Abstract
Objective
To investigate the relationship between anisotropic solute diffusion properties and tissue morphology in porcine temporomandibular joint (TMJ) discs.
Design
TMJ discs from eleven pigs aged 6–8 months were divided into five regions: anterior, intermediate, posterior, lateral, and medial. The transport properties and tissue morphology were investigated in three orthogonal orientations: anteroposterior, mediolateral, and superoinferior. The anisotropic diffusivity of fluorescein (332 Da) in the right discs was determined by the fluorescence recovery after photobleaching (FRAP) protocols. The tissue morphology in the left discs was quantified by scanning electron microscopy.
Results
The diffusivities of fluorescein in the TMJ disc were significantly anisotropic, except for the anterior region. In the medial, intermediate, and lateral regions, the diffusion along the fiber orientation (i.e., anteroposterior direction) was significantly faster than the diffusion in mediolateral and superoinferior directions. In the posterior region, the diffusion along the fiber orientation (i.e., mediolateral direction) was significantly faster than the diffusion in anteroposterior and superoinferior directions. The diffusion in the anterior region was mostly isotropic with the lowest degree of diffusion anisotropy, as well as collagen fiber alignment, likely due to the multi-directional fiber arrangement. The anterior region had the highest mean diffusivity [65.6 (49.3–81.8) μm2/s] in the disc, likely due to its high water content. The overall average diffusivity of fluorescein across the TMJ disc was 57.0 (43.0–71.0) μm2/s.
Conclusions
The solute diffusion in porcine TMJ discs was strongly anisotropic and inhomogeneous, which associated with tissue structure (i.e., collagen fiber alignment) and composition (e.g., water content).
Keywords: Temporomandibular joint (TMJ) disc, Anisotropic diffusion tensor, Fractional anisotropy, Fluorescence recovery after photobleaching (FRAP), Collagen fiber orientation, Coherency coefficient
INTRODUCTION
The temporomandibular joint (TMJ) is a synovial, bilateral joint with unique morphology and function1. The TMJ disc, a fibrocartilaginous tissue, is a major component of jaw function by providing stress distribution and lubrication in the joint2–3. Disc derangement (e.g., dislocation of the disc) is a common clinical finding in patients with TMJ disorders (affecting more than 35 million Americans with an annual health care cost of $4 billion)4–5. It has been suggested that degenerative processes predispose the disc to displacement and result in significant changes in disc morphology, biochemistry, function, and material properties6–7.
The TMJ disc has a distinctive extracellular matrix (ECM) composition and structure when compared to hyaline cartilage and other fibrocartilaginous tissues [e.g., the intervertebral disc (IVD)]. The TMJ disc tissue is composed primarily of water with a significant amount of collagen (mainly type I) and a very small amount of proteoglycan8–12. The human TMJ disc is a large avascular structure being wider mediolaterally than anteroposteriorly (approximately 19×13 mm)13. The nutrients required by disc cells for maintaining healthy matrix are supplied by synovial fluid at the margins of the disc as well as through nearby blood vessels at the connection to the posterior bilaminar zone13–14. The balance between the rate of nutrient transport through the matrix and the rate of consumption by disc cells establishes a concentration gradient across the disc. The concentration levels of essential nutrients, such as oxygen and glucose, can profoundly affect TMJ disc cell viability, matrix synthesis, and response to inflammatory factors15–16. This suggests that deviation from physiological nutrient levels in the TMJ disc due to the lack of nutrient supply may initiate tissue remodeling and matrix degradation. Although convection due to interstitial fluid flow induced by mechanical loading may affect large solute transport 17–19, the transport of small solutes (e.g., ions, oxygen, and glucose) within avascular cartilaginous tissues mainly depends on diffusion19–20. The rate of solute diffusion in tissue is governed by solute diffusivities which are affected by the composition and structure of the matrix, as well as mechanical strains on the tissue. Many studies have been conducted to determine diffusivities of various solutes in articular cartilage and IVD21. However, very few data of solute diffusivities are available for the TMJ disc. Recently, we examined the effect of mechanical loading on small ion diffusivity in TMJ discs using the electrical conductivity method. The results indicated that compressive strain significantly impeded solute transport in the TMJ disc22. Furthermore, the solute diffusivities of FITC-dextran (4k Da) in porcine TMJ discs were also measured by using a fluorescence recovery after photobleaching (FRAP) technique23. The results showed that the tissue matrix significantly hindered solute diffusion and the solute diffusivities were anisotropic (i.e., orientation dependent) and inhomogeneous (i.e., region dependent)23. A recent diffusion tensor imaging (DTI) study also showed the anisotropic and region-dependent behavior of water diffusion in porcine TMJ discs24.
The unique transport behavior in the TMJ disc tissue may be attributed to the complex tissue composition and structure (i.e., tissue morphology) of the disc. Differences in biochemical composition and structure distinguish the disc into three regions: the anterior band, intermediate zone, and posterior band13. Collagen fibers in the posterior band run primarily in the mediolateral direction and in the intermediate zone align predominately in an anteroposterior direction, while mixed fiber orientations have been found mainly in the anterior band25. Knowledge of transport properties for small nutrient molecules (e.g., oxygen and glucose) and their relationship with tissue morphology is important for elucidating the mechanism and pathways of nutrient transport in the TMJ disc and better understanding the etiology of TMJ disc degeneration. While several studies have been published about the effect of tissue morphology on anisotropic diffusion in articular cartilage26, ligament27, knee meniscus28, and IVD29, little is known regarding the relationship between solute diffusion properties and tissue structure of the TMJ disc.
Therefore, the objective of this study was to investigate the relationship between solute diffusion properties and tissue morphology in porcine TMJ disc. We hypothesized that solute diffusion in the TMJ disc is anisotropic and inhomogeneous, and transport properties are associated with tissue morphology. The diffusivities of fluorescein in five TMJ disc regions (anterior, medial, intermediate, lateral, and posterior) were determined by a FRAP technique previously developed in our lab23. The anisotropic diffusion behavior of fluorescein in the TMJ disc was investigated by measuring the diffusion coefficient in three orthogonal directions (medial-lateral, anterior-posterior, and superior-inferior) in each disc region. To investigate the relationship between tissue morphology and solute diffusivity, the three-dimensional (3D) collagen fiber structures in each disc region were examined by utilizing scanning electron microscopy (SEM). The anisotropy of fiber structures in five disc regions were quantified through the analyses of the SEM images.
