Abstract
The T-Box family of transcription factors plays fundamental roles in the generation of appropriate spatial and temporal gene expression profiles during cellular differentiation and organogenesis in animals. In this study we report that the Drosophila Tbx1 orthologue optomotor-blind-related-gene-1 (org-1) exerts a pivotal function in the diversification of circular visceral muscle founder cell identities in Drosophila. In embryos mutant for org-1, the specification of the midgut musculature per se is not affected, but the differentiating midgut fails to form the anterior and central midgut constrictions and lacks the gastric caeca. We demonstrate that this phenotype results from the nearly complete loss of the founder cell specific expression domains of several genes known to regulate midgut morphogenesis, including odd-paired (opa), teashirt (tsh), Ultrabithorax (Ubx), decapentaplegic (dpp) and wingless (wg). To address the mechanisms that mediate the regulatory inputs from org-1 towards Ubx, dpp, and wg in these founder cells we genetically dissected known visceral mesoderm specific cis-regulatory-modules (CRMs) of these genes. The analyses revealed that the activities of the dpp and wg CRMs depend on org-1, the CRMs are bound by Org-1 in vivo and their T-Box binding sites are essential for their activation in the visceral muscle founder cells. We conclude that Org-1 acts within a well-defined signaling and transcriptional network of the trunk visceral mesoderm as a crucial founder cell-specific competence factor, in concert with the general visceral mesodermal factor Biniou. As such, it directly regulates several key genes involved in the establishment of morphogenetic centers along the anteroposterior axis of the visceral mesoderm, which subsequently organize the formation of midgut constrictions and gastric caeca and thereby determine the morphology of the midgut.
Keywords: visceral mesoderm patterning, midgut morphogenesis, T-box transcription factor, combinatorial enhancer binding
Material and Methods
Drosophila strains
In this study the following strains were used: org-1OJ487, org-1OJ423 and UAS-org-1 (Schaub et al., 2012), tin346, bapDf (a Df(3R)eD7 chromosome carrying the tin genomic rescue construct tin Re28–58) (Azpiazu and Frasch, 1993), binR22, bap3-lacZ and bap3-GAL4 (Zaffran et al., 2001), UAS-tkvQ–D (Nellen et al., 1996), UAS-wg-HA (Bloomington Stock Center, Indiana), 2xPE-GAL4; how24B-GAL4 (Wang et al., 2005). GAL4/UAS induced overexpression was carried out at 28°C.
Construction of the reporter constructs
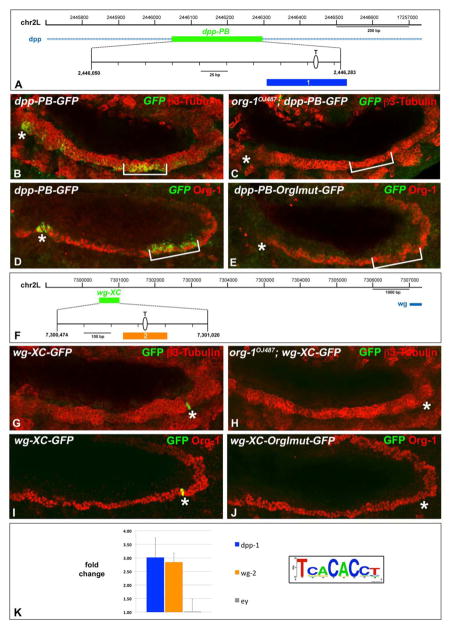
For the creation of the dpp-GFP and wg-GFP reporter lines the genomic regions chr2L:2446030…2446286 (dppPB, Manak et al., 1994) and chr2L:7300474…7301020 (wgXC, Grienenberger et al., 2003) according to R5.41 were amplified using yw genomic DNA as template. All fragments were cloned into EcoRI/KpnI of a modified pH-Stinger vector, which has AttB sequences inserted into its AvrII site (H. Jin and M. Frasch, unpublished). For the analogous creation of dppPB-OrgImut-GFP and wgXC-OrgImut-GFP reporters the Org-1 binding sites within the dppPB and wgXC sequences were predicted using Target Explorer (Sosinsky et al., 2003) searches with a positional weight matrix generated by SELEX with Org-1-GST (Schaub et al., 2012) and mutated via site directed mutagenesis as follows: dppPB TCACACCC → dppPB-OrgImut AAGCTTCC (Fig. 7E) and dppPB-OrgImut2 TCAATACC (with same result as with dppPB-OrgImut; CS and MF, unpublished data); wgXC TCGCACTT → WgXC-OrgImut-GFP TCAAGCTT (Fig. 7H). For transformation, the landing sites ZH-35B (Bischof et al., 2007) or AttP2 (Groth et al., 2004) were used.
Figure 7. Org-1 is a direct regulator of dpp and wg expression in the circular visceral muscle founder cell lineage.
A) Schematic diagram of the genomic region used for the generation of the dppPB reporter. The predicted T-Box binding site is indicated in the blow-up view. (B) dppPB driven GFP expression in parasegment (PS) 3 (asterisk) and PS6-7 (bracket) of a stage 11–12 embryo. (C) Stage 11–12 org-1 mutant embryo carrying dppPB-GFP showing complete absence of visceral GFP transcript. (D) The dppPB reporter (GFP) is coexpressed with Org-1 in visceral mesodermal PS3 (asterisk) and PS6-7 (bracket). (E) The dppPB-OrgImut-GFP enhancer construct displays nearly complete loss of reporter activity in PS3 and PS7. (F) Schematic diagram of the genomic region used for the generation of the wgXC reporter. The predicted T-Box binding site is indicated in the blow-up view. (G)The wgXC enhancer fragment drives GFP expression in the visceral founder cells of parasegment (PS) 8 (asterisk) of a stage 11–12 embryo. (H) Complete absence of visceral GFP expression in a stage 11–12 org-1 mutant embryo carrying wgXC. (I) wgXC reporter signal (GFP) is colocalized with Org-1 in visceral mesodermal PS8 (asterisk). (J) The wgXC-OrgImut-GFP reporter shows nearly complete loss of reporter activity in PS8. (K) Amplicons covering the T-Box binding sites (indicated in A and F) and an ey exonic amplicon as negative control were assayed by qPCR for enrichment in Anti-Org-1 ChIP. Three independent biological replicates were used to generate the average bar normalized to negative controls from the C15 gene. The Org-1 consensus motif identified by SELEX (Schaub et al., 2012) is visualized by its sequence logo.
Immunohistochemistry
Antibody staining and in situ hybridization were performed as described in (Azpiazu and Frasch, 1993; Knirr et al., 1999). The following primary antibodies and probes were used: rat anti-Org-1 (1:100, Schaub et al., 2012) mouse anti-Ubx monoclonal (1:50, White and Wilcox, 1985), rabbit anti-Teashirt (1:4000, Gallet et al., 1998), rat anti-Abd-A (1:500, Macias et al., 1990), mouse anti-Antp, mouse anti-FasciclinIII, mouse anti-Scr, mouse anti-Wg (all monoclonal, 1:20, Developmental Studies Hybridoma Bank, Univ. of Iowa), rabbit anti-Homothorax (1:1000, Kurant et al., 1998), rabbit anti-β3-Tubulin (1:3000, a gift from R. Renkawitz-Pohl, University of Marburg), rat anti-Tropomyosin (1:400, Brabraham Monoclonal Antibody Facility), rabbit anti-GFP (1:3000, Molecular Probes), mouse anti-GFP (1:200, Molecular Probes), rabbit anti-βGal (1:2000, Promega), mouse anti-βGal (40–1a, 1:20, Developmental Studies Hybridoma Bank, Univ. of Iowa) and digoxigenin labeled opa (Cimbora and Sakonju, 1995), dpp (St Johnston and Gelbart, 1987), GFP as well as pntP1 (Klambt, 1993) antisense RNA probes.
All mutant lines were balanced with lacZ or GFP balancers and mutant embryos were distinguished from wild type embryos by staining with β-galactosidase or GFP antibodies. Confocal pictures were taken with a Leica SP5 II (20×/1.3 PL APO Glycerol, 63×/1.3 PL APO Glycerol). Projections were done with Leica LAS AF.
Chromatin immunoprecipitation assays (ChIP)
Chromatin preparation using ChIP were performed using stage 11–14 yw embryo collections and Org-1 antibodies as described (Schaub et al., 2012). The precipitated DNA was quantified using FastStart Universal SYBR Green Master (Rox) (Roche Applied Science) on an Eco™ Real-Time PCR System (Illumina). In each experiment Real-Time PCR was performed in triplicate. Three independent precipitations per amplicon were analyzed.
Introduction
The musculatures of higher organisms can be subdivided into three major types: the skeletal, cardiac, and visceral muscles. These muscle types have distinct developmental origins and differ in terms of their morphological, physiological and molecular features including their contractile properties.
The visceral musculature of the Drosophila midgut, which is in the focus of this study, consists primarily of the musculature of the digestive tract and is composed of an inner layer of circular muscles and an outer layer of longitudinal muscles. Whereas the circular musculature derives from segmental portions of the dorsal mesoderm in the trunk under the inductive influence of decapentaplegic (dpp, a TGFβ signals (Frasch, 1995), the anlagen of the longitudinal muscles are located in the caudal visceral mesoderm (Nguyen and Xu, 1998; Kusch and Reuter, 1999; Ismat et al., 2010). The transcriptional response of the prospective trunk visceral mesoderm cells to the inductive cues of Dpp includes the induction of the NK homeobox genes tinman (tin), bagpipe (bap) and the FoxF family member biniou (bin) (Staehling-Hampton et al., 1994; Frasch, 1995; Zaffran et al., 2001). The segmental blocking of these inductive activities of Tin and Dpp in the dorsal mesoderm by the Wingless-induced repressor Sloppy-paired (Lee and Frasch, 2000; Lee and Frasch, 2005) leads to the expression and function of bap and bin exclusively in the metameric primordia of the circular visceral muscles of the midgut. Whereas tin and Dpp are required for the formation of all dorsal mesodermal derivatives (heart, dorsal body wall muscles, visceral muscles) (Azpiazu and Frasch, 1993; Bodmer, 1993; Zaffran et al., 2001; Lee and Frasch, 2005), bap and bin are both essential for the specification of the trunk visceral mesoderm and the formation of midgut muscles from it (Azpiazu and Frasch, 1993; Zaffran et al., 2001). Moreover, the sac-like appearance of the remnant of the midgut in bap and bin mutant embryos highlights the importance of the visceral mesoderm in shaping the proper morphogenesis of the midgut. In particular, it triggers the formation of three constrictions at defined positions along the developing midgut that evolve into gut loops and induces the formation of four blind-ending pouches termed gastric caeca at its anterior end (Tepass and Hartenstein, 1994; Campos-Ortega and Hartenstein, 1997).
