Abstract
The basolateral amygdala (BLA) modulates memory, particularly for arousing or emotional events, during post-training periods of consolidation. It strengthens memories whose substrates in part or whole are stored remotely, in structures such as the hippocampus, striatum and cerebral cortex. However, the mechanisms by which the BLA influences distant memory traces are unknown, largely because of the need for identifiable target mnemonic representations. Associative tuning plasticity in the primary auditory cortex (A1) constitutes a well-characterized candidate specific memory substrate that is ubiquitous across species, tasks and motivational states. When tone predicts reinforcement, the tuning of cells in A1 shifts toward or to the signal frequency within its tonotopic map, producing an over-representation of behaviorally important sounds. Tuning shifts have the cardinal attributes of forms of memory, including associativity, specificity, rapid induction, consolidation and long-term retention and are therefore likely memory representations. We hypothesized that the BLA strengthens memories by increasing their cortical representations. We recorded multiple unit activity from A1 of rats that received a single discrimination training session in which two tones (2.0 s) separated by 1.25 octaves were either paired with brief electrical stimulation (400 ms) of the BLA (CS+) or not (CS−). Frequency response areas generated by presenting a matrix of test tones (0.5–53.82 kHz, 0–70 dB) were obtained before training and daily for three weeks post-training. Tuning both at threshold and above threshold shifted predominantly toward the CS+ beginning on Day 1. Tuning shifts were maintained for the entire three weeks. Absolute threshold and bandwidth decreased, producing less enduring increases in sensitivity and selectivity. BLA-induced tuning shifts were associative, highly specific and long-lasting. We propose that the BLA strengthens memory for important experiences by increasing the number of neurons that come to best represent that event. Traumatic, intrusive memories might reflect abnormally extensive representational networks due to hyper-activity of the BLA consequent to the release of excessive amounts of stress hormones.
Keywords: Associative learning, Auditory cortex, Frequency receptive field, Memory, Memory modulation, Post-traumatic stress disorder, Tuning shift
1. Introduction
The mechanisms underlying the strength and persistence of memory fundamental issues in neuroscience. It is well known that emotionally arousing experiences generally create strong, long-lasting memories (McGaugh, 2003). Arousal-induced release of the epinephrine and cortisol (corticosterone in rats) plays a critical role modulating the consolidation of memories (McGaugh 1989, 2000; McGaugh & Roozendaal, 2009). For example, human memory is enhanced by post-learning administration of epinephrine or stimulation that induces the release of epinephrine (Cahill & Alkire, 2003; Cahill, Gorski, & Le, 2003). Similarly, post-training systemic administration of epinephrine or corticosterone to rats strengthens memory in many types of tasks (McGaugh 2002, 2004). Additionally, administration of β-adrenoceptor antagonists block the enhancing effects of emotional arousal on memory in both humans and rats (Cahill, Prins, Weber, & McGaugh, 1994; McGaugh, 2004; McGaugh & Roozendaal, 2002).
Several lines of evidence have implicated the basolateral amygdala (BLA) in the modulation of memory strength. For example, adrenal stress hormones influence memory that involves noradrenergic activation of the amygdala (McGaugh, 2004). Lesions or pharmacological inactivation of the BLA block the memory enhancing effects of peripherally administered epinephrine and corticosterone (Roozendaal & McGaugh, 1996). The release of norepinephrine (NE) within the BLA plays a critical role in modulating memory consolidation. Intra-BLA administration of β-adrenoceptor antagonists blocks the effects of epinephrine and corticosterone on memory (Quirarte, Roozendaal, & McGaugh, 1997; Roozendaal, Hui, Hui, Berlau, McGaugh, & Weinberger, 2006) and post-training intra-BLA infusions of NE, as well as noradrenergic agonists, enhance memory consolidation (Ferry & McGaugh, 1999, 2008; Roozendaal, Quirarte, & McGaugh, 1997). Importantly, arousal-induced training releases NE within the amygdala (Quirarte, Galvez, Roozendaal, & McGaugh, 1998) and the amount of increased NE correlates directly with subsequent retention performance (McIntyre, Hatfield, & McGaugh, 2002). Moreover, post-training administration of GABAergic and opioid peptidergic drugs (e.g., picrotoxin, naloxone) that strengthen memory also increase the release of NE in the amygdala, while drugs that impair memory (e.g., muscimol, β-endorphin) decrease the release of NE in the amygdala (Hatfield, Spanis, & McGaugh, 1999; Quirarte et al., 1998). Thus within the BLA, GABAergic and opioid drugs act “upstream” from norepinephrine.
BLA modulation of memory affects information stored elsewhere. For example, the memory enhancing effects of post-training administration of drugs into other brain regions, including the cingulate cortex (Malin, Ibrahim, Tu, & McGaugh, 2007), entorhinal cortex (Roesler, Roozendaal, & McGaugh, 2002), hippocampus (Packard & Chen, 1999) and nucleus accumbens (Setlow, Roozendaal, & McGaugh, 2000), are blocked by lesions or inactivation of the BLA or intra-BLA infusions of β-adrenoceptor antagonists (Roozendaal & McGaugh, 1997). The latter also prevents synaptic plasticity in other structures, e.g., the induction of LTP in the hippocampus and stress-induced influences on hippocampal and cortical LTP (Akirav & Richter-Levin, 1999, 2002; Ikegaya, Saito, & Abe, 1995; Li & Richter-Levin, 2012).
The BLA has other interactions with the cerebral cortex. For example, BLA stimulation enhances cortical LTP (Dringenberg, Kuo, & Tomaszek, 2004). But, how the BLA modulates memory in the cortex remains unknown. A target memory representation is needed so that its modulation by the BLA could be directly assessed. Fortunately, specific, associatively-induced representational plasticity in the primary auditory cortex constitutes a candidate memory substrate (Weinberger, 2004).
That primary sensory fields are critically involved in learning, memory and other cognitive processes is now well established, although by traditional accounts their function is restricted to stimulus analysis, behavioral significance being “assigned” to “higher” cortical fields (e.g., Campbell, 1905). Within the primary auditory cortex (A1), the representation of neurons is modified to emphasize the acoustic frequency of tones that gain predictive signal properties due to associative learning (Weinberger, 2004). For example, frequency receptive fields are shifted toward or even to the frequency of the conditioned stimulus (CS) (Bakin & Weinberger, 1990; Gao & Suga, 1998; Kisley & Gerstein, 2001; Weinberger, 1995).
These CS-directed tuning shifts have the same attributes as major forms of behavioral memory. They are associative, highly specific, discriminative, develop rapidly, consolidate (becomes stronger over days without further training), and exhibit long-term retention (tracked to 8 weeks) (Weinberger, 2007). Furthermore, such specific associative representational plasticity is ubiquitous. It develops in humans as well as animals (Molchan, Sunderland, McIntosh, Herscovitch, & Schreurs, 1994; Morris, Friston, & Dolan, 1998; Schreurs, McIntosh, Bahro, Herscovitch, Sunderland, & Molchan, 1997), and in a wide variety of both classical and instrumental conditioning tasks, for both positive and negative motivational valences (Weinberger, 2007). In toto, receptive field plasticity satisfies cardinal criteria for constituting a neural memory trace (Weinberger, 2004).
As the BLA modulates memories, including those stored at least in part in the cerebral cortex, and as associatively-induced CS-specific tuning shifts may constitute cortical substrates of memory, then the BLA may strengthen memories by promoting specific sensory cortical tuning shifts. This hypothesis has had an initial test: tone paired with electrical stimulation of the BLA in the anesthetized rat produces CS-directed tuning shifts in A1, suggesting that the BLA strengthens memories by enhancing their cortical representation (Chavez, McGaugh, & Weinberger, 2009). However, while these findings constitute a “proof-of-concept” demonstration, they leave open several critical questions. First, do BLA-induced specific cortical tuning shifts develop in the waking state? Second, if so, are they associative? Third, do they exhibit long-term retention? To address these issues, waking adult rats bearing chronically-implanted electrodes received a single two-tone discrimination training session, in which one tone was paired with BLA stimulation. Auditory cortical responses to a wide variety of tonal stimuli were recorded before training and daily for 21 days (3 weeks) post-training, to determine the specificity of any resultant remodeling of the cortical representation of sound frequencies.
2. Methods
2.1. Subjects
The subjects were 9 male Sprague–Dawley rats (250–300 g upon arrival, Charles River Laboratories, Wilmington, MA) individually housed in a vivarium (maintained at 22° C, 12/12 h light–dark cycle, on at 6:00 am) with ad libitum access to food and water. All procedures were performed in accordance with the University of California Irvine Animal Research Committee and the NIH Animal Welfare guidelines.
