Abstract
Resistance to chemotherapy and metastases are the major causes of breast cancer-related mortality. Moreover, cancer stem cells (CSCs) play critical roles in cancer progression and treatment resistance. Previously, it was found that CSC-like cells can be generated by aberrant activation of EMT, thereby making anti-EMT strategies a novel therapeutic option for treatment of aggressive breast cancers. Here, we report that the transcription factor FOXC2 induced in response to multiple EMT signaling pathways as well as elevated in stem cell-enriched factions is a critical determinant of mesenchymal and stem cell properties, in cells induced to undergo EMT and CSC-enriched breast cancer cell lines. More specifically, attenuation of FOXC2 expression using lentiviral short hairpin RNA led to inhibition of the mesenchymal phenotype and associated invasive and stem cell properties, which included reduced mammosphere forming ability and tumor initiation. Whereas, overexpression of FOXC2 was sufficient to induce CSC properties and spontaneous metastasis in transformed human mammary epithelial cells. Furthermore, a FOXC2-induced gene expression signature was enriched in the claudin-low/basal B breast tumor subtype that contains EMT and CSC features. Having identified PDGFR-β to be regulated by FOXC2, we demonstrate that the FDA-approved PDGFR inhibitor, sunitinib, targets FOXC2-expressing tumor cells leading to reduced CSC and metastatic properties. Thus, FOXC2 or its associated gene expression program may provide an effective target for anti-EMT based therapies for the treatment of claudin-low/basal B breast tumors or other EMT/CSC-enriched tumors.
Keywords: EMT; Metastasis; Cancer Stem Cells; Therapy resistance; PDGFR-beta, Sunitinib, Claudin-low tumors
Introduction
Despite the initial effectiveness of conventional therapies, recurrence and the emergence of metastases are major causes of therapeutic failure in cancer patients. These therapies are believed to target the differentiated and proliferative cells that comprise the bulk of the tumor. The relatively high relapse rate of patients with aggressive forms of breast cancer, including the recently identified triple-negative claudin-low/basal B subtype (for brevity referred to as claudin-low throughout), is attributed to a small population of cancer stem cells (CSCs) residing within the tumor. In addition to resistance to standard therapies, CSCs are reported to have inherently greater tumor-initiating potential, which is implicated in tumor relapse (1), driving primary tumor growth as well as the seeding and establishment of metastases (2–7). Therefore, therapeutic approaches that specifically target the CSC population, particularly when used in combination with conventional therapies, may provide a therapeutic strategy to substantially improve breast cancer patient outcome. The epithelial-to-mesenchymal transition (EMT) program has recently been linked to the generation of breast CSC-like cells (8) and has well documented roles in promoting an invasive and metastatic phenotype (9, 10). As such, the development of targeted therapeutics to inhibit the EMT program may provide significant clinical benefits in treating aggressive breast cancers for which current therapies are inadequate.
EMT was initially characterized as an important program during normal embryonic development (11, 12) however, more recent reports suggest that carcinoma cells are capable of reactivating the EMT program during tumor progression (9). Similar to cells that undergo EMT during normal development, carcinoma cells that undergo EMT lose cell-cell contacts, undergo major changes in their cytoskeleton and acquire a mesenchymal-like morphology endowing them with increased invasive and migratory abilities (11–13). Several signaling molecules present in the tumor microenvironment are capable of initiating EMT and metastasis (14, 15) and importantly, many of these same factors have also been found to be aberrantly activated and/or overexpressed in human cancers (16–20). Since cells undergoing EMT are shown to possess stem cell properties (8), and several recent studies independently demonstrated that CSCs as well as cells that have undergone EMT are relatively resistant to conventional chemo- and radio-therapies (1, 21–26), suggests the EMT program may provide a novel therapeutic window for inhibiting CSCs. However, due to the plethora of factors capable of inducing EMT and the hierarchy of the EMT programs, we sought to identify a central functional mediator of EMT independent of the initiating signal that may provide a novel target for anti-EMT based therapies. Here we report that FOXC2 serves as such a mediator. We show that it is induced by multiple factors as well as the expression of PDGFR-β is dependent on the expression of FOXC2, and thus serves as a potential therapeutic target for cells that have undergone EMT.
Materials and methods
Cell lines and antibodies
Immortalized human mammary epithelial cells (HMLE) and V12H-Ras transformed derivatives (HMLER), including cells expressing empty vector (pWZL), Snail, Twist, Goosecoid (GSC) or an activated form of TGF-β1 were maintained as previously described (8). Established human breast cancer cell lines were cultured in cell specific medium as outlined in the SI Materials and Methods. Antibodies used included; anti-β-actin (Abcam), FOXC2 (Dr. Naoyuki Miura), E-cadherin (BD Bioscience), Fibronectin (BD Bioscience), N-cadherin (BD Bioscience), Vimentin (NeoMarkers) and β-catenin (BD Bioscience).
Plasmids and viral transduction
The production of lentiviruses and amphotrophic retroviruses, and the transduction of target cells was followed as previously described (27). See SI Materials and Methods for brief description.
Mammosphere assay
Mammosphere assays were performed previously described (8). To test effects of sunitinib on mammosphere formation, 1000 cells were plated in 96-well ultra-low attachment plates in 100 µl mammosphere media. At 24 and 96 hours, media was replaced with media containing 10 µM sunitinib or vehicle control (DMSO). Spheres were quantified after 7 days.
Three dimensional (3D) laminin-rich extracellular matrix (lrECM) on-top cultures
The 3D lrECM on-top cultures were adapted from the procedures previously described (28). See SI Materials and Methods for brief description.
