Abstract
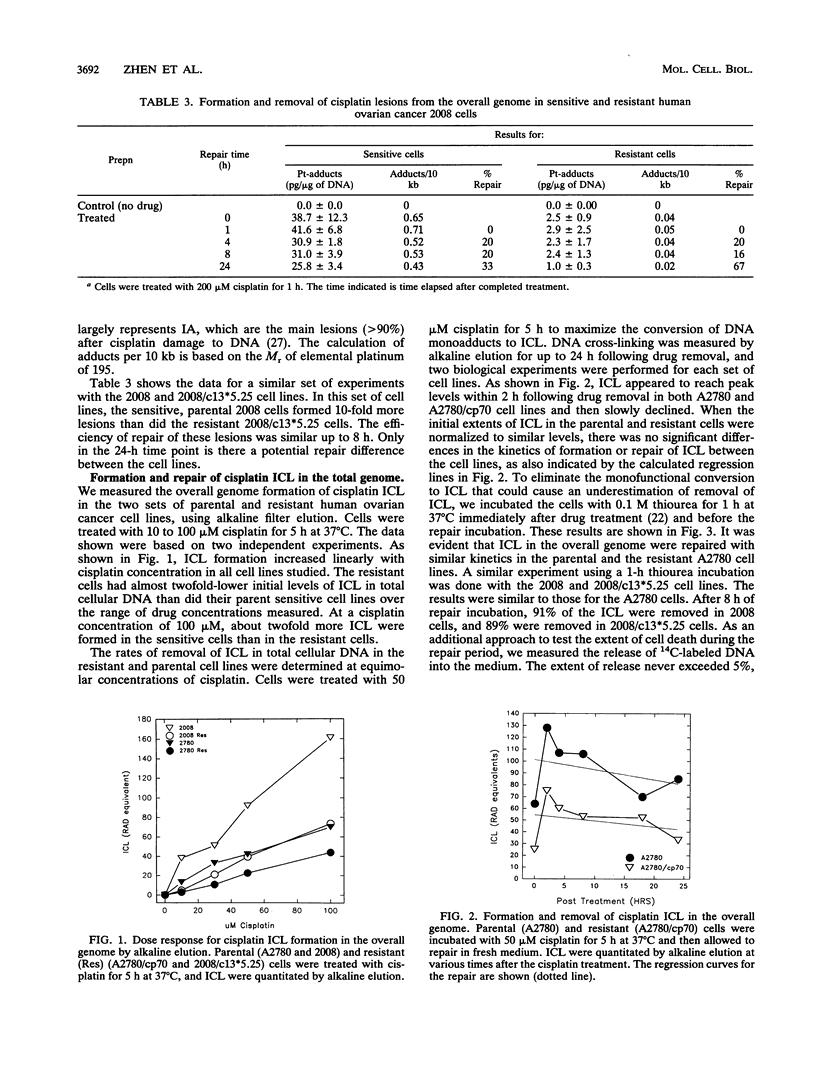
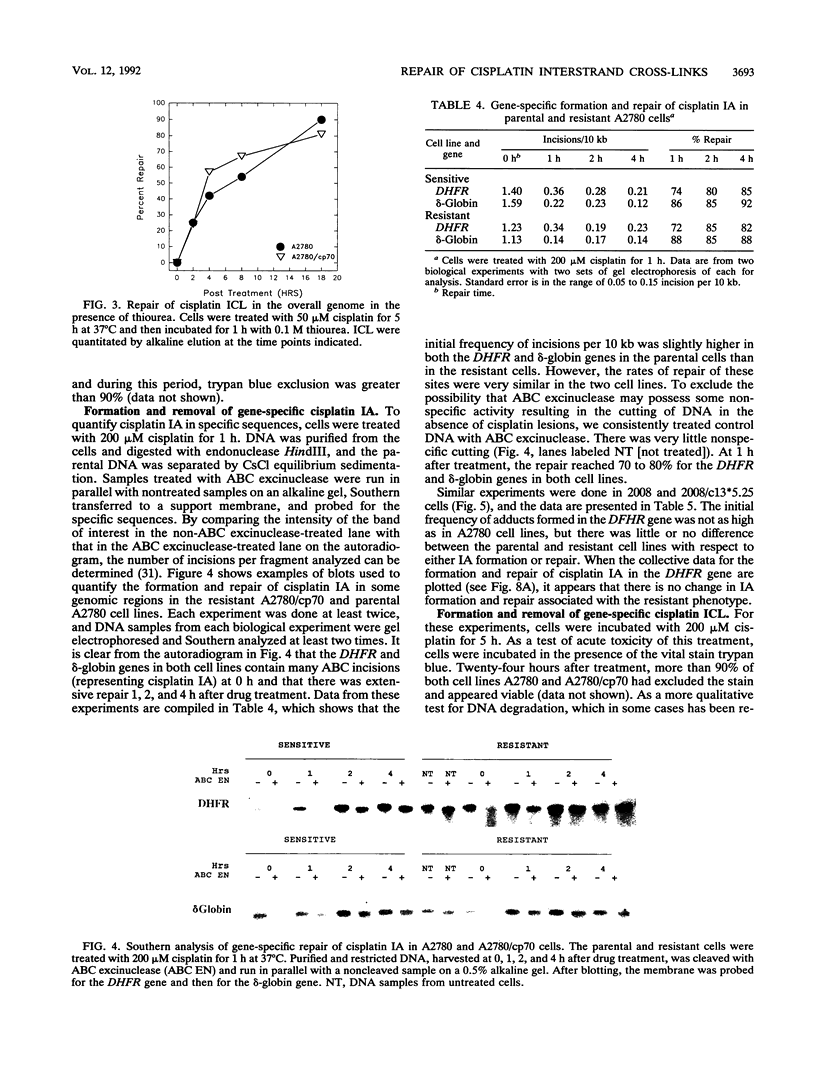
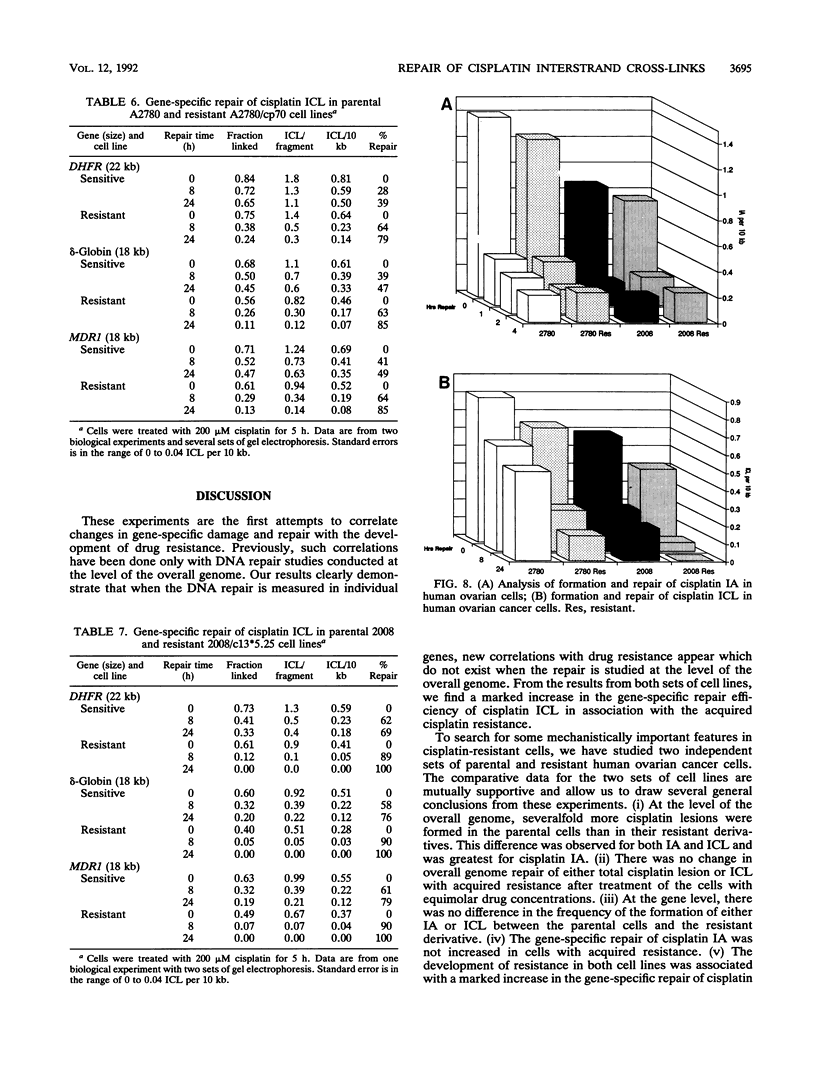
We have studied several aspects of DNA damage formation and repair in human ovarian cancer cell lines which have become resistant to cisplatin through continued exposure to the anticancer drug. The resistant cell lines A2780/cp70 and 2008/c13*5.25 were compared with their respective parental cell lines, A2780 and 2008. Cells in culture were treated with cisplatin, and the two main DNA lesions formed, intrastrand adducts and interstrand cross-links, were quantitated before and after repair incubation. This quantitation was done for total genomic lesions and at the level of individual genes. In the overall genome, the initial frequency of both cisplatin lesions assayed was higher in the parental than in the derivative resistant cell lines. Nonetheless, the total genomic repair of each of these lesions was not increased in the resistant cells. These differences in initial lesion frequency between parental and resistant cell lines were not observed at the gene level. Resistant and parental cells had similar initial frequencies of intrastrand adducts and interstrand cross-links in the dihydrofolate reductase (DHFR) gene and in several other genes after cisplatin treatment of the cells. There was no increase in the repair efficiency of intrastrand adducts in the DHFR gene in resistant cell lines compared with the parental partners. However, a marked and consistent repair difference between parental and resistant cells was observed for the gene-specific repair of cisplatin interstrand cross-links. DNA interstrand cross-links were removed from three genes, the DHFR, multidrug resistance (MDR1), and delta-globin genes, much more efficiently in the resistant cell lines than in the parental cell lines. Our findings suggest that acquired cellular resistance to cisplatin may be associated with increased gene-specific DNA repair efficiency of a specific lesion, the interstrand cross-link.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. A., Howell S. B. Cellular pharmacology of cisplatin: perspectives on mechanisms of acquired resistance. Cancer Cells. 1990 Feb;2(2):35–43. [PubMed] [Google Scholar]