2.2. Surgical preparation
Rats were anesthetized with Ketamine/Xylazine (1.1 mg/kg, i.p.) general anesthesia. Bronchial secretions were minimized by treatment with atropine sulfate (0.25 mg/kg, im) and body temperature was maintained at 37° C with a homeothermic heating blanket (Harvard Apparatus, Kent, England). A craniotomy over the right primary auditory cortex allowed for multiunit extracellular as well as EEG recordings with a sixteen-electrode array (400 μm separation) of tungsten microelectrodes (custom made), implanted directly through the dura mater. A second craniotomy was made to allow a bipolar stimulating electrode to be placed within the ipsilateral BLA (5 rats with concentric bipolar electrode CBCSH9, stainless steel, outer diameter 31 ga, inner diameter 75 μm, epoxylite-coated, FHC, Bowdoinham, ME; 4 rats with two 50 μm wires inside a canula outer diameter of 31 ga, custom made; A–P = −2.5 mm; M–L = 4.8 mm; D–V = −8.5 mm, Paxinos & Watson 2007). Animals recovered for at least 5 days prior to the commencement of the experiment.
2.3. Stimuli and electrophysiological recording
Neural activity was amplified (1000×), and bandpass filtered (0.3–3.0 kHz, Tucker–Davis technologies [TDT, Alachua, FL] RA16 Medusa Base Station) and monitored via Matlab custom software. Of the 16 electrodes in each array, four had been cut short so their tips were in layer 1 where no cellular discharges were recordable. These served as common reference electrodes, whose activity singly or in combination was subtracted from recordings from the depth electrodes, in order to eliminate movement and muscle artifact. Thus, a total of 108 recording electrodes were available to record multi-unit discharges. The electroencephalogram (EEG) was monitored with the same cortical electrodes, bandpass filtered at 10–100 Hz (TDT RA16 Medusa Base Station) and monitored through Matlab custom software. EEG activity for the analysis of gamma band activity (40–120 Hz) was restricted to a subset of the 108 cortical electrodes, due to channel limitations of the TDT workstation.
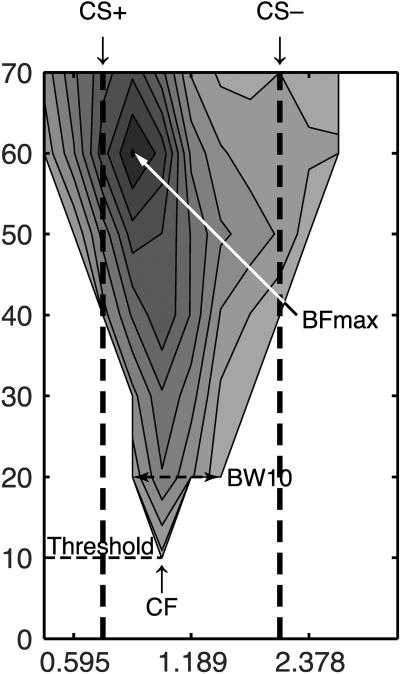
Acoustic stimuli were generated using TDT hardware and Matlab custom software. Pure tone bursts (50 ms duration, cosine-squared gate, rise/fall time of 10 ms) of 0.5–53.82 kHz were presented every 700 ms from 0 to 70 dB SPL in 10 dB steps (224 stimuli combinations) and repeated 15 times pseudo-randomly to determine frequency response. This combination of frequencies and stimulus levels yields a frequency response area (FRA), which provides a comprehensive profile of neural response to pure tone stimuli. Frequency response areas (FRA) were created offline (Matlab custom software) using evoked spike-time data, 6–40 ms following tone onset (Fig. 1).
Fig. 1.
Example of a frequency response area (FRA) recorded from the primary auditory cortex of the waking rat. Threshold is defined as the lowest sound level (dB SPL) that elicits a response. The frequency that elicits this response is the characteristic frequency (CF). The BFmax is defined as the frequency that elicits the greatest response (spikes per second above pre-tone activity). The breadth of tuning is measured at 10 dB above the evoked threshold (BW10).
Baseline recordings were taken during a 0–50 ms time window before tone presentation. Evoked activity was defined as activity 2.5 SE above mean spontaneous baseline activity. The characteristic frequency (CF) was the frequency that elicited the lowest threshold evoked discharge. In the rare case that two frequencies elicited the threshold response, their geometric mean was identified as the CF. The maximum best frequency (BFmax), was the frequency and level combination that produced the greatest evoked discharge within the FRA (Fig. 1).
2.4. Training
Prior to surgery, all subjects were handled daily for 2 days then acclimated to the recording cage for 1 hour daily for an additional 3 days. The recording cage was a wire mesh alley (30.5 × 5.8 × 6.0 cm) tilted at a 20° angle (which allows for little movement of the rat thereby providing acoustic stimulus control), placed inside a sound attenuated chamber with the overhead lights on and (see Headley & Weinberger, 2011). During the acclimation period, a calibrated electromagnetic speaker (FF1, TDT) was placed ~15 cm from the left ear of the subject (contralateral to recording electrodes) and acoustic stimuli was presented. The acoustic stimuli consisted of 224 different pure tone pips (50 ms duration, 0.50–53.82 kHz in 0.25 octave steps, 0–70 dB SPL in 10 dB SPL steps, cosine-squared gate, rise/fall time of 10 ms) repeated randomly 15 times with a 700 ms inter-tone interval.
After the three-day acclimation period, all subjects underwent surgical procedures (see Surgical Preparation above). Subjects then repeated the acclimation period except that neural data were collected during the presentation of the pure tones, for at least an additional 2 days. This data served as the pre-training neural data for each animal and ensured the stability of tuning at each electrode prior to training. 24 hours before training, following the acclimation period, the BLA was stimulated (400 ms train, 0.2 ms opposite polarity, 1.0 ms inter-pulse interval, delivered at 100 Hz) while the cortical EEG was visually monitored. The training current level chosen was the level that produced cortical desynchronization of the EEG during this period (40–120 μA). This stimulation did not produce the initiation of any discernable behavior or the interruption of ongoing behavior. On the day of training, a final pre-training recording was made and served as the pre-training baseline for all data analyses. Overhead lights were then turned off and remained off throughout the training session. Training consisted of 30 trials of a 2 s pure tone paired with BLA stimulation (CS+ trials) intermixed with 30 trials of a different 2 s pure tone presented alone (CS−) with an average inter-trial interval of 120 s (90–150 s). The CS+ and CS− were always 1.25 octaves from each other. Rats were returned to their home cage and the vivarium. Twenty-four hours after the single training session, subjects were placed back in the recording cage with the overhead lights on, and tuning data were collected from A1 as in the pre-training acclimation period. Acoustic testing and neural recording were repeated daily for the subsequent three weeks.
2.5. Neural data analysis
A1 was defined by a general progression of low to high characteristic frequencies along the posterior to anterior axis. Anterior placements that resulted in a reversal in the CF progression were defined as lying in the anterior auditory field and were excluded from analyses (Rutkowski, Miasnikov, & Weinberger, 2003). As noted above, post-training FRAs were obtained daily for 21 days and were analyzed for the development of any A1 tuning shifts. The post-training frequency responses were then compared to the pre-training responses to determine if a tuning shift had occurred. A shift index (SI) was used to quantify the extent and direction of shift at the CF (CF SI):
A positive SI indicates a shift towards the CS+, while a negative SI indicates a shift away from the CS+. A complete shift to the CS+ frequency after training would produce an SI = 1.0 while shifts away would produce negative scores. The maximum SI values were ± 1.6, i.e., shifts past CS frequencies (n = 1 each).
The same type of shift index was also determined for the largest magnitude response (best frequency/level combination, BFmax) within the FRA on every day (BFmax SI):
In addition to the changes in tuning, changes in sensitivity were measured by tracking the threshold Changes in the selectivity of the cortical response were also tracked by measuring changes in the bandwidth at 10 dB SPL above threshold (BW10). Missing data were interpolated using the missing values analysis package with SPSS statistical software and Type-I errors were reduced for multiple t-tests by using a Bonferonni correction set at an alpha level of p < 0.05.
2.6. Histology
Rats were sacrificed (Euthasol, 50 mg/kg, i.p.) and perfused with 0.9% saline, followed by 10% formalin. Brains were removed and placed into 20% sucrose solution until sectioned with a microtome. Brains were frozen and sliced into 50 μm slices, mounted on gelatinized slides and stained with thionine for visualization of BLA electrode placements. Placement of stimulating electrodes were determined under light microscope with the assistance of the Paxinos and Watson rat brain atlas (Paxinos & Watson, 2007).
3. Results
3.1. Histology
All stimulating electrodes were successfully placed in the BLA (Fig. 2).
Fig. 2.
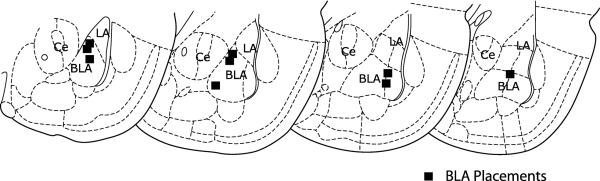
Histology: all stimulating electrodes were located in the basolateral amygdala (BLA). LA = lateral amygdala; Ce = central nucleus of the amygdala.
