Abstract
Objectives
First, we sought to determine if parents of children with cancer or a brain tumor had greater stress compared to parents of healthy children, and to evaluate the correlates of stress among parents of children with cancer or brain tumors. Second, we sought to examine the relationship between perceived stress and symptoms of stress, and how that relationship may differ for parents of children with cancer.
Methods
In-person interviewer-assisted surveys were administered to 73 case dyads (children with cancer or a brain tumor and their parents) and 133 comparison dyads (children without health problems and their parents from a community sample). Descriptive analyses and multivariable logistic regressions were performed for case-comparison and case-only analyses to distinguish correlates of parental stress.
Results
Parents of children with cancer exhibited higher levels of physiological symptoms of stress than parents of healthy children. Poor sleep quality and greater social stress (negative social interactions) were significant correlates of increased levels of stress in parents of children with cancer (odds ratio 4.23, 95% confidence interval 1.15–15.60; and odds ratio 1.07, 95% confidence interval 1.00–1.14, respectively). A subset of parents reported symptoms of stress but not perceived stress, and this discordance was more pronounced among cancer caregivers.
Conclusions
Implementation of screening tools that include symptoms of stress may help clinicians to comprehensively identify parents of children with cancer who are in need of additional services. Targeted stress-reduction interventions that address sleep quality and negative social interactions may mitigate the deleterious effects of caregiving, improving the psychosocial well-being of both parents and children with cancer.
Keywords: childhood cancer, implications for cancer survivors, parental caregivers, sleep, stress
INTRODUCTION
Treatments and survival rates for childhood cancer have greatly improved in recent years,1 yet survivors of childhood cancer face long-term risks to their health and well-being.2 Because childhood health conditions are major life stressors that affect the health of all family members, it is imperative to understand not only the impact of childhood cancer on the well-being of the child, but also on that of the entire family.
Previous studies comparing parents of children with cancer to those without cancer have evaluated the downstream health effects of caregiving. Caring for a child with cancer has been associated with poor health-related quality of life (QOL),3–5 depression,6,7 and posttraumatic stress symptoms.8–15 Importantly, our previous work has shown that stress is one pathway through which caring for a child with cancer may influence poor parental QOL.5 Stress is a process by which exposure to a stressor may lead to the perception of stress and subsequent physiological responses.16 Measuring how stress is manifested physiologically and psychologically (ie, measuring “symptoms of stress”) therefore may more fully capture the extent to which a parent of a child with a serious illness is experiencing stress.
To our knowledge, little work has evaluated the factors associated with stress among parents of children with cancer. Evaluating stress, which occurs upstream from and may contribute to poor health outcomes, can improve our understanding of which caregivers may be at the greatest risk of declines in health and QOL. Further, to our knowledge, no studies have evaluated the differences in parental report of perceived stress as compared to symptoms of stress among parents of children with cancer. Understanding discrepancies in the reporting of perceived stress and symptoms of stress may aid clinicians in screening, monitoring, and treating parents, and prevent poor health outcomes among parents of children with serious health conditions. The aims of our study were therefore 2-fold. First, this study aimed to determine if parents of children with cancer or a brain tumor had greater parental stress compared to parents of healthy children, and to evaluate the correlates of stress among parents of children with cancer or brain tumors. Second, we aimed to examine the relationship between perceived stress and symptoms of stress, and how that relationship may differ for parents of children with cancer. Together, the findings from this study will help in designing effective screening tools and interventions that could prevent the downstream effects associated with the stress of caring for a child with serious health problems.
METHODS
Study design, population, and data sources
Detailed information about study design and data collection has been published elsewhere and is briefly described below.5 The parent who was most involved in providing support and care to the child (defined as biological, step, adoptive, or foster parent, grandparent, or legal guardian) was recruited into the study.
Participants
Case dyads
Case dyads consisted of parents and their children living with cancer or a brain tumor (hereafter “cases”). Eligible families were invited to participate by staff at a local pediatric hematology and oncology clinic if they had an inpatient or outpatient visit, or attended an advisory board meeting, support group, or survivor reunion. In addition, clinical staff mailed an invitation letter to eligible families who could not be contacted in person. Of the 162 case families invited to participate in the study, 26 did not respond to the invitation, 46 declined to participate, 2 were ineligible, and 8 could not be scheduled to participate before the end of the study period. Eighty families ultimately participated. Of those, 7 surveys were missing data and were removed from this analysis, resulting in a final sample of 73 cases.
Comparison dyads
Comparison dyads consisted of healthy children and their parents (hereafter “comparisons”). Comparisons were selected from 2 community-based research registries, and their families were mailed an informational letter. Families in which 1 or more children reported having cancer, a brain tumor, a chronic condition, or an activity limitation or special healthcare need were excluded from the study. Of the 768 comparison families invited to participate in the study, 344 responded to the invitation. Of these, 122 declined to participate, 50 were not eligible, and 31 could not be scheduled to participate within the study timeframe. Ultimately, 141 comparison families participated. Eight participants were removed from this analysis due to missing data, resulting in a final sample of 133 comparisons.
