Abstract
Recent advances in the understanding of the molecular pathophysiology of facioscapulohumeral muscular dystrophy (FSHD) have identified potential therapeutic targets. Consequently, an accurate understanding of disease progression in FSHD is crucial for the design of future clinical trials. Data from 228 subjects in 3 clinical trials and 1 natural history study were compared to examine disease progression in FSHD. All studies utilized the same techniques for manual muscle testing and maximum voluntary isometric contraction testing. Both techniques yield a total strength score that can be followed over time as an indicator of disease progression. Whereas natural history data showed a decrease in strength over 1 year, there was an apparent increase in strength at 6 months in 2 of the 3 clinical trials in both the placebo and treatment groups, that persisted for up to 1 year for maximum voluntary isometric contraction testing. Variability estimates from the clinical trial data were consistent with those seen in the natural history data. Patients in clinical trials in FSHD may have better outcomes than those in natural history studies, regardless of treatment assignment, emphasizing the importance of placebo groups and the need for caution when interpreting the strength results of controlled and uncontrolled trials.
Keywords: 1. All neuromuscular disease, 2. Muscle disease, 3. Outcome research, 4. All clinical trials, 5. Clinical trials methodology/study design
Introduction
Facioscapulohumeral muscular dystrophy (FSHD) is one of the most common forms of muscular dystrophy along with Duchenne muscular dystrophy and myotonic muscular dystrophy type 1, with an estimated population prevalence of between 1:15,000 and 1:20,000[1-3]. This dominantly inherited disease is slowly progressive and causes significant lifetime morbidity, with up to 20% of patients eventually requiring wheelchair use. The clinical spectrum of disease severity is wide; however, the regional distribution of muscle weakness and the pattern of progression are rather distinctive. Clinically, muscle weakness typically starts in the face and shoulders with a descending progression to involve the distal lower extremity muscles before affecting the hip girdle muscles.
FSHD is caused by loss of a critical number of subtelomeric D4Z4 macrosatellite repeat units[4]. Whereas normal individuals have 11-100 repeats, individuals with FSHD have 1-10 repeats. Each D4Z4 repeat contains a copy of DUX4, a double homeodomain retrogene, making DUX4 a logical candidate FSHD gene[5-7]. Recent studies have provided evidence implicating aberrant reactivation of DUX4 as the cause of FSHD[8-10]. With high sensitivity RT-PCR assays, full length DUX4 mRNA can be demonstrated in FSHD muscle but not at comparable levels in controls. DUX4 expression is believed to interfere with myogenic differentiation, lead to apoptotic cell death and make cells more susceptible to oxidative stress[11-15]. This FSHD model provides a rational therapeutic target for FSHD. As therapeutic modalities are being explored, there is an urgent need to have in place standard clinical trial research tools to conduct efficient, high quality clinical trials.
Muscle strength, as measured by maximal voluntary isometric contraction testing (MVICT) and manual muscle testing (MMT), are outcome measures commonly used in clinical trials in neuromuscular disease including several in FSHD[16-19]. In a prospective, longitudinal natural history study of 81 FSHD patients, MVICT and MMT were used to generate global strength scores at baseline and every 6 months thereafter for up to 3 years[20]. A significant decrease in overall strength was detected at 12 months. Functional measures included in the natural history study (times to walk 30 feet, climb 4 stairs, and drink 6 oz of water through a straw) did not significantly change over time. The variability reported for MVICT and MMT was used for power and sample size calculations to plan subsequent clinical trials[21, 22]. Both MVICT and MMT were used to derive efficacy outcomes in the randomized controlled trials (RCTs) of albuterol and MYO-029 in FSHD[21, 23]. Both studies failed to show a difference between placebo and active treatment in these strength outcomes. A Dutch RCT evaluated strength training and albuterol, alone or in combination, versus placebo using a 2 × 2 factorial design with MVICT-derived strength in individual muscles as the primary outcome variables[22]. No improvements in isometric strength associated with strength training at 12 months were reported, but significant improvements were detected in several isolated muscles with albuterol.
