Abstract
Purpose
Accumulating evidence supports the existence of breast cancer stem cells (BCSCs), which are characterized by their capacity to self-renew and divide indefinitely, and resistance to conventional therapies. The Notch pathway is important for stem cell renewal, and is a potential target for BCSC-directed therapy.
Experimental Design
Using human breast tumorgraft studies, we evaluated the impact of gamma secretase inhibitors (GSI) on the BCSC population and the efficacy of combining GSI with docetaxel treatment. The mouse experimental therapy paralleled a concurrent clinical trial in advanced breast cancer patients, designed to determine the maximally tolerated dose of the GSI, MK-0752, administered sequentially with docetaxel, and to evaluate BCSC markers in serial tumor biopsies.
Results
Treatment with GSI reduced BCSCs in MC1 and BMC-2147 tumorgrafts by inhibition of the Notch pathway. GSI enhanced the efficacy of docetaxel in preclinical studies. In the clinical trial, 30 patients with advanced breast cancer were treated with escalating doses of MK-0752 plus docetaxel. Clinically meaningful doses of both drugs were possible, with manageable toxicity and preliminary evidence of efficacy. A decrease in CD44+/CD24−, ALDH+, and MSFE were observed in tumors of patients undergoing serial biopsies.
Conclusions
These preclinical data demonstrate that pharmacological inhibition of the Notch pathway can reduce BCSCs in breast tumorgraft models. The clinical trial demonstrates feasibility of combination GSI and chemotherapy, and together these results encourage further study of Notch pathway inhibitors in combination with chemotherapy in breast cancer.
Keywords: breast cancer, Phase I clinical trial, cancer stem cells, agents with other mechanisms of action, Notch inhibitors
INTRODUCTION
Current systemic therapies for breast cancer, such as chemotherapy and hormonal therapy, are partially effective in killing cancer cells and controlling tumor growth. Yet nearly all patients with metastatic breast cancer, and a quarter of those with early disease, will relapse despite initial response. In part, this may be due to inherent limitations in existing therapies that were selected for clinical development based on their effects on proliferative and apoptotic pathways, resulting in temporary therapy-induced shrinkage of cell lines, xenografts, and human breast cancers [1]. Accumulating evidence supports the existence of breast cancer stem cells (BCSCs), which are characterized by their capacity to self-renew and divide indefinitely, and their resistance to conventional therapies [2]. BCSC theory predicts that complete eradication of BCSCs is necessary to achieve a cure, but in patients with symptomatic metastatic disease, eliminating the small population of BCSCs would do little in the short term to reduce tumor burden and preserve organ function. Combinations of conventional, tumor-shrinking cytotoxics and BCSC-directed therapies have the potential to both treat patient symptoms and prevent future relapses of breast cancer.
We and others have hypothesized that Notch inhibition will result in control of advanced breast cancer through the elimination of BCSCs [3, 4]. Preclinical data indicate that the Notch pathway is dysregulated in a variety of cancers including T-cell acute lymphoblastic leukemia/lymphoma (T-ALL) [5], breast cancer [6], colon cancer [7], and several other cancers. The inappropriate activation of Notch signaling results in signals that stimulate proliferation, restrict differentiation, and prevent apoptosis in cancer cells (recently reviewed in [8]). In normal tissues, activation of the Notch pathway results in changes in cell fate, including self-renewal of stem cells or differentiation along a particular lineage [9]. Specific to the breast, the Notch pathway was shown to be involved in the normal development of the mammary gland and in carcinogenesis [10, 11]. Notch and other highly preserved developmental pathways, including Wnt and Hedgehog, have been hypothesized to be central for the maintenance of BCSCs [12]. For example, gene expression analysis of tumorigenic, mammosphere-forming human BCSCs versus non-tumorigenic cells implicated the Notch, Phosphatidylinositol 3-kinase (PI3K), and Hedgehog signaling pathways in regulating BCSC [13, 14].
MK-0752 is an experimental oral pharmaceutical under development for the treatment of solid tumor malignancies. In vitro, MK-0752 significantly inhibits gamma secretase, an aspartic protease required for activation of the Notch receptor [15]. Cleavage of the Notch receptor by gamma-secretase is required to release the Notch intracellular domain (NICD), which then translocates to the nucleus, turning on genes involved in cell differentiation and proliferation [9, 15]. Thus, gamma secretase inhibition results in a loss of Notch function in cells [16]. MK-0752 has been evaluated in solid tumor malignancies including refractory pediatric CNS tumors and various adult solid tumors [17, 18]. The recommended phase II dose from the pediatric trial was 260 mg/m2/dose once daily using a 3 days on followed by 4 days off schedule [17]. The adult trial saw pharmacodynamic and clinical activity limited to CNS gliomas at a dose of 1800 mg weekly [18].
We hypothesized based on preclinical data implicating Notch signaling in BCSC, that a combination of cytotoxic chemotherapy to address the proliferating, non-BCSC proportion of the breast tumors, together with a GSI, would result in better disease control than either therapy alone. Since combination chemotherapy and GSI therapy had never been attempted in humans before, we planned a Phase I trial to determine a tolerable dose and schedule. Concurrent with the Phase I trial, we performed human breast tumorgraft studies to evaluate the impact of gamma secretase inhibitors on the BCSC population and the efficacy of combining GSI with docetaxel treatment. Targeted anticancer therapies are best developed in conjunction with biomarkers that can identify patients with a higher chance of benefit from the treatment, and/or measure treatment efficacy. The ideal biomarker for pharmacodynamic evaluation of cancer stem cell therapies would be able to accurately measure the proportion of stem cells within the tumor, so as to select patients with a high proportion of stem cells for treatment, and to determine on pre- and post-therapy specimens whether the proportion of stem cells within the tumor decreased with the therapy. The proof of “stem cell-ness” requires that the cell population exhibit the archetypal stem cell properties of self-renewal and generation of differentiated progeny [19-21]. These properties are evaluated in vitro by determining the ability of a single cell to generate a differentiated tissue [13]. In breast cancer models, BCSCs can be identified by their ability to form non-adherent mammospheres in serum-free media (MSFE), and to initiate tumors upon re-transplantation. Although the MSFE and re-transplantation approaches to stem cell identification are possible to execute in a preclinical setting, these are impractical to carry out in most clinical situations. Breast cancer stem cells have been characterized by the cell surface marker phenotype (CD44+/CD24−/low) [22] and aldehyde dehydrogenase activity (ALDH+) [23, 24], which identifies these assays as potential surrogate biomarkers for BCSC directed therapies in clinical applications.
In summary, although previous studies have examined the effect of GSI on BCSCs in vitro and in cell lines, none have translated this work to clinical studies. In the studies reported herein, we performed a Phase I clinical trial to establish a safe and potentially efficacious combination of a GSI in combination with docetaxel chemotherapy in patients with advanced breast cancer. We tested GSI, docetaxel, and combination therapy modeled after the clinical trial regimen on mice bearing human tumorgrafts in order to evaluate the effects of treatment on tumor volume and/or BCSCs as determined by MSFE, re-transplantation, and the surrogate markers ALDH+ and CD44+/CD24−. In addition, we obtained serial biopsies on a subset of clinical trial participants in order to preliminarily evaluate the effect of GSI plus docetaxel therapy on BCSCs using ALDH+ and CD44+/CD24− in clinical samples.
MATERIALS AND METHODS
Preclinical Studies
For preclinical evaluation of BCSC inhibitors, the Chang laboratory in collaboration with Michael T. Lewis has developed stable breast cancer-in-mice xenograft models by transplanting human breast cancer tumor biopsies into the mammary gland fat pad of immune deficient mice (herein referred to as tumorgrafts). To evaluate the effectiveness of stem cell targeted agents in altering the tumorigenic BCSC population, the mice are treated with the agents and the tumor subsequently excised for rigorous evaluation in BCSC assays. These BCSC assays include: 1) flow cytometric analysis of BCSC cell-surface markers and aldehyde dehydrogenase activity (CD44+/CD24− and ALDH+, respectively); 2) mammosphere forming efficiency (MSFE); and 3) re-transplantation to measure the presence of tumor-initiating cells (TICs).
