Abstract
Testosterone administration increases hemoglobin levels and has been used to treat anemia of chronic disease. Erythrocytosis is the most frequent adverse event associated with testosterone therapy of hypogonadal men, especially older men. However, the mechanisms by which testosterone increases hemoglobin remain unknown.
Testosterone administration in male and female mice was associated with a greater increase in hemoglobin and hematocrit, reticulocyte count, reticulocyte hemoglobin concentration, and serum iron and transferring saturation than placebo. Testosterone downregulated hepatic hepcidin mRNA expression, upregulated renal erythropoietin mRNA expression, and increased erythropoietin levels. Testosterone-induced suppression of hepcidin expression was independent of its effects on erythropoietin or hypoxia-sensing mechanisms. Transgenic mice with liver-specific constitutive hepcidin over-expression failed to exhibit the expected increase in hemoglobin in response to testosterone administration. Testosterone upregulated splenic ferroportin expression and reduced iron retention in spleen. After intravenous administration of transferrin-bound 58Fe, the amount of 58Fe incorporated into red blood cells was significantly greater in testosterone-treated mice than in placebo-treated mice. Serum from testosterone-treated mice stimulated hemoglobin synthesis in K562 erythroleukemia cells more than that from vehicle-treated mice. Testosterone administration promoted the association of androgen receptor (AR) with Smad1 and Smad4 to reduce their binding to BMP-response elements in hepcidin promoter in the liver. Ectopic expression of AR in hepatocytes suppressed hepcidin transcription; this effect was blocked dose-dependently by AR antagonist flutamide. Testosterone did not affect hepcidin mRNA stability. Conclusion: Testosterone inhibits hepcidin transcription through its interaction with BMP-Smad signaling. Testosterone administration is associated with increased iron incorporation into red blood cells.
Introduction
Testosterone is an important regulator of erythropoiesis in men and women (Bhasin et al., 2010; Mirand EA, 1965). Circulating testosterone levels have been associated with hemoglobin levels in community-dwelling middle-aged and older men (Ferrucci L, 2006; Yeap et al., 2009) and in men with prostate cancer who are receiving androgen-deprivation therapy (Hara N, 2010). Before the advent of erythropoietin (EPO), androgens were used widely to treat anemia-associated with chronic disease, end stage renal disease, and aplastic anemia (Shahidi NT, 1959; Watkinson G, 1947). Erythrocytosis is the most frequent adverse event associated with testosterone therapy of hypogonadal men, especially in older men. However, the mechanisms by which testosterone stimulates erythropoiesis remain poorly understood. An understanding of these mechanisms has important implications for the therapeutic applications of androgens for the treatment of androgen deficiency, anemia, and sarcopenia associated with aging or chronic disease.
Several hypotheses have been proposed to explain the mechanisms by which testosterone increases hemoglobin and hematocrit, including stimulation of EPO, direct effects on erythroid progenitors, ferrokinetics, and red cell survival (Bachman E, 2010; Beran M, 1982; Moriyama Y, 1975); however, the evidence supporting these hypotheses is inconclusive (Coviello AD, 2008; Mirand EA, 1971). We show here that testosterone regulates hepcidin expression, in association with decreased splenic iron retention and increased iron incorporation into red cells. Additionally, we investigated the mechanisms by which testosterone regulates hepcidin expression; we provide multiple lines of evidence that testosterone, through activation of its nuclear receptor, interferes with bone morphogenetic protein (BMP)/Smad signaling to reduce hepcidin transcription. We also tested whether testosterone regulates hepcidin by stimulating EPO or by activating hypoxia-sensing mechanisms.
Results
1. The effects of testosterone on red cell indices
In adult female C57BL/6 mice, testosterone administration by subcutaneous implants was associated with a gradual increase in hematocrit after a lag of two days (Fig. 1A). Two weeks after initiation of testosterone administration, mice assigned to testosterone group had significantly higher levels of hemoglobin and hematocrit, and a trend towards higher mean corpuscular hemoglobin than those in the control group (Fig. 1B). Mean corpuscular volume did not differ between groups. Serum iron and transferrin saturation were significantly higher and total iron binding capacity significantly lower in testosterone-treated mice than in placebo-treated mice (Fig. 1C). Reticulocyte count and reticulocyte hemoglobin ratio (CHr), a sensitive marker of iron available for hemoglobin synthesis (Fishbane S, 1997), were also significantly higher in testosterone-treated mice than in placebo-treated mice (Fig. 1D).