Animal studies
NOD/SCID mice were purchased from Jackson Laboratory. All mouse procedures were approved by the Animal Care and Use Committees of M.D. Anderson Cancer Center and performed in accordance with Institutional policies. For xenograft tumor initiation studies, the indicated number of cells were suspended in 50 µl of Matrigel diluted 1:1 with DMEM and injected into the inguinal mammary gland of NOD/SCID mice. Tumor incidence was monitored for 12 weeks following orthotopic injection. To assess the spontaneous metastatic potential of cells, 2×106 HMLER-vector and HMLER-FOXC2 cells labeled with firefly luciferase were injected into the inguinal mammary gland of NOD/SCID mice. Mice were assessed weekly for metastasis via the intraperitoneal injection of D-Luciferin (Caliper LifeSciences) at 150 mg/kg in PBS and in vivo bioluminescence was assessed using the IVIS imaging system 200 series (Xenogen Corporation). Once mammary gland tumors reached 1.5 cm in diameter, mice were euthanized and organs were harvested for confirmation of metastatic tumor burden via bioluminescence.
To ascertain the effects of sunitinib on HMLER-FOXC2 tumor growth and animal survival, 1×106 firefly luciferase labeled HMLER-FOXC2 cells were injected into the mammary fat-pad of NOD/SCID mice as described above. After 24 hours, the mice were treated 5 days a week with 40 mg/kg sunitinib or vehicle (n=9) by oral gavage. Animal were sacrificed once the tumors reached 1.5 cm3 or 30 days following injection and the dissected organs were immediately analyzed for luciferase activity
Details of additional procedures can be found in SI Materials and Methods.
Results
FOXC2 is required for the maintenance of the mesenchymal phenotype following EMT induction in human mammary epithelial cells
In our previous study, enhanced expression of FOXC2 was observed following the induction of EMT by several factors in experimentally immortalized human mammary epithelial (HMLE) cells (17) strongly suggesting that FOXC2 may be a critical determinant of multiple EMT programs. To assess the functional role of FOXC2 during EMT, we employed shRNA mediated suppression of FOXC2 in HMLE cells that underwent EMT via ectopic expression of Snail, Twist or TGF-β1. The suppression of FOXC2 had no significant effect on cell growth, but substantially altered the in vitro morphology of all cell lines, including increased clustering of cells into epithelial-like islands with prominent cell-cell contacts and reduced fibroblastic morphology (Figure 1a). FOXC2 attenuation also led to reduced expression of mesenchymal markers vimentin, fibronectin and N-cadherin across all cell types tested as well as re-expression (both mRNA and protein levels) of the epithelial marker E-cadherin in HMLE-Snail and HMLE-TGF-β1 cells (Figure 1b and c). To examine whether FOXC2 could function in parallel to other EMT regulators we examined the expression of Snail, Twist and Slug in cells ectopically expressing FOXC2 and found only moderate upregulation of Twist but not Snail or Slug expression (Supplemental Figure 1) suggesting FOXC2 may not regulate expression of these factors.
Figure 1.
FOXC2 expression is necessary to maintain the mesenchymal and invasive properties induced by EMT in mammary epithelial cells. (a) Phase-contrast images of HMLE-Vector, - Snail, - Twist and -TGF-β1 cells expressing either control-shRNA (shCntrl) or FOXC2-shRNA (shFOXC2). Scale bar indicates 100 µm. (b) Expression of EMT marker mRNA by quantitative RT-PCR. GAPDH was used as an internal control. n=3; error bars indicate SEM. N.D. = not detected. (c) Western blot analysis of EMT marker protein expression upon FOXC2 suppression in HMLE-Snail, -Twist and -TGF-β1 cells, respectively. (d) Quantification of invasion in Matrigel Transwell chambers using HMLE-Snail-shCntrl and HMLE-Snail-shFOXC2 cells in response to basic fibroblast growth factor (bFGF) and platelet-derived growth factor-BB (PDGF-BB). n=3; error bars indicate SEM. * P<0.05. (e) Confocal microscopy images of HMLE-Snail-shCntrl and HMLE-Snail-shFOXC2 cells in 3D lrECM cultures. The integrity of the basement membrane was assessed using anti-Laminin V (red) with DAPI nuclear stain (blue). Scale bar indicates 100 µm.
The passage of cells through an EMT and the acquisition of mesenchymal properties is associated with increased migratory and invasive properties. Suppression of FOXC2 was observed to significantly inhibit the invasion of HMLE-Snail cells through matrigel using Transwell migration assays in response to the soluble chemotactic ligands basic fibroblast growth factor (bFGF) and platelet-derived growth factor-BB (PDGF-BB) (Figure 1d). Mammary cells possessing epithelial traits are known to form organized multicellular acini structures with an intact laminin positive basement membrane in 3D laminin-rich extracellular matrix matrigel cultures (3D lrECM) (29, 30). However, following the induction of EMT, cells became disorganized and gained invasive properties characterized by disrupted laminin staining (31, 32). Using 3D lrECM assays, HMLE-Snail control-shRNA cells grew as highly invasive stellate structures with disrupted basement membrane, displayed by disorganized laminin V staining (Figure 1e). In contrast, the HMLE-Snail FOXC2-shRNA cells formed non-invasive, multicellular structures with an intact basement membrane as depicted by a continuous laminin V layer (Figure 1e). Taken together, these results suggest that FOXC2 is necessary for the maintenance of the mesenchymal phenotype of mammary epithelial cells following passage through an EMT.