FSHD is a rare disease and there are a limited number of clinical studies that have been conducted. A better understanding of the expected change over time on strength measurements during clinical trials, along with the variability of change, are essential for planning future clinical trials. We examined data from the natural history study and the three RCTs referenced above, all of which utilized similar techniques for strength testing, to address this issue.
Methods
We compared data from 3 RCTs and 1 natural history study with a total of 277 participants, 228 of whom had post-baseline evaluations[20-23]. All clinical trials utilized the same techniques for strength testing described in the natural history study[18]. The primary variables for this analysis were the composite strength scores derived from MVICT and MMT. For the albuterol and MYO-029 studies, treatment groups and placebo groups were compared to the natural history cohort in terms of mean changes over time. The Dutch exercise/albuterol study was a two-phase design: strength training versus no strength training for 6 months in the first phase followed by randomization within each of these groups to albuterol or placebo (resulting in four groups: strength training plus placebo, no training plus placebo, strength training plus albuterol, no training plus albuterol) in the second phase (months 6 to 12).
Participants
For the natural history study and the albuterol RCT, patients met strict clinical diagnostic criteria for FSHD[24]. For the MYO-029 and the Dutch exercise/albuterol studies, patients met both clinical and molecular diagnostic criteria[25, 26]. All patients in the natural history study were ambulatory. For clinical trials, patients had to be at least 18 years old and able to walk independently. For the albuterol RCT, subjects had to have at least 8 testable muscles (MVICT > 20 newtons). For the MYO-029 study, subjects had to have strength on MMT ≥ 3- and ≤ 4+ in at least 8 muscle groups. For the Dutch exercise/albuterol study, subjects had to have at least 2 trainable muscles (MMT ≥ 3 for elbow flexion and ankle dorsiflexion > 0 degrees from neutral). In addition, for all studies, patients with contraindications to the study medications and pregnant women were excluded.
Measurements
MVICT
All studies used the Quantitative Muscle Assessment fixed myometry testing system (QMA, Gainesville, GA), with a force transducer attached by an inelastic strap to a metal frame. As previously published, standardized positions for isolated isometric force testing were used on 10 muscle groups: shoulder abductors, elbow flexors and extensors, and knee flexors and extensors on both sides[18]. Raw MVICT scores were standardized using regression equations based on normative data to yield the number of standard deviations from predicted normal strength, after accounting for age, gender, and height[24]. A composite MVICT score was created by averaging these standardized scores across the 10 muscles tested.
MMT
In addition to muscles tested by MVICT, the following muscles were also included: bilateral shoulder horizontal adductors, external rotators, hip flexors, and ankle dorsiflexors. All studies used a 13 point modified Medical Research Council scale, and individual muscle scores were averaged to create a composite MMT score[18], except for The Dutch exercise/albuterol study which did not collect MMT data.
Strength training in the Dutch exercise/albuterol study consisted of a progressive overload program which included dynamic and isometric exercises focusing on elbow flexors and ankle dorsiflexors. Patients trained at home for three days per week and were visited by a physical therapist every third week to optimize the exercises and to adjust the training weights. The follow up periods varied between studies from 6 months to 3 years. Fifteen subjects enrolled in the natural history study were subsequently enrolled in the albuterol RCT, so longitudinal data for these subjects were available for 2-7 years of follow-up. All subjects completed their involvement in the natural history study prior to enrollment in the albuterol RCT.