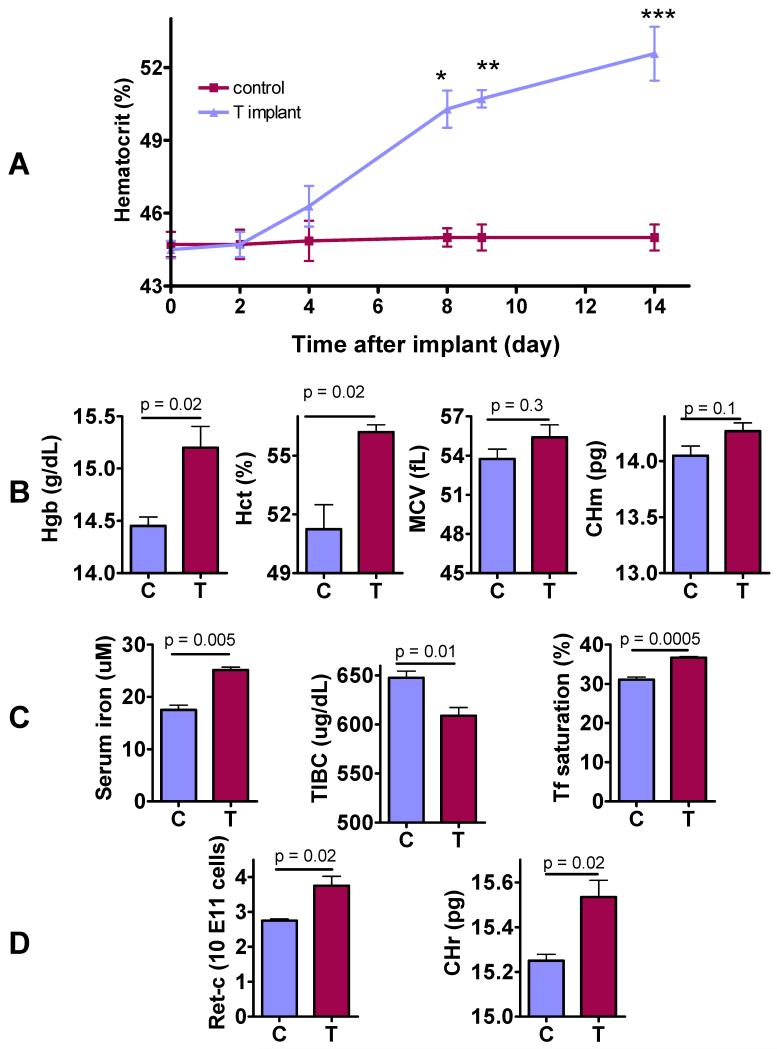
Figure 1. Effects of Testosterone on Red Cell Indices, Reticulocyte Count, Reticulocyte Hemoglobin Ratio, and Circulating Iron Indices.
A: Time course of hematocrit change after subcutaneous insertion of empty or testosterone implants in female mice (*p=0.0002, **p<0.0001, ***p=0.0002 for between-group comparison at each time). The slope of hematocrit change over time was 0.65 for testosterone group (p<0.0001) and 0.02 for control group (p=0.6121).
B: The mice treated with testosterone implants for 2-weeks had significantly higher hemoglobin (Hgb), hematocrit (Hct), and a trend towards higher mean corpuscular hemoglobin (CHm) than mice that received empty implants.
C: Testosterone-treated mice had significantly higher serum iron, lower total iron binding capacity (TIBC), and higher transferrin (Tf) saturation than controls 2 weeks after insertion of either empty (C) or testosterone implants (T).
D. Testosterone-treated mice (T) had significantly higher reticulocyte count (Retic-C) and higher reticulocyte hemoglobin ratio (CHr) than controls (C) after 2 weeks of treatment.
The data are mean±SEM, n=10-20 mice for each measurement.
Similar data were obtained in castrated male mice (supplementary Fig. s1). Thus, testosterone stimulates erythropoiesis in female and male mice and increases markers of iron bioavailability for erythropoiesis. Because the magnitude of change in hemoglobin and hematocrit was greater in female mice than in male mice, subsequent experiments were performed initially in female mice and key findings were confirmed in male mice.