- Andrews P. A., Murphy M. P., Howell S. B. Differential potentiation of alkylating and platinating agent cytotoxicity in human ovarian carcinoma cells by glutathione depletion. Cancer Res. 1985 Dec;45(12 Pt 1):6250–6253. [PubMed] [Google Scholar]
- Beecham E. J., Mushinski J. F., Shacter E., Potter M., Bohr V. A. DNA repair in the c-myc proto-oncogene locus: possible involvement in susceptibility or resistance to plasmacytoma induction in BALB/c mice. Mol Cell Biol. 1991 Jun;11(6):3095–3104. doi: 10.1128/mcb.11.6.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belt P. B., van Oosterwijk M. F., Odijk H., Hoeijmakers J. H., Backendorf C. Induction of a mutant phenotype in human repair proficient cells after overexpression of a mutated human DNA repair gene. Nucleic Acids Res. 1991 Oct 25;19(20):5633–5637. doi: 10.1093/nar/19.20.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr V. A., Chu E. H., van Duin M., Hanawalt P. C., Okumoto D. S. Human repair gene restores normal pattern of preferential DNA repair in repair defective CHO cells. Nucleic Acids Res. 1988 Aug 11;16(15):7397–7403. doi: 10.1093/nar/16.15.7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr V. A., Evans M. K., Fornace A. J., Jr DNA repair and its pathogenetic implications. Lab Invest. 1989 Aug;61(2):143–161. [PubMed] [Google Scholar]
- Bohr V. A. Gene specific DNA repair. Carcinogenesis. 1991 Nov;12(11):1983–1992. doi: 10.1093/carcin/12.11.1983. [DOI] [PubMed] [Google Scholar]
- Bohr V. A., Okumoto D. S., Hanawalt P. C. Survival of UV-irradiated mammalian cells correlates with efficient DNA repair in an essential gene. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3830–3833. doi: 10.1073/pnas.83.11.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr V. A., Smith C. A., Okumoto D. S., Hanawalt P. C. DNA repair in an active gene: removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell. 1985 Feb;40(2):359–369. doi: 10.1016/0092-8674(85)90150-3. [DOI] [PubMed] [Google Scholar]
- Eastman A., Schulte N. Enhanced DNA repair as a mechanism of resistance to cis-diamminedichloroplatinum(II). Biochemistry. 1988 Jun 28;27(13):4730–4734. doi: 10.1021/bi00413a022. [DOI] [PubMed] [Google Scholar]
