Abstract
In human islet transplantation, insulin independence decreases over time. We previously showed that amyloid deposition following transplantation of islets from human islet amyloid polypeptide (hIAPP) transgenic mice resulted in β-cell loss, and that rosiglitazone treatment decreased islet amyloid deposition and preserved β-cell area in the endogenous pancreas of hIAPP transgenic mice. Thus, we sought to determine if rosiglitazone treatment decreases islet amyloid deposition and the associated β-cell loss after islet transplantation.
Streptozocin-diabetic mice were transplanted with 100 islets from hIAPP transgenic (T) mice or non-transgenic (NT) littermates under the kidney capsule and received either rosiglitazone (R) in drinking water or plain drinking water (C). The resultant groups (NTC [n=11], NTR [n=9], TC [n=14] and TR [n=10]) were followed for 12 weeks after which the graft was removed and processed for histology.
Amyloid was detected in nearly all T islet grafts (TC=13/14, TR=10/10) but not in NT grafts. Rosiglitazone did not alter amyloid deposition (% graft area occupied by amyloid; TC: 2.15±0.7, TR: 1.72±0.66; p=0.86). % β-cell/graft area was decreased in the TC grafts compared to NTC (56.2±3.1 vs. 73.8±1.4; p<0.0001) but was not different between TC and TR groups (56.2±3.1 vs. 61.0±2.9; p=0.34). Plasma glucose levels before and after transplantation did not differ between NTC and TC groups and rosiglitazone did not affect plasma glucose levels post islet transplantation.
Rosiglitazone did not decrease amyloid deposition in hIAPP transgenic islet grafts. Therefore rosiglitazone treatment of recipients of amyloid forming islets may not improve transplantation outcomes.
INTRODUCTION
Islet transplantation is a potential therapy for humans with type 1 diabetes (1). Although it has a high short-term success rate (2), most grafts fail over time (3; 4). In addition to immune-mediated islet destruction, several non-immune factors have been suggested to contribute to graft failure (5). Amyloid deposits have been described in human islets transplanted into streptozocin-diabetic immune-deficient mice (6; 7) and more recently in the transplanted islets from four of five patients with type 1 diabetes (8; 9); suggesting that amyloid deposition could be a factor contributing to loss of β-cells in clinical islet transplantation.
To facilitate the study of islet amyloid, which is a hallmark of islet morphology in type 2 diabetes (10–12), transgenic mice have been developed, that produce the amyloidogenic human form of islet amyloid polypeptide (hIAPP) in their β-cells (13–16). These genetically modified mice are needed as mouse IAPP is not amyloidogenic (17). In the endogenous pancreas, islet amyloid develops in hIAPP transgenic mice over the course of a year (18; 19), and this is associated with impaired insulin secretion and β-cell loss in vivo. Following islet transplantation, however, amyloid formation is markedly accelerated, being detectable as early as one week after transplantation (20). Amyloid deposition in these grafts was associated with β-cell loss, increased β-cell apoptosis and decreased β-cell replication in transplanted hIAPP transgenic islets (20), supporting the concept that amyloid could be one factor contributing to islet transplant failure.
Islet amyloid can trigger a number of stress signaling pathways in β-cells, including oxidative stress (21) and JNK signaling (22). Recent studies have shown that amyloidogenic hIAPP can also elicit an inflammatory response from dendritic cells or macrophages (23; 24), which may provide an additional mechanism by which islet amyloid can elicit cytotoxicity following islet transplantation. Of note, hIAPP transgenic islets contain a human protein that is foreign to murine islet transplant recipients, thus whether an immune-mediated response is responsible for β-cell loss in hIAPP transgenic islet grafts remains unanswered.
Rosiglitazone is an insulin sensitizer used to treat type 2 diabetes (25; 26). We previously showed that treatment of hIAPP transgenic mice with rosiglitazone for one year was associated with reduced islet amyloid deposition and amelioration of amyloid-associated β-cell loss in vivo compared to untreated controls (27). Rosiglitazone has also been shown to preserve islet architecture in rodent models of diabetes (28; 29), and to protect cultured human (30) and mouse (31) islets from free fatty acid-induced β-cell secretory dysfunction or from toxicity elicited by direct application of hIAPP peptide (32). Rosiglitazone has also been suggested to be anti-inflammatory (33), which may underlie some of its positive effects on the islet. Thus, rosiglitazone can be effective both in decreasing amyloid formation and its toxic effects and in protecting against β-cell dysfunction. In the present study we determined whether rosiglitazone treatment can decrease amyloid formation in transplanted hIAPP transgenic islets and/or offset its toxic effects.
