Abstract
Purpose
Currently, there is no clinically validated test for the prediction of response to tubulin-targeting agents in non-small cell lung cancer (NSCLC). Here, we investigated the significance of nuclear expression of the mitotic checkpoint gene checkpoint with forkhead and ringfinger domains (CHFR) as predictor of response and overall survival (OS) with taxane-based first-line chemotherapy in advanced stage NSCLC.
Methods
We studied a cohort of 41 patients (median age 63 years) with advanced NSCLC treated at the Atlanta VAMC between 1999 and 2010. CHFR expression by immunohistochemistry (score 0–4) was correlated with clinical outcome using Chi-Square test and Cox proportional models. A cutoff score of ‘3’ was determined by ROC-analysis for “low” CHFR expression. Results were validated in an additional 20 patients who received taxane based chemotherapy at Emory University Hospital and the Atlanta VAMC.
Results
High expression (score = 4) of CHFR is strongly associated with adverse outcomes: the risk for progressive disease (PD) after first-line chemotherapy with carboplatin-paclitaxel was 52% in patients with CHFR-high vs. only 19% in those with CHFR-low tumors (p=0.033). Median OS was strongly correlated with CHFR expression status (CHFR low: 9.9 months; CHFR high: 6.2 months; p =0.002). After multivariate adjustment, reduced CHFR expression remained a powerful predictor of improved OS (HR 0.24 (95% CI 0.1–0.58, p=0.002). In the validation set, low CHFR expression was associated with higher likelihood of clinical benefit (p=0.03) and improved OS (p=0.038).
Conclusions
CHFR expression is a novel predictive marker of response and OS in NSCLC patients treated with taxane-containing chemotherapy.
Introduction
With 210,000 new cases and an estimated 170,000 annual deaths in the United States alone, lung cancer remains the most lethal malignancy(1). With the exception of the approximately 20% of patients with targetable driver mutations which confer “oncogene addiction”, the mainstay of therapy in patients with metastatic disease remains systemic chemotherapy. Taxanes, such as paclitaxel and docetaxel play a major role both in first- and second line therapy of NSCLC, but overall response rates remain disappointing(2, 3). The identification of biomarkers that are predictive for response serves two purposes: first, it may allow appropriate targeting of chemotherapy by selecting agents for individual patients with a high likelihood of response. Second, it may allow the development of novel targeted therapies to overcome associated mechanisms of resistance. Promising examples of such predictive markers exist for other commonly employed therapeutic agents in lung cancer patients where reduced expression of the ERCC1 and RRM1genes ‘predict sensitivity to platinum compounds and gemcitabine respectively(4–6). For taxanes however, such a promising or validated biomarker does not exist at present. Small case series have suggested that epigenetic silencing by promoter hypermethylation of the mitotic checkpoint gene ‘Checkpoint with forkhead and ringfinger domains’ (CHFR) may be associated with enhanced sensitivity to taxanes in gastric(7), cervical(8) cancer, but rigorous clinical evidence of CHFR’s usefulness as a predictive biomarker has been lacking. We and others have previously reported that CHFR is a frequent target for aberrant DNA methylation in cancer (9, 10) whereas inactivating mutations are uncommon(11). Preclinical evidence shows that CHFR is required for maintenance of the antephase checkpoint which regulates entry into mitosis. CHFR deficient cells show high levels of mitotic stress after exposure to microtubule-damaging agents such as taxanes or nocodazole(12). CHFR is a E3-ubiquitin-ligase (13) and acts as a key regulator for cell cycle entry into mitosis by controlling the activity of the aurora-kinase A (14) and the polo-like kinase 1 (15) and by excluding cyclin B1 from the nucleus (16). Mice deficient in CHFR develop spontaneous malignancies and are more susceptible to chemical carcinogenesis (17). Recently, a new zinc-finger motif was identified in the C-terminal region of CHFR as a poly-ADP-ribose-binding site(18). Interactions between CHFR and PARP1 regulate PARP1 levels and seem to be required for CHFR’s checkpoint function(19) in response to taxane-induced mitotic stress.
Given the extensive preclinical evidence of CHFR’s role in mediating the antephase checkpoint in response to taxane-induced microtubular damage, we performed a retrospective cohort study to investigate if either CHFR silencing by DNA methylation or CHFR protein levels could serve as a predictive marker for taxane sensitivity in NSCLC.
Materials and methods
Study design
The study was approved by the Institutional Review Board of Emory University and the Research and Development Committee of the Atlanta VAMC. Patients with stage IV NSCLC who received first-line treatment with a platinum-taxane combination between the years 1999–2010 were initially identified from the local cancer registry at the Atlanta VAMC. The registry data were then validated by review of the individual medical records. The following variables were recorded: Age, Sex, Race, chemotherapy regimen, number and type of subsequent therapies, clinical response at first restaging exam, ECOG performance status, tumor histology, date of first diagnosis and overall survival. Patients were further categorized based on the ECOG performance status into good(0 and 1) vs. poor(2 and 3) status. Response assessment was done by “Response Evaluation Criteria In Solid Tumors (RECIST 1.1)” criteria. Patients who had received at least 2 cycles of therapy and had available paraffin-embedded blocks with sufficient tumor tissue to cut at least 4 sections at 5uM thickness were eligible. After analysis of this original cohort, we assembled a validation cohort of 20 individuals who were either treated either at the Atlanta VAMC between 2011–2012 or Emory University Hospital between 2004–2012.
DNA extraction and methylation specific PCR
DNA was extracted from slides 3 and 4 using the E.Z.N.A™ FFPE DNA extraction kit from Omega Biotek (Norcross, GA). DNA content was quantified using an Eppendorf Biophotometer Plus (Eppendorf, Hauppauge, NJ) with Hellma Tray Cell (Hellma, Mullheim, Germany). Sodium bisulfite modification was performed on 250 ng of DNA using a commercially available kit (EZ DNA methylation kit; Zymo, Irvine, CA). This was followed by a 2-step methylation specific PCR for CHFR as previously described(9).