The circular visceral muscles (cVM) are built from the trunk visceral mesoderm by the fusion of circular visceral muscle founder cells (cFC) and visceral fusion competent myoblasts (FCM) into binucleated muscle fibers (Martin et al., 2001; Klapper et al., 2002). The founder cells are specified by spatially restricted Jelly belly (Jeb) signals acting via the Anaplastic lymphoma kinase (Alk) receptor tyrosine kinase along the ventral margin of the trunk visceral mesoderm, whereas the dorsally adjacent cells become fusion-competent myoblasts by default (Englund et al., 2003; Lee et al., 2003).
The morphogenetic events along the developing midgut are the readout of regulatory patterning networks that act within specific anteroposterior areas of the cVM. The networks integrate the spatial activities of homeotic selector genes and secreted growth factors, leading to the establishment of morphogenetic centers at defined positions. In the most anterior part of the midgut the gene products of Sex combs reduced (Scr) and decapentaplegic (dpp) are required for proper formation of the gastric caeca (Panganiban et al., 1990; Reuter and Scott, 1990). The formation of the anterior midgut constriction is initiated by the activity of Antennapedia (Antp) and its downstream targets, the zinc finger protein encoding genes odd-paired (opa) and teashirt (tsh) (Reuter and Scott, 1990; Mathies et al., 1994; Cimbora and Sakonju, 1995). The formation and placement of the central midgut constriction depends crucially on Ultrabithorax (Ubx), dpp and wingless (wg, a Wnt member), which act together in a regulatory feedback loop that has been subject to detailed dissection (Muller et al., 1989; Panganiban et al., 1990; Reuter et al., 1990; Hursh et al., 1993; Capovilla et al., 1994; Sun et al., 1995). teashirt (tsh), another Wg downstream gene, is additionally required for the formation of the central constriction and, independently of Wg, also for the anterior constriction (Mathies et al., 1994). The identification and analysis of cis-regulatory modules (CRMs) of Ubx, dpp and wg expression in the visceral mesoderm has further shown that all three genes are direct regulators of each other (Thuringer et al., 1993; Capovilla et al., 1994; Manak et al., 1994; Sun et al., 1995; Yang et al., 2000; Grienenberger et al., 2003) The formation of the posterior constriction is dependent on abdominal-A (abd-A) expression (Tremml and Bienz, 1989). For this event, Wg needs to activate the expression of the ETS transcription factor encoding gene pointed (pnt) in the cells of the anterior abd-A domain. In the cells of the same region, Dpp signals are essential to prevent Abd-A from activating the transcription of the zinc finger factor-encoding gene odd-paired (opa) (Cimbora and Sakonju, 1995; Bilder et al., 1998).
Because the above-described signals, Hox factors, and other transcriptional regulators are also present in tissues outside of the visceral mesoderm, the visceral mesoderm-specific establishment and maintenance of the various morphogenetic centers they participate in must additionally require tissue-specific cofactors. In this context, it was found that bin functions as a direct upstream regulator of dpp and is also required for wg expression at the prospective central constriction (Zaffran et al., 2001). However, the expression of Ubx in the visceral mesoderm was independent of bin (and bap), thus indicating a requirement for additional visceral mesodermal competence factors. Furthermore, it was found that the absence of cFCs in Alk mutant backgrounds prevents Ubx, dpp, and wg expression in the visceral mesoderm even though bin is not affected. This observation implied that the initial activation of these genes is either directly dependent on Alk signals or dependent on factor(s) induced transcriptionally by Jeb/Alk in circular muscle founder cells (Shirinian et al., 2007).
In the present study, we identify org-1, a Jeb/Alk-induced gene encoding the Drosophila Tbx1 ortholog (Lee et al., 2003), as a crucial factor required for the full activation and maintenance of opa, tsh, Ubx, dpp and wg expression in the founder cells of the circular visceral musculature. Consequently, the morphogenesis of midgut structures depending on these activities, namely the gastric caeca and the anterior as well as the central midgut constrictions, is disrupted in org-1 mutant embryos. We demonstrate that the activation of the respective visceral mesoderm specific enhancer elements of dpp and wg by org-1 requires T-box binding motifs and show in vivo occupancy of these elements by Org-1. Thus, by directly regulating dpp and wg, org-1 represents a component of the intrinsic founder cell program of the cFCs that helps implementing their anteroposterior diversification and organizing subsequent midgut morphogenesis.
Results
org-1 is expressed in the circular visceral muscles and their progenitors
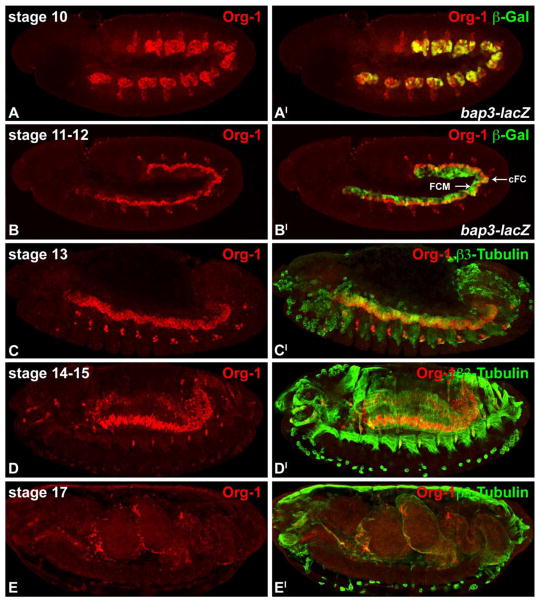
As described previously, org-1, the Drosophila orthologue of Tbx1, shows a highly restricted expression pattern in the cells of the developing somatic and visceral mesoderm (Lee et al., 2003; Schaub et al., 2012). In the dorsal mesoderm Org-1 expression is induced at stage 10 in all cells of the 11 bilateral patches of cells of the prospective trunk visceral mesoderm (TVM) (Fig. 1A,A′), showing an analogous expression pattern to bagpipe (bap) and biniou (bin) at this stage. During stages 11–12, the visceral Org-1 expression is refined and restricted to the progenitor cells, and after their division, the founder cells of the circular visceral musculature (cVM) (Fig. 1B,B′). By contrast, bap and bin expression is maintained in all TVM cells although bap expression ceases after stage 12 (Azpiazu and Frasch, 1993; Zaffran et al., 2001). Upon fusion of the circular visceral muscle founder cells (cFC) with visceral fusion competent myoblasts (FCM) at stage 13 (Fig. 1C,C′) until late embryogenesis (Fig 1D,D′,E,E′), Org-1 protein is found, like Biniou, in all nuclei of the cVM.
Figure 1. org-1 shows a highly dynamic expression pattern during visceral mesoderm development.
(A,AI) Org-1 protein (Org-1) is found during stage 10 in all cells of the trunk visceral mesoderm primordia, visualized by bap3-lacZ expression (β-Gal). (B,BI) At stage 11 the visceral mesodermal expression domains of Org-1 are narrowed down to the founder cells of the circular visceral musculature (cFC), whereas the visceral fusion competent myoblasts (FCM) have become negative for Org-1 protein. (C,CI) Visceral muscle fusion takes place in stage 13, leading to the activation of Org-1 expression in the newly incorporated nuclei in the binucleated muscle syncytia. (D,DI) From stage 14 until the end of embryogenesis (E,EI) all nuclei of the circular visceral musculature continue to express Org-1.
org-1 expression in the trunk visceral mesoderm is regulated by tinman and dpp but largely independent of bap and bin
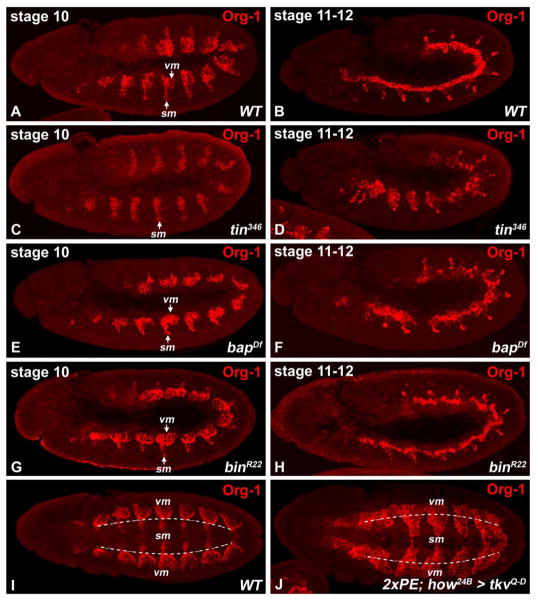
Previous work has shown that the expression domains of bap and bin in the 11 bilateral patches of the TVM are defined by the intersecting dorsal activities of dpp/tin, which act positively, and the segmental activities of wg/slp, which have repressing effects. (Azpiazu and Frasch, 1993; Staehling-Hampton et al., 1994; Frasch, 1995; Azpiazu et al., 1996; Riechmann et al., 1997; Lee and Frasch, 2000; Zaffran et al., 2001; Lee and Frasch, 2005). As shown in Fig. 2C,D (compare with A, B), like bap and bin, also org-1 requires tin activity for its full activation in the TVM. In a tin mutant background, only partial activation of org-1 expression in the visceral mesoderm primordia occurs (Fig. 2C; note that most of the residual striped expression corresponds to somatic mesodermal (sm) expression) and there is no refinement of org-1 expression to the two rows of cFCs (Fig. 2D). Apart from being downstream genes of Tin, bap and bin also regulate each other through a cross-regulatory feedback loop (Zaffran et al., 2001). To elucidate if additional inputs from Bap and Bin are necessary for org-1 regulation, we analyzed Org-1 expression in bapDf and binR22 genetic backgrounds. At stage 10, neither the loss of bap (Fig. 2E) nor of bin function (Fig. 2G) have any severe effects on the expression pattern of org-1, thus indicating that activation of org-1 in the trunk visceral mesoderm patches is largely independent of bap and bin. During stages 11–12, loss of bap results in perturbed restriction of Org-1 to the visceral muscle founder cells (if any are present; Fig. 2F), indicating some influence of bap on org-1 expression during later stages. Unlike bap mutants, binR22 mutant embryos show only slight aberrations with regard to Org-1 expression at this stage (Fig. 2H).