Preparation of Tumorgrafts
All animal protocols were reviewed and approved by the Animal Protocol Review Committee at Baylor College of Medicine or The Methodist Hospital Research Institute. Tumorgrafts were initially generated by transplantation of patient breast cancer tumor biopsies or a fragment of surgical specimens into the cleared fat-pad of SCID/Beige mice (Harlan Laboratories, Indiana, IN, USA) as previously described [20, 21, 25]. As previously described, MC1 human tumors were originally derived from a pleural effusion and are estrogen and progesterone receptor negative and HER-2 negative [22, 24]. BCM-2147 breast tumorgrafts were generated by transplantation of estrogen and progesterone receptor negative and HER-2 negative human breast tumor biopsy tissue into the cleared fat-pad of SCID/Beige mice. Xenografted tumors were maintained as tumor lines by serial passage of tumor tissue into the cleared fat-pad of SCID/Beige mice, without intervening culture. Currently thirty-eight stable tumor lines representing twenty-eight independent patients have been generated and rigorously characterized for quality control, including STR analysis to document retention of original human tumor tissue across multiple generations. These tumors consistently retain the biomarker status and morphology of the original patient tumor over multiple generations. Additionally, gene expression is consistent across multiple generations as confirmed by Affymetrix microarray, Reverse Phase Protein Assay, and Sequenome analysis (manuscript submitted). These tumor lines serve as a highly quality controlled source of human breast tumors for preclinical evaluation of novel treatment regimens. During the clinical trial, the procedures for developing primary tumorgrafts were applied to primary breast tumors samples from trial participants.
In vivo drug treatment
Gamma secretase inhibitor (GSI, MRK-003) was provided by Merck & Co, Inc (N.J., USA). Docetaxel was purchased from Sigma-Aldrich. Antibodies were purchased from BD Biosciences (C.A., USA), unless otherwise indicated below.
To generate enough cells for subsequent functional analysis, tumor fragments were transplanted into the cleared fat-pad (right abdominal) of 3 to 4-week-old SCID/Beige mice. Mice were equally distributed according to tumor size into 1 of 4 treatment groups including: 1) vehicle-control, 2) chemotherapy-docetaxel (10 or 20 mg/kg), 3) GSI (100 mg/kg), or 4) combination (docetaxel plus GSI). The GSI and combination group received GSI by oral gavage on days 1-3. The chemotherapy and combination group received docetaxel by intraperitoneal injection on day 8. The control grouped received vehicle corresponding to the GSI and chemotherapy treatment schedule. Total body weight and tumor volume were measured twice weekly. Tumor volume (mm3) was calculated as length (m) × width (m) × width (m) × 0.5.
Tumors were collected from the mice and dissociated as described [21] with minor changes. Briefly, tumors were minced, dissociated with 200-250 units of Type III Collagenase (Worthington Biochemical Co, N.J., USA) per ml of media for 1-3 hours. After filtering cells through a 70 micron filter, RBC were lysed by hypotonic shock and then tumor cells were washed with ice cold Hanks-buffered saline solution (HBSS). Cells were maintained on ice for all subsequent procedures. For re-transplantation assays, dissociated tumor cells were transplanted with matrigel (1:1) into the fat-pad of SCID/Beige mice.
Flow cytometry [20]
Dissociated tumor cells (1 million cells per 0.1 ml HBSS containing 2% fetal bovine serum (HBSS+)) were labeled with fluorophore-conjugated antibodies (1:10 CD44-APC, 1:40 custom ordered CD24-PECY7 or 1:10 CD24-FITC, 1:40 H2kD-PE to remove mouse cells) for 15 minutes on ice or labeled according to manufacturers’ recommendation with the Aldefluor kit for 45 minutes at 37°C (StemCell Technologies, B.C., Canada). After the 45 minute Aldefluor incubation, those cells were also labeled with H2kD-PE for 15 minutes on ice in 0.1mL HBSS+. After washing, propidium iodide (PI, 10 μg/mL) was added and cells were analyzed or sorted with a four laser FACS AriaII (BD Biosciences, San Jose, C.A., USA). Side scatter and forward scatter were used to eliminate debris and doublets, and PI staining was evaluated to remove dead cells. The remaining tumor cells (H2kD negative) were further analyzed for CD44 and CD24 expression or aldehyde dehydrogenase activity. Data analysis was performed with FACS Diva (BD Biosciences, San Jose, C.A., USA). Patient samples were processed similarly with the following exceptions. To remove lineage cells, patient samples were labeled with a cocktail of PE-conjugated lineage antibodies. Cells were analyzed using Dako MoFlo flow cytometry and data analysis was performed with FlowJo (Ashland, O.R., USA).
Mammosphere (MS) Assay
Dissociated tumor cells were filtered through 40 micron filter, counted with the Countess Automated Cell Counter (Invitrogen, USA), and plated 60,000 cells per ml of mammosphere media in low-adherent 6- or 24-well plates (Corning Life Sciences, USA). Mammosphere media (MEGM+) contained mammary epithelial growth medium (Lonza, Walkersville, M.D.) supplemented with B27, bFGF, and EGF (final concentration 20 ng/ml bFGF and 20 ng/ml EGF). MSs were imaged and counted using GelCount imaging and software system (Oxford Optronix Ltd, OX, U.K.). For secondary MSFE, MSs were collected, dissociated with trypsin (0.05%) for 5 minutes at 37°C, filtered through a 40 micron filter, counted, and replated at 60,000 cells per ml MEGM+.
Real Time RTqPCR
RNA was extracted from snap-frozen tissue using Qiagen Bead Tissue Lyser followed by Qiagen RNeasy Mini with DNase treatment on the column (Qiagen, Valencia, CA, USA). cDNA was synthesized with iScript (Biorad). cDNA from flow sorted cells was isolated with WT-Ovation One-Direct RNA Amplification System (Nugen Technologies, San Carlos, CA, USA). Real time PCR was performed on Applied Biosystem 7900HT Fast Instrument with Applied Biosystems Primers sets, Taqman Probes and Taqman Fast Universal PCR master mix according to the manufacturer’s protocols (Applied Biosystems, Foster City, CA, USA). Data were evaluated using ABI Prism RQ Manager 1.2 (Applied Biosystems, Foster City, CA, USA). The following adjustable analysis settings were used: automatic threshold (CT), automatic outlier removal, and relative quantification (RQ) min/max confidence 99%. All data were calibrated to pooled cDNA and each sample was normalized to 18S rRNA endogenous control. At least three independent tissue samples were evaluated per experimental group.
Protein extraction and western blot
Whole cell lysates were isolated with Cell Signaling Technologies (CST) Lysis Buffer (20 mM Tris-CCl (pH 7.5), 150 mM NaCl, 1mM Na2EDTA, 1mM EGTA, 1% Triton, 25 mM sodium pyrophosphate, 1 mM beta-glycerophosphate, 1 mM Na3VO4 and 1 ug/ml leupeptin, using QiagenTM TissueLyser. Twenty micrograms of protein were loaded on 3-8% Nupage Novex Mini Gels (Invitrogen) and transferred to PVDF membrane. Membranes were then blocked with 5% non-fat milk solution in TBS with Tween-20 (0.05%,TBST) at room temperature for 1 hour, followed by 4°C overnight incubation with primary antibodies 1:1000 dilutions in 5% BSA (Notch1 clone D1E11, CST#3608; Beta-Actin clone AC-15,CST#4967). After washing with TBST, secondary horseradish peroxidase-conjugated antibodies were incubated for 1 hour in 5% BSA solution (CST 1:2000 dilution). Luminata Classico Western HRP substrate (Millipore) was used for chemiluminescent detection using Image Quant LAS 4000 (GE Healthcare) hardware and software, according to manufacturer’s instructions. Quantification of bands intensity was measured using Image Quant TL (GE Healthcare) software; it determined a parameter called volume, resulting from multiply the size of the band by the light intensity. Statistical comparison and graphics were made with GraphPad Prism 5 software, by one-side analysis of variance and Bonferroni method.
