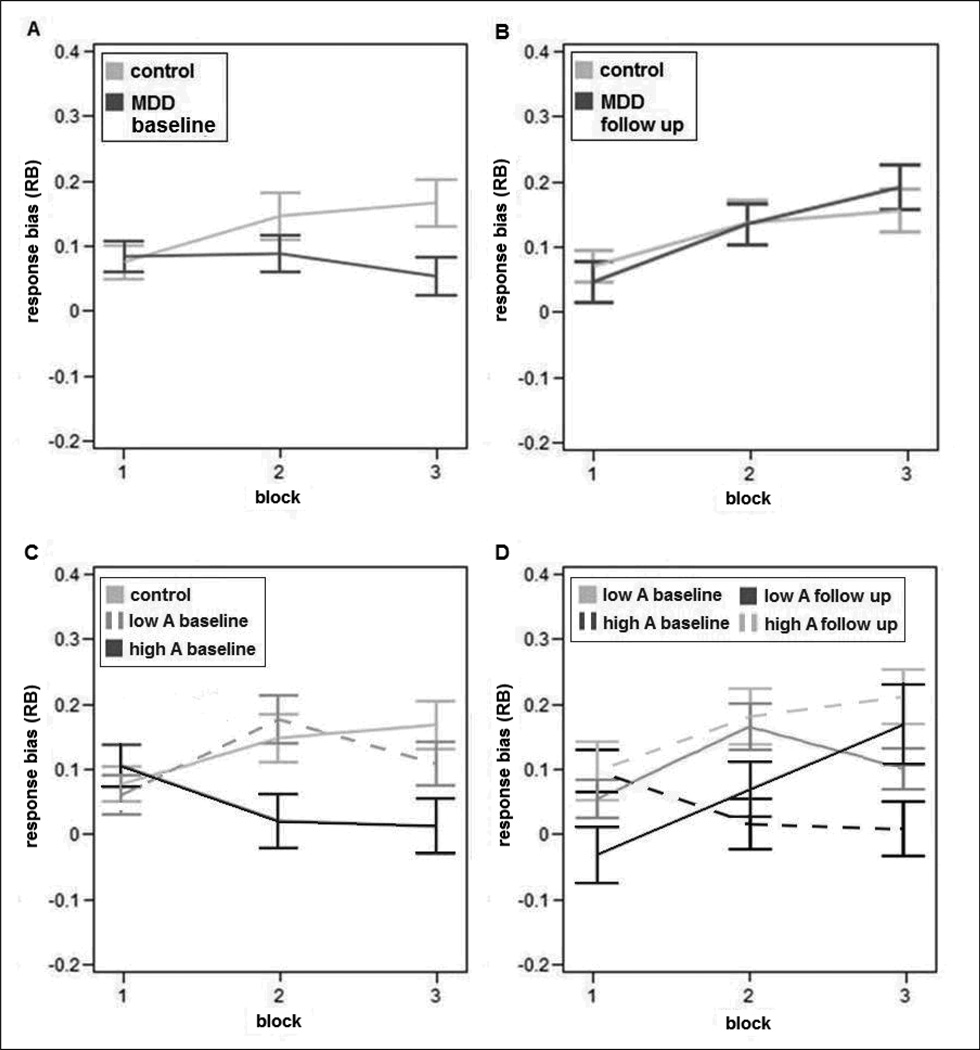
The ANOVA on RB scores revealed a significant Group*Block interaction (F(2,280) = 3.53, p = 0.03, ε = 0.95), due to a reduced reward learning ability in MDD patients compared to controls (Figure 2a). Unpaired t-tests showed that, relative to controls, MDD patients had significantly lower RB in Block 3 (t= 2.45, p= 0.01). Moreover, follow-up one-way ANOVAs showed that the control group had a significant increase of RB over time (Block effect: F(2,124) = 4.83, p = 0.01, ε = 0.91), due to significantly higher RB in Block 2 (t = 2.63, Tukey-Kramer adjusted (Adjp = 0.03) and Block 3 (t = 2.60, Adjp = 0.03) relative to Block 1. An analogous one-way ANOVA in the MDD group revealed no Block effect (p = 0.55), indicating that patients failed to develop a response bias towards the rich stimulus.
Figure 2.