MATERIALS AND METHODS
Specimen Preparation
A total of eleven pig heads (Yorkshire, male, aged 6~8 months) were collected from a local abattoir within 2 hours of slaughter. The entire TMJ with capsule intact was removed en bloc. Joints were opened under a sterile dissection hood; TMJ discs were then removed from peripheral attachments. All discs were assessed visually, and were not used if signs of degeneration were apparent. The transport properties and tissue morphology were investigated in three orthogonal orientations (i.e., mediolateral, anteroposterior, and superoinferior) for each of the five different regions (i.e., anterior, medial, intermediate, lateral, and posterior) (Fig. 1). Eleven TMJ discs of the right joints were used to determine the anisotropic diffusivities via FRAP protocols. Eleven TMJ discs of the corresponding left joints were used to examine the tissue morphology via SEM. When the specimens were observed under the microscopes, the material coordinates of the samples were fixed to the microscopy coordinates [i.e., mediolateral (ML) axis→X axis, anteroposterior (AP) axis→Y axis, and superoinferior (SI) axis→Z axis] in order to correlate the diffusion properties to the tissue morphology of the TMJ discs.
Figure 1.
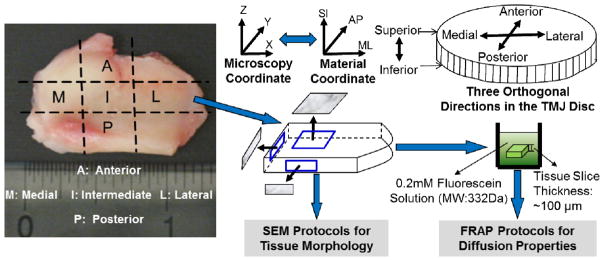
Schematic of specimen preparation and testing protocols. Each disc was examined in five regions: anterior, intermediate, posterior, lateral and medial. In each region, solute diffusion properties and tissue morphology of these specimens were investigated in three orthogonal planes (i.e., XY, ZX and ZY). The right side discs were tested by FRAP protocols for the diffusion properties and the corresponding left side discs were examined by SEM protocols for tissue morphology.
Diffusivity Measurement using FRAP Technique
Three tissue blocks were harvested from each region for the diffusivity measurements (Fig. 1). One tissue block was for the diffusivity measurement on the XY horizontal plane (i.e., ML-AP plane), one was for the ZX vertical plane (i.e., SI-ML plane), and the other one was for the ZY vertical plane (i.e., SI-AP plane). The disc tissue blocks were sectioned to 100μm slices using a microtome (SM24000, Leica Microsystems GmbH, Wetzlar, Germany) with a freezing stage (Model BFS-30, Physitemp, Clifton, NJ). The specimens were marked to identify the material coordinates. The tissue slices were then immersed in a phosphate buffered saline (PBS) solution (Sigma®, St. Louis, MO) with 0.2mM fluorescein (332 Da, λex 490 nm; λem 514 nm, Fluka-Sigma-Aldrich®, St. Louis, MO) for 48 hours. No significant tissue swelling occurred in this study. The specimen was sandwiched between two porous platens to prevent tissue swelling during the incubation. This method was successfully used in FRAP studies for IVD tissues29–30. The anisotropic diffusion tensors of fluorescein in the specimens on the sectioned plane were determined using a Spatial Fourier Analysis (SFA) based FRAP technique developed in our previous study23. The FRAP experiments were performed on a Leica TCS-SP5 confocal laser scanning microscope (Leica Microsystems, Inc., Exton, PA) at room temperature (22°C). Briefly, the specimens were photobleached using an Ar-488nm laser to create a circular bleached spot with a diameter of 48μm. For each FRAP measurement, 300 frames of recovery images of 128×128 pixels (387.5μm×387.5μm), plus 5 images prior to photobleaching, were acquired at a scanning rate of 0.355 seconds per frame. To minimize the contribution of the fluorescence emission of the background, pre-bleaching images were averaged and then subtracted from the post-bleaching image series. The confocal series images were analyzed by implementing custom written codes in MATLAB (MATLAB 7.0, The MathWorks Inc., Natick, MA) to calculate the 2D solute diffusion tensor in the specimen on the section plane. For the specimen sectioned on the XY horizontal plane (i.e., ML-AP plane), the diffusivities along the mediolateral (DML) and anteroposterior (DAP) orientations were determined. Similarly, for the specimen sectioned on the ZX vertical plane (i.e., SI-ML plane), the diffusivities along the superoinferior (DSI) and mediolateral (DML) were determined. For the specimen sectioned in the ZY vertical plane (i.e., SI-AP plane), the diffusivities along the superoinferior (DSI) and anteroposterior (DAP) orientations were determined. Finally, for each disc region, the diffusivities along the anteroposterior (DAP), mediolateral (DML), and superoinferior (DSI) orientations were calculated by averaging the corresponding diffusivities of the three specimens prepared from the same disc region in three orthogonal planes. The mean diffusivities [(DML+DAP+DSI)/3] in each region were calculated as well.
Fractional Anisotropy
To quantify the solute diffusion anisotropy, the fractional anisotropy (FA)31 was calculated for each disc region by following the equation:
Here, the diffusivities of DAP, DML, and DSI were used to represent the three eigenvalues of the 3D diffusion tensor. Our data showed that values of the off-diagonal components of the diffusivity tensor in the material coordinates were much smaller (at least one order of magnitude smaller) compared to the diagonal components (i.e., DAP, DML, and DSI). The FA is a scalar and rotationally invariant quantity that describes the degree of anisotropy of diffusion in a tissue sample. A FA value of zero means that diffusion is isotropic (i.e. it is equally restricted in all directions). In contrast, a FA value of one represents that diffusion occurs only along one axis and is fully restricted along all other directions.
Tissue Structure Examination using SEM
To determine the fiber structure on the horizontal plane (e.g., XY plane), a whole disc sample was microtomed to remove the concave shape of the disc and expose the collagen fiber structure on the horizontal plane which is parallel to the superior surface. To determine the fiber structure on the two vertical planes (e.g., ZX and ZY planes), the discs were cut mediolaterally or anteroposteriorly to uncover the tissue structure on the vertical planes. The specimens were marked to distinguish the different regions and identify the material coordinates. The specimens were then fixed with a solution of 2.5% glutaraldehyde in PBS at 4°C for 72 hours. The fixed tissues were dehydrated using a graded series of ethanol. Afterwards, the specimens were dried by immersion in hexamethyldisilazane (HMDS) solution. In order to enhance the contrast of SEM images, the specimens were coated with a layer (20μm thickness) of gold. Finally, the coated specimens were examined via SEM (Hitachi TM-1000) at 3000x magnification.