3.2. Tuning shifts at threshold
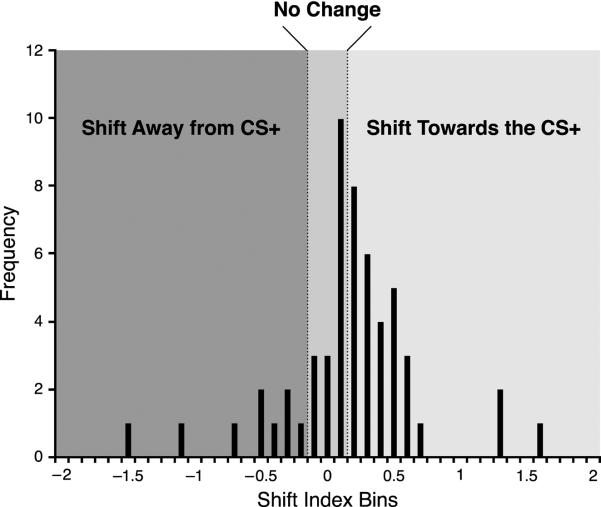
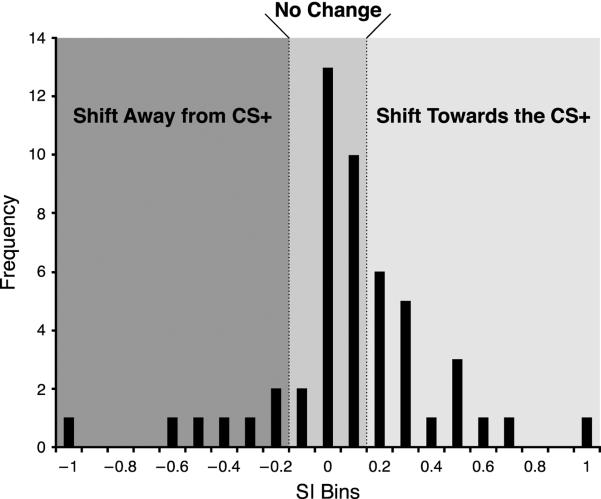
Previous studies have shown that associative tuning shifts in A1 during classical conditioning develop within 24 hours but may take up to 3 days to consolidate, i.e., attain maximum shifting; moreover, they endure for at least 10 days, the longest period tested (Galván & Weinberger, 2002). To detect BLA-induced non-transient shifts that may consolidate, we averaged the shift index at the characteristics frequency (CF SI) for each recording site across the first seven days following the single tone–BLA pairing session. A total of 55 electrodes yielded reliable and complete frequency response areas. A frequency distribution shows that most of the sites (30/55) developed tuning shifts toward or to the CS+ (Fig. 3). Thirteen did not change and 12 sites shifted away from the CS+.
Fig. 3.
Group frequency distribution of the Week 1 average post training shift index at CF. The central area represents recording sites categorized as “No Change” (shift index [SI] less than or equal to 0.1). Positive values indicate shift towards the CS+, negative values indicate shift away from the CS+.
3.2.1. Tuning shifts toward the CS+
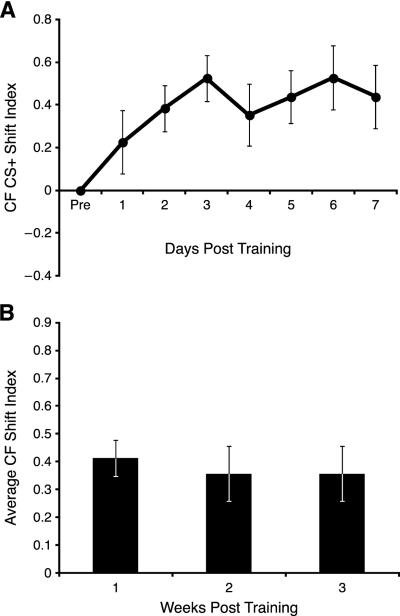
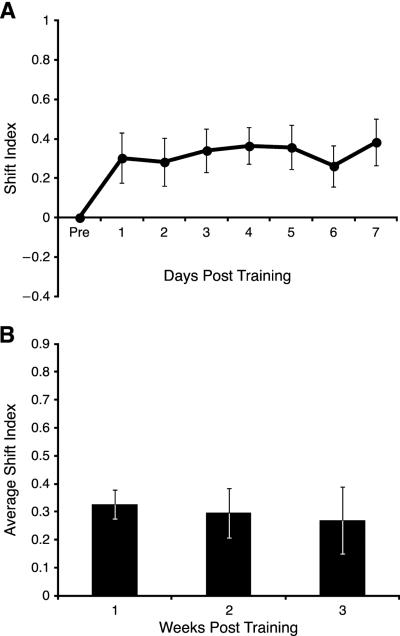
Shifts toward the CS+ developed over days at the beginning of Week 1, as indicated by a significant days effect (F(7, 203) = 2.150, p = 0.040; Fig. 4A). Although shifting was evident on the first day post-training, it did not attain significance until Day 2 (t = 3.582, p < 0.05, df = 29). The tuning shifts toward the CS+ were maintained throughout the three week period of recording as there was no significant days effects across 21 days post-training (F(21, 525) = 0.978, p = 0.489; Fig. 4B). The average amount of shifting was ~0.5, i.e., about 50% of the frequency distance from the pre-training characteristic frequency to the frequency of the CS+ tone.
Fig. 4.
Recording sites at threshold (CF) that shifted toward the CS+. (A) Week 1: note the increase in SI relative to the baseline (set at SI = 0) beginning on Day 1 and reaching asymptote on Day 3. (B) The weekly shift index revealed that the electrodes remained shifted for all three weeks post -training.
Recording electrodes within a single animal could span the tonotopic axis within A1. We had set the octave distance between the CS+ and CS− frequencies to be small (only 1.25 octaves), to make discrimination difficult and thereby study BLA effects under circumstances of a high degree of required specificity. Therefore, not all recording sites lay between the CS+ and the CS−. Thus, if the tuning of neurons were either above or below both the CS+ and CS− frequencies, then tuning shifts in their direction might be directed to either the CS+ or CS. To disambiguate this, we separated electrodes into three groups: (a) those tuned between the CS+ and CS−; for all electrodes in which both the CS+ and CS− were tuned higher or lower than the CS+, (b) those that were tuned nearer to the CS+ vs. the CS−, and (c) those tuned nearer to the CS− than the CS+.
Recording sites whose cells shifted towards the CS+ and were tuned between the CS+ and CS− (n = 12) had a significant linear trend to shift towards the CS+ across the first 3 days of training (F = 9.288, p = 0.011) and a significant shift towards the CS+ during Week 1 (t = 4.122, p < 0.05, df = 11). Again, across 21 days there was no significant days effect, indicating that the shift in tuning occurred rapidly and was maintained for 21 days (F(1, 12) = 1.002, p = 0.462). Sites not tuned between the CS+ and CS−, but nearer to the CS+ (n = 14) also had a significant linear trend to shift towards the CS+ in the first three days following training (F = 7.710, p = 0.017). This shift was also significant for the first week following training (t = 3.913, p < 0.05, df = 12), and there was no significant days effect across 21 days (F(21, 189) = 0.609, p = 0.909). There were too few sites tuned closer to the CS− (n = 4) for statistical analysis.
3.2.2. No change group
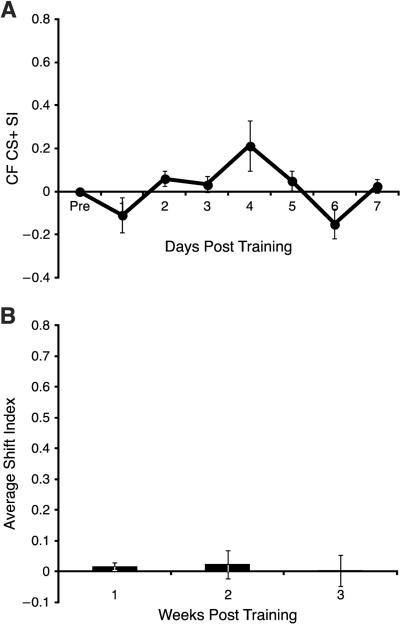
A1 sites in the No Change group did show a transient trend to shift towards the CS+ before returning to baseline during Week 1 (Days effect: F(7, 84) = 2.882, p = 0.010; quadratic trend: F(1, 12) = 6.622, p = 0.024; Fig. 5A). However, there was no significant increase or decrease in the shift index compared to baseline during Weeks 1, 2 or 3 (Week 1: t = 1.458, p = 0.171, df = 12; Week 2: t = 0.524, p = 0.611, df = 11; Week 3: t = 0.068, p = 0.947, df = 11) (Fig. 5B).
Fig. 5.
Sites categorized as No Change. (A) Week 1. There was no significant increase in S1. (B) There was no significant change across three weeks.