Procedures
This study was approved by the Health Sciences Institutional Review Board of the University of Wisconsin-Madison. Written informed consent was obtained from all participants, and case parents provided written Health Insurance Portability and Accountability Act Privacy Rule authorization for abstraction of the child’s medical record. All participating parents completed an in-person interviewer-assisted survey between September 2008 and July 2009.
Measures
Outcome measures
The caregivers’ perceptions of stress symptoms in the week before the initial interview were measured using the 56-item Calgary Symptoms of Stress Inventory (C-SOSI).17 The C-SOSI has 8 subscales (depression, anger, muscle tension, cardiopulmonary arousal, sympathetic arousal, neurological/gastrointestinal, cognitive disorganization, and upper respiratory symptoms) and explores both physiological and psychological manifestations of stress. Responses are recorded on a 5-point Likert scale ranging from 0 (never) to 4 (very frequently) and summed across subscales. Internal consistency for the subscales ranges from 0.80 to 0.92, with an overall Cronbach’s α of 0.95 for cancer patients.17 Higher scores indicate greater symptoms of stress. For the purpose of the multivariable logistic regression analyses, a trimmed mean of 21.0 was used as the cut point for the C-SOSI variable for the entire sample, with scores of 21 or higher classified as “high stress” and scores less than 21 classified as “low stress.” Various cut points for stress were evaluated as predictors of health-related QOL, as their association has previously been established,5 and the trimmed mean was selected for the best statistical fit and predictive value.
Perception of stress over the last month was measured using the 4-item version of the Perceived Stress Scale (PSS).18 Responses are recorded on a 5-point Likert scale ranging from 0 (never) to 4 (very often) and summed across the 4 items to provide a total score. An individual yielding a higher score is considered to perceive more stress. A trimmed mean of 4.0 was used as a cut point for the PSS variable, with scores of 4 or higher classified as “high perceived stress” and scores less than 4 classified as “low perceived stress,” chosen in the same fashion as for the C-SOSI variable.
Sociodemographics and health behaviors
Sociodemographic characteristics included the following: age, gender, marital/partner status (partner/married or no partner/unmarried), race (white or nonwhite), educational attainment (high school graduate or less, or GED; vocational college or some college; college degree; or professional or graduate degree), employment status (full-time work outside the home; part-time work outside of the home; or not working outside the home), relationship to the child (biological vs adoptive, foster, or step parent, or grandparent), number of people in the household, and family income. Parental health behaviors were also examined, including smoking (nonsmokers, current smokers, or former smokers); alcohol consumption (nondrinkers, moderate drinkers, or risky drinkers [8 or more drinks/week for women; 15 or more drinks/week for men]); and exercise (inactive, active-irregular, or active-regular).
In addition, parents were administered self-reported measures of diet, sleep quality,19 aging-related diseases, negative life events, and social support and social stress.20 Parent diet for the past month was collapsed into summary categories: meets national guidelines (at least 5 fruits or vegetables per day and less than 35% of calories from fat) or does not meet national guidelines. Poor parental sleep quality was defined as scoring greater than 5 on the Pittsburgh Sleep Quality Index for the month before.19 Body mass indexes and waist-to-hip ratios were also calculated from measurements of the parents’ height and weight, and waist and hip circumferences.
Parents reported their child’s age and gender. A trained and licensed clinician abstracted key diagnosis and treatment information from the child’s medical record, including: type of cancer (leukemia/lymphoma, central nervous system tumor, or non-central nervous system tumor), treatment status (active/maintenance treatment or off treatment), time since diagnosis (time between date of diagnosis and date of interview), types of treatment received (chemotherapy, radiation, surgery, and/or transplant), having 1 or more complications during treatment, and having a recurrence of the primary cancer. Parents also reported the severity of their child’s symptoms during cancer treatment on a 5-point Likert scale, ranging from 0 (no symptoms) to 4 (very severe symptoms).
Children’s activity limitation status was reported by case parents. Children were considered to have an activity limitation if they were “limited or prevented in their ability to do the things most children of the same age can do” because of a medical, behavioral or other health condition that had lasted or was expected to last for at least 12 months.
Parental report of children’s psychosocial problems was measured by the Columbia Impairment Scale, an instrument that includes 13 questions about a child’s emotions, engagement in everyday activities, and ability to get along with others.21
Analytic Approach
Chi-square analyses, t tests, and Kruskal-Wallis tests were used to examine differences in characteristics by case status. Descriptive statistics were also used to evaluate the frequency distribution of clinical characteristics among children with cancer.
Multivariable logistic regressions were conducted among cases and comparisons to evaluate the association between case-comparison status and symptoms of stress, controlling for other factors. Parsimonious models were constructed using logistic regression and a manual, forward, stepwise selection procedure. Specifically, single covariates that were statistically significant (P ≤ 0.05) or impacted the stress point estimate for case status (|change in estimate| ≥ 10%) were included in the final models. Age and gender were included in the models a priori. Multivariable logistic regression analyses were also conducted to evaluate the correlates of stress in case parents only. Parsimonious models were constructed as described above. Finally, 3-way cross-tabulations and correlation analyses were carried out to examine the relationship between perceived stress and symptoms of stress by case status. For the cross-tabulations, the PSS and C-SOSI were dichotomized at their cut points, as described above; for the correlation analysis, the PSS and C-SOSI were used as continuous variables.