- Fichtinger-Schepman A. M., van der Veer J. L., den Hartog J. H., Lohman P. H., Reedijk J. Adducts of the antitumor drug cis-diamminedichloroplatinum(II) with DNA: formation, identification, and quantitation. Biochemistry. 1985 Jan 29;24(3):707–713. doi: 10.1021/bi00324a025. [DOI] [PubMed] [Google Scholar]
- Hamilton T. C., Winker M. A., Louie K. G., Batist G., Behrens B. C., Tsuruo T., Grotzinger K. R., McKoy W. M., Young R. C., Ozols R. F. Augmentation of adriamycin, melphalan, and cisplatin cytotoxicity in drug-resistant and -sensitive human ovarian carcinoma cell lines by buthionine sulfoximine mediated glutathione depletion. Biochem Pharmacol. 1985 Jul 15;34(14):2583–2586. doi: 10.1016/0006-2952(85)90551-9. [DOI] [PubMed] [Google Scholar]
- Jones J. C., Zhen W. P., Reed E., Parker R. J., Sancar A., Bohr V. A. Gene-specific formation and repair of cisplatin intrastrand adducts and interstrand cross-links in Chinese hamster ovary cells. J Biol Chem. 1991 Apr 15;266(11):7101–7107. [PubMed] [Google Scholar]
- Kelley S. L., Basu A., Teicher B. A., Hacker M. P., Hamer D. H., Lazo J. S. Overexpression of metallothionein confers resistance to anticancer drugs. Science. 1988 Sep 30;241(4874):1813–1815. doi: 10.1126/science.3175622. [DOI] [PubMed] [Google Scholar]
- Link C. J., Jr, Burt R. K., Bohr V. A. Gene-specific repair of DNA damage induced by UV irradiation and cancer chemotherapeutics. Cancer Cells. 1991 Nov;3(11):427–436. [PubMed] [Google Scholar]
- Loehrer P. J., Einhorn L. H. Drugs five years later. Cisplatin. Ann Intern Med. 1984 May;100(5):704–713. doi: 10.7326/0003-4819-100-5-704. [DOI] [PubMed] [Google Scholar]
- Masuda H., Ozols R. F., Lai G. M., Fojo A., Rothenberg M., Hamilton T. C. Increased DNA repair as a mechanism of acquired resistance to cis-diamminedichloroplatinum (II) in human ovarian cancer cell lines. Cancer Res. 1988 Oct 15;48(20):5713–5716. [PubMed] [Google Scholar]
- Masuda H., Tanaka T., Matsuda H., Kusaba I. Increased removal of DNA-bound platinum in a human ovarian cancer cell line resistant to cis-diamminedichloroplatinum(II). Cancer Res. 1990 Mar 15;50(6):1863–1866. [PubMed] [Google Scholar]
- Micetich K., Zwelling L. A., Kohn K. W. Quenching of DNA:platinum(II) monoadducts as a possible mechanism of resistance to cis-diamminedichloroplatinum(II) in L1210 cells. Cancer Res. 1983 Aug;43(8):3609–3613. [PubMed] [Google Scholar]
