Abstract
Autocrine stimulation of S1PR2, a receptor for the lipid mediator sphingosine-1-phosphate (S1P), has been implicated in mast cell degranulation to IgE/antigen (Ag) although, paradoxically, its ligand cannot trigger substantial degranulation. Additionally, the in vivo role of S1PR2 in the overall allergic responses is unclear since S1PR2 was reported to be required for the onset of systemic anaphylaxis by IgE/Ag but, in apparent contradiction, also for the recovery from histamine-induced anaphylaxis in a mast cell independent manner. Here, we sought to clarify the role of S1PR2 in mast cell degranulation and in IgE and IgG-mediated anaphylaxis. Lack of S1PR2 reduced IgE/Ag-induced degranulation in in vitro experiments with mucosal mast cells, but had no effect on connective tissue type mast cells. This latter response correlated with a lack of involvement of S1PR2 in the onset of non-lethal IgE/Ag-mediated systemic and cutaneous anaphylaxis. However, S1pr2−/− mice were slow to recover (or did not recover) from FcεRI-mediated anaphylaxis, an outcome that mirrored their known impairment in histamine clearance due to defective vascular tone. A minor role for S1PR2 in mast cell degranulation was uncovered upon engagement of low affinity receptors for IgG and in the onset of IgG-mediated anaphylaxis. Our findings show that S1PR2 is dispensable for initiating IgE/Ag-mediated connective tissue mast cell degranulation and anaphylaxis, but it is required for normal recovery from anaphylaxis.
Keywords: Sphingosine-1-phosphate, S1PR2, mast cell degranulation, anaphylaxis, IgE, IgG
1. INTRODUCTION
Mast cells participate in innate and adaptive immune responses. These cells reside in tissues and have the potential to either amplify or suppress immune responses [1,2]. The versatility of mast cells and their phenotypic plasticity in response to the microenvironmental milieu in which they reside is largely endowed by the presence of multiple types of receptors, including receptors for innate, growth factor, cytokine, and adaptive immune stimuli; the latter primarily characterized by the presence of the high affinity receptor for IgE (FcεRI) and low affinity activating and inhibitory receptors for IgG (in mouse, FcγRIII and FcγRII respectively) [2,3].
These receptors provide the means by which mast cells can mediate specific effector functions, enhance or dampen their own as well as other immune responses or even modify the threshold of such responses [3]. G-protein coupled receptors (GPCRs) are known to modulate FcεRI-driven mast cell responses [4]. Sphingosine-1-phosphate (S1P) is a pleiotropic lipid that affects multiple cellular processes by binding five known GPCRs (S1PR1–5) on the cell surface or by acting on its intracellular targets [5]. In this family, S1PR1 and S1PR2, which are expressed on mast cells, were shown to modulate antigen-driven mast cell responses [3,6–8]. In immunity, S1P receptors have been implicated in lymphocyte trafficking in vivo [9], in allergic responses [6] [8], and in the modulation of other immune responses [10].
Evidence for the distinct roles of S1PRs in mast cell function was provided by the demonstration that silencing of S1PR1 in RBL-2H3 cells caused inhibition of chemotaxis towards antigen, whereas silencing of S1PR2 in these cells reduced FcεRI-mediated degranulation [6]. However, various reports have demonstrated that direct ligation of S1PRs by S1P, at concentrations sufficient for receptor engagement, does not induce considerable degranulation or calcium mobilization, and only at high concentrations (20–100 µM) mild effects were observed on these responses [6,8,11–13]. In contrast, optimal degranulation of skin-type human mast cells to S1P has been reported by one group [14,15] using concentrations as low as 1 nM, albeit without an apparent concentration-dependent response [15]. Yet, conflicting with the concept of a dependence on the autocrine engagement of S1PR2 for degranulation, inhibition of ABCC1-mediated S1P export to the extracellular medium did not affect FcεRI-induced degranulation while inhibiting chemotaxis to antigen (S1PR1-mediated) [16].
The role of S1PR2 in the allergic response in vivo is also incompletely understood. Consistent with a role for S1PR2 in mast cell degranulation Oskeritzian et al reported that S1pr2−/− mice had reduced anaphylactic reactions to an IgE/Ag challenge [15]. However, our previous work using a histamine-induced systemic anaphylaxis model revealed a strong requirement for S1PR2 in the recovery from anaphylaxis that was independent of the response of mast cells to antigen [17]. Because histamine is a major mediator driving IgE-induced anaphylaxis, this raises the conundrum of what conditions would require S1PR2 in the initiation of shock [15] versus a role for this receptor in the recovery of anaphylaxis. From a pharmacological perspective, addressing this question would be of importance for determining if S1PR2 antagonism or S1PR2 agonism is of potential therapeutic value in ameliorating anaphylaxis.
Herein we sought to clarify the in vivo role of S1PR2 in IgE/Ag-dependent mast cell degranulation and anaphylaxis. We find that S1PR2 is dispensable for the degranulation of mouse connective tissue type mast cells and it is not, in our experimental setting, involved in the onset of IgE/Ag-mediated anaphylaxis, local or systemic. We observed a minor delay in the onset of anaphylaxis in the S1pr2−/− mice when low affinity receptors for IgG were engaged alone at low occupancy or in conjunction with FcεRI. This may partly explain the differences with the previous report using high doses of IgE for induction of anaphylaxis, which may result from the combined engagement of IgE- and IgG-receptors. Nonetheless, a requirement for S1PR2 in recovery from IgE- or IgG-mediated anaphylaxis was prominent. Thus, our findings support the notion that specific agonism of S1PR2 after initiation of anaphylactic shock could be a potential alternative to epinephrine when considering patients who are at risk for this treatment.
2. METHODS
2.1. Mice and mast cell cultures
Mice were maintained and used in accordance with NIH guidelines and animal study proposal (A010-04-03) approved by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. S1pr2−/− and corresponding WT littermates were bred at Taconic Farms generated from heterozygous mating pairs and genotyped as previously described [18]. WT or S1pr2−/− bone marrow-derived mast cells (BMMC) and peritoneal-derived mast cells (PDMC) were obtained, respectively, from the tibia bone marrow and the peritoneal lavage of 6–8 week old mice and cultured at least for 6–8 weeks (BMMC) or 15–20 days (PDMC) as described previously [8,19]. BMMC and PDMC were cultured in RPMI media (Invitrogen) supplemented with 10% (BMMC) or 20% (PDMC) fetal calf serum (Invitrogen) and 20 ng/ml each of recombinant mouse IL-3 and stem cell factor (SCF) or IL-3 alone as indicated (Peprotech, Rocky Hill, NJ). Cells were used for studies when greater than 95% of the population expressed both FcεRI and Kit as determined by flow cytometry [20]. LAD2 cells were generously provided by Dr. A. Gilfillan (NIAID, NIH) and cultured as previously described [21].
2.2. Mast cell degranulation assays
Mast cells (106 cells) were sensitized with 1 µg/ml anti-DNP IgE (H1-DNP-ε-26.82) [22] in Tyrodes-BSA buffer (20 mM HEPES buffer (pH 7.4), 135 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, 1 mM MgCl, 5.6 mM glucose, and 0.05% bovine serum albumin (Sigma-Aldrich)) for 3 h at room temperature. After sensitization, cells were washed and distributed in the wells of a V-bottom 96-well plate (60,000 mast cells/well in 80 µl). MCs were stimulated for 30 min with the indicated concentrations of DNP-HSA. For human LAD2 degranulation, cells were sensitized with 100 ng/ml biotinylated IgE overnight and stimulated for 30 min with 100 ng/ml streptavidin (Ag) or the indicated concentrations of S1P. The release of β-hexosaminidase was determined using a colorimetric assay as previously described [8]. The extent of degranulation was calculated as a percent of the total content found in the supernatants following challenge. To measure IgG-mediated degranulation by activating Fcγ receptors, mast cells (106 cells) were incubated with 10 µg/ml rat anti-mouse anti-FcγRII/FcγRIII (anti-CD16/CD32; clone 2.4G2, BioXcell) in Tyrodes-BSA buffer for 1 h at 37°C. Cells were washed and stimulated with 4–10 µg/ml of goat anti-rat IgG (Thermo Scientific) for 30 min [23]. β-hexosaminidase released into the media was determined as described [8].
