Abstract
Ischemic cardiovascular disease remains the leading cause of death worldwide. Despite advances in the medical management of atherosclerosis over the past several decades, many patients require arterial revascularization to reduce mortality and alleviate ischemic symptoms. Technological advancements have led to dramatic increases in the use of percutaneous and endovascular approaches, yet surgical revascularization (bypass surgery) with autologous vein grafts remains a mainstay of therapy for both coronary and peripheral artery disease. Although bypass surgery is highly efficacious in the short-term, long-term outcomes are limited by relatively high failure rates as a result of intimal hyperplasia, which is a common feature of vein graft disease. The supply of native veins is limited, and many individuals require multiple grafts and repeat procedures. The need to prevent vein graft failure has led to great interest in gene therapy approaches to this problem. Bypass grafting presents an ideal opportunity for gene therapy, as surgically harvested vein grafts can be treated with gene delivery vectors ex vivo, thereby maximizing gene delivery while minimizing the potential for systemic toxicity and targeting the pathogenesis of vein graft disease at its onset. Here we will review the pathogenesis of vein graft disease and discuss vector delivery strategies and potential molecular targets for its prevention. We will summarize the preclinical and clinical literature on gene therapy in vein grafting and discuss additional considerations for future therapies to prevent vein graft disease.
INTRODUCTION
Atherosclerosis results in obstruction of the coronary and peripheral arteries and is a major cause of morbidity and mortality worldwide1. Pharmacological therapies help to control symptoms and slow disease progression in mild to moderate or chronic stable disease, but more invasive interventions, including percutaneous and surgical revascularization, are required in acute or severe cases. Although advances in percutaneous and endovascular approaches over the past two decades have resulted in substantial increases in utilization of these procedures, surgical revascularization remains a mainstay of therapy for atherosclerosis of the coronary and peripheral arteries. In the United States, over 400,000 patients per year still undergo coronary artery bypass grafting (CABG)1–4. Peripheral artery disease is now recognized to be nearly as common as coronary disease, with over 8 million affected individuals in the U.S.1 Although the rate of peripheral bypass surgery has fallen significantly, over 40,000 of these procedures are performed annually5. Arterial conduits such as the internal mammary artery are preferred when available, but venous conduits, primarily the saphenous vein, are still the most commonly used vascular grafts.
Post-operatively, vein grafts undergo "arterialization", in which the vessel wall thickens due to proliferation of vascular smooth muscle cells (SMC) and increases in extracellular matrix (ECM) deposition. This process is thought to be necessary for the vein to adapt to the high wall stress and shear force within the arterial circulation, but arterialization may also contribute to subsequent stenosis, accelerated atherosclerosis, and ultimate failure of the graft. In the coronary circulation the vein graft failure rate at 1 and 10 years is reported to be 15% and 50%, respectively6, 7. In the peripheral vasculature failure rates may be as high as 50% within 5 years8. Vein graft failure is a substantial health problem, as it leads to recurrent ischemic events, such as angina, myocardial infarction, and gangrene, necessitating repeat revascularization procedures or amputation. Vein graft failure is not only devastating to individual patients but it also contributes significantly to the healthcare costs of cardiovascular disease, which exceeded $286 billion dollars in the United States in 2007 alone9.
Medical therapies, including aspirin and other anti-platelet agents and aggressive lipid-lowering therapy, have proven successful in reducing saphenous vein graft (SVG) atherosclerosis, the need for revascularization procedures, and the incidence of major adverse cardiovascular events10–12. However, despite these advances, overall vein graft failure rates have remained fairly constant since the introduction of coronary artery bypass grafting in 196813–15. The inability of traditional approaches to effectively reduce vein graft failure rates has led to the investigation of alternative strategies to maintain long-term vein graft patency, including gene therapy. Here we will review the pathophysiology of vein graft disease (VGD) and discuss vector and gene delivery strategies for the prevention of VGD. We will then discuss targets for vein graft gene therapy based on the molecular mechanisms of VGD and address additional considerations for future studies, with a focus on translating the wealth of pre-clinical data into clinical applications.
PATHOPHYSIOLOGY OF VEIN GRAFT DISEASE
Although the vasculature is often perceived as a system of passive channels, blood vessels are themselves complex organs that consist of several cell types working in concert to regulate vascular function. Vessels larger than capillaries can be divided into three layers: the intima, media, and adventitia. The composition and thickness of some of these layers depends on the size of the vessel and varies considerably between arteries and veins. The intima is the innermost layer of the blood vessel and in all normal vessels is composed of a single, continuous layer of endothelial cells (EC) and basal lamina. Outside of the intima is the media, which, in veins, consists primarily of SMCs surrounded by ECM. The adventitia is the outermost layer of the vessel and contains connective tissue, nerves, and the vasa vasorum, the small vessels that vascularize the vessel wall.
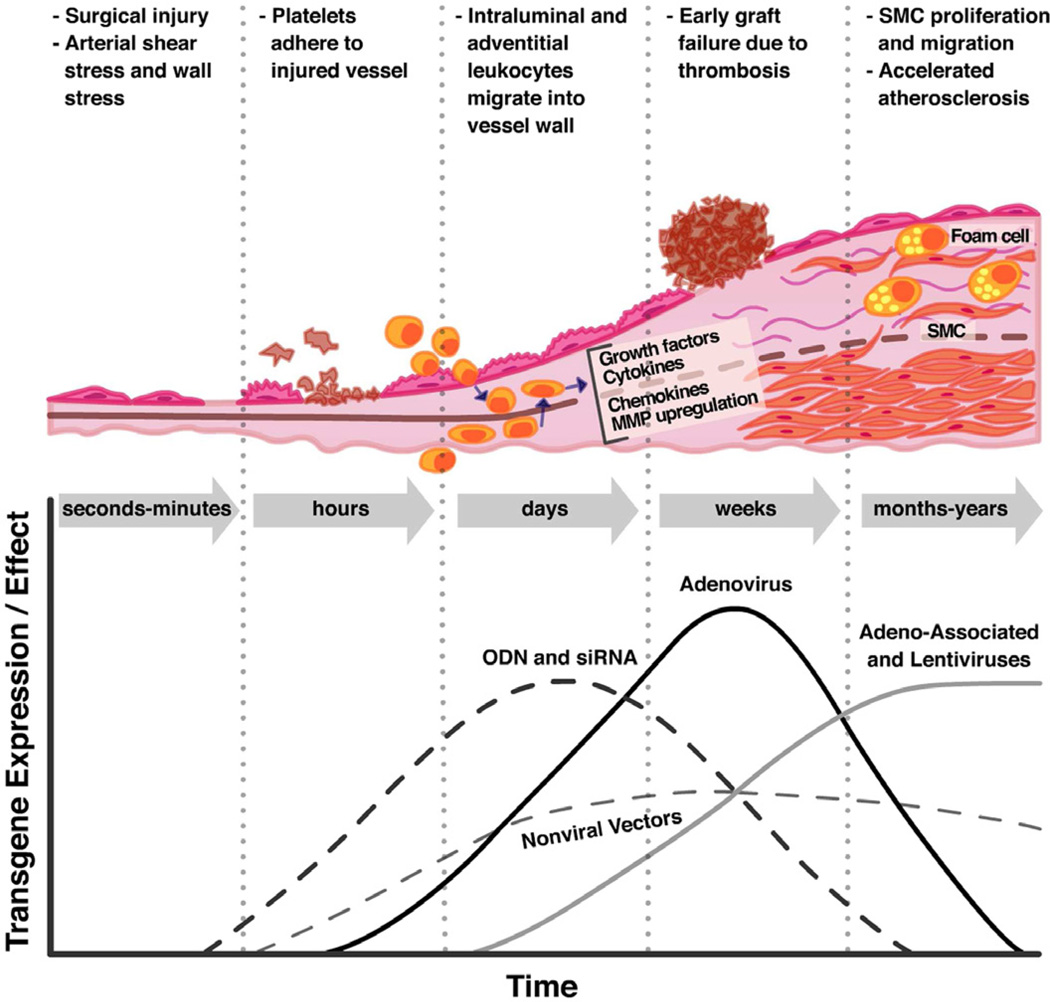
The molecular mechanisms that lead to vein graft disease are initiated as soon as the vein graft is surgically harvested (Fig 1). Removal of the vein from the circulation cuts off blood supply to the vasa vasorum, resulting in ischemia and subsequent cellular hypoxia, leaving the vein vulnerable to ischemia-reperfusion (IR) injury after engraftment (reviewed in16, 17). Physical manipulation during removal contributes to injury as well, and a “no-touch” technique for vein graft harvesting has been shown to improve patency rates (reviewed in18, 19). Once the vein is grafted into the arterial circulation, increased wall stretch and shear stress result in additional EC injury and, in some cases, death20. These altered hemodynamic forces induce gene expression changes in the remaining cells, which promote inflammation and cellular proliferation21–23. Damage to the endothelium results in platelet adhesion, activation, and aggregation; leukocyte recruitment; and activation of the coagulation cascade24–27. All of these events represent targets for molecular intervention and will be discussed in detail below. The common result of the vascular injury is local production of growth factors and hormones that stimulate a phenotypic change in SMCs in the vessel wall. Within 24 hours of vein graft implantation, vascular SMCs undergo a well-characterized shift from quiescent, contractile cells to proliferative, synthetic, and motile cells28, 29. By post-operative day 7, SMCs have migrated into the intima and begun to express ECM genes30. The thickening of the intima as a result of SMC proliferation, migration, and ECM protein production is known as intimal hyperplasia (IH) and is the hallmark of VGD. The so-called "neointima" also forms the nidus for accelerated atherosclerosis, which enhances VGD and can lead to plaque rupture and subsequent acute graft occlusion31.
Fig 1.
Schematic representation of the phases of vein graft injury and timeline of different gene therapy effects. Surgical harvesting induces injury of the vein graft endothelium, which is compounded by arterial shear force and wall stress after anastomosis. Rapid endothelial damage results in exposure of tissue factor and upregulation of adhesion molecules, increasing the risk of thrombosis and leading to adhesion and migration of inflammatory leukocytes into the vessel media. Upregulation of growth factors, cytokines, and matrix metalloproteinases (MMP) in the vein graft wall leads to SMC migration into the neointima, SMC proliferation, and resultant intimal hyperplasia and increased susceptibility to development of atherosclerosis. Onset and magnitude of transgene expression or effect varies depending on the gene delivery modality. Oligodeoxynucleotide (ODN) decoys and siRNA, which do not require gene expression, can act almost immediately; adenoviruses express high levels of transgene for brief periods; and adeno-associated viruses express moderate levels of transgene for extended periods.
The molecular mechanisms that underlie IH are complex and incompletely understood. A more detailed review of this process is beyond the scope of this article, however the reader is referred to several excellent reviews for more information28, 30, 32.
VECTORS AND DELIVERY STRATEGIES
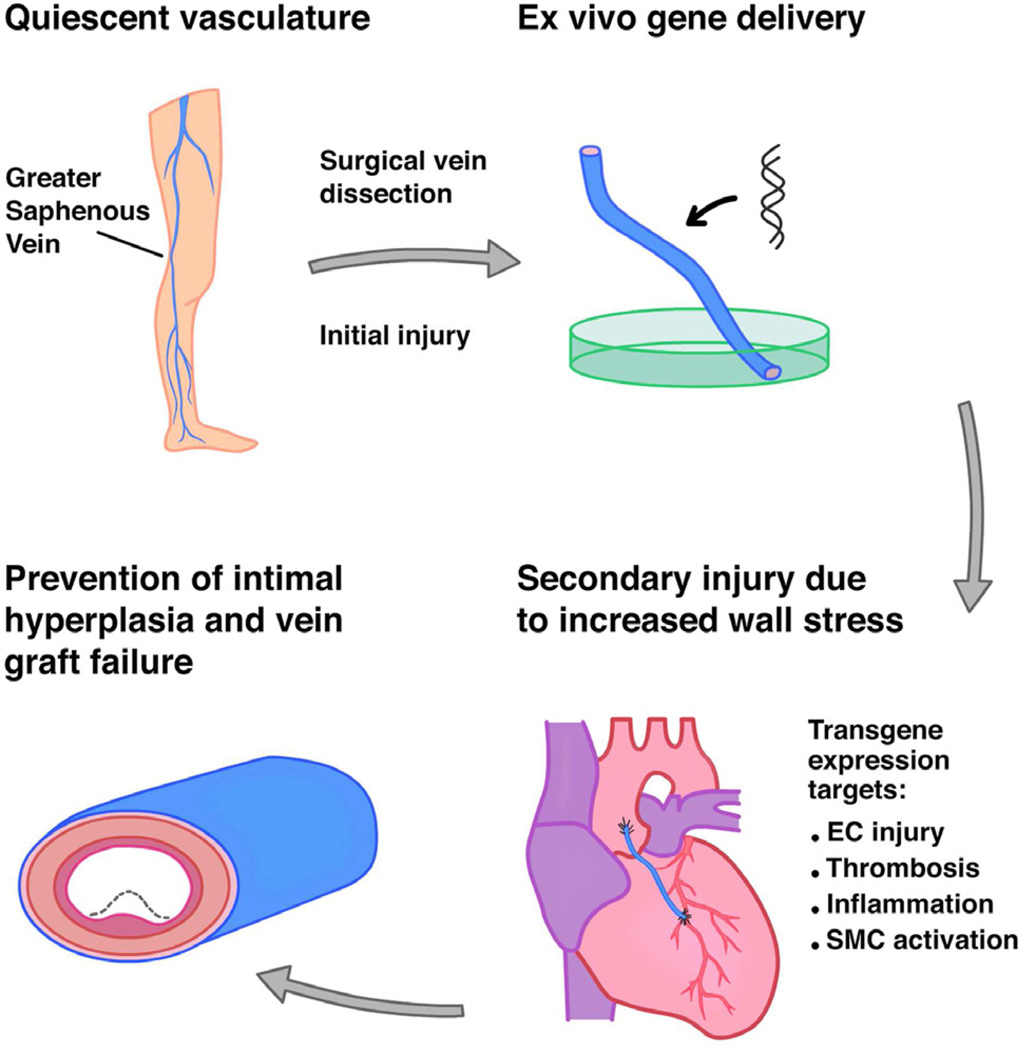
Vein graft disease is an ideal candidate for gene therapy for several reasons. First, because vein graft disease begins with the surgical dissection and removal of the vein graft from its native location, intraoperative gene therapy allows molecular reprogramming to occur at the time of the initial insult (Fig 2). Unlike many other conditions, gene delivery for prevention of VGD is one of the few scenarios in which pre-treatment is possible. Second, the time between harvesting the vein and surgical anastomosis into the arterial circulation provides a natural window for gene delivery. Third, gene delivery to vein grafts can be performed ex vivo, which substantially diminishes some of the concerns inherent in systemic gene therapy. After vector delivery to the vein graft lumen, unincorporated vector can be flushed from the graft, markedly decreasing the chances of systemic delivery. Accordingly, our group used ex vivo adenovirus delivery in a canine model of aortocoronary bypass in which the vein graft was gently flushed with heparinized saline following gene delivery, and the transgene was undetectable in multiple organs at the time of graft harvest 90 days later (Fig 3D)33.