Figure 2. Regulation of org-1 expression in the trunk visceral mesoderm.
(A,B) Stage 10 and stage 11–12 wildtype embryos stained for Org-1 protein. (C,D) Stage 10 and stage 11–12 tin346 embryos stained for Org-1 showing strongly reduced expression in the trunk visceral mesoderm and missing founder cell restriction. (E) Stage 10 bapDf mutant embryos display only slight reduction of Org-1 expression domains during early development. (F) bapDf embryos of stage 12 display missing founder cell restriction of Org-1. (G,H) Loss of Bin function has no influence on Org-1 expression at early stages as well as during stage 12. (I) Ventral view of a stage 12 wildtype embryo stained for Org-1. (J) Ventral view of a stage 12 embryo with forced panmesodermal expression of a constitutively activated version of the Dpp-receptor Thickveins (tkvQ–D). The Org-1 expression domains have been extended into the ventral somatic mesoderm. Abbreviations: (vm) visceral mesoderm, (sm) somatic mesoderm.
To test if Dpp signaling from the overlying ectoderm is involved in the induction of org-1 expression (which may explain the residual visceral mesodermal org-1 expression in tin mutant embryos), we overexpressed a constitutively-activated version of the Dpp receptor Thickveins (tkvQ–D) (Nellen et al., 1996) panmesodermally. As shown in Fig. 2J (compare with Fig. 2I), this leads to an expansion of Org-1 expression into the ventral somatic mesoderm, thus demonstrating direct or indirect activation of org-1 by Dpp signaling. Taken together, our data suggest a model in which org-1 expression is activated by tin and dpp in the visceral mesoderm primordia in parallel to and largely independently of bap and bin.
Org-1 is required for proper midgut morphogenesis, but not for the specification of the visceral musculature itself
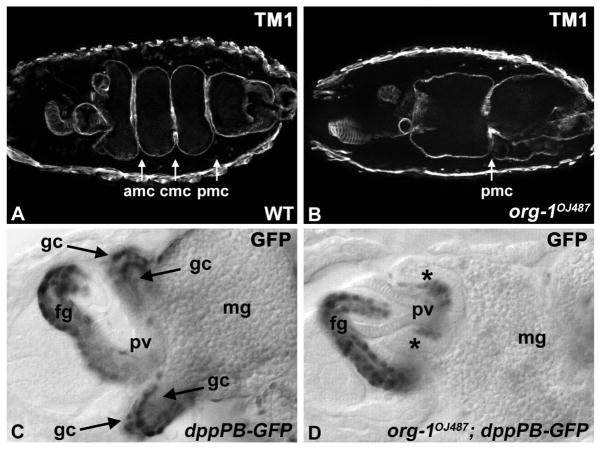
To analyze the function of org-1 during visceral mesoderm development, we examined the expression patterns of various markers in hemizygous embryos carrying the lethal org-1 null alleles org-1OJ487 or org-1OJ423 (Schaub et al., 2012) at different developmental time points. It is well documented that proper specification of the endodermal as well as the mesodermal cell layers of the embryonic midgut is required for normal morphogenesis and differentiation of the midgut (Azpiazu and Frasch, 1993; Reuter et al., 1993; Tepass and Hartenstein, 1994; Zaffran et al., 2001; Wolfstetter et al., 2009). To examine whether potential abnormalities in the visceral mesoderm of org-1 mutants affect the embryonic midgut we stained embryos with antibodies against Tropomyosin (Fig. 3A), which is expressed in all visceral muscles and provides a clear picture of midgut morphology. The analysis of stage 16 org-1OJ487 and org-1OJ423 mutant embryos revealed pronounced abnormalities in midgut morphology. In particular, the midgut fails to form the anterior and the central midgut constrictions whereas the remaining posterior constriction is displaced (Fig. 3B). In order to investigate gastric caeca differentiation, we visualized them by reporter staining in stage 17 dppPB-GFP embryos. Whereas in wild type embryos the two pairs of gastric caeca are clearly visible and show strong reporter signals (Fig. 3C), midguts of org-1 mutant late stage 17 embryos show an arrest of gastric caeca formation and only few cells with reporter signals (Fig. 3D, asterisks). These midgut phenotypes of the org-1 mutant alleles can be rescued by the expression of org-1 under the control of a bap3-GAL4 driver in the trunk visceral mesoderm of org-1 mutant embryos (data not shown), clearly demonstrating that the org-1 gene is required within the visceral mesoderm for proper midgut organogenesis.
Figure 3. org-1 is required for proper midgut morphogenesis.
(A,B) Comparison of midgut morphology of stage 16 WT and org-1OJ487 mutant embryos (stained with antibodies against Tropomyosin (TM1)) reveals the loss of the anterior (amc) and central midgut (cmg) constrictions as well as a mislocated posterior (pmc) constriction in the mutant. (C,D) Anterior midguts from late stage 17 dppPB-GFP embryos, stained against GFP. In wildtype background the reporter is active in the visceral musculature around the 2 pairs of gastric caeca (gc, arrows). In the org-1OJ487 genetic background gastric caeca morphogenesis is arrested (asterisks). Abbreviations: (fg) foregut, (pv) proventriculus, (mg) midgut.
To test whether the observed midgut phenotypes are due to the failure of TVM specification in the absence of functional org-1, the IgG-domain adhesion molecule Fasciclin III (FasIII) was used as an early marker for specified TVM and developing cVM (Patel et al., 1987). However, in stage 11 org-1 mutant embryos, no changes were observed in the expression pattern of FasIII and all other tested trunk visceral mesoderm markers (data not shown). Likewise, the formation of circular gut muscle founder cells (cFC) was not disrupted because, as shown with a bap-lacZ marker in the org-1 mutant background, the trunk visceral mesoderm precursors (including fusion-competent cells) are still strictly fated to contribute to midgut muscles (data not shown). By contrast, in genetic situations that prevent the formation of cFCs, fusion of the FCMs occurs with somatic founder cells, which causes their subsequent integration into the somatic musculature (Lee et al., 2003).
Org-1 is required for proper activation of distinct patterning genes in the founder cells of the developing circular midgut musculature
Previous work has shown that the formation and the placement of the gastric caeca and the three midgut constrictions depend on regulatory networks in the visceral muscle lineage that integrate the spatial and temporal inputs of specific transcriptional regulators and signaling activities. These include the homeotic selector genes Sex combs reduced (Scr), Antennapedia (Antp), Ultrabithorax (Ubx) and abdominal A (abd-A), as well as the secreted growth factors decapentaplegic (dpp) and wingless (wg), and lead to the establishment of distinct expression domains of the downstream effectors teashirt (tsh), pointed (pnt) and odd-paired (opa) in the midgut musculature (Tremml and Bienz, 1989; Immergluck et al., 1990; Panganiban et al., 1990; Reuter et al., 1990; Reuter and Scott, 1990; Andrew et al., 1994; Bienz, 1994; Mathies et al., 1994; Staehling-Hampton and Hoffmann, 1994; Cimbora and Sakonju, 1995; Bilder et al., 1998).
As the loss of org-1 function disrupts gastric caeca formation and causes the loss and misplacement of midgut constrictions (Fig. 3B,D), it was conceivable that org-1 represents a new and founder cell specific regulator within this network. If this were the case, then at least some members of the network would be expected to show founder cell specific expression domains. For Ubx, dpp, wg and Abd-A this has already been suggested (Martin et al., 2001; Shirinian et al., 2007).
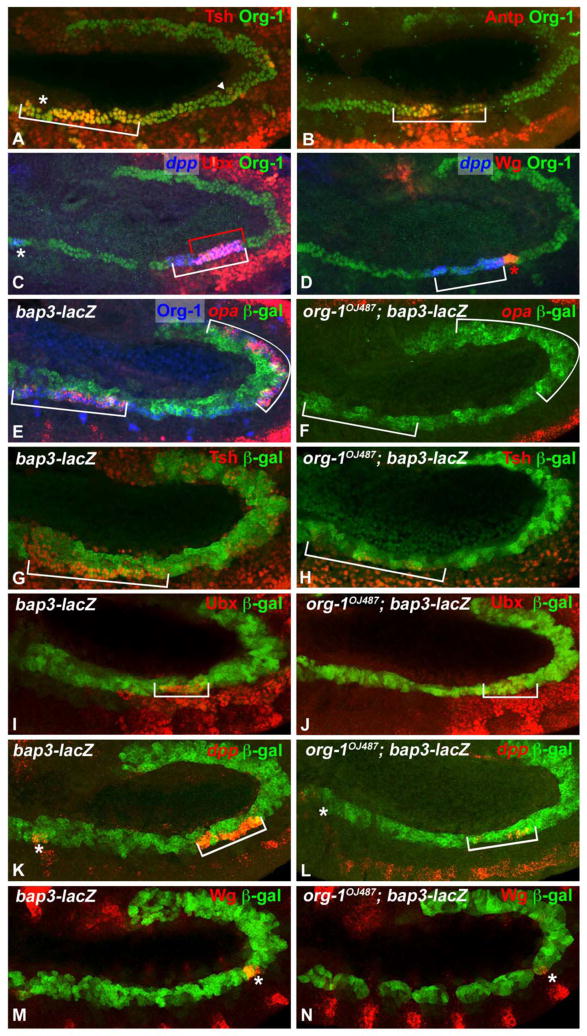
This notion was further underpinned by our colocalization analyses of the respective expression patterns in stage 11 embryos with Org-1. Although it has been described that the visceral expression of tsh and Antp starts in defined parasegments of stage 13 embryos (Reuter and Scott, 1990; Mathies et al., 1994), our analyses revealed that the expression of these genes in fact already initiates in the founder cells at stage 11 in the respective visceral mesodermal parasegments (Fig. 4A,B). Tsh expression is detected in the founder cells of PS4-5 as well as in the anterior part of PS6, with the expression in PS5-6 being very robust as compared to that in PS4 (Fig. 4A, bracket, asterisk). Early Tsh expression is still very weak in PS8 (Fig. 4A, arrowhead) where it is upregulated later as a response to Wg and Dpp signals (Mathies et al., 1994) (see also Fig. 6E, asterisk). Likewise, we demonstrate that that the visceral mesoderm specific expression domains of Ubx (Fig. 4C, in PS7, red bracket), dpp (Fig. 4C,D in PS3, white asterisk and PS6-7, white bracket) and wg (Fig. 4D, in PS8, red asterisk) as well as Abd-A (data not shown) in stage 11–12 embryos are founder cell specific. Interestingly, the dpp expression domain in the founder cells is broader than described for the visceral musculature (Panganiban et al., 1990). Instead of being expressed exclusively in PS7, it extends into the posterior part of PS6, whereas Ubx is expressed solely in PS7 (Fig. 4C, white bracket, compare to red bracket). Wg is expressed in the founder cells of PS8 posteriorly adjacent to the dpp domain (Fig 4D, white bracket and red asterisk). Whereas the data for the visceral musculature had indicated that wg is expressed in the entire PS8 (van den Heuvel et al., 1989), the smaller Wg domain in the founder cells indicates that, at least at stage 11, Wg expression is limited to the anterior part of PS8. Expression of the homeotic target gene opa has been documented in PS5 and PS9-12 of the visceral musculature (Cimbora and Sakonju, 1995) and we find that it is initiated already at stage 11 in the founder cells of visceral mesodermal PS3-5 (only weakly in PS3) and PS9-12 (Fig. 4E).
