Abstract
Purpose
Head and neck squamous cell carcinoma (HNSCC) is one of the ten most common cancers with a 50% five-year survival rate, which has remained unchanged for the past three decades. One of the major reasons for the aggressiveness of this cancer is that HNSCCs readily metastasize to cervical lymph nodes that are abundant in the head and neck region. Hence, discovering new molecules controlling the metastatic process as well as understanding their regulation at the molecular level are essential for effective therapeutic strategies.
Experimental Design
Rab25 expression level was analyzed in HNSCC tissue microarray. We used a combination of intravital microscopy in live animals and immunofluorescence in an in vitro invasion assay, to study role of Rab25 in tumor cells migration and invasion.
Results
In this study, we identified the small GTPase Rab25 as a key regulator of HNSCC metastasis. We observed that Rab25 is downregulated in HNSCC patients. Next, we determined that re-expression of Rab25 in a metastatic cell line is sufficient to block invasion in a 3D collagen matrix and metastasis to cervical lymph nodes in a mouse model for oral cancer. Specifically, Rab25 affects the organization of F-actin at the cell surface, rather than cell proliferation, apoptosis or tumor angiogenesis.
Conclusion
These findings suggest that Rab25 plays an important role in tumor migration and metastasis, and that understanding its function may lead to the development of new strategies to prevent metastasis in oral cancer patients.
Keywords: Rab25, metastasis, oral cancer, intravital microscopy, actin cytoskeleton
Introduction
The term head and neck cancer encompasses a series of cancers that arise from the oral and nasal cavity, salivary glands, paranasal sinuses, oropharynx, hypopharynx and larynx. More than 90% of these cancers are squamous cell carcinomas (SCC), which originate from the squamous mucosa lining the upper aerodigestive tract (1). Head and neck squamous cell carcinoma (HNSCC) is very aggressive and often invades the cervical lymph nodes and metastasizes to distant sites. Distant metastasis can adversely impact survival and significantly affect treatment planning. The combination of conventional surgery or radiotherapy and chemotherapy is still the treatment of choice for HNSCC patients (2). However, the five-year survival rate (50%) of these patients has not improved in the past three decades (3). Advancing our understanding of the molecular mechanisms regulating tumor invasion and metastasis for HNSCC may lead to more effective treatment options.
Invasion and metastasis are the result of a complex interplay of multiple processes including: angiogenesis of lymphatic and blood vessel, loss of cell polarity, loss of cell-cell contact, extracellular matrix breakdown mediated by specific metalloproteases, remodeling of the actin cytoskeleton, and tumor proliferation at the secondary sites (4, 5). Recent studies have demonstrated that molecules involved in intracellular membrane trafficking steps, endocytosis in particular, control some of these processes (6, 7). Among these molecules, members of the Rab family are deregulated in various cancers (8). Rab proteins are considered master regulators of membrane trafficking. With at least 70 members in the human genome, they constitute the largest family of small GTPases. However, Rabs share limited homology to each other (40%) and individual Rab family members play distinct biological roles (9, 10). Rabs regulate both endocytic and secretory pathways where they control different events including protein sorting, membrane lipid composition, organelle biogenesis, interaction with cytoskeletal elements, and membrane fission (11). Rab molecules that regulate the secretory pathway are elevated in different cancer types, such as Rab1 in human tongue cancer (12), Rab3 in cancers of the nervous system and neuroendocrine cells, Rab22b/31 in breast cancer (13), and Rab23 in hepatic and gastric cancer (14, 15). Endocytic Rabs are also deregulated in cancer. For example, Rab5a is elevated in hepatocellular carcinoma and thyroid adenoma (16, 17), Rab5b is decreased in metastatic melanoma, and Rab7 and Rab20 are upregulated in thyroid adenoma and pancreatic carcinoma, respectively (13).
Recently, more attention has been directed towards Rab25, a member of the Rab11 subfamily, which is exclusively expressed in epithelial cells. Rab25 has been proposed to regulate the recycling of proteins from the endosomes to the plasma membrane (18). However, the association between Rab25 deregulation and cancer is still a matter of debate. For example, Rab25 expression is elevated and correlated to poor prognosis in prostate and ovarian cancer. On the other hand, Rab25 has also been reported to be both up and downregulated in breast cancer (19-21). More recently, loss of Rab25 has been shown to promote intestinal neoplasia in mice and to correlate with human colorectal adenocarcinomas (22). These evidences indicate an important role of Rab25 in tumor progression and aggressiveness. Several mechanisms have been proposed to define the role of Rab25 in cancer. Cheng et al. reported that overexpression of Rab25 inhibits apoptosis and autophagy by increasing cellular bioenergetics (23). Others have suggested that Rab25, together with the chloride intracellular channel 3-(CLIC3) regulates tumor invasiveness and mediates recycling of α5β1-integrin to the plasma membrane from a late endosomal compartment (5). Though the mechanism remains unclear, these pieces of evidence strongly implicate Rab25 in tumor development, progression and aggressiveness.
In this study, we investigated the role of Rab25 and its regulation in HNSCC. First, we determined the Rab25 status in normal oral mucosa and HNSCC tissues of differing grade and stage. Next, we used a lentiviral expression system to modify Rab25 expression in SCC cells to study pathological functions of tumor cells. Finally, we investigated the role of Rab25 in tumor development and progression by using a combination of intravital two-photon microscopy and immunohistochemistry in a mouse model of oral cancer.
Materials and methods
Cells lines
Human cancer cell lines HN12, HeLa-O3, Cal27, UMCCC2 and UMSCC17B maintained as previously described (24) in Dulbecco’s Modified Eagle’s Media supplemented with 10% FBS, at 37°C in 95% air/5% CO2. ORL48 (25) and ORL150 were kindly gifts from Dr. Cheong Sok Ching, CARIF, Malaysia. HeLa-A, SiHa, HaCaT, C33A and A549 cell lines were obtained from ATCC (VA, USA) and HeLa-J (kindly gift from Dr. Julie Donaldson, NHLBI, NIH) maintained according to the company instruction. Human immortalized normal oral keratinocytes (HNOK) were established as described (26). All above cell lines underwent STR DNA authentication (Genetica DNA Laboratories, Inc., Cincinnati, OH) prior to the described experiments to ensure consistency in cell identity.
Plasmids constructs
Human Rab25 and Rab11a full-length cDNA clones were obtained from Mammalian Gene Collection (MGC, NIH) and subcloned into pENTR sfiI intermediate plasmid by fusing it with venus or RFP sequence at the c-terminal. The c-terminal hypervariable regions of Rab25 (amino acid 171-213) and Rab11 (amino acid 170-216) were generated by PCR-cloning and fused with the N-terminal regions of Rab11 (amino acid 1-169) and Rab25 (amino acid 1-170), respectively. Lifeact GFP was a gift from Tamas Balla (NICHD, NIH). Histone-GFP fusion (H2B-GFP) was obtained from Addgene (#11680) (27).
Antibodies and Reagents
The following antibodies were used in this study: rabbit polyclonal anti-Rab25 and cleaved-caspase3 (Cell Signaling Technology, Beverly, MA), rabbit polyclonal anti-Rab25 (recognized amino acid residues 131-145, Sigma -Aldrich, St. Louis, MO), rabbit polyclonal antisera against EGFR and tubulin (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit polyclonal anti-GFP and Lyve1 (Abcam, Cambridge, MA), rabbit polyclonal anti-Ki67 (Nova Castra, Leica microsystems, Buffalo Grove, IL), rat monoclonal anti-CD31 (BD Pharmingen, San Diego, CA). All antibodies were used for Western blot analysis or immunohistochemistry (IHC) at a dilution of 1:1000 or 1:100 respectively. AlexaFluor 488, 594 and 647 conjugated secondary antibodies for immunofluorescence were purchased from Invitrogen. Epidermal growth factor (EGF), Latrunculin A and Cytochalasin D were obtained from Sigma (St. Louis, MO).