Fig 2.
Vector delivery strategy to prevent vein graft disease. Prior to surgical harvesting of the vein graft, both the endothelium and underlying SMCs are in a quiescent state. Injury occurs within minutes as a result of ischemia and mechanical trauma and is exacerbated upon anastomosis into the arterial circulation. Transgene delivery is performed ex vivo and occurs rapidly and with a low risk of systemic transgene toxicity. Endothelial injury, thrombosis, inflammation, and SMC activation have been targeted for the prevention of vein graft intimal hyperplasia.
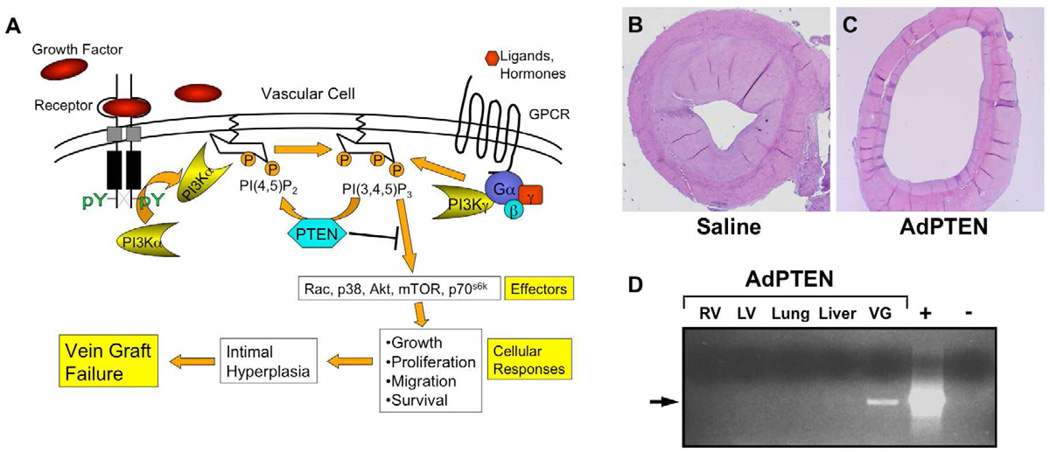
Fig 3.
Targeting vein graft intimal hyperplasia using PTEN overexpression. (A) Numerous growth factors and hormones activate different isoforms of phosphoinositide 3-kinase (PI3K) via receptor tyrosine kinases and G protein-coupled receptors (GPCR). PI3K activates numerous downstream effector molecules, which promote the cellular responses that collectively lead to intimal hyperplasia and vein graft failure. PTEN is a lipid phosphatase that specifically hydrolyzes the phospholipid product of PI3K, thereby inhibiting PI3K-mediated SMC growth and intimal thickening. (B–C) Adenovirus-mediated overexpression of PTEN (C) in a canine aorto-coronary bypass graft model resulted in inhibition of intimal hyperplasia at 90 days compared to saline-treated grafts (adapted from Hata et al33). (D) The PTEN transgene was detectable by PCR only in the AdPTEN-treated vein graft (VG) and not in other tissues at the time of harvest. RV, right ventricle; LV, left ventricle.
The development of gene transfer vectors is a major challenge in the field of gene therapy, but it is important to consider features of existing vectors and gene targeting modalities as they pertain to the pathophysiology of IH (Fig 1). For additional information on gene transfer strategies, the reader is referred to other reviews in this series.
Non-viral vectors
Non-viral vectors are attractive options for gene delivery because they are relatively safer than viral vectors and easier to prepare. However, non-viral vectors have historically been plagued by poor transfection efficiency. In the following section, we review non-viral vector-based gene delivery approaches to prevent VGD, including naked plasmid DNA, oligodeoxynucleotides (ODN), small interfering RNA (siRNA), and polymeric nanoparticles.
Naked plasmids, ODN, siRNA
Non-viral vectors are a promising strategy for VGD for several reasons. First, they can be optimized to efficiently transfect both ECs and SMCs34–37. Second, they can effect very early transgene expression. For example, Santel et al demonstrated uptake of fluorescently labeled siRNA in mouse endothelium 20 minutes following systemic delivery34. Intervention at the earliest stages of endothelial injury may promote EC integrity and help preserve the anti-inflammatory, anti-thrombotic effects of the healthy endothelium. In addition to targeting the early phases of VGD, non-viral vectors have induced sustained transgene expression for up to 8 weeks in a rabbit vein graft model36. Whereas siRNA and short hairpin RNA (shRNA) can be used to silence expression of a target gene within the vein graft wall, ODNs represent a unique approach to gene therapy. These gene-targeting tools are typically composed of short double-stranded DNA sequences that mimic transcription factor binding sites in the genome, thereby acting as "decoys" to block transcription of genes involved in cellular proliferation. Importantly, ex vivo delivery of ODNs and siRNA has been shown to be safe in both pre-clinical and clinical studies38.
Nanoparticles
Nanoparticles (NPs) are submicrometer-sized polymeric colloidal particles39. The physicochemical characteristics of NPs are ideally suited for genetic modulation of vein grafts, as they allow for efficient intracellular delivery of transgenes, can effect sustained gene delivery, and have an excellent safety profile. Successful therapeutic intervention for VGD is highly dependent on efficient intracellular gene delivery. NPs have demonstrated increased intracellular uptake compared to microparticles in both in vitro and in vivo models40, 41. Furthermore, certain polymeric formulations are able to escape lysosomal degradation via a reversal of surface charge, enabling entry into the cytosolic compartment42. Once within the cytosol the therapeutic agent of interest, such as a transgene, is slowly released from the polymeric matrix. In fact, polymer properties can be manipulated to regulate the release of their cargo over a predetermined time frame, thereby achieving the desired therapeutic effect43, 44, 41 Sustained gene delivery would be particularly helpful in suppressing SMC proliferation prior to re-endothelialization. Finally, unlike viral vectors, there is much less concern about the safety of NPs for gene delivery. The most extensively studied NP polymers are hydrolyzed to biologically compatible byproducts, which are eliminated by the citric acid cycle45.
Although no studies to date have used polymeric NPs for gene delivery to prevent VGD, there is evidence to support their use in this context. First, investigators have demonstrated efficient uptake of polymeric NPs by SMCs and ECs, the primary cell types responsible for development of the neointimal lesion42, 46. Additionally, Cohen-Sacks et al demonstrated that NP delivery of a PDGF receptor B antisense RNA inhibited neointimal hyperplasia in a rat carotid artery injury model47. Taken together, these studies support the use of NPs as a promising approach for gene delivery to prevent VGD.
Viral vectors
Viral vectors used in vein graft models include adenovirus (Ad), adeno-associated virus (AAV), and lentivirus. Each vector has distinct cell tropism, transgene expression profiles (Fig 1), and safety concerns that impact its potential utility for prevention of VGD.
Adenovirus
Adenoviruses (Ad) are non-enveloped double-stranded DNA viruses that are, in many respects, ideally suited for use in VGD. Ad vectors have large packing capacities, efficiently infect both dividing and quiescent cells, and begin to express the transgene within hours after infection33, 48. The ability to target non-dividing cells is a feature common to all of the vectors discussed here and is particularly important given that target cells within the vessel wall are typically quiescent and do not become activated until the time of graft harvest, when the vector will be delivered. However, because SMCs become activated rapidly after removal of the vein graft from its native location, the ability to infect proliferating cells is an advantage. However, there are some aspects of adenovirus biology that may limit its clinical use. Unlike the mostly seronegative animal models used in pre-clinical vein graft studies, approximately 60% of the human population is seropositive for antibodies against adenovirus49. Thus, if Ad vectors are delivered systemically in humans, it is likely that infection efficiency would be compromised. First-generation adenoviruses are also quite immunogenic as a result of their large (~30 kb) genome and concomitant expression of viral genes in infected cells. Cells expressing adenoviral proteins are aggressively eliminated by cytotoxic T cells, resulting in limitation of transgene expression, endothelial injury, and potential exacerbation of intimal hyperplasia50–52. Moreover, the viral promoters commonly used in gene therapy vectors are rapidly methylated, resulting in promoter silencing and inhibition of transgene expression53–55. In vivo gene expression with adenoviral vectors typically peaks by day 7 and is gone by day 2856. Recently, attempts to improve vascular gene therapy have utilized third-generation or helper-dependent adenoviruses (HDAd), in which all viral genes are deleted, thereby decreasing immunogenicity57, 58. HDAd vectors have demonstrated expression for up to 8 weeks after infection of rabbit carotid arteries but with significantly less inflammation and intimal hyperplasia than first-generation Ad59. To our knowledge, however, HDAd has not been tested in a vein graft model. Finally, there are legitimate safety concerns regarding the use of adenovirus in human patients stemming from a patient death during a clinical trial of gene therapy using direct intra-hepatic adenovirus administration, although there are important differences in the mode of gene delivery to vein grafts (discussed below)60, 61.
Adeno-associated virus
Adeno-associated viruses are single-stranded DNA viruses of the parvovirus family62. Several features of AAV suggest that it would be preferable to adenovirus for vascular gene therapy63, 64. AAVs effect long-term gene expression (months to years), making them particularly useful in targeting SMC proliferation, which continues for weeks to months following engraftment30, 65. Persistent AAV-mediated transgene expression and inhibition of SMC proliferation and migration may help prevent late graft failure, provided the target cells are infected at the time of gene delivery. Recombinant AAVs are deleted of all viral genes and therefore have substantially lower immunogenicity than adenovirus, and they have shown an excellent safety profile in pre-clinical and clinical trials66–69. Despite these qualities, most currently used AAV vectors are suboptimal for targeting vein grafts, primarily due to their lack of native tropism for ECs and SMCs, the primary cellular elements of the intimal lesion70, 71. This issue can be addressed by modifications of the AAV capsid, which dramatically enhance transduction of ECs and SMCs70–72. A potential limiting factor in AAV-mediated gene delivery for vein graft disease is the relatively slow onset of transgene expression compared to that of Ad vectors. Low levels of transgene expression during the first few weeks following EC injury may be insufficient to prevent progression of SMC growth and proliferation. This delay in gene expression arising from recombinant AAV vectors is due to the time required to convert its single-stranded DNA genome into a double-stranded form amenable to transcription. Delayed onset of AAV-mediated transgene expression can be circumvented by the use of self-complementary AAV vectors (scAAV, reviewed in73), which contain a DNA motif that enables folding into a double-stranded DNA genome without the requirement for DNA synthesis. To date, scAAV has not been evaluated for vascular gene delivery.
Lentivirus
Lentiviruses are derived from the retrovirus family. Lentiviral vectors efficiently transduce vascular tissues, have low immunogenicity, and yield long-lasting transgene expression74–76. However, the potential for insertional mutagenesis is a major safety concern, which dampens enthusiasm for the use of lentiviral vectors in humans77. Non-integrating vectors have been developed, but their safety and efficacy have yet to be evaluated in the vasculature78.
Vector delivery strategies
It is important to note that many of the concerns and/or weaknesses related to the use of viral vectors are circumvented in the case of vein graft treatment simply by virtue of the vector deliver strategy (Fig 2). Because vein grafts are typically treated ex vivo after removal of blood from the vein, there is a low likelihood of antibody-mediated clearance of the vector prior to infection of the vessel wall. Furthermore, systemic gene therapy is likely to require in vivo delivery of high titers of virus. Because vein graft treatment involves highly localized tissue infection, lower titers of virus can be used to effect efficient transgene expression, thus there is a reduced probability of systemic toxicity.