MATERIALS AND METHODS
Animals, Islet Isolation and Transplantation
Islet donors were 8–10 week old hemizygous transgenic mice expressing hIAPP in their pancreatic islet β-cells, and non-transgenic littermates (F1 C57BL/6 × DBA/2J). Islets were isolated by collagenase digestion as we have done previously (20; 34), and were cultured for 90 minutes in RPMI1640 medium supplemented with 10% fetal bovine serum prior to transplantation. Note that at this age, hIAPP transgenic mice do not have amyloid deposits in their islets (20; 34). Islet recipients were 8–10 week old syngeneic non-transgenic male mice rendered diabetic with streptozocin (STZ; 220 mg/kg). Islet transplantation was performed one week after induction of diabetes, whereby 100 hIAPP transgenic (T) or non-transgenic (NT) islets were transplanted under the renal capsule. Mice had ad libitum access to drinking water and a moderate fat diet containing 18% kcal from fat (PicoLab # 5058, Brentwood, MO, USA) that we have used previously (20; 27). This study was approved by the Institutional Animal Care and Use Committee at VA Puget Sound Health Care System.
Study Procedures
Immediately after islet transplantation, mice received plain drinking water (C) or drinking water containing rosiglitazone (R: 1.5 mg kg−1 day−1). Thus, four study groups were generated: non-transgenic control (NTC; n=11), non-transgenic rosiglitazone (NTR; n=9), transgenic control (TC; n=14) and transgenic rosiglitazone (TR; n=10). Mice were followed for 12 weeks after transplantation and body weights and non-fasting plasma glucose levels were monitored every 2–7 days (20). Water intake was determined from weeks 2–12 following transplantation to ensure adequate delivery of rosiglitazone. Average drug intake was 1.75±0.07 mg kg−1 day−1, close to the target dose. Nephrectomy of the graft bearing kidney was performed 12 weeks after transplantation and the graft harvested for morphological analysis. Mice were followed for another week after nephrectomy to assess the recurrence of diabetes, after which they were humanely euthanized. Plasma was taken at euthanasia from a subset of mice (n=5–10 per group) for determination of rosiglitazone levels by liquid chromatography tandem mass spectrometry (GSK Laboratories, Herts, UK) (35).
Initial success of islet transplantation was defined as mean non-fasting plasma glucose ≤250 mg/dl from 2 to 8 days after islet transplantation. Subsequent graft failure was defined by recurrence of hyperglycemia >250 mg/dl on two consecutive plasma glucose measurements occurring from two weeks following islet transplantation. Recurrence of diabetes after the nephrectomy of the graft bearing kidney was confirmed by the elevation of plasma glucose levels to 447±15 mg/dl.
Insulin Tolerance Tests
A subset of animals (TC, n=5; TR, n=7) underwent an insulin tolerance test 12 weeks after islet transplantation. Mice were fasted for 4 hours after which a baseline blood sample was taken via tail tip. Insulin was administered (1.0 unit/kg i.p.) and tail tip blood samples were taken after 15, 30, 45 and 60 minutes. Blood glucose levels determined using an AlphaTRAK monitoring system (Abbott Animal Health, IL, USA). Insulin sensitivity was calculated as the decremental area under curve from 0 to 60 minutes during the ITT (invAUCg 0–60).
Islet Graft Morphology
After nephrectomy, islet grafts (n=9–14 per group) were fixed and processed as described previously (20). Islet graft area, insulin positive area and amyloid area were determined using our computer-based quantitative system and amyloid severity and β-cell area were calculated as described previously (20). All histological assessments were performed in a blinded manner by a single observer.
T-Cell Proliferation Assay
Spleens were harvested from a subset of transplant recipients (TC: n=5; NTC: n=3), splenocytes (T-cells) were isolated by density gradient centrifugation and plated (2×105 cells/well) in RPMI-1640 with 10% FBS. T-cells were then incubated alone or with hIAPP transgenic or non-transgenic islet lysates, human insulin (1U/ml as a negative control), phytohemagglutinin (PHA, 10 μg/ml; positive mitogenic control) for five days (or three days with PHA). 3H-thymidine (1 µCi/well) was added and the cultures incubated for another 18 hours and 3H-thymidine incorporation determined by scintillation counting. Stimulation index (SI) was calculated as mean counts per minute (CPM) experimental wells / mean CPM for wells with T-cells alone. Positive proliferation was considered to be an SI>2.0.
Data Analysis and Statistical Methods
Data are expressed as mean±SEM. Two group comparisons were performed using the Mann-Whitney U test for non-normally distributed data and an unpaired Students t-test for normally distributed data. Multiple group comparisons were performed using ANOVA. Comparison of graft failure over time between the groups was evaluated by Kaplan-Meier analysis. A p<0.05 was considered significant for all statistical analyses.