FOXC2 is necessary for the stem cell-like properties generated via EMT in mammary epithelial cells
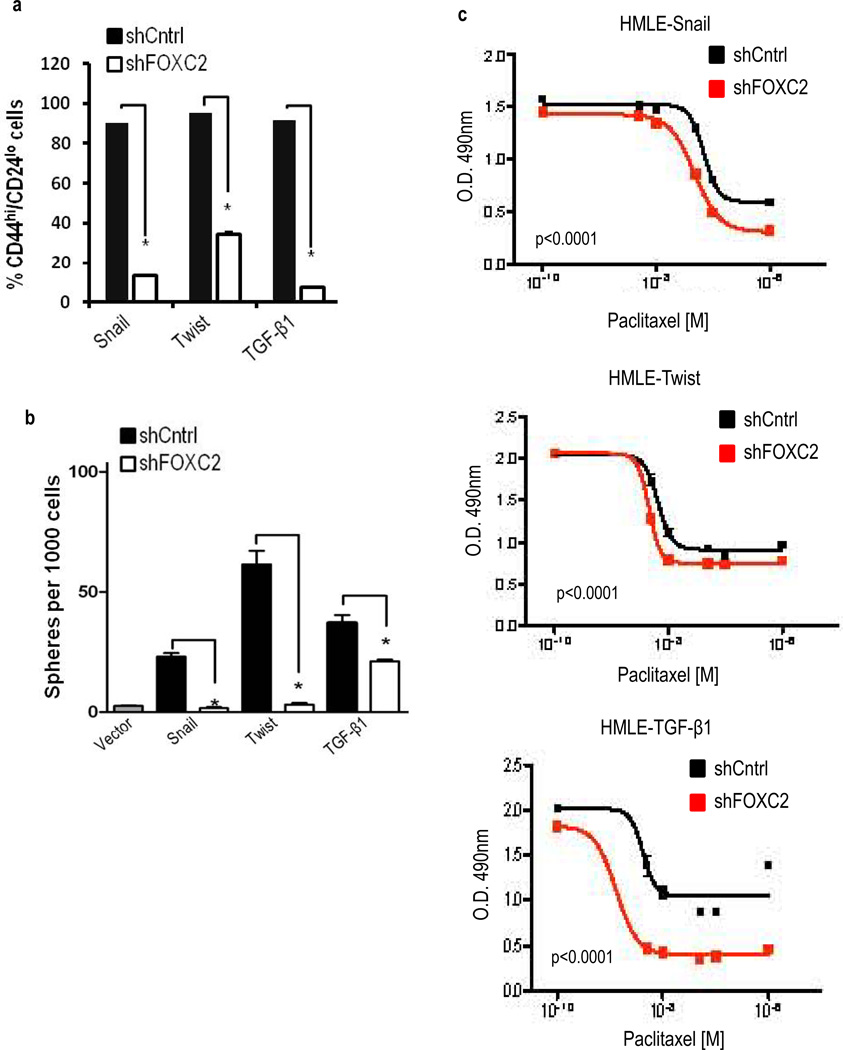
We previously reported that HMLE-Snail, -Twist and -TGF-β1 cells acquired properties similar to breast CSCs, including the CD44high/CD24low cell surface markers and an increased ability to form mammospheres (8). Here, we found that the attenuation of FOXC2 expression by shRNA in these cells reduced the number of cells with the CD44high/CD24low phenotype compared to the control-shRNA expressing cells (Figure 2a). In the same conditions, there was a marked decrease in the mammosphere forming ability (Figure 2b).
Figure 2.
FOXC2 expression is required for EMT-derived stem cell properties in mammary epithelial cells. (a) Quantification of CD44 (CD44-PE) and CD24 (CD24-FITC) expression by FACS analysis in HMLE-Snail, -Twist and -TGF-β1 cells expressing either shCntrl or shFOXC2. n=3; error bars indicated SEM. * P<0.05. (b). In vitro quantification of mammospheres formed by cells described in (a). n=3, error bars indicate SEM. * P<0.05. (c) Quantification of cell viability using an MTS assay in cells described in (a) following culture for 96 hours in increasing concentrations of Paclitaxel. Data is represented as the absorbance (O.D) at 490 nm. n=3, p < 0.005.
Next, the acquisition of stem cell properties and passage through an EMT has been reported to increase the resistance to chemotherapeutic agents (22, 23). In accordance with this, we found that suppression of FOXC2 expression in cells that have undergone EMT (HMLE-Snail, HMLE-Twist and HMLE-TGF-β1) sensitized them to paclitaxel (Figure 2c). Together, these results indicate FOXC2 expression is necessary for EMT-derived stem cell-like properties in mammary epithelial cells.
FOXC2 is elevated in CSC-enriched populations and is sufficient to promote the generation of CSCs and metastatic competence in transformed human mammary epithelial cells
Previously it has been demonstrated that FACS isolation of breast tumor cells with the CD44high/CD24low cell surface phenotype (3) as well as isolation of tumor cells from mammospheres (33) can enrich for populations of tumor initiating CSCs. Using this rationale we FACS isolated CD44high/CD24low and CD44low/CD24high cellular fractions from HMLER and SUM159 breast cancer cell lines and observed increased FOXC2 protein expression in the CD44high/CD24low stem cell fraction relative to the CD44low/CD24high fraction (Figure 3a). Increased FOXC2 protein expression was also observed in primary mammospheres isolated from HMLER, SUM159, HCC38, and SUM149 cells as compared to same cells grown in monolayer cultures (Figure 3b).
Figure 3.