Delivery of gene transfer vectors to vein grafts has been accomplished in a variety of ways. They have been applied extra-luminally, intra-luminally, or both, and delivered with or without pressurization of the vein. The primary approach to extra-luminal vector delivery is via application of a gel (e.g., poloxamer) containing the transgene and a transfection reagent to the adventitia. Diffusion of the transfection complex into the media and intima allows expression throughout the vessel wall. This technique has been used successfully in animal models of vein grafting to deliver siRNA targeting the proliferative proteins STAT-3 and c-myc and to deliver cDNA encoding platelet-derived endothelial cell growth factor (PD-ECGF)37, 79, 80. Extra-luminal delivery appears to be safe in animal models, and the application of gel lacking transgene does not exacerbate IH80. Intra-luminal delivery has been used for both viral and non-viral vectors. Following vein harvest, the gene transfer solution is infused into the vein lumen and the graft is typically incubated in buffer at room temperature for 20–30 minutes with a distension pressure of approximately 10 mmHg. Following infection, gentle flushing of the vein with heparinized saline removes the majority of unincorporated viral particles33, 81, 82. This strategy has demonstrated excellent transduction efficiency and safety, however there is some concern that higher distending pressures may worsen IH83. Non-distending pressure-mediated transfection is a hybrid approach that delivers viral or non-viral vectors both intra- and extra-luminally. The gene transfer solution is introduced into the lumen and also bathes the vein, which is incubated in a chamber that is pressurized to 300 mmHg for 10 minutes without distention. This method has been investigated extensively and was used to deliver an ODN decoy in the PREVENT trials, the only clinical trials to date of gene therapy for VGD14, 38, 84, 85. The PREVENT trials are discussed more extensively below.
POTENTIAL MOLECULAR THERAPEUTIC TARGETS IN VEIN GRAFT DISEASE
The pathogenesis of VGD presents many potential molecular targets for gene therapy. Vascular quiescence is driven largely by a healthy endothelium, which inhibits vascular inflammation, thrombosis, and pathological vascular SMC growth through the production of vasoprotective factors, and these processes are adversely affected as a result of vein graft harvesting and subsequent endothelial injury (Fig 1). Accordingly, pre-clinical and clinical studies have utilized gene delivery approaches to target molecular mediators of many different steps in the development and progression of vein graft disease. The primary cellular targets in most of these studies have been endothelial and vascular SMCs, however the relative importance of each cell type in the prevention of VGD remains unclear. In theory, maintenance of endothelial integrity would prevent thrombosis, inflammation, and subsequent SMC injury and neointima formation. Alternatively, interventions that target SMCs directly would prevent the injury-induced shift toward a more proliferative, synthetic phenotype, preventing neointima formation irrespective of endothelial damage and until re-endothelialization of the vein graft can occur. Although it is not known whether one approach is superior, it is important to consider the dynamic nature of different cells within the remodeling vein graft. At least one study has suggested that loss of Ad vector-mediated transgene expression is due in part to SMC proliferation within the neointima and gradual replacement of transduced cells86, which could affect the functional outcome of gene delivery. This section will review both pre-clinical and clinical studies of gene therapy for VGD in the context of these mechanistic considerations, and these studies are summarized in Table I.
Table I.
Summary of pre-clinical and clinical studies of gene delivery modalities for the prevention of vein graft disease.
| Pre-Clinical Studies | ||||||
|---|---|---|---|---|---|---|
| Target | Approach | Vector | Model | Delivery Modality | Summary of Results | Reference |
| EC Health | eNOS overexpression | HVJ-liposome | Canine femoral artery bypass | Intraluminal distending pressure-mediated transfection | >50% reduction in intimal thickness at 4 weeks | 94 |
| eNOS overexpression | HVJ-liposome | Hypercholesterolemic rabbit jugular vein-carotid artery bypass | Intraluminal distending pressure-mediated transfection | 30% reduction in intimal thickness at 4 weeks | 95 | |
| Reduction in neointimal macrophages at 4 weeks, decreased PCNA positive cells at 2 weeks | ||||||
| COX-1 (PGI2) overexpression | Adenovirus | Hypercholesterolemic rabbit jugular vein-carotid artery bypass | Intraluminal without distension | No difference in IH | 100, 101 | |
| Increased luminal area, blood flow, and PGI2 at 4 weeks | ||||||
| EC-SOD overexpression (in combination with 35K and TIMP-1) | Adenovirus | Rabbit jugular vein-carotid artery bypass | Intraluminal distending pressure-mediated transfection | Reduced I/M ratio at 2 weeks in combination with 35K and at 2 and 4 weeks with TIMP-1 | 108 | |
| Reduced macrophage infiltration and BrDU staining in combination with 35K at 4 weeks | ||||||
| Slight reduction in superoxide anion production at 2 weeks with EC-SOD alone | ||||||
| Thrombosis | Thrombomodulin overexpression | Adenovirus | Rabbit jugular-carotid artery bypass | Intraluminal distending pressure-mediated transfection | No difference in IH | 118 |
| Reduced thrombin generation at 7 days | ||||||
| tPA overexpression | Adenovirus | Porcine saphenous-carotid artery bypass | Intraluminal without distension | Reduction in flow-restricting thrombi at 1 day | 122 | |
| Inflammation | MCP-1 inhibition (7ND-MCP-1) | Naked plasmid | Hypercholesterolemic mouse donor IVC-carotid artery bypass | Systemic expression (IM injection and electroporation) | 51% decrease in IH at 4 weeks | 135 |
| No change in macrophage infiltration | ||||||
| MCP-1 inhibition (7ND-MCP-1) | Adenovirus | Canine jugular-carotid artery bypass | Submersion in adenoviral solution without pressurization | Decreased IH and lumen stenosis at 4 weeks | 136 | |
| Decreased PCNA positive cells and macrophages at 4 weeks | ||||||
| MCP-1 inhibition (CCR2 silencing) | Lentiviral shRNA | Hypercholesterolemic mouse donor IVC-carotid artery bypass | Adventitial application of bioprotein gel | 38% reduction in IH at 4 weeks | 137 | |
| CC-Chemokine inhibition (35K) | Adenovirus | Hypercholesterolemic mouse donor IVC-carotid artery bypass | Systemic expression (IV injection 2 days pre-op) | Reduced wall thickness, Ki67 positive cells, SMA positive cells, and macrophage infiltration at 2 and 4 weeks | 144 | |
| CC-Chemokine inhibition (35K) | Adenovirus | Rabbit jugular-carotid artery bypass | Intraluminal distending pressure-mediated transfection | Reduced I/M ratio at 2 weeks | 145 | |
| Reduced macrophage infiltration and BrdU incorporation at 4 weeks | ||||||
| NFκB inhibition | ODN | Canine aortocoronary bypass | Non-distending pressure-mediated transfection | Decreased intimal area and PCNA positive cells at 4 weeks | 139 | |
| NFκB inhibition | ODN | Rabbit hypercholesterolemic jugular vein-carotid artery bypass | Non-distended; pressure-mediated transfection | Decrease in IH at 4 weeks | 35 | |
| Improved endothelium-mediated vasorelaxation | ||||||
| PD-ECGF overexpression | Naked plasmid | Rabbit jugular-carotid artery bypass | Adventitial application of bioprotein gel | Reduced intimal thickness at 8 weeks | 79 | |
| Reduced PCNA positive SMC at 2 weeks | ||||||
| VSMC | Gβγ inhibition | Adenovirus | Canine aortocoronary bypass | Intraluminal pressure-mediated delivery | Reduced intimal area and I/M ratio at 3 months | 82 |
| PTEN overexpression | Adenovirus | Canine aortocoronary bypass | Intraluminal pressure-mediated delivery | Reduced intimal area and I/M ratio at 3 months | 33 | |
| TIMP-3 overexpression | Adenovirus | Porcine saphenous-carotid artery bypass | Intraluminal delivery without distention | 58% reduction in IH at 28 days | 152 | |
| TIMP-3 overexpression | Adenovirus | Porcine saphenous-carotid artery bypass | Intraluminal delivery without distention | Reduced IH at 3 months | 153 | |
| PCNA inhibition; cdc2 inhibition | HVJ liposome-ODN | Rabbit jugular-carotid artery bypass | Intraluminal; pressure-mediated transfection | Increased vasoresponsiveness to acetylcholine at 6 weeks | 156 | |
| E2F inhibition | ODN | Rabbit hypercholesterolemic jugular vein-carotid artery bypass | Non-distended; pressure-mediated transfection | Decrease in IH at 6 months | 166–169 | |
| Clinical Studies | ||||||
| Trial | Approach | Vector | Model | Delivery Modality | Summary of Results | Reference |
| PREVENT I (Phase I) | E2F inhibition | ODN | Human infrainguinal arterial bypass with autologous vein | Non-distended; pressure-mediated transfection | Decrease graft occlusions, revisions, or critical stenoses at 12 months | 38 |
| PREVENT II (Phase IIb) | E2F inhibition | ODN | Human coronary artery bypass grafting with autologous vein | Non-distended; pressure-mediated transfection | 30% relative reduction in critical stenosis at 12 months | 170 |
| PREVENT III (Phase III) | E2F inhibition | ODN | Human infrainguinal arterial bypass with autologous vein | Non-distended; pressure-mediated transfection | No significant difference in primary graft patency or limb salvage at 12 months | 171 |
| PREVENT IV (Phase III) | E2F inhibition | ODN | Human coronary artery bypass grafting with autologous vein | Non-distended; pressure-mediated transfection | No significant difference in critical graft stenosis at 12 months | 14 |
Abbreviations: CCR2, C-C chemokine receptor-2; COX-1, cyclooxygenase-1; EC, endothelial cell; EC-SOD, extracellular superoxide dismutase; eNOS, endothelial nitric oxide synthase; HVJ, hemagglutinating virus of Japan; IH, intimal hyperplasia; I/M, intima to media; IVC, inferior vena cava; MCP-1, monocyte chemoattractant protein-1; NFκB, nuclear factor κB; ODN, oligodeoxynucleotide; PCNA, proliferating cell nuclear antigen; PD-ECGF, platelet-derived endothelial cell growth factor; PGI2, prostaglandin I2; PREVENT, Project of Ex Vivo Vein Graft Engineering via Transfection; PTEN, phosphatase and tensin homology deleted on chromosome 10; SMA, smooth muscle actin; TIMP, tissue inhibitor of metalloproteinase; tPA, tissue plasminogen activator; VSMC, vascular smooth muscle cell.
Endothelial injury and loss of endothelial vasoprotective factors
Endothelial injury and denudation during vein graft harvest and arterial engraftment results in loss of the vasoprotective substances that are normally produced by healthy ECs. Two of these factors, the gas nitric oxide (NO) and the eicosanoid prostacyclin (PGI2), have been targeted using gene therapy approaches. Pharmacological approaches to modulating these pathways are reviewed in87 and88.
Endothelial nitric oxide synthase (eNOS), one of the enzymes that synthesizes NO, is constitutively expressed in normal vascular endothelium and is critical in modulating several aspects of vascular physiology. NO released from the endothelium induces vasodilation, prevents SMC proliferation, and inhibits adhesion of platelets and leukocytes to the endothelium89–91. Impaired release of NO from the damaged vein graft endothelium is therefore thought to contribute to the initiation of IH. This hypothesis is supported by studies of adenovirus-mediated overexpression of bovine eNOS (Ad-eNOS) in the endothelium and adventitia of human saphenous vein segments. Saphenous veins treated with Ad-eNOS demonstrated increased NO release and improved vascular function in a ring relaxation test92. In another study from the same group, human saphenous vein segments were cultured ex vivo and IH was assessed histologically. Vein segments treated with Ad-eNOS had reduced intima to media ratios compared to untreated vein segments93. These findings were applied in animal models of vein graft disease with moderate success. Intraluminal transfection of bovine eNOS cDNA encapsulated in hemagglutinating virus of Japan (HVJ)-liposomes reduced intimal thickness at 4 weeks post-graft in both canine and hypercholesterolemic rabbit models94, 95. However the reduction in intimal thickness was modest, particularly in the rabbit model (30% reduction vs. control). This may be due to damage to the endothelium and media resulting from the transfection process, which utilized distending pressure83.
PGI2 is also constitutively produced by healthy endothelium. PGI2 is a potent vasodilator and inhibitor of platelet aggregation, leukocyte adhesion, and SMC proliferation96–98. Cyclooxygenase-1 (COX-1), the rate-limiting enzyme in the PGI2 synthesis pathway, must be continuously expressed, as its catalytic mechanism results in its rapid degradation99. Adenoviral overexpression of COX-1 in a porcine carotid angioplasty model increased PGI2 production and prevented arterial thrombosis. However, treatment of rabbit vein grafts with adenovirus encoding human COX-1 had no effect on intimal thickness at four weeks despite improved PGI2 production and blood flow through the graft100, 101. Although COX-1 failed to prevent IH, these results highlight that, although intimal thickness is a useful indicator of vein graft disease, it does not always correlate with graft function (i.e., perfusion).
Even in intact ECs, endothelial function can be impaired due to generation of reactive oxygen species (ROS) following graft reperfusion (reviewed in16, 17, 88). The principle ROS studied in this context is superoxide (O2−), which reacts with NO to form the weaker vasodilator peroxynitrite (ONOO−). ONOO− formation further disrupts NO production by contributing to eNOS uncoupling and impairing PGI2 production through inhibitory tyrosine nitration of PGI2 synthase102, 103. The degradation of O2− is initiated by superoxide dismutases (SOD), which catalyze the conversion of O2− to hydrogen peroxide (H2O2), which is then degraded by the enzyme catalase. Several groups have demonstrated that, compared to SVGs, arterial grafts have greater SOD activity and NO release at the time of harvest104, 105. In a porcine jugular-carotid interposition model, vein grafts had increased O2− production, decreased SOD activity, and greater lipid accumulation compared to arterial grafts at 1 month106. These differences in ROS processing may contribute to the increased patency of arterial grafts. Intraoperative PEG-SOD treatment in a rabbit jugular-carotid interposition model reduced the I/M ratio and increased luminal area of the graft at 28 days, but no improvement in vasomotor function was observed107. Intraoperative delivery of adenovirus encoding extracellular-SOD (AdEC-SOD) reduced macrophage accumulation but not I/M ratio or BrdU incorporation at 4 weeks in a rabbit model108. However, combination of AdEC-SOD with other genes was more effective at reducing I/M ratio and proliferation in the graft wall than either therapy alone (see Table I). Catalase gene therapy improves liver function following IR injury in a mouse model, but has not been applied to models of VGD109. Likewise, other anti-oxidant enzymes (such as heme oxygenase-1 and glutathione peroxidase) have been used to reduce atherosclerosis and arterial neointimal hyperplasia in a number of animal models but have not been tested in VGD (reviewed in110, 111).