Statistical analysis- Preclinical
Comparison of categorical data i.e. numbers of tumor initiating cells among vehicle- and GSI-treated mice was done by Fischer’s exact test or χ2 test. A one-way ANOVA was used for significant differences among treatment groups in mammosphere assays with Bonferroni’s multiple comparison post-test for comparison of two specific groups. Generalized estimating equations (GEE) models from longitudinal data analysis were used to model the treatment and time effects on tumor volume. Interactions terms between treatment and time were tested; however, it was not statistically significant. Variables that did not follow a normal distribution were transformed appropriately. All preclinical statistical analyses were performed using STATA (Version 11, College Station, TX, USA).
Clinical Trial Design and Statistical Methods Patient Population
Eligible subjects included men or women with metastatic (Stage IV) breast cancer, or with locally advanced breast cancer (Stages IIIA > 10 cm, or stages IIIB and IIIC) that did not respond to first-line anthracycline-based chemotherapy. Participants could have received any number of prior anti-neoplastic regimens, including prior taxanes in the adjuvant or metastatic setting, but could not have disease that progressed on a taxane, and there must have been at least a 6 month interval since prior taxane therapy. Subjects had measurable or evaluable disease by RECIST 1.0. Participants were required to have a Zubrod Performance Status of 0-1 with at least a 3 month life expectancy, and normal hepatic, renal and hematologic laboratory studies. Baseline peripheral neuropathy could not exceed Grade 1 and participants could not have clinically significant cardiac disease. The clinical management of the patient could not include any concurrent antineoplastic therapy for breast cancer while on study. Subjects had to be capable of taking oral medications. Pregnant or nursing women were not allowed to participate in this trial because of the increased risk of fetal harm including fetal death from the chemotherapeutic agents. Subjects were informed of the investigational nature of this study and were required to provide written informed consent. The trial was approved by each local institutional review board and was performed in accordance with all institutional and federal guidelines.
Study Treatment
The study drug was MK-0752, chemical cis-4-[(4-chlorophenyl)sulfonyl]-4-(2,5-difluorophenyl) cyclohexanepropanoic acid sodium salt, provided by Merck, Sharp, and Dohme Corporation. The IND for this trial was held by the principal investigator (AFS), IND #100944. At the time of development of the clinical trial, the weekly dosing of MK-0752 had not yet been evaluated, but preliminary clinical data using the 3 days on, four days off schedule was available. Therefore, the Phase I study utilized a regimen of GSI (MK-0752) on days 1-3 in a dose determined by the dose escalation schema, followed by docetaxel on day 8 of each 21 day cycle. Docetaxel was administered in the standard 21 day dosing schedule, as a weekly docetaxel schedule would not allow sufficient time between docetaxel and MK-0752 to avoid the toxicity observed in the mice. Dose level assignment was done by the coordinating statistician (KAG) at the University of Michigan, after subject registration and confirmation of eligibility. Dose levels were: Level 1: 300 mg MK-0752 by mouth days 1-3; Level 2: 450 mg MK-0752 by mouth days 1-3; Level 3: 600 mg MK-0752 by mouth days 1-3; Level 4: 800 mg MK-0752 by mouth days 1-3. Docetaxel 80 mg/m2 IV was administered over 1 hour on day 8 of each cycle of therapy. Standard dexamethasone premedication was given at 8 mg every 12 hours for 3 doses beginning on the evening of day 7. Peg-filgrastim was administered on day 9, approximately 24 hours after docetaxel, to all participants. A cycle was defined as 21 days. Treatment was continued until disease progression, unacceptable toxicity, or symptomatic deterioration if deemed to be necessary by the patient or physician. If the patient went on to radiation or surgery of a target lesion, treatment was discontinued. The participant could decide to discontinue treatment at any time for any reason.
Toxicity monitoring
This study utilized the CTCAE (NCI Common Terminology Criteria for Adverse Events) Version 3.0 for toxicity and Serious Adverse Event reporting. Toxicity monitoring occurred at a clinic visit on day 1 of each cycle, or more often as clinically indicated. Monitoring of hematologic values and liver function tests was required on Day 1 and Day 8 of every cycle, and on Day 15 of Cycle 1.
MK-0752 doses were reduced by 1 dose level at a time in individual patients, for any Grade 3 or 4 non-hematologic toxicity that was possibly, probably, or definitely attributable to study drug, or for recurrent grade 2 toxicities attributable to study drug. If the dose would be reduced below 300 mg, the patient was required to come off study. Docetaxel dose reduction of 20% was mandated for emergence of abnormal liver function tests, Grade 2 neuropathy, and Grade 3 and 4 non-hematologic toxicities. For Grade 3 or higher neuropathy, the patient was required to come off study. Once a dose of MK-0752 or docetaxel was reduced, it was not re-escalated.
Efficacy monitoring
Patients were required to have baseline physical examination, x-rays, and CT scans as necessary to assess measurable and evaluable disease. Radiologic imaging and physical examination for tumor response was repeated every odd cycle until the time of disease progression or treatment discontinuation, whichever came first. Response was assessed using RECIST 1.0 criteria. Study patients with tumors amenable to biopsy had optional research core biopsies obtained before beginning study drug, after 1 cycle of therapy, after 3 cycles of therapy, and at the time of treatment discontinuation or completion of 6 cycles of therapy (whichever came first). For those patients with intact primary disease who proceeded to breast surgery, tissue was obtained at the time of surgery. Fresh tissue was used for fluorescence activated flow cytometry to determine the CD44+/CD24− population and the ALDH+ populations, for mouse tumorgraft transplantation, and for mammosphere studies.
Statistical Methods
The trial was monitored using a modification of the Continual Reassessment Method, called Time-to-Event CRM or TITE-CRM. The TITE-CRM method assumes a model for the time to occurrence of toxic response as a function of dose, and allows information from all patients enrolled in the trial to be employed when allocating a new patient to a dose level. Because this method is flexible in terms of the number of patients treated at each dose, subjects could be continuously recruited throughout the trial, without recruitment pauses, as long as patients were treated at a dose consistent with the current safety profile.
DLT was defined on toxicities possibly, probably, or definitely related to the study drug observed during the first cycle (first 21 days) as follows:
Non-hematologic toxicity Grade ≥3 by the NCI CTCAE version 3.0.
ANC<1000 for more than 7 days despite use of pegfilgrastim.
Platelet count <25,000 for more than 7 days, or associated with bleeding, or less than 10,000 at any time.
The target rate of acute toxicity for this trial was 20%, and this target rate defined the MTD of MK-0752 with 80 mg/m2 docetaxel. Expected rates of acute toxicity were estimated based upon previous treatment and clinical trial experience with docetaxel and clinical experience with the MK-0752 in healthy volunteer populations and limited cancer populations. The a priori estimates for dose levels 1-4 were 10%, 15%, 20%, and 30% respectively. These rates were re-evaluated throughout the conduct of this trial as treatment experience accrued. Implementation of the design was carried out using SAS Version 9.2 software (SAS institute, Cary NC) with statistical code and documentation written by the University of Michigan Comprehensive Cancer Center (UMCC) Biostatistical Unit and made publically available at (http://roadrunner.cancer.med.umich.edu/wiki/index.php/TITE-CRM).
Thirty patients were planned for estimation of the dose-toxicity function. During the treatment of the first 18 patients, accrual was limited to a maximum of 3 patients per calendar month in order to allow sufficient time for observation of toxicity. Following the completion of the acute toxicity observation period for the 18th subject, patients were then accrued at the natural rate possible for the three sites, until 30 patients were accrued and treated on protocol.
Final estimates for the probability of dose-limiting toxicity and 95% Bayesian confidence (credible) intervals for each dose level were calculated using Markov Chain Monte Carlo methods for those cases (N = 29) that either completed the acute observation period (first 21-day cycle) without dose-limiting toxicity or that experienced a DLT during that period.