Lentiviral expression system
cDNAs encoding for TagRFP, H2B-GFP, venus, venus-Rab25, venus-Rab25-11, venus-Rab11-25, RFP-Rab25 and Lifeact GFP were subcloned into the intermediate vector pENTRsfiI and transferred to the lentiviral expression vector pLESIP (28). Short hairpin RNAs (shRNAs) targeting non-silencing scramble sequence (pGIPZ sh-scramble) and three different sequences of Rab25 (pGIPZ sh-Rab25; clone ID V2LHS_38594, V3LHS_362078, V3LHS_362078) were obtained from Open Biosystem (Thermo Scientific, Rockford, IL). Lentiviral stocks were prepared and titrated with HEK-293T cells as packaging cells. Tumor cells were infected with the virus for 16 hours. After, cells were returned to normal growth medium and infected cells were isolated with fluorescent activated cell sorting (FACS) and maintained under puromycin (1 μg/ml) selection.
Tissue arrays immunohistochemistry (IHC)
Oral cavity squamous cell carcinoma and normal tissues high-density tissue microarray with grade and TNM (stage) (69 cases/208 cores, #OR 208) were purchased from US BioMax Inc. (Rockville, MD) and used for IHC. The formalin-fixed paraffin-embedded tumor tissue array slides were first deparaffinized in 100% xylene. The slides were hydrated through a series of graded alcohols (100%, 95%, 80% and 70%) for 5 minutes each, washed once in H2O for 5 minutes, incubated in sodium citrate buffer (pH6.0) for 2 minutes in microwave at full power, and then for 20 minutes at 10% power to unmask the antigen. The slides were then incubated in: 1) 3% hydrogen peroxide at room temperature for 10 minutes to quench endogenous peroxidase activity; 2) blocking serum (2% bovine serum albumin in PBS-1% Tween 20) for 1 hour; and 3) primary antibody in blocking buffer overnight at 4°C. The slides were washed in PBS three times, incubated with a biotin-conjugated secondary antibody (1:400 dilution) at room temperature for 30 minutes followed by the ABC complex (Vector Stain Elite, ABC kit, Vector Laboratories) for 30 min at room temperature. The slides were washed and developed in 3,3′-diaminobenzidine (Sigma FASTDAB tablet, Sigma Chemical) under microscopic observation. The reaction was stopped in tap water and the tissues were counterstained with Mayer’s hematoxylin, dehydrated, and mounted. The images were taken using Scanscope (Aperio, Vista, CA). The evaluation of the IHC was conducted blindly by AM and PA. The whole tissue array slides were examined and classified based on the staining intensity (1, weak staining; 2, moderate staining; and 3, strong staining) and the percentage of positive cells quantified as previously described (0, <10% of stained cells; 1, 10%-25%; 2, 25%-50%; 3, 50-75%; and 4, 75-100% of cells stained) (28, 29).
Three-dimensional collagen-matrix invasion assay
Collagen gels (rat tail collagen type I, BD Bioscience, Bedford, MA) were prepared in serum free DMEM at a final concentration of 2.0 mg/ml and added (500 μl volume) to transwell inserts (0.4 μm polycarbonated membrane) of 12-well plates (Costar, Corning, NY). Collagen was allowed to polymerize and equilibrate in culture incubator for 2h. After, 200 μl of serum-free media containing 0.4×106 tumor cells were added to each well. Serum-containing, serum-free media without or with 10 ng/ml Epidermal growth factor (EGF) were added into the bottom wells. When needed, inhibitors of the actin cytoskeleton were added in the transwell insert at the beginning of assay (1 μM Latrunculin A and 10 μm Cytochalasin D). Cells were allowed to invade in the 3D collagen gels for 8 h. Culture media was removed and the gels containing the tumor cells were fixed in 4% formaldehyde in PBS for 30 minutes. Z stack images were obtained using an inverted confocal Olympus microscope (water objective XLUMPFL20XW, NA 0.95). Fluorescent-labeled polystyrene microspheres (15 μm) (Invitrogen) were used to mark the top of the gel. To quantify tumor cells invasion, eight random fields per condition in at least two independent experiments were scanned and the distance of the invading cells from the top of the gel was measured. Data are reported as mean distance of invading cell (± S.D.).
Tongue tumor xenograft in athymic nu/nu or SCID mice
All the experiments were approved by the National Institute of Dental and Craniofacial Research Animal Care and Use Committee (National Institute of Health, Bethesda, MD, USA). Female athymic (nu/nu) nude mice (Harlan Sprague Dawley, Frederick, MD) and female SCID mice (National Cancer Institute at Frederick, Frederick, MD) 5-6 weeks old and 20-25 g, were used in the study. The mice were housed in appropriate sterile filter-capped cages, fed and watered ad libitum. Half a million of HN12 or HeLa-O3 cells were submucosally injected in the lateral anterior of the tongue. Animals were fed with soft dough diet from the day of injection. The tumor growth was monitored on a weekly basis. For each experiment, tumor-bearing animals were randomly divided into groups of 10 animals. All experiments were done in triplicated. At the indicated time points, animals were euthanized and tissues were collected. Tissues were lysed in protein lysis buffer for Western blot. Alternatively, they were fixed and embedded in paraffin, or frozen and embedded in OCT compound (Optimal cutting temperature, Tissue-Tek, Sakura Finetek, CA) for histopathological assessment and immunohistochemistry study.
Immunofluorescence
For immunofluorescence staining, OCT-embedded frozen tissues were cut (15μm) and put onto silanated glass slides, air-dried and stored at −80°C. Cryosections were thawed at room temperature, hydrated, washed with PBS, and incubated in blocking solution (10% heated-inactivated fetal bovine serum in 0.01% saponin PBS) for 1h followed by incubation with the first primary antibody diluted in blocking solutions at 4°C, overnight. After washing, slides were sequentially incubated with the appropriate fluorescent-conjugated secondary antibody (dilution, 1:750) for 30 min. The slides were washed and incubated for 10 min in Hoechst 33342 (Invitrogen) to label nuclei, and mounted in Fluoromount G (Southern Biotech, Birmingham, AL). Images were acquired and analyzed using a confocal microscope, as described below.
Tongue tumor imaging with two-photon (TPM) and confocal microscopy
Mice were anesthetized by intraperitoneal injections of ketamine (10 mg/kg) and xylazine (100 mg/kg). The animals were placed on a preheated stage with the mouth open and the tongue gently retracted using non-tooth forceps. The tongue was hold in a custom-made holder, secured with a glass coverslip (Fig. 4A). The preheated stage was moved close to the objective of a two-photon microscope and the body temperature was maintained at 37-38°C with a heated pad (Kent Scientific, Torrington, CT).