- O'Connor P. M., Kohn K. W. Comparative pharmacokinetics of DNA lesion formation and removal following treatment of L1210 cells with nitrogen mustards. Cancer Commun. 1990;2(12):387–394. doi: 10.3727/095535490820873949. [DOI] [PubMed] [Google Scholar]
- Parker R. J., Eastman A., Bostick-Bruton F., Reed E. Acquired cisplatin resistance in human ovarian cancer cells is associated with enhanced repair of cisplatin-DNA lesions and reduced drug accumulation. J Clin Invest. 1991 Mar;87(3):772–777. doi: 10.1172/JCI115080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. J., Friedlos F. Quantitative aspects of the formation and loss of DNA interstrand crosslinks in Chinese hamster cells following treatment with cis-diamminedichloroplatinum(II) (cisplatin). I. Proportion of DNA-platinum reactions involved in DNA crosslinking. Biochim Biophys Acta. 1981 Sep 28;655(2):146–151. doi: 10.1016/0005-2787(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Scanlon K. J., Kashani-Sabet M. Elevated expression of thymidylate synthase cycle genes in cisplatin-resistant human ovarian carcinoma A2780 cells. Proc Natl Acad Sci U S A. 1988 Feb;85(3):650–653. doi: 10.1073/pnas.85.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher B. A., Holden S. A., Kelley M. J., Shea T. C., Cucchi C. A., Rosowsky A., Henner W. D., Frei E., 3rd Characterization of a human squamous carcinoma cell line resistant to cis-diamminedichloroplatinum(II). Cancer Res. 1987 Jan 15;47(2):388–393. [PubMed] [Google Scholar]
- Thomas D. C., Morton A. G., Bohr V. A., Sancar A. General method for quantifying base adducts in specific mammalian genes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3723–3727. doi: 10.1073/pnas.85.11.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos J. M., Hanawalt P. C. Processing of psoralen adducts in an active human gene: repair and replication of DNA containing monoadducts and interstrand cross-links. Cell. 1987 Aug 28;50(5):789–799. doi: 10.1016/0092-8674(87)90337-0. [DOI] [PubMed] [Google Scholar]
- Zwelling L. A., Michaels S., Schwartz H., Dobson P. P., Kohn K. W. DNA cross-linking as an indicator of sensitivity and resistance of mouse L1210 leukemia to cis-diamminedichloroplatinum(II) and L-phenylalanine mustard. Cancer Res. 1981 Feb;41(2):640–649. [PubMed] [Google Scholar]