Thrombosis
In addition to the loss of endothelial vasoprotective substances, endothelial injury and subsequent denudation exposes the basement subendothelial matrix to circulating platelets, which initiates the coagulation cascade. The release of coagulation factors results in thrombosis and graft occlusion, and release of certain factors exacerbates IH by promoting SMC proliferation and migration112–114. Anticoagulation reduces the risk of early graft thrombosis, but does not eliminate it. Furthermore, systemic anticoagulation is associated with side effects, primarily bleeding complications115, 116, which is particularly dangerous in the post-operative period. Gene therapy to inhibit vein graft thrombosis would circumvent these systemic side effects.
There have been comparatively few studies using gene therapy to target thrombosis. The two primary targets have been the anti-coagulant proteins thrombomodulin (TM) and tissue plasminogen activator (tPA). TM binds to thrombin, inactivating its pro-coagulant effects and activating the antithrombotic protein C pathway. TM activity in vein grafts decreases dramatically within 90 minutes of engraftment into the arterial circulation117, 118. Cationic liposome-mediated delivery of TM improved graft thromboresistance in a rat model119. However, adenoviral delivery of human TM in a rabbit model had no effect on graft wall thickness at 6 weeks118. Similar results were obtained using pharmacological coagulation inhibitors. For example, aspirin applied locally to vein grafts attenuated acute thrombosis in a mouse model, and although systemic aspirin reduced 1-year clinical vein graft failure rates, it had no effect on long-term (3-year) graft patency120, 121. The other model tested the ability of tPA, the primary intravascular activator of plasminogen, to promote graft thrombolysis. Like TM, tPA expression is decreased after engraftment. Adenoviral overexpression of tPA in a porcine model decreased flow-restricting thrombi 1 day after engraftment122. However, the effect of tPA on IH is unclear. In a rabbit arterial injury model, Ad-tPA treatment promoted IH, possibly due to ECM degradation by tPA123. Conversely, in a rabbit vein graft model Ad-tPA reduced IH at 4 weeks124. These results may be explained by differences in the pathogenesis of IH in arteries and veins, and reinforce the importance of studying gene delivery in disease-specific models. However, further studies are required to ensure that tPA overexpression does not have adverse effects on IH in VGD models.
Inflammation
In the week following graft implantation, platelet activation at sites of endothelial damage initiates an inflammatory cascade in the vein graft wall that promotes SMC proliferation, intimal thickening, and accelerated atherosclerosis31. P-selectin glycoprotein ligand-1 (PSGL-1) on the surface of rolling leukocytes interacts with P-selectin on adherent platelets, activating leukocyte migration into the vessel wall125. Migration is mediated by the engagement of leukocyte transmembrane proteins with the endothelial counter-receptors ICAM-1 and VCAM-1. Activated leukocytes, ECs, and SMCs secrete a number of cytokines and growth factors that potentiate the inflammatory response, including MCP-1, IL-1, IL-6, IL-8, and TNFα. The molecular mechanisms of endothelial cell interaction with the adaptive and innate immune system are reviewed in detail elsewhere126–128. Many of these adhesion molecules and cytokines have been shown to play a role in the development of VGD, and several of these genes have been used in VGD gene therapy approaches.
In a rat VGD model, pharmacological macrophage depletion after engraftment decreased MCP-1 and TGFβ expression in the graft and decreased intimal thickness at 4 weeks129. This led to several approaches to block macrophage recruitment, with a focus on MCP-1, a potent monocyte/macrophage chemokine secreted by SMCs, ECs, fibroblasts, and leukocytes130. In humans, elevated MCP-1 (both circulating and localized to atherosclerotic lesions) correlates with increased risk of restenosis in patients undergoing percutaneous angioplasty131, 132. In rat vein graft models, MCP-1 is upregulated within hours of engraftment, peaks during the first week, and persists for at least 8 weeks129, 130. 7ND-MCP-1 is a dominant negative N-terminal deletion mutant of MCP-1 that binds to the MCP-1 receptor, CCR2, but does not promote monocyte/macrophage migration133, 134. Treatment of veins with naked plasmid DNA encoding 7ND-MCP-1 inhibited SMC proliferation in vitro and resulted in an approximately 50% reduction in IH in an ApoE3 Leiden mouse model and in cultured segments of human saphenous veins135. These results were confirmed in a canine model in which Ad-7ND-MCP-1 treatment reduced IH without impairing re-endothelialization136. Another innovative approach to blocking the MCP-1 pathway used lentivirus to deliver CCR2 shRNA to grafts in an ApoE3 Leiden mouse model. Treated veins had decreased SMC proliferation, macrophage recruitment, and graft wall thickness137.
The transcription of many of the inflammatory cytokines and adhesion molecules described above is upregulated by NFκB, a transcription factor that is activated by inflammatory stimuli and other cell stressors. The role of NFκB in vascular inflammation (with a focus on atherosclerosis) has been reviewed previously138. Pressure-mediated transfection of the lumen and adventitia of vein grafts with an NFκB decoy ODN in canine and hypercholesterolemic rabbit models decreased intimal thickness and VSMC proliferation35, 139. The reduction in the intima to media (I/M) ratio in the rabbit study was modest, particularly in light of the increased medial area in the NFκB ODN-treated group, but the treatment did significantly inhibit macrophage recruitment to the graft wall. Interestingly, the NFκB decoy-treated grafts also displayed improved acetylcholine-induced vasodilation at 4 weeks post-implantation. This relaxation was inhibited by the potent nitric oxide synthase inhibitor L-NAME, indicating that the NFκB ODN may preserve some of the vasoprotective functions of the surviving endothelium35.
Another novel approach to broadly inhibiting the inflammatory response utilized the vaccinia virus protein, 35K. 35K is a soluble poxvirus protein that binds to CC chemokines such as members of the MCP family and RANTES, blocks their interactions with chemokine receptors, and prevents their physiological effects in vitro and in vivo140–142. Intravenous injection of adenovirus encoding 35K (Ad35K) decreased systemic atherosclerosis in ApoE−/− mice fed a high fat diet and reduced vein graft wall thickness, proliferation, and macrophage infiltration in ApoE−/− mice fed standard chow143, 144. The results of the vein graft study are striking at 2 weeks post-implantation but seem to diminish between 2 and 4 weeks, possibly due to the use of an adenoviral expression vector. Similar results were reported in a rabbit vein graft model, in which intraluminal infection of the graft with Ad35K resulted in modest decreases in I/M ratio but dramatic decreases in macrophage infiltration145. A membrane-targeted mutant of 35K, m35K, improved CC chemokine inhibition in murine peritonitis and hepatitis models, but has not been tested in a vein graft model, in which sequestration of m35K to the membrane may prove particularly useful146.
Finally, several studies have demonstrated that inflammation of the adventitia contributes to neointimal thickening in arterial balloon injury models147–149 (reviewed in150). Using an "outside-in" approach, genes that antagonize growth factor signaling and ECM remodeling were delivered directly to the adventitia and successfully reduced IH after arterial injury. In a rabbit vein graft model, poloxamer hydrogel-mediated delivery of the gene encoding PD-ECGF to the graft adventitia resulted in efficient transgene expression and decreased intimal thickness at 8 weeks79. Several reports discussed in this review utilized protocols that involve submersing grafts in vector-containing solution during gene delivery, resulting in gene transfer to the adventitia as well as the lumen, but to our knowledge no studies have utilized perivascular gene transfer to target adventitial inflammation exclusively for the prevention of vein graft IH.
Smooth muscle cell proliferation and migration
SMCs are the predominant cells within the neointimal lesion. The goal of gene therapy targeting SMCs is to restore cellular quiescence and the contractile phenotype. In the quiescent vasculature, SMC proliferation and migration are inhibited in part by integrin-mediated contacts with the ECM151. Following SMC activation, matrix-degrading metalloproteinases (MMPs) digest the ECM, facilitating SMC proliferation and migration into the neointima. George et al demonstrated that overexpression of tissue inhibitor of metalloproteinase (TIMP)-3 significantly reduced IH in both short-term (28-day) and long-term (3-month) porcine vein graft models.152, 153
There is a significant body of literature demonstrating that, following vein graft anastomosis, increased wall stress upregulates fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF)154, both of which are potent SMC mitogens and chemoattractants155. Increased arterial wall stress and shear forces also directly activate growth factor receptors21. The ultimate effect of increased growth factor signaling is to promote cell cycle progression and SMC proliferation, thereby enhancing intimal hyperplasia. Mann et al targeted SMC proliferation in a rabbit model of jugular vein-carotid artery interposition grafting by inhibiting expression of the cell cycle regulatory genes proliferating cell nuclear antigen (PCNA) and cdc2 kinase using antisense ODN156. HVJ-liposomes were used to deliver ODN complexes to the vein graft wall by pressure-mediated transfection, resulting in a reduction in intimal hyperplasia and inhibition of diet-induced atherosclerosis. Many of the mitogens responsible for SMC proliferation act via G protein-coupled receptors (GPCRs). Members of our group used a first-generation adenovirus to express a dominant negative peptide inhibitor derived from the carboxyl terminus of the β-adrenergic receptor kinase (βARKct) in a canine model of aortocoronary bypass surgery. Inhibition of Gβγ signaling with this approach significantly reduced IH at 90 days.82
Recently, our group has focused on the phosphoinositide 3-kinase (PI3K) pathway as a target for inhibition of intimal hyperplasia in models of both arterial injury and vein graft disease33, 157, 158. PI3K is activated by most growth factor receptors and GPCRs and acts through multiple downstream effector molecules to promote cellular growth, proliferation, migration, and survival159, 160, all of which play a role in intimal hyperplasia and vein graft disease (Fig 3A). The therapeutic benefit of targeting the PI3K pathway has been proven in clinical trials of sirolimus-eluting stents, which potently inhibit intimal hyperplasia and restenosis following percutaneous coronary intervention161, 162. Sirolimus (rapamycin) inhibits mammalian target of rapamycin (mTOR), which is an important PI3K effector molecule that regulates protein translation and cell growth163. We reasoned that inhibition of PI3K signaling more broadly would have greater effects on intimal hyperplasia than targeting only one of PI3K's many effector molecules. Therefore, we generated a first-generation adenovirus encoding PTEN (phosphatase and tensin homology deleted on chromosome 10), a lipid phosphatase that is a natural PI3K antagonist (Fig 3A)164. PTEN hydrolyzes the 3-phosphoinositide lipid products of PI3K, which are potent signaling molecules, thereby abrogating all PI3K signaling and cellular responses involved in intimal hyperplasia. We first demonstrated that adenovirus-mediated overexpression of PTEN markedly inhibited intimal hyperplasia in a rat carotid injury model as a result of increased apoptosis and decreased proliferation of SMCs158. We subsequently showed that AdPTEN delivery to vein grafts significantly inhibited intimal hyperplasia in a 90-day canine aorto-coronary bypass graft model (Fig 3B–C)33. Notably, the PTEN transgene could be detected by PCR only in the treated vein graft but not in any of a number of other tissues, demonstrating that ex vivo delivery of AdPTEN poses a low risk of systemic infection (Fig 3D).
THE PREVENT TRIALS
As we have highlighted in this review, there is a substantial body of work demonstrating pre-clinical efficacy of a variety of gene therapy approaches in different animal models. However, only one strategy, the E2F decoy edifoligide, has been evaluated in clinical trials. E2F is a transcription factor responsible for the upregulation of several genes associated with cell cycle progression165. Edifoligide is a 14-base pair double-stranded ODN designed from the E2F binding site in its target genes. When delivered to cells or tissues, edifoligide acts as a decoy binding site, sequestering E2F from genomic DNA and thereby preventing target gene expression and cellular proliferation36. Pre-clinical studies in rabbit models and human vein segments demonstrated that edifoligide treatment inhibited SMC proliferation, preserved endothelial function, limited the development of atherosclerosis, and significantly reduced IH166–169. These exciting data led to the development of a series of clinical trials that were part of the Project of Ex Vivo Vein Graft Engineering via Transfection (PREVENT) trials. PREVENT I was a phase I trial that demonstrated the safety and biologic efficacy of E2F decoy gene delivery in 41 patients undergoing lower extremity bypass surgery38. In PREVENT II, a phase IIb trial of 200 patients undergoing coronary artery bypass grafting, the treatment group demonstrated a 30% relative reduction in vein graft critical stenosis by angiography and a 30% reduction in total wall volume by intravascular ultrasound170. Based on these positive results, the E2F decoy strategy was investigated in two separate phase III trials of vein graft disease: PREVENT III in lower extremity bypass surgery and PREVENT IV in coronary artery bypass grafting. Unfortunately, neither study demonstrated a significant difference in the primary endpoints of primary graft patency and critical graft stenosis at 1 year14, 171.