An inverted confocal microscope (model IX81, Olympus America, Center Valley, PA) was modified to perform TPM, as previously described (30). As a laser source, a tunable Ti:sapphire femtosecond laser (Chameleon Ultra II, Coherent Laser Group, Santa Clara, CA) was used, and the power was modulated using a combination of neutral density filters (Chroma Technology, Rockingham, VT). A beam expander (LSM Technologies, Stewartstown, PA) was used to modulate the size of the beam that was then directed into a scanning head (Fluoview 1000, Olympus America). The emitted signal was aimed into a custom-made set of three non-descanned detectors (LSM Technologies). Two dichroic mirrors and the barrier filters were purchased from Chroma Technology, and three cooled photo multipliers (PMTs) were purchased from Hamamatsu (Bridgewater, NJ). The first PMT (510-nm dichroic mirror, 400- to 480-nm barrier filter) detected the endogenous fluorescence and the second harmonic signal. GFP, venus and Alexa 488 signal were detected with the second PMT (570-nm dichroic mirror, 505- to 560-nm barrier filter). TagRFP and Alexa 594 were detected on the third PMT (590- to 650-nm barrier filter). For time-lapse imaging of the live animals, the acquisition speed was set to 0.3 frames/s. All images and movies were acquired using a UPLSAPO x60, numerical aperture (NA) 1.2, a XLUMPFL20XW x20, NA 0.95 and a XLPlanN x25, NA 1.05 water immersion objectives (Olympus America).
Image processing
The image background noise was reduced by applying a 2 × 2 pixel lowpass filter to each image for one or two rounds using Metamorph software (Molecular Devices). Brightness, contrast, and gamma correction were applied. For the movies, the alignment of each frame was adjusted using ImageJ (W. Rasband, National Institutes of Health) with Stackreg plug-in. Volume rendering was performed using Imaris 7.4 64-bit (Bitplane). The final preparation of the images was managed with Adobe Photoshop CS. Movies were assembled with Metamorph and compressed with Quicktime Pro.
Western Blotting
Cells or small pieces of tissues were rinsed with PBS and rapidly lysed with protein lysis buffer (62.5mM Tris-HCl (pH 6.8), 2% SDS, 10% glycerol, 50 mM DTT), and transferred immediately to microcentrifuge tubes and sonicated for 20 sec. Protein yield was quantified using the Quick start Bradford protein assay (Bio-Rad, Hercules, CA). Equivalent amounts protein (80-100μg) were separated by SDS-PAGE, transferred to PVDF membranes, and immunodetection performed with tubulin as loading control.
In vitro wound scratch assay
Tumor cells were seeded uniformly (2×105 cells/well) onto a silicone culture insert (Ibidi, Verona, WI) and grown to 100% confluence as described by the manufacturer. After 24 hours, the inserts were removed. Images of the wounds were acquired 0, 3, 6, 9, and 12 h after the onset by a microscope (Olympus,) equipped with a CCD camera. The wound closure distance was measured and reported.
Cell cycle analysis
Cell cycle analysis was performed as described (24). Briefly, cells were harvested in DMEM with 1% fetal bovine serum, fixed in 70% ethanol, and stained with 50 μg/mL of propidium iodide and 0.1 μg/mL of RNaseA in PBS. DNA content of cells was quantified on a Becton Dickinson FACScan and analyzed using Cell Quest software (Immunocytometry system; Becton Dickinson).
Tumor microvessel density analysis
Tumor microvessels were identified by CD31 and Lyve1 immunofluorescent staining of blood and lymphatic vessels, respectively. Images were captured from random areas in each section and morphometric analysis was performed using the MetaMorph 4.0 Imaging system (Molecular Devices Corporation). Microvessel density was calculated by dividing the total perimeter of microvessels by the number area counted. Values reported for each experimental condition correspond to the average values obtained from eight random field images in two independent experiments.
Immunofluorescence analysis for tumor cell proliferation and apoptosis
Tumor cell proliferation and apoptosis was estimated by using anti Ki67 and cleaved caspase-3 antibody respectively. Images were captured from at least five different areas in each tissue slide. After adjusting the fluorescent signal-noise threshold of the images, the total area presenting fluorescent signal was measured using ImageJ (NIH). Values reported correspond to the mean ± SE of values obtained from four samples for each experimental condition.
Analysis of F-actin levels at the plasma membrane in vivo
The levels of F-actin at the plasma membrane were evaluated in two experimental conditions. First, in cryosections from xenografts of either Hela-O3-v or Hela-O3-vRab25 those were labeled with Alexa 594-phalloidin. Alternatively, in xenograft of Hela-O3 cells expressing RFP-lifeact and either venus or vRab25. Images of individual cells were acquired using confocal microscope as described above. The shape of the cells was determined by the expression of venus or vRab25. A region of interest was delineated around the cell surface (approximately 1 μm from each side of the cell border) and the integrated fluorescence intensity was calculated by using Image J. At least 8-10 cells per experimental conditions were evaluated.
Statistical analysis
The statistical analysis was performed using Prism 5 (GraphPad Software, La Jolla, CA). For each experiment, the statistical tests were indicated in the result sections. Unpaired t-test (two-tailed) and One-way ANOVA were used to analyze the immunohistochemistry staining scores and the 3D invasion assays. Fisher’s exact test and Chi square test were used for the tongue cancer metastasis in vivo. Statistical significance was calculated at a 95% confidence level.
Results
Rab25 is downregulated in head and neck squamous cell carcinoma
Genetic evidence supports the role of Rab25 in the development and progression of various cancers in human and mouse models including ovarian, breast and prostate cancers (20, 21, 23, 31, 32). Recently, loss of Rab25 was reported to increase the incidence of colorectal carcinoma in mice (33). Therefore, we performed immunohistochemistry on human HNSCC tissue arrays to determine whether Rab25 was abnormally expressed. In healthy individuals, we found that the staining intensity of Rab25 correlated with the degree of cell differentiation. Indeed, there is stronger immunoreaction of Rab25 in the upper mucosal layers (more differentiated cells, Fig.1 asterisk) than in the basal and suprabasal layers (less differentiated cells, Fig. 1, arrowhead). Moreover, we found that Rab25 expression was significantly lower in both advanced and metastatic tumors when compared to tissues from healthy individuals (Fig 1A and 1B). However, Rab25 expression was not significantly different among TNM stages. Moreover, we determined the expression of CLIC3, a protein linked to Rab25 in pancreatic cancer (32). Surprisingly, we found high levels of CLIC3 expression in tumors and normal tissue regardless of the Rab25 expression (Supplementary Fig. S1A). These findings suggest that Rab25 may operate in HNSCC through a different mechanism from those proposed for other tumors. Finally, we found that Rab25 expression was downregulated in cervical cancer in a similar fashion to HNSCC tissues (Supplementary Fig. S1B).
Figure 1. Rab25 expression in human head and neck squamous cell carcinoma (HNSCC).