RESULTS
Effect of rosiglitazone on plasma glucose, graft failure and body weight
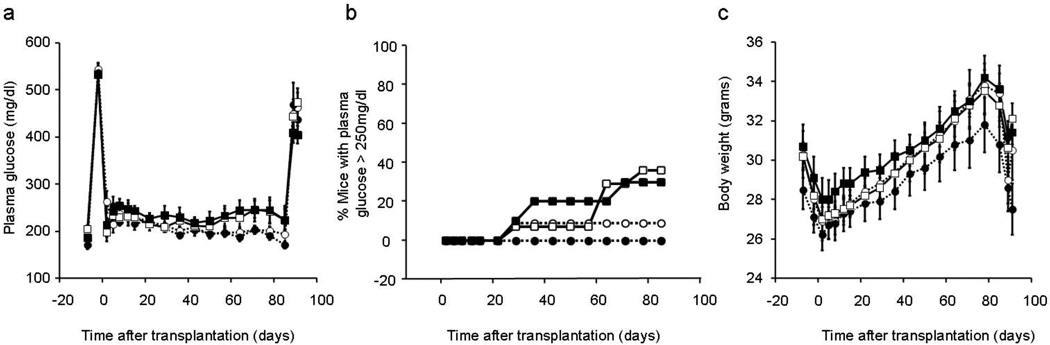
Plasma glucose levels after streptozocin (STZ), and prior to and during the first week after islet transplantation were not different between study groups (Figure 1a). During the subsequent 12 weeks of follow-up, graft failure rate was 9% (1/11) in mice that received non-transgenic islets and plain drinking water compared to 36% (5/14) in hIAPP islet recipients that received plain water (p=0.15) (Figure 1b). Amongst hIAPP islet transplant recipients, rosiglitazone treatment did not significantly improve graft failure rate (TC: 36% [5/14] and TR: 30% [3/10]). Rosiglitazone also did not affect the graft failure rate in non-transgenic islet recipients (NTR: 0% [0/9] and NTC: 9% [1/11]). Nephrectomy of the graft-bearing kidney at the end of this period resulted in the recurrence of diabetes in all the islet transplant recipients (Figure 1a). Body weights in the islet transplant recipients prior to STZ injection and throughout the study period did not differ in the four groups of mice (Figure 1c).
Figure 1.
Plasma glucose levels (a) and body weight (c) in mice that received 100 non-transgenic islets (circles) or 100 hIAPP transgenic islets (squares) and plain drinking water (open) or rosiglitazone in drinking water (closed) (NTC: n=11, NTR: n=9, TC: n=14 and TR: n=10). Graft failure (b), defined as recurrence of hyperglycemia >250 mg/dl on two consecutive plasma glucose measurements, occurred in 36% and 30% of hIAPP transgenic islet recipients and in 9% and 0% of non-transgenic islet recipients treated with water or rosiglitazone respectively.
Effect of rosiglitazone on amyloid deposition and β-cell area
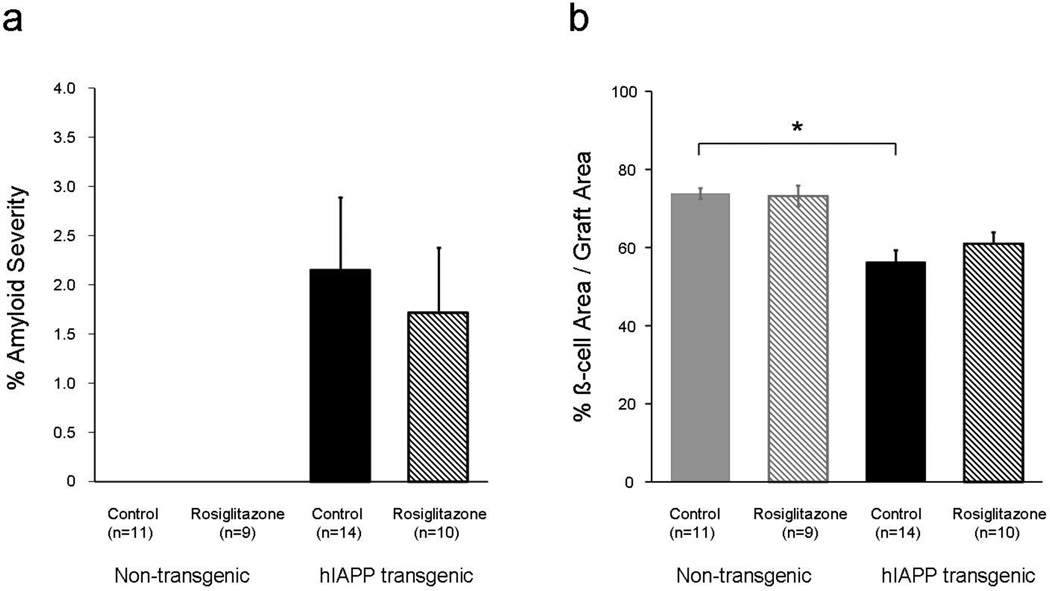
Amyloid deposits were detected in 13 of 14 (93%) and 10 of 10 (100%) hIAPP transgenic islet grafts of recipients treated with water and rosiglitazone respectively. However, contrary to expectation, rosiglitazone treatment of hIAPP islet recipients did not alter graft amyloid deposition (Figure 2a). Twelve weeks after islet transplantation, and as expected, amyloid deposits were not detected in any non-transgenic islet grafts. β-cell area was decreased by 24% in the hIAPP transgenic islet grafts compared to non-transgenic islet grafts of recipients treated with plain drinking water (p<0.0001). Rosiglitazone did not preserve β-cell area in the amyloid forming hIAPP transgenic islet grafts (p=0.33) and did not change it in the non-transgenic islet grafts (p=0.97).