Collagen Fiber Alignment Measurement
The SEM images in each disc region were used to assess the degree of collagen fiber alignment. The fiber alignment measurement was characterized by using an ImageJ (Version 1.45s, by Wayne Rasband, National Institutes of Health, USA) plug-in (OrientationJ) that calculates the directional coherency coefficient of the collagen fibers32–33. The coherency coefficient indicates if the local fibers are aligned or not. A coherency coefficient close to one demonstrates a significantly coherent orientation of the local fibers in one direction. A coherency coefficient close to zero represents no dominant orientation of the fibers32.
Statistical Analysis
One-way ANOVA and Tukey’s post hoc tests were performed on DML, DAP and DSI to examine in the anisotropy of solute diffusion in each disc region. The mean solute diffusivities, diffusion fractional coefficients, and fiber coherency in the five disc regions were also analyzed using the same statistical methods to examine the regional effect on the solute diffusion rate, diffusional anisotropy, and fiber alignment in porcine TMJ discs. SPSS 16.0 software (SPSS Inc., Chicago, IL) was used for all statistical analyses and significant differences were reported at p-values < 0.05.
RESULTS
Solute Diffusivities in the TMJ Discs (FRAP)
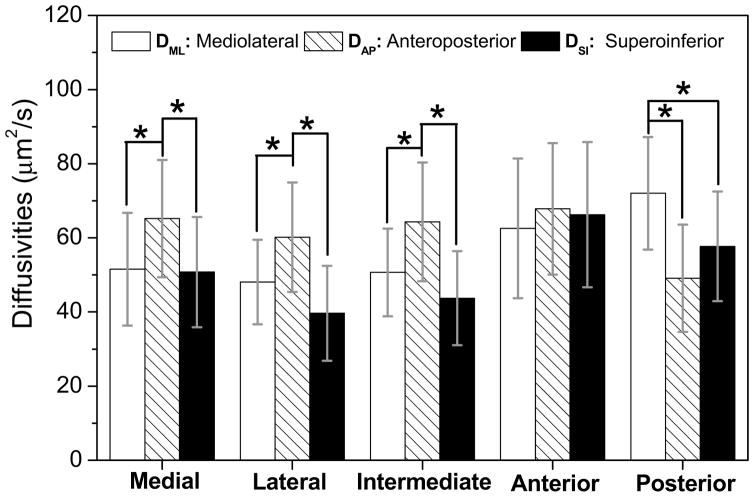
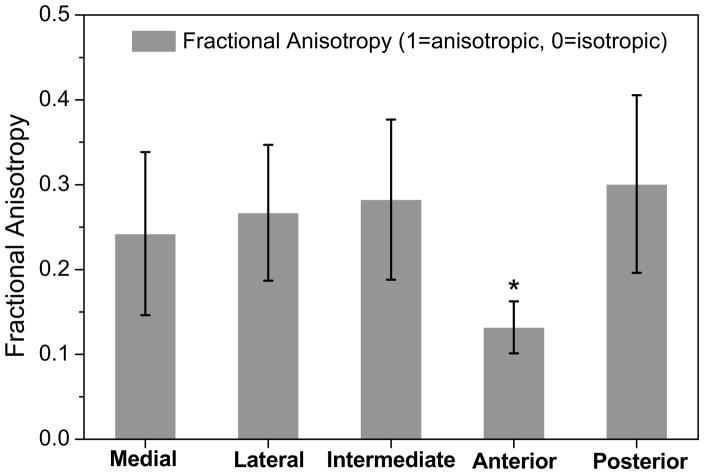
The diffusivities of fluorescein in each TMJ disc region were significantly anisotropic, except the anterior region (Fig. 2). In the medial region, anteroposterior diffusivity [mean, 95% confidence interval (CI), 65.2 (49.4–81.1) μm2/s] was significantly larger than the diffusivities in the mediolateral [51.6 (36.4–66.7) μm2/s, p = 0.031] and superoinferior [50.8 (35.9–65.7) μm2/s, p = 0.023] directions. In the lateral region, anteroposterior diffusivity [60.2 (45.4–75.0) μm2/s] was also significantly larger than the diffusivities in the mediolateral [48.1 (36.7–59.5) μm2/s, p = 0.023] and superoinferior [39.7 (26.9–52.5) μm2/s, p < 0.0001] directions. Similarly, in the intermediate region, anteroposterior diffusivity [64.3 (48.3–80.4) μm2/s] was significantly larger than the diffusivities in the mediolateral [50.7 (38.8–62.5) μm2/s, p = 0.013] and superoinferior [43.7 (31.1–56.4) μm2/s, p = 0.0001] directions. In contrast, in the posterior region, mediolateral diffusivity [72.0 (56.8–87.2) μm2/s] was significantly larger than anteroposterior [49.1 (34.7–63.6) μm2/s, p = 0.0003] and superoinferior diffusivity [57.7 (42.9–72.5) μm2/s, p = 0.04]. However, in the anterior region, there were no significant differences in diffusivity between anteroposterior [67.8 (50.1–85.6) μm2/s], mediolateral [62.6 (43.7–81.4) μm2/s], and superoinferior [66.3 (46.3–81.8) μm2/s] directions (ANOVA, p = 0.66). The degree of the diffusional anisotropy was quantified by the fractional anisotropy in the five disc regions (Fig. 4). The value of fractional anisotropy in the anterior region [0.13 (0.10–0.16)] was significantly lower than those of the medial [0.24 (0.15–0.34)], lateral [0.26 (0.19–0.35)], intermediate [0.28 (0.19–0.38)], and posterior [0.30 (0.20–0.41)] regions (ANOVA, p = 0.0002).
Figure 2.