In order to examine if the findings held among those who were no longer receiving active or maintenance treatment, all analyses were also conducted restricting the case sample to parents of children who were off treatment.
RESULTS
The final sample included 73 cases and 133 comparisons (Table 1). Several important differences were identified between case and comparison parents. Case parents were significantly younger, less educated, of lower income, more likely to be current smokers and have poor sleep quality, and less likely to engage in regular exercise. Case parents also reported less social support and more negative life events and were more likely to report high stress than comparison parents.
Table 1.
Characteristics of parents and their children with and without cancer or brain tumors
| Casea | Comparisona | ||
|---|---|---|---|
|
| |||
| % / Mean(SD) | % / Mean(SD) | P-value | |
| Number of participants (%) | 73 (35.4%) | 133 (64.6%) | |
|
| |||
| Parent characteristics | |||
| Sociodemographic factors | |||
| Age, mean (SD) | 41.03 (6.48) | 42.82 (5.92) | 0.05 |
| Gender, % | 0.4 | ||
| Male | 8.2% | 12.0% | |
| Female | 91.8% | 88.0% | |
| Marital/Partner status, % | 0.1 | ||
| Partner/married | 93.1% | 97.7% | |
| No partner/unmarried | 6.9% | 2.3% | |
| Race, % | 0.38 | ||
| White | 94.5% | 97.0% | |
| Non-white | 5.5% | 3.0% | |
| Highest education level achieved, % | 0.004 | ||
| Some high school or less/ High school graduate or GED | 13.7% | 4.5% | |
| Vocational college or some college | 34.3% | 19.5% | |
| College degree | 30.1% | 42.9% | |
| Professional or graduate degree | 21.9% | 33.1% | |
| Current employment status, % | 0.74 | ||
| Full-time work outside of the home | 45.2% | 46.6% | |
| Part-time work outside of the home | 35.6% | 38.4% | |
| Not working outside the home | 19.2% | 15.0% | |
| Relationship to child, % | 0.68 | ||
| Biological parent | 95.9% | 97.0% | |
| Adoptive/foster parent, stepparent, or grandparent | 4.1% | 3.0% | |
| Number of people in household, mean (SD) | 4.30 (1.15) | 4.26 (0.87) | 0.78 |
| Income, mean (SD) | 83,468 (44,866) | 122,406 (118,080) | 0.007 |
| Health behaviors | |||
| Smoking status, % | 0.002 | ||
| Non-smoker | 64.4% | 80.4% | |
| Current smoker | 17.8% | 3.8% | |
| Former smoker | 17.8% | 15.8% | |
| Alcohol consumption, % | 0.31 | ||
| Non-drinker | 11.0% | 5.3% | |
| Moderate drinker | 84.9% | 89.4% | |
| Risky drinker | 4.1% | 5.3% | |
| Exercise, % | 0.02 | ||
| Inactive | 4.1% | 1.5% | |
| Active-irregular | 49.3% | 32.3% | |
| Active-regular | 46.6% | 66.2% | |
| Diet, % | 0.35 | ||
| Meets national guidelines | 24.7% | 30.8% | |
| Does not meet national guidelines | 75.3% | 69.2% | |
| Body Mass Index | 28.31 (6.59) | 27.01 (5.57) | 0.14 |
| Waist to Hip Ratio | 0.85 (0.07) | 1.01 (1.17) | 0.24 |
| Sleep, % | <0.0001 | ||
| Poor sleep quality (in the past month)b | 53.4% | 22.6% | |
| Good sleep quality (in the past month)b | 46.6% | 77.4% | |
| Aging-related disease | 0.08 | ||
| Presence of aging related disease | 43.8% | 31.6% | |
| No presence of aging related disease | 56.2% | 68.4% | |
| Psychosocial factors | |||
| Negative life events, mean (SD)c | 10.23 (10.56) | 4.50 (5.21) | <0.0001 |
| Social support and stress, mean (SD)d | |||
| Total social support | 67.56 (18.84) | 73.58 (16.42) | 0.02 |
| Total social stress | 21.48 (12.48) | 21.66 (12.59) | 0.92 |
| Stress | |||
| High stress (C-SOSI ≥ 21) | 71.2% | 33.1% | <0.0001 |
| Low stress (C-SOSI < 21) | 28.8% | 66.9% | |
| Symptoms of stress, mean (SD)e | 37.29 (24.07) | 18.13 (13.97) | <0.0001 |
| Depression | 5.37 (5.39) | 1.38 (2.08) | <0.0001 |
| Anger | 6.85 (5.21) | 4.10 (3.61) | <0.0001 |
| Muscle tension | 8.23 (7.12) | 3.45 (4.42) | <0.0001 |
| Sympathetic arousal | 7.86 (6.36) | 4.53 (4.46) | <0.0001 |
| Upper respiratory | 3.44 (4.25) | 2.29 (3.44) | 0.04 |
| Cognitive disorganization | 2.64 (2.93) | 1.59 (2.13) | 0.005 |
| Cardiopulmonary Arousal | 1.53 (2.26) | 0.38 (1.03) | <0.0001 |
| Neurological/GI | 1.36 (2.10) | 0.41 (1.11) | <0.0001 |
| Child characteristics | |||
| Sociodemographic factors | |||
| Age, mean (SD) | 10.00 (4.75) | 9.41 (3.97) | 0.35 |
| Gender, % | 0.36 | ||
| Male | 54.8% | 48.1% | |
| Female | 45.2% | 51.9% | |
| Health characteristics | |||
| Diagnosis, % | |||
| Leukemia/lymphoma | 49.3% | ||
| CNS tumor | 34.3% | ||
| Non-CNS tumor | 16.4% | ||
| Treatment status, % | |||
| Active/maintenance | 39.7% | ||
| Off treatment | 60.3% | ||
| Time since diagnosis, % | |||
| <1 year | 23.3% | ||
| 1–2 years | 16.4% | ||
| 3–4 years | 26.0% | ||
| 5–9 years | 23.3% | ||
| 10 or more years | 11.0% | ||
| Type of treatment, %† | |||
| Chemotherapy | 89.0% | ||
| Radiation | 21.9% | ||
| Surgery | 49.3% | ||
| Transplant | 8.2% | ||
| Had 1 or more treatment complications | 75.3% | ||
| Had a recurrence of cancer | 12.3% | ||
| Symptom severityf | 2.58 (0.97) | ||
| Activity limitationg | 56.2% | ||
| Psychosocial problemsh | 17.8% | 3.8% | 0.0006 |
Note: “Case” refers to parents of children with cancer or a brain tumor.