The reasons underlying the failure to translate the pre-clinical success of edifoligide into clinically relevant endpoints are not entirely clear. However, there are several factors that may have contributed. First, E2F is not a single transcription factor, but rather a family of 8 isoforms (E2F1-8). E2F1-3 are transcriptional activators, while E2F4-8 are repressors. E2F biology is reviewed in detail in172. Edifoligide was designed to bind to the activating E2F isoforms, but may have inadvertently bound inhibitory E2F isoforms due to their highly conserved DNA binding domains36. Moreover, even traditionally activating isoforms can act as context-dependent transcriptional repressors173. Another surprising result from the PREVENT IV trial was the higher than expected incidence of the primary end point of angiographic vein graft failure, defined as ≥75% stenosis at 12–18 months. Compared to the historical graft failure rate of 15–20% at one year, 45.2% of grafts in the edifoligide group and 46.3% in the placebo group reached the primary end point174. Interestingly, the study lacked a "standard of care" control group; both the treatment and placebo groups were exposed to 310 mmHg of non-distending pressure, which may have exacerbated endothelial injury14. The high failure rate in both groups raises concerns that the pressure-mediated delivery system contributed to the development of IH. Finally, there is accumulating evidence that graft extrinsic cells play a large role in formation of the neointimal lesion175, 176. The treatment strategy in the PREVENT trials, and in many of the pre-clinical trials discussed above, primarily targets graft intrinsic cells.
ADDITIONAL CONSIDERATIONS AND CONCLUSIONS
Cardiovascular disease remains the leading cause of morbidity and mortality in the developed world1,161, and surgical revascularization with autologous veins is likely to remain a mainstay of therapy for patients with arterial occlusive disease until such time as improved therapies for atherosclerosis become available. Therefore, it is critical that advances in our mechanistic understanding of VGD are efficiently translated into the clinic. Although numerous gene therapy approaches have effectively reduced IH and other aspects of VGD in animal models, the pre-clinical success of edifoligide did not translate into clinically meaningful reductions in VGD. In addition to potential explanations for failure of the PREVENT trials already discussed, additional concerns must be addressed before moving promising treatment strategies into human trials of vein graft disease.
First, to properly assess the promise of pre-clinical studies, endpoints in animal models must correlate with clinically relevant endpoints in patients. Many of the published pre-clinical studies assessed IH as the primary endpoint, but this may not be the most clinically meaningful endpoint. Analysis of PREVENT IV demonstrates that critical graft stenosis is associated with an increased need for repeat vascularization but not an increased risk of death and/or myocardial infarction177. Other readouts such as graft atherosclerosis, measures of systemic inflammation, and plaque stability may be more meaningful, particularly in late graft failure178. Focusing exclusively on IH in animal models may exclude promising therapies that may have modest effects on IH but dramatic effects on other aspects of VGD (for example macrophage recruitment). Moreover, many of the studies reviewed here demonstrate that increased graft wall thickness does not necessarily reflect poor graft function.
Second, although several large and small animal models recapitulate the pathogenesis of VGD on both gross and histological levels179, more work must be done to define the molecular pathways responsible for VGD in humans to determine the extent to which animal models accurately represent these molecular mechanisms. For example, while there is a wealth of data on inflammatory pathways in mouse models of VGD, there are significant differences between humans and mice in the cellular expression patterns of adhesion molecules, inflammatory cytokines, and receptors126. These and similar issues may lead to disappointing results when animal studies are translated into the clinic.
Finally, even if gene therapy for VGD is successfully applied in the clinic, it is unlikely that targeting a single pathway will be successful in all patients. Moreover, targeting different pathways and cell types simultaneously may be synergistic, as in the case of SOD, TIMP-1, and 35K combination gene therapy discussed above108, 145. Similarly, combining gene therapy with other approaches may yield better outcomes than either approach alone. Potential examples include genetic modification of progenitor cells used to seed bioengineered grafts; genetic modification of grafts to promote progenitor cell homing (used successfully in a rat model of chronic hind limb ischemia180); the use of sheaths to provide structural support for vein grafts treated while serving as gene delivery vehicles; and the use of biological scaffolds for long-term delivery of gene therapy to engrafted veins.
Although the PREVENT trials did not lead to a clinical treatment, they demonstrated that gene therapy for VGD is feasible and generally safe. Continued efforts to characterize the molecular mechanisms of VGD in animal models and humans, combined with the development of more sensitive and clinically meaningful endpoints, will allow for more efficient translation of pre-clinical studies into successful therapies for VGD.
ACKNOWLEDGMENTS
The authors wish to thank Duane A. Mitchell, M.D., Ph.D., for helpful discussions; and Jenny Y. Kim and Matthew J. Kan for generation of graphic images. This work was supported in part by grants R01-HL083385 and R21-HL095795 from the NIH/NHLBI and by grant UL1-RR024128-04S1, an administrative supplement to Duke University's CTSA grant from NIH/NCRR, to CDK, DEB, and CAM. KWS was supported in part by a Research Fellowship Award from the Association for Academic Surgery Foundation. SBF was supported in part by the Duke University Medical Scientist Training Program (T32-GM007171) and a Pre-Doctoral Fellowship Award (12PRE11380009) from the Mid-Atlantic Affiliate of the American Heart Association.
Abbreviations
- Ad
adenovirus
- AAV
adeno-associated virus
- CABG
coronary artery bypass grafting
- CCR2
C-C chemokine receptor-2
- COX-1
cyclooxygenase-1
- EC
endothelial cell
- ECM
extracellular matrix
- eNOS
endothelial nitric oxide synthase
- HDAd
helper-dependent adenovirus
- H2O2
hydrogen peroxide
- HVJ
hemagglutinating virus of Japan
- IH
intimal hyperplasia
- IL
interleukin
- I/M
intima to media
- IR
ischemia-reperfusion
- IVC
inferior vena cava
- MCP-1
monocyte chemoattractant protein-1
- mTOR
mammalian target of rapamycin
- NFκB
nuclear factor κB
- NO
nitric oxide
- NP
nanoparticle
- O2−
superoxide
- ODN
oligodeoxynucleotide
- ONOO−
peroxynitrite
- PCNA
proliferating cell nuclear antigen
- PD-ECGF
platelet-derived endothelial cell growth factor
- PDGF
platelet-derived growth factor
- PGI2
prostaglandin I2
- PI3K
phosphoinositide 3-kinase
- PREVENT
Project of Ex Vivo Vein Graft Engineering via Transfection
- PTEN
phosphatase and tensin homology deleted on chromosome 10
- scAAV
self-complementary AAV
- ROS
reactive oxygen species
- siRNA
small interfering RNA
- SMA
smooth muscle actin
- SMC
smooth muscle cell
- SOD
superoxide dismutase
- STAT
signal transducer and activator of transcription
- SVG
saphenous vein graft
- TGFβ
transforming growth factor β
- TIMP
tissue inhibitor of metalloproteinase
- TM
thrombomodulin
- tPA
tissue plasminogen activator
- VCAM
vascular cell adhesion molecule
- VEGF
vascular endothelial growth factor
- VGD
vein graft disease
- VSMC
vascular smooth muscle cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors have read the journal's policy on disclosure of potential conflicts of interest, and they declare that they have no financial conflicts of interest to disclose.
REFERENCES
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Executive summary: heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 2.Bittl JA. Advances in coronary angioplasty. N Engl J Med. 1996;335:1290–1302. doi: 10.1056/NEJM199610243351707. [DOI] [PubMed] [Google Scholar]
- 3.Haider SN, Kavanagh EG, Forlee M, et al. Two-year outcome with preferential use of infrainguinal angioplasty for critical ischemia. J Vasc Surg. 2006;43:504–512. doi: 10.1016/j.jvs.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 4.Adam DJ, Beard JD, Cleveland T, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–1934. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- 5.Goodney PP, Beck AW, Nagle J, Welch HG, Zwolak RM. National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. J Vasc Surg. 2009;50:54–60. doi: 10.1016/j.jvs.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 6.Hata M, Sezai A, Niino T, et al. What is the optimal management for preventing saphenous vein graft diseases?: early results of intravascular angioscopic assessment. Circ J. 2007;71:286–287. doi: 10.1253/circj.71.286. [DOI] [PubMed] [Google Scholar]
- 7.Parang P, Arora R. Coronary vein graft disease: pathogenesis and prevention. Can J Cardiol. 2009;25:e57–e62. doi: 10.1016/s0828-282x(09)70486-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conte MS, Lorenz TJ, Bandyk DF, Clowes AW, Moneta GL, Seely BL. Design and rationale of the PREVENT III clinical trial: edifoligide for the prevention of infrainguinal vein graft failure. Vasc Endovascular Surg. 2005;39:15–23. doi: 10.1177/153857440503900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The effect of aggressive lowering of low-density lipoprotein cholesterol levels and low-dose anticoagulation on obstructive changes in saphenous-vein coronary-artery bypass grafts. The Post Coronary Artery Bypass Graft Trial Investigators. N Engl J Med. 1997;336:153–162. doi: 10.1056/NEJM199701163360301. [DOI] [PubMed] [Google Scholar]
- 11.Shah SJ, Waters DD, Barter P, et al. Intensive lipid-lowering with atorvastatin for secondary prevention in patients after coronary artery bypass surgery. J Am Coll Cardiol. 2008;51:1938–1943. doi: 10.1016/j.jacc.2007.12.054. [DOI] [PubMed] [Google Scholar]
- 12.Domanski M, Tian X, Fleg J, et al. Pleiotropic effect of lovastatin, with and without cholestyramine, in the post coronary artery bypass graft (Post CABG) trial. Am J Cardiol. 2008;102:1023–1027. doi: 10.1016/j.amjcard.2008.05.053. [DOI] [PubMed] [Google Scholar]
- 13.Fitzgibbon GM, Kafka HP, Leach AJ, Keon WJ, Hooper GD, Burton JR. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol. 1996;28:616–626. doi: 10.1016/0735-1097(96)00206-9. [DOI] [PubMed] [Google Scholar]
- 14.Alexander JH, Hafley G, Harrington RA, et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294:2446–2454. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 15.Favaloro RG. Saphenous vein autograft replacement of severe segmental coronary artery occlusion: operative technique. Ann Thorac Surg. 1968;5:334–339. doi: 10.1016/s0003-4975(10)66351-5. [DOI] [PubMed] [Google Scholar]
- 16.Eltzschig HK, Collard CD. Vascular ischaemia and reperfusion injury. British Medical Bulletin. 2004;70:71–86. doi: 10.1093/bmb/ldh025. [DOI] [PubMed] [Google Scholar]
- 17.Shuvaev VV, Muzykantov VR. Targeted modulation of reactive oxygen species in the vascular endothelium. J Control Release. 2011;153:56–63. doi: 10.1016/j.jconrel.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dashwood MR, Tsui JC. 'No-touch' saphenous vein harvesting improves graft performance in patients undergoing coronary artery bypass surgery: A journey from bedside to bench. Vascul Pharmacol. 2012 doi: 10.1016/j.vph.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Sepehripour AH, Jarral OA, Shipolini AR, McCormack DJ. Does a 'no-touch' technique result in better vein patency? Interact Cardiovasc Thorac Surg. 2011;13:626–630. doi: 10.1510/icvts.2011.281998. [DOI] [PubMed] [Google Scholar]
- 20.Davies MG, Klyachkin ML, Dalen H, Massey MF, Svendsen E, Hagen PO. The integrity of experimental vein graft endothelium--implications on the etiology of early graft failure. Eur J Vasc Surg. 1993;7:156–165. doi: 10.1016/s0950-821x(05)80756-x. [DOI] [PubMed] [Google Scholar]
- 21.Cheng J, Du J. Mechanical stretch simulates proliferation of venous smooth muscle cells through activation of the insulin-like growth factor-1 receptor. Arterioscler Thromb Vasc Biol. 2007;27:1744–1751. doi: 10.1161/ATVBAHA.107.147371. [DOI] [PubMed] [Google Scholar]