Statistical Considerations
Analysis was performed using SAS version 9.3. Pooled estimates of variance were obtained using a one-way analysis of variance model with treatment assignment as the group factor. Mean changes over time were compared among the following groups: natural history, albuterol treatment, albuterol placebo, MYO-029 treatment, MYO-029 placebo, and the four groups from the Dutch exercise/albuterol study (training plus placebo, no training plus placebo, training plus albuterol, no training plus albuterol). No imputation was performed for missing data. A one-way analysis of variance model was used to test for differences in mean baseline strength among the studies, with pair-wise comparisons of means performed utilizing a Bonferroni correction. A two-sample Student's t-test was used to test the hypothesis that there were no differences in mean change at 6 and 12 months between the natural history cohort and the placebo-treated subjects pooled from the albuterol and MYO-029 RCTs. A linear mixed effects model with change in strength as the dependent variable, years of follow-up as the independent variable (with random intercept and slope), and center as a fixed effect was used to obtain an estimate of the average slope as an indicator of disease progression in the 15 subjects who participated in both the natural history study and the baseline visit of the albuterol RCT 2-5 years later.
Results
For all studies combined there were 277 participants enrolled at baseline (81 in the natural history study, 90 in the albuterol RCT, 42 in the MYO-029 RCT, and 64 in the Dutch exercise/albuterol study). The gender distribution ranged from 43.2% to 72.7% male and the mean age ranged from 35.4 to 43.1 years across studies (Table 1).
Table 1.
Baseline characteristics of study participants
| Natural History Study[20] | Albuterol RCT[21] | MYO-029 RCT[23] | Dutch Exercise/Albuterol Study[22] | |
|---|---|---|---|---|
| Male Gender (%) | 43.2 | 54.4 | 72.2 | 62.5 |
| Age (years) | 39.0 (13.4) | 35.4 (10.4) | 43.1 (10.6) | 37.2 (10.3) |
| Composite MVICT Score | -5.50 (4.2) | -6.57 (3.7) | -5.67 (3.0) | -4.87 (3.6) |
| Composite MMT Score | 3.82 (0.8) | 3.81 (0.5) | 3.52 (0.5) | - |
Values are mean (standard deviation) unless otherwise indicated.
The average composite MMT score at baseline ranged from 3.5 to 3.8 across studies. Subjects in the MYO-029 study were slightly weaker than those in the albuterol RCT (3.5 versus 3.8, P=0.002). The average composite MVICT score ranged from -4.9 to -6.6 standard deviations (SDs) below predicted normal strength across studies. On average, subjects entering the albuterol RCT were weaker (-6.6 SD) than subjects entering the Dutch exercise/albuterol study (-4.9 SD, P=0.005).
Follow up data were available for a total of 228 participants. Six-month follow-up data were available for 48 participants (59%) in the natural history study, 85 participants (94%) in the albuterol RCT (29 placebo [97%]), and 31 participants (74%) in the MYO-029 RCT (8 placebo [73%]). Twelve-month follow-up data were available for 50 participants (62%) in the natural history study and 84 participants (93%) in the albuterol RCT (29 placebo [97%]). The Dutch exercise/albuterol study had follow-up data from 64 participants (100%) at both the 6-month and 12-month visits.
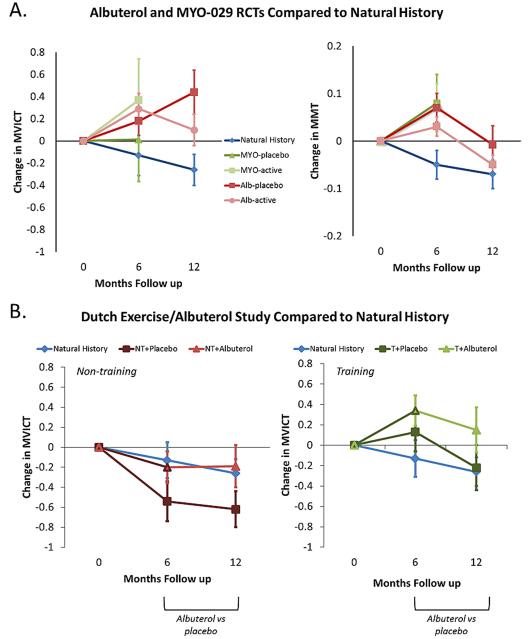
For both placebo and treatment groups, and therefore regardless of treatment assignment, there was a mean increase in strength over 6 months in the albuterol and MYO-029 RCTs as measured by either the composite MVICT score or the MMT score, compared to the average loss of strength observed in the natural history study (Figure 1A). From the MVICT score, at 12 months the mean increase in strength persisted in the albuterol RCT, particularly in the placebo group. Placebo-treated participants (combined among the albuterol and MYO-029 RCTs at 6 months) had a higher mean change in composite MVICT score compared to the natural history cohort that was statistically significant at 12 months (group difference 0.70, 95% confidence interval [CI] 0.22 to 1.18, P= 0.005). The group difference in mean change was not statistically significant at 6 months (group difference 0.27, 95% CI -0.21 to 0.75, P= 0.27).