2.3. Passive cutaneous-, passive systemic-anaphylaxis and vascular permeability measurements
Passive cutaneous anaphylaxis (PCA) was preformed as previously described [24]. Mice were passively sensitized with an intradermal injection of 75 ng of DNP-specific mouse IgE (20 µl) into the right ear, while the contralateral ear was injected with 20 µl of PBS as a negative control. After 24 h, mice were challenged intravenously with 250 µg of antigen (Ag, DNP-HSA) in PBS containing 1% Evans Blue (200 µl). Mice were euthanized with CO2, 30 minutes after the Ag injection. The right and left ears were cut and minced. Evans Blue dye from the ears was extracted using 700 µl of formamide at 55°C for 2 h and quantified by absorbance at 620 nm after clearing the extracts by centrifugation or filtration.
For IgE-induced passive systemic anaphylaxis (PSA), mice were sensitized (i.v.) with 3 µg of DNP-specific IgE (H1-DNP-ε-26.82) in 200 µl of phosphate buffered saline (PBS) and challenged after 24 h with 250 µg of Ag (DNP36-HSA) in 200 µl of PBS (Sigma-Aldrich) [17]. Alternatively, mice were sensitized with 35 to 75 µg of DNP-specific IgE (SPE-7 clone; Sigma-Aldrich) (i.p.) in 100 µl of PBS, and after 16 h challenged with 100 µg of Ag in 100 µl; conditions previously described [15] with the exception of the IgE clone used. The SPE-7 clone IgE was dialyzed against PBS (3 changes of buffer, at least 500X dilution each time) to remove sodium azide from the preparation and all types of IgE were centrifuged for 1 h at 100,000×g to remove large immune complexes and aggregates before injection into the mice. Protein concentration was determined after dialysis and centrifugation. To block binding to Fcγ receptors, 50–75 µg of clone 2.4G2 (anti-mouse CD16/CD32) was injected (i.p.) 90 min before IgE sensitization. The PAF antagonist CV-6209 (Santa Cruz Biotechnology) (70 µg) was injected (i.v.) in 100 µl of PBS just prior to Ag challenge. For IgG-induced PSA, mice were injected with 200 µg of 2.4G2 rat-anti mouse antibody (i.p.) (100 µl). Body temperature changes were monitored using implantable electronic transponders as described [17].
To measure histamine released into the circulation during PSA, mice were euthanized in a CO2 chamber 90 s after challenge with Ag (i.v.). Blood was immediately withdrawn by cardiac puncture in heparinized syringes and plasma histamine was measured with a competitive histamine immunoassay kit (Beckman Coulter). To measure changes in vascular permeability during PCA or PSA, mice were injected (i.v.) with Ag in a 1% Evans Blue dye solution (100 µl) and euthanized after 30–35 min. Ears were harvested (PCA) and Evans Blue was extracted with formamide as previously described [17] or the peritoneal lavage (PSA) was obtained after injecting 2 ml PBS in the peritoneal cavity (centrifuged for 10 min at 3000×g). The amount of Evans blue in the ear or lavage supernatants was quantified by absorbance at 620 nm.
2.4. Statistical Analysis
Statistical significance was determined using a two-tailed Student’s t test, and statistical differences between genotypes or treatments during the course of anaphylactic responses (measured as body temperature changes) were determined by two-way ANOVA, with the exception of Figure 5B, which showed significant differences (t test) only at indicated points. A p value of less than 0.05 was considered significant. Data are shown as mean ± SEM.
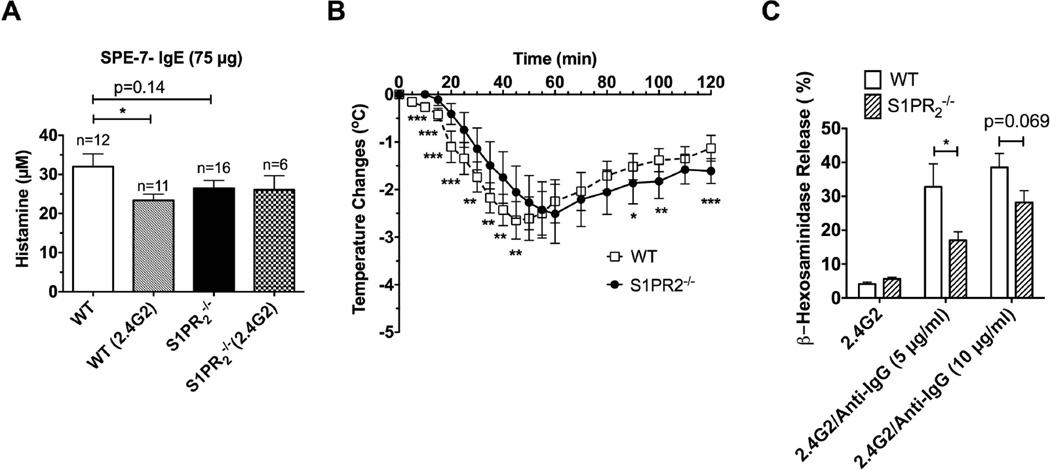
Fig. 5. Blocking of IgG receptors in S1pr2−/− mice reduces histamine release and delays onset of SPE-7 IgE/Ag-induced anaphylaxis and mast cells from S1pr2−/− mice have reduced degranulation.
(A) Mice (WT and S1pr2−/−) were injected (i.p.) with SPE-7 IgE (SPE-7) (75 µg) and subsequently challenged as in Fig. 3. FcγR was blocked by 50–75 µg anti CD16/32 antibody (2.4G2 clone) where indicated. Mice were euthanized 90 s after Ag challenge and plasma histamine was measured. (B) Anaphylaxis was induced by injection (i.p.) of 200 µg anti CD16/32 antibody (2.4G2 clone). Significant differences between WT (n=11) and S1pr2+/− (n=11) were observed at the indicated time points using a two-tailed Student’s t test. * P<0.05** P<0.01, *** P<0.001. (C) Degranulation of peritoneal mast cells by engagement of IgG receptors. Peritoneal mast cells from WT or S1PR2-deficient mice were incubated for 1 h with 10 µg/ml of rat 2.4G2 anti-mouse CD16/CD32 antibody, washed and crosslinked with the indicated concentrations of goat anti rat IgG antibody for 30 min. β-Hexosaminidase released to the media was measured as described in methods.