The results of anisotropic solute diffusivities in porcine TMJ discs (n=11). (The data shown were means and the error bar was a 95% confidence interval.) In the medial (* ANOVA, p = 0.013), lateral (* ANOVA, p < 0.0001) and intermediate regions (* ANOVA, p = 0.0002), anteroposterior diffusivity was significantly larger than the diffusivities in the other two directions. The mediolateral diffusion predominated in the posterior region (*ANOVA, p = 0.001). In the anterior region, there were no significant differences for diffusivity between the orientations (ANOVA, p = 0.66) likely due to the multi-directional fiber arrangement.
Figure 4.
The results of solute diffusional anisotropy in five regions of the TMJ disc (n=11). (The data shown were means and the error bar was a 95% confidence interval.) The results of fractional anisotropy showed the region-dependent solute diffusion properties in the TMJ disc. The anterior region had lower FA than other four regions (*ANOVA, p = 0.0002).
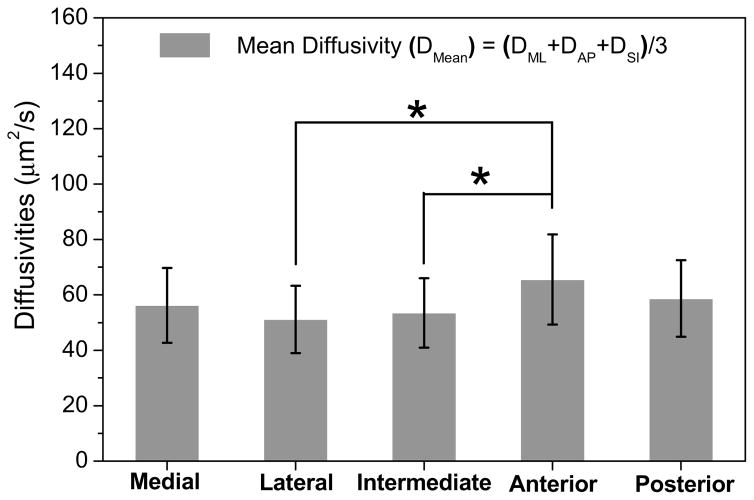
The mean diffusivities of fluorescein in porcine TMJ discs were region-dependent (ANOVA, p = 0.013). The anterior region [65.6 (49.3–81.8) μm2/s] had a significantly higher mean diffusivity than the intermediate region [53.5 (41.0–66.0) μm2/s, p = 0.048] and lateral region [51.2 (39.0–63.3) μm2/s, p = 0.01]. Moreover, the anterior region also had a higher value of mean diffusivity than the medial region [56.2 (42.7–69.7) μm2/s] and posterior region [58.7 (44.9–72.5) μm2/s], although the differences were not statistically significant (Fig. 3). The overall average diffusivity of fluorescein across the TMJ disc was 57.0 (43.0–71.0) μm2/s.
Figure 3.
The results of inhomogenous solute diffusivities in porcine TMJ discs (n=11). (The data shown were means and the error bar was a 95% confidence interval.) Region-dependent diffusion properties were found in the TMJ disc. The anterior region had higher mean diffusivity than the lateral and intermediate regions (* ANOVA, p = 0.013 compared to anterior) probably due to the higher water content and random fiber arrangement in this region.
Collagen Fiber Orientations (SEM)
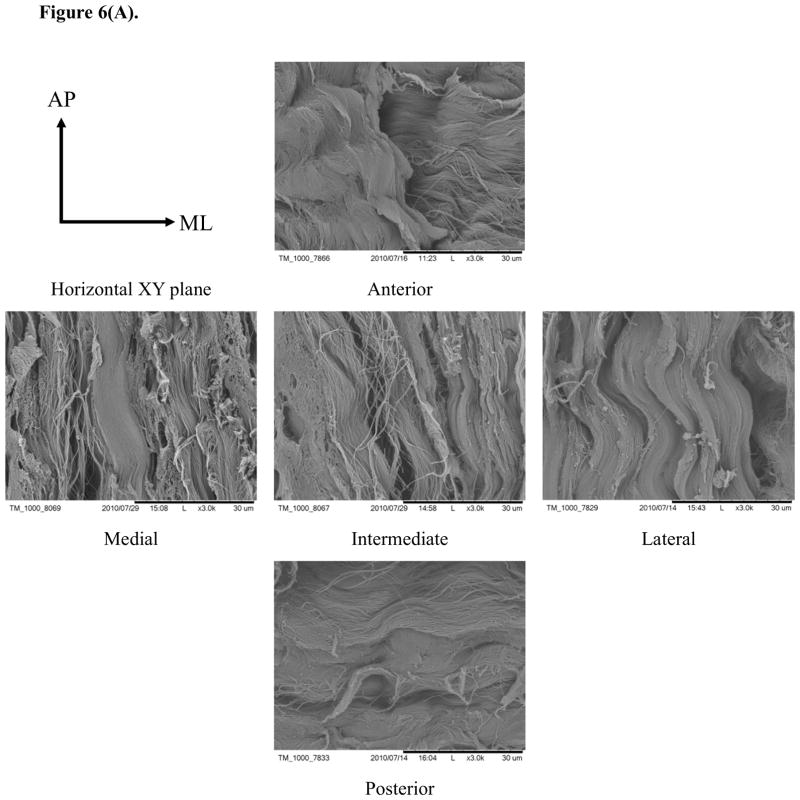
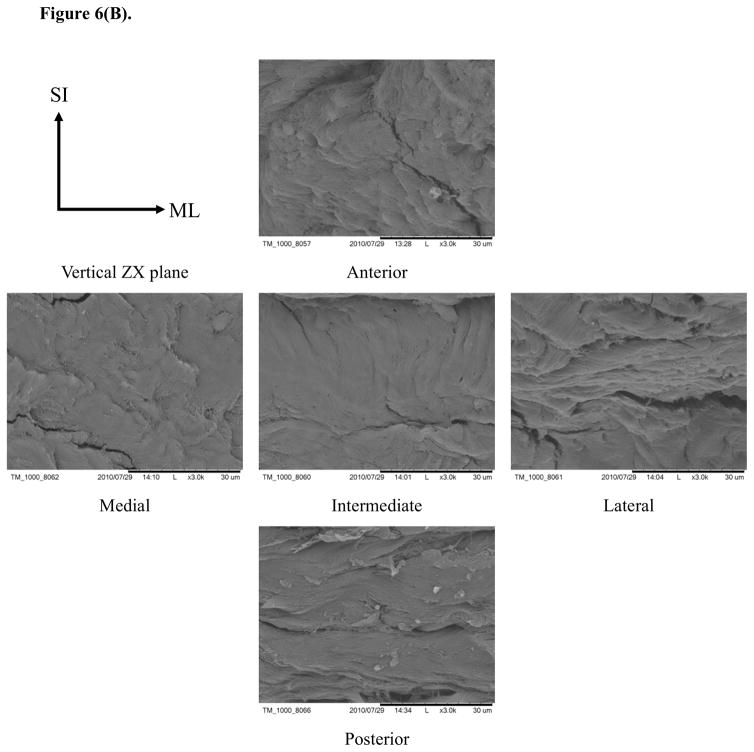
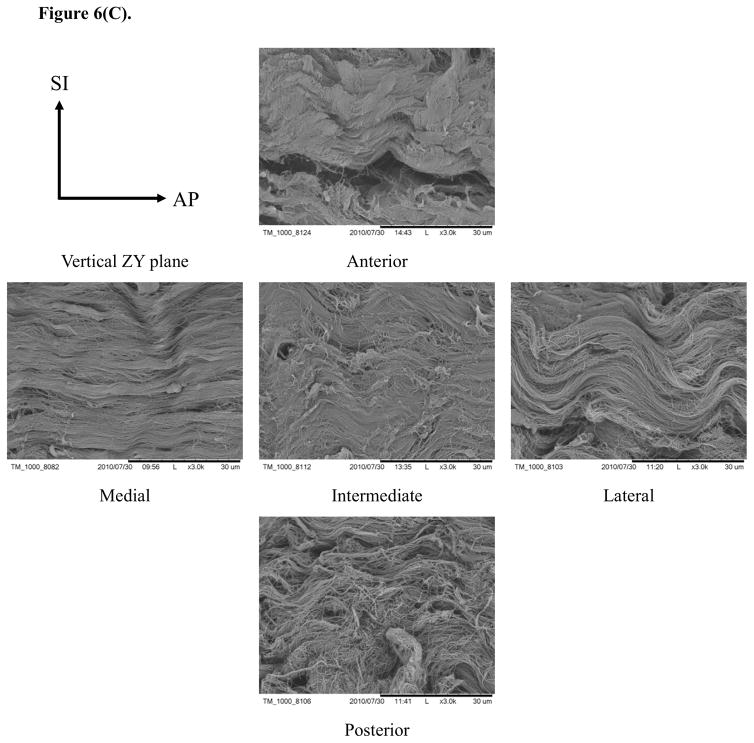
Typical SEM images of porcine TMJ discs in the horizontal XY, vertical ZX, and vertical ZY sections (Fig. 6) showed differences in the organization of collagen fibers among five disc regions. In the horizontal XY plane (i.e., ML-AP plane), the collagen fibers aligned anteroposteriorly in the medial, intermediate, and lateral regions while fibers were aligned mediolaterally in the posterior region. Interestingly, fibers