Figure 1.
A. Comparison of mean changes in composite strength scores among groups from the natural history study and the albuterol and MYO-029 RCTs. Results (mean ± standard error) are presented for composite MVICT score (left) and composite MMT score (right). B. Comparison of mean changes in composite MVICT scores among groups from the natural history study and the Dutch exercise/albuterol study. Results (mean ± standard error) are presented for subjects assigned to non-training (left) and training (right) compared to subjects in the natural history study.
Alb = albuterol; MYO = MYO-029; NT = non-training; T = training
For MMT measurement, strength decreased in both treatment and placebo groups, and hence regardless of treatment assignment, in the albuterol RCT over months 6 to 12. Placebo-treated subjects (combined among the albuterol and MYO-029 RCTs at 6 months) had a significantly higher mean change in composite MMT score compared to the natural history cohort at 6 months (group difference 0.12, 95% CI 0.04 to 0.19, P= 0.002). This difference attenuated by month 12 (group difference 0.06, 95% CI -0.04 to 0.16, P= 0.25).
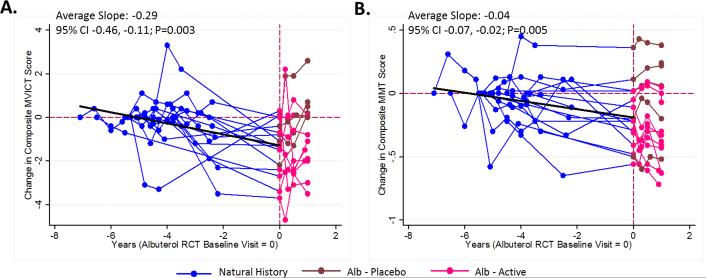
The Dutch exercise/albuterol study had a two-phase design: strength training versus no strength training for 6 months in the first phase followed by randomization within each of these groups to albuterol or placebo for the second phase. For the training group there was a mean increase in the composite MVICT score at 6 months that attenuated over months 6-12, though the attenuation was somewhat less for subjects treated with albuterol (Figure 1B). The progression in the non-training group closely paralleled that seen in the natural history study. Data were available for 15 subjects who were enrolled in the natural history study prior to entering the albuterol RCT. There was a gradual loss of strength over time (MVICT slope = -0.29, 95% CI -0.46 to -0.11, P= 0.003; MMT slope = -0.04, 95% CI -0.07 to -0.02, P= 0.005) over the 2-7 year follow-up period (Figure 2).
Figure 2.
Progression of disease as measured by composite MVICT score (A) and composite MMT score (B) for 15 subjects entered in the natural history study (blue) that later participated in the albuterol RCT (pink=active, maroon=placebo). The slope is estimated from a linear mixed effects model (see text for details) and includes measurements up to the albuterol RCT baseline visit. The time scale on the X-axis is in relation to the baseline visit in the albuterol RCT (time zero). Alb = albuterol; CI = Confidence interval.
The pooled estimates of variability in the RCTs and the natural history study are presented in Table 2. The combined RCT estimates at 6 and 12 months for both MMT (18 overlapping muscles) and MVICT (10 muscles) are comparable to the estimates from the Natural History study, with the exception of the Dutch study which appeared to have lower variance. The overall pooled SDs for MVICT and MMT are slightly lower at 12 months than those seen in the natural history study alone (MVICT: 0.96 versus 0.98; MMT: 0.19 versus 0.23).