3. RESULTS
3.1. The involvement of S1PR2 in FcεRI-induced mast cell degranulation is dependent on the tissue origin of the cell
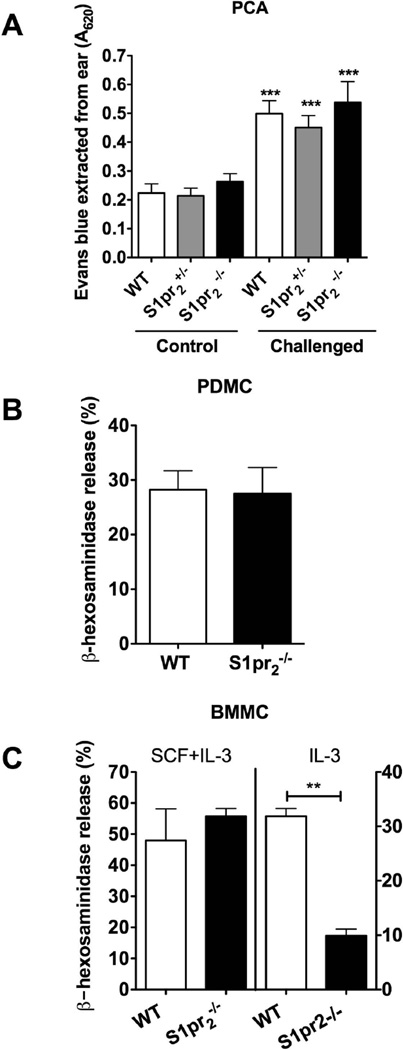
We first explored the in vivo role of S1PR2 in mast cell degranulation by assessing changes in vascular permeability during PCA, a model of a type I allergic reaction that is dependent on the local response of mast cells. As shown in Fig. 1A, vascular permeability was considerably increased upon an IgE/Ag challenge of the ears in all three genotypes tested. However, no significant difference in vascular permeability was found between S1pr2−/−, S1pr2+/− and WT mice. We then asked if other connective type tissue mast cells were also independent of S1PR2 for FcεRI-mediated degranulation. Consistent with the skin mast cell responses in vivo, PDMC, mature peritoneal connective tissue type mast cells from S1pr2−/− mice showed no differences in the degranulation responses to IgE/Ag when compared to WT cells (Fig. 1B). Similarly, as shown in Supplemental Fig. 1, the skin-like human mast cell line, LAD2, had no degranulation response to S1P and siRNA silencing of S1PR2 showed minimal, statistically not significant inhibition of IgE/Ag-induced degranulation (Supplemental Fig. 1B). MCs derived from the bone marrow (BMMC) are considered immature intermediates, mostly of a mucosal type, with the ability to further differentiate into more mature MC [25]. When cultured in the presence of IL-3 and SCF, BMMC are still immature MC with some traits of a connective tissue mast cell type [25,26,27], including the pattern of expression of mMCP-1, -2 and -4 (Supplemental Fig. 1C). Degranulation responses of BMMC grown in the presence of IL3 and SCF, as those of PDMC, were also unaffected by the absence of S1PR2 (Fig. 1C). In contrast, BMMCs cultured in the presence of IL-3 alone, which have a more mucosal-like phenotype [25,26,27] (Supplemental Fig. 1C), were dependent on S1PR2 for FcεRI-induced degranulation (Fig. 1C). These results indicate that S1PR2 is dispensable for degranulation responses in connective tissue type mast cells in vivo and in vitro, while it may play a role in mucosal-like mast cells.
Fig. 1. Connective tissue type mast cells, but not mucosal-like mast cells, are independent of S1PR2 in IgE/Ag-induced degranulation.
(A) Passive cutaneous anaphylaxis in WT, S1pr2+/− and S1pr2−/− mice. Anti-DNP IgE (H1-DNP-ε-26.82) (75 ng) was injected intradermally into one ear of the mouse (challenged) while the contralateral ear was injected with PBS (control). After 24 h, Evans blue dye (200 µl of 0.1%) and DNP-BSA (250 µg, Ag) were injected (i.v.). Extravasation of plasma fluids and Evans Blue into tissues as a consequence of IgE-mediated degranulation was measured by absorbance at 620 nm. ***p<0.001 compared to the respective control ears. B, C) Degranulation to H1-DNP-ε-26.82 IgE and Ag (25 ng/ml) in peritoneal derived mast cells (PDMC) (B) or bone marrow derived mast cells (BMMC) (C) grown in the presence of SCF and IL3 (connective tissue type mast cells; white bars) or only IL3 (mucosal-type mast cells; grey bars) from WT and S1pr2−/− mice. β-hexosaminidase release was measured from supernatants of cells challenged with 25 ng/ml of antigen as explained in the methods section. ** P<0.01 compared to WT mice, n=4 independent cultures.
3.2. Passive systemic anaphylaxis induced by IgE/Ag is more severe in S1pr2−/− mice
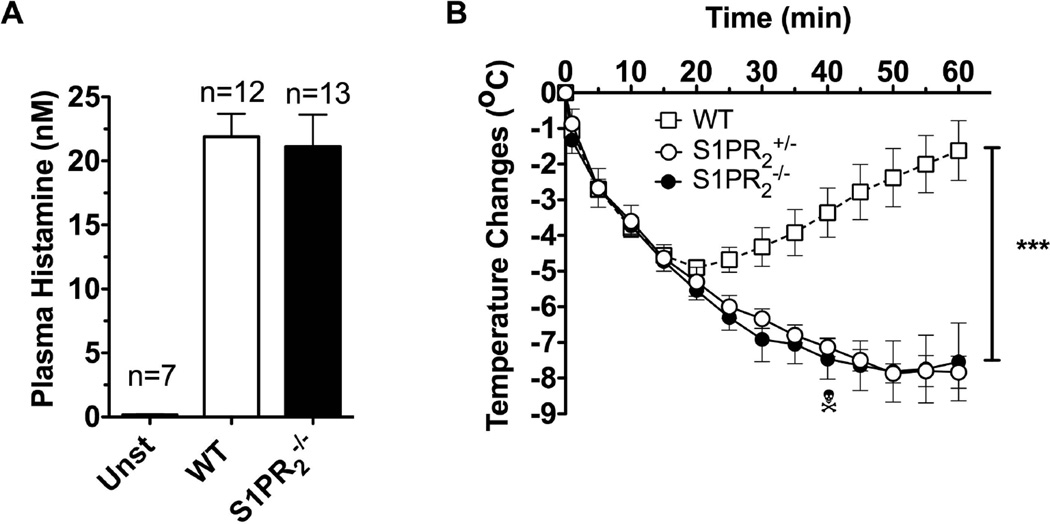
To determine if S1PR2 was required for the onset (initiated by mast cell degranulation) of PSA we measured the amount of histamine released to the circulation shortly after challenge (90 sec) in WT and S1pr2−/− mice. No differences were observed (Fig 2A), consistent with the similar extent of vascular leakage observed for these genotypes during cutaneous anaphylaxis (Fig 1A). However, body temperature measurements revealed a marked drop associated with anaphylaxis in S1pr2−/− mice and in S1pr2+/− mice relative to the rapid recovery seen in WT mice, suggesting a dominant effect of this receptor in controlling the recovery from anaphylaxis instead of its onset (Fig 2B). Consistent with our previous work on histamine-induced shock [17], the presence of S1PR2 in peripheral tissues seems to be critical for the recovery from IgE-induced anaphylaxis.
Fig. 2. S1PR2 is dispensable for mast cell degranulation but indispensable for recovery from anaphylactic shock induced by IgE/Ag challenge.
Mice (WT, S1pr2+/− and S1pr2−/−) were injected (i.v.) with DNP-specific IgE (H1-DNP-ε-26.82) (3 µg) and subsequently challenged 24 h later with Ag (250 µg) to induce systemic anaphylaxis. (A) Mice were euthanized 90 s following challenge and histamine in the plasma was measured. (B) Body temperature changes during the anaphylactic shock induced by IgE/Ag. *** P<0.001 between WT (n=5) and S1pr2+/− (n=3) or S1pr2−/− (n=8).