Figure 2.
Amyloid severity (a) and β-cell/graft area (b) in non-transgenic (grey bars) and hIAPP transgenic islet recipients (black) treated with plain drinking water (closed bars) or rosiglitazone in drinking water (hatched bars). *p<0.05 for mice transplanted with hIAPP transgenic islets vs. non-transgenic islets, both treated with plain drinking water.
Plasma rosiglitazone levels in islet transplant recipients
Rosiglitazone was detected in the plasma in 5 of 5 (100%) non-transgenic islet transplant recipients and in 6 of 7 (86%) hIAPP transgenic islet transplant recipients treated with rosiglitazone (NTR: 172±61, TR: 133±85 ng/ml). No rosiglitazone was detected in the plasma of islet transplant recipients that received plain drinking water (NTC: 0 of 10 and TC: 0 of 6).
Effect of rosiglitazone on insulin sensitivity of hIAPP islet transplant recipients
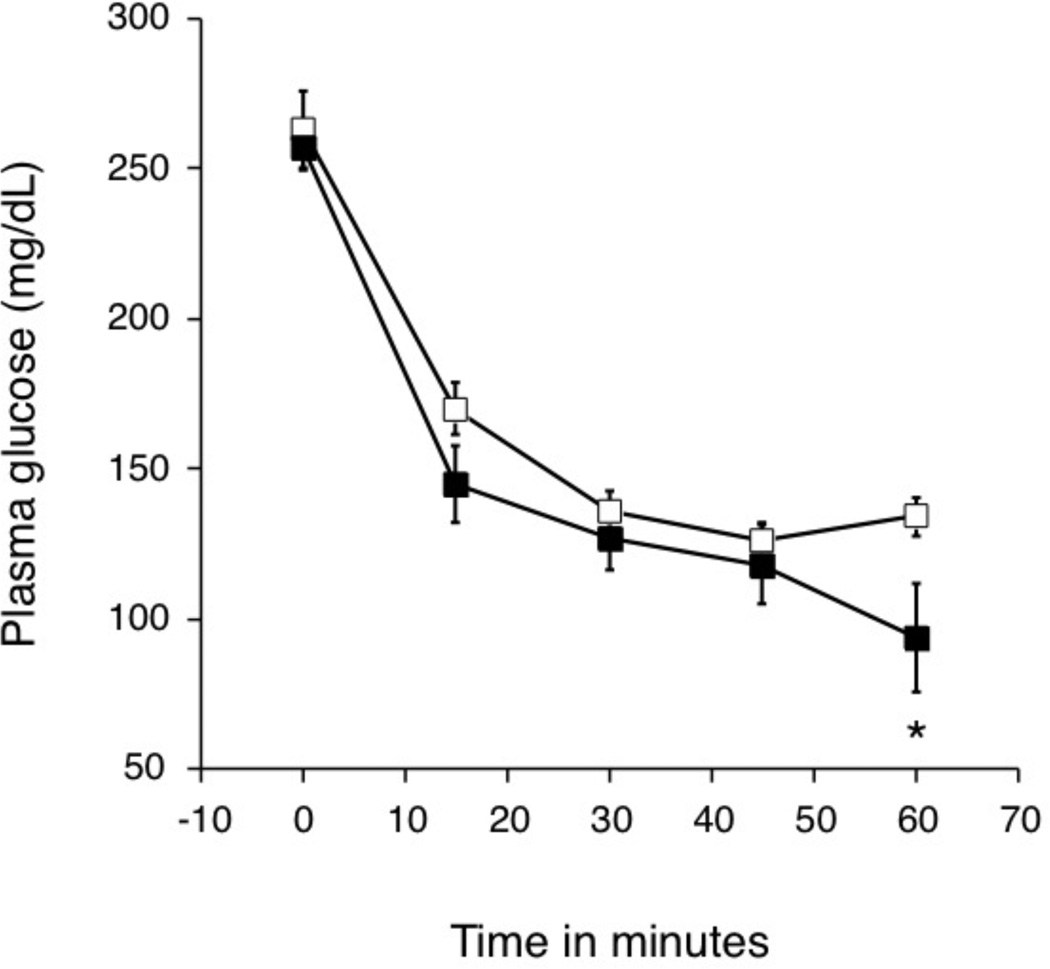
In hIAPP islet transplant recipients that received an insulin tolerance test (ITT; TC: n=5 and TR: n=7), body weight and non-fasting plasma glucoses were similar before and during the 12 weeks following the transplant (data not shown). Blood glucose levels before and after the insulin injection as part of the ITT were not different between the groups (Figure 3). Glucose levels decreased to a similar extent in both groups during the 60 minutes following insulin injection (invAUC glucose0–60: 6296±439 vs. 6926±647 mg/dl/min, p=0.5).
Figure 3.