Pathologic review and immunohistochemistry
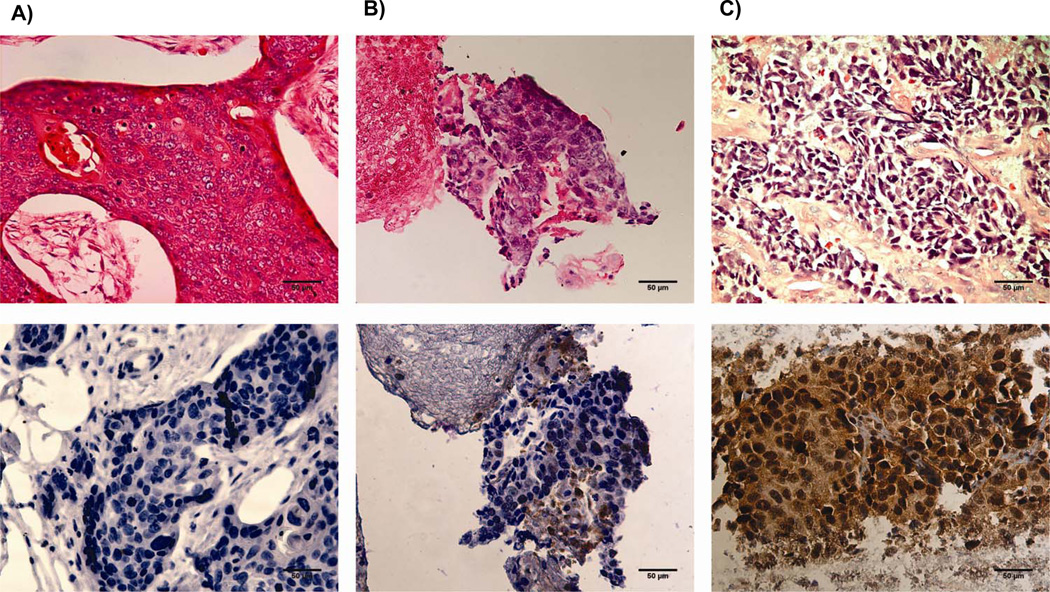
Paraffin blocks were sectioned at 5um thickness. The first slide of each block was stained with hematoxilyn and eosin (H&E) to confirm original diagnosis and specimen adequacy. The second slide was used for the detection of CHFR protein by immunohistochemistry (IHC). IHC was performed by the Cancer Tissue and Pathology Shared Resource of the Winship Cancer Institute using a monoclonal-rabbit CHFR antibody (Clone, D40H6;Cell Signaling Technology, Danvers, MA) in a 1:200 dilution. Staining occurred on a fully automated stainer after standard antigen retrieval steps as previously described. A horseradish-peroxidase labeled secondary anti-rabbit antibody was used in 1: 1000 dilution. CHFR staining was scored both for nuclear and cytoplasmic staining based on intensity (0=no staining, 1=weak staining, 2=strong staining) and percentage of cells staining (0 < 10%; 1: 10–50%; 2>50%)(20) [Figure2]. Scores for intensity and percentage of stained cells were added for a maximum score of ‘4’. Receiver operator characteristics (ROC) were used determine the optimal cut-off value [supplemental Figure 1]. Scores of ‘4’ were considered “high” expression, while all others were “reduced” expression [Figure 1]. H&E stained slides and immunohistochemistry were reviewed for accuracy of diagnosis and for scoring by a dedicated lung cancer pathologist (G.S.) who was blinded to the clinical outcomes of the patients.
Figure 2.
nuclear CHFR expression was analyzed by immunohistochemistry. Shown are samples representative of scores of ‘0’ (A), ‘2’ (B), and ‘4’ (C). Tumors with nuclear expression scores of less than ‘4’ are classified as samples with reduced nuclear CHFR expression.
Figure 1.
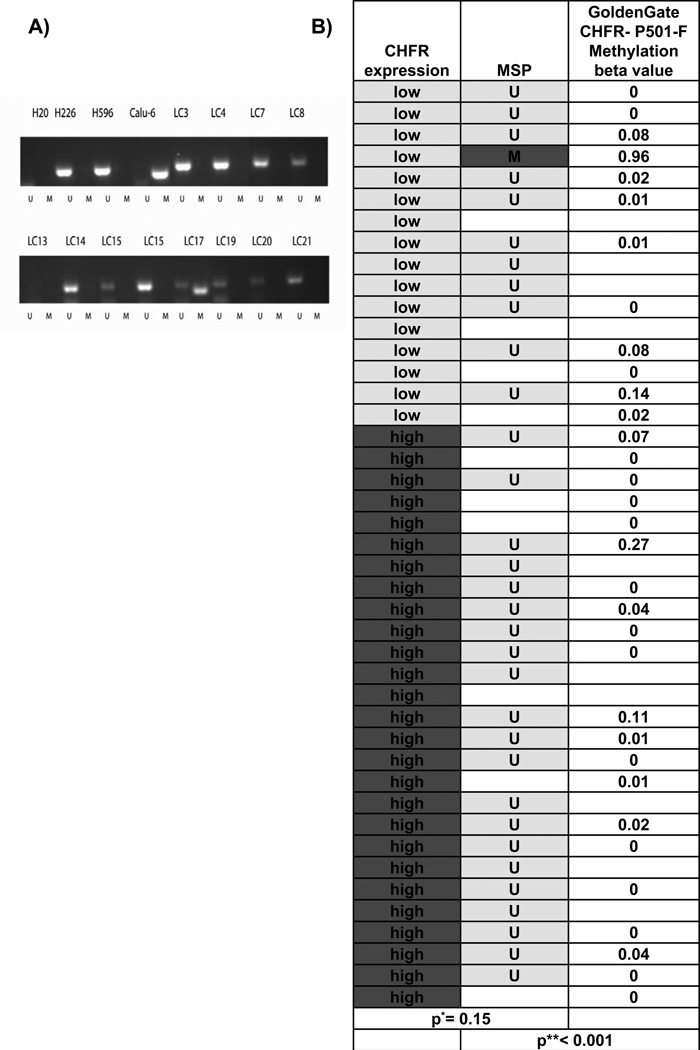
A) CHFR promoter methylation was analyzed by MSP. H226 and H596 lung cancer cell lines were used as controls for unmethylated (U) CHFR, while the Calu-6 cell lines served as control for methylated (M) CHFR. Only 1 sample (LC17) showed CHFR promoter methylation. B) CHFR expression by immunohistochemistry was compared to CHFR methylation using Fisher’s exact test*. CHFR methylation by MSP was compared to methylation beta-values derived from an Illumina GoldenGate methylation microarray using student’s t-test**.