“Comparison” refers to parents of children without cancer or a brain tumor.
Poor sleep quality was defined as having a total score greater than 5, measured by the Pittsburgh Sleep Quality Index
Higher scores (Life Events Questionnaire) indicate more negative life events
Higher scores (Duke Social Support and Stress Scale) indicate more support or stress
Higher scores (Calgary Symptoms of Stress Inventory score for the past week) indicate greater symptoms of stress
Higher scores indicate more severe symptoms
Child was considered to have an activity limitation if the parent indicated that the child was “limited or prevented in their ability to do the things most children of the same age can do.”
Child was considered to have psychosocial problems if they had a total score greater than 15 on the Columbia Impairment Scale
SD=standard deviation; CNS=Central Nervous System;
Note: categories are not mutually exclusive
Children with cancer or a brain tumor were 10 years old, on average, and slightly more than half were boys. Nearly half of the children’s diagnoses were leukemia or lymphoma, and almost two-thirds of the children had been diagnosed in the last 5 years. Approximately 40% were receiving active or maintenance treatment. Most children (almost 90%) had received chemotherapy, while about 50% had received surgery, nearly 22% had received radiotherapy, and less than 9% had received a transplant. Many children received more than one type of treatment. Three-quarters of the children had experienced 1 or more treatment complications, and 12% experienced a recurrence of their cancer. The parent-reported severity of the child’s symptoms during treatment averaged 2.6, or moderate to severe symptoms. Finally, more than half of children had an activity limitation, while 17.8% of children reported psychosocial problems.
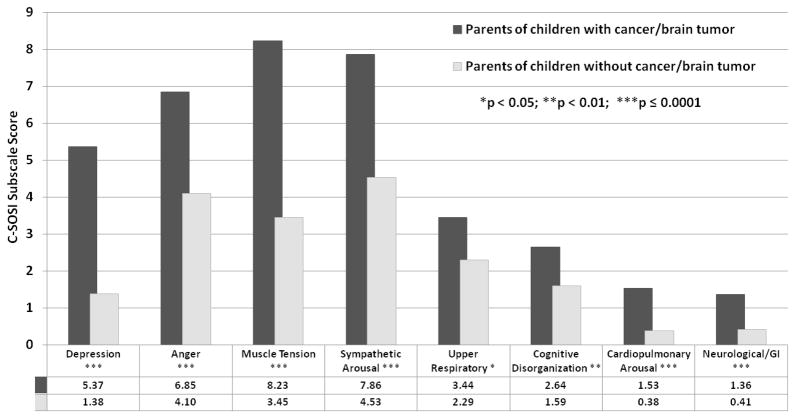
Figure 1 shows the unadjusted symptoms of stress among cases and comparisons. Overall, case parents had significantly higher levels of stress than comparisons (P < 0.0001), and significantly higher scores for all C-SOSI subscales. Cases reported the greatest absolute difference from comparisons on the C-SOSI scale in the categories of muscle tension (8.23 vs 3.45), depression (5.37 vs 1.38), sympathetic arousal (7.86 vs 4.53), and anger (6.85 vs 4.10). They also reported more cardiopulmonary arousal (1.53 vs 0.38), upper respiratory symptoms (3.44 vs 2.29), cognitive disorganization (2.64 vs 1.59), and neurological/gastrointestinal symptoms (1.36 vs 0.41).
Figure 1. Mean Calgary Symptoms of Stress Inventory (C-SOSI) subscale scores, by case status.
Unadjusted mean C-SOSI subscale scores among parents of children with cancer (dark gray bars) and parents of healthy children (light gray bars) are provided. Group mean values are presented below the chart.