Blood glucose levels during an ITT in mice that received hIAPP transgenic islets and either plain drinking water (TC: n=5, open squares) or rosiglitazone (TR: n=7, closed squares) (b). *p<0.05
Effect of hIAPP to elicit a T-cell proliferative response in islet recipients
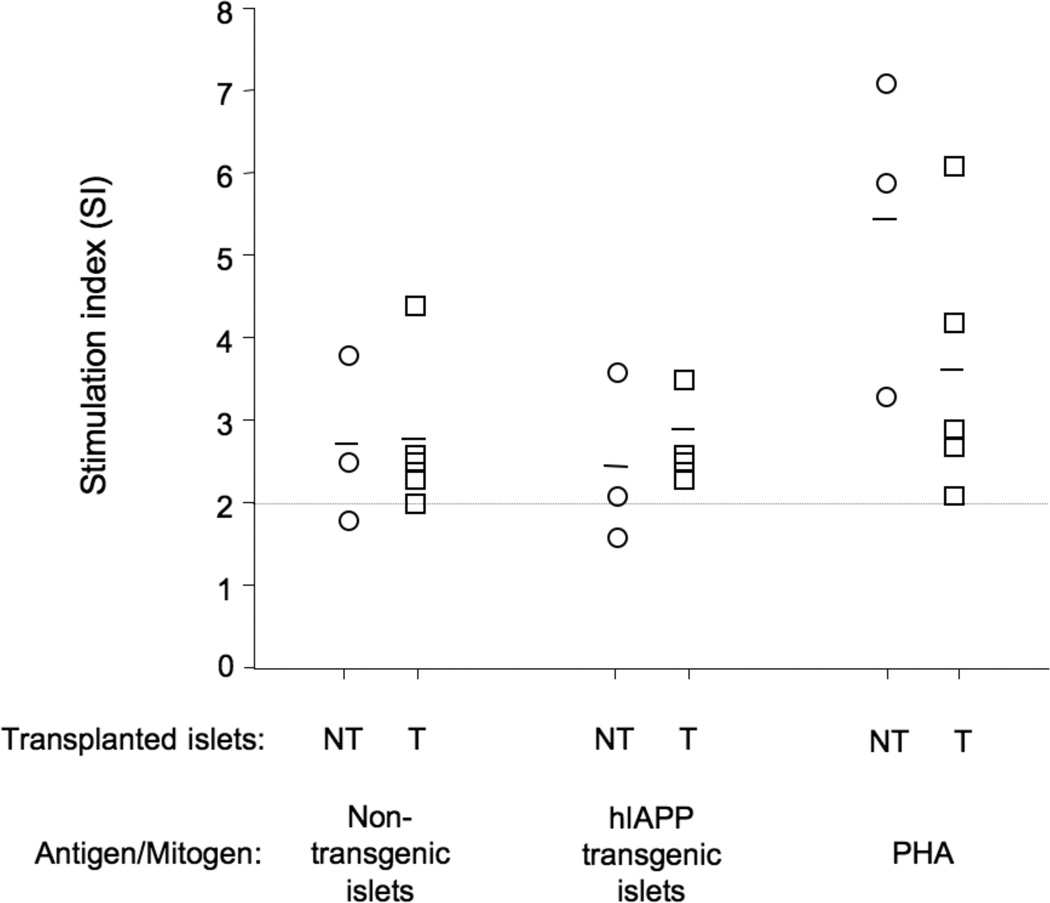
Splenocytes (T-cells) from hIAPP transgenic islet transplant recipients (n=5) exhibited a similar proliferative response to both hIAPP transgenic islet and non-transgenic islet lysates (Figure 4). T-cells isolated from non-transgenic recipients (n=3) also responded similarly to both hIAPP transgenic and non-transgenic islet lysates. Human insulin, a negative control, did not elicit a proliferative response in either T-cell population (data not shown), while T-cells from both groups of mice were responsive to the mitogen PHA.
Figure 4.
T-cell proliferation responses of non-transgenic islet recipients (NTC: n=3, open circles) and hIAPP transgenic islet recipients (TC: n=5, open squares) to lysates from non-transgenic islets, hIAPP transgenic islets and the mitogen PHA. Responses are shown in SI units and the dotted line represents an SI of 2.0, the value above which a response is considered to be positive. Individual responses are depicted as separate symbols and the mean SI for each response by the solid line.
DISCUSSION
We sought to determine if rosiglitazone treatment is an effective therapeutic strategy to decrease amyloid formation in islet grafts and thereby improve islet transplantation outcomes. Surprisingly, rosiglitazone treatment of hIAPP islet transplant recipients did not decrease graft amyloid deposition or abrogate its associated β-cell toxicity compared to untreated controls. We observed a trend towards the occurrence of graft failure in the hIAPP transgenic islet recipient controls compared to the non-transgenic islet recipient controls, consistent with our previous findings (20). However, rosiglitazone did not affect glycemic control post islet transplantation in hIAPP islet recipients, and therefore did not improve graft failure rates amongst these recipients.