3.3. The anaphylactic response of S1pr2−/− mice differs depending on the IgE clone used for passive sensitization
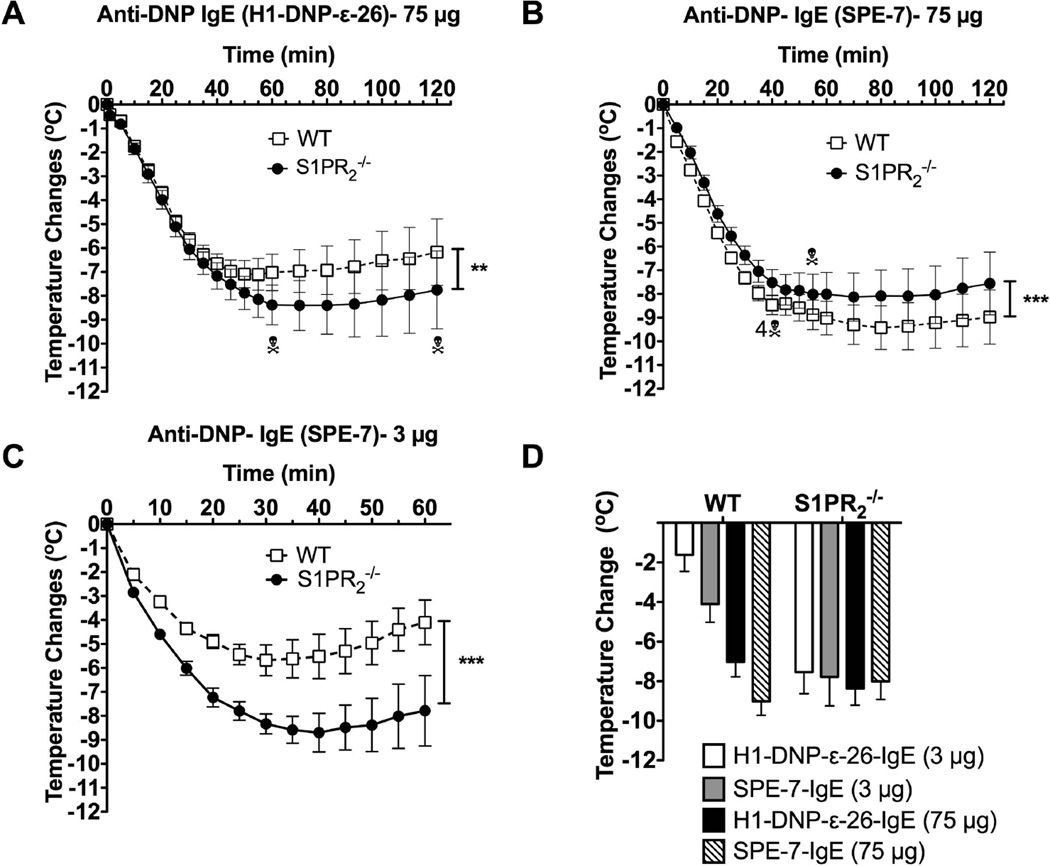
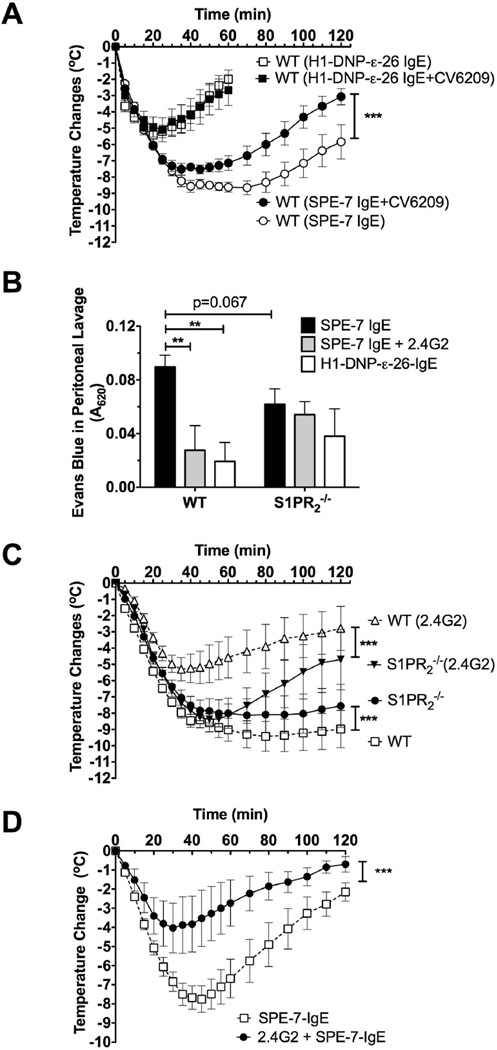
In a previous study [15], the authors found that the anaphylactic response of S1pr2−/− mice was reduced apparently due to defective degranulation of S1pr2−/− mast cells in response to IgE/Ag stimulation. Since we only found reduced responses to Ag in a particular MC population derived from these mice, a possible explanation for the apparent discrepancy with our study is the high concentration of IgE (75 µg, instead of 3 µg in our study) used in this prior study [15]. We postulated that this might result in the activation of a broader spectrum of mast cell populations (including mucosal-type mast cells), thus, potentially affecting the onset of anaphylaxis. To test this possibility, we conducted experiments at the higher concentration of IgE (75 µg) using the reported experimental conditions [15]. In our hands, the deficiency of S1PR2 was still detrimental for the recovery of shock, with a 20% lethality rate, and did not affect its onset (Fig 3A). Nonetheless, anaphylaxis induced by high concentration of IgE caused a more severe drop in body temperature (compare WT responses Fig 2B, 3C versus 3A). This temperature drop was longer lasting (recovery was minimal by 2 h) in both WT and S1pr2−/− mice, and the difference in the rate of recovery between the two genotypes was not as pronounced as with the lower dose of IgE (Fig. 2B versus 3A). This is probably due to an increased amount of mediators released by the higher levels of IgE-crosslinking and the possible overload in the clearance systems in both types of mice. Another potential variable was the type of IgE used for passive sensitization. To test whether IgE’s derived from different hybridoma clones could elicit different responses we tested DNP-specific IgE’s from the H1-DNPε-26.82 [22] and the SPE-7 hybridomas [28]. These IgE’s were shown to differ in their ability to elicit mast cell signaling and effector responses when bound to FcεRI (termed cytokinergic ability). SPE-7 –derived IgE was shown to be a highly cytokinergic whereas H1-DNPε-26.82 had a moderate cytokinergic ability [29]. As shown in Fig 3B, and in contrast to Fig. 3A, 75 µg SPE-7 induced a more acute response and increased lethality in WT mice compared to S1pr2−/− mice. Interestingly, this modest protective effect of S1PR2 deficiency was evident only at high concentrations of the SPE-7 IgE since at 3 µg S1pr2−/− mice demonstrated a more severe shock as compared to WT mice (Fig. 3C). Importantly, while the maximal temperature drop and the duration of hypothermia in WT mice showed a dose- and IgE type- dependence (Fig. 3D), the maximal temperature drop and duration in S1pr2−/− mice appeared to reach a plateau regardless of the dose or the type of IgE (Fig 3D); a result consistent with the defective handling of histamine [17]. Thus, we find that regardless of the dose of IgE (although less evident at high concentrations) a dominant consequence for the lack of S1PR2 is the poor recovery from anaphylaxis. Only when a high concentration of a very cytokinergic IgE (SPE-7) was used was a mild protective effect observed in the outcome of anaphylaxis. Furthermore, the findings argue that this apparent improvement in S1pr2−/− mice may be attributed to differences in the onset of anaphylaxis as both WT and S1pr2−/− mice show a poor temperature recovery under these conditions (Fig 3D).
Fig. 3. Differences in recovery of WT and S1pr2−/− mice from IgE-induced anaphylactic shock is dependent on dose and type of IgE.