A. Representative core tissues from a human oral cancer tissue array stained using an antibody directed against human Rab25. Tissues were grouped according to the TNM classification (T1, T2, T3, T4 and Met = metastasis). Rab25 immunoreaction in more differentiated (asterisk) and less differentiated (arrowhead) cells. The insets show higher magnification of the staining pattern of cancer cells. B. Quantitative analysis of Rab25 expression from human normal and oral cancer tissue array. *** p< 0.0001; One-way ANOVA, n= number of tissue cores
Rab25 downregulation promotes cell invasion in a 3D microenvironment
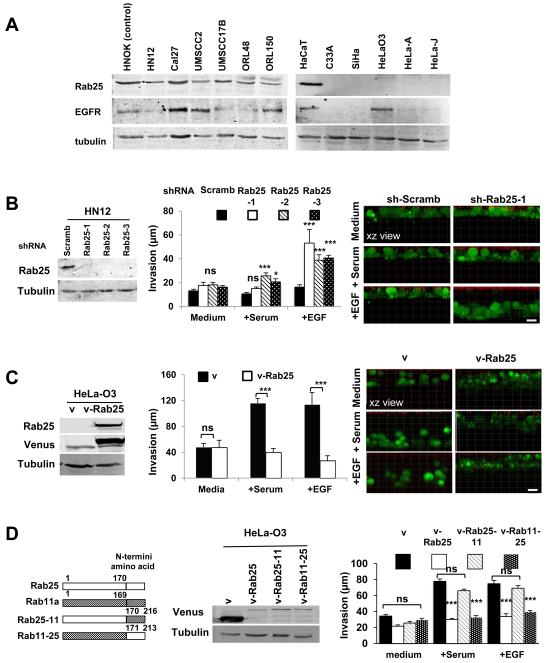
In order to investigate the role of Rab25 downregulation in HNSCC, we screened a panel of human oral squamous carcinoma cell lines for Rab25 expression (Fig. 2A). All the tested cell lines expressed Rab25 at levels comparable to normal oral keratinocytes (Fig. 2A). Therefore, we engineered HN12 cells to stably expressed three different shRNAs targeting Rab25 (HN12-shRab25) or a scrambled shRNA (HN12-shScramb) as a control (Fig. 2B). This cell line was chosen since it forms tumors in orthotopic models of oral cancer (28). Downregulation of Rab25 did not affect either cell proliferation or motility when cultured on different substrates in 2D (data not shown). However, HN12-shRab25 invaded a three-dimensional collagen matrix in the presence of EGF, whereas HN12-shScramb and HN12 parental line did not (Fig. 2B). To further characterize this finding, we screened additional human cancer cell lines for the expression of Rab25 (Fig. 2A). Among the cell lines that did not express Rab25, we selected HeLa-O3 cells, which are highly metastatic when injected into floor of the mouth of immunocompromised mice (this is a variant of HeLa adenocarcinoma cells formerly mistaken for an oral cancer cell line (OSCC3) (34)). This cell line also expressed high EGFR level, which is commonly upregulated in HNSCC (Fig. 2A) (35). HeLa-O3 cells were engineered to stably express either Rab25 fused with the fluorescent protein Venus (HeLa-O3-vRab25) or Venus alone (HeLa-O3-v) (Fig. 2C). Notably, we observed that HeLa-O3-v cells migrated through a 3D collagen matrix when stimulated by either serum or EGF without effecting cell proliferation, whereas HeLa-O3-vRab25 did not (Fig. 2C). Rab25 and Rab11a share significant sequence homology with the exception of the c-terminal hypervariable region, which contains the targeting information for the Rab proteins (6, 9, 10). In order to confirm that this effect is specific for Rab25, we constructed two chimeras fused with venus: one, in which the c-terminus of Rab25 was replaced with Rab11a c-terminus (vRab25-11), and the other, in which the c-terminus of Rab11a was replaced with Rab25 c-terminus (vRab11-25) (Fig. 2D). Notably, cells expressing vRab11-25 (Hela-O3-vRab11-25) were impaired to migrate through the 3D collagen matrix, whereas cells expressing vRab25-11 (Hela-O3 v-Rab25-11) were not (Fig. 2D). These results suggest that altering Rab25 expression is sufficient to change the invasive behavior of tumor cells in an in vitro 3D microenvironment.
Figure 2. Rab25 controls tumor cell invasion in a three-dimensional in vitro model.
A. The protein levels of RAB25 and EGFR were determined by Western blot in a cancer cells panel using human normal oral keratinocytes as control. Tubulin was used as loading control. B, C. Invasion assay in collagen type I matrix. HN12 cells were engineered to stably express shRNA for RAB25 (sh-Rab25) or control scramble shRNA (sh-Scramb) (B, Western blot - left panels), whereas Hela-O3 cells were engineered to express venus-RAB25 (v-Rab25) or venus (v) as control (C, Western blot - left panels). D. Western blot and collagen matrix invasion assay of Hela-O3 cells expressing v, v-Rab25, vRab25-11 and vRab11-25. EGF (10 ng/ml) or serum was used as chemoattractants. The volume rendering from Z-stacks was performed using Imaris (Bitplane). The extent of invasion was assessed by measuring the distance of the cell front from the top of the matrix that was marked by fluorescent beads (red). Data represent mean ± SEM from eight random fields (ns: not statistically significant, * and ***; p<0.01 and p<0.0001 respectively; One-way ANOVA in B and D and unpaired t-test in C). Scale bars, 20 μm
Rab25 re-expression inhibits metastasis to cervical lymph nodes in a mouse model for oral cancer
To assess whether Rab25 is involved in the development and progression of SCC in vivo, we used a previously established tumor xenograft model in which tumor cells are injected into the tongue of immunocompromised mice (36). HN12 cells formed primary tumors but did not invade lymph nodes even after 60 days (Fig. 3A). On the other hand, HeLa-O3 cells formed a visible tumor mass within 7-10 days (Fig 3B, upper panel) and invaded the cervical lymph nodes in more than 80% of the mice within 3 weeks (Fig 3B, lower panels). When these cells were injected into the flank of immunocompromised mice, tumor formation occurred but they did not invade or metastasize (data not shown). Interestingly, other variants of HeLa cells did not metastasize when transplanted into the tongue (Fig. 3B) or in other areas, such as the flank or the cervix (data not shown).
Figure 3. Rab25 controls tongue cancer metastases in vivo.
Tumor cells lines (HN12 in A; HeLa-O3 and HeLa-A in B; HeLa-O3-v; HeLa-O3-vRab25 in C and Hela-O3-v, -v-Rab25, -v-Rab25-11 and, -v-Rab11-25 in D) were transplanted into the lateral tongues of immunocompromised mice. Mice were euthanized after 60 days. Tongue primary tumors and cervical lymph nodes were removed for histopathological evaluation. Histology of whole tongue tumors and metastasis in cervical lymph node are depicted from representative tissue for each tumor cell (A. HN12 B. HeLa-O3 and C. HeLa-O3-v). Broken lines indicate the tumor mass border. High magnification insets are in the right panels. Quantitative data represent percentage of mice with tumor burden or cervical lymph nodes metastasis (LN met= lymph nodes metastasis, n = number of mice). Quantitative analysis of tumor burden and LN metastasis are shown in graphs (ns: not statistically significant, ** and *** p< 0.001 and p< 0.0001 respectively; Fisher’s exact test in A, B and C and; Chi-square test in D.
Next, we transplanted HeLa-O3-vRab25 or HeLa-O3-v cells into the mouse tongue to investigate whether Rab25 has a role in the observed cervical lymph nodes metastasis. The expression of Venus or Venus-Rab25 did not affect the ability of HeLa-O3 cells to form primary tumors in the tongues of immunocompromised mice (Fig. 3C). Strikingly, the metastasis incidence in HeLa-O3-vRab25 tumors was significantly reduced compared to the incidence in HeLa-O3-v tumors (Fig. 3C). This reduction was not due to differences in tumor growth (assessed by tumor weight and volume), cell proliferation (assessed by Ki67 staining) or apoptosis (assessed by cleaved caspase3 staining) (Supplementary Fig S2A, and S2C, respectively). Moreover, the expression of Rab25 did not affect the formation of blood and lymphatic vessels, in contrast to a previous report in breast cancer (Supplementary Fig S2B) (19). The effects from Rab25 were specific, as shown by the transplantation of Hela-O3-vRab11-25 and Hela-O3-vRab25-11. Indeed, the expression of vRab11-25 reduced the metastatic incidence in the tongue xenograft tumor, whereas the expression of vRab25-11 recuperated the cervical metastasis incidence in nearly 50% of the mice (Fig. 3D).