FOXC2 expression is increased in stem cell enriched populations and is sufficient to promote phenotypes associated with CSCs. (a) Western blot analysis of FOXC2 expression in the stem cell enriched CD44hi/CD24lo (44hi/24lo) and more differentiated CD44lo/CD24hi (44lo/24hi) cellular fractions isolated by FACS from HMLER and SUM159 cell lines. (b) Western blot analysis of FOXC2 expression in cells cultured in monolayer culture (2D) and stem cell enriched mammosphere cultures (MS) for the indicated breast cancer cell lines. (c) FACS analysis of CD44 and CD24 expression in HMLER-Vector and HMLER-FOXC2 cells. Representative FACS plots are shown. (d) In vitro quantification of mammospheres formed by 1000 cells described in (c) (n = 3); error bars indicate SEM. * P<0.05. (e) Quantification of cell viability by MTS assay using HMLER cells expressing either Vector or FOXC2 cDNA following culture for 96 hours in increasing concentrations of Paclitaxel. Data represented is the mean absorbance (O.D) at 490 nm, (n = 3). (f) Tumor incidence of FOXC2 expressing HMLER cells injected into the mammary fat pad of NOD/SCID mice in limiting dilutions. (g) Tumor growth quantification of luciferase labelled HMLER-Vector and HMLER-FOXC2 xenografts in vivo using bioluminescence after 28 days of inoculation into the mammary fat pad of NOD/SCID mice. (n = 5). (h) Ex vivo bioluminescence images of the indicated organs of mice carrying HMLER-Vector and HMLER-FOXC2 xenografts after 28 days.
Previously, we have reported that ectopic expression of FOXC2 induced partial EMT in Madin-Darby canine kidney (MDCK) epithelial cells, and was sufficient to promote the metastasis of EpRas murine mammary carcinoma cells (17). However, whether FOXC2 expression alone can induce EMT and generate CSC-like properties remains unknown. We report here for the first time that ectopic expression of FOXC2 in HMLER cells is sufficient to induce a robust EMT and subsequent CSC-like properties, both in vitro and in vivo. This was displayed as a characteristic switch of morphology to a fibroblastic appearance with a reduction in epithelial specific E-cadherin, in conjunction with increased expression of mesenchymal markers vimentin, fibronectin and N-cadherin at the mRNA and protein levels (Supplemental Figure 2a–c). Furthermore, HMLER-FOXC2 cells had increased CSC-like properties, including a switch to the CD44high/CD24low cell surface phenotype (Figure 3c), enhanced mammosphere-forming efficiency (Figure 3d) and increased resistance to paclitaxel (Figure 3e). Notably, overexpression of FOXC2 led to increased tumor formation of HMLER cells in the mammary fat-pad (Figure 3f). In fact, as few as 1×103 HMLER-FOXC2 cells could robustly initiate tumors (7/8 sites), while at least 2×106 HMLER-Vector control cells were required to get a 54% tumor take rate (7/13 sites) within the same 12 week time frame (Figure 3f).
To assess the effect of FOXC2 expression on the orthotopic growth and spontaneous metastasis of HMLER cells, we injected 2×106 luciferase-labeled HMLER-FOXC2 or HMLER-Vector cells into the mammary fat-pad of NOD/SCID mice and analyzed for metastases using bioluminescent imaging once per week until the tumors either reached a volume of 1.5 cm3 (28 days for HMLER-FOXC2) or until the end of the experiment after 12 weeks (HMLER-Vector). FOXC2 expression promoted aggressive growth (Figure 3g) and metastasis of HMLER cells to the lungs, liver, hind leg bone, and most strikingly, to the brain (Figure 3h) as soon as 28 days post-injection. In contrast HMLER-Vector cells did not metastasize to any of the organs examined (Figure 3h) even at the end of the experiment (Data not shown). Collectively, these findings suggest that FOXC2 expression is sufficient to promote EMT and CSC properties, including chemoresistance, tumor initiation and metastatic competence in transformed human mammary epithelial cells.
A FOXC2-associated gene expression signature is enriched in claudin-low human breast tumors
We next investigated whether FOXC2 activity could be observed in metastatic tumors in vivo. As a measure of FOXC2 activity, we generated using microarray data, a FOXC2 gene expression signature (GES) by comparing the gene expression profile of HMLER cells overexpressing FOXC2 to vector-transduced counterparts. Applying this GES to data from two human xenograft models, MDA-MB-231 and CN34, we observed that the FOXC2 GES was significantly higher in metastases compared with the primary tumors in both datasets (Figure 4a and b). Collectively, these data indicate that the FOXC2-associated signature is enriched in metastases relative to primary tumors.
Figure 4.
FOXC2 derived gene signature is enriched in claudin-low human breast cancer samples and can accurately predict claudin-low human tumors. (a,b) Measurement of FOXC2 gene expression signature (GES) in: (a) MDA-MB231 (GSE12237) and (b) CN34 (GSE12237) xenograft models consisting of the parental tumors and brain metastases. The box plots show the mean, 5%, and 95% distribution of the level of FOXC2 signature (42) in GSE12237. Predicted activation of FOXC2 between the primary tumors and metastases was compared and a p-value was calculated using a Student’s t-test. (c,d) The FOXC2 GES was scored in (c) tumors (GSE18229) and (d) established breast cancer cell lines (E-TABM-157). The box plots represent the mean, 5%, and 95% distribution of the FOXC2 signature scores across the (c) breast tumor subtypes data derived from GSE18229 (35) and (d) an expression dataset of 51 breast cancer cell lines described in (43) (E-TABM-157; ArrayExpress). The one-way ANOVA significance for each plot was p < 0.0001. (e) Western blot analysis of FOXC2 expression in a panel of established breast cancer cell lines representing luminal, basal and claudin-low subtypes.
We previously reported that elevated FOXC2 expression correlated with basal-like breast cancers (17). However, using gene expression profiling, an aggressive claudin-low group has been found within this subtype (34, 35). We found the FOXC2 GES to be significantly enriched in claudin-low human breast tumors (Figure 4c), as well as in claudin-low cell lines (Figure 4d) compared to other subtypes. To verify this finding, we performed a western blot analysis and found that all claudin-low cell lines analyzed (6/6) expressed FOXC2 at varying degrees, while none of the other cell lines expressed significant levels of FOXC2 (Figure 4e).