aligned in both anteroposterior and mediolateral directions were found in the anterior region [Fig. 6(A)]. In the vertical ZX plane (i.e., SI-ML plane), there was no clear fiber orientation in the medial, intermediate and lateral regions, likely due to their orientation perpendicular to the sectioned plane. There was also no clear fiber orientation in the anterior region. Conversely, the fibers clearly aligned in the mediolateral direction in the posterior region [Fig. 6(B)]. In the vertical ZY plane (i.e., SI-AP plane), anteroposterior fibers were found again in the medial, intermediate, and lateral regions while fibers with two orientations existed in the anterior region. There was no clear fiber orientation in the posterior region, likely due to fibers being oriented perpendicular to the section plane [Fig. 6(C)].
Figure 6.
SEM images of collagen fiber orientations in the TMJ disc. (A) In the XY plane, the collagen fibers aligned anteroposteriorly in the medial, intermediate and lateral regions. The collagen fibers run mediolaterally in the posterior region and fibers in both directions were found in the anterior region. (B) In the ZX plane, there was no clear fiber orientation in the anterior, medial, intermediate and lateral regions, but the fibers in the posterior region aligned mediolaterally. (C) In the ZY plane, anteroposterior fibers were found again in the medial, intermediate and lateral regions, and fibers with both orientations existed in the anterior region and were randomly distributed in the posterior region.
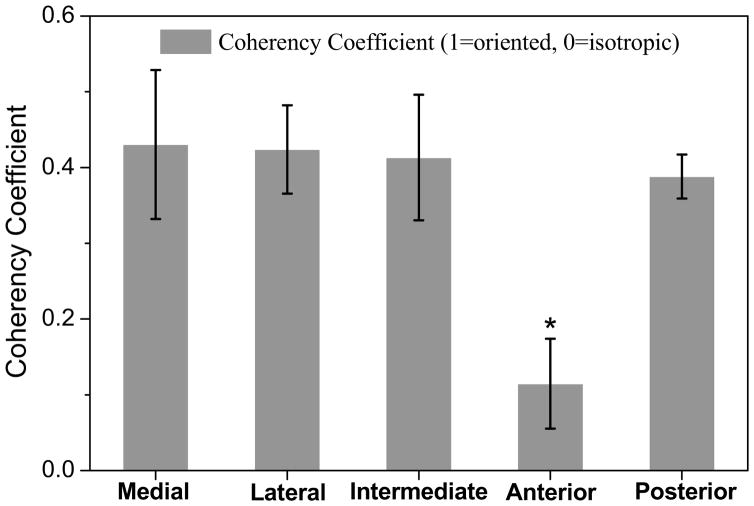
The degree of collagen fiber alignment was quantified by the coherency coefficient in the five disc regions (Fig. 5). The results indicated that the values of the coherency coefficient were region-dependent in the TMJ disc. The collagen fibers highly aligned in the medial [0.43 (0.33–0.53)], lateral [0.42 (0.37–0.48)], intermediate [0.41 (0.33–0.50)], and posterior [0.39 (0.36–0.42)] regions. In contrast, the anterior [0.12 (0.06–0.17)] region had a significant lower coherency coefficient than the other four regions (ANOVA, p < 0.0001), likely due to the multi-directional fiber arrangement in this region.
Figure 5.
The results of collagen fiber alignment anisotropy in five regions of the TMJ disc (n=11). (The data shown were means and the error bar was a 95% confidence interval.) The measurements of coherency coefficient demonstrated the region-dependent collagen fiber alignment properties in the TMJ disc. The anterior region had lower coherency coefficient than other four regions (* ANOVA, p < 0.0001) likely caused by the random fiber arrangement in the anterior region.