Statistical analysis
Statistical analysis was done using SAS Version 9.3 or SAS JMP9. Descriptive data, mean, median and range were generated to describe the overall sample and the CHFR high and low expression groups. Differences between CHFR high vs. low expression were assessed using ANOVA for numerical covariates and Chi-square test or Fisher’s exact test for categorical variables, where appropriate. Overall survival was estimated using the Kaplan-Meier method and the difference between groups was assessed using a log-rank test.
Univariate survival analysis for each variable was carried out using the Cox proportional hazards model. The proportional hazard assumption was also checked for each variable. The multivariate survival analysis was conducted by entering sex, race, ECOG performance status, age, histology, and additional lines of treatment into a Cox model and using a backward variable selection method with an alpha = .20 removal criteria. CHFR was forced in the model. The model was stratified by treatment regimen and time of diagnosis since they were shown to be time dependent covariates.
Results
Patient characteristics
Between the years from 1999 to 2010 a total of 178 patients received platinum plus taxane-based chemotherapy for stage IV NSCLC at the Atlanta VAMC. Of these, 106 had a biopsy confirmation of disease at our center and had received at least 2 cycles of chemotherapy. Sixty six blocks of paraffin embedded tissue were retrieved from the pathology archives of which 41 had sufficient tumor content (at least 20% to be used in this study). The vast majority of the patients were males which is representative of the Veterans’ Health administration hospital patient population (40). All patients received carboplatin (CBCDA) and paclitaxel (TAX) with or without bevacizumab (physician decision). All enrolled patients had died at the time of data analysis.
CHFR promoter methylation in NSCLC
Previous reports have suggested that CHFR expression undergoes epigenetic silencing by DNA methylation in 14–18% of NSCLC (21, 22). Since epigenetic silencing of CHFR expression has been described in various malignancies and linked to taxane sensitivity in some, we hypothesized that CHFR promoter methylation would serve as a predictive marker for taxane sensitivity in lung cancer. We analyzed CHFR promoter methylation by qualitative methylation specific PCR (MSP)(9). We successfully amplified bisulfite modified DNA in 32 samples but observed DNA methylation in only one sample (3.1%, 95%CI (0.06–15.4%))[Figure 1A]. We compared these data to CHFR methylation data derived from a methylation microarray (Illumina Goldengate) of the same sample set and found high concordance between MSP and methylation beta-values (p < 0.001), indicating that the low frequency of CHFR methylation is unlikely to be explained by technical error[Figure 1B]. We also compared these data to those derived from a separate cohort of patients with previously resected NSCLC from the Johns Hopkins Hospital. Here, CHFR methylation was observed in 6/65 patients (9%; 95%CI (2.17–16.03%)) (data not shown). These methylation percentages are also statistically not significantly different than the results of most other studies in the literature: 18% (95% CI 5.6–30.4%)(23), 14% (95% CI 9.28–18.7%)(21), 14% (95% CI −1.2–29%)(24), 10% (95% CI −3.5%–23.5%)(25). A significantly higher methylation percentage was only found in only one study, in which circulating cell free DNA and not primary tumor samples were analyzed (26). Together data from both cohorts indicate that CHFR silencing by promoter methylation is a rare event in NSCLC.
Nuclear CHFR expression predicts response and survival
Next, we investigated if CHFR protein expression by IHC can be reduced in lung cancer, potentially due to mechanisms other than DNA methylation. Since CHFR is a checkpoint gene which primarily affects nuclear processes(12), we focused on nuclear staining patterns for our correlative analysis. Reduced nuclear staining for CHFR was observed in 16 of 41 patient samples (supplemental Table1). Baseline characteristics for age, gender, race, treatment regimen (CBCDA/TAX vs. CBCDA/TAX/Bevacizumab), histology and date of diagnosis (before or after 2005) were not significantly different between patient groups with high or low CHFR expression [Table1A]. The year 2005 was chosen as cutoff because it was around this time when second line therapy with pemetrexed(27) and antiangiogenic therapy with bevacizumab(3) emerged, resulting in improved overall survival rates(28). The subgroup of patients with low nuclear CHFR expression had trends towards having a better ECOG performance status (0 and 1 vs. 2 and 3), p=0.058 and towards a higher rate of subsequent therapies (44% vs. 32%, p=0.446).