FOXC2 is required for the mesenchymal and CSC-like properties of claudin-low breast cancer cells
As FOXC2 expression and activity was found to be associated with claudin-low breast tumors we tested whether FOXC2 expression was required for the mesenchymal and invasive properties of SUM159, MDA-MB231 and HMLER-Snail cells that have a claudin-low gene expression phenotype (35). The suppression of FOXC2 resulted in a less fibroblastic morphology with increased epithelial-like cell clustering (Figure 5a), decreased expression of mesenchymal markers fibronectin and N-cadherin (Figure 5b) and re-expression of E-cadherin in HMLER-Snail cells (Figure 5b). Furthermore, FOXC2 suppression significantly reduced Transwell migration of both SUM159 and HMLER-Snail cells (Figure 5c and d) (p<0.05). In a similar fashion, SUM159 Control-shRNA cells grew in 3D lrECM cultures with a characteristic stellate morphology (36) and extended multicellular protrusions invading into the surrounding Matrigel (Figure 5e). The suppression of FOXC2 resulted in a dramatic reduction in the invasive morphology of SUM159 cells (SUM159 FOXC2-shRNA) compared to control cells (Figure 5e).
Figure 5.
Attenuation of FOXC2 expression reduces the mesenchymal and stem cell properties of breast cancer cell lines with a claudin-low phenotype. (a) Phase-contrast images of SUM159 and HMLER-Snail cells expressing either a control-shRNA (shCntrl) or FOXC2-shRNA (shFOXC2). Scale bar indicates 100 µm. (b) Western blot analysis of EMT marker protein expression upon FOXC2 suppression in SUM159 and HMLER-Snail cells. (c,d) Quantification of Transwell cell migration for SUM159 (c) and HMLER-Snail (d) cells expressing either a control-shRNA (shCntrl) or FOXC2-shRNA (shFOXC2) in response to epidermal growth factor (EGF). Columns represent the average cell migration (n = 6) relative to that induced in shCntrl cells by serum free media alone (SFM). Error bars indicate SEM. * P<0.05. (e) Confocal microscopy images of SUM159-shCntrl and SUM159-shFOXC2 cells in 3D lrECM cultures. Cell invasion was qualitatively assessed using anti-vimentin (green) and F-actin detected with TRITC-conjugated phalloidin (red). Nuclei were stained with DAPI (blue). Scale bar indicates 250 µm. (f) Quantification of FACS analysis of CD44 (CD44-PE) and CD24 (CD24-FITC) expression in SUM159, MDA-MB-231 and HMLER-Snail cells expressing either shCntrl or shFOXC2, (n = 3) error bars indicated SEM. * P<0.05. (g) In vitro quantification of mammospheres formed by 1000 cells described in (f), (n = 3), error bars indicate SEM. * P<0.05. (h) Tumor incidence of SUM159 and HMLER-Snail cells expressing shCntrl or shFOXC2 injected into the mammary fat pad of NOD/SCID mice in limiting dilutions and measured as palpable tumors after 12 weeks.
We next assessed the effect of FOXC2 suppression on stem cell properties on the same three cell lines. Suppression of FOXC2 expression substantially reduced the percentage of cells displaying the CD44high/CD24low CSC-like cell surface profile (Figure 5f) as well as significantly abrogating the mammosphere forming ability (Figure 5g) as compared to the Control-shRNA expressing cells. Given that suppression of FOXC2 expression diminished stem cell properties in vitro, we next examined if FOXC2 knockdown affected the tumor-initiating potential of SUM159 as well as HMLER-Snail cells using limiting dilution tumor-initiation assays. FOXC2-shRNA or Control-shRNA expressing cells were introduced into the mammary fat-pad of NOD/SCID mice, and we found that the suppression of FOXC2 expression decreased tumor initiation frequency relative to control cells of both SUM159 and HMLER-Snail xenografts (Figure 5h). In summary, these findings suggest FOXC2 may be an important functional mediator of the mesenchymal and CSC properties of claudin-low breast cancer cells.
FOXC2 regulates PDGFR-β expression
We recently reported that cells induced to undergo EMT by multiple factors upregulated the expression of PDGFR-β (CD140b) (37) similar to FOXC2. Thus we hypothesized that PDGFR-β might provide a druggable downstream target in FOXC2 expressing cells. We first confirmed that PDGFR-β protein expression was upregulated in a panel of EMT-derived cells (Figure 6a, i and ii). As observed for FOXC2 (Figure 3a and b), the expression of PDGFR-β was elevated in the stem cell-enriched CD44high/CD24low subpopulation of HMLER cells as well as in SUM159 cells cultured as mammospheres, compared to controls (Figure 6a, iii and iv). Furthermore, the expression pattern of PDGFR-β and FOXC2 correlated strongly across a panel of established cell lines with increased expression observed in claudin-low cell lines SUM159 and Hs578T (Figure 6b). Reflecting the increased levels of PDGFR-β in EMT-derived and claudin-low cell lines, the addition of the ligand PDGF-BB significantly elevated Transwell cell migration in all cells tested (Figure 6c) and led to the further enhancement of the invasive phenotype of HMLER-FOXC2 cells in 3D lrECM cultures (Figure 6d).
Figure 6.