Figure 4. Lineage specific odd paired, teashirt, Ultrabithorax, decapentaplegic and wingless expression in the founder cells of the circular visceral musculature depends on Org-1.
(A) At stage 11 Teashirt (Tsh) shows expression within the circular visceral muscle founder cells of parasegments (PS) 4–6 (bracket), albeit being weaker in PS4 (asterisk) than in PS5-6, and traces of expression in PS8 (arrowhead). (B) In an embryo of the same stage Antennapedia (Antp) is detected in the founder cells of PS5-6 (bracket). (C) In the visceral mesoderm, Ultrabithorax (Ubx) is solely expressed in the founders of PS7 (red bracket), whereas decapentaplegic (dpp) transcript can be found in the anterior founders of PS3 (asterisk), posterior PS6 and PS7 (bracket). (D) Some founder cells of PS8 are positive for Wingless (Wg) (red asterisk), whereas the adjacent founders of posterior PS6 and PS7 are positive for dpp transcript (bracket). (E) odd-paired (opa) transcript can be found in the founders of PS3-5 (weak in PS3) and PS9-12 (brackets). (F) In org-1 loss of function background opa expression in the visceral mesoderm is absent. (G) Tsh shows a strong expression domain in the founder cells of PS4-6 (bracket). (H) Org-1 loss of function leads to the nearly complete abolishment of Tsh in these founder cells. (I) In a stage 11–12 wildtype embryo Ubx is detected in the visceral founder cells of PS 7 (bracket). (J) In org-1 mutant background Ubx expression in the nuclei of the founder cells of PS7 is absent. (K) Dpp is expressed in wildtype embryos in the founders of PS3 (asterisk) and PS6-7 (bracket) whereas (L) the loss of Org-1 leads to heavy reduction of visceral mesodermal dpp transcription in PS3 and PS6-7. (M) The visceral expression domain of Wg is seen in founder cells of PS8 (asterisk). (N) The removal of functional Org-1 leads to the nearly complete loss of Wg expression in the founders of PS8. The founder cells are visualized by stainings with Org-1 antibodies. The visceral mesoderm and musculature are visualized by bap3-lacZ reporter stainings (β-Gal).
Figure 6. Org-1 is required for proper anteroposterior patterning of the developing visceral musculature.
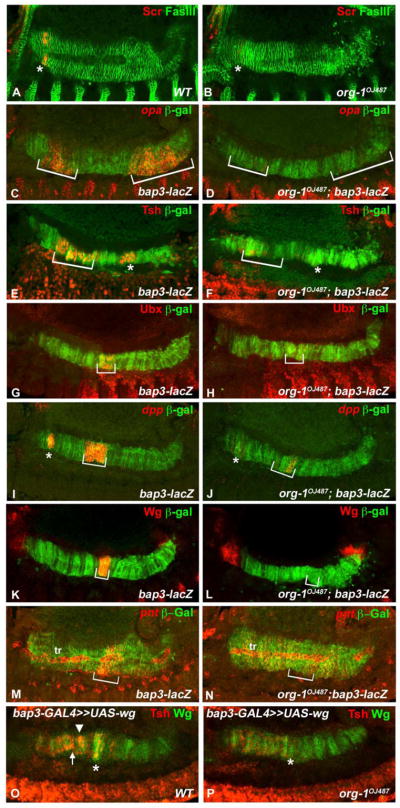
(A) Sex combs reduced (Scr) expression is initiated in the visceral musculature (visualized by Fasciclin III (FasIII) staining) of stage 13 embryos in parasegment (PS) 4 (asterisk). (B) In org-1 mutant background visceral mesodermal Scr expression is nearly completely abolished. (C) Visceral muscle specific odd-paired (opa) expression can be detected in PS5 (asterisk) and PS10-12 (bracket). (D) In an org-1 mutant embryo both expression domains are absent. (E) Teashirt (Tsh) shows two expression domains in the visceral musculature: one in PS4-6 (bracket) and another one in PS8 (asterisk). (F) Loss of Org-1 expression leads to abolishment of both expression domains of visceral mesodermal Tsh. (G) Stage 13 wildtype embryo showing Ubx expression in PS7 of the developing visceral musculature (bracket). (H) In the org-1OJ487 genetic background Ubx expression in PS7 is completely abolished. (I) Dpp shows strong expression domains in PS3 (asterisk) and PS6-7 (bracket) of the developing visceral musculature. (J) Only weak dpp expression can be detected in PS3 and PS7 in the org-1 mutant background. (K) In a stage 13 wildtype embryo, Wg can be detected in PS8 (bracket) of the developing visceral musculature. (L) In an org-1 mutant embryo Wg expression can no longer be detected in PS8. (M) In a stage 13 embryo pointed (pnt) transcript is detectable in PS8-9 of the developing visceral musculature (bracket) and the trachea adjacent to it (tr). (N) In an org-1 mutant embryo the visceral musculature specific expression of pnt is not present. (O) Overexpression of Wg in the whole visceral mesoderm of wildtype embryos via bap-Gal4 induces between PS4-6 (bracket) and PS8 (asterisk) a strong ectopic Tsh expression domain (arrowhead). (P) In an org-1OJ487 embryo, forced induction of Wg expression in the visceral mesoderm causes only faint Tsh expression anterior to PS8 (asterisk). The visceral musculature is visualized by bap3-lacZ reporter stainings (β-Gal).
To investigate potential regulatory inputs from Org-1 towards these genes that are expressed in localized domains within the visceral muscle founder cells we tested their expression patterns in org-1 loss and gain of function backgrounds. As shown in Fig. 4F,H,J,L,N loss of org-1 function causes near absence of the expression of opa, tsh, Ubx, dpp and wg within the founder cells of the visceral musculature (compare to Fig. 4E,G,I,K,M). By contrast, Antp and abd-A expression in the founder cells is not affected by the loss of org-1 function (data not shown). In light of this result the loss of founder cell specific opa and Tsh expression was surprising, because it has been shown that Antp regulates both, tsh in PS4-6 and opa expression in PS4-5, and that abd-A is required for opa activation in PS9-12 of the visceral musculature (Mathies et al., 1994; Cimbora and Sakonju, 1995). Therefore we conclude that org-1 is an essential regulator of opa and tsh expression in these visceral founder cells in parallel with Antp and abd-A respectively.
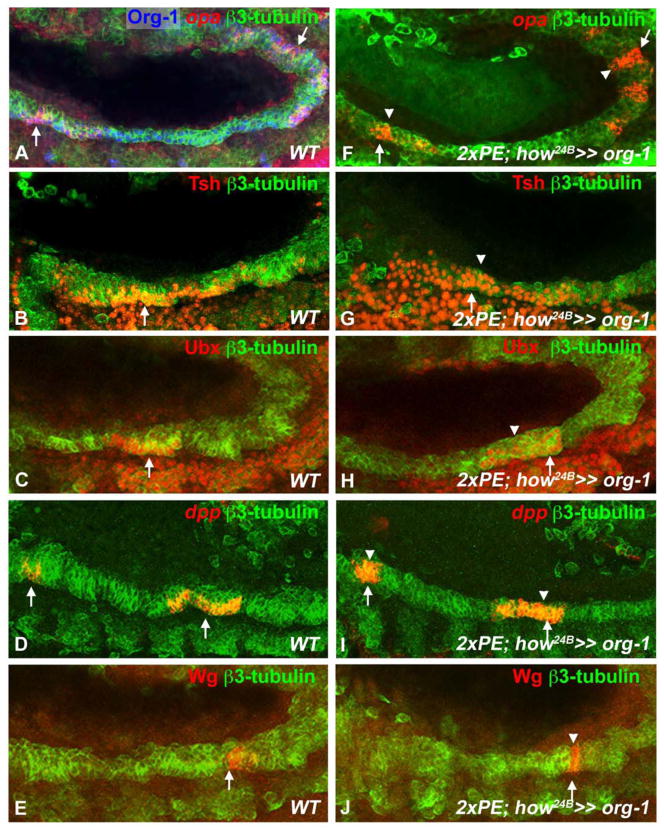
To test whether the founder cell-specific expression of org-1 is limiting the induction of the patterning genes to the founder cells in the TVM we expressed org-1 ectopically. Forced org-1 expression in the whole trunk visceral mesoderm, including the fusion-competent cells of the TVM, causes an expansion of the opa, tsh, Ubx, dpp and wg expression domains into these FCMs (Fig. 5F,G,H,I,J compare to Fig. 5A,B,C,D,E). Hence, org-1 expression is required and in the proper tissue context sufficient for visceral lineage specific opa, tsh, Ubx, dpp and wg expression.
Figure 5. Org-1 is sufficient for visceral opa, tsh, Ubx, dpp and wg expression in the proper visceral mesodermal context.
(A,B,C,D,E) Wildtype stage 12 embryos showing founder cell specific expression of (A) opa in PS3-5 and PS9-12 PS, (B) Tsh in PS4-6 (C) Ubx in parasegment (PS) 7, (D) dpp in PS3 and PS7 and (E) Wg in PS8 in the visceral founder cells (arrows). (F,G,H,I,J) Forced expression of Org-1 in all cells of the visceral mesoderm leads to the expansion of (F) opa, (G) Tsh, (H) Ubx, (I) dpp and (J) Wg expression domains from the visceral founder cells (arrows) into the visceral fusion competent myoblasts (arrowheads). The visceral mesoderm is visualized by 3-Tubulin stainings.