RESULTS
Treatment with GSI reduced the BCSC population in patient-derived breast tumorgrafts
To evaluate the ability of GSI to target BCSCs, we performed a series of preclinical studies with mice bearing human breast tumorgrafts. Similar to the inclusion criteria for the clinical trial, these tumorgrafts were derived from advanced and/or chemotherapy-resistant breast cancer. MC1 was derived from metastatic breast tumor cells in pleural effusate and is docetaxel sensitive [22, 24, 26] while BCM-2147 was derived from docetaxel-resistant triple negative invasive breast cancer. To retain the characteristics of the original patient tumors, these tumors were maintained by passage in mice and never under cell culture conditions. Maintenance of patient biomarkers and histopathology was confirmed by immunohistochemical analysis, and genetic stability across multiple transplant generations was confirmed by Affymetrix gene expression, Reverse Phase Protein Assay, and Sequenome analysis (manuscript submitted).
Our treatment approach was to target BCSCs with GSI, while reducing tumor bulk with a standard chemotherapy. We carried out short-term studies to determine the immediate effects of GSI treatment on BCSCs (Fig 1). Mice with MC1 or BCM-2147 tumorgrafts were stratified to equally distribute tumor size amongst two treatment groups and treated as follows: i) the vehicle control group received GSI vehicle at corresponding treatment time points; ii) the GSI group received GSI (100 mg/kg) by oral gavage for 3 days. To allow for turnover of tumor cells in response to treatment in vivo, the tumors were collected 72 hours after the final treatment, dissociated to single cell suspensions for BCSC assays including flow cytometric analysis for BCSC markers, MSFE, and tumor-initiation in mice.
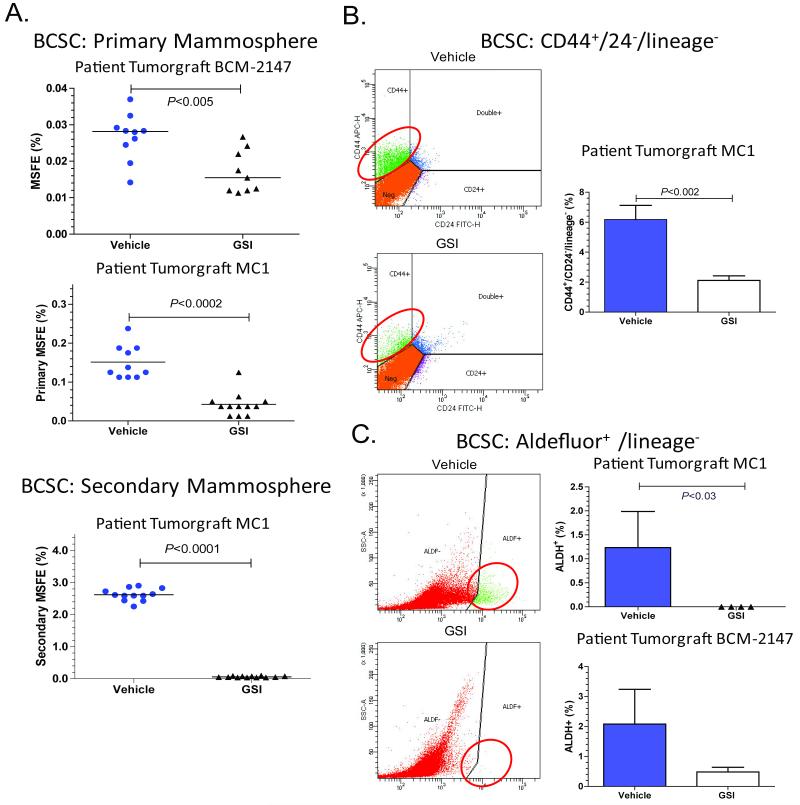
Figure 1. Treatment with GSI reduced the BCSC population in patient-derived breast tumorgrafts.
Mice with tumors were treated for 3 days with GSI (100 mg/kg) or vehicle, and then tumors were collected 72 hours after the last treatment. A. Treatment with GSI reduced mammosphere forming efficiency (MSFE). Tumors were treated in vivo and subsequently plated under MS conditions. To determine secondary MSFE, primary MS were collected, dissociated, and replated under MS conditions. The Mann-Whitney two-tailed t-test was used to calculate p-values. Top figure, each symbol represents an individual BCM-2147 tumor. Middle panel, MC1 tumors cells were pooled for each treatment group. Each symbol represents a well of MS. Bottom panel, MC1 secondary MSFE. B and C. Treatment with GSI reduced BCSC populations according to flow cytometric analysis of BCSC markers, CD44+/CD24− and ALDH+. Treated tumors were dissociated, incubated with antibodies and/or Aldefluor reagents and analyzed by flow cytometry. The Mann-Whitney two-tailed t-test was used to calculate p-values. B. Left column contains representative flow cytometry images. Right column, MC1 CD44/CD24 data (Vehicle n=8, GSI n= 6). C. Left column contains representative flow cytometry images. Top graph MC1 ALDH+ data (Vehicle n=3, GSI n=4). Bottom graph, BCM-2147 ALDH+ data (Vehicle n=9, GSI n=10).
Consistent with decreased BCSCs, primary and secondary MSFE were significantly decreased in GSI-treated tumors compared to vehicle-treated tumors (Fig. 1A). In MC1 tumorgrafts, treatment with GSI reduced the CD44+/CD24− and ALDH+ subpopulations 72h post treatment as measured by flow cytometry (Figs. 1B and 1C). With BCM-2147, GSI treatment reduced the ALDH+ population compared to vehicle (Fig. 1B). BCM-2147 does not have a CD44-positive population for evaluation, therefore only ALDH was evaluated by flow cytometry. To determine whether treatment had reduced BCSCs, we re-transplanted the MC1 tumor cells from each group into mice and monitored tumor development (Table 1). The vehicle-treated tumor cells regenerated tumors with 50-55% tumor incidence. Importantly, there were no regenerated tumors in the GSI-treated group (P<0.011), indicating GSI reduced tumor cells capable of tumor initiation.
Table 1. Re-Transplantation of Treated MC1 Tumor Cells.
| 15,000 cells |
Tumor Incidence Decreased |
45,000 Cells |
Tumor Incidence Decreased |
Total | |
|---|---|---|---|---|---|
| Vehicle | 7/14 (50%) | 5/9 (55%) | 12/23 (52%) | ||
| GSI | 0/14 (0%) | P<0.006 | 0/10 (0%) | P<0.011 | 0/24 (0%) |
MC1 tumors from vehicle- and GSI-treated mice were dissociated and pooled. 15,000 or 45,000 cells from each group were transplanted into the mammary gland fat pad of 4-6 week old mice. Tumor incidence is reported at 10 weeks post-transplantation. Data was analyzed by pairwise comparisons with Fischer’s Exact Test.
GSI inhibited the Notch pathway
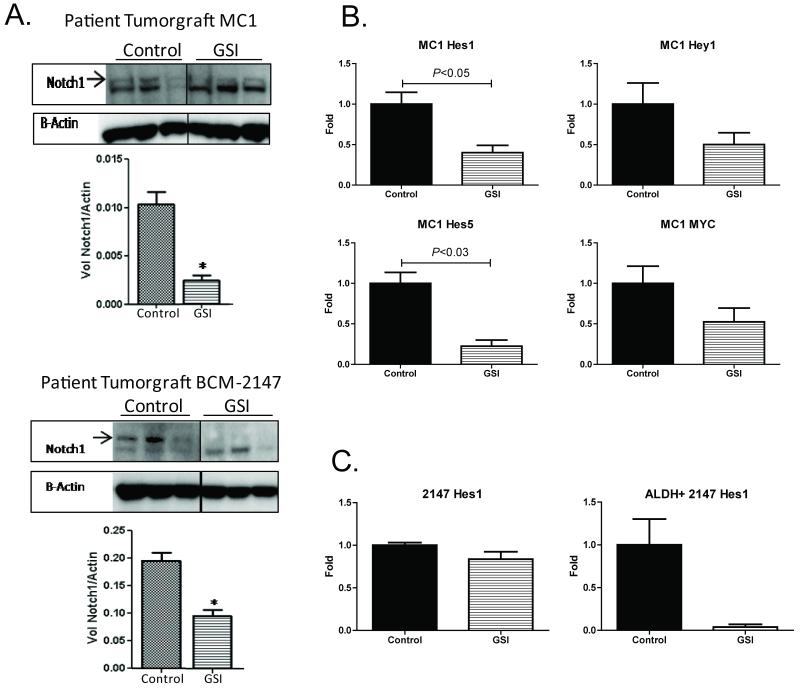
To ensure that GSI was inhibiting the Notch pathway in our tumorgrafts, we evaluated expression of the Notch intracellular domain (NICD) and downstream targets of the Notch pathway in response to GSI treatment in MC1 and BCM-2147 tumors (Fig 2). Quantification of western blot data confirmed downregulation of NICD with GSI treatment in MC1 and BCM-2147 tumors (Fig 2A). In MC1 tumors, Hes1, Hey1, Hes5, and myc were reduced to 40%, 50%, 25%, and 40% respectively, in the GSI-treated tumors relative to controls (Fig 2B). In BCM-2147 tumors, Hes1 and other Notch targets were not significantly decreased in the whole tumor (Fig 2B and data not shown); thus, we collected treated tumors and flow sorted for the ALDH+ BCSC population. Hes1 expression was markedly reduced to less than 5% of control within the ALDH+ population, further supporting the role of the Notch pathway in BCSCs. Because the ALDH+ population is less than 2% of the whole tumor, the change in expression was undetectable in the whole tumor. Collectively, these data indicate that GSI inhibited activation of NICD and reduced Notch pathway targets.