Table 1.
| A: Patient characteristics by CHFR Expression | |||||
|---|---|---|---|---|---|
| Total | CHFR low |
CHFR high |
p- value* |
||
| N=41 (%) |
N=16 (%) |
N=25 (%) |
|||
| Age (years) | Median | 63 | 65 | 62 | 0.13 |
| Mean | 64 | 66 | 62.4 | ||
| SD | 7.7 | 8.8 | 6.7 | ||
| Race | Caucasian | 28 (68) | 12 (75) | 16 (64) | 0.46 |
| African American | 13 (32) | 4 (25) | 9 (36) | ||
| Sex | M | 40 (98) | 15 (94) | 25(100) | 0.39 |
| F | 1 (2) | 1 (6) | 0 | ||
| Treatment | CBCDA/TAX | 32 (78) | 12 (75) | 20 (80) | 0.72 |
| Bev/CBCDA/TAX | 9 (22) | 4 (25) | 5 (20) | ||
| ECOG PS | 0 | 11 (27) | 5 (31) | 6 (24) | 0.22 |
| 1 | 15 (37) | 8 (50) | 7 (28) | ||
| 2 | 6 (15) | 2 (13) | 4 (16) | ||
| 3 | 9 (22) | 1 (6) | 8 (32) | ||
| Response | CR | 1 (2) | 1 (6) | 0(0) | 0.034 |
| PR | 12 (29) | 4 (25) | 8 (32) | ||
| SD | 12 (29) | 8 (50) | 4 (16) | ||
| PD | 16 (39) | 3 (19) | 13 (52) | ||
| Squamous cell | |||||
| Histology | carcinoma (SCC) non-SCC |
9 (22) 32 (78) |
3 (19) 13 (81) |
6 (24) 19 (76) |
1.00 |
| Number of lines of additional therapy |
0 | 26 (63) | 9 (56) | 17 (68) | 0.22 |
| 1 | 10 (24) | 3 (19) | 7 (28) | ||
| 2 | 4 (10) | 3 (19) | 1 (4) | ||
| 3 | 1 (2) | 1 (6) | 0 (0) | ||
| Age-category | >=65 | 17 (41) | 8 (50) | 9 (36) | 0.38 |
| <65 | 24(59) | 8 (50) | 16 (64) | ||
| ECOG PS- category |
good | 26 (63) | 13 (81) | 13 (52) | 0.06 |
| poor | 15(37) | 3 (19) | 12 (48) | ||
| Response- category |
Clinical benefit | 25 (61) | 13 (81) | 12 (48) | 0.033 |
| Progression | 16 (39) | 3 (19) | 13 (52) | ||
| Time of diagnosis |
before 2005 | 14(34) | 5(31) | 9 (36) | 0.75 |
| 2005 and later | 27(66) | 11 (69) | 16 (64) | ||
| 2 or more lines of treatment |
no | 26 (63) | 9 (56) | 17 (68) | 0.446 |
| yes | 15 (37) | 7 (44) | 8 (32) | ||
| B: Patient characteristics of the validation set | |||||
|---|---|---|---|---|---|
| total | CHFR low | CHFR high | p- value* |
||
| 20 | 7 (%) | 13 (%) | |||
| Age | Mean | 67.4 | 61.5 | 0.3 | |
| Median | 69 | 66 | |||
| Race | C | 12 (60) | 4 (58) | 8 (62) | 1 |
| AA | 6 (30) | 2 (28) | 4 (31) | ||
| unknown | 2 (10) | 1 (14) | 1 (7) | ||
| Sex | M | 16 (80) | 5 (71.4) | 11 (84.6) | 0.48 |
| F | 4 (20) | 2 (28.6) | 2 (15.4) | ||
| Treatment | CDDP/TAX | 17 (85) | 5 (71) | 12 (70) | 0.33 |
| CDDP/TAX/Avastin | 3 (15) | 2 (29) | 1 (30) | ||
| ECOG PS | 0 | 4 (20) | 2 (29) | 2 (15) | 0.84 |
| 1 | 6 (30) | 2 (29) | 4 (31) | ||
| 2 | 6 (30) | 2 (29) | 4 (31) | ||
| unknown | 4 (20) | 1 (15) | 3 (23) | ||
| Histology | SCC | 1 (5) | 0 (0) | 1 (7.7) | 0.34 |
| non-SCC | 19 (95) | 7 (100) | 12 (92.3) | ||
| Clinical benefit |
yes | 11 (55) | 7 (100) | 4 (31) | 0.03 |
| non | 8 (40) | 0 (0) | 8 (62) | ||
| Unknown | 1 (5) | 0 (0) | 1 (7) | ||
| Time of diagnosis |
Before 2005 | 2 (10) | 1(14.3) | 1 (7.7) | 0.64 |
| After 2005 | 18 (80) | 6 (85.7) | 12 (92.3) | ||
| 2 or more lines of therapy |
yes | 3 (15) | 2(29) | 1(8) | |
| no | 17 (85) | 5(71) | 12(92) | 0.22 | |
The p-value is calculated by ANOVA for age in years; Fisher’s exact test for gender, treatment, ECOG PS, response, histology, and additional lines of treatment; Chi-sqaure test for the remaining categorical covariates; and log-rank test for survival.