- 22.Song H, Mowbray AL, Sykes MC, Jo H. Emerging role of IGF-1R in stretch-induced neointimal hyperplasia in venous grafts. Arterioscler Thromb Vasc Biol. 2007;27:1679–1681. doi: 10.1161/ATVBAHA.107.148189. [DOI] [PubMed] [Google Scholar]
- 23.Kozai T, Eto M, Yang Z, Shimokawa H, Luscher TF. Statins prevent pulsatile stretch-induced proliferation of human saphenous vein smooth muscle cells via inhibition of Rho/Rho-kinase pathway. Cardiovasc Res. 2005;68:475–482. doi: 10.1016/j.cardiores.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka H, Sukhova GK, Swanson SJ, et al. Sustained activation of vascular cells and leukocytes in the rabbit aorta after balloon injury. Circulation. 1993;88:1788–1803. doi: 10.1161/01.cir.88.4.1788. [DOI] [PubMed] [Google Scholar]
- 25.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 26.Lee MS, David EM, Makkar RR, Wilentz JR. Molecular and cellular basis of restenosis after percutaneous coronary intervention: the intertwining roles of platelets, leukocytes, and the coagulation–fibrinolysis system. The Journal of Pathology. 2004;203:861–870. doi: 10.1002/path.1598. [DOI] [PubMed] [Google Scholar]
- 27.Huynh TT, Davies MG, Thompson MA, Ezekowitz MD, Hagen P, Annex BH. Local treatment with recombinant tissue factor pathway inhibitor reduces the development of intimal hyperplasia in experimental vein grafts. J Vasc Surg. 2001;33:400–407. doi: 10.1067/mva.2001.111989. [DOI] [PubMed] [Google Scholar]
- 28.Davies MG, Hagen PO. Pathobiology of intimal hyperplasia. Br J Surg. 1994;81:1254–1269. doi: 10.1002/bjs.1800810904. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz SM. Smooth muscle migration in atherosclerosis and restenosis. J Clin Invest. 1997;100:S87–S89. [PubMed] [Google Scholar]
- 30.Mitra AK, Gangahar DM, Agrawal DK. Cellular, molecular and immunological mechanisms in the pathophysiology of vein graft intimal hyperplasia. Immunol Cell Biol. 2006;84:115–124. doi: 10.1111/j.1440-1711.2005.01407.x. [DOI] [PubMed] [Google Scholar]
- 31.Lardenoye JH, de Vries MR, Lowik CW, et al. Accelerated atherosclerosis and calcification in vein grafts: a study in APOE*3 Leiden transgenic mice. Circ Res. 2002;91:577–584. doi: 10.1161/01.res.0000036901.58329.d7. [DOI] [PubMed] [Google Scholar]
- 32.Wan S, George SJ, Berry C, Baker AH. Vein graft failure: current clinical practice and potential for gene therapeutics. Gene Ther. 2012;19:630–636. doi: 10.1038/gt.2012.29. [DOI] [PubMed] [Google Scholar]
- 33.Hata JA, Petrofski JA, Schroder JN, et al. Modulation of phosphatidylinositol 3-kinase signaling reduces intimal hyperplasia in aortocoronary saphenous vein grafts. J Thorac Cardiovasc Surg. 2005;129:1405–1413. doi: 10.1016/j.jtcvs.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 34.Santel A, Aleku M, Keil O, et al. A novel siRNA-lipoplex technology for RNA interference in the mouse vascular endothelium. Gene therapy. 2006;13:1222–1234. doi: 10.1038/sj.gt.3302777. [DOI] [PubMed] [Google Scholar]
- 35.Miyake T, Aoki M, Shiraya S, et al. Inhibitory effects of NFkappaB decoy oligodeoxynucleotides on neointimal hyperplasia in a rabbit vein graft model. J Mol Cell Cardiol. 2006;41:431–440. doi: 10.1016/j.yjmcc.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Morishita R, Gibbons GH, Horiuchi M, et al. A gene therapy strategy using a transcription factor decoy of the E2F binding site inhibits smooth muscle proliferation in vivo. Proc Natl Acad Sci U S A. 1995;92:5855–5859. doi: 10.1073/pnas.92.13.5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun J, Zheng J, Ling KH, et al. Preventing intimal thickening of vein grafts in vein artery bypass using STAT-3 siRNA. Journal of Translational Medicine. 2012;10:2. doi: 10.1186/1479-5876-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mann MJ, Whittemore AD, Donaldson MC, et al. Ex-vivo gene therapy of human vascular bypass grafts with E2F decoy: the PREVENT single-centre, randomised, controlled trial. Lancet. 1999;354:1493–1498. doi: 10.1016/S0140-6736(99)09405-2. [DOI] [PubMed] [Google Scholar]
- 39.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2012 doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 40.Desai MP, Labhasetwar V, Walter E, Levy RJ, Amidon GL. The mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependent. Pharmaceutical research. 1997;14:1568–1573. doi: 10.1023/a:1012126301290. [DOI] [PubMed] [Google Scholar]
- 41.Desai MP, Labhasetwar V, Amidon GL, Levy RJ. Gastrointestinal uptake of biodegradable microparticles: effect of particle size. Pharmaceutical research. 1996;13:1838–1845. doi: 10.1023/a:1016085108889. [DOI] [PubMed] [Google Scholar]
- 42.Panyam J, Zhou WZ, Prabha S, Sahoo SK, Labhasetwar V. Rapid endo-lysosomal escape of poly(DL-lactide-co-glycolide) nanoparticles: implications for drug and gene delivery. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2002;16:1217–1226. doi: 10.1096/fj.02-0088com. [DOI] [PubMed] [Google Scholar]
- 43.Guzman LA, Labhasetwar V, Song C, et al. Local intraluminal infusion of biodegradable polymeric nanoparticles. A novel approach for prolonged drug delivery after balloon angioplasty. Circulation. 1996;94:1441–1448. doi: 10.1161/01.cir.94.6.1441. [DOI] [PubMed] [Google Scholar]
- 44.Labhasetwar V, Bonadio J, Goldstein SA, Levy JR. Gene transfection using biodegradable nanospheres: results in tissue culture and a rat osteotomy model. Colloid Surface B. 1999;16:281–290. [Google Scholar]
- 45.Hanafusa S, Matsusue Y, Yasunaga T, et al. Biodegradable plate fixation of rabbit femoral shaft osteotomies. A comparative study. Clinical orthopaedics and related research. 1995:262–271. [PubMed] [Google Scholar]
- 46.Davda J, Labhasetwar V. Characterization of nanoparticle uptake by endothelial cells. International journal of pharmaceutics. 2002;233:51–59. doi: 10.1016/s0378-5173(01)00923-1. [DOI] [PubMed] [Google Scholar]
- 47.Cohen-Sacks H, Najajreh Y, Tchaikovski V, et al. Novel PDGFbetaR antisense encapsulated in polymeric nanospheres for the treatment of restenosis. Gene therapy. 2002;9:1607–1616. doi: 10.1038/sj.gt.3301830. [DOI] [PubMed] [Google Scholar]
- 48.Walther W, Stein U. Viral vectors for gene transfer: a review of their use in the treatment of human diseases. Drugs. 2000;60:249–271. doi: 10.2165/00003495-200060020-00002. [DOI] [PubMed] [Google Scholar]
- 49.Schulick AH, Vassalli G, Dunn PF, et al. Established immunity precludes adenovirus-mediated gene transfer in rat carotid arteries. Potential for immunosuppression and vector engineering to overcome barriers of immunity. J Clin Invest. 1997;99:209–219. doi: 10.1172/JCI119149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Y, Nunes FA, Berencsi K, Furth EE, Gonczol E, Wilson JM. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci U S A. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Channon KM, Qian HS, Youngblood SA, et al. Acute host-mediated endothelial injury after adenoviral gene transfer in normal rabbit arteries: impact on transgene expression and endothelial function. Circ Res. 1998;82:1253–1262. doi: 10.1161/01.res.82.12.1253. [DOI] [PubMed] [Google Scholar]
- 52.Newman KD, Dunn PF, Owens JW, et al. Adenovirus-mediated gene transfer into normal rabbit arteries results in prolonged vascular cell activation, inflammation, and neointimal hyperplasia. J Clin Invest. 1995;96:2955–2965. doi: 10.1172/JCI118367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brooks AR, Harkins RN, Wang P, Qian HS, Liu P, Rubanyi GM. Transcriptional silencing is associated with extensive methylation of the CMV promoter following adenoviral gene delivery to muscle. The journal of gene medicine. 2004;6:395–404. doi: 10.1002/jgm.516. [DOI] [PubMed] [Google Scholar]
- 54.Doerfler W, Hoeveler A, Weisshaar B, et al. Promoter inactivation or inhibition by sequence-specific methylation and mechanisms of reactivation. Cell Biophys. 1989;15:21–27. doi: 10.1007/BF02991576. [DOI] [PubMed] [Google Scholar]
- 55.Groudine M, Eisenman R, Weintraub H. Chromatin structure of endogenous retroviral genes and activation by an inhibitor of DNA methylation. Nature. 1981;292:311–317. doi: 10.1038/292311a0. [DOI] [PubMed] [Google Scholar]
- 56.Guzman RJ, Lemarchand P, Crystal RG, Epstein SE, Finkel T. Efficient gene transfer into myocardium by direct injection of adenovirus vectors. Circ Res. 1993;73:1202–1207. doi: 10.1161/01.res.73.6.1202. [DOI] [PubMed] [Google Scholar]
- 57.Kochanek S, Clemens PR, Mitani K, Chen HH, Chan S, Caskey CT. A new adenoviral vector: Replacement of all viral coding sequences with 28 kb of DNA independently expressing both full-length dystrophin and beta-galactosidase. Proc Natl Acad Sci U S A. 1996;93:5731–5736. doi: 10.1073/pnas.93.12.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parks RJ, Chen L, Anton M, Sankar U, Rudnicki MA, Graham FL. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc Natl Acad Sci U S A. 1996;93:13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wen S, Graf S, Massey PG, Dichek DA. Improved vascular gene transfer with a helper-dependent adenoviral vector. Circulation. 2004;110:1484–1491. doi: 10.1161/01.CIR.0000141574.78032.A9. [DOI] [PubMed] [Google Scholar]
- 60.Marshall E. Gene therapy death prompts review of adenovirus vector. Science. 1999;286:2244–2245. doi: 10.1126/science.286.5448.2244. [DOI] [PubMed] [Google Scholar]
- 61.Lehrman S. Virus treatment questioned after gene therapy death. Nature. 1999;401:517–518. doi: 10.1038/43977. [DOI] [PubMed] [Google Scholar]
- 62.Wasala NB, Shin J-H, Duan D. The evolution of heart gene delivery vectors. The Journal of Gene Medicine. 2011;13:557–565. doi: 10.1002/jgm.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eslami MH, Gangadharan SP, Sui X, Rhynhart KK, Snyder RO, Conte MS. Gene delivery to in situ veins: differential effects of adenovirus and adeno-associated viral vectors. J Vasc Surg. 2000;31:1149–1159. [PubMed] [Google Scholar]
- 64.Gruchala M, Bhardwaj S, Pajusola K, et al. Gene transfer into rabbit arteries with adeno-associated virus and adenovirus vectors. The journal of gene medicine. 2004;6:545–554. doi: 10.1002/jgm.535. [DOI] [PubMed] [Google Scholar]
- 65.Svensson EC, Marshall DJ, Woodard K, et al. Efficient and stable transduction of cardiomyocytes after intramyocardial injection or intracoronary perfusion with recombinant adeno-associated virus vectors. Circulation. 1999;99:201–205. doi: 10.1161/01.cir.99.2.201. [DOI] [PubMed] [Google Scholar]
- 66.Wagner JA, Reynolds T, Moran ML, et al. Efficient and persistent gene transfer of AAV-CFTR in maxillary sinus. Lancet. 1998;351:1702–1703. doi: 10.1016/S0140-6736(05)77740-0. [DOI] [PubMed] [Google Scholar]
- 67.Kaplitt MG, Feigin A, Tang C, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 68.Finn JD, Nichols TC, Svoronos N, et al. The efficacy and the risk of immunogenicity of FIX Padua (R338L) in hemophilia B dogs treated by AAV muscle gene therapy. Blood. 2012 doi: 10.1182/blood-2012-06-440123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pankajakshan D, Makinde TO, Gaurav R, et al. Successful transfection of genes using AAV-2/9 vector in swine coronary and peripheral arteries. J Surg Res. 2012;175:169–175. doi: 10.1016/j.jss.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Work LM, Buning H, Hunt E, et al. Vascular bed-targeted in vivo gene delivery using tropism-modified adeno-associated viruses. Mol Ther. 2006;13:683–693. doi: 10.1016/j.ymthe.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 71.Work LM, Nicklin SA, Brain NJ, et al. Development of efficient viral vectors selective for vascular smooth muscle cells. Molecular therapy : the journal of the American Society of Gene Therapy. 2004;9:198–208. doi: 10.1016/j.ymthe.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 72.Nicklin SA, Buening H, Dishart KL, et al. Efficient and selective AAV2-mediated gene transfer directed to human vascular endothelial cells. Mol Ther. 2001;4:174–181. doi: 10.1006/mthe.2001.0424. [DOI] [PubMed] [Google Scholar]
- 73.McCarty DM. Self-complementary AAV vectors; advances and applications. Molecular therapy : the journal of the American Society of Gene Therapy. 2008;16:1648–1656. doi: 10.1038/mt.2008.171. [DOI] [PubMed] [Google Scholar]
- 74.Cefai D, Simeoni E, Ludunge KM, et al. Multiply attenuated, self-inactivating lentiviral vectors efficiently transduce human coronary artery cells in vitro and rat arteries in vivo. J Mol Cell Cardiol. 2005;38:333–344. doi: 10.1016/j.yjmcc.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 75.Naldini L, Blomer U, Gallay P, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 76.Qian Z, Haessler M, Lemos JA, et al. Targeting vascular injury using Hantavirus-pseudotyped lentiviral vectors. Mol Ther. 2006;13:694–704. doi: 10.1016/j.ymthe.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 77.Sakuma T, Barry MA, Ikeda Y. Lentiviral vectors: basic to translational. Biochem J. 2012;443:603–618. doi: 10.1042/BJ20120146. [DOI] [PubMed] [Google Scholar]