Electrodes whose cells did not shift (No Change group) and were nearest to the CS+ exhibited no significant effects (n = 9) (Week 1: F(7, 56) = 1.407, p = 0.221; 21 days: F(21, 147) = 0.794, p = 0.723). Too few recording sites were located between the CS+ and CS− and sites tuned nearer the CS− (n = 1 and 3, respectively) for statistical analysis.
3.2.3. Tuning shifts away from the CS+
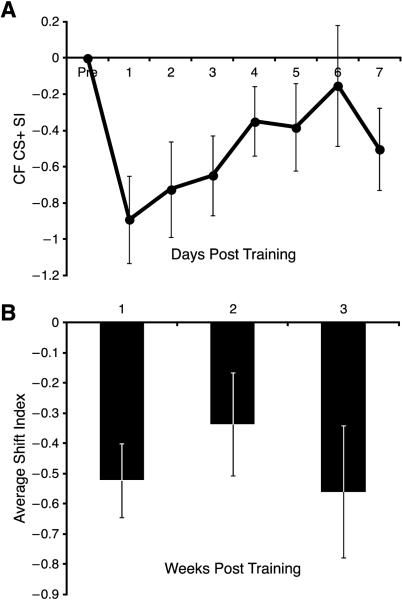
Electrodes whose neurons shifted away from the CS+ did exhibit a significant decrease in the shift index during the first week (t = 4.266, p < 0.05, df = 11). However, upon closer inspection, this shift was transient and driven by the first day post-training (t = 3.704, p < 0.05, df = 11). Day 2 post-training was no longer significantly different from baseline (t = 2.734, p > 0.05, df = 11, Fig. 6A). Moreover, there were no significant days effects either during Week 1 (F(7, 77) = 1.809, p = 0.098) or across the entire three weeks (F(21, 210) = 1.552, p = 0.064) (Fig. 6B).
Fig. 6.
Recording sites that shifted away from the CS+ across the first week post training. (A) There was a transient decrease in SI. (B) This shift away became highly variable across the Weeks 2 and 3, and was not significantly different from baseline.
Sites that shifted away from the CS+ and were tuned between the CS+ and CS− (n = 6) showed a significant, but transient, shift away from the CS+ for the first week (cubic trend, F(1, 5) = 7.742, p = 0.039); weeks two and three were not significantly different from baseline (t = 2.174, p > 0.05, df = 5; t = 2.144, p > 0.05, df = 5, respectively). There were an insufficient number of sites tuned near the CS− (n = 2) and the CS+ (n = 4) for statistical analysis.
3.2.4. Overview of tuning at threshold
The predominant tuning shifts were toward the CS+ (30/55 sites). Shifting developed over the first few post-training days, was significantly above baseline during the first post-training week and was maintained for the three week recording period. This pattern developed whether neurons were tuned between the CS+ and CS− or tuned lower or higher than these frequencies if tuned closer to the CS+ than CS−. In contrast to shifts toward the CS+, cells that shifted away from the CS+ exhibited highly transient effects that returned to baseline within the first week and remained non-significant for the three weeks of daily recording. Recording sites classified as “no change” or “shifts away from the CS+” did not have these characteristics. Thus, shifts away were transient rather than enduring for three weeks.
Given the different tuning outcomes for the three groups of recording sites, we asked if there were any differences between them prior to the single training session. We investigated two potential sources of inadvertent bias: octave distance from the CS+ frequency and characteristic frequency. However, we found no effect of distance from the CS+ on the shift index (F(2,52 = 2.412, p = 0.100). We also failed to find any relationship to the pre-training CF (F(2, 52) = 0.600, p = 0.553). Therefore, BLA activation is effective regardless of these factors.
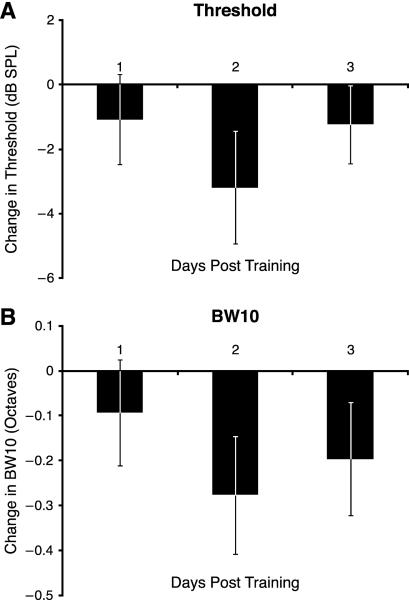
3.3. Threshold and bandwidth
A change in threshold indicates a modification of sensitivity; a decrease indicates a gain of sensitivity while an increase indicates a loss of sensitivity Recording sites whose tuning shifted toward the CS+ developed a significant decreasing linear trend in threshold across the first week (F(1,29) = 4.677, p = 0.039). This trend extended over Weeks 1 and 2 (F(1, 25) = 6.431, p = 0.018). The maximum increase in sensitivity was 6 dB (SPL) on day 13. Thereafter, there was a rapid return to baseline (Fig. 7A). Recording sites that did not change tuning also had no change in threshold (Week 1: F(7,84) = 0.479, p = 0.847; 21 Days: F(21, 231) = 0.824, p = 0.688). Sites that shifted away from the CS+ also had no change in threshold (Week 1: F(7, 77) = 1.596, p = 0.149; 21 days: F(21, 210) = 1.072, p = 0.380).
Fig. 7.
(A) Threshold at CF across the three weeks of recording for cortical sites that shifted toward the CS+ (significant mean increased SI during Week 1). There was a significant linear trend for decrease (increasing sensitivity) during Weeks 1 and 2, with return to the baseline during Week 3. (B) Bandwidth (BW10) for recording sites that shifted toward the CS+. There was a significant quadratic trend for decrease, indicating greatest increase in selectivity during Week 2.
Bandwidth 10 dB above threshold (BW10) indexes selectivity near threshold. A decrease in BW10 reflects an increase in selectivity and vice-versa for increasing bandwidth. Neurons at sites that shifted toward the CS+ also developed increased selectivity, although in a variable manner. Decreased BW10 was noticeable on Day 2, returned toward baseline after Day 3 and decreased again from Days 7–10, attaining a maximum increase of selectivity of 0.5 octaves; thereafter, it returned toward baseline while maintaining an increase in selectivity of ~0.2 octaves. Overall, these changes were significant: (quadratic trend, F(1, 25) = 5.238, p = 0.031) (Fig. 7B). Sites that showed no change in tuning also had no change in BW10 (Week 1: F(7, 77) = 1.044, p = 0.408; 21 Days: F(21, 231) = 1.452, p = 0.096). Finally, cells that shifted tuning away from the CS+ developed no changes in bandwidth (Week 1: F(7, 70) = 0.829, p = 0.566; 21 Days: F(21, 210) = 1.080, p = 0.371).
3.4. Tuning shifts at maximum response (BFmax)
The frequency distribution of shift index (SI) values for the maximum response in the FRA (BFmax) is presented in Fig. 8. Nineteen recording sites shifted toward the CS+, 21 sites did not change and 10 sites shifted away from the CS+.
Fig. 8.
Group frequency distribution of BFmax SI scores averaged across the first week post training. Central area indicates “No Change” sites. Positive values denote shifts toward CS+. Negative values indicate shifts away from CS+.
As in the case of threshold tuning, shifts toward the CS+ were evident on Day 1 and remained elevated during Week 1 (Fig. 9A) (linear trend across the first week, F(1, 18) = 4.649, p = 0.045). This shift was long lasting as there were no days effects across the 21 days (F(21, 357) = 0.837, p = 0.674; Fig. 9B). Recording sites in the No Change group did not show any days effects (Week 1: F(7, 140) = 0.458, p = 0.863; 21 days: F(21, 378) = 0.683, p = 0.850). Neither did the Decrease group (Week 1: F(7, 63) = 1.688, p = 0.128; 21 days: F(21, 147) = 1.460, p = 0.100).
Fig. 9.
Recording sites at maximum response above threshold (BFmax) that shifted towards the CS+. (A) Week 1: there was a significant shift towards the CS+. (B) This shift was maintained for three weeks.
It is clear that the proportion of tuning shifts toward the CS+ frequency was less for BFmax (19/50, 38%) that at threshold (30/55, 55%; see section 3.2). This discrepancy is caused by a much greater proportion of sites that did not change at BFmax: BFmax, 21/50 = 42%; threshold, 13/55 = 24%. Considering only those sites that developed tuning shift, the percentage of shifts toward the CS+ are much closer: BFmax, toward = 19, away = 10, 19/29 = 66%; shifts at threshold, toward = 30, away = 12, 30/42 = 71%.
Insofar at CS+ directed tuning shifts were comparable at BFmax to shifts at threshold, we also investigated potential predisposing biases that might have contributed to CS+ shifts for the former. Analysis of octave distance from the CS+ frequency revealed no effect of this variable on BFmax shift indices for the three groups: shifts toward or away from the CS+, or no change (F(2, 47) = 1.517, p = 0.230). An analysis of the potential contributions of pre-training CF also failed to find any relationship (F(2, 47) = 1.783, p = 0.179).