Response bias as a function of blocks for (A) control and MDD group at baseline; (B) control and MDD group at follow up (after 8 weeks); (C) control, low anhedonic (low A) and high anhedonia (high A) group at baseline; and (D) Low A and high A group at baseline, as well as at follow up (after 8 weeks). Error bars denote SEM.
Discriminability
When considering DIS, no significant effect emerged suggesting that controls and MDD subjects performed equally well in discriminating the short from the long mouth (all Fs < 0.56, all ps> 0.55). Moreover, one-way ANOVA on DIS scores revealed no significant effect of Block for either groups (all Fs <0.37, all ps> 0.67).
3.2.2 Within depressed Group (low anhedonia, high anhedonia) comparisons at baseline
Response Bias
High anhedonic patients showed a significantly reduced reward learning over time compared to low anhedonic patients (MDD Group*Block interaction: F(2,152) = 5.15, p = 0.009, ε = 0.94) (Figure 2c). Follow-up one-way ANOVA in the low anhedonic patients revealed significant learning over time (Block effect: F(2,68) = 3.34, p = 0.046, ε = 0.93), due to higher RB in Block 2 relative to Block 1 (t = 2.85, Adjp = 0.02). In contrast, the main effect of Block was not significant in high anhedonic patients, indicating blunted reward learning. Additionally, unpaired t-tests in each Block showed a significant difference between high anhedonic group compared to controls in Block 2 (t= 2.31, p= 0.02) and Block 3 (t= 2.76, p= 0.007). Low anhedonic patients did not differ from controls when comparing RB in each block.
Discriminability
No significant effects emerged (all Fs < 1.63, ps> 0.2), indicating that the two MDD subgroups found the task equally difficult. Similarly, one-way ANOVA on DIS scores revealed no significant effect over time for both depressed subgroups (all Fs < 1.86, ps> 0.17). We also found no group differences, for DIS scores, between controls and low as well as high anhedonic patients (all Fs < 2.20, ps> 0.11).
As shown in Table 3, low and high anhedonic patients were matched in their demographic variables, but the high anhedonic group had more severe depression. To evaluate whether the reward learning difference between the MDD subgroups was specifically related to anhedonia and not simply due to depression severity, we conducted a regression analysis (stepwise selection) of RB in Block 1, 2 and 3, as well as reward learning across the 300 trials ( =RBBlock 3 – RBBlock 1) and 200 trial (= RBBlock 2 – RBBlock 1), with HDRS scores and MDD subgroup (high anhedonia and low anhedonia) as independent variables. Findings revealed that MDD subgroup was the unique predictor of RB in Block 2 (R2 = 0.09, p = 0.008), ΔRB over 200 trials (R2 = 0.13, p = 0.001) and ΔRB over 300 trials (R2 = 0.04, p = 0.07). HDRS scores were not related to the performances on the reward task.
3.2.3 Relationship between reward learning and anhedonic symptoms within MDD group at baseline
For the depressed group, overall reward learning (= RBBlock 3 – RBBlock 1) did not correlate with anhedonic symptoms, as measured by the SHAPS scale. When considering RB learning after 200 trials (= RBBlock 2 – RBBlock 1) in a post hoc analysis, SHAPS scores in the MDD group were significantly related to reward learning in the expected direction (r = −0.33, p = 0.003).
In order to better understand the nature of the relationship between anhedonic symptoms and reward learning, we conducted a linear stepwise regression analysis with ΔRB2-1 as dependent variable to test whether anhedonic symptoms (considered as continuum) predicted reward learning when accounting for depression severity and anxiety comorbidity. To this end, SHAPS and HDRS scores and the presence of an anxiety disorder (dummy coded) were entered in the model. SHAPS scores were the unique predictor of ΔRB2-1 (β = 0.33, t = 3.08, p = 0.003), revealing a significant overall effect (R2 = 0.11, F(1,76) = 9.47, p = 0.003).
3.2.4 Between Group (MDD (follow up), control (baseline)) comparisons
Response bias
When comparing the control group (baseline) with the depressed patients after 8 weeks of treatment, we found no significant group differences (Group*Block interaction: F(2,242) = 0.94, p = 0.40, ε= 0.97), indicating a performance normalization in the MDD group at follow-up (Figure 2b). After treatment, depressed patients showed a significant reward learning effect (Block effect: F(2,118) = 9.33, p = 0.0002, ε= 0.97), due to a significant increase in RB from Block 1 to Block 2 and 3 (all Adjps< 0.03). Similarly, no significant Group or Group*Block effects emerged when comparing RB for the control group (assessed at baseline) with the high and low anhedonic group at follow up (all Fs < 1.82, all ps > 0.17).