The p-value is calculated by ANOVA for age in years; Fisher’s exact test for gender, treatment, ECOG PS, clinical benefit, histology
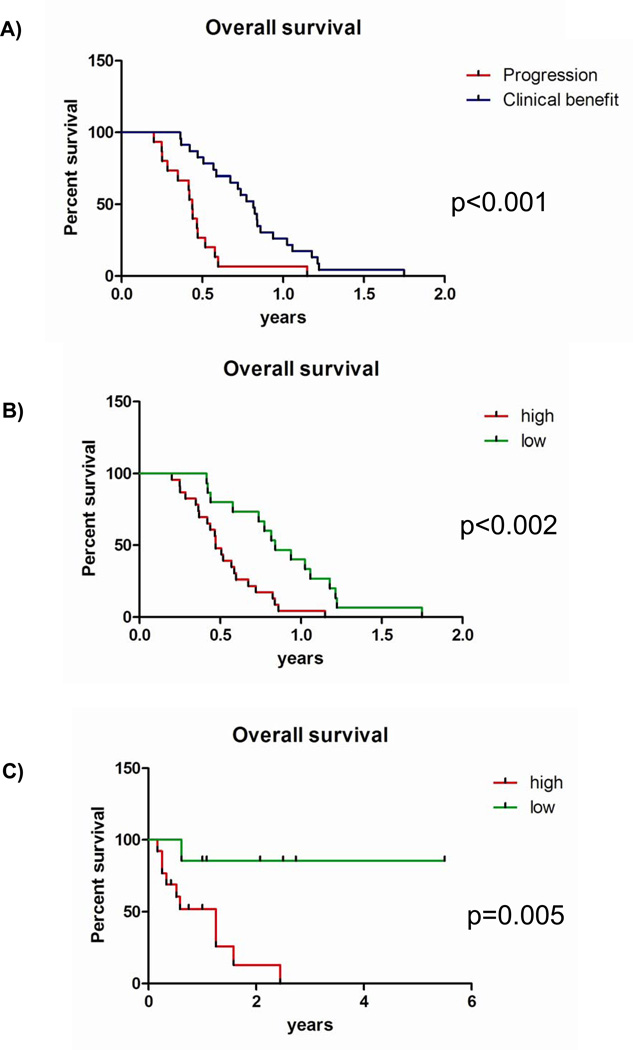
Reduced nuclear CHFR expression in 16 patients (37%) showed a statistically significant association with response to therapy as determined at first restaging (19% progression vs. 52% progression, p=0.033) [Table1A]. Kaplan-Meier analysis and univariate Cox models showed a strong correlation between clinical benefit at first restaging and overall survival (Median survival 9.4 months vs. 5.1 months, HR 0.28 (95%CI 0.14–0.56), p<.001), [Figure 3A]. Low nuclear CHFR expression was also strongly predictive of improved survival (median survival 9.9 months vs. 5.7 months, HR 0.32 (95%CI 0.16–0.67, p=0.002) [Figure 3B]. To account for potential confounders of these results, particularly in light of the slightly uneven distribution of patients with good vs. poor performance status, we performed a multivariate Cox proportional hazard analysis. After multivariate adjustment, reduced nuclear CHFR expression emerged as an even more powerful predictor of survival (HR 0.24 (95% CI 0.1–0.58, p=0.002) [Table2]. Second line of treatment was the only other covariate which was significantly associated with survival. These results were validated in our second cohort: low CHFR expression was associated with a higher likelihood of achieving a clinical benefit (100% vs. 31%, p= 0.03) [Table 1B] and improved overall survival (median survival CHFR high: 1.25 years vs. CHFR low- not yet reached HR 0.09 (95% CI 0.004–0.5), p= 0.006) [Figure 3C]. This association was confirmed after multivariate adjustment (HR 0.11(95%CI 0.01–0.88), p =0.038) [Table 2].
Figure 3.
Overall survival analysis by Kaplan-Meier in the original VAMC cohort by A) response to treatment and B) CHFR expression levels. C) Overall survival in the validation cohort by CHFR expression level. All p-values were determined by the log-rank test.
Table 2.
Univariate and multivariate adjusted hazard ratio for overall survival
| Atlanta VAMC cohort | Validation cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| Crude HR (95%CI) |
p-value | Multivariate adjusted HR* (95% CI) |
p-value | Crude HR (95%CI) |
p-value | Multivariate adjusted HR*(95% CI) |
p- value |
|
| CHFR nuclear stain high vs. low |
3.09 (1.5–6.4) |
0.001 |
4.18 (1.71–10.18) |
0.002 |
10.9 (1.36–88) |
0.006 |
9.15 (1.14–104.84) |
0.038 |
| Age (<65 vs. >=65) | 1.60 (0.82–3.12) |
0.16 | 1.96 (0.83–4.65) |
0.125 | 1.4 (0.40–4.76) |
0.58 | 1.37 (0.32–5.88) |
0.67 |
| Gender (Male vs. female) | 1.5 (0.7–25.7) |
0.69 | † | 2.15 (0.4–39.7) |
0.45 | † | ||
| Race (AA vs. C) | 1.07 (0.54–2.09) |
0.85 | † | 1.27 (0.36–4.27) |
0.70 | † | ||
| Treatment | ||||||||
| (CBCDA/TAX vs. CBCDA/TAX/Bevacizumab |
1.405 (0.65–3.01) |
0.38 | * | 2.67 (0.50–49.2) |
0.31 | * | ||
| Histology (SCC vs non- SCC) |
1.6 (0.73–3.36) |
0.25 | † | 4.21 (0.22–28.5) |
0.16 | † | ||
| Performance status (good vs. poor) |
0.57 (0.29–1.11) |
0.09 | † | 0.51 (0.12–4.58) |
0.31 | † | ||
| Time of diagnosis (before 2005 vs. later) |
0.95 (0.48–1.86) |
0.87 | * | 0.28 (0.06–1.96) |
0.10 | * | ||
| Second line treatment (no vs. yes) |
3.254 (1.59–6.66) |
<0.001 |
6.32 (2.58–15.51) |
<0.001 | 4.16 (0.75–78) |
0.15 | 2.21 (0.24–20.5) |
0.49 |
Stratified by treatment and time of diagnosis.
Variable dropped through backward selection (p-value > 0.20).