The phenotypes in the developing visceral musculature of org-1 mutant embryos with regard to the expression of various patterning genes resembles those seen in stage 11–12 embryos. The expression domains of opa, tsh, Ubx, dpp and wg are nearly abolished (Fig. 6D,F,H,J,L compare to Fig. 6C,E,G,I,K). In addition, the visceral mesoderm expression domain of Scr, which is normally induced at stage 13 in PS4 of the developing midgut musculature, is barely detectable in org-1 mutant embryos (Fig. 6B compare to A). This effect could explain the observed defects in the formation of gastric caeca in org-1 mutant embryos because Scr is known to be indispensible for the development of these sacs (Reuter and Scott, 1990). Other defects in the developing midgut musculature of stage 13–14 org-1 mutant embryos include the lack of expression of the zinc finger factor encoding gene tsh in PS8 (Fig. 6F, asterisk) and the ETS domain factor encoding gene pointed in PS8-9 (Fig. 6N). It has been reported that, in the normal situation, both pnt and tsh (in PS8) require Wg for their induction. To clarify if the absence of tsh and pointed expression at the future central midgut constriction are solely due to the loss of sufficient wg expression in org-1 mutants or if there are additional regulatory inputs from Org-1 we forced wg expression in the whole visceral mesoderm in the org-1 mutant background. We found that, in this situation, pnt expression is re-initiated (data not shown), whereas tsh (Fig. 6P) is only slightly activated, demonstrating that even in the presence of sufficient doses of activating factors and signals the full activation of tsh transcription in PS8 of the visceral musculature is dependent on Org-1 function.
Taken together, our analysis provides strong evidence that org-1 is required for the lineage specific activation of opa, tsh, Ubx, dpp and wg in the visceral muscle founder cells and of Scr in the developing visceral musculature. These findings implicate org-1 as an additional tissue-specific regulator in the regulatory network controlling midgut morphogenesis.
Org-1 is a direct upstream regulator of dpp and wg expression in the visceral mesoderm
Previous work has shown that the expression patterns of dpp, Ubx and wg in PS7/8 of the visceral mesoderm are established through a regulatory feedback loop which integrates the direct inputs of all three genes together with Biniou as a tissue specific activator (Muller et al., 1989; Panganiban et al., 1990; Reuter et al., 1990; Hursh et al., 1993; Manak et al., 1994; Sun et al., 1995; Yu et al., 1996; Zaffran et al., 2001; Grienenberger et al., 2003). Our current data raise the question of whether Org-1 is an additional direct upstream regulator of Ubx, dpp and wg that acts together with Biniou but in this case exclusively in the founder cells of the circular visceral muscles. To test this possibility we dissected visceral mesoderm specific cis-regulatory modules (CRMs) of dpp (dppPB, Fig 7A) and wingless (wgXC, Fig. 7F) which are known to mediate the signal integration of these two genes in PS7 and PS8, respectively (Manak et al., 1994; Grienenberger et al., 2003). The activity of both enhancers at stage 11–12 is indeed restricted to founder cells in the TVM, with the dppPB-GFP reporter being initiated in the cFCs of PS3 and PS7 of the visceral mesoderm (Fig. 7B) and wgXC-GFP in cFCs of PS8 (wgXC-GFP, Fig. 7G). By contrast, in org-1 loss of function genetic backgrounds the dppPB-GFP (Fig. 7C) and wgXC-GFP (Fig. 7H) enhancer constructs show a loss of the reporter signals, which mimics the effects on dpp and wg expression upon loss of functional Org-1.
To address if Org-1 contributes directly to the activation of these enhancers, we searched their sequences for putative Org-1 binding motifs (Schaub et al., 2012) and identified one good match in dppPB and one within wgXC. The sequences spanning the predicted T-Box motifs were tested for in vivo binding of Org-1 by anti-Org-1 ChIP experiments, which demonstrated significant binding of Org-1 to the tested sites in the enhancer fragments in stage 11–14 embryos (Fig. 7K). To address their functionality in vivo we introduced 2–3 base pair changes in the T-Box motifs at positions known to be important for T-box protein binding (see Materials & Methods) (Muller and Herrmann, 1997) These mutations had dramatic effects as neither the dppPB-OrgImut-GFP reporter (Fig. 7E) nor the wgXC-OrgImut-GFP reporter (Fig. 7J) allowed any GFP expression in the cFCs of transgenic embryos during stages 11–12. Both mutant constructs did become active in the cVM of stage 13 embryos, albeit with strongly reduced intensities (data not shown). Altogether, these data strongly suggest that the normal activation of dpp and wg in the visceral mesoderm requires Org-1 binding to the T-box binding motifs within the dppPB and wgXC CRM sequences.
Discussion
The analysis of org-1 expression and function during visceral mesoderm development defined this gene as a new and essential lineage specific regulator of circular visceral muscle founder cell identities and midgut patterning in Drosophila. Our data add new insights into the developmental regulatory mechanisms responsible for the diversification of the circular visceral muscle founder cell lineage and midgut morphogenesis.
Regulation and functions of org-1 in trunk visceral mesoderm primordia versus circular visceral muscle founder cells
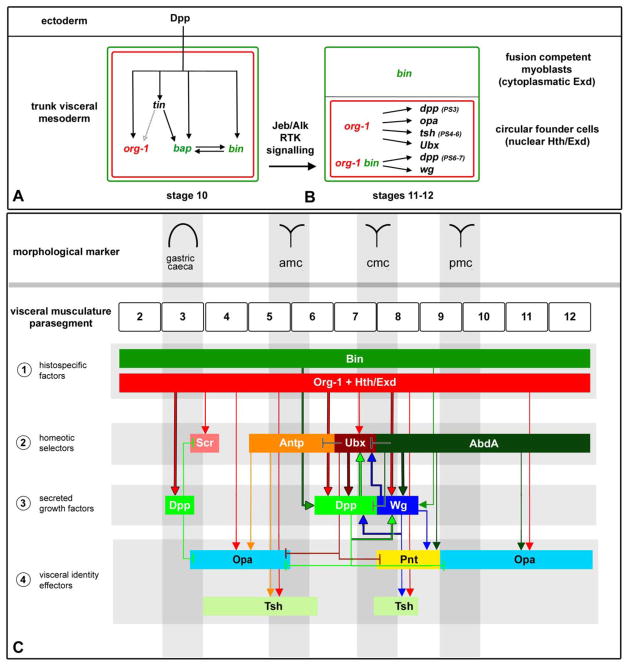
The initial expression of org-1 occurs in the segmented TVM, where it is coexpressed with tin, bap, bin and Alk (Azpiazu and Frasch, 1993; Zaffran et al., 2001; Loren et al., 2003). It has been documented that the induction of tin and bap in the dorsal mesoderm involves the combined binding of Smad proteins (Medea and Mad) and Tin to Dpp-responsive enhancers of the tin and bap genes (Xu et al., 1998; Lee and Frasch, 2005), whereas the segmental repression of bap is mediated by binding of the sloppy paired (slp) gene product (Lee and Frasch, 2005). Our genetic analysis of org-1 has shown that org-1 is activated downstream of tin but independently of bap and bin, and that dpp provides the key signals for its induction. This suggests a regulatory mechanism analogous to that of bap, in which the combined binding of Smads and Tin activates a Dpp-responsive org-1 enhancer, whereas Wg activated Slp is required for its mutual segmental repression (Fig. 8A).
Figure 8. Regulatory interactions during trunk visceral mesoderm specification, founder cell diversification and midgut differentiation.
(A) At stage 10 all cells of the trunk visceral mesoderm primordia initiate the expression of tinman (tin), bagpipe (bap), binou (bin) and org-1 due to inductive decapentaplegic (dpp) cues from the ectoderm (only positive inputs are shown). (B) Whereas bap expression diminishes during stage 11, bin expression persists in all cells of the visceral mesoderm and org-1 expression is maintained under the influence of receptor tyrosine kinase signaling (RTK) from the Anaplastic lymphoma kinase (Alk) only in the founder cells of the visceral musculature. Alk signaling induces nuclear translocation of Homothorax/Extradenticle (Hth/Exd) that activate together with Org-1 in the visceral founder cells of PS3 dpp, of PS3-5 and PS9-12 opa, of PS4-6 tsh, and of PS7 Ubx, and together with Org-1 and Bin the founder cell specific expression of dpp in PS6-7 and of wg in PS8. This process results in the diversification of visceral muscle founder cell fates along the anteroposterior axis. (C) After muscle fusion the spatial expression patterns of opa, tsh, Ubx, dpp and wg in the founder cells expand to the respective muscle fibers. Additionally Org-1 initiates the expression of Sex combs reduced (Scr) in the visceral musculature and is required in addition to Wg for the activation of tsh expression in PS8. abd-A, wg and dpp initiate the expression domains of Pointed (pnt) in PS8-9. Demonstrated direct regulation is indicated by arrows outlined in black.
The similarities in the early expression patterns of bap, bin, Alk and org-1 in the trunk visceral mesoderm primordia raise the question of the contribution of org-1 to the early development of the TVM as such. Whereas bap and bin are crucially required for the specification of the trunk visceral mesoderm and visceral musculature (Azpiazu and Frasch, 1993; Zaffran et al., 2001), loss of org-1 function, like the loss of Alk (Englund et al., 2003), has no obvious impact on the specification of the early TVM. Therefore, it is notable that during the subdivision of the visceral mesoderm primordia into founder and fusion-competent myoblasts (cFCs and FCMs), org-1 expression is extinguished in the FCMs and only sustained in the cFC lineage of the circular visceral musculature. This lineage-specific restriction and maintenance of org-1 expression crucially depends on Jeb mediated Alk/Ras/MAPK signaling (Lee et al., 2003) and points towards a possible cFC lineage specific function of org-1. Our genetic analysis demonstrates that org-1 is not required for cFC specification, but plays a decisive role in the induction of the visceral mesoderm specific expression of patterning genes in the founder cells of the circular musculature. Thus, org-1 is critical for the processes of cell fate diversification that provide individual fields of cells along the anteroposterior axis of the visceral mesoderm with their specific identities (Fig. 8B).
org-1 as a founder cell-specific regulator of visceral mesodermal patterning genes
Proper anteroposterior patterning of the trunk visceral mesoderm and the formation of localized organizer fields are prerequisites for eliciting the morphogenetic events that shape the midgut. The formation of these organizer fields depends on the appropriate spatial expression domains of the homeotic selectors Scr, Antp, Ubx and abd-A, the secreted factors dpp and wg, as well as the zinc finger proteins opa and tsh, which are required for the formation of the midgut constrictions as well as the gastric caeca (Bienz and Tremml, 1988; Tremml and Bienz, 1989; Immergluck et al., 1990; Panganiban et al., 1990; Reuter and Scott, 1990; Masucci and Hoffmann, 1993; Mathies et al., 1994; Cimbora and Sakonju, 1995). The regulatory mechanisms responsible for the establishment of the spatial, temporal and tissue-specific expression patterns of these genes in the TVM are only partially understood. Genetic and molecular analyses with the FoxF gene bin, which is expressed in all trunk visceral mesoderm precursors and their descendents, have demonstrated that bin is a direct upstream regulator of dpp in PS7 and is also required for the expression of wg in PS8 of the TVM (Zaffran et al., 2001). Thus, Bin serves as an essential TVM-specific competence factor in conjunction with the dpp/wg signaling feedback loop. Our current findings have defined Org-1 as an additional tissue-specific regulator with an even broader range of downstream patterning genes in the TVM, but with a narrower spatial range of action. We have shown that org-1 acts specifically within the visceral muscle founder cell lineage as a positive regulator upstream of opa, tsh, Ubx, dpp as well as wg.