FOXC2 regulates the expression of PDGFR-β. (a) Western blot analysis of PDGFR-β expression in normal (i) and transformed (ii) mammary epithelial cells following EMT induction by multiple factors as well as in stem cell enriched (44hi/24lo) relative to (44lo/24hi) (iii) and mammosphere cultures (iv). (b) Western blot analysis of PDGFR-β expression in a panel of established breast cancer cell lines representing luminal, basal and claudin-low subtypes. (c) Quantification of Transwell cell migration for the indicated cell lines in response to the PDGFR-β ligand, platelet-derived growth factor-BB (PDGF-BB) (20 ng/ml), (n = 6) error bars indicate SEM, * P<0.05. (d) Phase contrast images of HMLER-Vector and HMLER-FOXC2 cells in 3D lrECM cultures in the presence and absence of PDGF-BB. Scale bar indicates 50 µm. (e,f) Western blot analysis of PDGFR-β expression in cells induced to undergo EMT by ectopic expression of Twist, Snail or TGF-β1 in HMLE derived cells (e) as well as in transformed cell lines (SUM159 and HMLER-Snail) (f) expressing either shCntrl or shFOXC2. (g) Quantification of Transwell cell migration for the indicated cell lines expressing either a control-shRNA (shCntrl) or FOXC2-shRNA (shFOXC2) in response to PDGF-BB (20 ng/ml), (n = 6) error bars indicate SEM, * P<0.05. (h) Quantification of binding of FOXC2 to the PDGFR-β promoter by ChIP assays, (n = 3) error bars represent the SEM. Capitalized nucleotides indicate the predicted FOXC2 binding sites at the indicated chromosomal locations.
We next investigated if PDGFR-β expression is dependent on FOXC2 expression. Across a panel of cells, the expression of PDGFR-β was found to be substantially decreased upon suppression of FOXC2 (Figures 6e and f; Supplemental Figure 3). Consequently, the suppression of FOXC2 in HMLE-Snail, HMLE-TGF-β1, HMLER-Snail and SUM159 cells compromised the ability of these cells to migrate towards PDGF-BB (Figure 6g). To examine the possibility of FOXC2 directly regulating PDGFR-β transcription, we performed a chromatin immunoprecipation (ChiP) assay using HMLER-FOXC2 cells. FOXC2 preferentially bound to two regions at 2.7 kb (−2.7 kb) and 1.3 kb (−1.3 kb) upstream of the PDGFR-β transcription start site (Figure 6h), thus demonstrating that FOXC2 may be a direct transcriptional regulator of PDGFR-β expression.
Sunitinib targets FOXC2-expressing tumors
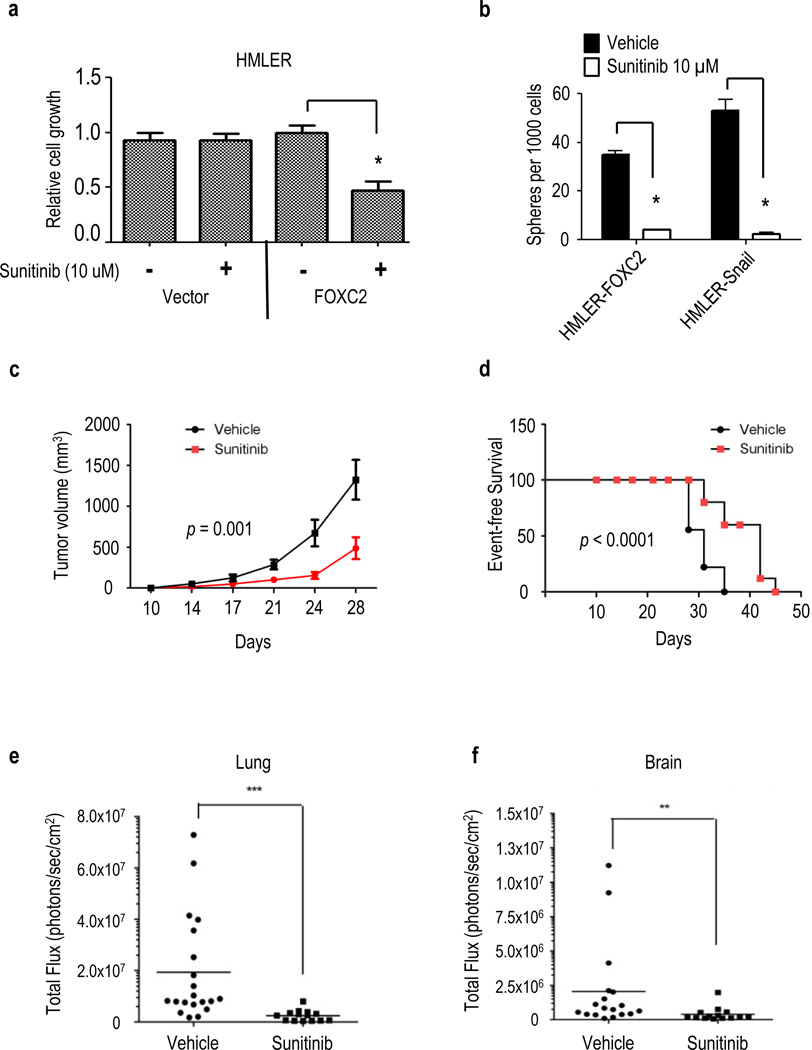
Since we found that expression of PDGFR-β is regulated by FOXC2, we tested whether sunitinib, a small molecule inhibitor of PDGFR-β, could suppress the stem-like and metastatic properties of FOXC2-expressing cells. Sunitinib treatment was found to specifically inhibit the cell growth of HMLER-FOXC2 but not HMLER-Vector control cells in monolayer cultures (Figure 7a). The treatment of HMLER-FOXC2 and HMLER-Snail cells with sunitinib was found to significantly decrease mammosphere formation by >8-fold and >20-fold, respectively (Figure 7b), as compared to vehicle (DMSO) treated cells. Furthermore, cells expressing endogenous FOXC2 also display increased sensitivity to sunitinib as evidenced by both MTS assay and reduction in mammosphere formation (Supplemental Figure 4a–c).