Discussion
This is the first report to show a robust and statistically significant correlation between nuclear CHFR expression levels and two important clinical outcomes measures (response and overall survival) after first-line therapy with carboplatin and paclitaxel in NSCLC. Our results support the ample preclinical evidence for the role of the CHFR controlled antephase checkpoint in response to microtubular damage(12): in cells with intact CHFR expression, the antephase checkpoint delays entry into mitosis, prevents nuclear translocation of cyclin D1 and allows cells to repair taxane induced microtubular damage (12). Cells which are deficient in CHFR expression enter mitosis without delay and undergo mitotic catastrophy, ultimately resulting in apoptosis. Our findings have two-fold direct clinical implications: first, taxane based chemotherapy could emerge as molecularly targeted therapy for patients with metastatic NSCLC whose tumors show reduced expression of CHFR but lack actionable driver mutations. The feasibility of biomarker-directed therapy in NSCLC has been well established as exemplified by treatment selections based on the presence of EGFR(29)- and more recently k-ras mutations(30), ALK-translocations(31) and expression levels of ERCC1(32) and RRM1(33).
The second clinical consequence is the identification of high nuclear CHFR expression as a mechanism of resistance against taxane-based first line therapy in NSCLC. Strategies to target CHFR’s function could thus lead to dramatic improvements in response rates to taxane-based first line therapy in metastatic NSCLC. One such approach would be to exploit the interaction between CHFR and PARP1, which is required, at least in vitro, for CHFR’s checkpoint function(18, 19).
Given its retrospective nature, several limitations of our study need to be considered. This is a single cohort of US veterans who were treated at a single institution over a long period of time. The median overall survival of slightly over 7 months in our cohort is significantly shorter than overall survival rates reported in recent chemotherapy trials in metastatic NSCLC(2, 3). Possible explanations for this are higher rates of associated comorbidities in US veterans, poorer performance status of this ‘real-world cohort’ compared to patients eligible for clinical trials, and the fact the observation period started in 1999, when significantly less treatment options were available for NSCLC. It is well established that survival times in lung cancer improved over the last decade with an increasing number of therapeutic options(28). In patients without oncogenic driver mutations, the most significant developments were the introduction of antiangiogenic therapy with bevacizumab in the first-line setting, the development of active second-line therapies with docetaxel and pemetrexed and maintenance therapy with pemetrexed. In our analysis, we observed an equal distribution of patients treated before and after 2005 in both cohorts. Date of diagnosis was stratified into the multivariate survival analysis to take this effect into account. In our analysis we focused on response and overall survival as the primary endpoints. Progression-free survival, which is heavily dependent on uniform timing of the restaging exams was not considered in this retrospective analysis where patients did not receive treatment according to a uniform schedule. Overall survival can be influenced by the number and duration of subsequent treatment regimens. Indeed, reduced CHFR expression in our cohort was associated with trend towards a higher chance of receiving subsequent second line therapy. However, there are two lines of evidence in this cohort to support CHFR’s direct role in mediating sensitivity of taxane-based chemotherapy independent from subsequent therapies: first, we observed a correlation between reduced CHFR expression and a significantly higher likelihood of response at first restaging exam. This finding could not have been affected by additional therapies and is likely to have a direct effect on overall survival(34). In addition, our multivariate analysis revealed that both reduced CHFR expression levels and second line therapies were both independent predictors of improved overall survival. Due to the demographics of lung cancer patients in the Veteran’s Health Administration, our cohort is overwhelmingly male and it is impossible to exclude a confounding effect of gender on the observed association between reduced nuclear CHFR expression and clinical outcomes.
This exciting observation is supported by preclinical mechanistic data that support our hypothesis. In a uniformly treated cohort like ours it is not possible to distinguish between prognostic or predictive markers or to prove that CHFR levels and platinum sensitivity are completely unrelated. However, ample preclinical and clinical evidence exists linking low CHFR expression specifically to taxane- but not platinum-sensitivity: first, overexpression or knockdown of CHFR in vitro is strongly associated with altered response to taxanes(10, 12, 13) but not DNA damaging agents (35, 36). Second, clinical studies have failed to show an association between CHFR methylation and response to platinum /non-taxane based combination-therapy (37). Finally, in chemonaive patients with resected NSCLC, reduced CHFR expression is associated with a more aggressive phenotype and inferior survival, ruling out the possibility that our findings could be due to an inherently favorable prognosis of patients whose tumors have reduced CHFR expression (24).
We have clearly shown that epigenetic silencing by promoter DNA methylation does not account for all instances of reduced CHFR expression, a finding that is supported by at least one other report in the literature(24). The molecular basis for CHFR repression in non-epigenetically silenced cancers will require further study.
In summary, we have shown for the first time a robust association between reduced nuclear CHFR expression and response and survival after first-line platinum-taxane combination therapy in NSCLC. We expect that these findings will aid the design of prospective studies to evaluate the role of taxanes as molecularly targeted therapy in patients whose tumors show reduced CHFR expression and help to develop novel therapies to target CHFR in order to overcome taxane resistance.
Supplementary Material
Supplemental Figure1: Receiver operator characteristics (ROC) were determined for various cutoff values in order to predict ‘clinical benefit’. Best test performance was achieved with a cutoff <=3.
Statement of translational relevance.
Our study represents a correlative biomarker study in which protein expression of the mitotic checkpoint gene CHFR (checkpoint with forkhead and ringfinger domains) is correlated with clinical outcomes after first-line therapy with carboplatin and paclitaxel in patients with advanced NSCLC. A strong correlation between response and improved survival is observed with reduced CHFR expression, making this a potentially very powerful biomarker to predict outcomes after taxane based therapy in NSCLC.
Acknowledgements
Grant support:
Veterans’ Health Administration Career Development Award 1IK2BX001283-01 to JCB; NCI- 5 P50 CA128613-02 Career Development Project to JCB; CHEST Foundation / Lungevity Foundation Clinical Lung Cancer Research Award to JCB; Suntrust Scholar Award to JCB. SSR, TKO and FRK are Georgia Cancer Coalition Distinguished Cancer Scholars.