As seen in Table 2, multivariable analysis showed that case parents were significantly more likely to report high symptoms of stress, controlling for confounders. Parents who were white, met national dietary guidelines, reported poor sleep quality, had more social stress (negative social interactions), or had more negative life events were more likely to have high stress than their counterparts, while those with more social support were less likely to have high levels of stress. Among cases only, parents with poor sleep quality and greater social stress had an increased odds of elevated stress (Table 2). Total social support and negative life events were of borderline statistical significance. Other parental sociodemographic factors and child clinical and diagnostic characteristics were tested in the model, but they did not affect these results and therefore were not retained in the final model.
Table 2.
Multivariable models of physiological and psychological symptoms of stressa, by characteristics of parents of children with and without cancer or brain tumors
| All Parents (n=206) | Parents of Children with Cancer (n=73) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Unadjusted
|
Full model
|
Unadjusted
|
Full model
|
|||||
| OR | 95% Confidence Limits | OR | 95% Confidence Limits | OR | 95% Confidence Limits | OR | 95% Confidence Limits | |
| Case/comparison status (reference= case)b | 5.01 | [2.69, 9.33] | 4.83 | [2.07, 11.26] | ||||
| Parent characteristics | ||||||||
| Sociodemographic factors and health behaviors | ||||||||
| Age | 0.98 | [0.94, 1.03] | 0.99 | [0.93, 1.06] | 0.96 | [0.89, 1.05] | 1.00 | [0.91, 1.11] |
| Gender (reference = Male) | 0.86 | [0.35, 2.08] | 0.60 | [0.17, 2.06] | 1.26 | [0.21, 7.48] | 1.01 | [0.14, 7.40] |
| Race (reference = non-white) | 2.71 | [0.53, 13.76] | 9.15 | [1.43, 58.65] | -- | -- | -- | -- |
| Meets national diet guidelines | 1.54 | [0.84, 2.82] | 2.97 | [1.26, 6.70] | -- | -- | -- | -- |
| Poor sleep qualityc | 8.14 | [4.14, 16.03] | 7.01 | [3.00,16.39] | 4.34 | [1.44, 13.07] | 4.23 | [1.15, 15.60] |
| Measures of family functioning and social support | ||||||||
| Social support and stressd | ||||||||
| Total social supporte | 0.96 | [0.94, 0.98] | 0.97 | [0.94, 0.99] | 0.97 | [0.94, 0.99] | 1.00 | [0.96, 1.04] |
| Total social stress | 1.05 | [1.02, 1.07] | 1.05 | [1.02, 1.09] | 1.08 | [1.02, 1.14] | 1.07 | [1.00, 1.14] |
| Negative life eventsf | 1.14 | [1.08, 1.21] | 1.08 | [1.01, 1.16] | 1.08 | [1.01, 1.17] | 1.05 | [0.96, 1.15] |
Calgary Symptoms of Stress Inventory score greater than 21
Note: “Case” refers to parents of children with cancer or a brain tumor; “Comparison” refers to parents of children without cancer or a brain tumor.
Poor sleep quality was defined as having a total score greater than 5, measured by the Pittsburgh Sleep Quality Index
Higher scores (Duke Social Support and Stress Scale) indicate more support or stress
Centered at the minimum (9.1)
Higher scores (Life Events Questionnaire) indicate more negative life event
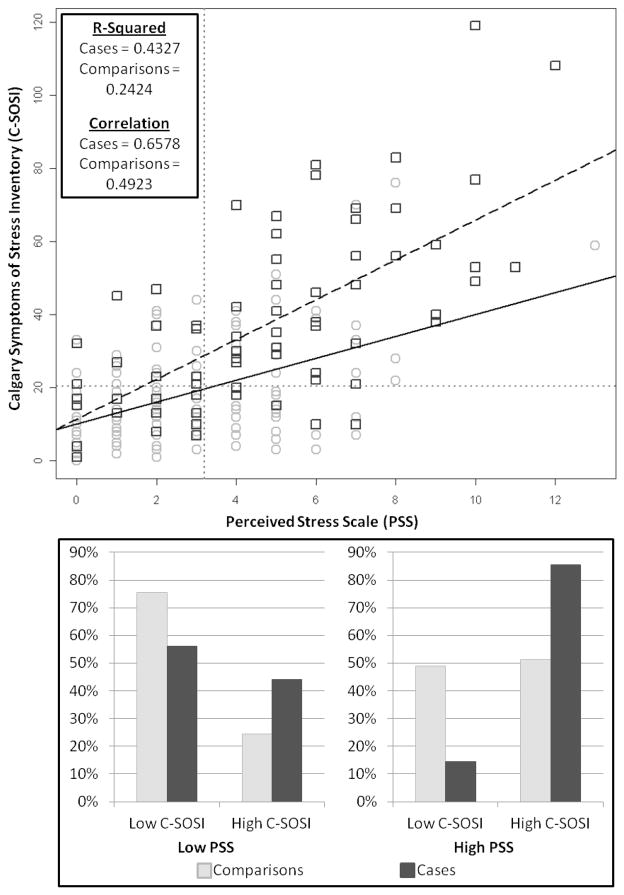
Figure 2 includes a scatter plot and bar charts of responses from PSS and C-SOSI, by case status. The PSS and C-SOSI were more highly correlated in cases than comparisons (Pearson’s correlation 0.66 vs 0.49, respectively). As seen in the bar charts, most participants reported concordant levels of perceived stress and symptoms of stress (ie, among those who reported high perceived stress, most cases and comparisons also reported high symptoms of stress, and among those who reported low perceived stress, most cases and comparisons also reported low symptoms of stress). However, 44.0% of cases reporting low perceived stress also reported discordantly high symptoms of stress, compared to only 24.4% of comparisons.