3.5. Gamma band changes during training
Given that the BLA can shift tuning in the primary auditory cortex toward the CS+, some insight into the mechanisms may be gleaned from analysis of gamma band oscillations (~40–120 Hz) in the auditory cortical EEG during training trials. This range of oscillations has been linked to synchronous or coordinated neuronal activity during several cognitive processes, including attention, learning and memory (Lutzenberger, Ripper, Busse, Birbaumer, & Kaiser, 2002; Osipova, Takashima, Oostenveld, Fernández, Maris, & Jensen, 2006). A subset of electrodes yielded EEG/gamma activity (n = 26/55, 9 animals, Methods, section 2.3) during the 30 each CS+ and CS− trials during the single training session. Nineteen of these twenty-six recording sites shifted their tuning toward the CS+. (There were too few recording sites in the No Change and Decrease groups for statistical analysis.) We compared mean gamma activity elicited by the CS+ and the CS− during the temporal window of 0.5–1.5 sec after tone onset. This period avoided contamination from the onset evoked potential (which contains power in the gamma band) and artifact caused by stimulation of the BLA. Gamma band activity following BLA stimulation was not analyzed because CS+ trials contain both tone and stimulation produced gamma, the effects of which cannot be separated.
For recording sites that shifted towards the CS+ (n = 19), there appeared to be greater tone induced gamma to the CS+ than to the CS− (Fig. 10). This impression was verified statistically (t = 2.091, p = 0.05, df = 18) (Fig. 11).
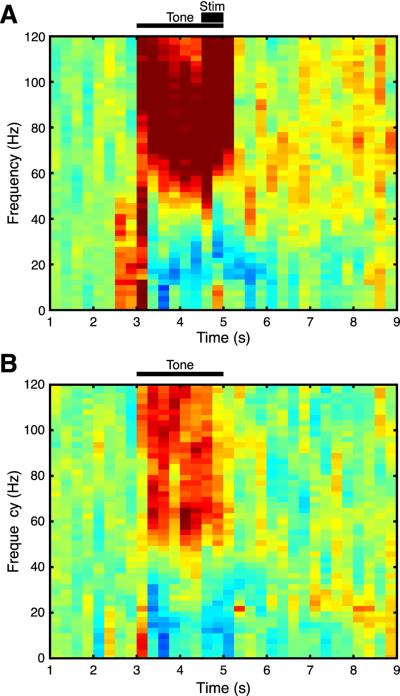
Fig. 10.
Average EEG spectral activity during presentation of (A) the CS+ and (B) the CS− during the training session (30 trials each CS+ and CS−). Tone presentation (2 s) is indicated by thin horizontal bar (A and B); BLA stimulation is denoted by short thick bar (A only). (Extremely intensive gamma activity (dark red) during and immediately following BLA stimulation reflects artifact.) Vertical color bar indicates amount of activity across the EEG spectrum. Gamma band = 40–120 Hz. Note the greater amount of gamma band activity to the CS+ (A) than the CS− (B), especially in “high gamma” band (60–120 Hz).
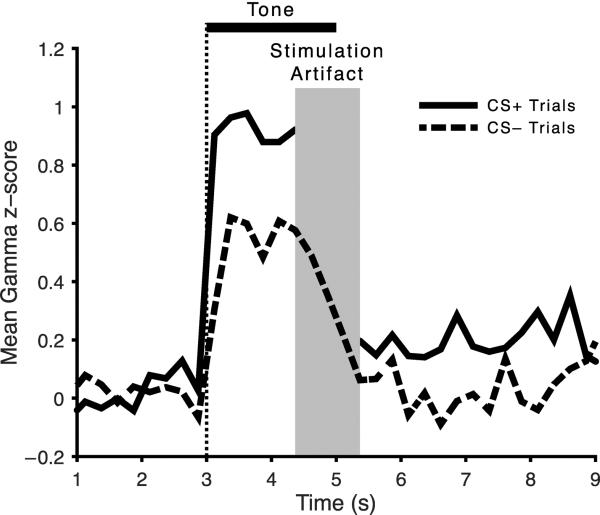
Fig. 11.
Quantification of tone elicited conditioned gamma band activity (“high gamma”, 60–120 Hz). Note that the CS+ elicits significantly greater gamma activity than the CS−.
The increase in gamma seemed to be specific for high gamma (60–120 Hz) vs. low gamma (40–60 Hz) bands. This was confirmed statistically: high gamma, t(18) = 2.188, p = 0.042, df = 18; low gamma, t(18) = 0.747, p = 0.464. These findings suggest that when tuning is shifted toward the CS+, cells in A1 increase their synchronous activity.
4. Discussion
4.1. Precis of issues, answers and relevance
This study addressed three questions about BLA induced tuning shifts in auditory cortical representations: (a) Do they occur in adult, waking animals? (b) Are they associative? (c) How long do they endure? The answers to the first two are “yes” and to the third: they endure (on a daily basis) for at least 3 weeks, the length of the study.
What is the relevance of these findings to brain substrates of memory? Briefly, although BLA modulation of memory is well established, the means by which it can strengthen memory are unknown. Auditory cortical RF plasticity, i.e., CS-directed tuning shifts, provide a targeted substrate, particularly as they have the attributes of associative memory and representational enhancement is known to predict the strength of memory. Therefore, the findings support the hypothesis that the BLA strengthens memory by enhancing specific sensory cortical representations. In the next section, we address detailed aspects of the novel approach and findings.
4.2. Resume of findings
Tone paired with brief electrical stimulation of the basolateral amygdala can shift the tuning of cells in the primary auditory cortex toward the frequency of this CS+, compared to a non-paired tone (CS−). This functional reorganization of A1 occurs both at threshold (characteristic frequency, CF) and above threshold at the maximum response (BFmax) within the matrix of frequencies and sound levels that comprise the frequency response area. Perhaps most importantly, tuning shifts favoring the CS+ developed rapidly and were maintained for at least three weeks, the duration of this study. Of note, the current findings appear to provide the most detailed record of associatively induced neural plasticity over the longest continuous period of time yet reported. Thus, any variations in post-training dynamics would have been detected. Therefore, the conclusion that rapidly-developing specific cortical neural plasticity was maintained continuously can be held with confidence.
Additionally, decreased threshold and decreased bandwidth developed during a portion of the three-week post-training recording period together with shifts toward the CS+. Such related specific plasticity in tuning, sensitivity and selectivity has not been reported previously. Neither of these measures had the same time course as tuning shifts or each other. Threshold decreased during the first two weeks and then returned to baseline; bandwidth was reduced mainly during Week 2. As novel findings, there is not enough parametric information to understand these individual temporal profiles, but the findings indicate subtleties in associative representational plasticity. Detection of tuning, threshold and bandwidth plasticity y underscores the value of using sensory neurophysiological measures in studies of learning and memory.
To test the effects of BLA activation under conditions of a high degree of specificity, we used a discrimination paradigm in which the CS+ and CS− tones were relatively close together, 1.25 octaves. Nonetheless, BLA stimulation favored tuning shifts toward the tone with which it was paired. Thus, the results demonstrate a high level of specificity, which is needed in natural learning, in which memory modulation by the BLA does exhibit stimulus specificity (Roozendaal, Castello, Vedana, Barsegyan, & McGaugh, 2008). Additionally, such specificity indicates that the effects of BLA stimulation in this study are also associative, again an important property in natural learning and memory modulation (Malin & McGaugh, 2006).
Insofar as most of the recording sites that shifted their tuning favored the CS+, (71% at threshold, 66% at BFmax), it might be the case that inadvertent bias was involved. There might have been predisposing differences between groups of recording sites that shifted toward, away from, or did not change tuning. For example, if cells shifting toward the CS+ had been tuned more closely to the CS+ than cells that did not shift to this frequency, then “recruitment” of cells to a stimulus paired with BLA activation would be severely limited, i.e., to “local neurons”. However, an analysis of octave distance from the CS+ failed to reveal any such difference for either tuning shifts at threshold or at BFmax. Similarly, if cells that had “gravitated” toward the CS+ had come from a population with only low or high frequency tuning, then the domain of effects of BLA activation would be markedly constrained: it could strengthen memories only for a narrow range of auditory stimuli. But as was the case of octave distance, there was no relationship of pre-training tuning either at threshold (CF) or above threshold (BFmax). Therefore, the effectiveness of BLA activation on cortical tuning appears to be general for acoustic frequency in the primary auditory cortex.
The current results replicate and greatly extend prior findings in the anesthetized preparation. Tone paired with BLA stimulation in the urethane anesthetized rat was found to produce tuning shifts toward the CS tone at BFmax (Chavez et al., 2009). The prior observations were necessarily limited to a relatively brief post-training period of tens of minutes. Nonetheless, tuning shifts directed to the signal tone were seen to develop within minutes following a single training session (100 trials, ~30 min training period), and continue to shift during the next hour of testing.