Our combination of genetic data and functional enhancer analyses provides convincing evidence that both dpp and wg are direct transcriptional targets of Org-1 in the cFCs. Prior dissections of the dpp visceral mesoderm (VM) enhancer had shown that it is also regulated by the direct binding of Ubx, Exd, dTCF (a Wg effector) and Bin, and that minimal synthetic variants that contain only the binding motifs for Ubx, Exd, Bin, and dTCF within conserved sequence contexts (which happen to include the Org-1 motif) are active as VM enhancers (Manak et al., 1994; Yang et al., 2000; Zaffran et al., 2001; Johnson et al., 2008). Likewise, the wgXC enhancer fragment integrates Org-1 with the direct regulatory inputs of Abd-A as well as CREB and Smad (Mad/Medea) proteins mediating Dpp signaling (Grienenberger et al., 2003).
Org-1 is the first transcription factor known to be required for Ubx expression in PS7 of the visceral musculature. Extensive work on an Ubx visceral mesoderm CRM (UbxRP) indicated that dpp and wg regulate Ubx through indirect autoregulation (Thuringer and Bienz, 1993; Thuringer et al., 1993). Of note, in bin embryos, which also lack visceral mesodermal dpp and wg expression, Ubx is still expressed (Zaffran et al., 2001). Our genetic data show that the UbxRP element, while requiring org-1, is not directly regulated by Org-1 as mutation of its four predicted T-Box binding sites did not have any effects (CS and MF, unpublished data). Taking into account that we were not able to detect UbxRP reporter activity in the cFCs at pre-fusion stages, we suggest that UbxRP represents a late enhancer element and responds to dpp and wg only after they are activated by Org-1 in the founder cells. To clarify whether the regulation of Ubx by Org-1 is direct or indirect, the identification and dissection of a founder cell specific CRM will be required.
tsh and opa were described as homeotic target genes of Antp in PS4-6 (tsh) and PS4-5 (opa) as well as of abd-A in PS8 (tsh) and PS9-12 (opa) of the visceral musculature (Mathies et al., 1994; Cimbora and Sakonju, 1995). Our data show that tsh and opa expression is already activated in the respective cFCs of the visceral parasegments where it requires org-1. The later activation of tsh in PS8 during muscle fusion follows the org-1 dependent founder cell specific initiation of wg in PS8, which acts upstream of tsh (Mathies et al., 1994). Thus it was conceivable that the regulation of tsh by org-1 is indirect. However, ectopic activation of wg in an org-1 loss of function background is not able to rescue tsh expression and Antp and abd-A expression is not altered upon loss of org-1. These observations suggest that Org-1 acts directly on tsh and opa, e.g., via functional cooperation with Antp and Abd-A, respectively, during the early activation of tsh and opa in the founder cells.
Functional relationships between org-1 and of Jeb/Alk signals during visceral mesoderm patterning
It was reported that the absence of Jeb/Alk signaling causes loss of dpp expression in the founder cells in PS7 of the visceral mesoderm (Shirinian et al., 2007). In light of our current findings that org-1 loss-of-function produces a similar phenotype, and of our previous demonstration that org-1 expression is downstream of Jeb/Alk, this observation could simply be explained by the action of a linear regulatory cascade from Jeb/Alk via org-1 towards dpp. Alternatively, Jeb/Alk may provide additional inputs towards dpp (and other patterning genes) in parallel to org-1, which could explain the slightly stronger phenotype of Alk as compared to org-1 mutations with respect to dpp. A possible candidate for an additional effector of Jeb/Alk signals in this pathway is extradenticle (exd), which is known to be required for normal dpp expression in PS7 of the visceral mesoderm, presumably through direct binding of Exd in a complex with Hox proteins and Homothorax (Hth) to a PS7-specific enhancer element (a derivative of which was used herein) (Rauskolb and Wieschaus, 1994; Ryoo et al., 1999; Stultz et al., 2006). Like org-1, exd is also needed for the expression of tsh and wg in the visceral mesoderm (Additionally, it represses dpp in PS4-6 through sequences not contained in the minimal PS7 enhancer). It is thought that Exd complexed with Hox proteins and Hth increases the binding preference of these Hox complexes for specific binding sites within visceral mesodermal enhancers of their target genes (Rauskolb and Wieschaus, 1994; Mann and Chan, 1996; Grieder et al., 1997; Slattery et al., 2011).
As exd is expressed in both founder and fusion-competent cells in the visceral mesoderm, it is unlikely that it fulfils its roles in the regulation of dpp, wg, and tsh in the founder cells as a downstream gene of org-1. However, it is known that Exd requires nucleocytoplasmic translocation for it to be functional (Mann and Abu-Shaar, 1996; Aspland and White, 1997) and, interestingly, Shirinan et al. (2007) showed that Jeb/Alk signals trigger nuclear localization of Exd specifically in the cFCs of the visceral mesoderm. Because nuclear Exd appears to be hyperphosphorylated as compared to cytoplasmic Exd (Stultz et al., 2006), nuclear translocation of Exd may be triggered by Alk-mediated phosphorylation, as proposed by Shirinan et al. (Shirinian et al., 2007). Alternatively, Jeb/Alk signals may induce the expression of hth in the cFCs and Hth could then translocate Exd to the nuclei, as it has been shown in other contexts (Rieckhof et al., 1997; Pai et al., 1998; Abu-Shaar et al., 1999; Berthelsen et al., 1999). This would be compatible with our observation that Hth is upregulated in the founder cells in an org-1-independent manner (CS and MF, unpublished data).
The combined data show that Jeb/Alk signals exert at least two parallel inputs towards patterning genes in the cFCs, which are the induction of org-1 and the nuclear translocation of Exd. Taken altogether, we suggest a model in which combinatorial binding of Org-1, nuclear Exd/Hth and the homeotic selector proteins to the corresponding visceral mesoderm specific CRMs is required for the initiation of lineage specific expression of opa, tsh, dpp, Ubx and wg in the founder cells of the respective parasegments. As shown in the examples of dpp (PS7) and wg (PS8), accessory Bin is required for the activation as a general visceral mesodermal competence factor, whereas Dpp and Wg effectors mediate autoregulatory stabilization of their expression (Fig. 8B, C).
Muscle identity factors in somatic versus visceral muscle founder cells
Extensive work has shown that during somatic muscle development individual founder myoblasts acquire distinct identities, which are adopted by the newly incorporated nuclei upon myoblast fusion, thus leading to the morphological and physiological diversification of the differentiating muscles (Abmayr et al., 1995; de Joussineau et al., 2012). We propose that the same principle is active during visceral muscle development. In this view, Org-1 acts as a muscle identity factor in both the somatic and visceral mesoderm. In the visceral mesoderm, Org-1 helps diversifying founder cell identities and, after myoblast fusion, their differential identities are transmitted to the respective differentiating circular gut muscles. The activation of downstream targets of this identity factor in the developing muscles leads to the observed morphogenetic differentiation events of the midgut and the establishment of the signaling center in PS7/8 that is also required for Dpp and Wg mediated induction of labial in the endodermal germ layer (Fig. 8C). As is the case for identity factors in the somatic muscle founders, Org-1 in the visceral mesoderm acts in concert with other, spatially restricted activities such as Hox factors and signaling effectors to achieve region-specific outputs. The main difference is that, in the trunk visceral mesoderm, Org-1 is present in all founder cells whereas in the somatic mesoderm this identity factor (like others) is expressed in a particular subset of founder myoblasts. Thus, in contrast to the somatic mesoderm, the spatial expression of Org-1 does not contribute to its function in visceral muscle diversification and instead, it solely relies on spatially-restricted co-regulators during this process.
The pool of trunk visceral mesodermal fusion-competent cells contributes to the formation of both circular and longitudinal midgut muscles, depending on whether they fuse with resident founder cells of the trunk visceral mesoderm or with founders that migrated in from the caudal visceral mesoderm. The restricted expression of the identity factor Org-1 in the founder myoblasts in the trunk visceral mesoderm and its exclusion from the FCMs represents an elegant mechanism to ensure that the respective patterning events only occur in the developing circular musculature but not in the longitudinal muscle fibers, which extend as multinucleate syncytia throughout the length of the midgut.
Highlights.
Circuit of Hox, dpp, and wg regulation is established in visceral founder myoblasts
Org-1 is a tissue-specific co-factor during Drosophila visceral mesoderm patterning
Org-1 is needed for activation of majority of known patterning genes in founder cells
It is required for visceral muscle founder diversification and midgut morphogenesis
Org-1 functions via direct activation of dpp and wg enhancers in vm founder cells
Acknowledgments
We are grateful for receiving fly stocks from the Bloomington Stock Center (Indiana, USA), antibodies from Adi Salzberg, Thomas Kaufman, Renate Renkawitz-Pohl, Rob White and the Developmental Studies Hybridoma Bank (Univ. of Iowa, USA) as well as the PntP1 and PntP2 plasmids from Christian Klämbt. We gratefully acknowledge funding from the National Institutes of Health (NIDDK and NICHD) and Deutsche Forschungsgemeinschaft (DFG).