Footnotes
Presented at the ASCO 2012 annual meeting; J Clin Oncol 30, 2012 (suppl; abstr 10625)
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. Epub 2008/02/22. [DOI] [PubMed] [Google Scholar]
- 2.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. The New England journal of medicine. 2002;346(2):92–98. doi: 10.1056/NEJMoa011954. Epub 2002/01/11. [DOI] [PubMed] [Google Scholar]
- 3.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. The New England journal of medicine. 2006;355(24):2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 4.Lord RV, Brabender J, Gandara D, Alberola V, Camps C, Domine M, et al. Low ERCC1 expression correlates with prolonged survival after cisplatin plus gemcitabine chemotherapy in non-small cell lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2002;8(7):2286–2291. Epub 2002/07/13. [PubMed] [Google Scholar]
- 5.Olaussen KA, Dunant A, Fouret P, Brambilla E, Andre F, Haddad V, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. The New England journal of medicine. 2006;355(10):983–991. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- 6.Zheng Z, Chen T, Li X, Haura E, Sharma A, Bepler G. DNA synthesis and repair genes RRM1 and ERCC1 in lung cancer. The New England journal of medicine. 2007;356(8):800–808. doi: 10.1056/NEJMoa065411. [DOI] [PubMed] [Google Scholar]
- 7.Koga Y, Kitajima Y, Miyoshi A, Sato K, Sato S, Miyazaki K. The significance of aberrant CHFR methylation for clinical response to microtubule inhibitors in gastric cancer. Journal of gastroenterology. 2006;41(2):133–139. doi: 10.1007/s00535-005-1732-7. [DOI] [PubMed] [Google Scholar]
- 8.Banno K, Yanokura M, Kawaguchi M, Kuwabara Y, Akiyoshi J, Kobayashi Y, et al. Epigenetic inactivation of the CHFR gene in cervical cancer contributes to sensitivity to taxanes. International journal of oncology. 2007;31(4):713–720. [PubMed] [Google Scholar]
- 9.Brandes JC, van Engeland M, Wouters KA, Weijenberg MP, Herman JG. CHFR promoter hypermethylation in colon cancer correlates with the microsatellite instability phenotype. Carcinogenesis. 2005;26(6):1152–1156. doi: 10.1093/carcin/bgi058. [DOI] [PubMed] [Google Scholar]
- 10.Toyota M, Sasaki Y, Satoh A, Ogi K, Kikuchi T, Suzuki H, et al. Epigenetic inactivation of CHFR in human tumors. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(13):7818–7823. doi: 10.1073/pnas.1337066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mariatos G, Bothos J, Zacharatos P, Summers MK, Scolnick DM, Kittas C, et al. Inactivating mutations targeting the chfr mitotic checkpoint gene in human lung cancer. Cancer research. 2003;63(21):7185–7189. Epub 2003/11/13. [PubMed] [Google Scholar]
- 12.Scolnick DM, Halazonetis TD. Chfr defines a mitotic stress checkpoint that delays entry into metaphase. Nature. 2000;406(6794):430–435. doi: 10.1038/35019108. [DOI] [PubMed] [Google Scholar]
- 13.Chaturvedi P, Sudakin V, Bobiak ML, Fisher PW, Mattern MR, Jablonski SA, et al. Chfr regulates a mitotic stress pathway through its RING-finger domain with ubiquitin ligase activity. Cancer research. 2002;62(6):1797–1801. [PubMed] [Google Scholar]
- 14.Yu X, Minter-Dykhouse K, Malureanu L, Zhao WM, Zhang D, Merkle CJ, et al. Chfr is required for tumor suppression and Aurora A regulation. Nature genetics. 2005;37(4):401–406. doi: 10.1038/ng1538. [DOI] [PubMed] [Google Scholar]
- 15.Kang D, Chen J, Wong J, Fang G. The checkpoint protein Chfr is a ligase that ubiquitinates Plk1 and inhibits Cdc2 at the G2 to M transition. The Journal of cell biology. 2002;156(2):249–259. doi: 10.1083/jcb.200108016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Summers MK, Bothos J, Halazonetis TD. The CHFR mitotic checkpoint protein delays cell cycle progression by excluding Cyclin B1 from the nucleus. Oncogene. 2005;24(16):2589–2598. doi: 10.1038/sj.onc.1208428. [DOI] [PubMed] [Google Scholar]
- 17.Alao JP, Stavropoulou AV, Lam EW, Coombes RC, Vigushin DM. Histone deacetylase inhibitor, trichostatin A induces ubiquitin-dependent cyclin D1 degradation in MCF-7 breast cancer cells. Molecular cancer. 2006;5:8. doi: 10.1186/1476-4598-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahel I, Ahel D, Matsusaka T, Clark AJ, Pines J, Boulton SJ, et al. Poly(ADPribose)- binding zinc finger motifs in DNA repair/checkpoint proteins. Nature. 2008;451(7174):81–85. doi: 10.1038/nature06420. Epub 2008/01/04. [DOI] [PubMed] [Google Scholar]
- 19.Kashima L, Idogawa M, Mita H, Shitashige M, Yamada T, Ogi K, et al. CHFR protein regulates mitotic checkpoint by targeting PARP-1 protein for ubiquitination and degradation. The Journal of biological chemistry. 2012;287(16):12975–12984. doi: 10.1074/jbc.M111.321828. Epub 2012/02/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi C, Oda Y, Takahira T, Izumi T, Kawaguchi K, Yamamoto H, et al. Aberrant expression of CHFR in malignant peripheral nerve sheath tumors. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2006;19(4):524–532. doi: 10.1038/modpathol.3800548. [DOI] [PubMed] [Google Scholar]