DISCUSSION
The 3D solute diffusion properties and tissue morphology in porcine TMJ discs were determined in this study by using FRAP and SEM techniques, respectively. The results of the FRAP measurements demonstrated that the solute diffusion in porcine TMJ discs was anisotropic. Moreover, the degrees of the diffusional anisotropy and main orientations of diffusion were region-dependent. The SEM results showed that the collagen fiber structure in the TMJ discs was highly organized and the degree and orientation of collagen fiber alignment were region-dependent as well. This study suggested that the 3D anisotropic diffusion properties were associated with tissue morphology (i.e., collagen fiber structure) in each TMJ disc region.
The collagen fiber orientations observed by SEM in this study were in agreement with the experimental results reported in the literature. Detamore et al. found collagen fibers inside the porcine TMJ discs have a ring-like structure around the periphery and are oriented anteroposteriorly through the intermediate zone25. The collagen fibers primarily run in a mediolateral direction within the posterior region. However, the anterior band has the merging of anteroposterior fibers with mediolateral fibers, since central anteroposterior fibers extend somewhat into the anterior band. The collagen fibers in human and other animal TMJ discs34–37 have similar structures as the results shown in this study.
The mean diffusivity of fluorescein across the TMJ disc measured in this study was comparable to the values in other cartilaginous tissues (e.g., intervertebral disc, meniscus, and articular cartilage) (Table I). The diffusivity of fluorescein was lower than that of ions (Na+ and Cl−)22, but higher than that of 4k Da FITC-dextran23 in porcine TMJ discs determined in our previous studies (Table I). This was expected since the fluorescein molecular weight lies in between the other solute weights. The results of this study demonstrated that the solute diffusion in the TMJ disc was highly anisotropic, which was also found in our previous study examining 2D anisotropic diffusion of a larger solute (4k Da FITC-dextran) in the horizontal X-Y plane23. This study of 3D anisotropic solute diffusion further indicated that the main diffusion directions were associated with the main collagen fiber orientations. In the medial, intermediate, and lateral regions, the fluorescein diffusion along the fiber orientation (i.e., anteroposterior direction) was significantly faster than the diffusion transverse to the fiber orientation (i.e., mediolateral and superoinferior directions). In the posterior regions (i.e., posterior band), the fluorescein diffusion along the fiber orientation (i.e., mediolateral direction) was significantly faster than the diffusion transverse to the fiber orientation (i.e., anteroposterior and superoinferior directions) as well. In contrast, the diffusion in the anterior region was mostly isotropic, likely due to the multi-directional fiber arrangements.
Table I.
The diffusivities (mean ± standard deviation) of different solutes in various cartilaginous tissues (unit: μm2/s). Standard deviations are not available for annulus fibrosus, meniscus, and articular cartilage.
| Type of tissues and species | Type of Solute | Diffusivities | Reference |
|---|---|---|---|
| Porcine TMJ disc | Chloride ion (35Da) | 439.5 ± 88.6 | 22 |
| Sodium ion (23 Da) | 316.7 ± 63.8 | ||
| Porcine TMJ disc | FITC-Dextran (4k Da) | 26.1 ± 4.3 | 23 |
| Porcine TMJ disc | Fluorescein (322 Da) | 57.0 ± 20.9 | Present study |
| Bovine AF | Fluorescein (322 Da) | 110.2 | 28 |
| Human AF | Fluorescein (322 Da) | 153.0 | 29 |
| Bovine meniscus | Fluorescein (322 Da) | 101.5 | 28 |
| Porcine articular cartilage | FITC-Dextran (3k Da) | 75 | 26 |
| FITC-Dextran (40k Da) | 56 |
Furthermore, the degree of diffusional anisotropy in five disc regions has a similar trend as the degree of collagen fiber alignment. The collagen fibers were highly aligned in the medial, lateral, intermediate, and posterior regions. However, the anterior region had a significantly lower coherency coefficient than the other four regions (Fig. 5).
Correspondingly, the value of fractional anisotropy in the anterior region was significantly lower than those of the other disc regions (Fig. 4). Similar results were also found by DTI measurements of 3D water diffusion in porcine TMJ discs24. However, the fractional anisotropy of water diffusion (0.36–0.55) in the DTI study was higher than the fluorescein diffusion (0.13–0.30) in this study. The possible reason for this difference might be the age difference of the animals used between the DTI study (12–16 months) and this study (6–8 months). The existence of more aligned collagen fibers in matured pigs may cause a relatively higher fractional anisotropy. The studies on bovine28 and human29 annulus fibrosus (AF) also showed highly anisotropic fluorescein diffusion properties exist because of the well-organized tissue structures.
The mean diffusivity of fluorescein in the TMJ disc is about 10% of its diffusivity in water, indicating the solute diffusion is significantly hindered by the ECM of the tissue. In addition to the tissue structure, the tissue composition also could influence the solute diffusion properties in hydrated soft tissues21. The comparison of mean diffusivities between different regions showed the inhomogeneous solute diffusion properties in the TMJ disc. The anterior region had a higher mean diffusivity of fluorescein than the other disc regions probably due to the high water content, although the tissue water content was not measured in this study. Our previous study showed that the ion diffusivities were highly associated with the tissue water content in porcine TMJ discs22. The anterior region has the highest ion diffusivities as well as tissue water content. The higher diffusivity may be needed to meet the higher nutrient demands in the anterior region. Our previous study has shown that the anterior region has the highest cell density in the disc38. Studies29–30, 39 on other cartilaginous tissues also showed that the solute diffusivity is positively related to the tissue water content. The diffusivity was lower in the TMJ disc when compared to other cartilaginous tissues (Table I) likely due to the low water content in the TMJ disc. Considering the higher cell density and nutrient consumption rate of TMJ disc cells38, the lower solute diffusivity may result in a steeper nutrient gradient in the TMJ disc compared to other cartilaginous tissues. Such a nutrient gradient may be vulnerable to any pathological event that can impede nutrient supply, and ultimately result in tissue degeneration.