Unfortunately, we could not use HN12-shRab25 cells in vivo, since Rab25 protein expression returned back to its level after injection into the tongue in spite of stable expression of the shRNA (data not shown).
Next, we determined whether Rab25 would affect invasion and metastasis in vivo, similar to what we observed in an in vitro 3D assay. To this aim, we used intravital microscopy, which enables imaging the dynamics of biological processes in live animals (37). In addition to the other cell lines, we generated HN12 and HeLa-O3 cells stably expressing histone 2B fused with GFP (H2B-GFP) for a better visualization of the tumor mass. Moreover, we developed a tongue-holder device to image tumors daily and to minimize the motion artifacts from the heartbeat and respiration (Fig. 4A). This approach has two main advantages: first, it does not require invasive surgical procedures, thus making possible to perform longitudinal studies, and second, it facilitates repeated imaging in the same area. We acquired images of primary tumors located 100-200 μm below the tongue surface by using a combination of two-photon microscopy and second harmonic generation (Fig. 4B, Supplementary Movie 1 and 2). The tumors edges were imaged once a day for 15 to 25 consecutive days. Both the depth and the orientation of the tumors were determined by reference to collagen fibers (Fig. 4B), which have a very slow turnover and their pattern is maintained over the monitoring period (38) (Fig. 5A, arrows). Interestingly, we observed the tumors expanding in size and individual cells departing from the tumor mass (Fig. 5A, arrowheads). In order to catch the movement of these cells, the tumors edges were imaged by consecutive Z-scans at 5 minutes intervals (Fig. 5B). Strikingly, we observed some cells moving away from the edges of the tumors at a speed of approximately 1-2 μm/min (Fig. 5B, arrowheads, and Supplementary Movie 3), whereas the majority of the cells did not migrate within the observation period. Later, these tumor cells were either found near or inside lymphatic vessels, as revealed by intra-organ injections of fluorescent dextran (Fig. 5C and D) (39). Later, tumor cells were either completely encapsulated once they reached and colonized the cervical lymph nodes (Fig. 5E) or they scattered near large blood vessels (Fig. 5F).
Figure 4. Intravital imaging of the tongue cancer model.
A. Schematic drawings of the tongue holding device, animal set up for intravital imaging, and primary tumor mass growing in the tongue. B. Intravital microscopy of tongue cancer at the primary site. Hela-O3 cells expressing H2B-GFP were transplanted into the tongue submucosa. Before imaging, a 70 kDa dextran was injected systemically to reveal stromal cells. A Z-scan was performed by using two-photon microscopy (60× water immersion lens, NA 1.2, Olympus). The same area was imaged using either 750 nm (upper panels) or 930 nm (lower panels) as excitation wavelengths. Both conditions revealed tumor cells (green) and stromal cells (red). However, the excitation at 750 nm revealed the tongue parenchyma (cyan), whereas the excitation at 930 nm revealed components of the extracellular matrix and myosin filaments from the muscle fibers (cyan). Scale bars, 20 μm.
Figure 5. Long-term imaging of the tongue cancer.
(A-F) Hela-O3 cells expressing either venus (A, B) or H2B-GFP (C-F) were transplanted into the tongue submucosa, as described in Material and Methods. A. After 7 days from the injection, the edges of the tumor were imaged on a daily basis by using two-photon microscopy (excitation wavelength of 930 nm) to reveal collagen fibers (red) and the tumor mass (green). Z-stacks were acquired at day 7 (D7), 8 (D8), and 9 (D9) by using a 25X water lens (N.A. 1.05, Olympus). 3D reconstructions (upper panels, yz view) and maximal projections of the xy view were performed by using Imaris (Bitplane) (middle and lower panels). The extracellular matrix at the surface of the tongue was used as a reference point (middle panels, arrows). Individual tumor cells were observed migrating from the tumor mass (arrowheads). Bar 50 μm. B After 14 days, the edge of the tumor was imaged as described above by performing Z-stacks for two hours at 5 min interval. Individual cells were observed leaving the tumor mass (insets, red arrowheads). Bar, 20 μm. C, D. Texas Red-dextran (70 kDa) was injected in the tongue in order to map the lymphatic vessels, and Z-scan were performed, as described above. Maximal projections show tumor cells expressing H2B-GFP (green) either in proximity (C) or inside lymphatic vessels (D). Note collagen fibers in C (cyan). E, F. Dextran (70 kDa) was intravenously injected to label blood vessels and stromal cells, and cervical lymph nodes were exposed and imaged as described above. E. Tumor cells (green) colonized a cervical lymph node: stromal cells (red) and collagen fibers (cyan). F. Tumor cells (green) are in close proximity to a blood vessel (red). Bars, 50 μm
In order to determine whether expression of Rab25 directly affected the motility of the tumor cells in vivo, we co-injected HeLa-O3 cells stably expressing the red fluorescent protein RFP (HeLa-O3-RFP) with HeLa-O3-vRab25 and imaged the tumor edge either on a daily basis or at shorter intervals (every 5 minutes). Both cell lines were equally distributed in the tumor mass in the early stage (Fig. 6A). However, HeLa-O3-RFP cells migrated from the primary sites, which became enriched in HeLa-O3-vRab25 cells at later time points (Fig. 6B). Moreover, we could not observe any HeLa-O3-vRab25 cells leave the primary site (data not shown). Consistent with this finding, HeLa-O3-RFP cells were the only cells that invaded the lymphatic system and metastasized to the cervical lymph nodes (Fig. 6C).
Figure 6. RAB25 expression inhibits lymphatic metastasis in tongue cancer.
HeLa-O3 cells expressing RFP (red) or venus-Rab25 (green) were co-transplanted into the lateral tongue of immunocompromised mice. A, B. The edges of the tumor were imaged on a daily basis by intravital two-photon microscopy, as described in Fig. 5. A. Tumor mass after 9 days (D9). B. Maximal projections of the tumor mass imaged at day 17 (D17), 18 (D18) and 19 (D19). Note that the tumor cells lacking Rab25 (red) migrate from the tumor mass that is mainly formed by cells expressing Rab25 (green). Bars, 50 μm. C. After 30 days, tumors and cervical lymph nodes were removed, fixed and processed as described in Material and Methods. Tissue cryosections were labeled with an antibody directed against Lyve-1, a marker for lymphatic vessels (cyan). Primary tumors are shown in the left and the middle panel. Note that only cell lacking Rab25 are able to invade the lymphatic vessels (middle panel) and colonize the cervical lymph node (right panel)
Rab25 re-expression blocks the formation of actin-rich protrusive structures
As shown above, re-expression of Rab25 negatively regulates the ability of tumor cells to metastasize to the cervical lymph nodes in our mouse model of oral cancer. Our data also suggest that expression of Rab25 inhibits the motility of a subpopulation of tumor cells. To gain some insight on the machinery regulated by Rab25 we imaged both HeLa-O3-v and HeLa-O3-vRab25 tumors at higher resolution and faster acquisition speed. Rab25 was clearly localized in small and dynamic vesicles scattered in the cytoplasm, confirming that its expression did not elicit any toxic cellular effect (Fig. 7A, Supplementary Movie 4). Interestingly, HeLa-O3-v showed a series of dynamic cell protrusions that were not detected in HeLa-O3-vRab25 (Fig. 7A, Supplementary Movie 4). Since the actin cytoskeleton has been implicated in the formation of various plasma membrane structures involved in cell migration (5), we investigated whether F-actin associated with these protrusions. Indeed, actin was enriched at the plasma membrane, particularly in the protrusions, in both 3D collagen matrix and live animals (Fig. 7B and 7C, Supplementary movie 5). Notably, cells expressing Rab25 had a substantial reduction in the levels of F-actin at the plasma membrane (Fig. 7B, 7C, 7D and 7E, Suppl. movie 5). Moreover, we found that knocking down Rab25 in HN12 cells also resulted in the actin-enriched protrusion formation in 3D collagen matrix (data not shown). To address whether the actin cytoskeleton affected tumor cells invasion, we used two potent F-actin disrupting drugs (cytochalasin D and latrunculin A) in the 3D invasion assays. Both inhibitors significantly affected tumor cells invasion independently of the Rab25 levels (Fig. 7F). Interestingly, modulations of Rab25 levels in both HeLa-O3 and HN12 cells did not change the arrangement of the cytoskeleton when cells were grown in two-dimensional substrates suggesting that the 3D architecture and the tissue microenvironment play a fundamental role in this process (data not shown).