- 21.Koga T, Takeshita M, Yano T, Maehara Y, Sueishi K. CHFR hypermethylation and EGFR mutation are mutually exclusive and exhibit contrastive clinical backgrounds and outcomes in non-small cell lung cancer. Int J Cancer. 128(5):1009–1017. doi: 10.1002/ijc.25447. Epub 2010/05/18. [DOI] [PubMed] [Google Scholar]
- 22.Mizuno K, Osada H, Konishi H, Tatematsu Y, Yatabe Y, Mitsudomi T, et al. Aberrant hypermethylation of the CHFR prophase checkpoint gene in human lung cancers. Oncogene. 2002;21(15):2328–2333. doi: 10.1038/sj.onc.1205402. Epub 2002/04/12. [DOI] [PubMed] [Google Scholar]
- 23.Mizuno K, Osada H, Konishi H, Tatematsu Y, Yatabe Y, Mitsudomi T, et al. Aberrant hypermethylation of the CHFR prophase checkpoint gene in human lung cancers. Oncogene. 2002;21(15):2328–2333. doi: 10.1038/sj.onc.1205402. [DOI] [PubMed] [Google Scholar]
- 24.Takeshita M, Koga T, Takayama K, Kouso H, Nishimura-Ikeda Y, Yoshino I, et al. CHFR expression is preferentially impaired in smoking-related squamous cell carcinoma of the lung, and the diminished expression significantly harms outcomes. Int J Cancer. 2008;123(7):1623–1630. doi: 10.1002/ijc.23673. Epub 2008/07/16. [DOI] [PubMed] [Google Scholar]
- 25.Corn PG, Summers MK, Fogt F, Virmani AK, Gazdar AF, Halazonetis TD, et al. Frequent hypermethylation of the 5' CpG island of the mitotic stress checkpoint gene Chfr in colorectal and non-small cell lung cancer. Carcinogenesis. 2003;24(1):47–51. doi: 10.1093/carcin/24.1.47. [DOI] [PubMed] [Google Scholar]
- 26.Salazar F, Molina MA, Sanchez-Ronco M, Moran T, Ramirez JL, Sanchez JM, et al. First-line therapy and methylation status of CHFR in serum influence outcome to chemotherapy versus EGFR tyrosine kinase inhibitors as second-line therapy in stage IV non-small-cell lung cancer patients. Lung Cancer. 72(1):84–91. doi: 10.1016/j.lungcan.2010.07.008. Epub 2010/08/14. [DOI] [PubMed] [Google Scholar]
- 27.Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-smallcell lung cancer previously treated with chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(9):1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 28.Owonikoko TK, Ramalingam SS, Kanterewicz B, Balius TE, Belani CP, Hershberger PA. Vorinostat increases carboplatin and paclitaxel activity in non-small-cell lung cancer cells. Int J Cancer. 126(3):743–755. doi: 10.1002/ijc.24759. Epub 2009/07/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. The New England journal of medicine. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. Epub 2009/08/21. [DOI] [PubMed] [Google Scholar]
- 30.Janne PA, Shaw AT, Pereira JR, Jeannin G, Vansteenkiste J, Barrios C, et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. The lancet oncology. 2012 doi: 10.1016/S1470-2045(12)70489-8. Epub 2012/12/04. [DOI] [PubMed] [Google Scholar]
- 31.Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. The New England journal of medicine. 2010;363(18):1693–1703. doi: 10.1056/NEJMoa1006448. Epub 2010/10/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cobo M, Isla D, Massuti B, Montes A, Sanchez JM, Provencio M, et al. Customizing cisplatin based on quantitative excision repair cross-complementing 1 mRNA expression: a phase III trial in non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(19):2747–2754. doi: 10.1200/JCO.2006.09.7915. Epub 2007/07/03. [DOI] [PubMed] [Google Scholar]
- 33.Simon G, Sharma A, Li X, Hazelton T, Walsh F, Williams C, et al. Feasibility and efficacy of molecular analysis-directed individualized therapy in advanced non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(19):2741–2746. doi: 10.1200/JCO.2006.08.2099. Epub 2007/07/03. [DOI] [PubMed] [Google Scholar]
- 34.Shanafelt TD, Loprinzi C, Marks R, Novotny P, Sloan J. Are chemotherapy response rates related to treatment-induced survival prolongations in patients with advanced cancer? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(10):1966–1974. doi: 10.1200/JCO.2004.08.176. Epub 2004/04/28. [DOI] [PubMed] [Google Scholar]
- 35.Ogi K, Toyota M, Mita H, Satoh A, Kashima L, Sasaki Y, et al. Small interfering RNA-induced CHFR silencing sensitizes oral squamous cell cancer cells to microtubule inhibitors. Cancer biology & therapy. 2005;4(7):773–780. doi: 10.4161/cbt.4.7.1896. [DOI] [PubMed] [Google Scholar]
- 36.Satoh A, Toyota M, Itoh F, Sasaki Y, Suzuki H, Ogi K, et al. Epigenetic inactivation of CHFR and sensitivity to microtubule inhibitors in gastric cancer. Cancer research. 2003;63(24):8606–8613. [PubMed] [Google Scholar]
- 37.Hamilton JP, Sato F, Greenwald BD, Suntharalingam M, Krasna MJ, Edelman MJ, et al. Promoter methylation and response to chemotherapy and radiation in esophageal cancer. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2006;4(6):701–708. doi: 10.1016/j.cgh.2006.03.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure1: Receiver operator characteristics (ROC) were determined for various cutoff values in order to predict ‘clinical benefit’. Best test performance was achieved with a cutoff <=3.