Figure 7.
Sunitinib inhibits the growth and metastasis of FOXC2-expressing tumors. (a) Quantification of HMLER-Vector and HMLER-FOXC2 cell viability in the presence of sunitinib (10 µM) relative to vehicle (DMSO), (n = 3), error bars indicate SEM, * P<0.05. (b) In vitro quantification of mammospheres formed by 1000 HMLER-FOXC2 and HMLER-Snail cells in the presence of sunitinib (10 µM) or DMSO, (n = 3), error bars indicate SEM, * P<0.05. (c) Tumor volume of HMLER-FOXC2 cells injected into the mammary fat pad of NOD/SCID mice and treated with 40 mg/kg of sunitinib or vehicle (n = 9) daily for the indicated number of days. (d) Event-free survival of mice with orthotopic HMLER-FOXC2 xenografts treated daily with sunitinib (40 mg/kg, n = 7) or vehicle (n = 10). Mice were euthanized once tumors reached 1.5 cm3. (e,f) Thirty days following initiation of sunitinib or vehicle treatment, mice were euthanized and the organs, (e) lung and (f) brain, were dissected and analysed for metastatic tumor burden using bioluminescence imaging. The luminescent signal of tumor cells is represented as the total photon flux detected in each organ from individual mice with the bar indicating the average. ***P < 0.001, **P< 0.05 compared to the vehicle control group.
To investigate whether sunitinib could inhibit FOXC2-expressing tumors in vivo, we orally administered sunitinib to mice following orthotopic injection of luciferase labelled HMLER-FOXC2 cells into the mammary fat-pad. In accordance with our in vitro observations, sunitinib treatment reduced primary tumor growth (Figure 7c) and extended event-free survival of mice carrying FOXC2 tumors compared to vehicle-treated control mice (Figure 7d). We also analyzed the lungs (Figure 7e) and brain (Figure 7f) of sunitinib-treated mice for the presence of HMLER-FOXC2 metastases and found that the sunitinib-treated group had significantly reduced metastatic burden compared to the vehicle-treated mice as evidenced by significantly lower photon counts in these organs following dissection (Figure 7e and f). Taken together, these results indicate FOXC2-expressing tumor cells are sensitive to PDGFR-targeted therapies and suggest sunitinib may be an effective means of targeting FOXC2-expressing cell populations, which may include EMT-derived CSC-like and metastatic phenotypes.
Discussion
Many recent studies support the emerging dogma that CSCs are responsible for chemotherapy resistance, tumor relapse and metastatic competence (reviewed in (38)). We and others have shown that CSCs and cells that have undergone EMT share many functional and molecular traits, with the corollary that engagement of the EMT program within a tumor may lead to the de novo generation and/or expansion of CSCs (8, 39). Moreover, it suggests that perturbing or targeting EMT signaling pathways may provide an effective therapeutic strategy to deplete the EMT/CSC populations within a tumor. While this seems like a rational approach, the sheer number and diversity of EMT-inducing stimuli that elicit EMT in different tumor contexts will likely hinder the development of universally-applicable therapeutics. Based on the increased expression of FOXC2 following experimental induction of EMT by numerous EMT-inducing factors as well as in stem cell-enriched fractions (CD44high/CD24low population and mammospheres), we hypothesized that FOXC2 lies at the crossroads of EMT and stem cell properties. Indeed, we found that FOXC2 expression is critical for stem cell properties, including resistance to chemotherapeutics and tumor initiation, using multiple EMT models and claudin-low breast cancer cell lines. These data clearly demonstrate that targeting FOXC2 and the associated pathways may provide an additional strategy for diminishing the CSC pool or at least those that arose via EMT.
It was surprising to see re-expression of E-cadherin in the FOXC2 depleted HMLE-Snail or TGF-β1 cells but not in Twist cells even though both Snail and Twist are continuously expressed from a retroviral expression vector and known to function similarly. While these data does not explain the failure of Snail to suppress E-cadherin expression in the absence of FOXC2, we speculate that this could be mostly due to differences in epigenetic alterations.
Claudin-low tumors account for between 25 to 39% of triple-negative breast cancers (ER−/PR−/HER2−); shown to resemble most closely with mammary epithelial stem cells; and are also enriched for markers of EMT and CSCs (35, 40). The enrichment of FOXC2 expression and its associated GES in claudin-low tumors (1, 21, 34, 41) provides the first evidence that FOXC2 transcriptional activity may play an important functional role for this molecular subtype.
We speculate that targeting the FOXC2 pathway may be an effective therapeutic strategy for tumors with enriched EMT/CSC properties. Future studies to assess the protein expression of FOXC2 pathway members in clinical specimens will be critical. As transcription factors can be difficult to directly inhibit therapeutically, a potential target for FOXC2-expressing tumors is PDGFR-β, which has FDA approved small molecule inhibitors, such as sunitinib. However, as sunitinib is a multi-targeted tyrosine kinase inhibitor, future studies using more specific pharmacological inhibitors of PDGFR-β or RNAi approaches will be required to determine if PDGFR-β is a key functional mediator of FOXC2 in EMT-derived cells and claudin-low tumors. Nevertheless, our results suggest that sunitinib and other PDGFR inhibitors may be effective in patients with claudin-low or therapy resistant tumors displaying elevated FOXC2 expression.
Supplementary Material
Acknowledgements
We acknowledge Michael Lewis, Thomas Westbrook, Jenny Chang and Charles Perou for insightful discussions and valuable advice. This work was supported by NIH/NCI CA155243-01 (SAM) and Susan G. Komen Postdoctoral Fellowship (BGH/SJW). JTC is supported by NIH R00LM009837 and grant R1006 from the Cancer Prevention & Research Institute of Texas. Flow cytometry, confocal microscopy, and animal imaging were in part funded by the Cancer Center Support Grant from the National Cancer Institute (CA16672).