Figure 2. Perceived stress scale (PSS) scores versus Calgary Symptoms of Stress Inventory (C-SOSI) scores by case status.
Scatter plot shows the continuous PSS (x-axis) and C-SOSI (y-axis) scores for parents of children with cancer (dark gray squares) and parents of healthy children (light gray circles). The dashed black line indicates the linear relationship between these 2 scores for case parents, while the solid black line represents the linear relationship between these 2 scores for comparison parents. Gray dotted lines indicate the cut points for the PSS and C-SOSI. The bar chart depicts the proportion of case (dark gray bars) and comparison (light gray bars) parents who report low versus high C-SOSI scores among those who report low PSS scores (left chart) and high PSS scores (right chart).
When the case sample was restricted to parents of children who were not currently receiving treatment, these findings remained but were somewhat attenuated. Parents of children who were not receiving treatment had greater stress than parents of healthy children (odds ratio 4.13, 95% confidence interval 1.61–10.59). Among cases, only the estimate for poor sleep quality remained elevated (odds ratio 2.74, 95% confidence interval 0.64–11.73; data not shown), although this finding was not statistically significant, likely as a result of the small sample size. The PSS and C-SOSI were also slightly more highly correlated among those whose children were not receiving treatment (Pearson’s correlation 0.52) than among comparisons, and discordance was found among 35.3% of these parents (data not shown).
DISCUSSION
To our knowledge, this is the first study to show that parents of children with cancer or brain tumors are significantly more likely to report high symptoms of stress than parents of healthy children. Poor sleep quality and negative social interactions were significantly associated with increased levels of stress in both parents of children with cancer and healthy children. Notably, some parents who reported low perceived stress also reported experiencing high symptoms of stress, underscoring the importance of measuring both perceived and physiological symptoms of stress.
Poor parental sleep quality and social stress (ie, negative social interactions) were identified as strong correlates of high levels of stress for parents of children with and without cancer. Although this study could not evaluate the directionality of these associations, which may be even cyclic, these factors may prove to be important for reducing levels of stress among parental caregivers. Parents of children with cancer commonly report sleep disturbances 22 and are more likely to experience sleep problems compared with their noncaregiving counterparts.23 Interventions focused on sleep behavior are feasible and may improve sleep quality in caregivers; 24–26 such interventions should be tested among parents of children with cancer or chronic conditions. Negative social interactions may also contribute to stress and lead to worse parental health and greater anxiety and depression.27 Preventing or reducing such interactions may also be an important avenue for improving symptoms of stress in parents of children with cancer.
Importantly, this study identified discrepancies in how parents report their stress. We observed an important subset of parents who reported high symptoms of stress but discordantly low levels of perceived stress. This discrepancy was more pronounced among parents of children with cancer compared with parents of healthy children. Parents of children with cancer may be underreporting their levels of perceived stress. This phenomenon may be a result of parents engaging in the caregiver role (ie, being strong for the family) or because of adaptation to stress. Nevertheless, measurement of symptoms of stress reveals that many of these parents are physiologically affected by stress.
This study has important implications for both clinicians and parents of children with cancer. First, parents and clinicians should monitor and attempt to reduce levels of stress among parents. Parents who are stressed tend to display more negative interactions with their children.28 Further, stress may influence parents’ decision making,29,30 which may affect several domains of the child’s life, including the medical care they receive. Reducing parental stress, therefore, stands to improve the care, health outcomes, and QOL of both parents and their children. Improving sleep quality and preventing or reducing negative social interactions may contribute to reduced levels of stress, leading to better health outcomes for parents and children.
This study suggests that caregivers may more readily report symptoms of stress (eg, heart palpitations, headaches, or nausea) than perceived stress. Screening of parental stress using only perceptions could result in the misclassification of an important subset of parents. In this sample, measuring perceived stress alone would have misclassified almost 20% of those with high stress as having low stress. By examining both physiological and psychological manifestations of stress, clinicians and researchers will be better able to identify caregivers who may be at risk for stress-induced health problems and in need of additional services. These findings also suggest new avenues for interventions that may decrease parental stress and improve parental health outcomes, such as massage or biofeedback training; additional research is needed to determine the feasibility and effectiveness of such interventions.
Our findings were largely unchanged when the case sample was restricted to parents of children not receiving treatment. This indicates that families may remain at risk for poor outcomes and require screening for elevated parental stress even after the child completes treatment. Although social stress does not seem to be a correlate of symptoms of stress in this subgroup, sleep may still be an important avenue for preventing or improving parental stress outcomes. Further, it appears that the discrepancies between perceived stress and symptoms of stress are reduced, but still evident, among this subgroup. Clinicians should therefore continue to monitor both perceived stress and symptoms of stress in these parents, even after their child has completed treatment.