Figure 7. RAB25 expression reduces actin-rich protrusion in tumor cells.
A. Intravital two-photon microscopy of tongue tumors from HeLa-O3 expressing venus (left panel) or venus-Rab25 (right panel). Cells lacking Rab25 exhibit dynamics protrusions (arrowheads and Supplementary Movie 4). B. HeLa-O3 cells expressing venus (left) or venus-Rab25 (right) were allowed to invade a 3D collagen matrix, fixed, and labeled with phalloidin. In cells lacking Rab25, actin-rich protrusions are clearly visible at the cell surface. C. Mouse tongue xenograft tissues from HeLa-O3 expressing venus (left) or venus-Rab25 (right) were stained with phalloidin (overlay) and phalloidin alone (lower panel). Broken lines indicated the approximate tumor cell borders. Arrowheads indicate the thick actin structures at cell protrusions. D. Intravital imaging of tumor cells expressing lifeactGFP in HeLa-O3 expressing RFP (left) or RFPRab25 (right) in tongue tumors. Arrowheads indicate the thick actin structures at cell protrusions. Scale Bars, 20 μm. E. Quantification of F-actin at the plasma membrane. Actin expression (phalloidin staining and Lifeact expression) from HeLa-O3 and HeLa-O3-Rab25 cells was analyzed by ImageJ software as described in the Materials and Methods. The graphs represent the pixel intensity at the plasma membrane of each cell (mean±SE) (* and ***; p<0.01 and p<0.0001 respectively; unpaired t-test). F. Collagen invasion assay of HeLa-O3-v cells stimulated with EGF (10 ng/ml) in the presented or absent of actin polymerization inhibitors (10 μM of cytochalasin D-cytoD and 1 μM of latrunculin A-latA). Cell invasion quantification was conducted as described in Fig 2. Data represent mean ± SEM from eight random fields (ns: not statistically significant, * and ***; p<0.01 and p<0.0001 respectively; One-way ANOVA)
Discussion
Several evidences suggest that deregulation of molecules involved in endocytosis plays a fundamental role in human diseases including cancer. Indeed, endocytosis coordinates multiple steps that may affect cancer progression, such as receptor-mediated signaling from the plasma membrane, cell-to-cell contact, and cell motility (7). Rab proteins are considered master regulators of endocytosis and often deregulated in various cancers. Among the Rab family members, Rab25 has been implicated in different cancers although its mechanism of action has been controversial. Indeed, both upregulation and downregulation of Rab25 have been associated with tumor growth, differentiation, and poor prognosis. Moreover, Rab25 has been involved in several cellular processes such as proliferation, apoptosis, angiogenesis, cell cycle, trafficking of adhesion molecules and cell migration (19-21, 31, 40-43). In this study, we determine the role of Rab25 in HNSCC formation and metastasis. To this aim, we used a series of approaches, which include expression analysis of HNSCC patient tissues, analysis of cellular function in 3D microenvironments in vitro, and tumor cells dynamics in vivo using intravital microscopy in a tongue cancer model.
First, we found a significant reduction in Rab25 expression in HNSCC patient tissues, although without any correlation between Rab25 expression and tumor stage. These findings would favor a model in which Rab25 acts as a tumor suppressor, as suggested for other cancers (19, 22). However, loss of Rab25 alone may not be sufficient to initiate tumorigenesis since Rab25 knockout mice do not develop any spontaneous tumors (33). In addition, we detected high levels of CLIC3 expression in both normal and HNSCC tissues independent of Rab25 expression. This observation is in contrast to a report suggesting that CLIC3 and Rab25 synergistically drive tumor progression in pancreatic ductal adenocarcinoma (32). Our data strongly suggests that Rab25 may operate in HNSCC differently from what was described for other cancers (19-21, 23, 31, 32, 40, 44, 45). Indeed, we found that Rab25 downregulation promotes cell invasion and metastasis in HNSCC. The main evidence comes from an invasion assay in a 3D collagen matrix and in a tongue cancer mouse model. We used two cancer cell lines; HN12 (Rab25 levels comparable to normal oral keratinocytes) and Hela-O3 (expression of Rab25 below the detection levels) that were engineered to knockdown Rab25 or express fluorescently tagged Rab25, respectively. Both cell lines were chosen since they form aggressive tumors when implanted in the tongue or the floor of mouth in immunodeficient mouse (34, 36). We found that alteration in Rab25 expression did not affect cell proliferation or apoptosis both in vitro and in vivo. However, cells lacking Rab25 migrated through 3D collagen matrix when stimulated by EGF or serum. Consistent with this finding, HeLa-O3 cells metastasized to cervical lymph nodes when transplanted in the tongue or in floor of mouth (34), whereas other HeLa variants, which lacked both Rab25 and EGFR, did not. This observation suggests a possible link between Rab25 and EGFR signaling in cell migration and metastasis. Moreover, we ruled out the roles of Rab25, in tumor growth or vascular angiogenesis regulation, as reported in breast cancer (19, 42).
To gain further insight on how Rab25 regulate invasion and metastasis in vivo, we used intravital microscopy. We observed that a subpopulation of cells lacking Rab25 migrated from the tumor mass, whereas Rab25-expressing cells were confined to the primary site. Consistent with this observation, only Rab25-depleted cells reached lymphatic vessels, intravasated, and spread to the distal lymph nodes. These observations suggest that tumor invasion occurs through single-cell rather than collective migration in this model (46) and this process is facilitated by the lack of Rab25. Finally, we observed that re-expression of Rab25 induced significant changes in cell shape. During cell migration, the actin cytoskeleton is rearranged at the cell surface and forms a series of structures such as invadopodia, podosomes, lamellipodia and ruffles that interact with the extracellular matrix components (46). Both in vivo and in a 3D matrix, cells lacking Rab25 displayed actin-rich protrusions, which has similar morphology to invadopodia (47, 48). Rab25 expression significantly reduced the number of actin-rich structures and the overall levels of F-actin at the plasma membrane, suggesting a possible involvement in actin dynamics regulation. Moreover, we found that the invasion of tumor cells in a 3D collagen assay is significantly inhibited by the pharmacological disruption of the actin cytoskeleton. Thus, we can hypothesize that Rab25 may regulate trafficking of molecules that negatively regulate actin assembly (e.g. signaling molecules, integrin, or cholesterol-rich membranes) (5, 47, 48), although we cannot rule out other mechanisms. This idea is supported by the role of FIP3, a protein that simultaneously interacts with the small GTPase Arf6 and Rab25/Rab11, which controls the motility of breast cancer cells by regulating Rac1 and actin dynamics (49). Based on the fact that Rab25 is localized in intracellular vesicles and that Rab25 has been implicated in endosomal recycling (50), we speculated that the lack of Rab25 might prevent these actin regulators from reaching the plasma membrane and divert them to degradation pathway. Indeed, a similar mechanism has been suggested in breast and pancreatic cancer (6, 32). However, in these models, overexpression of Rab25 enhanced cell migration and invasion by increasing the recycling of integrin β1 from a late endosomal compartment to the plasma membrane (6, 32). These differences in the modality of action of Rab25 may be explained by the fact that the trafficking of molecules involved in migration may occur through different pathways in which Rab25 may interact with a completely different set of molecules.