Figure 2. GSI inhibited the Notch pathway.
A. Western blot analysis confirmed inhibition the Notch Intracellular Domain (NICD) cleavage. Western blots were incubated with primary antibody against Notch1 or Beta-Actin. Cropped versions of the blots are shown. Image Quant LAS 4000 (GE Healthcare) hardware and software were used to capture multiple digital images over time so that band intensity could be quantitated in the linear dynamic range. The bar graph shows quantification of band intensity to determine NICD relative to Beta-Actin expression. Top panel, MC1; bottom panel, BCM-2147. B. GSI suppressed Notch pathway targets in MC1 tumors. Mice were treated with GSI (n=4) or vehicle (n=4) for two days before collection of tumor tissue on the third day. RTqPCR was performed using ABI Taqman Probes and Primer sets on the ABI 7900HT FAST instrument. Relative gene expression was calculated using the Relative Quantification (RQ) Method, comparing gene expression to 18S ribosomal RNA for each gene. Data is presented relative to the vehicle control. C. GSI suppressed the Notch pathway in the BCSC population. Left panel, relative Hes1 expression in whole tumor (n=3 per group). Right panel, relative Hes1 expression in sorted ALDH+ cells from BCM-2147 tumors (n=2 per group).
Treatment with GSI enhanced efficacy of docetaxel and reduced BCSCs
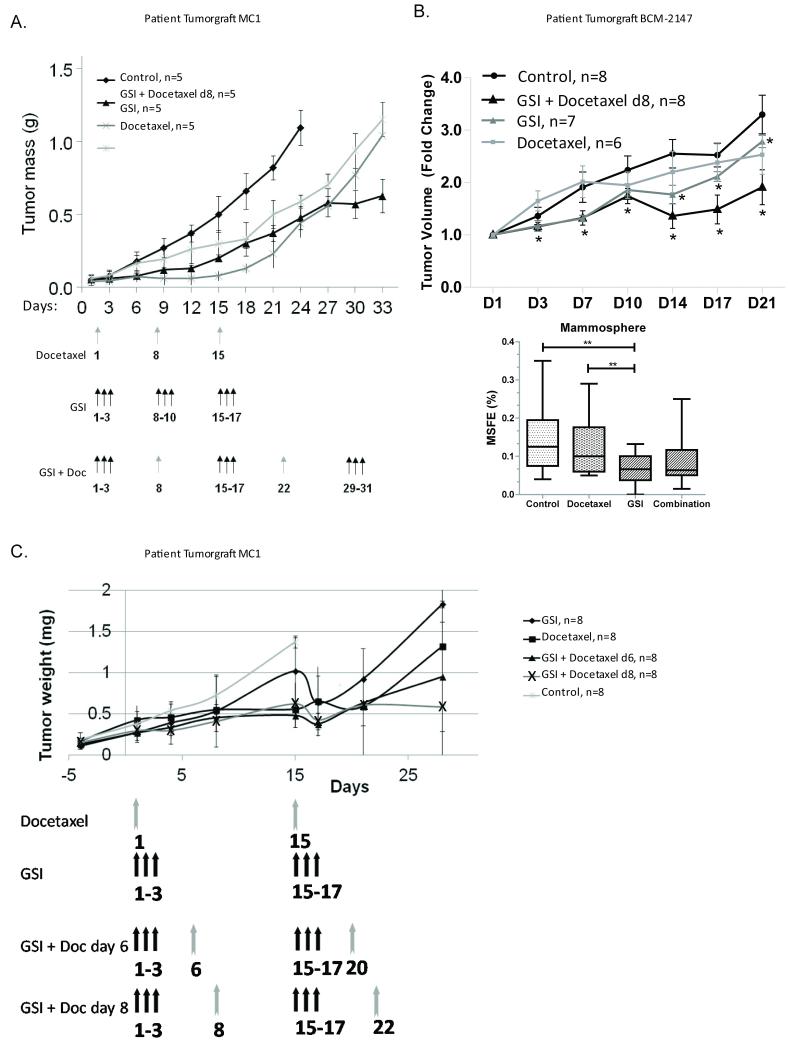
To evaluate in a preclinical setting the GSI and docetaxel treatment regimen used in the Phase I clinical trial, mice bearing MC1 or BCM-2147 tumors were stratified by tumor size to treatment groups: docetaxel (10 mg/kg), GSI (100 mg/kg), or a combination of both and compared to animals treated with vehicle alone. The doses were chosen based on toxicity and efficacy in our animal model. Single agent treatment with either GSI or docetaxel delayed MC1 tumor progression, but eventually, after treatment was stopped, tumors reached sizes equivalent to control mice (Fig 3A). As expected, treatment with docetaxel did not significantly reduce tumor volume in docetaxel-resistant BCM-2147, while GSI alone and GSI with docetaxel reduced tumor growth compared to vehicle (Fig 3B). GSI alone also reduced mammosphere forming efficiency (MSFE) compared to vehicle- and docetaxel-treated tumors (Fig 3B). The combination of GSI and docetaxel decreased tumor size more than the single agents in both MC1 and BCM-2147 tumors (Fig 3). Regimens proposed to be used in human patients were used to assess efficacy of treatment. The difference in tumor growth between mice treated with docetaxel alone and combination of GSI and docetaxel at either 3- or 5-day intervals is significant (Fig 3C; P< 0.01 and 0.05, respectively). GSI alone slows tumor growth significantly compared to control (P<0.03). There was no significant difference between administration of docetaxel 3 days after GSI compared to 5 days after GSI (P<0.09) Administration of docetaxel earlier than 3 days post GSI or concomitant administration of docetaxel and GSI showed significant toxicity in mice, consisting of diarrhea, dehydration and severe weight loss. In summary, GSI treatment reduced BCSC and enhanced the efficacy of docetaxel.
Figure 3. Treatment with GSI enhanced efficacy of docetaxel and reduced BCSCs.
Mice bearing MC1 or BCM-2147 tumors were stratified by tumor size to treatment groups: docetaxel (10 mg/kg, gray arrows), GSI (100 mg/kg, black arrows), or a combination of both and compared to animals treated with vehicle alone. The tumor growth in different groups of treatment (5 mice per group in A, 6-8 mice per group in B, and 8 mice per group in C) is shown as tumor weight in grams over time or tumor volume fold change. A. The combination of GSI and docetaxel prevented tumor growth more than the single agents. B. GSI alone and with docetaxel reduced tumor growth compared to vehicle [P<0.030 (coefficient = −0.132, 95% confidence interval = −0.252, −0.012); P<0.008 (coefficient = −0.287, 95% confidence interval = −0.498, −0.075, respectively)]. The * indicate statistical differences compared to vehicle. Bottom panel, GSI alone reduced mammosphere forming efficiency (MSFE) compared to vehicle- and docetaxel-treated tumors. Tumors were treated in vivo and subsequently plated under MS conditions. Each symbol represents a well of MS. Square root transformed data were analyzed by with One-Way ANOVA with Bonferonni multiple comparison post test (P<0.05). C. Regimens proposed to be used in human patients were used to assess efficacy of treatment. The difference in tumor growth between mice treated with docetaxel alone and combination of GSI and docetaxel at 3 or 5 days interval is significant (P< 0.01 and 0.05 respectively). GSI alone slows tumor growth significantly compared to control (P<0.03). There was no significant difference between administration of docetaxel 3 days after GSI compared to 5 days after GSI (P<0.09).