This study has several potential limitations. First, because the data used in this study were cross-sectional, the directionality of the associations could not be determined. In addition, the measures used in this study covered differing time frames. Future studies should incorporate longitudinal data and include larger sample sizes to better understand the direction and implications of these relationships. Second, our participation rate was somewhat low. However, it is likely that those who did not participate were experiencing the greatest stress, likely leading to conservative estimates of the correlates of stress. Our results should nevertheless be interpreted with caution. Third, this study recruited the parent who was the most involved in providing support and care to the child, frequently the child’s mother; our sample therefore primarily consisted of women. Fourth, there was significant variety in the cancer population demographics in our sample, which may lead to heterogeneity in our results. Furthermore, our sample was drawn from a large Midwestern area, and therefore these findings may not be generalizable to other populations of parents of children with cancer. The metropolitan area from which the comparison sample was drawn tends to be wealthier and better educated than the rest of the state. Differences between cases and comparisons were controlled for in the statistical analyses; nevertheless, residual confounding by such differences may have influenced our results. As such, additional studies should seek to determine if these findings are consistent across other populations.
The present study has important methodological and substantive strengths. This is the first study to examine the correlates of stress among parents of children with cancer. In addition, a comprehensive definition of stress that assessed both physiological and psychological manifestations of symptoms of stress was used for the outcome in this study. Finally, a community comparison group was recruited and known confounders were controlled for in the analyses.
CONCLUSION
This study shows that parents of children with cancer or a brain tumor experience greater stress than parents of healthy children. Further, we were the first to identify sleep quality and negative social interactions as key correlates of stress in parents of children with cancer or a brain tumor. This study also identified a subset of parents who reported symptoms of stress but not perceived stress, and this discordance was more pronounced among case parents. This information is critical for effectively intervening with families to reduce stress for parents of children with cancer, improving the overall health of parents and families of children with cancer.
What’s New.
Poor sleep quality and greater negative social interactions were significant correlates of increased symptoms of stress in parents of children with cancer. A subset of parents reported high symptoms of stress but low perceived stress, indicating discordance in reporting.
Acknowledgments
We would like to thank the families who participated in this study, as well as the clinical and research staff who were instrumental to study recruitment. The follow-up portion of this study was administered by Survey Research Shared Services of the University of Wisconsin Carbone Cancer Center (UWCCC). This research was supported by a grant from the National Institute of Child Health and Human Development (HD049533, W. P. Witt, Principal Investigator and Waisman Center P30 HD03352, M. M. Seltzer, Principal Investigator), UWCCC Investigator Initiated Trial, the UW Care for Kids Foundation, the UW Graduate School, and the National Science Foundation (DBI-1063085). We would also like to thank the anonymous reviewers for their helpful comments and suggestions.
Footnotes
Disclosure: None of the authors have any conflicts of interest to disclose.
References
- 1.American Cancer Society. Cancer facts & figures 2012. Atlanta: American Cancer Society; 2012. [Google Scholar]
- 2.Robison LL, Green DM, Hudson M, et al. Long-term outcomes of adult survivors of childhood cancer. Cancer. 2005;104(S11):2557–2564. doi: 10.1002/cncr.21249. [DOI] [PubMed] [Google Scholar]
- 3.Klassen AF, Klaassen R, Dix D, et al. Impact of caring for a child with cancer on parents’ health-related quality of life. Journal of Clinical Oncology. 2008;26(36):5884–5889. doi: 10.1200/JCO.2007.15.2835. [DOI] [PubMed] [Google Scholar]
- 4.Litzelman K, Catrine K, Gangnon R, Witt WP. Quality of life among parents of children with cancer or brain tumors: the impact of child characteristics and parental psychosocial factors. Quality of Life Research. 2011;20(8):1261–1269. doi: 10.1007/s11136-011-9854-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Witt WP, Litzelman K, Wisk LE, et al. Stress-mediated quality of life outcomes in parents of childhood cancer and brain tumor survivors: a case-control study. Quality of Life Research. 2010;19(7):995–1005. doi: 10.1007/s11136-010-9666-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merrill RM, Brown RJ, Alder S, et al. Psychological disorders among children and the parents of children undergoing cancer workup. Journal of Psychosocial Oncology. 2007;25(3):1–18. doi: 10.1300/J077v25n03_01. [DOI] [PubMed] [Google Scholar]
- 7.Fotiadou M, Barlow JH, Powell LA, Langton H. Optimism and psychological well-being among parents of children with cancer: an exploratory study. Psycho-Oncology. 2008;17(4):401–409. doi: 10.1002/pon.1257. [DOI] [PubMed] [Google Scholar]