2. The effects of testosterone on hemoglobin and hematocrit in transgenic mice with liver-specific hepcidin over-expression
Hepcidin is an important regulator of iron availability for erythropoiesis (Ganz T., 2011). Hepcidin binds to ferroportin, an iron transporter present on the duodenal cells and macrophages, which promotes internalization and degradation of ferroportin, preventing iron entry into plasma to lower transferrin saturation and reduce iron availability.
We determined whether testosterone administration suppresses hepcidin expression and whether testosterone increases hemoglobin and hematocrit in hepcidin transgenic mice. Testosterone administration was associated with a significant suppression of hepatic hepcidin mRNA expression, compared to vehicle-treated mice; 96-hours after treatment initiation, hepcidin mRNA expression was ~70% lower in testosterone-treated mice than in vehicle-treated mice and this effect was sustained for the two-week treatment duration (Fig. 2A).
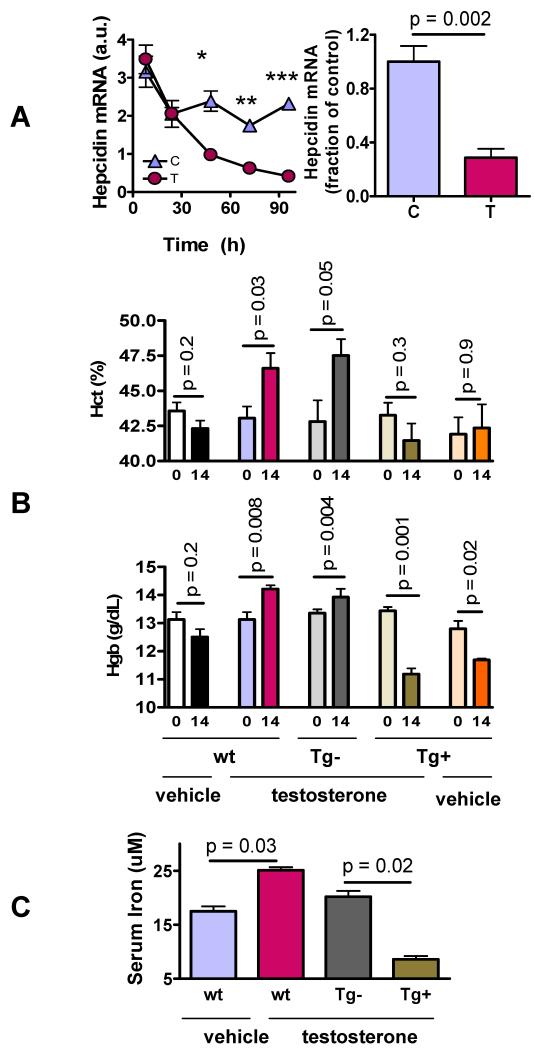
Figure 2. The effects of testosterone on hepcidin mRNA expression and on hematocrit, hemoglobin, and serum iron in wild type and hepcidin transgenic mice.
A. Testosterone suppresses hepatic hepcidin expression. Testosterone administration was associated with a greater decrease in hepatic hepcidin mRNA expression than vehicle after 96h (left panel, *p<0.0001, **p< 0.0001, ***p<0.0001 for between-group comparison at each time) and 2 weeks of treatment (right panel).
B. Hematocrit (Hct) (upper panel) and hemoglobin (Hgb) (lower panel) in wild type (wt) and transgenic female mice that carried either a silent (Tg−) or a constitutively active (Tg+) hepcidin transgene at baseline (day 0) or on day 14 after administration of either empty or testosterone implants. Wt and Tg− mice both displayed higher Hgb and Hct on day 14 compared to baseline in response to testosterone administration, whereas Tg+ mice displayed a slight decrease in Hgb and Hct. Wild-type mice treated with empty implants displayed a small decrease in Hgb and Hct likely due to repeated blood drawing.
C. Serum iron levels in female wild type (wt) and mice carrying silent (Tg−) or constitutively active (Tg+) hepcidin transgene. Testosterone treatment for 2 weeks was associated with significantly higher serum iron levels in wt and Tg− mice than in vehicle-treated controls and in testosterone-treated Tg+ mice. Tg+ mice had significantly lower serum iron than all other groups.