In this study, fluorescein was utilized as a molecular probe to investigate the relationship between solute diffusion properties and tissue morphology. The molecular weight of fluorescein (332 Da) is about two times higher than the molecular weight of glucose (180 Da). Generally, the tissue ECM has a greater impact on the diffusivity of larger solutes21. Therefore, it is necessary to determine the impact of tissue ECM on the diffusivities of other essential solutes, such as oxygen and glucose. In this study, the measurement of 3D anisotropic diffusivities was achieved by measuring 2D diffusion tensors in three orthogonal tissue sections. However, this tissue sectioning process might alter the collagen fiber alignment. Therefore, it is necessary to develop a FRAP technique to measure local 3D solute diffusion tensor without tissue sectioning. Although pigs have been considered as a good animal model to study human TMJ disc mechanics, the determination of the solute diffusivity on human samples is also needed.
In summary, this study investigated the relationship between small solute diffusion properties and tissue morphology in porcine TMJ discs. Both FRAP and SEM techniques were utilized to determine the 3D solute diffusion properties and collagen fiber orientations, respectively. It was found that the diffusion of fluorescein in the TMJ disc was anisotropic and inhomogeneous. This suggests that tissue structure (i.e., collagen fiber alignment) and composition (e.g., water content) could be key factors affecting the solute diffusion properties within TMJ discs. The fluorescein diffusion along the fiber orientation was significantly faster than the diffusion transverse to the fiber orientation. Interestingly, the quantitative measurements showed the degree of diffusional anisotropy and the degree of collagen fiber alignment have a similar trend in five disc regions, which indicated the anisotropic diffusion properties were associated with tissue morphology. Furthermore, this study also demonstrated that the regional difference in tissue composition resulted in the region-dependent solute diffusion properties. This study has provided a baseline investigation on the relationship between solute transport properties and tissue morphology. These findings are important for understanding transport properties as well as further developing numerical models on nutritional supply within the TMJ disc.
Acknowledgments
This project was supported by NIH grants DE021134, DE018741, and AR055775, a NSF RII grant predoctoral fellowship (EPS-00903795) to CS, and a NIH T32 predoctoral fellowship (DE017551) to GJW.
Footnotes
AUTHOR CONTRIBITIONS
The authors made substantial contributions in designing the study (CS, HY), gathering and analyzing the data (CS, GJW, CLE, ADB, HY), and drafting the article (CS, HY).
CONFLICT OF INTEREST
None of the authors of this paper have a conflict of interest that might be construed as affecting the conduct or reporting of the work presented.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Werner JA, Tillmann B, Schleicher A. Functional anatomy of the temporomandibular joint. A morphologic study on human autopsy material. Anat Embryol(Berl) 1991;183:89–95. doi: 10.1007/BF00185839. [DOI] [PubMed] [Google Scholar]
- 2.Nickel JC, McLachlan KR. In vitro measurement of the frictional properties of the temporomandibular joint disc. Arch Oral Biol. 1994;39:323–331. doi: 10.1016/0003-9969(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 3.Nickel JC, McLachlan KR. In vitro measurement of the stress-distribution properties of the pig temporomandibular joint disc. Arch Oral Biol. 1994;39:439–448. doi: 10.1016/0003-9969(94)90175-9. [DOI] [PubMed] [Google Scholar]
- 4.Dworkin SF, Huggins KH, LeResche L, Von Korff M, Howard J, Truelove E, et al. Epidemiology of signs and symptoms in temporomandibular disorders: clinical signs in cases and controls. J Am Dent Assoc. 1990;120:273–281. doi: 10.14219/jada.archive.1990.0043. [DOI] [PubMed] [Google Scholar]
- 5.Gatchel RJ, Stowell AW, Wildenstein L, Riggs R, Ellis E., 3rd Efficacy of an early intervention for patients with acute temporomandibular disorder-related pain: a one-year outcome study. J Am Dent Assoc. 2006;137:339–347. doi: 10.14219/jada.archive.2006.0183. [DOI] [PubMed] [Google Scholar]
- 6.Stegenga B. Osteoarthritis of the temporomandibular joint organ and its relationship to disc displacement. J Orofac Pain. 2001;15:193–205. [PubMed] [Google Scholar]
- 7.Stegenga B, de Bont LG, Boering G. Osteoarthrosis as the cause of craniomandibular pain and dysfunction: a unifying concept. J Oral Maxillofac Surg. 1989;47:249–256. doi: 10.1016/0278-2391(89)90227-9. [DOI] [PubMed] [Google Scholar]
- 8.Berkovitz BK, Robertshaw H. Ultrastructural quantification of collagen in the articular disc of the temporomandibular joint of the rabbit. Arch Oral Biol. 1993;38:91–95. doi: 10.1016/0003-9969(93)90161-e. [DOI] [PubMed] [Google Scholar]
- 9.Gage JP, Virdi AS, Triffitt JT, Howlett CR, Francis MJ. Presence of type III collagen in disc attachments of human temporomandibular joints. Arch Oral Biol. 1990;35:283–288. doi: 10.1016/0003-9969(90)90044-b. [DOI] [PubMed] [Google Scholar]
- 10.Nakano T, Scott PG. A quantitative chemical study of glycosaminoglycans in the articular disc of the bovine temporomandibular joint. Arch Oral Biol. 1989;34:749–757. doi: 10.1016/0003-9969(89)90082-4. [DOI] [PubMed] [Google Scholar]
- 11.Nakano T, Scott PG. Changes in the chemical composition of the bovine temporomandibular joint disc with age. Arch Oral Biol. 1996;41:845–853. doi: 10.1016/s0003-9969(96)00040-4. [DOI] [PubMed] [Google Scholar]
- 12.Sindelar BJ, Evanko SP, Alonzo T, Herring SW, Wight T. Effects of intraoral splint wear on proteoglycans in the temporomandibular joint disc. Arch Biochem Biophys. 2000;379:64–70. doi: 10.1006/abbi.2000.1855. [DOI] [PubMed] [Google Scholar]
- 13.Rees LA. The structure and function of the mandibular joint. Br Dent J. 1954;96:125–133. [Google Scholar]