Table 2.
Pooled estimates of the variability (standard deviation, SD) of changes in composite strength scores by study.
| Composite MVICT Score | Composite MMT Score | |||||||
|---|---|---|---|---|---|---|---|---|
| 6 months | 12 months | 6 months | 12 months | |||||
| n | SD | n | SD | n | SD | n | SD | |
| Natural History Study[20] | 48 | 1.23 | 50 | 0.98 | 48 | 0.19 | 47 | 0.23 |
| Albuterol RCT[21] | 85 | 1.00 | 84 | 1.04 | 85 | 0.16 | 84 | 0.17 |
| MYO-029 RCT[23] | 30 | 1.53 | - | - | 31 | 0.22 | - | - |
| Dutch Exercise/Albuterol Study[22] | 64 | 0.71 | 64 | 0.84 | - | - | - | - |
| Pooled | 227 | 1.10 | 198 | 0.96 | 164 | 0.18 | 131 | 0.19 |
Discussion
Both MVICT and MMT can be used to create composite muscle strength scores to track disease progression or measure response to treatment in RCTs in patients with muscular dystrophy. In FSHD, a slow progression of disease was demonstrated over 12 months by both methods (mean change of -0.29 on MVICT and mean change of -0.07 on MMT)[20]. Here we report an increase in strength at 6 months in both MVICT and MMT associated with participation in the RCTs (albuterol or MYO-029) as compared to the natural history group, which is observed equally in both placebo and active treatment groups. For the MVICT this effect persists for up to 12 months, but falls back by 12 months on MMT testing. Overall, these data demonstrate the possible effects that participation in a RCT has on patient strength data. One explanation for this difference is the more extensive screening process involved to enter a clinical trial resulting in participants who may be less likely to progress compared to those who enrolled in the natural history study. The main strength criteria for the clinical trials included here were the ability to ambulate independently and having a majority of muscles with some weakness on MMT but within a testable range by MVICT (> 20 Newtons). Although there were small differences in baseline strength among the studies, all studies utilized the same technique for strength testing and the range of average composite MMT scores was somewhat limited (3.5 to 3.8).
In addition to the studies included in this analysis, one other FSHD clinical trial, a 6-month randomized, double-blind, placebo-controlled trial of albuterol in France, evaluated participants with quantitative myometry and manual muscle testing but used different measurement techniques[27]. Similar to our findings, however, the mean MVICT and MMT composite scores both increased at 3 and 6 months regardless of treatment assignment, thereby emphasizing that it appears to be the involvement in a study which seems to have the main effect. Both MVICT and MMT are effort dependent. Some of the increase in average strength seen in these clinical trials may be due to increased effort related to the optimism of being part of a clinical trial. The natural history study showed a slow decline in strength over 3 years[20]. This modest decline is supported by our data from extended 2-7 year follow-up of 15 subjects (Figure 2). In this small cohort we see both principles illustrated in this study: a slow but steady loss of strength over periods up to 7 years; which appears to reverse once entering the albuterol RCT, most notable over months 0-6 for MMT, and which persists for QMT. It may be that an inherently effort-dependent test on a fairly stable clinical background is particularly susceptible to the optimism presented by a clinical trial. Interestingly, in the Dutch exercise/albuterol study, for patients who had been followed for 6 months in the non-training group, the transition to placebo at Month 6 was not accompanied by a commensurate increase in strength, although the number of subjects in this subgroup was quite small (n = 16). For diseases where the natural history of disease progression is slow and the change in measurement is small but significant at 1 year, an effect related to being involved in a clinical trial can be fairly dramatic. In a recent study of ascorbic acid in Charcot Marie Tooth disease Type 1A, both the placebo and treatment arms progressed at a slower rate than would have been predicted from prior natural history data[28]. There has been considerable research into placebo effects in Parkinson's Disease, a chronic disease with faster yearly progression, where 50% improvements in the Unified Parkinson's Disease Rating Scale having been reported with placebo treatment[29].