Two different models [15,17] and two types of IgE were used for IgE/Ag-mediated anaphylaxis in WT or S1pr2−/−. Mice were injected (i.p.) with 5 µg of H1-DNP-ε-26.82 (A,D) or SPE-7 clone anti-DNP IgE (B,D) and challenged with 100 µg of Ag (A,B,D) 16 h later [15], or with 3 µg of SPE-7 anti-DNP IgE (C, D) or H1-DNP-ε-26.82 (D) as in Fig. 2 and challenged with 250 µg of Ag 24 h later [17]. Body temperature during the anaphylactic shock was monitored. ** P<0.01, *** P<0.001 between WT and S1pr2−/− (n=10/group in A, n=16 in B and n=6 in C) using a two-way ANOVA. (D) Temperature drop at 60 min post-PSA induction in WT and S1pr2−/− induced by the indicated concentrations of SPE-7 or H1-DNP-ε-26.82–IgE as in A,B,C and Fig. 2. Number of mice and time of deaths are indicated in A and B.
3.4. High concentrations of SPE-7 IgE induce an anaphylactic response mediated by both IgE and IgG receptors
We further explored if the potential difference in the onset of anaphylaxis was solely FcεRI–mediated (due to the higher crosslinking of IgE receptors and also involvement of additional mast cell subtypes) or if other mechanisms might be involved. SPE-7 IgE has an intrinsic ability to interact with various epitopes (other than DNP) and form aggregates [29–32]. IgE aggregates can bind the low affinity receptors for IgG (FcγRII/III and FcγRIV) [33], two of which (FcγRIII and FcγRIV) can induce degranulation from mast cells, basophils and neutrophils and can contribute to anaphylaxis [34,35]. Engagement of IgG receptors can induce an anaphylactic response that is more dependent on platelet activating factor (PAF) than that resulting from engagement of the high affinity IgE receptor [36,37]. As shown in Fig. 4A, hypothermia induced by 3 µg H1-DNP-ε-26.82 IgE was not altered by the PAF receptor antagonist CV8209. In contrast, anaphylaxis induced by 75 µg of SPE-7 IgE was significantly ameliorated by PAF receptor antagonism suggesting the involvement of IgG receptors. To explore whether 75 µg IgE could induce the activation of low affinity receptors for IgG that contribute to anaphylaxis we injected (i.p.) the FcγRII/III antibody (rat anti mouse CD16/CD32, clone 2.4G2) as a blocking agent [38,39] at low concentrations (up to 75 µg) in WT and S1pr2−/− mice, prior to the administration of IgE. As shown in Supplemental Fig. 2A, i.p. injection of up to 75 µg’s of 2.4G2 did not elicit anaphylaxis. The blocking of IgG receptors in WT mice markedly reduced the anaphylaxis induced by high concentrations of SPE-7 IgE (Fig. 4B) while it did not affect anaphylaxis induced after sensitization with 3 µg of H1-DNP-ε-26.82 IgE (Supplemental Fig. 2B). Consistent with a contribution of IgG receptors in SPE-7 IgE-mediated anaphylaxis, vascular permeability in the peritoneum, was more pronounced when compared to H1-DNP-ε-26.82 IgE-mediated anaphylaxis (Fig. 4C) [40]. Indeed, blocking of FcγR’s reverted the SPE-7-induced anaphylaxis to a response that was similar to that of H1-DNP-ε-26.82 IgE (Fig 4C). To clarify what FcγR (FcγRII or FcγRIII) was involved in SPE-7-induced anaphylaxis, we tested the effect of the FcγRII/FcγRIII blocking antibody in FcγRII-deficient mice. As shown in Fig. 4D, 2.4G2 partially blocked anaphylaxis induced by a high dose of SPE-7, demonstrating that anaphylaxis in this model is mediated by FcεRI in conjunction with FcγRIII.
Fig. 4. Anaphylaxis induced by intraperitoneal injection of high doses of SPE-7 IgE is partly mediated by low affinity IgG receptors.
(A) The PAF antagonist CV-6209 was injected (i.p.) before Ag challenge in mice sensitized (i.p.) with 75 µg SPE-7 IgE (SPE-7 clone) (n=10–16/group), or mice sensitized (i.v.) with 3 µg H1-DNP-ε-26.82 IgE (n=6/group) as described in Fig. 2. (B) Fcγ receptors were blocked by (i.p.) injection of 50–75 µg anti CD16/32 antibody (2.4G2 clone) prior to sensitization with 75 µg SPE-7 IgE and Ag challenge (n=5–8/group). *** P<0.001 by two-way ANOVA. The curves for WT and S1PR2 without 2.4G2 treatment are shown in 3B. (C) Vascular permeability into the peritoneum was measured, as explained in methods, 35 min after Ag challenge in 1% Evans Blue using similar experimental settings as in Fig.3. Statistical significance between indicated groups (n=5–10/group), ** P<0.01. (D) Mice (WT and FcγRII−/−) were injected (i.p.) with 50 µg anti CD16/32 antibody (2.4G2 clone) 3 h prior to sensitization with 35 µg SPE-7 IgE (n=4/group) and subsequently challenged 16 h later with Ag (100 µg) to induce systemic anaphylaxis. Body temperature changes during the anaphylactic shock were followed for 2 h. *** P<0.001 between the groups.
In contrast to WT mice, hypothermia (Fig. 4B) and vascular permeability (Fig 4C) in S1pr2−/− mice (which was marginally reduced compared to WT) were modestly or not affected, respectively, by FcγR blocking 2.4G2. This suggested an impaired IgG receptor involvement in these mice. Importantly, even when IgG receptors were blocked, poor recovery of S1pr2−/− mice from anaphylaxis was evident (compare WT and S1pr2−/− mice pretreated with 2.4G2 in Fig 4B and WT and S1pr2−/− responses in Figs. 2B and 3C). Altogether, these findings show that anaphylaxis induced by high concentrations of IgE, particularly highly cytokinergic IgE, is mediated by FcεRI as well as FcγRIII. Moreover, S1pr2−/− mice appear defective in IgG-mediated responses, while their defect in the recovery from shock is partly masked when the systemic anaphylaxis is very severe.
3.5. FcγR-induced anaphylaxis is delayed in S1pr2−/− mice and their mast cells have diminished FcγR-mediated degranulation
We investigated the underlying mechanism causing the modest reduction of anaphylactic symptoms for S1pr2−/− mice relative to WT mice induced by high concentrations of SPE-7 IgE. S1pr2−/− mice appeared to have reached saturation in the ability to recover from shock regardless of the model of anaphylaxis used (Fig 3D), suggesting that any differences in temperature were likely related to the onset of anaphylaxis (i.e, degranulation). When sensitized with SPE-7 IgE (75µg), S1pr2−/− mice showed a strong trend towards decreased histamine release (albeit not significant) upon antigen challenge (in 90 s) when compared to WT mice (Fig.5A). When WT mice were first pre-treated with 2.4G2 and then sensitized with SPE-7 IgE, histamine release to antigen was similar to that of S1pr2−/− mice (Fig. 5A). In contrast to WT mice, SPE-7 IgE sensitized S1pr2−/− mice did not differ in their release of histamine upon blocking of Fcγ receptors with 2.4G2 (Fig. 5A). Together with the experiments in Fig. 4D, these results show that FcγRIII contributes to the release of histamine to the circulation when WT, but not S1pr2−/−, mice are sensitized with high doses of SPE-7 IgE.