We studied the effect of testosterone in transgenic mice that exhibit liver-specific over-expression of a hepcidin transgene (TgN(tTALAP)5Uh;TgN(TRE.Hepc1) or “Tg+”) (Roy CN, 2007). Littermates carrying a silent hepcidin transgene (TgN(TRE.Hepc1 or “Tg-”) were used as controls. We reasoned that constitutive hepcidin over-expression in Tg+ mice would not be regulated by testosterone. Therefore, if hepcidin suppression were essential for mediating testosterone’s effects on hemoglobin, testosterone administration would not increase hemoglobin in Tg+ mice. We confirmed using quantitative PCR that hepcidin mRNA expression was 3-fold higher in Tg+ mice than in Tg− and wild type mice (supplementary Fig. s2). In comparison to vehicle-treated wild type controls, which experienced a small nonsignificant decrease in hemoglobin and hematocrit, testosterone-treated wild type as well as Tg− female mice exhibited significant increases in hemoglobin and hematocrit compared to baseline (Fig. 2B). In contrast, testosterone administration failed to induce significant increases in hemoglobin and hematocrit in Tg+ mice in which hepcidin transgene remained over-expressed in spite of testosterone treatment (Fig. 2B, supplementary Fig. s2). After two-weeks of testosterone treatment, wild-type and Tg− mice that received testosterone had higher concentrations of hemoglobin, hematocrit, and serum iron than testosterone-treated hepcidin Tg+ mice (Fig. 2B, 2C). Similarly, in castrated male mice, transgenic expression of hepcidin (Tg+) blunted testosterone-induced increase in hematocrit and completely blocked the testosterone-induced increase in hemoglobin (supplementary Fig. s3). These data indicate that regulation of hepcidin by testosterone is important for mediating testosterone-induced increase in hemoglobin.
3. Testosterone upregulates ferroportin expression in spleen, and reduces splenic ferric iron retention
The iron used in erythropoiesis is derived either from dietary iron or from recycling of red cell iron by macrophages, particularly in the spleen. The latter is a quantitatively more important source of iron for erythropoiesis over short durations (Ganz T., 2007). We found a significantly higher expression of ferroportin in the spleens of testosterone-treated mice than in vehicle-treated mice (Fig. 3A). These findings are consistent with reports showing that hepcidin reduces ferroportin expression in macrophages (Chaston T, 2008). As ferroportin promotes iron transport out of splenic macrophages, increased ferroportin expression in testosterone-treated mice would be expected to increase the iron recycled by splenic macrophages back into blood and thereby reduce splenic iron stores. Indeed, Prussian blue staining revealed significantly lower splenic iron stores in testosterone-treated mice than in vehicle-treated mice (Fig. 3B).
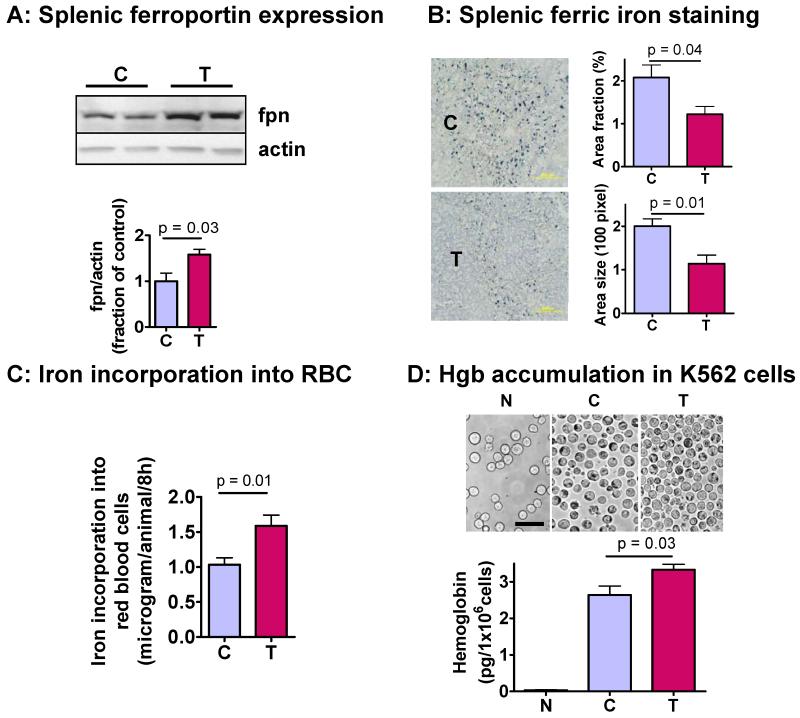
Figure 3. Effect of testosterone on splenic ferroportin expression and ferric iron staining, hemoglobin accumulation in K562 erythroleukemia cells, and on 58Fe incorporation into red blood cells.