The duration of BLA-induced specific representational enhancement was extended from 75 min in the anesthetized state to 3 weeks (21 days) in the waking state. The first post-training data were obtained ~24 h after the conclusion of training (30 each CS+ and CS− trials, 60 trials total). Therefore the detailed temporal dynamics during the first day after training remain to be studied. However, as tuning had shifted by the first day, it is likely that consolidation processes had been initiated during training and continued to promote tuning shifts thereafter. The present observations did reveal continued increase in the shift index over days. Thus, the magnitude of the shift at threshold was clearly greater on Day 1 than baseline; it did not attain statistical significance until Day 2 (section 3.2.1; Fig. 4). Such gradual shifting was less evident above threshold (section 3.4; Fig. 9).
4.3. Possible role of acetylcholine and the nucleus basalis
The basolateral amygdala is promiscuous in its outputs to other areas of the brain. Thus, it is not possible at this time to delineate the pathways by which activation of the BLA induces tuning shifts in A1. However, it is likely that acetylcholine (ACh) is critically involved in BLA mediated memory modulation, as both systemic and intra-amygdala post-training administration of cholinergic agents affect memory consolidation (Power, McIntyre, Litmanovich, & McGaugh, 2003; Vazdarjanova & McGaugh, 1999). Cholinergic effects on memory strength act “downstream” of NE actions in the BLA because blockade of β-adrenoceptors within the BLA does not prevent the memory enhancement induced by systemic intra-amygdala infusions of the muscarinic cholinergic agonist oxotremorine. Moreover, intra-amygdala administration of atropine blocks NE-induced memory enhancement (Dalmaz, Introini-Collison, & McGaugh, 1993; Introini-Collison, Dalmaz, & McGaugh, 1996). Additionally, intra-amygdala infusions of atropine also block the memory enhancing effects of systemic or intra-amygdala administration of glucocorticoid receptor agonists (Power, Roozendaal, & McGaugh, 2000).
The nucleus basalis of the basal forebrain is the major source of ACh to the cerebral cortex (Mesulam, Mufson, Levey, & Wainer, 1983) and the BLA projects to the NB, both directly and indirectly via the central nucleus of the amygdala (Jolkkonen, Miettinen, Pikkarainen, & Pitkänen, 2002; Krettek & Price, 1978). In view of the evidence that acetylcholine acts downstream from NE actions within the amygdala, as expected, memory consolidation is impaired by NB lesions, and intra-BLA infusions of oxotremorine or the muscarinic agonist physostigmine attenuate this impairment of memory (Power & McGaugh, 2002).
Additional evidence implicating ACh and the nucleus basalis in BLA induced RF plasticity is that stimulation of the NB shifts the tuning of A1 cells to favor the CS frequency of the tone with which it is paired (Bakin & Weinberger, 1996, Bjordahl, Dimyan, & Weinberger, 1998; Dimyan & Weinberger, 1999) and can thereby enlarge its representational area in the tonotopic map (Kilgard & Merzenich, 1998). Furthermore, activation of the NB implants specific memory for its paired tone, as indicated by behavioral changes that are indistinguishable from associatively induced natural memory (McLin, Miasnikov, & Weinberger, 2002). Implanted memory also has the attributes of natural memory: it is associative, highly specific, develops rapidly, consolidates (i.e., become stronger over time without additional training) and exhibits long-term retention (Miasnikov, Chen, & Weinberger, 2006, 2009, 2011; Weinberger, Miasnikov, & Chen, 2006, 2009). Moreover, NB stimulation that implants specific memory is motivationally neutral (Miasnikov, Chen, Gross, Poytress, & Weinberger, 2008) and thus likely to be downstream from the amygdala. Also, NB implanted memory is dependent on central muscarinic cholinergic receptors (Miasnikov, Chen, & Weinberger, 2008). Further, stimulation of the BLA produces cortical activation mediated by acetylcholine release in the cortex (Dringenberg, Saber, & Cahill, 2001; Dringenberg & Vanderwolf, 1996) and dependent upon an intact nucleus basalis (Dringenberg, Sparling, Frazer, & Murdoch, 2006). Additionally, it should be noted that pairing tone with NB stimulation produces tone-elicited conditioned increase of cortical gamma band activity (McLin, Miasnikov, & Weinberger, 2003). And, as reported in this study, tone paired with BLA stimulation also produces conditioned increased gamma, in particular greater to the CS+ than to the CS−.
4.4. Strengthening memory by increasing stimulus representation
Although the route(s) by which the BLA modulates frequency representations in A1 remain(s) to be determined, this study has provided some clues as to how it may strengthen memory during or for a time-limited period in acquisition. We propose that at least part of the process requires that the BLA increases the number of neurons that are “recruited” to the representation of behaviorally important stimuli. The findings come from the primary auditory cortex, which provides the most extensive body of relevant studies. However, our hypothesis is intended as a general mechanism, across sensory modalities and from simple through complex network-based representations of experience.
The rationale is as follows. First, associative learning is accompanied by specific tuning shifts that enhance the representation of a signal stimulus relative to other stimuli (tones). Second, such receptive field plasticity viewed across A1 produces an increased number of cells that better represent such a signal stimulus, resulting in a gain in the area of its representation in the tonotopic map (Bieszczad & Weinberger, 2010a). Third, the greater the importance of a tone for an animal: the greater the gain in representational area (Rutkowski & Weinberger, 2005). Fourth, the greater the gain in representational area: the greater the strength of behavioral memory (i.e., the greater its resistance to extinction) (Bieszczad & Weinberger, 2010b). Fifth, the greater the weakening of a memory (i.e., the greater its extinction): the greater the loss in gain of representational area (Bieszczad & Weinberger, 2012). When taken together with the fact that the BLA can increase the strength of memory, the current BLA effects of tuning shifts converge with the Literature for studies of the primary auditory cortex.
The mechanism of shifting tuning to more strongly represent a behaviorally important signal stimulus was hypothesized for both the amygdala and the nucleus basalis in a broader model of learning-induced cortical plasticity (Weinberger, 1998; Weinberger, Ashe, Metherate, McKenna, Diamond, & Bakin, 1990). The present findings provide support for this model. More recent developments require a refinement of such a model in a rapidly developing field of research. One of these concerns gamma band activity, which is thought to index the coordinated activity of cortical cells, thus producing a greater downstream effect that the same amount of unsynchronized activity. Recently, it has been shown that conditioned increases in gamma band activity predict both the acquisition of simple associations (tone–shock pairing, “auditory fear conditioning”) and auditory cortical tuning shift plasticity (Headley & Weinberger, 2011). The present results show that the BLA can produce a conditioned increase in gamma activity that is specific to a CS+ (section 4). Therefore, we suggest that the BLA strengthens memory, at least in part, by both increasing the number of cells that represent the relevant stimulus (experience) and inducing them to act in a more coordinated manner, thus increasing the effect on downstream structures and increasing the likelihood of behavioral expression.
4.5. Future directions
The approach and findings of the present experiment constitute one of the initial steps in a new line of research. As such, they, with the prior study in anesthetized animals (Chavez et al., 2009), constitute “proof of concept” demonstrations rather than reductionistic investigations. Clearly, future studies need to be directed to the neural bases of amygdala modulation of memory, especially for the context presented above. Issues include, but are in no sense limited to (a) the relevant circuitry, (b) the involved transmitters and (c) the involved molecular mechanisms. At a network/systems level, it will be critical to understand how cells interact to effectuate an increase in memory strength. Use of gamma band activity as a convenient index of neural interactions should be helpful.
More generally, the concepts advanced by the current findings should be applied to other sensory regions of the cortex and to other aspects of memory. It may well be that at least the initial strengthening of memory involves increasing the participation of greater numbers of neurons as an elemental mechanism. Moreover, the findings may be relevant to memory disorders, including PTSD. For example, traumatic memories are invasive. They might be represented by too many neurons, which might occur with BLA hyper-modulation of extremely emotional experiences consequent to release of abnormally high levels of stress hormones during trauma. In turn, this could produce an abnormally enlarged representational network. Such extensive representations would be more likely to be activated by a similar “recall” stimulus than a normal smaller network. This would produce uncontrollable recall and intrusiveness of the traumatic experience. Thus, the current findings should be applicable far beyond the primary auditory cortex, which while proven to be an excellent model system for the study of memory strength and memory modulation, is simply an opportune entry point into the broader pursuit of normal and abnormal mnemonic brain substrates.
Highlights
The basolateral amygdala strengthens remotely stored memories.
Auditory cortical tuning plasticity serves as memory representations.
Waking rats receive 1 session of tone paired with BLA stimulation.
Stimulation rapidly induced specific tuning shifts, maintained for at least 3 weeks.
Thus, BLA may strengthen memory by increasing number of its representative cells.