For the low anhedonic group, a Time (baseline, follow-up) × Block ANOVA on RB revealed no significant Time effect. However, for the high anhedonic group, the Time (F(2,76)=6.25, p=0.015, ε = 0.99) and Time* Block (F(2,152)=5.27, p=0.0061, ε = 0.99) effects were significant, due to increased RB after 8 weeks of treatment (Figure 2d).
Discriminability
Group*Block ANOVAs on DIS scores at follow up revealed no significant effect (all ps > 0.42).
3.2.5 Relationship between reward learning and MDD diagnosis after 8 weeks of treatment
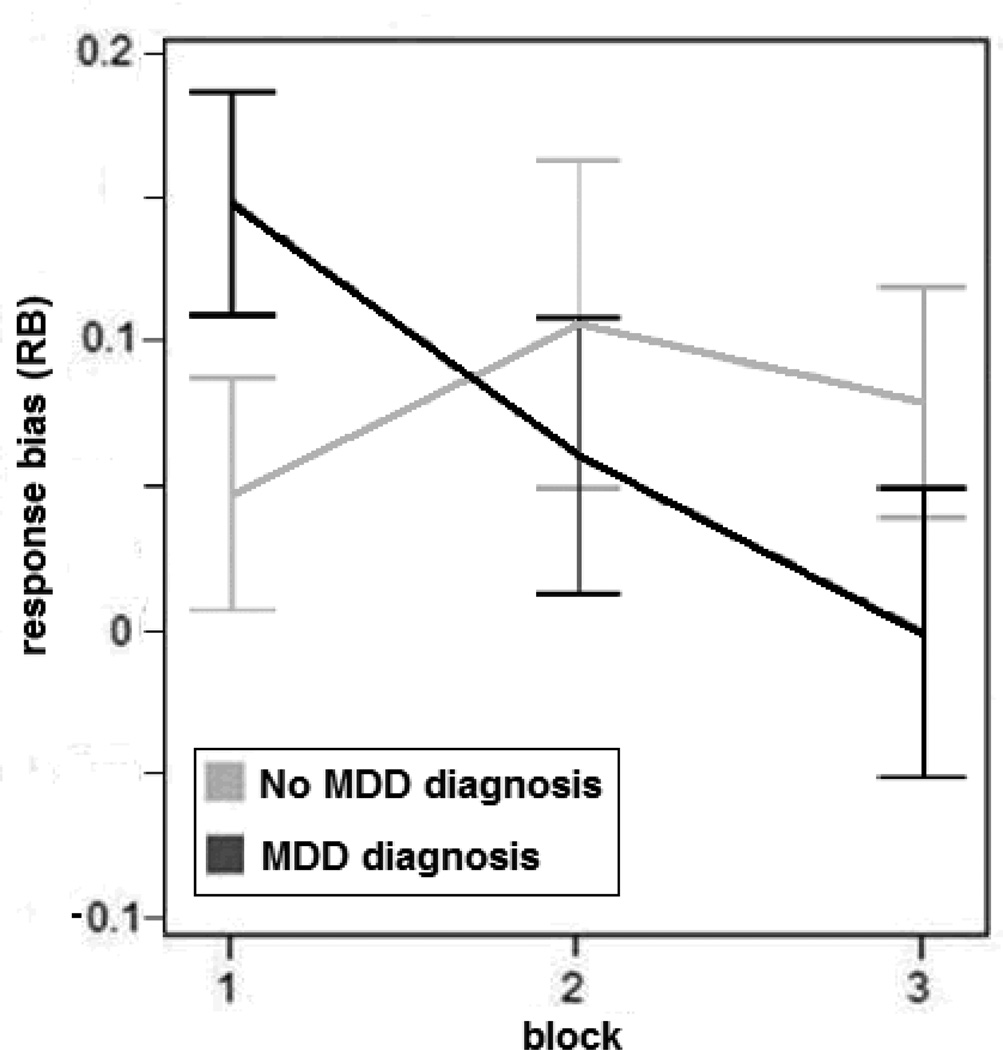
At the follow-up assessment after 8 weeks, 52.5% of patients were still diagnosed with MDD. A logistic regression model was run to predict the persisting diagnosis of MDD after 8 weeks of treatment (YES or NO). The overall reward learning at baseline (calculated as RBBlock 3 – RBBlock 1) was entered as the independent variable in the model. Baseline HDRS scores, anxiety comorbidity, age and gender were entered as covariates. Results indicated that reduced reward learning at baseline was a unique predictor of MDD diagnosis at 8 weeks (OR = 7.84, CI: 1.17 – 52.42, p=0.03). These findings remained when correcting for the type of psychopharmacological treatment at week 8. The relationship between reward learning and MDD status after 8 weeks is summarized in Figure 3.