To determine if S1pr2−/− mice had a defect in IgG-mediated anaphylaxis, we induced anaphylaxis by directly injecting a high concentration (200 µg (i.p.)) of the 2.4G2 monoclonal antibody. High concentrations of this antibody are known to induce anaphylaxis [23,36], in contrast to the lower concentrations used for blocking FcγR (Supplemental Fig. 2A). 2.4G2-induced anaphylaxis was modestly delayed in S1pr2−/− mice demonstrating a slower onset in FcγR-induced anaphylactic shock in these mice (Fig. 5B). One hr after Ag challenge, S1pr2−/− mice responded similarly to WT mice or in some cases showed a diminished recovery from shock. The diminished recovery from shock was marked at higher concentrations of 2.4G2 (data not shown). Thus, the findings show a defective onset of anaphylaxis in S1pr2−/− mice when Fcγ receptors or a combination of FcεRI/FcγR are engaged, but not when FcεRI is primarily engaged.
We further investigated if Fcγ receptor engagement of mast cells from WT and S1pr2−/− mice resulted in differences in mast cell degranulation. Mast cells from the peritoneum of WT or S1pr2−/− mice were assayed for their degranulation response ex vivo to 2.4G2/anti-IgG immune complexes [23]. As shown in Fig. 5C, Fcγ receptor crosslinking resulted in a reduced response that reached statistical significance at suboptimal concentrations and showed a strong trend for reduced responses at optimal concentrations, thus arguing for a delayed FcγR-mediated anaphylactic response in S1pr2−/− mice. These findings promote the view that the mild protective effect of S1PR2-deficiency on anaphylaxis, observed at high concentrations of SPE-7 IgE, is a consequence of defective mast cell FcγR function. Regardless, the collective data strongly supports the argument that S1PR2 plays a dominant role in the recovery from IgE-mediated anaphylaxis and not in the initiation of this response.
4. DISCUSSION
Systemic anaphylaxis is an immediate hyperacute reaction that is initiated by the release of bioactive mediators mostly from (but not restricted to [35]) mast cells [41,42]. These mediators (i.e., histamine) may cause severe hypotension among other circulatory, respiratory, skin and gastrointestinal symptoms. Histamine is a vasomediator that in mice reproduces closely the symptoms of IgE-mediated anaphylaxis [43]. In a histamine-induced mouse model of anaphylaxis, we recently found that S1PR2 in peripheral tissues (independent of mast cells) controlled histamine clearance [17]. Mice lacking S1PR2 or sphingosine kinase 1 (which produces S1P) had more pronounced hypotension, and a more severe and longer lasting anaphylactic response. Injection of S1P shortly after shock in WT, but not in S1pr2−/− mice, promoted histamine clearance and recovery comparably to an epinephrine injection [17]; the first line of treatment for anaphylaxis in humans. In contrast, others [15] have reported diminished IgE/Ag-induced passive systemic anaphylaxis in S1pr2−/− mice; a result attributed to a role for S1PR2 in promoting mast cell degranulation. Thus, exploring the balance between these two possible roles for S1PR2 in anaphylaxis would be important when considering the potential targeting of S1PR2 as a treatment option in anaphylaxis.
Here we find that the previously reported role of S1PR2 in mediating IgE/Ag-induced mast cell degranulation in vitro [6] is not seen in murine connective tissue type mast cells and is seemingly restricted to mucosal-like mast cells (RBL-2H3 or BMMC differentiated in the presence of IL3). Our in vivo studies are consistent with our previous findings using a histamine-induced anaphylaxis model [17] and demonstrate that during FcεRI-mediated systemic anaphylaxis, the predominant role of S1PR2 is the recovery, presumably by controlling vascular tone and histamine clearance in a mast cell independent manner [17]. Furthermore, our data indicates no role for S1PR2 in the initiation of FcεRI-induced anaphylaxis, local or systemic, a result that contrast with another report [15]. The reasons for the discrepancies with this prior study are not entirely clear. However, a potential explanation drawn form our results is that high concentrations of IgE, particularly some types of IgE that are highly cytokinergic, induce an anaphylaxis mediated by both IgE and IgG receptors and the onset of the latter is affected by S1PR2 deficiency. Furthermore, regardless of the clone of IgE used in the studies, it should be cautioned that high concentrations of IgE can contain considerable amounts of aggregates (some of which can be removed by ultracentrifugation as done in our study), which could trigger IgE-dependent or IgG-dependent (through binding to low affinity FcγRs) mast cell degranulation when given hours in advance of Ag challenge. This could reduce the subsequent anaphylactic response to an Ag challenge, potentially more markedly in S1pr2−/− than in WT mice when considering that S1pr2−/− mice have defects in vascular tone and permeability [17,44] and delayed recovery from anaphylaxis [15], and thus may not be able to demonstrate a full response to the Ag challenge. This is consistent with a less marked body temperature drop (3–5°C) seen in the prior study [15], following Ag challenge, when compared to the drop (7–9°C) seen in our study when both studies used 75 µg of IgE for in vivo sensitization. It should also be noted that the use of 75 µg of IgE for sensitization translates into a concentration of 25 µg/ml (generously assuming 3 ml total blood volume in circulation and tissues) in mice; concentrations that are not achieved even under circumstances of hyper IgE levels during Th2-mediated disease in mice [45], whereas the use of 3 µg results in a pathophysiological meaningful concentration of 1 µg/ml seen in such diseases [45] and is approximately 10 fold greater than that in the circulation of WT mice. Moreover, the use of presumed specific inhibitors for S1PR2 (such as JTE-013) to query its in vivo function [15] may be flawed as we have found that JTE-013 effectively inhibits the degranulation of S1pr2−/− peritoneal-derived mast cells (Supplemental Fig. 3), suggesting it has potent off target effects.
Another interesting finding is that IgE derived from different hybridoma clones (or potentially the same clone containing varied amounts of aggregates) can elicit a phenotypically different anaphylactic response. This is a consequence of their relative abilities to engage both the high affinity IgE receptor and low affinity receptors for IgG in vivo. Notably, the highly cytokinergic SPE-7 IgE induced an anaphylactic shock response that was mediated by IgE and FcγRIII receptors, and thus more severe than that seen with the more moderate cytokinergic H1-DNPε-26.82 IgE. This more marked drop in body temperature revealed a modest protective effect of S1PR2 deficiency in SPE-7 IgE-sensitized mice that appears to result from reduced IgG-mediated mast cell degranulation and a defective FcγR contribution to the anaphylactic response in these mice. The participation of IgG receptors in IgE-mediated anaphylaxis has been shown [46]. IgE engagement of the low affinity receptor FcγRIII required large amounts of IgE and/or Ag, reflecting a need for formation of large immune complexes [46,47]. The demonstrated ability of SPE-7 IgE to assume multiple conformations that endow it with the ability to interact with multiple cross-reactive antigens (or epitopes) may promote increased aggregate size [29–32] and thus increase the probability of binding FcγRIII and IV [33,35,48]. This view is consistent with our finding (Fig. 3C) that a lower concentration (3 µg) of SPE-7 did not result in the mild in vivo protective effect (mediated by FcγR engagement) seen at the high concentration of IgE in S1pr2−/− mice. In vitro experiments have also shown that when high concentrations of SPE-7 IgE are added to mast cells the spectrum of responses elicited differs when compared to the same concentration of H1-DNPε-26.82 IgE [29]. Collectively, the multiple findings support the view that a mild defect in the onset of anaphylaxis is manifested in S1PR2-deficient mice (Fig. 5A) when coaggregation of stimulatory IgE and IgG receptors occurs or when IgG receptors are engaged at low occupancy; yet even under these circumstances recovery from anaphylaxis is still affected (see recovery phase in Fig. 5B).