Acknowledgements
We thank Drew B. Headley, Gabriel K. Hui and Jacquie Weinberger for assistance. This study was funded by the NIDCD grant #DC-05592 (NMW), NIMH grant #MH-12526 (JLM) and the APA/DPN fellowship #5-T32-MH-18882 (CMC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akirav I, Richter-Levin G. Biphasic modulation of hippocampal plasticity by behavioral stress and basolateral amygdala stimulation in the rat. Journal of Neuroscience. 1999;19(23):10530–10535. doi: 10.1523/JNEUROSCI.19-23-10530.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akirav I, Richter-Levin G. Mechanisms of amygdala modulation of hippocampal plasticity. Journal of Neuroscience. 2002;22(22):9912–9921. doi: 10.1523/JNEUROSCI.22-22-09912.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Research. 1990;536(1–2):271–286. doi: 10.1016/0006-8993(90)90035-a. [DOI] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(20):11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieszczad KM, Weinberger NM. Remodeling the cortex in memory: Increased use of a learning strategy increases the representational area of relevant acoustic cues. Neurobiology of Learning and Memory. 2010a;94(2):127–144. doi: 10.1016/j.nlm.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieszczad KM, Weinberger NM. Representational gain in cortical area underlies increase of memory strength. Proceedings of the National Academy of Sciences of the United States of America. 2010b;107(8):3793–3798. doi: 10.1073/pnas.1000159107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieszczad KM, Weinberger NM. Extinction reveals that primary sensory cortex predicts reinforcement outcome. European Journal of Neuroscience. 2012;35(4):598–613. doi: 10.1111/j.1460-9568.2011.07974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjordahl TS, Dimyan MA, Weinberger NM. Induction of long-term receptive field plasticity in the auditory cortex of the waking guinea pig by stimulation of the nucleus basalis. Behavioral Neuroscience. 1998;112(3):467–479. doi: 10.1037//0735-7044.112.3.467. [DOI] [PubMed] [Google Scholar]
- Cahill L, Alkire MT. Epinephrine enhancement of human memory consolidation: Interaction with arousal at encoding. Neurobiology of Learning and Memory. 2003;79(2):194–198. doi: 10.1016/s1074-7427(02)00036-9. [DOI] [PubMed] [Google Scholar]
- Cahill L, Gorski L, Le K. Enhanced human memory consolidation with post-learning stress: Interaction with the degree of arousal at encoding. Learning and Memory. 2003;10(4):270–274. doi: 10.1101/lm.62403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Prins B, Weber M, McGaugh JL. β-adrenergic activation and memory for emotional events. Nature. 1994;371(6499):702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- Campbell AW. Histological studies on the localisation of cerebral function. Cambridge University Press; Cambridge, UK: 1905. [Google Scholar]
- Chavez CM, McGaugh JL, Weinberger NM. The basolateral amygdala modulates specific sensory memory representations in the cerebral cortex. Neurobiology of Learning and Memory. 2009;91(4):382–392. doi: 10.1016/j.nlm.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmaz C, Introini-Collison IB, McGaugh JL. Noradrenergic and cholinergic interactions in the amygdala and the modulation of memory storage. Behavioural Brain Research. 1993;58(1–2):167–174. doi: 10.1016/0166-4328(93)90101-u. [DOI] [PubMed] [Google Scholar]
- Dimyan MA, Weinberger NM. Basal forebrain stimulation induces discriminative receptive field plasticity in the auditory cortex. Behavioral Neuroscience. 1999;113(4):691–702. doi: 10.1037//0735-7044.113.4.691. [DOI] [PubMed] [Google Scholar]
- Dringenberg HC, Kuo MC, Tomaszek S. Stabilization of thalamo-cortical long-term potentiation by the amygdala: Cholinergic and transcription-dependent mechanisms. European Journal of Neuroscience. 2004;20(2):557–565. doi: 10.1111/j.1460-9568.2004.03515.x. [DOI] [PubMed] [Google Scholar]
- Dringenberg HC, Saber AJ, Cahill L. Enhanced frontal cortex activation in rats by convergent amygdaloid and noxious sensory signals. NeuroReport. 2001;12(11):2395–2398. doi: 10.1097/00001756-200108080-00022. [DOI] [PubMed] [Google Scholar]
- Dringenberg HC, Sparling JS, Frazer J, Murdoch J. Generalized cortex activation by the auditory midbrain: Mediation by acetylcholine and subcortical relays. Experimental Brain Research. 2006;174(1):114–123. doi: 10.1007/s00221-006-0427-5. [DOI] [PubMed] [Google Scholar]
- Dringenberg HC, Vanderwolf CH. Cholinergic activation of the electrocorticogram: An amygdaloid activating system. Experimental Brain Research. 1996;108(2):285–296. doi: 10.1007/BF00228101. [DOI] [PubMed] [Google Scholar]
- Ferry B, McGaugh JL. Clenbuterol administration into the basolateral amygdala post-training enhances retention in an inhibitory avoidance task. Neurobiology of Learning and Memory. 1999;72(1):8–12. doi: 10.1006/nlme.1998.3904. [DOI] [PubMed] [Google Scholar]
- Ferry B, McGaugh JL. Involvement of basolateral amygdala α2-adrenoceptors in modulating consolidation of inhibitory avoidance memory. Learning and Memory. 2008;15(4):238–243. doi: 10.1101/lm.760908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván VV, Weinberger NM. Long-term consolidation and retention of learning-induced tuning plasticity in the auditory cortex of the guinea pig. Neurobiology of Learning and Memory. 2002;77(1):78–108. doi: 10.1006/nlme.2001.4044. [DOI] [PubMed] [Google Scholar]
- Gao E, Suga N. Experience-dependent corticofugal adjustment of midbrain frequency map in bat auditory system. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(21):12663–12670. doi: 10.1073/pnas.95.21.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield T, Spanis C, McGaugh JL. Response of amygdalar norepinephrine to footshock and GABAergic drugs using in vivo microdialysis and HPLC. Brain Research. 1999;835(2):340–345. doi: 10.1016/s0006-8993(99)01566-8. [DOI] [PubMed] [Google Scholar]
- Headley DB, Weinberger NM. Gamma-band activation predicts both associative memory and cortical plasticity. Journal of Neuroscience. 2011;31(36):12748–12758. doi: 10.1523/JNEUROSCI.2528-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegaya Y, Saito H, Abe K. High-frequency stimulation of the basolateral amygdala facilitates the induction of long-term potentiation in the dentate gyrus in vivo. Neuroscience Research. 1995;22(2):203–207. doi: 10.1016/0168-0102(95)00894-7. [DOI] [PubMed] [Google Scholar]
- Introini-Collison IB, Dalmaz C, McGaugh JL. Amygdala β-noradrenergic influences on memory storage involve cholinergic activation. Neurobiology of Learning and Memory. 1996;65(1):57–64. doi: 10.1006/nlme.1996.0006. [DOI] [PubMed] [Google Scholar]
- Jolkkonen E, Miettinen R, Pikkarainen M, Pitkänen A. Projections from the amygdaloid complex to the magnocellular cholinergic basal forebrain in rat. Neuroscience. 2002;111(1):133–149. doi: 10.1016/s0306-4522(01)00578-4. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279(5357):1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Kisley MA, Gerstein GL. Daily variation and appetitive conditioning-induced plasticity of auditory cortex receptive fields. European Journal of Neuroscience. 2001;13(10):1993–2003. doi: 10.1046/j.0953-816x.2001.01568.x. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. Journal of Comparative Neurology. 1978;178(2):225–254. doi: 10.1002/cne.901780204. [DOI] [PubMed] [Google Scholar]
- Li Z, Richter-Levin G. Stimulus intensity-dependent modulations of hippocampal long-term potentiation by basolateral amygdala priming. Frontiers in Cellular Neuroscience. 2012;6(21):1–9. doi: 10.3389/fncel.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutzenberger W, Ripper B, Busse L, Birbaumer N, Kaiser J. Dynamics of gamma-band activity during an audiospatial working memory task in humans. Journal of Neuroscience. 2002;22(13):5630–5638. doi: 10.1523/JNEUROSCI.22-13-05630.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin EL, Ibrahim DY, Tu JW, McGaugh JL. Involvement of the rostral anterior cingulate cortex in consolidation of inhibitory avoidance memory: Interaction with the basolateral amygdala. Neurobiology of Learning and Memory. 2007;87(2):295–302. doi: 10.1016/j.nlm.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin EL, McGaugh JL. Differential involvement of the hippocampus, anterior cingulate cortex, and basolateral amygdala in memory for context and footshock. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(6):1959–1963. doi: 10.1073/pnas.0510890103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. Involvement of hormonal and neuromodulatory systems in the regulation of memory storage. Annual Review of Neuroscience. 1989;12:255–287. doi: 10.1146/annurev.ne.12.030189.001351. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory — A century of consolidation. Science. 2000;287(5451):248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory consolidation and the amygdala: A systems perspective. Trends in Neurosciences. 2002;25(9):456–461. doi: 10.1016/s0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory and emotion: The making of lasting memories. Columbia University Press; New York: 2003. [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Current Opinion in Neurobiology. 2002;12(2):205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. Drug enhancement of memory consolidation: Historical perspective and neurobiological implications. Psychopharmacology. 2009;202(1–3):3–14. doi: 10.1007/s00213-008-1285-6. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Hatfield T, McGaugh JL. Amygdala norepinephrine levels after training predict inhibitory avoidance retention performance in rats. European Journal of Neuroscience. 2002;16(7):1223–1226. doi: 10.1046/j.1460-9568.2002.02188.x. [DOI] [PubMed] [Google Scholar]