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature
- Abmayr SM, Erickson MS, Bour BA. Embryonic development of the larval body wall musculature of Drosophila melanogaster. Trends Genet. 1995;11:153–159. doi: 10.1016/s0168-9525(00)89030-7. [DOI] [PubMed] [Google Scholar]
- Abu-Shaar M, Ryoo HD, Mann RS. Control of the nuclear localization of Extradenticle by competing nuclear import and export signals. Genes Dev. 1999;13:935–945. doi: 10.1101/gad.13.8.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew DJ, Horner MA, Petitt MG, Smolik SM, Scott MP. Setting limits on homeotic gene function: restraint of Sex combs reduced activity by teashirt and other homeotic genes. EMBO J. 1994;13:1132–1144. doi: 10.1002/j.1460-2075.1994.tb06362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspland SE, White RA. Nucleocytoplasmic localisation of Extradenticle protein is spatially regulated throughout development in Drosophila. Development. 1997;124:741–747. doi: 10.1242/dev.124.3.741. [DOI] [PubMed] [Google Scholar]
- Azpiazu N, Frasch M. tinman and bagpipe: two homeo box genes that determine cell fates in the dorsal mesoderm of Drosophila. Genes Dev. 1993;7:1325–1340. doi: 10.1101/gad.7.7b.1325. [DOI] [PubMed] [Google Scholar]
- Azpiazu N, Lawrence PA, Vincent JP, Frasch M. Segmentation and specification of the Drosophila mesoderm. Genes Dev. 1996;10:3183–3194. doi: 10.1101/gad.10.24.3183. [DOI] [PubMed] [Google Scholar]
- Berthelsen J, Kilstrup-Nielsen C, Blasi F, Mavilio F, Zappavigna V. The subcellular localization of PBX1 and EXD proteins depends on nuclear import and export signals and is modulated by association with PREP1 and HTH. Genes Dev. 1999;13:946–953. doi: 10.1101/gad.13.8.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M. Homeotic genes and positional signalling in the Drosophila viscera. Trends Genet. 1994;10:22–26. doi: 10.1016/0168-9525(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Bienz M, Tremml G. Domain of Ultrabithorax expression in Drosophila visceral mesoderm from autoregulation and exclusion. Nature. 1988;333:576–578. doi: 10.1038/333576a0. [DOI] [PubMed] [Google Scholar]
- Bilder D, Graba Y, Scott MP. Wnt and TGFbeta signals subdivide the AbdA Hox domain during Drosophila mesoderm patterning. Development. 1998;125:1781–1790. doi: 10.1242/dev.125.9.1781. [DOI] [PubMed] [Google Scholar]
- Bischof J, Maeda RK, Hediger M, Karch F, Basler K. An optimized transgenesis system for Drosophila using germ-line-specific ϕC31 integrases. Proc Natl Acad Sci USA. 2007;104:3312–3317. doi: 10.1073/pnas.0611511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer R. The gene tinman is required for specification of the heart and visceral muscles in Drosophila. Development. 1993;118:719–729. doi: 10.1242/dev.118.3.719. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega J, Hartenstein V. The Embryonic Development of Drosophila melanogaster. Berlin: Springer-Verlag; 1997. [Google Scholar]
- Capovilla M, Brandt M, Botas J. Direct regulation of decapentaplegic by Ultrabithorax and its role in Drosophila midgut morphogenesis. Cell. 1994;76:461–475. doi: 10.1016/0092-8674(94)90111-2. [DOI] [PubMed] [Google Scholar]
- Cimbora DM, Sakonju S. Drosophila midgut morphogenesis requires the function of the segmentation gene odd-paired. Dev Biol. 1995;169:580–595. doi: 10.1006/dbio.1995.1171. [DOI] [PubMed] [Google Scholar]
- de Joussineau C, Bataille L, Jagla T, Jagla K. Diversification of muscle types in Drosophila: upstream and downstream of identity genes. Curr Top Dev Biol. 2012;98:277–301. doi: 10.1016/B978-0-12-386499-4.00011-2. [DOI] [PubMed] [Google Scholar]
- Englund C, Loren CE, Grabbe C, Varshney GK, Deleuil F, Hallberg B, Palmer RH. Jeb signals through the Alk receptor tyrosine kinase to drive visceral muscle fusion. Nature. 2003;425:512–516. doi: 10.1038/nature01950. [DOI] [PubMed] [Google Scholar]
- Frasch M. Induction of visceral and cardiac mesoderm by ectodermal Dpp in the early Drosophila embryo. Nature. 1995;374:464–467. doi: 10.1038/374464a0. [DOI] [PubMed] [Google Scholar]
- Gallet A, Erkner A, Charroux B, Fasano L, Kerridge S. Trunk-specific modulation of Wingless signalling in Drosophila by Teashirt binding to Armadillo. Curr Biol. 1998;8:893–902. doi: 10.1016/s0960-9822(07)00369-7. [DOI] [PubMed] [Google Scholar]
- Grieder NC, Marty T, Ryoo HD, Mann RS, Affolter M. Synergistic activation of a Drosophila enhancer by HOM/EXD and DPP signaling. EMBO J. 1997;16:7402–7410. doi: 10.1093/emboj/16.24.7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grienenberger A, Merabet S, Manak J, Iltis I, Fabre A, Berenger H, Scott MP, Pradel J, Graba Y. Tgfbeta signaling acts on a Hox response element to confer specificity and diversity to Hox protein function. Development. 2003;130:5445–5455. doi: 10.1242/dev.00760. [DOI] [PubMed] [Google Scholar]
- Groth AC, Fish M, Nusse R, Calos MP. Construction of transgenic Drosophila by using the site-specific integrase from phage ϕC31. Genetics. 2004;166:1775–1782. doi: 10.1534/genetics.166.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh DA, Padgett RW, Gelbart WM. Cross regulation of decapentaplegic and Ultrabithorax transcription in the embryonic visceral mesoderm of Drosophila. Development. 1993;117:1211–1222. doi: 10.1242/dev.117.4.1211. [DOI] [PubMed] [Google Scholar]
- Immergluck K, Lawrence PA, Bienz M. Induction across germ layers in Drosophila mediated by a genetic cascade. Cell. 1990;62:261–268. doi: 10.1016/0092-8674(90)90364-k. [DOI] [PubMed] [Google Scholar]
- Ismat A, Schaub C, Reim I, Kirchner K, Schultheis D, Frasch M. HLH54F is required for the specification and migration of longitudinal gut muscle founders from the caudal mesoderm of Drosophila. Development. 2010;137:3107–3117. doi: 10.1242/dev.046573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Zhao Y, Golden K, Barolo S. Reverse-engineering a transcriptional enhancer: a case study in Drosophila. Tissue Engin Part A. 2008;14:1549–1559. doi: 10.1089/ten.tea.2008.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klambt C. The Drosophila gene pointed encodes two ETS-like proteins which are involved in the development of the midline glial cells. Development. 1993;117:163–176. doi: 10.1242/dev.117.1.163. [DOI] [PubMed] [Google Scholar]
- Klapper R, Stute C, Schomaker O, Strasser T, Janning W, Renkawitz-Pohl R, Holz A. The formation of syncytia within the visceral musculature of the Drosophila midgut is dependent on duf, sns and mbc. Mech Dev. 2002;110:85–96. doi: 10.1016/s0925-4773(01)00567-6. [DOI] [PubMed] [Google Scholar]
- Knirr S, Azpiazu N, Frasch M. The role of the NK-homeobox gene slouch (S59) in somatic muscle patterning. Development. 1999;126:4525–4535. doi: 10.1242/dev.126.20.4525. [DOI] [PubMed] [Google Scholar]
- Kurant E, Pai CY, Sharf R, Halachmi N, Sun YH, Salzberg A. Dorsotonals/Homothorax, the Drosophila homologue of meis1, interacts with Extradenticle in patterning of the embryonic PNS. Development. 1998;125:1037–1048. doi: 10.1242/dev.125.6.1037. [DOI] [PubMed] [Google Scholar]
- Kusch T, Reuter R. Functions for Drosophila brachyenteron and forkhead in mesoderm specification and cell signalling. Development. 1999;126:3991–4003. doi: 10.1242/dev.126.18.3991. [DOI] [PubMed] [Google Scholar]
- Lee HH, Frasch M. Wingless effects mesoderm patterning and ectoderm segmentation events via induction of its downstream target sloppy paired. Development. 2000;127:5497–5508. doi: 10.1242/dev.127.24.5497. [DOI] [PubMed] [Google Scholar]
- Lee HH, Frasch M. Nuclear integration of positive Dpp signals, antagonistic Wg inputs and mesodermal competence factors during Drosophila visceral mesoderm induction. Development. 2005;132:1429–1442. doi: 10.1242/dev.01687. [DOI] [PubMed] [Google Scholar]
- Lee HH, Norris A, Weiss JB, Frasch M. Jelly belly protein activates the receptor tyrosine kinase Alk to specify visceral muscle pioneers. Nature. 2003;425:507–512. doi: 10.1038/nature01916. [DOI] [PubMed] [Google Scholar]
- Loren CE, Englund C, Grabbe C, Hallberg B, Hunter T, Palmer RH. A crucial role for the Anaplastic lymphoma kinase receptor tyrosine kinase in gut development in Drosophila melanogaster. EMBO Rep. 2003;4:781–786. doi: 10.1038/sj.embor.embor897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias A, Casanova J, Morata G. Expression and regulation of the abd-A gene of Drosophila. Development. 1990;110:1197–1207. doi: 10.1242/dev.110.4.1197. [DOI] [PubMed] [Google Scholar]
- Manak JR, Mathies LD, Scott MP. Regulation of a decapentaplegic midgut enhancer by homeotic proteins. Development. 1994;120:3605–3619. doi: 10.1242/dev.120.12.3605. [DOI] [PubMed] [Google Scholar]
- Mann RS, Abu-Shaar M. Nuclear import of the homeodomain protein Extradenticle in response to Wg and Dpp signalling. Nature. 1996;383:630–633. doi: 10.1038/383630a0. [DOI] [PubMed] [Google Scholar]
- Mann RS, Chan SK. Extra specificity from Extradenticle: the partnership between HOX and PBX/EXD homeodomain proteins. Trends Genet. 1996;12:258–262. doi: 10.1016/0168-9525(96)10026-3. [DOI] [PubMed] [Google Scholar]
- Martin BS, Ruiz-Gomez M, Landgraf M, Bate M. A distinct set of founders and fusion-competent myoblasts make visceral muscles in the Drosophila embryo. Development. 2001;128:3331–3338. doi: 10.1242/dev.128.17.3331. [DOI] [PubMed] [Google Scholar]