Our finding of a lack of an effect of rosiglitazone to affect glycemic control post-transplantation is in contrast to a study where rosiglitazone treatment of STZ-diabetic C57BL/6 mice for 9 or 31 days post islet transplantation was associated with decreased rates of diabetes at both time points (36). However, a major difference between this study and our own is that in the study with C57BL/6 mouse islet transplantation, mice did not to attain euglycemia after transplantation while in our study they did so. Rosiglitazone therefore was effective in treating post-transplant diabetes when non-amyloid forming mouse islets were transplanted (36), but did not have a beneficial effect on amyloid formation in susceptible islet grafts under euglycemic conditions.
The lack of rosiglitazone effect to decrease graft amyloid deposition is also in contrast to our previously published in vivo study (27). One possible reason for the lack of effect of rosiglitazone on amyloid deposition following islet transplantation was the difference in treatment durations between these studies (12 weeks in the present study vs. 12 months in the in vivo study). Of note, however, is the substantial difference in the timeframe over which amyloid deposition occurs in these two models. In the endogenous pancreas, amyloid deposition is detectable at 12 months (18; 19; 27), with only minimal amyloid deposition being present at 6 months (34). In contrast, following islet transplantation, amyloid deposition is detectable as early as one week with increased amyloid deposition occurring over 6–12 weeks (20). Thus, we believe that 12 weeks of treatment in the present study should have been sufficient to observe an effect of rosiglitazone treatment on amyloid deposition. We also ruled out the fact that the lack of effect of rosiglitazone was due to inadequate dosing. Measurement of water intake demonstrated that mice drank sufficient water to reach the target treatment dose, and rosiglitazone was detectable in plasma from 11 of 12 mice that received the drug. While these plasma drug levels may not reflect steady state levels, since they were drawn following an overnight fast in diabetic mice following removal of the islet graft, they do indicate that rosiglitazone was successfully administered to the mice that were designed to receive this intervention via the drinking water.
In order to evaluate the pharmacodynamic efficacy of rosiglitazone treatment, we measured insulin sensitivity of hIAPP islet transplant recipients by performing an ITT in mice that had received either rosiglitazone or plain drinking water for 12 weeks post transplantation. Contrary to our expectation, rosiglitazone did not improve insulin sensitivity in these mice. It has been previously shown that the presence of obesity/insulin resistance is required for an effective response to rosiglitazone in tissues that normally respond to insulin (37), and we observed marked differences in weight gain in the current study and our previous in vivo study (27). In the current study the net weight gain after 12 weeks of islet transplantation was at most 2 to 3 grams per mouse, in contrast to our previously published findings where the hIAPP transgenic mice had a mean weight gain of 17.5 grams per mouse after three months of treatment (27). Thus, the lack of the expected treatment effect of rosiglitazone on graft amyloid deposition could be due to lack of its effect on insulin sensitivity in these relatively lean islet transplant recipients.
The use of hIAPP transgenic islets in our islet transplant model introduces a foreign protein (hIAPP) to islet transplant recipients that could potentially elicit an immune response. We addressed this by determining T-cell proliferation in response to hIAPP transgenic or non-transgenic islet lysates. Interestingly transplant recipients receiving either syngeneic hIAPP transgenic or non-transgenic islets, demonstrated a small T-cell proliferative response to both islet types. This absence of a difference in the immune response to the transplanted islets, irrespective of the presence of hIAPP, suggests that the loss of β cells in hIAPP islet grafts is not likely due to an immune response, but more likely due to the toxic effects of amyloid formation.
Finally, hIAPP in its oligomeric/fibrillar form has been shown to stimulate the production of proinflammatory cytokines and chemokines from macrophages and dendritic cells (23; 24). Given the anti-inflammatory properties of rosiglitazone (33), it is possible that this drug could exert a beneficial effect to limit this particular adverse consequence of islet amyloid deposition. However, given that rosiglitazone had no detectable effect to alter amyloid deposition, β-cell area or glycemic status in hIAPP transgenic islet transplant recipients, we believe it unlikely that such a beneficial effect occurred.
In summary, we have demonstrated that 12 weeks after islet transplantation, amyloid deposition is associated with β-cell loss, which is in keeping with our previously published finding (20). Furthermore, rosiglitazone treatment of hIAPP islet transplant recipients is not associated with a decrease in amyloid deposition or preservation of β-cell area in these islet grafts. This lack of treatment effect may be due to a lack of significant weight gain and obesity in islet transplant recipients as evidenced by the inability of rosiglitazone to improve insulin sensitivity in these non-obese, hIAPP islet transplant recipients. Therefore, rosiglitazone treatment in unlikely to improve outcomes of islet transplantation in insulin sensitive individuals but may still benefit obese, insulin resistant individuals with Type 1 diabetes who undergo islet transplantation.
ACKNOWLEDGEMENTS
We thank J. Willard, M. Watts, M. Peters, B. Barrow, C. Braddock, M. Cone and R. Bhatti for excellent technical support.