A. Western analysis of splenic ferroportin (fpn) expression (N=5 for each group). Ferroportin protein level, normalized by actin expression, was significantly higher in spleens of testosterone-treated (T) mice than in controls (C).
B. Prussian blue staining for iron in the spleen of mice treated with either empty (C) or testosterone-containing (T) implants (N=3 for each group). Ferric iron stains greenish-blue and background tissue is stained with hematoxylin. Testosterone treatment significantly reduced splenic ferric iron, measured either as area of iron staining (upper left panel) or as the size of iron stains (lower left panel). This result is consistent with the increased expression of iron-exporter ferroportin in this tissue.
C. Iron incorporation into red blood cells. Female mice were injected testosterone twice weekly for 2 weeks. 58Fe/transferrin complex was injected into tail vein at 10 ng/g body weight. After 8 h, blood was taken to measure 58Fe/56Fe ratio using MC-ICPMASS. The amount of iron incorporated into red cells was calculated from specific activity of 58Fe in steady-state serum iron pool size.
D. Effects of testosterone on hemoglobin accumulation in K562 cells. Erythroid differentiation was induced by sodium butyrate (0.5 mM) for 48 h. Cells were incubated with 10% serum from female mice pre-treated with vehicle (C) or testosterone (T). Serum iron concentrations were 17.5±0.9uM and 25.1±0.6uM and transferrin saturation 31.0±0.7% and 36.7±0.3% for the control and T-treated mice, respectively. A negative control was incubated with serum-free medium (N). Cells were harvested after 24 h and stained with benzidine (upper panel). Cells were lysed with 0.2% Triton and hemoglobin was measured after benzidine staining at OD600.
Since diferric transferrin-bound iron is physiologically the primary iron source for heme/hemoglobin synthesis in erythroid cells (Ponka P., 1997), our findings of increased serum iron, increased transferrin saturation and decreased splenic iron retention would be expected to favor iron incorporation into red blood cells for heme/hemoglobin synthesis. Accordingly, we determined the effects of testosterone on iron incorporation into red blood cells, after a bolus i.v. injection of transferrin-bound 58Fe tracers. Female mice were injected with testosterone or placebo for two weeks. The 58Fe/transferrin complex was injected into mice via tail vein at a dose of 10 ng/g body weight, and 8 hours later, blood was drawn from cheek vein and washed with saline to remove serum and nonerythroid cells. This time point was selected based on previous studies showing that iron is linearly incorporated into red blood cells within 24 hour of administration (Srivastava et al., 1979). The amount of iron incorporated into red blood cells was determined from steady-state total iron pool size in serum and in red blood cells. As expected, the amount of iron incorporated into red blood cells was significantly greater in testosterone-treated mice than in vehicle-treated mice (Fig. 3C).
4. Sera from testosterone-treated mice increase hemoglobin accumulation in differentiating K562 erythroleukemia cells to a greater extent than those from vehicle-treated mice
To determine whether increased iron availability in testosterone-treated mice is associated with increased hemoglobin synthesis, K562 erythroleukemia cells were induced to differentiation into erythroid lineage by sodium butyrate (0.5 mM) for 48h (Kawasaki et al., 1996). The cells were washed and incubated with serum from female mice treated with either vehicle or testosterone for two weeks. The cells incubated in serum-free medium were included as an additional negative control. The cells were harvested after 24h and stained with benzidine (Fig. 3D, upper panel). Additionally, the hemoglobin accumulation in the cells was measured using a benzidine assay. Incubation of differentiating K562 cells with serum from testosterone-treated mice was associated with significantly greater hemoglobin accumulation than with serum from vehicle-treated mice (Fig. 3D).