Figure 3.
Response bias as a function of blocks for patients with (MDD diagnosis) vs. without (No MDD diagnosis) MDD diagnosis at follow up. Error bars denote SEM.
4. Discussion
The overarching goal of this study was to test the hypotheses that: 1) patients with MDD – particularly those with high anhedonic symptoms – are characterized by an inability to adapt behavior as a function of rewards, and 2) reduced reward learning before treatment predicted a persisting diagnosis of MDD. Both hypotheses were confirmed. Specifically, using a probabilistic reward task that allowed us to objectively assess reward learning, we found that depressed patients showed a reduced ability to integrate reinforcement history over time compared to controls. Moreover, this difference was driven by patients with high anhedonic symptoms. Depressed patients reporting high anhedonic symptoms showed more blunted reward learning in comparison with patients with less anhedonic symptoms. After 8 weeks of treatment, the reward learning ability of the MDD group normalized to healthy controls’ levels. Critically, and highlighting the specificity of the current findings, reward learning ability before treatment onset predicted a persisting diagnosis of MDD after 8 weeks, even after controlling for anxiety comorbidity and HDRS scores at time of inclusion.
Of note, our findings were not due to general impairment in task performance or difficulty, since we found no group differences in discriminability, indicating that reduced reward learning in the MDD sample was not associated with global cognitive deficits. Also, among the MDD group, discriminability scores at follow-up did not significantly change compared to baseline, suggesting there was no learning effect of the task.
Overall, the present findings are in line with prior reports highlighting reduced reactivity (26) and attention (27) to positive stimuli, blunted reward-related decision making, as well as a failure to develop a reward bias in MDD (7,28). A blunted response to positive reinforcement probably represents a deficit in the approach-related system and may result in low motivational drive, reduced goal-directed behaviour and diminished engagement in pleasurable behaviour and/or anhedonia, which are presumed to be important risk factors for MDD (29,30). Importantly, by showing that (a) treatment normalize blunted reward learning and (b) a decreased ability to modulate behavior as a function of reward before treatment predicts the persistence of an MDD diagnosis, our findings extend these prior data in two critical ways.
The current findings that reward learning was most disrupted in MDD patients reporting elevated anhedonic symptoms is consistent with prior findings in clinical (6) and non-clinical (17,31) studies reporting significant correlations between anhedonic symptoms and blunted reward learning in the same task, and is in support of conceptualizations highlighting the need to identify more homogenous subgroups of patients which might be characterized by a distinct pathophysiology (32). Of note, in the current study, a relationship between anhedonic symptoms and reward learning emerged only over the first 200 trials, rather than the entire 300 trials. The reason for this difference between studies is not clear and warrants further study.
In addition, our findings provided – we believe for the first time – direct evidence that reduced reward learning is predictive of a persisting diagnosis of MDD 8 weeks later, even when accounting for baseline depression severity and anxiety comorbidity. These data extend prior studies highlighting the predictive ability of positive affect for treatment outcome (4,33), residual symptoms (34,35) and course of illness (36) in depression. The fact that reward learning deficits predicted treatment outcome is intriguing given the fact that anhedonia is a particularly difficult symptom to treat with current pharmacotherapy (e.g., SSRIs) (37,38). There is some recent evidence on the influence of different antidepressant medication (e.g., SSRI, SNRI) on reward processing in the brain (39, 40, 41). However, it has also been hypothesized that motivational and reward-related deficits are not adequately addressed in current treatment (42) and neurobiological evidence is emerging on the involvement of a dopamine (DA) dysfunction in MDD (43,44). Notably, in a recent dynamic PET study, we found that the development of a response bias in the task used in the current study was associated with dopamine release in extrastriatal dopaminergic regions in healthy volunteers (45). Furthermore, a single dose of a DA agonist – hypothesized to activate DA autoreceptors and thus reduce DA release – blunted reward responsiveness (29) and altered reward-related dorsal ACC activation (46) in healthy volunteers. Collectively, these findings raise the possibility that persistent MDD diagnosis is associated with reduced DA transmission.