- 78.Philippe S, Sarkis C, Barkats M, et al. Lentiviral vectors with a defective integrase allow efficient and sustained transgene expression in vitro and in vivo. Proc Natl Acad Sci U S A. 2006;103:17684–17689. doi: 10.1073/pnas.0606197103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Handa M, Li W, Morioka K, Takamori A, Yamada N, Ihaya A. Adventitial delivery of platelet-derived endothelial cell growth factor gene prevented intimal hyperplasia of vein graft. J Vasc Surg. 2008;48:1566–1574. doi: 10.1016/j.jvs.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 80.Wang J, Liu K, Shen L, Wu H, Jing H. Small interfering RNA to c-myc inhibits vein graft restenosis in a rat vein graft model. J Surg Res. 2011;169:e85–e91. doi: 10.1016/j.jss.2011.03.060. [DOI] [PubMed] [Google Scholar]
- 81.Petrofski JA, Hata JA, Gehrig TR, et al. Gene delivery to aortocoronary saphenous vein grafts in a large animal model of intimal hyperplasia. J Thorac Cardiovasc Surg. 2004;127:27–33. doi: 10.1016/j.jtcvs.2003.07.032. [DOI] [PubMed] [Google Scholar]
- 82.Petrofski JA, Hata JA, Williams ML, et al. A Gβγ inhibitor reduces intimal hyperplasia in aortocoronary saphenous vein grafts. The Journal of Thoracic and Cardiovascular Surgery. 2005;130:1683–1690. doi: 10.1016/j.jtcvs.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 83.Khaleel MS, Dorheim TA, Duryee MJ, et al. High-pressure distention of the saphenous vein during preparation results in increased markers of inflammation: a potential mechanism for graft failure. Ann Thorac Surg. 2012;93:552–558. doi: 10.1016/j.athoracsur.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 84.Miyake T, Aoki M, Shiraya S, et al. Inhibitory effects of NFκB decoy oligodeoxynucleotides on neointimal hyperplasia in a rabbit vein graft model. Journal of Molecular and Cellular Cardiology. 2006;41:431–440. doi: 10.1016/j.yjmcc.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 85.Mann MJ, Gibbons GH, Hutchinson H, et al. Pressure-mediated oligonucleotide transfection of rat and human cardiovascular tissues. Proc Natl Acad Sci U S A. 1999;96:6411–6416. doi: 10.1073/pnas.96.11.6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vassalli G, Agah R, Qiao R, Aguilar C, Dichek DA. A mouse model of arterial gene transfer: antigen-specific immunity is a minor determinant of the early loss of adenovirus-mediated transgene expression. Circ Res. 1999;85:e25–e32. [PubMed] [Google Scholar]
- 87.Sugimoto M, Yamanouchi D, Komori K. Therapeutic approach against intimal hyperplasia of vein grafts through endothelial nitric oxide synthase/nitric oxide (eNOS/NO) and the Rho/Rho-kinase pathway. Surg Today. 2009;39:459–465. doi: 10.1007/s00595-008-3912-6. [DOI] [PubMed] [Google Scholar]
- 88.Weaver H, Shukla N, Ellinsworth D, Jeremy JY. Oxidative stress and vein graft failure: a focus on NADH oxidase, nitric oxide and eicosanoids. Curr Opin Pharmacol. 2012;12:160–165. doi: 10.1016/j.coph.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 89.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 90.Lamas S, Marsden PA, Li GK, Tempst P, Michel T. Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci U S A. 1992;89:6348–6352. doi: 10.1073/pnas.89.14.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gkaliagkousi E, Ferro A. Nitric oxide signalling in the regulation of cardiovascular and platelet function. Front Biosci. 2011;16:1873–1897. doi: 10.2741/3828. [DOI] [PubMed] [Google Scholar]
- 92.Cable DG, O'Brien T, Kullo IJ, Schwartz RS, Schaff HV, Pompili VJ. Expression and function of a recombinant endothelial nitric oxide synthase gene in porcine coronary arteries. Cardiovasc Res. 1997;35:553–559. doi: 10.1016/s0008-6363(97)00161-2. [DOI] [PubMed] [Google Scholar]
- 93.Cable DG, Caccitolo JA, Caplice N, et al. The role of gene therapy for intimal hyperplasia of bypass grafts. Circulation. 1999;100:II392–II396. doi: 10.1161/01.cir.100.suppl_2.ii-392. [DOI] [PubMed] [Google Scholar]
- 94.Matsumoto T, Komori K, Yonemitsu Y, et al. Hemagglutinating virus of Japan-liposome-mediated gene transfer of endothelial cell nitric oxide synthase inhibits intimal hyperplasia of canine vein grafts under conditions of poor runoff. J Vasc Surg. 1998;27:135–144. doi: 10.1016/s0741-5214(98)70300-3. [DOI] [PubMed] [Google Scholar]
- 95.Ohta S, Komori K, Yonemitsu Y, Onohara T, Matsumoto T, Sugimachi K. Intraluminal gene transfer of endothelial cell-nitric oxide synthase suppresses intimal hyperplasia of vein grafts in cholesterol-fed rabbit: a limited biological effect as a result of the loss of medial smooth muscle cells. Surgery. 2002;131:644–653. doi: 10.1067/msy.2002.124878. [DOI] [PubMed] [Google Scholar]
- 96.Belton O, Byrne D, Kearney D, Leahy A, Fitzgerald DJ. Cyclooxygenase-1 and -2-dependent prostacyclin formation in patients with atherosclerosis. Circulation. 2000;102:840–845. doi: 10.1161/01.cir.102.8.840. [DOI] [PubMed] [Google Scholar]
- 97.Crutchley DJ, Conanan LB, Que BG. Effects of prostacyclin analogs on the synthesis of tissue factor, tumor necrosis factor-alpha and interleukin-1 beta in human monocytic THP-1 cells. J Pharmacol Exp Ther. 1994;271:446–451. [PubMed] [Google Scholar]
- 98.Hara S, Morishita R, Tone Y, et al. Overexpression of prostacyclin synthase inhibits growth of vascular smooth muscle cells. Biochem Biophys Res Commun. 1995;216:862–867. doi: 10.1006/bbrc.1995.2701. [DOI] [PubMed] [Google Scholar]
- 99.Wu KK. Regulation of prostaglandin H synthase-1 gene expression. Adv Exp Med Biol. 1997;400A:121–126. doi: 10.1007/978-1-4615-5325-0_17. [DOI] [PubMed] [Google Scholar]
- 100.Eichstaedt HC, Liu Q, Chen Z, et al. Gene transfer of COX-1 improves lumen size and blood flow in carotid bypass grafts. J Surg Res. 2010;161:162–167. doi: 10.1016/j.jss.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 101.Zoldhelyi P, McNatt J, Xu XM, et al. Prevention of arterial thrombosis by adenovirus-mediated transfer of cyclooxygenase gene. Circulation. 1996;93:10–107. doi: 10.1161/01.cir.93.1.10. [DOI] [PubMed] [Google Scholar]
- 102.Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest. 2002;109:817–826. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hein TW, Qamirani E, Ren Y, Kuo L. C-reactive protein impairs coronary arteriolar dilation to prostacyclin synthase activation: role of peroxynitrite. J Mol Cell Cardiol. 2009;47:196–202. doi: 10.1016/j.yjmcc.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schmalfuss CM, Chen LY, Bott JN, Staples ED, Mehta JL. Superoxide Anion Generation, Superoxide Dismutase Activity, and Nitric Oxide Release in Human Internal Mammary Artery and Saphenous Vein Segments. J Cardiovasc Pharmacol Ther. 1999;4:249–257. doi: 10.1177/107424849900400406. [DOI] [PubMed] [Google Scholar]
- 105.Hamilton CA, Berg G, McIntyre M, McPhaden AR, Reid JL, Dominiczak AF. Effects of nitric oxide and superoxide on relaxation in human artery and vein. Atherosclerosis. 1997;133:77–86. doi: 10.1016/s0021-9150(97)00114-7. [DOI] [PubMed] [Google Scholar]
- 106.Shi Y, Patel S, Davenpeck KL, et al. Oxidative stress and lipid retention in vascular grafts: comparison between venous and arterial conduits. Circulation. 2001;103:2408–2413. doi: 10.1161/01.cir.103.19.2408. [DOI] [PubMed] [Google Scholar]
- 107.Huynh TT, Davies MG, Trovato MJ, Barber L, Safi HJ, Hagen PO. Reduction of lipid peroxidation with intraoperative superoxide dismutase treatment decreases intimal hyperplasia in experimental vein grafts. J Surg Res. 1999;84:223–232. doi: 10.1006/jsre.1999.5647. [DOI] [PubMed] [Google Scholar]
- 108.Turunen P, Puhakka HL, Heikura T, et al. Extracellular superoxide dismutase with vaccinia virus anti-inflammatory protein 35K or tissue inhibitor of metalloproteinase-1: Combination gene therapy in the treatment of vein graft stenosis in rabbits. Hum Gene Ther. 2006;17:405–414. doi: 10.1089/hum.2006.17.405. [DOI] [PubMed] [Google Scholar]
- 109.He SQ, Zhang YH, Venugopal SK, et al. Delivery of antioxidative enzyme genes protects against ischemia/reperfusion-induced liver injury in mice. Liver Transpl. 2006;12:1869–1879. doi: 10.1002/lt.21001. [DOI] [PubMed] [Google Scholar]
- 110.Van-Assche T, Huygelen V, Crabtree MJ, Antoniades C. Gene therapy targeting inflammation in atherosclerosis. Curr Pharm Des. 2011;17:4210–4223. doi: 10.2174/138161211798764799. [DOI] [PubMed] [Google Scholar]
- 111.Durante W. Targeting heme oxygenase-1 in vascular disease. Curr Drug Targets. 2010;11:1504–1516. doi: 10.2174/1389450111009011504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pakala R. Coagulation factor Xa synergistically interacts with serotonin in inducing vascular smooth muscle cell proliferation. Cardiovasc Radiat Med. 2003;4:69–76. doi: 10.1016/s1522-1865(03)00144-6. [DOI] [PubMed] [Google Scholar]
- 113.Bretschneider E, Braun M, Fischer A, Wittpoth M, Glusa E, Schror K. Factor Xa acts as a PDGF-independent mitogen in human vascular smooth muscle cells. Thromb Haemost. 2000;84:499–505. [PubMed] [Google Scholar]
- 114.Pyo RT, Sato Y, Mackman N, Taubman MB. Mice deficient in tissue factor demonstrate attenuated intimal hyperplasia in response to vascular injury and decreased smooth muscle cell migration. Thromb Haemost. 2004;92:451–458. doi: 10.1160/TH04-02-0122. [DOI] [PubMed] [Google Scholar]
- 115.Collen D, Lu HR, Stassen JM, et al. Antithrombotic effects and bleeding time prolongation with synthetic platelet GPIIb/IIIa inhibitors in animal models of platelet-mediated thrombosis. Thromb Haemost. 1994;71:95–102. [PubMed] [Google Scholar]
- 116.Belch JJ, Lowe GD, Pollock JG, Forbes CD, Prentice CR. Low dose heparin in the prevention of deep-vein thrombosis after aortic bifurcation graft surgery. Thromb Haemost. 1980;42:1429–1433. [PubMed] [Google Scholar]
- 117.Gosling M, Golledge J, Turner RJ, Powell JT. Arterial flow conditions downregulate thrombomodulin on saphenous vein endothelium. Circulation. 1999;99:1047–1053. doi: 10.1161/01.cir.99.8.1047. [DOI] [PubMed] [Google Scholar]
- 118.Kim AY, Walinsky PL, Kolodgie FD, et al. Early loss of thrombomodulin expression impairs vein graft thromboresistance: implications for vein graft failure. Circ Res. 2002;90:205–212. doi: 10.1161/hh0202.105097. [DOI] [PubMed] [Google Scholar]
- 119.Tabuchi N, Shichiri M, Shibamiya A, et al. Non-viral in vivo thrombomodulin gene transfer prevents early loss of thromboresistance of grafted veins. Eur J Cardiothorac Surg. 2004;26:995–1001. doi: 10.1016/j.ejcts.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 120.Torsney E, Mayr U, Zou Y, Thompson WD, Hu Y, Xu Q. Thrombosis and neointima formation in vein grafts are inhibited by locally applied aspirin through endothelial protection. Circ Res. 2004;94:1466–1473. doi: 10.1161/01.RES.0000129570.06647.00. [DOI] [PubMed] [Google Scholar]
- 121.Goldman S, Copeland J, Moritz T, et al. Long-term graft patency (3 years) after coronary artery surgery. Effects of aspirin: results of a VA Cooperative study. Circulation. 1994;89:1138–1143. doi: 10.1161/01.cir.89.3.1138. [DOI] [PubMed] [Google Scholar]
- 122.Thomas AC, Wyatt MJ, Newby AC. Reduction of early vein graft thrombosis by tissue plasminogen activator gene transfer. Thromb Haemost. 2009;102:145–152. doi: 10.1160/TH08-11-0772. [DOI] [PubMed] [Google Scholar]
- 123.Hilfiker PR, Waugh JM, Li-Hawkins JJ, et al. Enhancement of neointima formation with tissue-type plasminogen activator. J Vasc Surg. 2001;33:821–828. doi: 10.1067/mva.2001.112323. [DOI] [PubMed] [Google Scholar]
- 124.Jiang X, Liu X, Zhang K, et al. Experimental study of tissue-type plasminogen activator gene to prevent vein grafts stenosis. J Huazhong Univ Sci Technolog Med Sci. 2006;26:314–316. doi: 10.1007/BF02829561. [DOI] [PubMed] [Google Scholar]