- 8.Lindahl Norberg A, Pöder U, von Essen L. Early avoidance of disease-and treatment-related distress predicts post-traumatic stress in parents of children with cancer. European Journal of Oncology Nursing. 2011;15(1):80–84. doi: 10.1016/j.ejon.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Norberg AL, Boman KK. Mothers and fathers of children with cancer: loss of control during treatment and posttraumatic stress at later follow-up. Psycho-Oncology. 2011 Oct 21; doi: 10.1002/pon.2091. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Dunn MJ, Rodriguez EM, Barnwell AS, et al. Posttraumatic stress symptoms in parents of children with cancer within six months of diagnosis. Health Psychology. 2012;31(2):176–185. doi: 10.1037/a0025545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jurbergs N, Long A, Ticona L, Phipps S. Symptoms of posttraumatic stress in parents of children with cancer: Are they elevated relative to parents of healthy children? Journal of Pediatric Psychology. 2009;34(1):4–13. doi: 10.1093/jpepsy/jsm119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pöder U, Ljungman G, von Essen L. Posttraumatic stress disorder among parents of children on cancer treatment: a longitudinal study. Psycho-Oncology. 2008;17(5):430–437. doi: 10.1002/pon.1263. [DOI] [PubMed] [Google Scholar]
- 13.Davis GL, Parra GR, Phipps S. Parental Posttraumatic Stress Symptoms Due to Childhood Cancer and Child Outcomes: Investigation of the Role of Child Anger Regulation. Children’s Health Care. 2010;39(3):173–184. [Google Scholar]
- 14.Phipps S, Larson S, Long A, Rai SN. Adaptive style and symptoms of posttraumatic stress in children with cancer and their parents. Journal of Pediatric Psychology. 2006;31(3):298–309. doi: 10.1093/jpepsy/jsj033. [DOI] [PubMed] [Google Scholar]
- 15.Fuemmeler BF, Mullins LL, Van Pelt J, Carpentier MY, Parkhurst J. Posttraumatic stress symptoms and distress among parents of children with cancer. Children’s Health Care. 2005;34(4):289–303. [Google Scholar]
- 16.Cohen S, Kessler RC, Gordon LU. Strategies for measuring stress in studies of psychiatric and physical disorders. In: Cohen S, Kessler RC, Gordon LU, editors. Measuring stress: a guide for health and social scientists. Vol. 3 Oxford: Oxford University Press; 1995. [Google Scholar]
- 17.Carlson LE, Thomas BC. Development of the Calgary Symptoms of Stress Inventory (C-SOSI) International journal of behavioral medicine. 2007;14(4):249–256. doi: 10.1007/BF03003000. [DOI] [PubMed] [Google Scholar]
- 18.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of health and social behavior. 1983 Dec;24(4):385–396. [PubMed] [Google Scholar]
- 19.Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. Journal of psychosomatic research. 1998 Jul;45(1):5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- 20.Parkerson GRJ, Broadhead WE, Tse CK. Validation of the Duke Social Support and Stress Scale. Family Medicine. 1991;23(5):357–360. [PubMed] [Google Scholar]
- 21.Bird HR, Shaffer D, Fisher P, et al. The Columbia-Impairment-Scale (CIS) – Pilot Findings on a Measure of Global Impairment for Children and Adolescents. Int J Method Psych. 1993 Oct;3(3):167–176. [Google Scholar]
- 22.Zupanec S, Jones H, Stremler R. Sleep habits and fatigue of children receiving maintenance chemotherapy for ALL and their parents. Journal of Pediatric Oncology Nursing. 2010;27(4):217–228. doi: 10.1177/1043454209358890. [DOI] [PubMed] [Google Scholar]
- 23.Wright M. Children receiving treatment for cancer and their caregivers: A mixed methods study of their sleep characteristics. Pediatric Blood & Cancer. 2011;56(4):638–645. doi: 10.1002/pbc.22732. [DOI] [PubMed] [Google Scholar]
- 24.Simpson C, Carter PA. Pilot study of a brief behavioral sleep intervention for caregivers of individuals with dementia. Research in gerontological nursing. 2010 Jan;3(1):19–29. doi: 10.3928/19404921-20090731-02. [DOI] [PubMed] [Google Scholar]
- 25.Carter PA. A brief behavioral sleep intervention for family caregivers of persons with cancer. Cancer nursing. 2006 Mar-Apr;29(2):95–103. doi: 10.1097/00002820-200603000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Carter PA, Mikan SQ, Simpson C. A feasibility study of a two-session home-based cognitive behavioral therapy-insomnia intervention for bereaved family caregivers. Palliative and Supportive Care. 2009;7(02):197–206. doi: 10.1017/S147895150900025X. [DOI] [PubMed] [Google Scholar]
- 27.Rauktis ME, Koeske GF, Tereshko O. Negative social interactions, distress, and depression among those caring for a seriously and persistently mentally III relative. American Journal of Community Psychology. 1995;23(2):279–299. doi: 10.1007/BF02506939. [DOI] [PubMed] [Google Scholar]
- 28.Webster-Stratton C. Stress: A potential disruptor of parent perceptions and family interactions. Journal of Clinical Child Psychology. 1990;19(4):302–312. [Google Scholar]
- 29.Keinan G. Decision making under stress: scanning of alternatives under controllable and uncontrollable threats. Journal of personality and social psychology. 1987 Mar;52(3):639–644. doi: 10.1037//0022-3514.52.3.639. [DOI] [PubMed] [Google Scholar]
- 30.Starcke K, Brand M. Decision making under stress: A selective review. Neuroscience and Biobehavioral Reviews. 2012;36(4):1228–1248. doi: 10.1016/j.neubiorev.2012.02.003. [DOI] [PubMed] [Google Scholar]