An important question that remains incompletely answered is whether there is a role for S1PR2 in human mast cell responses. Previous studies in human mast cells (using siRNA to silence S1PR2 expression) showed that a deficiency in S1PR2 reduced degranulation and conversely in one study a robust degranulation was observed when human mast cells were treated with S1P [15]. However, other reports [12] and our own experiments in the LAD2 human mast cell line (Supplemental Fig. 1) and in peripheral blood-derived CD34+ cultured human mast cells (data not shown) show that S1P added exogenously had minimal or no effect on degranulation. Moreover, silencing of S1PR2 expression by siRNA had a minimal effect on the degranulation of LAD2 cells (Supplemental Fig. 1). In general, however, the difficulty of interpreting studies on cultured primary human mast cells or human mast cell lines is that these may not necessarily represent the mast cell phenotype seen in resident tissues and may well differ in their cell surface expression of S1PRs.
4.1. Conclusions
In summary, our findings demonstrate that the global lack of S1PR2 in the mouse predominantly affects the recovery from FcεRI -induced anaphylaxis, without generally affecting the initiation of this response. In contrast, when FcγR receptors are also engaged (by IgE immune complexes or by IgG immune complexes) S1PR2 shows a modest contribution to the onset of anaphylaxis and it is required for the full extent of IgG-mediated mast cell degranulation. Our findings show that S1PR2 is dispensable in IgE-mediated degranulation of connective tissue mast cells, but are suggestive of a role in mucosal mast cell responses. These new findings serve to clarify the role of S1PR2 in mast cell function and allergic responses and promote the view that the pursuit of specific S1PR2 agonists as an alternative to epinephrine in the treatment of anaphylaxis merits further consideration.
Supplementary Material
HIGHLIGHTS.
The role of mast cell S1PR2 in IgE/Ag-mediated degranulation is restricted to mucosal-like mast cells and it is generally inconsequential for the initiation of cutaneous and systemic IgE-mediated anaphylaxis.
High doses of highly cytokinergic IgE hybridomas induce anaphylaxis via FcεRI with a contribution of FcγRIII receptors.
A minor role for S1PR2 is observed in IgG-induced mast cell degranulation correlating with a slight delay in the initiation of IgG-induced anaphylaxis at low receptor occupancy.
The predominant role for S1PR2 in allergy is in promoting recovery from systemic anaphylaxis.
Our results suggest that agonism, but not antagonism of this receptor, may hold promise as secondary treatment for systemic anaphylaxis since its effects on initiation are minimal.
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health (NIH). We gratefully acknowledge the contributions of the Office of Science and Technology (Flow Cytometry and Laboratory Animal Care and Use Sections), NIAMS.
ABBREVIATIONS USED
- Ag
Antigen
- ABCC1
Mutidrug resistance-associated protein 1
- BMMC
Bone marrow derived mast cell
- GPCRs
G-protein coupled receptors
- DNP
2,4-dinitrophenyl
- FcεRI
High affinity receptor for IgE
- IL-3
Interleukin 3
- LAD2
skin-like human mast cell line
- PAF
Platelet activating factor
- PCA
Passive cutaneous anaphylaxis
- PDMC
Peritoneal derived mast cell
- PSA
Passive systemic anaphylaxis
- RBL-2H3
Rat basophilic leukemia cells-clone 2H3
- S1P
Sphingosine-1-phosphate
- S1PR
Sphingosine-1-phosphate receptor
- 2.4G2
Anti-FcγRII/FcγRIII (anti-CD16/CD32)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Galli SJ, Tsai M. Mast cells: versatile regulators of inflammation, tissue remodeling, host defense and homeostasis. J Dermatol Sci. 2008;49:7–19. doi: 10.1016/j.jdermsci.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsai M, Grimbaldeston M, Galli SJ. Mast cells and immunoregulation/immunomodulation. Adv Exp Med Biol. 2011;716:186–211. doi: 10.1007/978-1-4419-9533-9_11. [DOI] [PubMed] [Google Scholar]
- 3.Gilfillan AM, Peavy RD, Metcalfe DD. Amplification mechanisms for the enhancement of antigen-mediated mast cell activation. Immunol Res. 2009;43:15–24. doi: 10.1007/s12026-008-8046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuehn HS, Gilfillan AM. G protein-coupled receptors and the modification of FcepsilonRI-mediated mast cell activation. Immunol Lett. 2007;113:59–69. doi: 10.1016/j.imlet.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strub GM, Maceyka M, Hait NC, Milstien S, Spiegel S. Extracellular and intracellular actions of sphingosine-1-phosphate. Adv Exp Med Biol. 2010;688:141–155. doi: 10.1007/978-1-4419-6741-1_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, Rivera J, et al. Transactivation of sphingosine-1-phosphate receptors by FcepsilonRI triggering is required for normal mast cell degranulation and chemotaxis. J Exp Med. 2004;199:959–970. doi: 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olivera A, Rivera J. Sphingolipids and the balancing of immune cell function: lessons from the mast cell. J Immunol. 2005;174:1153–1158. doi: 10.4049/jimmunol.174.3.1153. [DOI] [PubMed] [Google Scholar]
- 8.Olivera A, Mizugishi K, Tikhonova A, Ciaccia L, Odom S, Proia RL, et al. The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity. 2007;26:287–297. doi: 10.1016/j.immuni.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 10.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008;8:753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olivera A, Urtz N, Mizugishi K, Yamashita Y, Gilfillan AM, Furumoto Y, et al. IgE-dependent activation of sphingosine kinases 1 and 2 and secretion of sphingosine 1-phosphate requires Fyn kinase and contributes to mast cell responses. J Biol Chem. 2006;281:2515–2525. doi: 10.1074/jbc.M508931200. [DOI] [PubMed] [Google Scholar]
- 12.Bansal G, DiVietro JA, Kuehn HS, Rao S, Nocka KH, Gilfillan AM, et al. RGS13 controls g protein-coupled receptor-evoked responses of human mast cells. J Immunol. 2008;181:7882–7890. doi: 10.4049/jimmunol.181.11.7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuehn HS, Beaven MA, Ma HT, Kim MS, Metcalfe DD, Gilfillan AM. Synergistic activation of phospholipases Cgamma and Cbeta: a novel mechanism for PI3K-independent enhancement of FcepsilonRI-induced mast cell mediator release. Cell Signal. 2008;20:625–636. doi: 10.1016/j.cellsig.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oskeritzian CA, Alvarez SE, Hait NC, Price MM, Milstien S, Spiegel S. Distinct roles of sphingosine kinases 1 and 2 in human mast-cell functions. Blood. 2008;111:4193–4200. doi: 10.1182/blood-2007-09-115451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oskeritzian CA, Price MM, Hait NC, Kapitonov D, Falanga YT, Morales JK, et al. Essential roles of sphingosine-1-phosphate receptor 2 in human mast cell activation, anaphylaxis, and pulmonary edema. J Exp Med. 2010;207:465–474. doi: 10.1084/jem.20091513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci U S A. 2006;103:16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olivera A, Eisner C, Kitamura Y, Dillahunt S, Allende L, Tuymetova G, et al. Sphingosine kinase 1 and sphingosine-1-phosphate receptor 2 are vital to recovery from anaphylactic shock in mice. J Clin Invest. 2010;120:1429–1440. doi: 10.1172/JCI40659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kono M, Belyantseva IA, Skoura A, Frolenkov GI, Starost MF, Dreier JL, et al. Deafness and stria vascularis defects in S1P2 receptor-null mice. J Biol Chem. 2007;282:10690–10696. doi: 10.1074/jbc.M700370200. [DOI] [PubMed] [Google Scholar]