- 125.Cerletti C, Tamburrelli C, Izzi B, Gianfagna F, de Gaetano G. Platelet-leukocyte interactions in thrombosis. Thromb Res. 2012;129:263–266. doi: 10.1016/j.thromres.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 126.Pober JS, Tellides G. Participation of blood vessel cells in human adaptive immune responses. Trends Immunol. 2012;33:49–57. doi: 10.1016/j.it.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vestweber D. Adhesion and signaling molecules controlling the transmigration of leukocytes through endothelium. Immunol Rev. 2007;218:178–196. doi: 10.1111/j.1600-065X.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- 128.Williams MR, Azcutia V, Newton G, Alcaide P, Luscinskas FW. Emerging mechanisms of neutrophil recruitment across endothelium. Trends Immunol. 2011;32:461–469. doi: 10.1016/j.it.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wolff RA, Tomas JJ, Hullett DA, Stark VE, van Rooijen N, Hoch JR. Macrophage depletion reduces monocyte chemotactic protein-1 and transforming growth factor-beta1 in healing rat vein grafts. J Vasc Surg. 2004;39:878–888. doi: 10.1016/j.jvs.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 130.Stark VK, Hoch JR, Warner TF, Hullett DA. Monocyte chemotactic protein-1 expression is associated with the development of vein graft intimal hyperplasia. Arterioscler Thromb Vasc Biol. 1997;17:1614–1621. doi: 10.1161/01.atv.17.8.1614. [DOI] [PubMed] [Google Scholar]
- 131.Cipollone F, Marini M, Fazia M, et al. Elevated circulating levels of monocyte chemoattractant protein-1 in patients with restenosis after coronary angioplasty. Arterioscler Thromb Vasc Biol. 2001;21:327–334. doi: 10.1161/01.atv.21.3.327. [DOI] [PubMed] [Google Scholar]
- 132.Hokimoto S, Oike Y, Saito T, et al. Increased expression of monocyte chemoattractant protein-1 in atherectomy specimens from patients with restenosis after percutaneous transluminal coronary angioplasty. Circ J. 2002;66:114–116. doi: 10.1253/circj.66.114. [DOI] [PubMed] [Google Scholar]
- 133.Zhang YJ, Rutledge BJ, Rollins BJ. Structure/activity analysis of human monocyte chemoattractant protein-1 (MCP-1) by mutagenesis. Identification of a mutated protein that inhibits MCP-1-mediated monocyte chemotaxis. J Biol Chem. 1994;269:15918–15924. [PubMed] [Google Scholar]
- 134.Zhang Y, Rollins BJ. A dominant negative inhibitor indicates that monocyte chemoattractant protein 1 functions as a dimer. Mol Cell Biol. 1995;15:4851–4855. doi: 10.1128/mcb.15.9.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schepers A, Eefting D, Bonta PI, et al. Anti-MCP-1 gene therapy inhibits vascular smooth muscle cells proliferation and attenuates vein graft thickening both in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2006;26:2063–2069. doi: 10.1161/01.ATV.0000235694.69719.e2. [DOI] [PubMed] [Google Scholar]
- 136.Tatewaki H, Egashira K, Kimura S, Nishida T, Morita S, Tominaga R. Blockade of monocyte chemoattractant protein-1 by adenoviral gene transfer inhibits experimental vein graft neointimal formation. J Vasc Surg. 2007;45:1236–1243. doi: 10.1016/j.jvs.2007.01.066. [DOI] [PubMed] [Google Scholar]
- 137.Eefting D, Bot I, de Vries MR, et al. Local lentiviral short hairpin RNA silencing of CCR2 inhibits vein graft thickening in hypercholesterolemic apolipoprotein E3-Leiden mice. J Vasc Surg. 2009;50:152–160. doi: 10.1016/j.jvs.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 138.Pamukcu B, Lip GY, Shantsila E. The nuclear factor--kappa B pathway in atherosclerosis: a potential therapeutic target for atherothrombotic vascular disease. Thromb Res. 2011;128:117–123. doi: 10.1016/j.thromres.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 139.Shintani T, Sawa Y, Takahashi T, et al. Intraoperative transfection of vein grafts with the NFkappaB decoy in a canine aortocoronary bypass model: a strategy to attenuate intimal hyperplasia. Ann Thorac Surg. 2002;74:1132–1137. doi: 10.1016/s0003-4975(02)03921-8. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 140.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 141.Alcami A, Symons JA, Collins PD, Williams TJ, Smith GL. Blockade of chemokine activity by a soluble chemokine binding protein from vaccinia virus. J Immunol. 1998;160:624–633. [PubMed] [Google Scholar]
- 142.Bursill CA, Cai S, Channon KM, Greaves DR. Adenoviral-mediated delivery of a viral chemokine binding protein blocks CC-chemokine activity in vitro and in vivo. Immunobiology. 2003;207:187–196. doi: 10.1078/0171-2985-00228. [DOI] [PubMed] [Google Scholar]
- 143.Bursill CA, Choudhury RP, Ali Z, Greaves DR, Channon KM. Broad-spectrum CC-chemokine blockade by gene transfer inhibits macrophage recruitment and atherosclerotic plaque formation in apolipoprotein E-knockout mice. Circulation. 2004;110:2460–2466. doi: 10.1161/01.CIR.0000145122.58420.CO. [DOI] [PubMed] [Google Scholar]
- 144.Ali ZA, Bursill CA, Hu Y, et al. Gene transfer of a broad spectrum CC-chemokine inhibitor reduces vein graft atherosclerosis in apolipoprotein E-knockout mice. Circulation. 2005;112:I235–I241. doi: 10.1161/CIRCULATIONAHA.104.526129. [DOI] [PubMed] [Google Scholar]
- 145.Puhakka HL, Turunen P, Gruchala M, et al. Effects of vaccinia virus anti-inflammatory protein 35K and TIMP-1 gene transfers on vein graft stenosis in rabbits. In Vivo. 2005;19:515–521. [PubMed] [Google Scholar]
- 146.Bursill CA, Cash JL, Channon KM, Greaves DR. Membrane-bound CC chemokine inhibitor 35K provides localized inhibition of CC chemokine activity in vitro and in vivo. J Immunol. 2006;177:5567–5573. doi: 10.4049/jimmunol.177.8.5567. [DOI] [PubMed] [Google Scholar]
- 147.Gotoh R, Suzuki J, Kosuge H, et al. E-selectin blockade decreases adventitial inflammation and attenuates intimal hyperplasia in rat carotid arteries after balloon injury. Arterioscler Thromb Vasc Biol. 2004;24:2063–2068. doi: 10.1161/01.ATV.0000145942.31404.20. [DOI] [PubMed] [Google Scholar]
- 148.Jabs A, Okamoto E, Vinten-Johansen J, Bauriedel G, Wilcox JN. Sequential patterns of chemokine- and chemokine receptor-synthesis following vessel wall injury in porcine coronary arteries. Atherosclerosis. 2007;192:75–84. doi: 10.1016/j.atherosclerosis.2006.05.050. [DOI] [PubMed] [Google Scholar]
- 149.Okamoto E, Couse T, De Leon H, et al. Perivascular inflammation after balloon angioplasty of porcine coronary arteries. Circulation. 2001;104:2228–2235. doi: 10.1161/hc4301.097195. [DOI] [PubMed] [Google Scholar]
- 150.Siow RC, Churchman AT. Adventitial growth factor signalling and vascular remodelling: potential of perivascular gene transfer from the outside-in. Cardiovasc Res. 2007;75:659–668. doi: 10.1016/j.cardiores.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 151.Newby AC. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev. 2005;85:1–31. doi: 10.1152/physrev.00048.2003. [DOI] [PubMed] [Google Scholar]
- 152.George SJ, Lloyd CT, Angelini GD, Newby AC, Baker AH. Inhibition of Late Vein Graft Neointima Formation in Human and Porcine Models by Adenovirus-Mediated Overexpression of Tissue Inhibitor of Metalloproteinase-3. Circulation. 2000;101:296–304. doi: 10.1161/01.cir.101.3.296. [DOI] [PubMed] [Google Scholar]
- 153.George SJ, Wan S, Hu J, MacDonald R, Johnson JL, Baker AH. Sustained Reduction of Vein Graft Neointima Formation by Ex Vivo TIMP-3 Gene Therapy. Circulation. 2011;124:S135–S142. doi: 10.1161/CIRCULATIONAHA.110.012732. [DOI] [PubMed] [Google Scholar]
- 154.Wilson E, Mai Q, Sudhir K, Weiss RH, Ives HE. Mechanical strain induces growth of vascular smooth muscle cells via autocrine action of PDGF. J Cell Biol. 1993;123:741–747. doi: 10.1083/jcb.123.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dashwood MR, Loesch A. Surgical damage of the saphenous vein and graft patency. J Thorac Cardiovasc Surg. 2007;133:274–275. doi: 10.1016/j.jtcvs.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 156.Mann MJ, Gibbons GH, Kernoff RS, et al. Genetic engineering of vein grafts resistant to atherosclerosis. Proc Natl Acad Sci U S A. 1995;92:4502–4506. doi: 10.1073/pnas.92.10.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Huang J, Kontos CD. Inhibition of vascular smooth muscle cell proliferation, migration, and survival by the tumor suppressor protein PTEN. Arterioscler Thromb Vasc Biol. 2002;22:745–751. doi: 10.1161/01.atv.0000016358.05294.8d. [DOI] [PubMed] [Google Scholar]
- 158.Huang J, Niu XL, Pippen AM, Annex BH, Kontos CD. Adenovirus-mediated intraarterial delivery of PTEN inhibits neointimal hyperplasia. Arterioscler Thromb Vasc Biol. 2005;25:354–358. doi: 10.1161/01.ATV.0000151619.54108.a5. [DOI] [PubMed] [Google Scholar]
- 159.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 160.Rameh LE, Cantley LC. The role of phosphoinositide 3-kinase lipid products in cell function. J Biol Chem. 1999;274:8347–8350. doi: 10.1074/jbc.274.13.8347. [DOI] [PubMed] [Google Scholar]
- 161.Sousa JE, Costa MA, Abizaid AC, et al. Sustained suppression of neointimal proliferation by sirolimus-eluting stents: one-year angiographic and intravascular ultrasound follow-up. Circulation. 2001;104:2007–2011. doi: 10.1161/hc4201.098056. [DOI] [PubMed] [Google Scholar]
- 162.Sousa JE, Costa MA, Abizaid A, et al. Sirolimus-eluting stent for the treatment of in-stent restenosis: a quantitative coronary angiography and three-dimensional intravascular ultrasound study. Circulation. 2003;107:24–27. doi: 10.1161/01.cir.0000047063.22006.41. [DOI] [PubMed] [Google Scholar]
- 163.Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001;15:807–826. doi: 10.1101/gad.887201. [DOI] [PubMed] [Google Scholar]
- 164.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 165.Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 166.Ehsan A, Mann MJ, Dell'Acqua G, Dzau VJ. Long-term stabilization of vein graft wall architecture and prolonged resistance to experimental atherosclerosis after E2F decoy oligonucleotide gene therapy. J Thorac Cardiovasc Surg. 2001;121:714–722. doi: 10.1067/mtc.2001.111204. [DOI] [PubMed] [Google Scholar]
- 167.Ehsan A, Mann MJ, Dell'Acqua G, Tamura K, Braun-Dullaeus R, Dzau VJ. Endothelial healing in vein grafts: proliferative burst unimpaired by genetic therapy of neointimal disease. Circulation. 2002;105:1686–1692. doi: 10.1161/01.cir.0000013775.02396.93. [DOI] [PubMed] [Google Scholar]
- 168.Mann MJ, Gibbons GH, Tsao PS, et al. Cell cycle inhibition preserves endothelial function in genetically engineered rabbit vein grafts. J Clin Invest. 1997;99:1295–1301. doi: 10.1172/JCI119288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mann MJKR, Dzau VJ. Vein graft gene therapy using E2F decoy oligonucleotides: target gene inhibition in human veins and long term resistance to atherosclerosis in rabbits. Surg Forum. 1997:242–244. [Google Scholar]
- 170.Late-Breaking Clinical Trial Abstracts. Circulation. 2001;104:1b–4b. doi: 10.1161/CIR.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 171.Conte MS, Bandyk DF, Clowes AW, et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43:742–751. doi: 10.1016/j.jvs.2005.12.058. discussion 51. [DOI] [PubMed] [Google Scholar]
- 172.Attwooll C, Lazzerini Denchi E, Helin K. The E2F family: specific functions and overlapping interests. EMBO J. 2004;23:4709–4716. doi: 10.1038/sj.emboj.7600481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chong JL, Wenzel PL, Saenz-Robles MT, et al. E2f1-3 switch from activators in progenitor cells to repressors in differentiating cells. Nature. 2009;462:930–934. doi: 10.1038/nature08677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation. 1998;97:916–931. doi: 10.1161/01.cir.97.9.916. [DOI] [PubMed] [Google Scholar]
- 175.Zhang L, Freedman NJ, Brian L, Peppel K. Graft-extrinsic cells predominate in vein graft arterialization. Arterioscler Thromb Vasc Biol. 2004;24:470–476. doi: 10.1161/01.ATV.0000116865.98067.31. [DOI] [PubMed] [Google Scholar]
- 176.Fogelstrand P, Osterberg K, Mattsson E. Reduced neointima in vein grafts following a blockage of cell recruitment from the vein and the surrounding tissue. Cardiovasc Res. 2005;67:326–332. doi: 10.1016/j.cardiores.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 177.Lopes RD, Mehta RH, Hafley GE, et al. Relationship between vein graft failure and subsequent clinical outcomes after coronary artery bypass surgery. Circulation. 2012;125:749–756. doi: 10.1161/CIRCULATIONAHA.111.040311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Owens CD, Ridker PM, Belkin M, et al. Elevated C-reactive protein levels are associated with postoperative events in patients undergoing lower extremity vein bypass surgery. J Vasc Surg. 2007;45:2–9. doi: 10.1016/j.jvs.2006.08.048. discussion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Thomas AC. Animal models for studying vein graft failure and therapeutic interventions. Curr Opin Pharmacol. 2012;12:121–126. doi: 10.1016/j.coph.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 180.Kuliszewski MA, Kobulnik J, Lindner JR, Stewart DJ, Leong-Poi H. Vascular gene transfer of SDF-1 promotes endothelial progenitor cell engraftment and enhances angiogenesis in ischemic muscle. Mol Ther. 2011;19:895–902. doi: 10.1038/mt.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]