In any clinical trial or natural history study patients come in for repeated clinical visits and have repeated measurements to monitor outcomes. In a clinical trial, however, these repeated visits and measurements occur in the setting of the excitement and expectations of any new therapy. There is a rapidly growing literature related to placebo effects in clinical trials. These effects are complex and include psychosocial expectations, neurochemical changes, and even changes on neuroimaging[30-32]. A Cochrane review of studies that included both a placebo group and a non-treated group, however, was unable to identify a significant difference between these groups when looking at relative risk for binary outcomes or standardized mean difference for continuous outcomes in disorders such as pain, smoking, nausea, and depression[33]. When planning clinical trials in FSHD, it seems prudent to anticipate a lack of decline, or even an improvement, in strength over time in the placebo group. In particular, the use of “natural history” controls or no controls at all in early-phase trials may yield results that are quite misleading with respect to the effects of treatment on strength. Other outcome measures like muscle MRI may increase the sensitivity of strength testing in future FSHD trials by identifying muscles which appear structurally at risk for targeted strength testing.
Estimates of variance from previous natural history studies and RCTs are important for power and sample size calculations for subsequent RCTs. We pooled SD estimates from treatment groups in three clinical trials, and combined these with estimates from the natural history study to obtain overall pooled SDs for composite MMT and MVICT scores. These pooled estimates are slightly lower than the estimate from the natural history study alone. For clinical trial planning, which SD to use may depend on how conservative an estimate is feasible. The pooled SD estimates provided here are useful for trials using composite strength scores comprised of a similar number and composition of tested muscles and derived from identical testing procedures. Alternatively, a range of SD scores from the various studies can be used to calculate a best case/worst case sample size required to detect a given difference in strength with a specified power.
Acknowledgements
Dr. Statland is funded by the MDA Clinical Research Training Grant and NIH Experimental Therapeutics in Neurological Disorders grant # T32 NS07338-20.
Dr. Heatwole receives funding through the National Institutes of Health; specifically through the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) NIAMS grant # 1K23AR055947.
Dr. van der Kooi received a government grant of the Netherlands Organization for Scientific Research (NOW) and the Health Research and Development Council of the Netherlands (ZON-MW), and received research support from the Prinses Beatrix Fonds (the Dutch Public Fund for Neuromuscular Disorders), and the Dutch FSHD Foundation.
Dr. Tawil received research support from the NIH NINDS 1P01NS069539-01 (co-PI), NIH R01 AR054366 (co-investigator), NIH 2U54NS048843-08 (co-investigator), R01 NS061795-01A2 (co-PI) and the Fields Center for FSHD and Neuromuscular Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
William Martens has no disclosures to report.
Shree Pandya has no disclosures to report.
Dr. McDermott has no disclosures to report.
Dr. Kissel has no disclosures to report.
Dr. Wagner has no disclosures to report.