The present study had several limitations: First, MDD patients were recruited from different psychiatric wards in an academic hospital and all depressed patients were already medicated at the time of inclusion. Psycho(pharmaco)logical treatment was not standardized and patients received treatment as clincally appropriate during follow up. The fact that antidepressants potentially influence reward learning in a negative way, and compliance is known to decrease during recovery of MDD, may have impacted the results. Second, depression severity scores ranged broadly but, on average, HDRS scores at time of inclusion were moderate. This indicates that reduced reward sensitivity is not restricted to severe depression, but is also relevant in mild to moderate MDD, replicating prior findings in unmedicated, non-treatment seeking MDD participants recruited from the community (6). Future studies should evaluate the generalizability of our findings to more severe inpatient samples. Third, even though we attempted to take into account most relevant clinical differences between the high and low anhedonic subgroups, we realize that these control analyses are probably incomplete due to limitations of the rating scales and/or lack of sampling of other clinical features. Fourth, controls were not followed longitudinally. Although this was not essential to test our main hypothesis, the lack of a second assessment for controls prevented us to investigate potential learning effects. Fifth, only one type of incentive manipulation was used. Future studies should evaluate whether depressed patients also show deficits to other types of incentive learning (e.g., punishment feedback).
In sum, our results indicate that depressed inpatients, particularly those with high anhedonic symptoms, have an impaired ability to modulate behaviour as a function of rewards. Critically, reward learning deficits predicted treatment outcome above and beyond baseline depression severity and anxiety comorbidity. These findings suggest that blunted reward learning might contribute to the persistence of MDD or treatment resistance. Moreover, they underscore the relevance of developing specific assessment methods in MDD, based on the knowledge of more homogeneous subdimensions of the disease. More adequate assessments in MDD will not only benefit clinical research in the identification of risk factors and development of new treatments, but also assist clinical practice in refining the diagnosis, predicting response and outcome, as well as selecting complementary treatment options.
Table 2.
Demographic and clinical features of the depressed group at follow up (after 8 weeks) (n=60).
| Mean | SD | |
|---|---|---|
| MDD diagnosisa | 32/28 | - |
| Anxiety disorder (%)b | 28.7 | |
| Medication (YES/NO)c | 60/0 | |
| HDRS | 10.8 | 6.6 |
| SHAPS | 4.5 | 4.1 |
MDD diagnosis at follow up with SCID-I: number of patients Yes/No
Anxiety disorder= SCID diagnosis of panic disorder and/or agoraphobia (n = 11), social phobia (n = 5) and/or OCD (n = 1).
Medication: number of patients taking psychopharmacology yes/no (35.0% SSRI; 40.0 % SNRI; 25.0 % others (e.g., tricyclic, mirtazepine, bupropion)).
HDRS: Hamilton Depression Rating Scale; SHAPS: Snaith Hamilton Pleasure Scale.
Acknowledgments
This project was supported by a research grant from Johnson and Johnson, by a research grant (OT06/60) from the University of Leuven (KUL) and by a grant (ELG-B5588-G.0193.07) from the Fund for Scientific Research, Flanders, Belgium (FWO). Prof. Claes is Senior Clinical Investigator of the FWO, has received consulting and/or speaker’s fees from Servier, Lundbeck, Eli Lilly, J&J and AstraZeneca. Dr. Pizzagalli was supported by NIMH grant R01MH68376 and R21MH078979 and over the past 3 years has received consulting fees from Shire, AstraZeneca, Ono Pharma USA, and Johnson & Johnson for projects unrelated to the current study.
Footnotes
Financial disclosure
Dr Schmidt and de Boer are employees of Janssen Research and Development. Prof. Demytennaere reports no conflict of interest directly related to the submitted manuscript.
The other authors declare no competing financial interests.