- Masucci JD, Hoffmann FM. Identification of two regions from the Drosophila decapentaplegic gene required for embryonic midgut development and larval viability. Dev Biol. 1993;159:276–287. doi: 10.1006/dbio.1993.1240. [DOI] [PubMed] [Google Scholar]
- Mathies LD, Kerridge S, Scott MP. Role of the teashirt gene in Drosophila midgut morphogenesis: secreted proteins mediate the action of homeotic genes. Development. 1994;120:2799–2809. doi: 10.1242/dev.120.10.2799. [DOI] [PubMed] [Google Scholar]
- Muller CW, Herrmann BG. Crystallographic structure of the T domain-DNA complex of the Brachyury transcription factor. Nature. 1997;389:884–888. doi: 10.1038/39929. [DOI] [PubMed] [Google Scholar]
- Muller J, Thuringer F, Biggin M, Zust B, Bienz M. Coordinate action of a proximal homeoprotein binding site and a distal sequence confers the Ultrabithorax expression pattern in the visceral mesoderm. EMBO J. 1989;8:4143–4151. doi: 10.1002/j.1460-2075.1989.tb08599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nellen D, Burke R, Struhl G, Basler K. Direct and long-range action of a Dpp morphogen gradient. Cell. 1996;85:357–368. doi: 10.1016/s0092-8674(00)81114-9. [DOI] [PubMed] [Google Scholar]
- Nguyen HT, Xu X. Drosophila mef2 expression during mesoderm development is controlled by a complex array of cis-acting regulatory modules. Dev Biol. 1998;204:550–566. doi: 10.1006/dbio.1998.9081. [DOI] [PubMed] [Google Scholar]
- Pai CY, Kuo TS, Jaw TJ, Kurant E, Chen CT, Bessarab DA, Salzberg A, Sun YH. The Homothorax homeoprotein activates the nuclear localization of another homeoprotein, Extradenticle, and suppresses eye development in Drosophila. Genes Dev. 1998;12:435–446. doi: 10.1101/gad.12.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panganiban GE, Reuter R, Scott MP, Hoffmann FM. A Drosophila growth factor homolog, Decapentaplegic, regulates homeotic gene expression within and across germ layers during midgut morphogenesis. Development. 1990;110:1041–1050. doi: 10.1242/dev.110.4.1041. [DOI] [PubMed] [Google Scholar]
- Patel NH, Snow PM, Goodman CS. Characterization and cloning of fasciclin III: a glycoprotein expressed on a subset of neurons and axon pathways in Drosophila. Cell. 1987;48:975–988. doi: 10.1016/0092-8674(87)90706-9. [DOI] [PubMed] [Google Scholar]
- Rauskolb C, Wieschaus E. Coordinate regulation of downstream genes by extradenticle and the homeotic selector proteins. EMBO J. 1994;13:3561–3569. doi: 10.1002/j.1460-2075.1994.tb06663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter R, Grunewald B, Leptin M. A role for the mesoderm in endodermal migration and morphogenesis in Drosophila. Development. 1993;119:1135–1145. doi: 10.1242/dev.119.4.1135. [DOI] [PubMed] [Google Scholar]
- Reuter R, Panganiban GE, Hoffmann FM, Scott MP. Homeotic genes regulate the spatial expression of putative growth factors in the visceral mesoderm of Drosophila embryos. Development. 1990;110:1031–1040. doi: 10.1242/dev.110.4.1031. [DOI] [PubMed] [Google Scholar]
- Reuter R, Scott MP. Expression and function of the homoeotic genes Antennapedia and Sex combs reduced in the embryonic midgut of Drosophila. Development. 1990;109:289–303. doi: 10.1242/dev.109.2.289. [DOI] [PubMed] [Google Scholar]
- Riechmann V, Irion U, Wilson R, Grosskortenhaus R, Leptin M. Control of cell fates and segmentation in the Drosophila mesoderm. Development. 1997;124:2915–2922. doi: 10.1242/dev.124.15.2915. [DOI] [PubMed] [Google Scholar]
- Rieckhof GE, Casares F, Ryoo HD, Abu-Shaar M, Mann RS. Nuclear translocation of Extradenticle requires Homothorax, which encodes an Extradenticle-related homeodomain protein. Cell. 1997;91:171–183. doi: 10.1016/s0092-8674(00)80400-6. [DOI] [PubMed] [Google Scholar]
- Ryoo HD, Marty T, Casares F, Affolter M, Mann RS. Regulation of Hox target genes by a DNA bound Homothorax/Hox/Extradenticle complex. Development. 1999;126:5137–5148. doi: 10.1242/dev.126.22.5137. [DOI] [PubMed] [Google Scholar]
- Schaub C, Nagaso H, Jin H, Frasch M. Org-1, the Drosophila ortholog of Tbx1, is a direct activator of known identity genes during muscle specification. Development. 2012;139:1001–1012. doi: 10.1242/dev.073890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirinian M, Varshney G, Loren CE, Grabbe C, Palmer RH. Drosophila Anaplastic Lymphoma Kinase regulates Dpp signalling in the developing embryonic gut. Differentiation. 2007;75:418–426. doi: 10.1111/j.1432-0436.2006.00148.x. [DOI] [PubMed] [Google Scholar]
- Slattery M, Riley T, Liu P, Abe N, Gomez-Alcala P, Dror I, Zhou T, Rohs R, Honig B, Bussemaker HJ, Mann RS. Cofactor binding evokes latent differences in DNA binding specificity between Hox proteins. Cell. 2011;147:1270–1282. doi: 10.1016/j.cell.2011.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosinsky A, Bonin CP, Mann RS, Honig B. Target Explorer: An automated tool for the identification of new target genes for a specified set of transcription factors. Nucleic Acids Res. 2003;31:3589–3592. doi: 10.1093/nar/gkg544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Johnston RD, Gelbart WM. Decapentaplegic transcripts are localized along the dorsal-ventral axis of the Drosophila embryo. EMBO J. 1987;6:2785–2791. doi: 10.1002/j.1460-2075.1987.tb02574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehling-Hampton K, Hoffmann FM. Ectopic decapentaplegic in the Drosophila midgut alters the expression of five homeotic genes, dpp, and wingless, causing specific morphological defects. Dev Biol. 1994;164:502–512. doi: 10.1006/dbio.1994.1219. [DOI] [PubMed] [Google Scholar]
- Staehling-Hampton K, Hoffmann FM, Baylies MK, Rushton E, Bate M. Dpp induces mesodermal gene expression in Drosophila. Nature. 1994;372:783–786. doi: 10.1038/372783a0. [DOI] [PubMed] [Google Scholar]
- Stultz BG, Jackson DG, Mortin MA, Yang X, Beachy PA, Hursh DA. Transcriptional activation by extradenticle in the Drosophila visceral mesoderm. Dev Biol. 2006;290:482–494. doi: 10.1016/j.ydbio.2005.11.041. [DOI] [PubMed] [Google Scholar]
- Sun B, Hursh DA, Jackson D, Beachy PA. Ultrabithorax protein is necessary but not sufficient for full activation of decapentaplegic expression in the visceral mesoderm. EMBO J. 1995;14:520–535. doi: 10.1002/j.1460-2075.1995.tb07028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass U, Hartenstein V. Epithelium formation in the Drosophila midgut depends on the interaction of endoderm and mesoderm. Development. 1994;120:579–590. doi: 10.1242/dev.120.3.579. [DOI] [PubMed] [Google Scholar]
- Thuringer F, Bienz M. Indirect autoregulation of a homeotic Drosophila gene mediated by extracellular signaling. Proc Natl Acad Sci USA. 1993;90:3899–3903. doi: 10.1073/pnas.90.9.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuringer F, Cohen SM, Bienz M. Dissection of an indirect autoregulatory response of a homeotic Drosophila gene. EMBO J. 1993;12:2419–2430. doi: 10.1002/j.1460-2075.1993.tb05896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremml G, Bienz M. Homeotic gene expression in the visceral mesoderm of Drosophila embryos. EMBO J. 1989;8:2677–2685. doi: 10.1002/j.1460-2075.1989.tb08408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M, Nusse R, Johnston P, Lawrence PA. Distribution of the wingless gene product in Drosophila embryos: a protein involved in cell-cell communication. Cell. 1989;59:739–749. doi: 10.1016/0092-8674(89)90020-2. [DOI] [PubMed] [Google Scholar]
- Wang J, Tao Y, Reim I, Gajewski K, Frasch M, Schulz RA. Expression, Regulation, and Requirement of the Toll Transmembrane Protein during Dorsal Vessel Formation in Drosophila melanogaster. Mol Cell Biol. 2005;25:4200–4210. doi: 10.1128/MCB.25.10.4200-4210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RA, Wilcox M. Distribution of Ultrabithorax proteins in Drosophila. EMBO J. 1985;4:2035–2043. doi: 10.1002/j.1460-2075.1985.tb03889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfstetter G, Shirinian M, Stute C, Grabbe C, Hummel T, Baumgartner S, Palmer RH, Holz A. Fusion of circular and longitudinal muscles in Drosophila is independent of the endoderm but further visceral muscle differentiation requires a close contact between mesoderm and endoderm. Mech Dev. 2009;126:721–736. doi: 10.1016/j.mod.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Xu X, Yin Z, Hudson JB, Ferguson EL, Frasch M. Smad proteins act in combination with synergistic and antagonistic regulators to target Dpp responses to the Drosophila mesoderm. Genes Dev. 1998;12:2354–2370. doi: 10.1101/gad.12.15.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, van Beest M, Clevers H, Jones T, Hursh DA, Mortin MA. decapentaplegic is a direct target of dTcf repression in the Drosophila visceral mesoderm. Development. 2000;127:3695–3702. doi: 10.1242/dev.127.17.3695. [DOI] [PubMed] [Google Scholar]
- Yu X, Hoppler S, Eresh S, Bienz M. decapentaplegic, a target gene of the Wingless signalling pathway in the Drosophila midgut. Development. 1996;122:849–858. doi: 10.1242/dev.122.3.849. [DOI] [PubMed] [Google Scholar]
- Zaffran S, Kuchler A, Lee HH, Frasch M. biniou (FoxF), a central component in a regulatory network controlling visceral mesoderm development and midgut morphogenesis in Drosophila. Genes Dev. 2001;15:2900–2915. doi: 10.1101/gad.917101. [DOI] [PMC free article] [PubMed] [Google Scholar]