References
- 1.Flanigan KM, Coffeen CM, Sexton L, Stauffer D, Brunner S, Leppert MF. Genetic characterization of a large, historically significant Utah kindred with facioscapulohumeral dystrophy. Neuromuscul Disord. 2001;11:525–9. doi: 10.1016/s0960-8966(01)00201-2. [DOI] [PubMed] [Google Scholar]
- 2.Mostacciuolo ML, Pastorello E, Vazza G, et al. Facioscapulohumeral muscular dystrophy: epidemiological and molecular study in a north-east Italian population sample. Clin Genet. 2009;75:550–5. doi: 10.1111/j.1399-0004.2009.01158.x. [DOI] [PubMed] [Google Scholar]
- 3.Padberg GW. Thesis. University of Leiden; Leiden, the Netherlands: 1982. Facioscapulohumeral disease. [Google Scholar]
- 4.van der Maarel SM, Tawil R, Tapscott SJ. Facioscapulohumeral muscular dystrophy and DUX4: breaking the silence. Trends Mol Med. 2011;17:252–8. doi: 10.1016/j.molmed.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clapp J, Mitchell LM, Bolland DJ, et al. Evolutionary conservation of a coding function for D4Z4, the tandem DNA repeat mutated in facioscapulohumeral muscular dystrophy. Am J Hum Genet. 2007;81:264–79. doi: 10.1086/519311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabriels J, Beckers MC, Ding H, et al. Nucleotide sequence of the partially deleted D4Z4 locus in a patient with FSHD identifies a putative gene within each 3.3 kb element. Gene. 1999;236:25–32. doi: 10.1016/s0378-1119(99)00267-x. [DOI] [PubMed] [Google Scholar]
- 7.Lyle R, Wright TJ, Clark LN, Hewitt JE. The FSHD-associated repeat, D4Z4, is a member of a dispersed family of homeobox-containing repeats, subsets of which are clustered on the short arms of the acrocentric chromosomes. Genomics. 1995;28:389–97. doi: 10.1006/geno.1995.1166. [DOI] [PubMed] [Google Scholar]
- 8.Dixit M, Ansseau E, Tassin A, et al. DUX4, a candidate gene of facioscapulohumeral muscular dystrophy, encodes a transcriptional activator of PITX1. Proc Natl Acad Sci U S A. 2007;104:18157–62. doi: 10.1073/pnas.0708659104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lemmers RJ, van der Vliet PJ, Klooster R, et al. A unifying genetic model for facioscapulohumeral muscular dystrophy. Science. 2010;329:1650–3. doi: 10.1126/science.1189044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snider L, Geng LN, Lemmers RJ, et al. Facioscapulohumeral dystrophy: incomplete suppression of a retrotransposed gene. PLoS Genet. 2010;6:e1001181. doi: 10.1371/journal.pgen.1001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosnakovski D, Daughters RS, Xu Z, Slack JM, Kyba M. Biphasic myopathic phenotype of mouse DUX, an ORF within conserved FSHD-related repeats. PLoS One. 2009;4:e7003. doi: 10.1371/journal.pone.0007003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kowaljow V, Marcowycz A, Ansseau E, et al. The DUX4 gene at the FSHD1A locus encodes a pro-apoptotic protein. Neuromuscul Disord. 2007;17:611–23. doi: 10.1016/j.nmd.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Snider L, Asawachaicharn A, Tyler AE, et al. RNA transcripts, miRNA-sized fragments and proteins produced from D4Z4 units: new candidates for the pathophysiology of facioscapulohumeral dystrophy. Hum Mol Genet. 2009;18:2414–30. doi: 10.1093/hmg/ddp180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vanderplanck C, Ansseau E, Charron S, et al. The FSHD atrophic myotube phenotype is caused by DUX4 expression. PLoS One. 2011;6:e26820. doi: 10.1371/journal.pone.0026820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wuebbles RD, Long SW, Hanel ML, Jones PL. Testing the effects of FSHD candidate gene expression in vertebrate muscle development. Int J Clin Exp Pathol. 2010;3:386–400. [PMC free article] [PubMed] [Google Scholar]
- 16.Brooke MH, Griggs RC, Mendell JR, Fenichel GM, Shumate JB, Pellegrino RJ. Clinical trial in Duchenne dystrophy. I. The design of the protocol. Muscle Nerve. 1981;4:186–97. doi: 10.1002/mus.880040304. [DOI] [PubMed] [Google Scholar]