References
- 1.Clark DA, Beck AT, Beck JS. Symptom differences in major depression, dysthymia, panic disorder, and generalized anxiety disorder. Am J Psychiatry. 1994;151(2):205–209. doi: 10.1176/ajp.151.2.205. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnosic and statistical manual of mental disorders. 4th ed., text revision. Washington, DC: American Psychiatric Press; 2000. [Google Scholar]
- 3.Spijker J, Bijl RV, de Graaf R, Nolen WA. Determinants of poor 1-year outcome of DSM-III-R major depression in the general population: results of the Netherlands Mental Health Survey and Incidence Study (NEMESIS) Acta Psychiatr Scand. 2001;103(2):122–130. doi: 10.1034/j.1600-0447.2001.103002122.x. [DOI] [PubMed] [Google Scholar]
- 4.Davidson RJ. Affective neuroscience and psychophysiology: toward a synthesis. Psychophysiology. 2003;40(5):655–665. doi: 10.1111/1469-8986.00067. [DOI] [PubMed] [Google Scholar]
- 5.Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35(3):537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res. 2008;43(1):76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eshel N, Roiser JP. Reward and punishment processing in depression. Biol Psychiatry. 2010;68(2):118–124. doi: 10.1016/j.biopsych.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 8.Forbes EE, Shaw DS, Dahl RE. Alterations in reward-related decision making in boys with recent and future depression. Biol Psychiatry. 2007;61(5):633–639. doi: 10.1016/j.biopsych.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi T, Oono H, Inoue T, Boku S, Kako Y, Kitaichi Y, Kusumi I, Masui T, Nakagawa S, Suzuki K, Tanaka T, Koyama T, Radford MH. Depressive patients are more impulsive and inconsistent in intertemporal choice behavior for monetary gain and loss than healthy subjects--an analysis based on Tsallis' statistics. Neuro Endocrinol Lett. 2008;29(3):351–358. [PubMed] [Google Scholar]
- 10.Must A, Szabó Z, Bódi N, Szász A, Janka Z, Kéri S. Sensitivity to reward and punishment and the prefrontal cortex in major depression. J Affect Disord. 2006;90(2–3):209–215. doi: 10.1016/j.jad.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Smoski MJ, Lynch TR, Rosenthal MZ, Cheavens JS, Chapman AL, Krishnan RR. Decision-making and risk aversion among depressive adults. J Behav Ther Exp Psychiatry. 2008;39(4):567–576. doi: 10.1016/j.jbtep.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58(11):843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 13.Forbes EE, Christopher May J, Siegle GJ, Ladouceur CD, Ryan ND, Carter CS, Birmaher B, Axelson DA, Dahl RE. Reward-related decision-making in pediatric major depressive disorder: an fMRI study. J Child Psychol Psychiatry. 2006;47(10):1031–1040. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH. Neural responses to monetary incentives in major depression. Biol Psychiatry. 2008;63(7):686–692. doi: 10.1016/j.biopsych.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166(6):702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR, Dichter GS. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. J Affect Disord. 2009;118(1–3):69–78. doi: 10.1016/j.jad.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pizzagalli DA, Jahn AL, O'Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2005;57(4):319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992;49(8):624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995;167(1):99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- 21.Franken IH, Rassin E, Muris P. The assessment of anhedonia in clinical and non-clinical populations: further validation of the Snaith-Hamilton Pleasure Scale (SHAPS) J Affect Disord. 2007;99(1–3):83–89. doi: 10.1016/j.jad.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 22.Bogdan R, Pizzagalli DA. Acute stress reduces reward responsiveness: implications for depression. Biol Psychiatry. 2006;60(10):1147–1154. doi: 10.1016/j.biopsych.2006.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pizzagalli DA, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166(6):702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pizzagalli DA, Goetz E, Ostacher M, Iosifescu DV, Perlis RH. Euthymic patients with bipolar disorder show decreased reward learning in a probabilistic rewardtask. Biol Psychiatry. 2008;64(2):162–168. doi: 10.1016/j.biopsych.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCarthy D, Davison M. Signal probability, reinforcement and signal detection. J Exp Anal Behav. 1979;32(3):373–386. doi: 10.1901/jeab.1979.32-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sloan DM, Strauss ME, Wisner KL. Diminished response to pleasant stimuli by depressed women. J Abnorm Psychol. 2001;110(3):488–493. doi: 10.1037//0021-843x.110.3.488. [DOI] [PubMed] [Google Scholar]