In conclusion, we have shown that the downregulation of Rab25 plays a significant role in HNSCC and suggested that Rab25 may regulate tumor invasion and metastasis through actin remodeling at the plasma membrane. We believe that identification of the molecules interacting with Rab25 and elucidation of the molecular machinery underlying its action may provide new strategies to prevent metastasis in oral cancer patients.
Supplementary Material
Statement of Translational Relevance.
In this study, we identified the small GTPase Rab25 as a key player in the invasion and metastasis in head and neck squamous cell carcinoma (HNSCC). Prompted by the observation that Rab25 is downregulated at the protein level in HNSCC patients, we exploited a recently developed xenograft model in the tongue of immunocompromised mice to further elucidate its role in HNSCC. Strikingly, we observed that re-expression of Rab25 is sufficient to block invasion and metastasis to locoregional lymph nodes. Moreover, by using high-resolution intravital microscopy and immunofluorescence in a 3D model, we determined that Rab25 expression affects cell migration by altering the actin cytoskeleton arrangement at the cell surface, rather than affecting cell proliferation, apoptosis or tumor angiogenesis.
These findings indicates that Rab25 may be a promising therapeutic target and that unraveling its function may lead to the development of new strategies to prevent metastasis in oral cancer patients.
Acknowledgement
This research was supported by the Intramural Research Program of National Institute of Dental and Craniofacial Research, NIH. We would like to thank Dr. Porat-Shliom, and Mr. Mohibullah Tora for critical reading of the manuscript.
Footnotes
Disclosure of Potential Conflicts of Interest: The authors indicate no disclosures of potential conflicts of interest.
References
- 1.Gale NPB, Sidransky D, Westra W, Califano J. Tumours of the hypopharynx, larynx and trachea (Epithelial precursor lesions) In: Barnes LEJ, Reichart P, Sidransky D, editors. World Health Organization Classification of Tumours Pathology & genetics Head and neck tumours International Agency for Research on Cancer (IARC) IARC Press; Lyon: 2005. pp. 140–3. [Google Scholar]
- 2.Haddad RI, Shin DM. Recent Advances in Head and Neck Cancer. New England Journal of Medicine. 2008;359:1143–54. doi: 10.1056/NEJMra0707975. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 4.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–92. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ridley AJ. Life at the Leading Edge. Cell. 2011;145:1012–22. doi: 10.1016/j.cell.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Caswell P, Norman J. Endocytic transport of integrins during cell migration and invasion. Trends in Cell Biology. 2008. 18:257–63. doi: 10.1016/j.tcb.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Mosesson Y, Mills GB, Yarden Y. Derailed endocytosis: an emerging feature of cancer. Nat Rev Cancer. 2008;8:835–50. doi: 10.1038/nrc2521. [DOI] [PubMed] [Google Scholar]
- 8.Mitra S, Cheng KW, Mills GB. Rab GTPases implicated in inherited and acquired disorders. Semin Cell Dev Biol. 2011;22:57–68. doi: 10.1016/j.semcdb.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colicelli J. Human RAS Superfamily Proteins and Related GTPases. Science Signaling. 2004;2004:re13. doi: 10.1126/stke.2502004re13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pereira-Leal JB, Seabra MC. Evolution of the rab family of small GTP-binding proteins. Journal of Molecular Biology. 2001;313:889–901. doi: 10.1006/jmbi.2001.5072. [DOI] [PubMed] [Google Scholar]
- 11.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–25. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 12.Shimada K, Uzawa K, Kato M, Endo Y, Shiiba M, Bukawa H, et al. Aberrant expression of RAB1A in human tongue cancer. Br J Cancer. 2005;92:1915–21. doi: 10.1038/sj.bjc.6602594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chia WJ, Tang BL. Emerging roles for Rab family GTPases in human cancer. Biochim Biophys Acta. 2009;1795:110–6. doi: 10.1016/j.bbcan.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Hou Q, Wu YH, Grabsch H, Zhu Y, Leong SH, Ganesan K, et al. Integrative genomics identifies RAB23 as an invasion mediator gene in diffuse-type gastric cancer. Cancer Res. 2008;68:4623–30. doi: 10.1158/0008-5472.CAN-07-5870. [DOI] [PubMed] [Google Scholar]
- 15.Liu YJ, Wang Q, Li W, Huang XH, Zhen MC, Huang SH, et al. Rab23 is a potential biological target for treating hepatocellular carcinoma. World J Gastroenterol. 2007;13:1010–7. doi: 10.3748/wjg.v13.i7.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukui K, Tamura S, Wada A, Kamada Y, Igura T, Kiso S, et al. Expression of Rab5a in hepatocellular carcinoma: Possible involvement in epidermal growth factor signaling. Hepatology Research. 2007;37:957–65. doi: 10.1111/j.1872-034X.2007.00143.x. [DOI] [PubMed] [Google Scholar]
- 17.Croizet-Berger K, Daumerie C, Couvreur M, Courtoy PJ, van den Hove M-F. The endocytic catalysts, Rab5a and Rab7, are tandem regulators of thyroid hormone production. Proceedings of the National Academy of Sciences. 2002;99:8277–82. doi: 10.1073/pnas.122187699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calhoun BC, Goldenring JR. Rab proteins in gastric parietal cells: evidence for the membrane recycling hypothesis. Yale J Biol Med. 1996;69:1–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng JM, Volk L, Janaki DK, Vyakaranam S, Ran S, Rao KA. Tumor suppressor function of Rab25 in triple-negative breast cancer. Int J Cancer. 2010;126:2799–812. doi: 10.1002/ijc.24900. [DOI] [PubMed] [Google Scholar]
- 20.Caswell PT, Spence HJ, Parsons M, White DP, Clark K, Cheng KW, et al. Rab25 associates with alpha5beta1 integrin to promote invasive migration in 3D microenvironments. Dev Cell. 2007;13:496–510. doi: 10.1016/j.devcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Cheng KW, Lu Y, Mills GB. Assay of Rab25 function in ovarian and breast cancers. Methods Enzymol. 2005;403:202–15. doi: 10.1016/S0076-6879(05)03017-X. [DOI] [PubMed] [Google Scholar]
- 22.Goldenring JR, Nam KT. Rab25 as a tumour suppressor in colon carcinogenesis. Br J Cancer. 2011;104:33–6. doi: 10.1038/sj.bjc.6605983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng KW, Agarwal R, Mitra S, Lee JS, Carey M, Gray JW, et al. Rab25 increases cellular ATP and glycogen stores protecting cancer cells from bioenergetic stress. EMBO Mol Med. 2012;4:125–41. doi: 10.1002/emmm.201100193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amornphimoltham P, Patel V, Sodhi A, Nikitakis NG, Sauk JJ, Sausville EA, et al. Mammalian target of rapamycin, a molecular target in squamous cell carcinomas of the head and neck. Cancer Res. 2005;65:9953–61. doi: 10.1158/0008-5472.CAN-05-0921. [DOI] [PubMed] [Google Scholar]