- 17.Merkies IS, Faber CG. Outcome measures in Duchenne muscular dystrophy: are we ready for the new therapeutic era? Neuromuscul Disord. 2009;19:447. doi: 10.1016/j.nmd.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Personius KE, Pandya S, King WM, Tawil R, McDermott MP. Facioscapulohumeral dystrophy natural history study: standardization of testing procedures and reliability of measurements. The FSH DY Group. Phys Ther. 1994;74:253–63. doi: 10.1093/ptj/74.3.253. [DOI] [PubMed] [Google Scholar]
- 19.A comparison of muscle strength testing techniques in amyotrophic lateral sclerosis. Neurology. 2003;61:1503–7. doi: 10.1212/01.wnl.0000095961.66830.03. [DOI] [PubMed] [Google Scholar]
- 20.FSH-DY A prospective, quantitative study of the natural history of facioscapulohumeral muscular dystrophy (FSHD): implications for therapeutic trials. The FSH-DY Group. Neurology. 1997;48:38–46. doi: 10.1212/wnl.48.1.38. [DOI] [PubMed] [Google Scholar]
- 21.Kissel JT, McDermott MP, Mendell JR, et al. Randomized, double-blind, placebo-controlled trial of albuterol in facioscapulohumeral dystrophy. Neurology. 2001;57:1434–40. doi: 10.1212/wnl.57.8.1434. [DOI] [PubMed] [Google Scholar]
- 22.van der Kooi EL, Vogels OJ, van Asseldonk RJ, et al. Strength training and albuterol in facioscapulohumeral muscular dystrophy. Neurology. 2004;63:702–8. doi: 10.1212/01.wnl.0000134660.30793.1f. [DOI] [PubMed] [Google Scholar]
- 23.Wagner KR, Fleckenstein JL, Amato AA, et al. A phase I/IItrial of MYO-029 in adult subjects with muscular dystrophy. Ann Neurol. 2008;63:561–71. doi: 10.1002/ana.21338. [DOI] [PubMed] [Google Scholar]
- 24.Tawil R, McDermott MP, Mendell JR, Kissel J, Griggs RC. Facioscapulohumeral muscular dystrophy (FSHD): design of natural history study and results of baseline testing. FSH-DY Group. Neurology. 1994;44:442–6. doi: 10.1212/wnl.44.3_part_1.442. [DOI] [PubMed] [Google Scholar]
- 25.Orrell RW, Tawil R, Forrester J, Kissel JT, Mendell JR, Figlewicz DA. Definitive molecular diagnosis of facioscapulohumeral dystrophy. Neurology. 1999;52:1822–6. doi: 10.1212/wnl.52.9.1822. [DOI] [PubMed] [Google Scholar]
- 26.van Deutekom JC, Bakker E, Lemmers RJ, et al. Evidence for subtelomeric exchange of 3.3 kb tandemly repeated units between chromosomes 4q35 and 10q26: implications for genetic counselling and etiology of FSHD1. Hum Mol Genet. 1996;5:1997–2003. doi: 10.1093/hmg/5.12.1997. [DOI] [PubMed] [Google Scholar]
- 27.Payan CA, Hogrel JY, Hammouda EH, et al. Periodic salbutamol in facioscapulohumeral muscular dystrophy: a randomized controlled trial. Arch Phys Med Rehabil. 2009;90:1094–101. doi: 10.1016/j.apmr.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 28.Pareyson D, Reilly MM, Schenone A, et al. Ascorbic acid in Charcot-Marie-Tooth disease type 1A (CMT-TRIAAL and CMT-TRAUK): a double-blind randomised trial. Lancet Neurol. 2011;10:320–8. doi: 10.1016/S1474-4422(11)70025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mellone S, Tacconi C, Chiari L. Validity of a Smartphone-based instrumented Timed Up and Go. Gait Posture. 2012 doi: 10.1016/j.gaitpost.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Benedetti F, Carlino E, Pollo A. How placebos change the patient's brain. Neuropsychopharmacology. 2011;36:339–54. doi: 10.1038/npp.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet. 2010;375:686–95. doi: 10.1016/S0140-6736(09)61706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oken BS. Placebo effects: clinical aspects and neurobiology. Brain. 2008;131:2812–23. doi: 10.1093/brain/awn116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hrobjartsson A, Gotzsche PC. Placebo interventions for all clinical conditions. Cochrane Database Syst Rev. 2010:CD003974. doi: 10.1002/14651858.CD003974.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]