- 27.Wang CE, Brennen T, Holte A. Decreased approach motivation in depression. Scand J Psychol. 2006;47(6):505–511. doi: 10.1111/j.1467-9450.2006.00525.x. [DOI] [PubMed] [Google Scholar]
- 28.Henriques JB, Glowacki JM, Davidson RJ. Reward fails to alter response bias in depression. J Abnorm Psychol. 1994;103(3):460–466. doi: 10.1037//0021-843x.103.3.460. [DOI] [PubMed] [Google Scholar]
- 29.Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical Conditioning II: Current Research and Theory. New York: Appleton Century Crofts; 1972. pp. 64–99. [Google Scholar]
- 30.Bylsma LM, Morris BH, Rottenberg J. A meta-analysis of emotional reactivity in major depressive disorder. Clin Psychol Rev. 2008;28(4):676–691. doi: 10.1016/j.cpr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Pizzagalli DA, Bogdan R, Ratner KG, Jahn AL. Increased perceived stress is associated with blunted hedonic capacity: potential implications for depression research. Behav Res Ther. 2007;45(11):2742–2753. doi: 10.1016/j.brat.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29(10):1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- 33.Kasch KL, Rottenberg J, Arnow BA, Gotlib IH. Behavioral activation and inhibition systems and the severity and course of depression. J Abnorm Psychol. 2002;111(4):589–597. doi: 10.1037//0021-843x.111.4.589. [DOI] [PubMed] [Google Scholar]
- 34.Hundt NE, Nelson-Gray RO, Kimbrel NA, Mitchell JT, Kwapil TR. The interaction of reinforcement sensitivity and life events in the prediction of anhedonic depression and mixed anxiety-depression symptoms. Pers. Indiv. Diff. 2007;43(5):1001–1012. [Google Scholar]
- 35.Kimbrel NA, Nelson-Gray RO, Mitchell JT. Reinforcement sensitivity and maternal style as predictors of psychopathology. Pers. Indiv. Diff. 2007;42(7):1139–1149. [Google Scholar]
- 36.McFarland BR, Shankman SA, Tenke CE, Bruder GE, Klein DN. Behavioral activation system deficits predict the six-month course of depression. J Affect Disord. 2006;91(2–3):229–234. doi: 10.1016/j.jad.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 37.Shelton RC, Tomarken AJ. Can recovery from depression be achieved? Psychiatr Serv. 2001;52(11):1469–1478. doi: 10.1176/appi.ps.52.11.1469. [DOI] [PubMed] [Google Scholar]
- 38.Price J, Cole V, Goodwin GM. Emotional side-effects of selective serotonin reuptake inhibitors: qualitative study. Br J Psychiatry. 2009;195(3):211–217. doi: 10.1192/bjp.bp.108.051110. [DOI] [PubMed] [Google Scholar]
- 39.McCabe C, Mishor Z. Antidepressant medications reduce subcortical-cortical resting-state functional connectivity in healthy volunteers. Neuroimage. 2011;57(4):1317–1323. doi: 10.1016/j.neuroimage.2011.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seymour B, Daw ND, Roiser JP, Dayan P, Dolan R. Serotonin selectively modulates reward value in human decision-making. J Neurosci. 2012;32(17):5833–5842. doi: 10.1523/JNEUROSCI.0053-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rogers RD. The roles of dopamine and serotonin in decision making: evidence from pharmacological experiments in humans. Neuropsychopharmacology. 2011;36:114–132. doi: 10.1038/npp.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nutt D, Demyttenaere K, Janka Z, Aarre T, Bourin M, Canonico PL, Carrasco JL, Stahl S. The other face of depression, reduced positive affect: the role of catecholamines in causation and cure. J Psychopharmacol. 2007;21(5):461–471. doi: 10.1177/0269881106069938. [DOI] [PubMed] [Google Scholar]
- 43.Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59(12):1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 44.Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64(3):327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- 45.Vrieze E, Ceccarini J, Pizzagalli DA, Bormans G, Vandenbulcke M, Demyttenaere K, Van Laere K, Claes S. Measuring extrastriatal dopamine release during a reward learning task. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21456. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santesso DL, Evins AE, Frank MJ, Schetter EC, Bogdan R, Pizzagalli DA. Single dose of a dopamine agonist impairs reinforcement learning in humans: evidence from event-related potentials and computational modeling of striatal-cortical function. Hum Brain Mapp. 2009;30(7):1963–1976. doi: 10.1002/hbm.20642. [DOI] [PMC free article] [PubMed] [Google Scholar]