Phase IB Clinical Trial of the GSI, MK-0752, with Docetaxel
The clinical trial was designed with the overall goals of determining a safe and potentially efficacious dose and schedule of combination MK0752 with docetaxel in patients with locally advanced or metastatic breast cancer, and to explore for an effect on BCSCs. At the time of study initiation, MK-0752 was in clinical development for the treatment of Alzheimer’s disease and other conditions, and therefore its toxicology and dose limiting toxicities in a non-cancer patient population were known. Limited single agent Phase I data in cancer patients were available at the time of study initiation.
The trial was initiated and sponsored by the investigators, and supported financially and with drug supply by Merck, Sharp, and Dohme. Patients were recruited from three institutions: the University of Michigan, Baylor College of Medicine, and the Dana Farber Cancer Institute and its affiliate hospitals. The primary objective of this Phase Ib clinical trial was to determine the maximally tolerated dose (MTD) of MK-0752 administered in a sequential combination regimen with fixed dose docetaxel, in patients with locally advanced or metastatic breast cancer. Secondary objectives were to describe the safety profile of the combination therapy, to determine the effect of this treatment regimen on the proportion of BCSCs and MSFE in the subset of patients with tumors amenable to biopsy pre- and post-therapy, and to seek preliminary evidence of anti-tumor activity of the combination.
30 patients were enrolled on the study and received escalating doses of MK-0752. Patient disease characteristics are presented in Table 2. All 30 patients were evaluable for toxicity. Specific dose limiting toxicities (DLT) included pneumonitis, hand-foot syndrome, LFT elevation, and diarrhea. Participant-level information on dose level, response, and DLTs experienced is presented in the Supplementary Material, Table 1. A single participant enrolled at dose level #1, died following Cycle #2 of therapy. This individual had evidence of lymphangitic spread of cancer at the time of study enrollment, and developed clinical worsening during Cycle #2 that led to discontinuation of treatment. Although arguably this event could have been attributed to disease progression, it was conservatively coded as Grade 5 toxicity to combination MK-0752 and docetaxel, especially as docetaxel is known to cause pneumonitis. No other events of this nature were observed during the remainder of the trial. The final estimates and confidence intervals for the probability of dose limiting toxicity at each dose level are summarized in the Supplementary Materials, Table 2, and toxicities and their grades are presented in Table 3. Dose level 3 was selected for further study based on its tolerability in combination with docetaxel in this schedule.
Table 2. Patient Characteristics (N = 30).
| No. of Patients | Percentage | |
|---|---|---|
|
| ||
| Number eligible | 30 | 100 |
|
| ||
| Age, years | ||
| 34-49 | 11 | 37 |
| 50-64 | 14 | 46 |
| 65-88 | 5 | 17 |
|
| ||
| Metastatic sites (multiple sites possible) | ||
| Bone | 12 | 46 |
| Lung/Pleura | 16 | 62 |
| Liver | 13 | 50 |
| Lymph nodes | 11 | 42 |
| Skin | 5 | 19 |
| Other | 7 | 27 |
|
| ||
| Number of metastatic sites | ||
| 0 | 4 | 13 |
| 1 | 6 | 20 |
| 2 | 6 | 20 |
| ≥ 3 | 14 | 47 |
|
| ||
| Tumor hormone receptor status | ||
| ER positive and/or PgR positive | 18 | 60 |
| ER negative and PgR negative | 12 | 40 |
|
| ||
| HER-2/neu status | ||
| Negative | 28 | 93 |
| Positive | 2 | 7 |
|
| ||
| Prior therapy for metastatic disease (N=26) | ||
| None | 3 | 12 |
| CT | 7 | 27 |
| HT | 0 | 0 |
| CT/HT | 16 | 61 |
|
| ||
| Number of prior metastatic CT regimens (N=26) | ||
| 0 | 3 | 11 |
| 1 | 2 | 8 |
| 2+ | 21 | 81 |
ER = estrogen receptor
PgR = progesterone receptor
CT = chemotherapy
HT = hormonal therapy
Table 3. Toxicities experienced by patients in the clinical trial.
| Number of Patients with Adverse Events Possibly, Probably, or Likely Related to study therapy (Maximum AE Grade Per Patient ) |
|||||
|---|---|---|---|---|---|
| CTCAE Group | 1 | 2 | 3 | 4 | 5 |
| Gastrointestinal | 11 | 6 | 2 | 0 | 0 |
| Pain | 7 | 2 | 0 | 0 | 0 |
| Metabolic/Laboratory | 9 | 6 | 3 | 0 | 0 |
| Allergy/Immunology | 4 | 0 | 0 | 0 | 0 |
| Dermatology/Skin | 7 | 6 | 2 | 0 | 0 |
| Constitutional Symptoms | 9 | 12 | 0 | 0 | 0 |
| Pulmonary/Upper Respiratory | 4 | 2 | 2 | 0 | 1* |
| Lymphatics | 1 | 1 | 0 | 0 | 0 |
| Blood/Bone marrow | 7 | 6 | 1 | 0 | 0 |
| Musculoskeletal/Soft Tissue | 1 | 1 | 0 | 0 | 0 |
| Infection | 0 | 2 | 1 | 0 | 0 |
| Ocular/Visual | 3 | 1 | 0 | 0 | 0 |
| Neurology | 3 | 2 | 1 | 0 | 0 |
| Total | 66 | 46 | 12 | 0 | 1 |
The single observed Grade 5 pulmonary toxicity was seen in a patient who also had progressive lymphangitic spread of cancer. It was coded “possibly related” to study therapy by the treating investigator.
26/30 participants entered the study with measurable disease by RECIST criteria. However, two of these individuals were not evaluable for response due to DLT in the first cycle that led to a change in treatment prior to re-assessment of disease. Of the 24 participants evaluable for response, we observed 11 PR, 9 SD, and 3 PD (Table 1, supplementary data). The four individuals with non-measurable disease had evaluable disease in skin, bone, and/or pleura. These efficacy data should be interpreted with caution, as all participants received a known active therapy with docetaxel, and therefore these responses cannot be solely attributed to study drug.
Treatment with MK0752 plus docetaxel reduced BCSCs in some patient tumors after multiple rounds of treatment
Our approach for the clinical trial was to reduce tumor bulk with docetaxel and concurrently target BCSCs with GSI. Tumor biopsies were optional for participants on study, and were performed most frequently in those individuals with locally advanced tumors for whom surgical management following initial chemotherapy treatment was planned. Biopsies were attempted in 10/30 participants at baseline, and 7/10 of the baseline biopsies contained tissue adequate for the BCSC assays (Supplementary Table 1). Repeat biopsies were obtained in 6/30 participants following cycles 1, 3, and at the time of treatment discontinuation or definitive breast surgery. One individual with adequate baseline tissue refused the repeat biopsies.