Grant support:
This work was supported by the Department of Veterans Affairs, VA Puget Sound Health Care System (Seattle, WA, USA), an investigator-initiated research grant from GlaxoSmithKline (GJT), DK74404 (RLH), DK80945 (SZ), DK017047 (University of Washington Diabetes Research Center, Cellular and Molecular Imaging Core), Juvenile Diabetes Research Foundation Postdoctoral Fellowship (JU), American Diabetes Association Mentor-Based Fellowship (KAM), University of Washington McAbee Fellowship (KAM) and NIH-training grant DK007247 (SLS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jayalakshmi Udayasankar, Email: judaya@u.washington.edu.
Sakeneh Zraika, Email: zraikas@u.washington.edu.
Kathryn Aston-Mourney, Email: k.astonmourney@deakin.edu.au.
Barbara M Brooks-Worrell, Email: bbrooks@u.washington.edu.
Gerald J Taborsky, Email: taborsky@u.washington.edu.
Rebecca L Hull, Email: rhull@u.washington.edu.
References
- 1.Robertson RP. Successful islet transplantation for patients with diabetes--fact or fantasy? N Engl J Med. 2000;343:289–290. doi: 10.1056/NEJM200007273430409. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 3.Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. The New England journal of medicine. 2006;355:1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 5.Bertuzzi F, Ricordi C. Prediction of clinical outcome in islet allotransplantation. Diabetes Care. 2007;30:410–417. doi: 10.2337/dc06-1233. [DOI] [PubMed] [Google Scholar]
- 6.Westermark P, Eizirik DL, Pipeleers DG, et al. Rapid deposition of amyloid in human islets transplanted into nude mice. Diabetologia. 1995;38:543–549. doi: 10.1007/BF00400722. [DOI] [PubMed] [Google Scholar]
- 7.Potter KJ, Abedini A, Marek P, et al. Islet amyloid deposition limits the viability of human islet grafts but not porcine islet grafts. Proc Natl Acad Sci U S A. 2010;107:4305–4310. doi: 10.1073/pnas.0909024107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westermark GT, Westermark P, Berne C, et al. Widespread amyloid deposition in transplanted human pancreatic islets. N Engl J Med. 2008;359:977–979. doi: 10.1056/NEJMc0802893. [DOI] [PubMed] [Google Scholar]
- 9.Westermark GT, Davalli AM, Secchi A, et al. Further evidence for amyloid deposition in clinical pancreatic islet grafts. Transplantation. 2012;93:219–223. doi: 10.1097/TP.0b013e31823e46ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Opie EL. The Relation Oe Diabetes Mellitus to Lesions of the Pancreas. Hyaline Degeneration of the Islands Oe Langerhans. J Exp Med. 1901;5:527–540. doi: 10.1084/jem.5.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westermark P. Quantitative studies on amyloid in the islets of Langerhans. Ups J Med Sci. 1972;77:91–94. doi: 10.1517/03009734000000014. [DOI] [PubMed] [Google Scholar]
- 12.Clark A, Wells CA, Buley ID, et al. Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res. 1988;9:151–159. [PubMed] [Google Scholar]
- 13.Janson J, Soeller WC, Roche PC, et al. Spontaneous diabetes mellitus in transgenic mice expressing human islet amyloid polypeptide. Proc Natl Acad Sci U S A. 1996;93:7283–7288. doi: 10.1073/pnas.93.14.7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Alessio DA, Verchere CB, Kahn SE, et al. Pancreatic expression and secretion of human islet amyloid polypeptide in a transgenic mouse. Diabetes. 1994;43:1457–1461. doi: 10.2337/diab.43.12.1457. [DOI] [PubMed] [Google Scholar]
- 15.Fox N, Schrementi J, Nishi M, et al. Human islet amyloid polypeptide transgenic mice as a model of non-insulin-dependent diabetes mellitus (NIDDM) FEBS Lett. 1993;323:40–44. doi: 10.1016/0014-5793(93)81444-5. [DOI] [PubMed] [Google Scholar]
- 16.Hoppener JW, Verbeek JS, de Koning EJ, et al. Chronic overproduction of islet amyloid polypeptide/amylin in transgenic mice: lysosomal localization of human islet amyloid polypeptide and lack of marked hyperglycaemia or hyperinsulinaemia. Diabetologia. 1993;36:1258–1265. doi: 10.1007/BF00400803. [DOI] [PubMed] [Google Scholar]
- 17.Westermark P, Engstrom U, Johnson KH, et al. Islet amyloid polypeptide: pinpointing amino acid residues linked to amyloid fibril formation. Proc Natl Acad Sci U S A. 1990;87:5036–5040. doi: 10.1073/pnas.87.13.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verchere CB, D'Alessio DA, Palmiter RD, et al. Islet amyloid formation associated with hyperglycemia in transgenic mice with pancreatic beta cell expression of human islet amyloid polypeptide. Proc Natl Acad Sci U S A. 1996;93:3492–3496. doi: 10.1073/pnas.93.8.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hull RL, Andrikopoulos S, Verchere CB, et al. Increased dietary fat promotes islet amyloid formation and beta-cell secretory dysfunction in a transgenic mouse model of islet amyloid. Diabetes. 2003;52:372–379. doi: 10.2337/diabetes.52.2.372. [DOI] [PubMed] [Google Scholar]