- 25.Hamid S, Lim KP, Zain RB, Ismail SM, Lau SH, Mustafa WM, et al. Establishment and characterization of Asian oral cancer cell lines as in vitro models to study a disease prevalent in Asia. Int J Mol Med. 2007;19:453–60. [PubMed] [Google Scholar]
- 26.Patel V, Iglesias-Bartolome R, Siegele B, Marsh CA, Leelahavanichkul K, Molinolo AA, et al. In: Cellular Systems for Studying Human Oral Squamous Cell Carcinomas Human Cell Transformation. Rhim JS, Kremer R, editors. Springer; New York: 2012. pp. 27–38. [DOI] [PubMed] [Google Scholar]
- 27.Kanda T, Sullivan KF, Wahl GM. Histone,ÄìGFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells. Current Biology. 1998;8:377–85. doi: 10.1016/s0960-9822(98)70156-3. [DOI] [PubMed] [Google Scholar]
- 28.Amornphimoltham P, Leelahavanichkul K, Molinolo A, Patel V, Gutkind JS. Inhibition of Mammalian target of rapamycin by rapamycin causes the regression of carcinogen-induced skin tumor lesions. Clin Cancer Res. 2008;14:8094–101. doi: 10.1158/1078-0432.CCR-08-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charafe-Jauffret E, Tarpin C, Bardou VJ, Bertucci F, Ginestier C, Braud AC, et al. Immunophenotypic analysis of inflammatory breast cancers: identification of an ‘inflammatory signature’. J Pathol. 2004;202:265–73. doi: 10.1002/path.1515. [DOI] [PubMed] [Google Scholar]
- 30.Masedunskas A, Weigert R. Intravital two-photon microscopy for studying the uptake and trafficking of fluorescently conjugated molecules in live rodents. Traffic. 2008;9:1801–10. doi: 10.1111/j.1600-0854.2008.00798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng KW, Lahad JP, Kuo WL, Lapuk A, Yamada K, Auersperg N, et al. The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nat Med. 2004;10:1251–6. doi: 10.1038/nm1125. [DOI] [PubMed] [Google Scholar]
- 32.Dozynkiewicz MA, Jamieson NB, Macpherson I, Grindlay J, van den Berghe PV, von Thun A, et al. Rab25 and CLIC3 collaborate to promote integrin recycling from late endosomes/lysosomes and drive cancer progression. Dev Cell. 2012;22:131–45. doi: 10.1016/j.devcel.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nam KT, Lee HJ, Smith JJ, Lapierre LA, Kamath VP, Chen X, et al. Loss of Rab25 promotes the development of intestinal neoplasia in mice and is associated with human colorectal adenocarcinomas. J Clin Invest. 2010;120:840–9. doi: 10.1172/JCI40728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henson B, Li F, Coatney DD, Carey TE, Mitra RS, Kirkwood KL, et al. An orthotopic floor-of-mouth model for locoregional growth and spread of human squamous cell carcinoma. Journal of Oral Pathology & Medicine. 2007;36:363–70. doi: 10.1111/j.1600-0714.2007.00549.x. [DOI] [PubMed] [Google Scholar]
- 35.Molinolo AA, Amornphimoltham P, Squarize CH, Castilho RM, Patel V, Gutkind JS. Dysregulated molecular networks in head and neck carcinogenesis. Oral Oncology. 2009;45:324–34. doi: 10.1016/j.oraloncology.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel V, Marsh CA, Dorsam RT, Mikelis CM, Masedunskas A, Amornphimoltham P, et al. Decreased Lymphangiogenesis and Lymph Node Metastasis by mTOR Inhibition in Head and Neck Cancer. Cancer Res. 2011;71:7103–12. doi: 10.1158/0008-5472.CAN-10-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weigert R, Sramkova M, Parente L, Amornphimoltham P, Masedunskas A. Intravital microscopy: a novel tool to study cell biology in living animals. Histochem Cell Biol. 2010;133:481–91. doi: 10.1007/s00418-010-0692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown E, McKee T, diTomaso E, Pluen A, Seed B, Boucher Y, et al. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat Med. 2003;9:796–800. doi: 10.1038/nm879. [DOI] [PubMed] [Google Scholar]
- 39.Melancon MP, Wang Y, Wen X, Bankson JA, Stephens LC, Jasser S, et al. Development of a macromolecular dual-modality MR-optical imaging for sentinel lymph node mapping. Investigative radiology. 2007;42:569–78. doi: 10.1097/RLI.0b013e31804f5a79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan Y, Xin XY, Chen BL, Ma X. Knockdown of RAB25 expression by RNAi inhibits growth of human epithelial ovarian cancer cells in vitro and in vivo. Pathology. 2006;38:561–7. doi: 10.1080/00313020601024037. [DOI] [PubMed] [Google Scholar]
- 41.Bigelow RL, Williams BJ, Carroll JL, Daves LK, Cardelli JA. TIMP-1 overexpression promotes tumorigenesis of MDA-MB-231 breast cancer cells and alters expression of a subset of cancer promoting genes in vivo distinct from those observed in vitro. Breast Cancer Res Treat. 2009;117:31–44. doi: 10.1007/s10549-008-0170-7. [DOI] [PubMed] [Google Scholar]
- 42.Yin Y, Shen F, Pei H, Ding Y, Zhao H, Zhao M, et al. Increased expression of Rab25 in breast cancer correlates with lymphatic metastasis. Tumor Biology. :1–7. doi: 10.1007/s13277-012-0412-5. [DOI] [PubMed] [Google Scholar]
- 43.Senga K, Mostov KE, Mitaka T, Miyajima A, Tanimizu N. Grainyhead-like 2 regulates epithelial morphogenesis by establishing functional tight junctions through the organization of a molecular network among claudin3, claudin4, and Rab25. Mol Biol Cell. 2012 doi: 10.1091/mbc.E12-02-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng JM, Ding M, Aribi A, Shah P, Rao K. Loss of RAB25 expression in breast cancer. Int J Cancer. 2006;118:2957–64. doi: 10.1002/ijc.21739. [DOI] [PubMed] [Google Scholar]
- 45.Cheng KW, Lahad JP, Gray JW, Mills GB. Emerging role of RAB GTPases in cancer and human disease. Cancer Res. 2005;65:2516–9. doi: 10.1158/0008-5472.CAN-05-0573. [DOI] [PubMed] [Google Scholar]
- 46.Friedl P, Alexander S. Cancer Invasion and the Microenvironment: Plasticity and Reciprocity. Cell. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 47.Murphy DA, Courtneidge SA. The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol. 2011;12:413–26. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Destaing O, Block MR, Planus E, Albiges-Rizo C. Invadosome regulation by adhesion signaling. Current Opinion in Cell Biology. 2011;23:597–606. doi: 10.1016/j.ceb.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 49.Jing J, Tarbutton E, Wilson G, Prekeris R. Rab11-FIP3 is a Rab11-binding protein that regulates breast cancer cell motility by modulating the actin cytoskeleton. Eur J Cell Biol. 2009;88:325–41. doi: 10.1016/j.ejcb.2009.02.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Casanova JE, Wang X, Kumar R, Bhartur SG, Navarre J, Woodrum JE, et al. Association of Rab25 and Rab11a with the apical recycling system of polarized Madin-Darby canine kidney cells. Mol Biol Cell. 1999;10:47–61. doi: 10.1091/mbc.10.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.