- 14.Leonardi R, Lo ML, Bernasconi G, Caltabiano C, Piacentini C, Caltabiano M. Expression of vascular endothelial growth factor in human dysfunctional temporomandibular joint discs. Arch Oral Biol. 2003;48:185–192. doi: 10.1016/s0003-9969(02)00207-8. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi A, Tojyo I, Yoshida H, Fujita S. Role of hypoxia and interleukin-1beta in gene expressions of matrix metalloproteinases in temporomandibular joint disc cells. Arch Oral Biol. 2005;50:81–87. doi: 10.1016/j.archoralbio.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Tojyo I, Yamaguchi A, Nitta T, Yoshida H, Fujita S, Yoshida T. Effect of hypoxia and interleukin-1beta on expression of tenascin-C in temporomandibular joint. Oral Dis. 2008;14:45–50. doi: 10.1111/j.1601-0825.2006.01344.x. [DOI] [PubMed] [Google Scholar]
- 17.O’Hara BP, Urban JP, Maroudas A. Influence of cyclic loading on the nutrition of articular cartilage. Ann Rheum Dis. 1990;49:536–539. doi: 10.1136/ard.49.7.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia AM, Frank EH, Grimshaw PE, Grodzinsky AJ. Contributions of fluid convection and electrical migration to transport in cartilage: relevance to loading. Arch Biochem Biophys. 1996;333:317–325. doi: 10.1006/abbi.1996.0397. [DOI] [PubMed] [Google Scholar]
- 19.Evans RC, Quinn TM. Solute convection in dynamically compressed cartilage. J Biomech. 2006;39:1048–1055. doi: 10.1016/j.jbiomech.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 20.Yao H, Gu WY. Convection and diffusion in charged hydrated soft tissues: a mixture theory approach. Biomechan Model Mechanobiol. 2006;6:63–72. doi: 10.1007/s10237-006-0040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson A, Gu W. Transport Properties of Cartilaginous Tissues. Curr Rheumatol Rev. 2009;5:40–50. doi: 10.2174/157339709787315320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuo J, Wright GJ, Bach DE, Slate EH, Yao H. Effect of mechanical loading on electrical conductivity in porcine TMJ discs. Journal of dental research. 2011;90:1216–1220. doi: 10.1177/0022034511415275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi C, Kuo J, Bell PD, Yao H. Anisotropic solute diffusion tensor in porcine TMJ discs measured by FRAP with spatial Fourier analysis. Ann Biomed Eng. 2010;38:3398–3408. doi: 10.1007/s10439-010-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benavides E, Bilgen M, Al-Hafez B, Alrefae T, Wang Y, Spencer P. High-resolution magnetic resonance imaging and diffusion tensor imaging of the porcine temporomandibular joint disc. Dentomaxillofac Radiol. 2009;38:148–155. doi: 10.1259/dmfr/19195745. [DOI] [PubMed] [Google Scholar]
- 25.Detamore MS, Orfanos JG, Almarza AJ, French MM, Wong ME, Athanasiou KA. Quantitative analysis and comparative regional investigation of the extracellular matrix of the porcine temporomandibular joint disc. Matrix Biol. 2005;24:45–57. doi: 10.1016/j.matbio.2004.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leddy HA, Guilak F. Site-specific molecular diffusion in articular cartilage measured using fluorescence recovery after photobleaching. Annals of Biomedical Engineering. 2003;31:753–760. doi: 10.1114/1.1581879. [DOI] [PubMed] [Google Scholar]
- 27.Leddy HA, Haider MA, Guilak F. Diffusional anisotropy in collagenous tissues: fluorescence imaging of continuous point photobleaching. Biophys J. 2006;91:311–316. doi: 10.1529/biophysj.105.075283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Travascio F, Zhao W, Gu WY. Characterization of anisotropic diffusion tensor of solute in tissue by video-FRAP imaging technique. Ann Biomed Eng. 2009;37:813–823. doi: 10.1007/s10439-009-9655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Travascio F, Jackson AR, Brown MD, Gu WY. Relationship between solute transport properties and tissue morphology in human annulus fibrosus. J Orthop Res. 2009;27:1625–1630. doi: 10.1002/jor.20927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Travascio F, Gu WY. Anisotropic diffusive transport in annulus fibrosus: experimental determination of the diffusion tensor by FRAP technique. Ann Biomed Eng. 2007;35:1739–1748. doi: 10.1007/s10439-007-9346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 32.Fonck E, Feigl GG, Fasel J, Sage D, Unser M, Rufenacht DA, et al. Effect of aging on elastin functionality in human cerebral arteries. Stroke. 2009;40:2552–2556. doi: 10.1161/STROKEAHA.108.528091. [DOI] [PubMed] [Google Scholar]
- 33.Rezakhaniha R, Agianniotis A, Schrauwen JT, Griffa A, Sage D, Bouten CV, et al. Experimental investigation of collagen waviness and orientation in the arterial adventitia using confocal laser scanning microscopy. Biomech Model Mechanobiol. 2012;11:461–473. doi: 10.1007/s10237-011-0325-z. [DOI] [PubMed] [Google Scholar]
- 34.Shengyi T, Xu Y. Biomechanical properties and collagen fiber orientation of TMJ discs in dogs: Part 1. Gross anatomy and collagen fiber orientation of the discs. J Craniomandib Disord. 1991;5:28–34. [PubMed] [Google Scholar]
- 35.Mills DK, Fiandaca DJ, Scapino RP. Morphologic, microscopic, and immunohistochemical investigations into the function of the primate TMJ disc. J Orofac Pain. 1994;8:136–154. [PubMed] [Google Scholar]
- 36.Scapino RP, Canham PB, Finlay HM, Mills DK. The behaviour of collagen fibres in stress relaxation and stress distribution in the jaw-joint disc of rabbits. Arch Oral Biol. 1996;41:1039–1052. doi: 10.1016/s0003-9969(96)00079-9. [DOI] [PubMed] [Google Scholar]
- 37.Minarelli AM, Del Santo JM, Liberti EA. The structure of the human temporomandibular joint disc: a scanning electron microscopy study. J Orofac Pain. 1997;11:95–100. [PubMed] [Google Scholar]
- 38.Kuo J, Shi C, Cisewski S, Zhang L, Kern MJ, Yao H. Regional Cell Density Distribution and Oxygen Consumption Rates in Porcine TMJ Discs: An Explant Study. Osteoarthritis Cartilage. 2011 doi: 10.1016/j.joca.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fetter NL, Leddy HA, Guilak F, Nunley JA. Composition and transport properties of human ankle and knee cartilage. J Orthop Res. 2006;24:211–219. doi: 10.1002/jor.20029. [DOI] [PubMed] [Google Scholar]