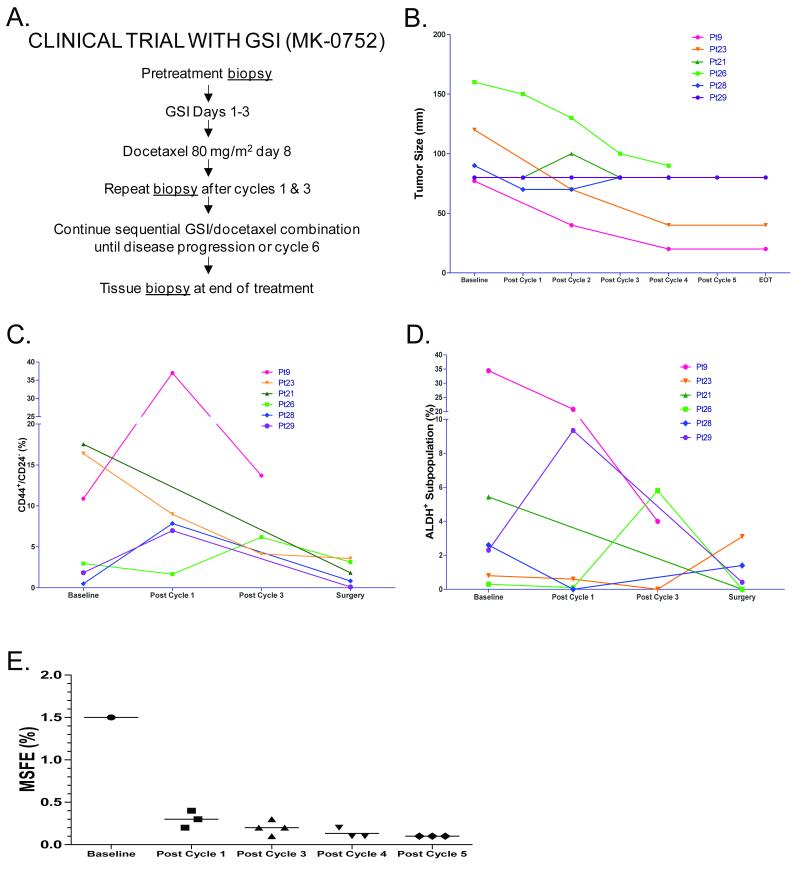
The BCSC assay results for participants (n=6) who successfully underwent serial repeat biopsies are reported here (Fig 4). Of these 6 individuals, 4 had PR and 2 had SD with all but one receiving the highest dose level 3 at 600mg (Fig 4B and Supplementary Table 1). CD44+/CD24− initially increased in 3/6 patients from baseline to post-cycle, but subsequently decreased from baseline to surgery in 3/5 patients, consistent with the stem cell hypothesis. ALDH+ increased in 1/6 patients from baseline to post-cycle 1 and, again, decreased from baseline to surgery in 3/5. The increase of CD44+/CD24− from baseline with subsequent decrease is consistent with the concept that Notch inhibition would cause BCSCs to transition into a proliferative state that is more susceptible to cytotoxic therapy, and indicates that multiple treatment cycles are necessary to effectively reduce BCSCs. A decrease in CD44+/CD24−, ALDH+, and MSFE (Fig 4C, 4D, and 4E) was observed from baseline to end of treatment even within this small subset of patients, consistent with the ability of GSI to reduce BCSCs as seen in our preclinical human breast tumorgraft experiments. Multiple cycles were required to see the cumulative benefits of this treatment regimen in reducing BCSC and tumor burden.
Figure 4. Treatment in human clinical trials reduced BCSCs in some patients after multiple rounds of treatment.
A. Clinical Trial Design. B. Tumor size of patients that received biopsies for BCSC. Each patient is represented in the same color line across all graphs in B, C, and D. C. Flow cytometry data for CD44+/CD24− at baseline, post cycle 1, post cycle 3, and end of treatment (EOT). Note pleural effusate was collected for Pt#9 rather than biopsies, and the line representing Pt#9 is discontinuous based on the break in the y-axis to accommodate larger numbers. D. Flow cytometry data for aldehyde dehydrogenase activity (ALDH+). Note: pleural effusate was collected for Pt#9 rather than biopsies, and the line representing Pt#9 is discontinuous based on the break in the y-axis to accommodate larger numbers. E. Patient MSFE decreased after GSI treatment in combination with docetaxel. Pt#9 tumor cells were collected from pleural effusate at baseline and after 4 treatment cycles. Lineage negative tumor cells (<1% of total cells) were sorted into non-adherent plates with mammosphere media for evaluation of MSFE. Each point represents an individual well. MSFE was not evaluated after cycle 2 or cycle 6.
DISCUSSION
Our preclinical data demonstrate that pharmacological inhibition of the Notch signaling pathway can reduce human BCSCs in breast tumorgraft models and enhance the efficacy of docetaxel. The effects of GSI treatment were evident in multiple measures of BCSC capacity including: 1) reduction of the cell populations with CD44+/CD24− phenotype and/or aldehyde dehydrogenase activity, 2) reduction of mammosphere forming efficiency, and 3) absence of tumor regeneration according to re-transplantation studies. Based on the controversies surrounding the various CSC markers and assays, our approach has been to carry out as many of the analyses as possible within in the confines of limited tumor cell number from patient biopsies. The reduction of BCSC capacity in the samples from patients in the Phase Ib clinical trial warrants expansion to a larger cohort of patients to confirm this effect, and to identify the patient population that will receive the most benefit from BCSC-targeted therapies.
Our data indicate that using traditional chemotherapy to reduce the tumor bulk in combination with BCSC-targeted therapy is a viable treatment strategy [3, 6]. Our current approach utilized Notch pathway inhibition to target BCSCs. Emerging evidence further supports the hypothesis that Notch pathway inhibition reduces BCSC capacity in breast tumors. Both DAPT (N-[N-(3,5-difluorophenacetyl-L-alanyl)]-S-phenylglycine t-butyl ester, another gamma secretase inhibitor) and Notch 4-neutralizing antibody reduce MSFE in DCIS [3, 11]. In UM-PE13 metastatic breast tumorgraft from pleural effusate, an antibody targeting the Notch ligand, Dll4 (delta-like ligand) in combination with high dose paclitaxel reduces cancer stem cell frequency [27]. Notch inhibition reduces BCSC capacity of MCF7 and MDA-MB-231 cell lines [28]. Finally, the data presented here provide robust collaborative evidence that pharmacological inhibition of Notch signaling in both primary human and clinical samples reduces BCSCs.
Despite strong evidence that BCSC are responsive to Notch pathway inhibition, a residual BCSC subpopulation remains unaffected by inhibition of the Notch signaling pathway in most tumors in our tumorgraft studies, mandating the use of additional BCSC-targeted inhibitors to eradicate breast tumors. Molecular analysis of the BCSCs that remain after GSI treatment should identify pathways of treatment resistance, pinpointing additional pathways that regulate BCSCs and potential mechanisms for therapeutic intervention. Targeting additional signaling pathways with a “cocktail” of BCSC inhibitors may improve treatment by inhibiting survival and self-renewal of all BCSC populations, ultimately preventing tumor recurrence and metastasis and thus eradicating the disease.
The clinical trial described here is the first of its kind combining a gamma secretase inhibitor with chemotherapy. We hypothesized that a successful BCSC-directed therapy in the advanced disease setting would require both cytotoxic therapy to shrink the tumor, as well as BCSC-directed therapy to deplete the BCSC component and reduce subsequent repopulation of the tumor mass. The first step in the development of such a strategy is to define a safe combination for further testing. The tested clinical regimen demonstrated a favorable safety profile with positive preliminary clinical outcomes. Full dosing of docetaxel was possible, and doses of MK0752 associated with pharmacodynamic effect were possible. The response rate was good (though confounded by expected effect of docetaxel), but perhaps more interestingly, some patients experienced very long disease stabilization (Supplementary Material, Table 1). Pharmacodynamic studies of tumor biopsies in a subset of participants suggested an effect of combined therapy on the tumor stem cell component in the breast tumors.
Where do we go from here? At the current time, patients who achieve excellent tumor regressions with chemotherapy in the metastatic setting are often continued on the chemotherapy, with all of its inherent toxicities, until the chemotherapy fails to control their disease any longer. Indeed, a recent meta-analysis found that longer treatment results in significantly better progression free survival, and marginally better overall survival [29]. Alternatively, patients and/or their doctors may advocate for a chemotherapy “holiday” to avoid the toxicity of therapy for some number of weeks or months before their disease progresses again. If stem cell targeted therapies are effective in reducing the tumor initiating cell population, then the addition of such therapies to standard chemotherapy has the potential to delay or prevent disease progression after chemotherapy is stopped, with low toxicity. This hypothesis requires further testing in the clinic in a randomized clinical trial. We propose that a randomized Phase II trial of gamma secretase inhibitors in combination with docetaxel, to include both efficacy and pharmacodynamic endpoints, is warranted to test this hypothesis.