- 19.Charles N, Watford WT, Ramos HL, Hellman L, Oettgen HC, Gomez G, et al. Lyn kinase controls basophil GATA-3 transcription factor expression and induction of Th2 cell differentiation. Immunity. 2009;30:533–543. doi: 10.1016/j.immuni.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saitoh S, Arudchandran R, Manetz TS, Zhang W, Sommers CL, Love PE, et al. LAT is essential for Fc(epsilon)RI-mediated mast cell activation. Immunity. 2000;12:525–535. doi: 10.1016/s1074-7613(00)80204-6. [DOI] [PubMed] [Google Scholar]
- 21.Kirshenbaum AS, Akin C, Wu Y, Rottem M, Goff JP, Beaven MA, et al. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcepsilonRI or FcgammaRI. Leukemia research. 2003;27:677–682. doi: 10.1016/s0145-2126(02)00343-0. [DOI] [PubMed] [Google Scholar]
- 22.Liu FT, Bohn JW, Ferry EL, Yamamoto H, Molinaro CA, Sherman LA, et al. Monoclonal dinitrophenyl-specific murine IgE antibody: preparation, isolation, and characterization. J Immunol. 1980;124:2728–2737. [PubMed] [Google Scholar]
- 23.Falanga YT, Chaimowitz NS, Charles N, Finkelman FD, Pullen NA, Barbour S, et al. Lyn but not Fyn kinase controls IgG-mediated systemic anaphylaxis. J Immunol. 2012;188:4360–4368. doi: 10.4049/jimmunol.1003223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serra-Pages M, Olivera A, Torres R, Picado C, de Mora F, Rivera J. E-prostanoid 2 receptors dampen mast cell degranulation via cAMP/PKA-mediated suppression of IgE-dependent signaling. Journal of leukocyte biology. 2012 doi: 10.1189/jlb.0212109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurish MF, Austen KF. Developmental origin and functional specialization of mast cell subsets. Immunity. 2012;37:25–33. doi: 10.1016/j.immuni.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Rennick D, Hunte B, Holland G, Thompson-Snipes L. Cofactors are essential for stem cell factor-dependent growth and maturation of mast cell progenitors: comparative effects of interleukin-3 (IL-3), IL-4, IL-10, and fibroblasts. Blood. 1995;85:57–65. [PubMed] [Google Scholar]
- 27.Pejler G, Abrink M, Ringvall M, Wernersson S. Mast cell proteases. Adv Immunol. 2007;95:167–255. doi: 10.1016/S0065-2776(07)95006-3. [DOI] [PubMed] [Google Scholar]
- 28.Eshhar Z, Ofarim M, Waks T. Generation of hybridomas secreting murine reaginic antibodies of anti-DNP specificity. J Immunol. 1980;124:775–780. [PubMed] [Google Scholar]
- 29.Kitaura J, Song J, Tsai M, Asai K, Maeda-Yamamoto M, Mocsai A, et al. Evidence that IgE molecules mediate a spectrum of effects on mast cell survival and activation via aggregation of the FcepsilonRI. Proc Natl Acad Sci U S A. 2003;100:12911–12916. doi: 10.1073/pnas.1735525100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruhns P, Fremont S, Daeron M. Regulation of allergy by Fc receptors. Curr Opin Immunol. 2005;17:662–669. doi: 10.1016/j.coi.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 31.James LC, Roversi P, Tawfik DS. Antibody multispecificity mediated by conformational diversity. Science. 2003;299:1362–1367. doi: 10.1126/science.1079731. [DOI] [PubMed] [Google Scholar]
- 32.James LC, Tawfik DS. The specificity of cross-reactivity: promiscuous antibody binding involves specific hydrogen bonds rather than nonspecific hydrophobic stickiness. Protein science : a publication of the Protein Society. 2003;12:2183–2193. doi: 10.1110/ps.03172703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mancardi DA, Iannascoli B, Hoos S, England P, Daeron M, Bruhns P. FcgammaRIV is a mouse IgE receptor that resembles macrophage FcepsilonRI in humans and promotes IgE-induced lung inflammation. J Clin Invest. 2008;118:3738–3750. doi: 10.1172/JCI36452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jonsson F, Daeron M. Mast cells and company. Front Immunol. 2012;3:16. doi: 10.3389/fimmu.2012.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jonsson F, Mancardi DA, Zhao W, Kita Y, Iannascoli B, Khun H, et al. Human FcgammaRIIA induces anaphylactic and allergic reactions. Blood. 2012;119:2533–2544. doi: 10.1182/blood-2011-07-367334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strait RT, Morris SC, Yang M, Qu XW, Finkelman FD. Pathways of anaphylaxis in the mouse. J Allergy Clin Immunol. 2002;109:658–668. doi: 10.1067/mai.2002.123302. [DOI] [PubMed] [Google Scholar]
- 37.Finkelman FD. Anaphylaxis: lessons from mouse models. J Allergy Clin Immunol. 2007;120:506–515. doi: 10.1016/j.jaci.2007.07.033. quiz 516-507. [DOI] [PubMed] [Google Scholar]
- 38.Unkeless JC. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979;150:580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurlander RJ, Ellison DM, Hall J. The blockade of Fc receptor-mediated clearance of immune complexes in vivo by a monoclonal antibody (2.4G2) directed against Fc receptors on murine leukocytes. J Immunol. 1984;133:855–862. [PubMed] [Google Scholar]
- 40.Tsujimura Y, Obata K, Mukai K, Shindou H, Yoshida M, Nishikado H, et al. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity. 2008;28:581–589. doi: 10.1016/j.immuni.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Metcalfe DD, Peavy RD, Gilfillan AM. Mechanisms of mast cell signaling in anaphylaxis. J Allergy Clin Immunol. 2009;124:639–646. doi: 10.1016/j.jaci.2009.08.035. quiz 647-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khan BQ, Kemp SF. Pathophysiology of anaphylaxis. Curr Opin Allergy Clin Immunol. 2011;11:319–325. doi: 10.1097/ACI.0b013e3283481ab6. [DOI] [PubMed] [Google Scholar]
- 43.Makabe-Kobayashi Y, Hori Y, Adachi T, Ishigaki-Suzuki S, Kikuchi Y, Kagaya Y, et al. The control effect of histamine on body temperature and respiratory function in IgE-dependent systemic anaphylaxis. J Allergy Clin Immunol. 2002;110:298–303. doi: 10.1067/mai.2002.125977. [DOI] [PubMed] [Google Scholar]
- 44.Skoura A, Hla T. Regulation of vascular physiology and pathology by the S1P2 receptor subtype. Cardiovasc Res. 2009;82:221–228. doi: 10.1093/cvr/cvp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Charles N, Hardwick D, Daugas E, Illei GG, Rivera J. Basophils and the T helper 2 environment can promote the development of lupus nephritis. Nat Med. 2010;16:701–707. doi: 10.1038/nm.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ujike A, Ishikawa Y, Ono M, Yuasa T, Yoshino T, Fukumoto M, et al. Modulation of immunoglobulin (Ig)E-mediated systemic anaphylaxis by low-affinity Fc receptors for IgG. J Exp Med. 1999;189:1573–1579. doi: 10.1084/jem.189.10.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ishikawa R, Tsujimura Y, Obata K, Kawano Y, Minegishi Y, Karasuyama H. IgG-mediated systemic anaphylaxis to protein antigen can be induced even under conditions of limited amounts of antibody and antigen. Biochemical and biophysical research communications. 2010;402:742–746. doi: 10.1016/j.bbrc.2010.10.098. [DOI] [PubMed] [Google Scholar]
- 48.Jonsson F, Mancardi DA, Kita Y, Karasuyama H, Iannascoli B, Van Rooijen N, et al. Mouse and human neutrophils induce anaphylaxis. J Clin Invest. 2011;121:1484–1496. doi: 10.1172/JCI45232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.