- McLin DE, 3rd, Miasnikov AA, Weinberger NM. Induction of behavioral associative memory by stimulation of the nucleus basalis. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(6):4002–4007. doi: 10.1073/pnas.062057099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLin DE, 3rd, Miasnikov AA, Weinberger NM. CS-specific gamma, theta, and alpha EEG activity detected in stimulus generalization following induction of behavioral memory by stimulation of the nucleus basalis. Neurobiology of Learning and Memory. 2003;79(2):152–176. doi: 10.1016/s1074-7427(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: Cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (Substantia innominata), and hypothalamus in the rhesus monkey. Journal of Comparative Neurology. 1983;214(2):170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Gross N, Poytress BS, Weinberger NM. Motivationally neutral stimulation of the nucleus basalis induces specific behavioral memory. Neurobiology of Learning and Memory. 2008;90(1):125–137. doi: 10.1016/j.nlm.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Weinberger NM. Rapid induction of specific associative behavioral memory by stimulation of the nucleus basalis in the rat. Neurobiology of Learning and Memory. 2006;86(1):47–65. doi: 10.1016/j.nlm.2005.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Weinberger NM. Specific auditory memory induced by nucleus basalis stimulation depends on intrinsic acetylcholine. Neurobiology of Learning and Memory. 2008;90(2):443–454. doi: 10.1016/j.nlm.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Weinberger NM. Behavioral memory induced by stimulation of the nucleus basalis: Effects of contingency reversal. Neurobiology of Learning and Memory. 2009;91(3):298–309. doi: 10.1016/j.nlm.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Weinberger NM. Consolidation and long-term retention of an implanted behavioral memory. Neurobiology of Learning and Memory. 2011;95(3):286–295. doi: 10.1016/j.nlm.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molchan SE, Sunderland T, McIntosh AR, Herscovitch P, Schreurs BG. A functional anatomical study of associative learning in humans. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(17):8122–8126. doi: 10.1073/pnas.91.17.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Dolan RJ. Experience-dependent modulation of tonotopic neural responses in human auditory cortex. Proceedings of the Royal Society B: Biological Sciences. 1998;265(1397):649–657. doi: 10.1098/rspb.1998.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osipova D, Takashima A, Oostenveld R, Fernández G, Maris E, Jensen O. Theta and gamma oscillations predict encoding and retrieval of declarative memory. Journal of Neuroscience. 2006;26(28):7523–7531. doi: 10.1523/JNEUROSCI.1948-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Chen SA. The basolateral amygdala is a cofactor in memory enhancement produced by intrahippocampal glutamate injections. Psychobiology. 1999;27(3):377–385. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. Academic Press; Burlington, MA: 2007. [Google Scholar]
- Power AE, McGaugh JL. Phthalic acid amygdalopetal lesion of the nucleus basalis magnocellularis induces reversible memory deficits in rats. Neurobiology of Learning and Memory. 2002;77(3):372–388. doi: 10.1006/nlme.2001.4030. [DOI] [PubMed] [Google Scholar]
- Power AE, McIntyre CK, Litmanovich A, McGaugh JL. Cholinergic modulation of memory in the basolateral amygdala involves activation of both m1 and m2 receptors. Behavioural Pharmacology. 2003;14(3):207–213. doi: 10.1097/00008877-200305000-00004. [DOI] [PubMed] [Google Scholar]
- Power AE, Roozendaal B, McGaugh JL. Glucocorticoid enhancement of memory consolidation in the rat is blocked by muscarinic receptor antagonism in the basolateral amygdala. European Journal of Neuroscience. 2000;12(10):3481–3487. doi: 10.1046/j.1460-9568.2000.00224.x. [DOI] [PubMed] [Google Scholar]
- Quirarte GL, Galvez R, Roozendaal B, McGaugh JL. Norepinephrine release in the amygdala in response to footshock and opioid peptidergic drugs. Brain Research. 1998;808(2):134–140. doi: 10.1016/s0006-8993(98)00795-1. [DOI] [PubMed] [Google Scholar]
- Quirarte GL, Roozendaal B, McGaugh JL. Glucocorticoid enhancement of memory storage involves noradrenergic activation in the basolateral amygdala. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(25):14048–14053. doi: 10.1073/pnas.94.25.14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesler R, Roozendaal B, McGaugh JL. Basolateral amygdala lesions block the memory-enhancing effect of 8-Br-cAMP infused into the entorhinal cortex of rats after training. European Journal of Neuroscience. 2002;15(5):905–910. doi: 10.1046/j.1460-9568.2002.01924.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Castello NA, Vedana G, Barsegyan A, McGaugh JL. Noradrenergic activation of the basolateral amygdala modulates consolidation of object recognition memory. Neurobiology of Learning and Memory. 2008;90(3):576–579. doi: 10.1016/j.nlm.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Hui GK, Hui IR, Berlau DJ, McGaugh JL, Weinberger NM. Basolateral amygdala noradrenergic activity mediates corticosterone-induced enhancement of auditory fear conditioning. Neurobiology of Learning and Memory. 2006;86(3):249–255. doi: 10.1016/j.nlm.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. The memory-modulatory effects of glucocorticoids depend on an intact stria terminalis. Brain Research. 1996;709(2):243–250. doi: 10.1016/0006-8993(95)01305-9. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. Glucocorticoid receptor agonist and antagonist administration into the basolateral but not central amygdala modulates memory storage. Neurobiology of Learning and Memory. 1997;67(2):176–179. doi: 10.1006/nlme.1996.3765. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Quirarte GL, McGaugh JL. Stress-activated hormonal systems and the regulation of memory storage. Annals of the New York Academy of Sciences. 1997;821:247–258. doi: 10.1111/j.1749-6632.1997.tb48284.x. [DOI] [PubMed] [Google Scholar]
- Rutkowski RG, Miasnikov AA, Weinberger NM. Characterisation of multiple physiological fields within the anatomical core of rat auditory cortex. Hearing Research. 2003;181(1–2):116–130. doi: 10.1016/s0378-5955(03)00182-5. [DOI] [PubMed] [Google Scholar]
- Rutkowski RG, Weinberger NM. Encoding of learned importance of sound by magnitude of representational area in primary auditory cortex. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(38):13664–13669. doi: 10.1073/pnas.0506838102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs BG, McIntosh AR, Bahro M, Herscovitch P, Sunderland T, Molchan SE. Lateralization and behavioral correlation of changes in regional cerebral blood flow with classical conditioning of the human eyeblink response. Journal of Neurophysiology. 1997;77(4):2153–2163. doi: 10.1152/jn.1997.77.4.2153. [DOI] [PubMed] [Google Scholar]
- Setlow B, Roozendaal B, McGaugh JL. Involvement of a basolateral amygdala complex–nucleus accumbens pathway in glucocorticoid-induced modulation of memory consolidation. European Journal of Neuroscience. 2000;12(1):367–375. doi: 10.1046/j.1460-9568.2000.00911.x. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, McGaugh JL. Basolateral amygdala is involved in modulating consolidation of memory for classical fear conditioning. Journal of Neuroscience. 1999;19(15):6615–6622. doi: 10.1523/JNEUROSCI.19-15-06615.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Dynamic regulation of receptive fields and maps in the adult sensory cortex. Annual Review of Neuroscience. 1995;18:129–158. doi: 10.1146/annurev.ne.18.030195.001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Tuning the brain by learning and by stimulation of the nucleus basalis. Trends in Cognitive Sciences. 1998;2(8):271–273. doi: 10.1016/s1364-6613(98)01200-5. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Specific long-term memory traces in primary auditory cortex. Nature Reviews Neuroscience. 2004;5(4):279–290. doi: 10.1038/nrn1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Associative representational plasticity in the auditory cortex: A synthesis of two disciplines. Learning and Memory. 2007;14(1–2):1–16. doi: 10.1101/lm.421807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM, Ashe JH, Metherate R, McKenna TM, Diamond DM, Bakin J. Retuning auditory cortex by learning: A preliminary model of receptive field plasticity. Concepts in Neuroscience. 1990;1(1):91–132. [Google Scholar]
- Weinberger NM, Miasnikov AA, Chen JC. The level of cholinergic nucleus basalis activation controls the specificity of auditory associative memory. Neurobiology of Learning and Memory. 2006;86(3):270–285. doi: 10.1016/j.nlm.2006.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM, Miasnikov AA, Chen JC. Sensory memory consolidation observed: Increased specificity of detail over days. Neurobiology of Learning and Memory. 2009;91(3):273–286. doi: 10.1016/j.nlm.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]