Footnotes
Disclosure: The authors are inventors of a patent application based on the work described here.
References
- 1.Creighton CJ, Li X, Landis M, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A. 2009;106:13820–13825. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham BK, Fritz P, McClellan M, Hauptvogel P, Athelogou M, Brauch H. Prevalence of CD44+/CD24−/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin Cancer Res. 2005;11:1154–1159. [PubMed] [Google Scholar]
- 3.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu R, Wang X, Chen GY, et al. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med. 2007;356:217–226. doi: 10.1056/NEJMoa063994. [DOI] [PubMed] [Google Scholar]
- 6.Sheridan C, Kishimoto H, Fuchs RK, et al. CD44+/CD24− breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res. 2006;8:R59. doi: 10.1186/bcr1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138:822–829. doi: 10.1016/j.cell.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 10.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- 12.Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Savagner P, Boyer B, Valles AM, Jouanneau J, Thiery JP. Modulations of the epithelial phenotype during embryogenesis and cancer progression. Cancer Treat Res. 1994;71:229–249. doi: 10.1007/978-1-4615-2592-9_12. [DOI] [PubMed] [Google Scholar]
- 14.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 15.Moustakas A, Heldin CH. Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci. 2007;98:1512–1520. doi: 10.1111/j.1349-7006.2007.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J, Mani SA, Donaher JL, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Mani SA, Yang J, Brooks M, et al. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc Natl Acad Sci U S A. 2007;104:10069–10074. doi: 10.1073/pnas.0703900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blanco MJ, Moreno-Bueno G, Sarrio D, et al. Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene. 2002;21:3241–3246. doi: 10.1038/sj.onc.1205416. [DOI] [PubMed] [Google Scholar]
- 19.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Ugolini F, Charafe-Jauffret E, Bardou VJ, et al. WNT pathway and mammary carcinogenesis: loss of expression of candidate tumor suppressor gene SFRP1 in most invasive carcinomas except of the medullary type. Oncogene. 2001;20:5810–5817. doi: 10.1038/sj.onc.1204706. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Lewis MT, Huang J, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 22.Hollier BG, Evans K, Mani SA. The epithelial-to-mesenchymal transition and cancer stem cells: a coalition against cancer therapies. J Mammary Gland Biol Neoplasia. 2009;14:29–43. doi: 10.1007/s10911-009-9110-3. [DOI] [PubMed] [Google Scholar]
- 23.Gupta PB, Onder TT, Jiang G, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008;10:R25. doi: 10.1186/bcr1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu F, Yao H, Zhu P, et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 26.Woodward WA, Chen MS, Behbod F, Alfaro MP, Buchholz TA, Rosen JM. WNT/beta-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc Natl Acad Sci U S A. 2007;104:618–623. doi: 10.1073/pnas.0606599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart SA, Dykxhoorn DM, Palliser D, et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA. 2003;9:493–501. doi: 10.1261/rna.2192803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee GY, Kenny PA, Lee EH, Bissell MJ. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods. 2007;4:359–365. doi: 10.1038/nmeth1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci U S A. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol. 2001;3:785–792. doi: 10.1038/ncb0901-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HJ, Litzenburger BC, Cui X, et al. Constitutively active type I insulin-like growth factor receptor causes transformation and xenograft growth of immortalized mammary epithelial cells and is accompanied by an epithelial-to-mesenchymal transition mediated by NF-kappaB and snail. Mol Cell Biol. 2007;27:3165–3175. doi: 10.1128/MCB.01315-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viloria-Petit AM, David L, Jia JY, et al. A role for the TGFbeta-Par6 polarity pathway in breast cancer progression. Proc Natl Acad Sci U S A. 2009;106:14028–14033. doi: 10.1073/pnas.0906796106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dontu G, Abdallah WM, Foley JM, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herschkowitz JI, Simin K, Weigman VJ, et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8:R76. doi: 10.1186/gb-2007-8-5-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prat A, Parker JS, Karginova O, et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12:R68. doi: 10.1186/bcr2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kenny PA, Lee GY, Myers CA, et al. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol Oncol. 2007;1:84–96. doi: 10.1016/j.molonc.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Battula VL, Evans KW, Hollier BG, et al. Epithelial-mesenchymal transition-derived cells exhibit multilineage differentiation potential similar to mesenchymal stem cells. Stem Cells. 2010;28:1435–1445. doi: 10.1002/stem.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.May CD, Sphyris N, Evans KW, Werden SJ, Guo W, Mani SA. Epithelial-mesenchymal transition and cancer stem cells: a dangerously dynamic duo in breast cancer progression. Breast Cancer Res. 13:202. doi: 10.1186/bcr2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taube JH, Herschkowitz JI, Komurov K, et al. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc Natl Acad Sci U S A. 2010;107:15449–15454. doi: 10.1073/pnas.1004900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prat A, Parker JS, Karginova O, et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 12:R68. doi: 10.1186/bcr2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang JT, Gatza ML, Lucas JE, Barry WT, Vaughn P, Nevins JR. SIGNATURE: a workbench for gene expression signature analysis. BMC Bioinformatics. 2011;12:443. doi: 10.1186/1471-2105-12-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pavon-Eternod M, Gomes S, Geslain R, Dai Q, Rosner MR, Pan T. tRNA over-expression in breast cancer and functional consequences. Nucleic Acids Res. 2009;37:7268–7280. doi: 10.1093/nar/gkp787. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.