- 20.Udayasankar J, Kodama K, Hull RL, et al. Amyloid formation results in recurrence of hyperglycaemia following transplantation of human IAPP transgenic mouse islets. Diabetologia. 2009;52:145–153. doi: 10.1007/s00125-008-1185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zraika S, Hull RL, Udayasankar J, et al. Oxidative stress is induced by islet amyloid formation and time-dependently mediates amyloid-induced beta cell apoptosis. Diabetologia. 2009;52:626–635. doi: 10.1007/s00125-008-1255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Subramanian SL, Hull RL, Zraika S, et al. cJUN N-terminal kinase (JNK) activation mediates islet amyloid-induced beta cell apoptosis in cultured human islet amyloid polypeptide transgenic mouse islets. Diabetologia. 2012;55:166–174. doi: 10.1007/s00125-011-2338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masters SL, Dunne A, Subramanian SL, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westwell-Roper C, Dai DL, Soukhatcheva G, et al. IL-1 blockade attenuates islet amyloid polypeptide-induced proinflammatory cytokine release and pancreatic islet graft dysfunction. Journal of immunology. 2011;187:2755–2765. doi: 10.4049/jimmunol.1002854. [DOI] [PubMed] [Google Scholar]
- 25.Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 26.Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 27.Hull RL, Shen ZP, Watts MR, et al. Long-term treatment with rosiglitazone and metformin reduces the extent of, but does not prevent, islet amyloid deposition in mice expressing the gene for human islet amyloid polypeptide. Diabetes. 2005;54:2235–2244. doi: 10.2337/diabetes.54.7.2235. [DOI] [PubMed] [Google Scholar]
- 28.Smith SA, Lister CA, Toseland CD, et al. Rosiglitazone prevents the onset of hyperglycaemia and proteinuria in the Zucker diabetic fatty rat. Diabetes Obes Metab. 2000;2:363–372. doi: 10.1046/j.1463-1326.2000.00099.x. [DOI] [PubMed] [Google Scholar]
- 29.Finegood DT, McArthur MD, Kojwang D, et al. Beta-cell mass dynamics in Zucker diabetic fatty rats. Rosiglitazone prevents the rise in net cell death. Diabetes. 2001;50:1021–1029. doi: 10.2337/diabetes.50.5.1021. [DOI] [PubMed] [Google Scholar]
- 30.Lupi R, Del Guerra S, Marselli L, et al. Rosiglitazone prevents the impairment of human islet function induced by fatty acids: evidence for a role of PPARgamma2 in the modulation of insulin secretion. Am J Physiol Endocrinol Metab. 2004;286:E560–E567. doi: 10.1152/ajpendo.00561.2002. [DOI] [PubMed] [Google Scholar]
- 31.Abaraviciene SM, Lundquist I, Salehi A. Rosiglitazone counteracts palmitate-induced beta-cell dysfunction by suppression of MAP kinase, inducible nitric oxide synthase and caspase 3 activities. Cell Mol Life Sci. 2008;65:2256–2265. doi: 10.1007/s00018-008-8100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin CY, Gurlo T, Haataja L, et al. Activation of peroxisome proliferator-activated receptor-gamma by rosiglitazone protects human islet cells against human islet amyloid polypeptide toxicity by a phosphatidylinositol 3'-kinase-dependent pathway. J Clin Endocrinol Metab. 2005;90:6678–6686. doi: 10.1210/jc.2005-0079. [DOI] [PubMed] [Google Scholar]
- 33.Mohanty P, Aljada A, Ghanim H, et al. Evidence for a potent antiinflammatory effect of rosiglitazone. J Clin Endocrinol Metab. 2004;89:2728–2735. doi: 10.1210/jc.2003-032103. [DOI] [PubMed] [Google Scholar]
- 34.Zraika S, Hull RL, Udayasankar J, et al. Identification of the amyloid-degrading enzyme neprilysin in mouse islets and potential role in islet amyloidogenesis. Diabetes. 2007;56:304–310. doi: 10.2337/db06-0430. [DOI] [PubMed] [Google Scholar]
- 35.Muxlow AM, Fowles S, Russell P. Automated high-performance liquid chromatography method for the determination of rosiglitazone in human plasma. J Chromatogr B Biomed Sci Appl. 2001;752:77–84. doi: 10.1016/s0378-4347(00)00519-3. [DOI] [PubMed] [Google Scholar]
- 36.Hsu BR, Fu SH, Ku KW, et al. Enhancing islet engraftment with rosiglitazone. Transplant Proc. 2005;37:245–247. doi: 10.1016/j.transproceed.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 37.Edvardsson U, Bergstrom M, Alexandersson M, et al. Rosiglitazone (BRL49653), a PPARgamma-selective agonist, causes peroxisome proliferator-like liver effects in obese mice. J Lipid Res. 1999;40:1177–1184. [PubMed] [Google Scholar]