Supplementary Material
TRANSLATIONAL RELEVANCE.
This manuscript describes the combined preclinical, clinical, and clinical-translational studies of gamma secretase inhibitors, which inhibit the Notch pathway, in combination with chemotherapy in human breast cancer. Basic laboratory work previously identified a therapy-resistant and tumor-initiating population of breast cancer cells within heterogeneous breast tumors, coined “breast cancer stem cells”. Elimination of breast cancer stem cells has the potential to reduce the incidence of breast cancer relapse. Mechanistic studies suggest the Notch pathway to be a potential target for this cell population. Herein we describe the effect of gamma secretase inhibitors plus docetaxel on the cancer stem cell population in human breast tumorgrafts, a model with significant translational relevance. These preclinical studies were extended into a Phase Ib clinical trial of the gamma secretase inhibitor, MK-0752, in combination with docetaxel, in patients with advanced breast cancer. The clinical trial included serial biopsies to complete the bench-to-bedside-to-bench cycle.
ACKNOWLEDGEMENTS
We would like to thank Melissa Mietzel for coordinating the multisite clinical trial. We also thank Dr. Michael Lewis for his intellectual input and Dr. Lisardo Ugidos de la Varga, Wei Qian and Dr. Yi Liu for their research support.
Grant Support
This work was supported by Merck, Sharpe, and Dohme (Grant: IISP #31841 (University of Michigan: AF Schott, PI)). Preclinical work was also supported by the National Institute of Health (1R01CA138197 and U54 1 U54CA149196-01).
Footnotes
Potential conflicts of interest: Dr. Krop serves as a consultant to Pfizer at an amount <$10,000 per year; Dr. Wicha is a co-founder and holds equity in Oncomed Pharmaceuticals, which is also testing Notch inhibitory antibodies.
REFERENCES
- 1.Chang JC, Wooten EC, Tsimelzon A, Hilsenbeck SG, Gutierrez MC, Tham YL, et al. Patterns of resistance and incomplete response to docetaxel by gene expression profiling in breast cancer patients. Journal of Clinical Oncology. 2005;23(6):1169–1177. doi: 10.1200/JCO.2005.03.156. [DOI] [PubMed] [Google Scholar]
- 2.Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004;23(43):7274–7282. doi: 10.1038/sj.onc.1207947. [DOI] [PubMed] [Google Scholar]
- 3.Farnie G, Clarke RB. Mammary stem cells and breast cancer--role of Notch signalling. Stem Cell Reviews. 2007;3(2):169–175. doi: 10.1007/s12015-007-0023-5. [DOI] [PubMed] [Google Scholar]
- 4.Song LL, Miele L. Cancer stem cells--an old idea that’s new again: implications for the diagnosis and treatment of breast cancer. Expert Opinion on Biological Therapy. 2007;7(4):431–438. doi: 10.1517/14712598.7.4.431. [DOI] [PubMed] [Google Scholar]
- 5.Weng AP, Ferrando AA, Lee W, Morris JPt, Silverman LB, Sanchez-Irizarry C, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306(5694):269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 6.Pece S, Serresi M, Santolini E, Capra M, Hulleman E, Galimberti V, et al. Loss of negative regulation by Numb over Notch is relevant to human breast carcinogenesis. J Cell Biol. 2004;167(2):215–221. doi: 10.1083/jcb.200406140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435(7044):959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 8.Rizzo P, Osipo C, Foreman K, Golde T, Osborne B, Miele L. Rational targeting of Notch signaling in cancer. Oncogene. 2008;27(38):5124–5131. doi: 10.1038/onc.2008.226. [DOI] [PubMed] [Google Scholar]
- 9.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284(5415):770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 10.Smith GH, Gallahan D, Diella F, Jhappan C, Merlino G, Callahan R. Constitutive expression of a truncated INT3 gene in mouse mammary epithelium impairs differentiation and functional development. Cell Growth Differ. 1995;6(5):563–577. [PubMed] [Google Scholar]
- 11.Farnie G, Clarke RB, Spence K, Pinnock N, Brennan K, Anderson NG, et al. Novel cell culture technique for primary ductal carcinoma in situ: role of Notch and epidermal growth factor receptor signaling pathways. J Natl Cancer Inst. 2007;99(8):616–627. doi: 10.1093/jnci/djk133. [DOI] [PubMed] [Google Scholar]
- 12.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434(7035):843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 13.Mumm JS, Kopan R. Notch signaling: from the outside in. Dev Biol. 2000;228(2):151–165. doi: 10.1006/dbio.2000.9960. [DOI] [PubMed] [Google Scholar]
- 14.Bolos V, Grego-Bessa J, de la Pompa JL. Notch signaling in development and cancer. Endocr Rev. 2007;28(3):339–363. doi: 10.1210/er.2006-0046. [DOI] [PubMed] [Google Scholar]
- 15.Kopan R, Ilagan MXG. Gamma-secretase: proteasome of the membrane? Nature Reviews Molecular Cell Biology. 2004;5(6):499–504. doi: 10.1038/nrm1406. [DOI] [PubMed] [Google Scholar]
- 16.Miele L. Notch signaling. Clinical Cancer Research. 2006;12(4):1074–1079. doi: 10.1158/1078-0432.CCR-05-2570. [DOI] [PubMed] [Google Scholar]
- 17.Fouladi M, Stewart CF, Olson J, Wagner LM, Onar-Thomas A, Kocak M, et al. Phase I trial of MK-0752 in children with refractory CNS malignancies: a pediatric brain tumor consortium study. Journal of Clinical Oncology. 2011;29(26):3529–3534. doi: 10.1200/JCO.2011.35.7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krop I, Demuth T, Guthrie T, Wen PY, Mason WP, Chinnaiyan P, et al. Phase I Pharmacologic and Pharmacodynamic Study of the Gamma Secretase (Notch) Inhibitor MK-0752 in Adult Patients With Advanced Solid Tumors. Journal of Clinical Oncology. 2012;30(19):2307–2313. doi: 10.1200/JCO.2011.39.1540. [DOI] [PubMed] [Google Scholar]
- 19.Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65(13):5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100(9):672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 21.Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A. 2009;106(33):13820–13825. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dontu G, Wicha MS. Survival of mammary stem cells in suspension culture: implications for stem cell biology and neoplasia. J Mammary Gland Biol Neoplasia. 2005;10(1):75–86. doi: 10.1007/s10911-005-2542-5. [DOI] [PubMed] [Google Scholar]
- 24.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1(5):555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeOme KB, Medina D. A new approach to mammary tumorigenesis in rodents. Cancer. 1969;24(6):1255–1258. doi: 10.1002/1097-0142(196912)24:6<1255::aid-cncr2820240632>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 26.Dave B, Landis MD, Tweardy DJ, Chang JC. Selective small molecule Stat3 inhibitor reduces breast cancer tumor-initiating cells and improves recurrence free survival in a human xenograft model. PLoS One. 2012 doi: 10.1371/journal.pone.0030207. accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoey T, Yen WC, Axelrod F, Basi J, Donigian L, Dylla S, et al. DLL4 blockade inhibits tumor growth and reduces tumor-initiating cell frequency. Cell Stem Cell. 2009;5(2):168–177. doi: 10.1016/j.stem.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 28.Harrison H, Farnie G, Howell SJ, Rock RE, Stylianou S, Brennan KR, et al. Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Res. 2010;70(2):709–718. doi: 10.1158/0008-5472.CAN-09-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gennari A, Stockler M, Puntoni M, Sormani M, Nanni O, Amadori D, et al. Duration of Chemotherapy for Metastatic Breast Cancer: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Journal of Clinical Oncology. 2011;29:2144–2149. doi: 10.1200/JCO.2010.31.5374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.