5. Role of EPO in mediating testosterone’s effects on hepcidin
As EPO plays an important role in hepcidin regulation (Lainé F, 2011; Pak M, 2006), we assessed whether EPO is causally involved in testosterone-induced hepcidin regulation. Testosterone administration was associated with a rapid but transient increase in renal EPO mRNA expression and serum EPO levels (Fig. 4A). Hepatic hepcidin mRNA expression was suppressed by testosterone administration (Fig. 2A) lagging behind the rise of renal EPO mRNA expression (Fig. 4A).
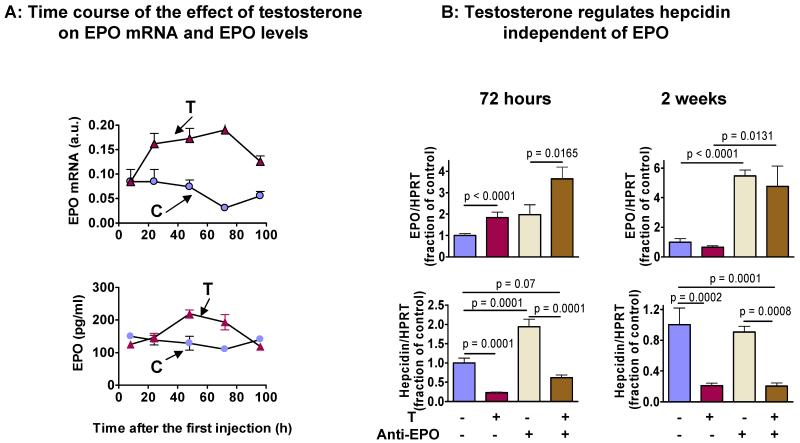
Figure 4. Erythropoietin is not essential for testosterone-mediated suppression of hepcidin.
A: Time-course of testosterone-induced changes in renal EPO mRNA expression (upper panel) and serum EPO concentrations (lower panel) in female mice. Results are mean±SEM, N=8 for each group. Testosterone administration induced a rapid increase in renal EPO mRNA expression which was followed by an increase in serum EPO levels.
B: To determine whether EPO is the mediator of testosterone-induced hepcidin suppression, we treated female mice with testosterone (T) with and without an anti-EPO neutralizing antibody (anti-EPO). EPO and hepcidin mRNA expression levels were assessed 72h (left panel) and 2 weeks (right panel) after treatment initiation. Testosterone administration was associated with a 2 fold increase in renal EPO mRNA expression at 72 h and ~75% decrease in hepatic hepcidin mRNA expression. The administration of anti-EPO antibody alone resulted in a 2 fold increase in renal EPO mRNA. Combined administration of testosterone and anti-EPO antibody resulted in greater increase in renal EPO mRNA expression than either intervention alone. The administration of anti-EPO antibody resulted in a nearly 2 fold increase in hepatic hepcidin mRNA expression. However, co-treatment with anti-EPO did not prevent testosterone-induced suppression of hepcidin either at 72h or 2 weeks. Thus, EPO is not essential for mediating the inhibitory effects of testosterone on hepcidin expression. Results are mean±SEM, N=5 for each group.
To investigate whether EPO is a mediator of testosterone-induced hepcidin suppression, we treated wild type mice with testosterone with and without a monoclonal anti-EPO neutralizing antibody. Testosterone administration was associated with a two-fold increase in renal EPO mRNA expression at 72h, which returned to baseline after two-weeks of testosterone administration (Fig. 4B, upper panel). The administration of anti-EPO antibody alone increased renal EPO mRNA at both time points tested, likely as a compensatory mechanism activated by the inhibition of erythropoiesis. Consistent with reports that EPO can suppress hepcidin expression (Pak et al., 2006), administration of anti-EPO antibody increased hepatic hepcidin mRNA expression by nearly 2-fold within 72h. This effect was transient as no difference in hepcidin expression was detected after 2 weeks between mice treated with anti-EPO and those treated with vehicle only. Remarkably, even in the presence of anti-EPO antibody, testosterone administration caused a sustained suppression of hepcidin (Fig. 4A). These data indicate that EPO is not essential for mediating the effects of testosterone on hepcidin expression.
5. Effects of testosterone on hypoxia-sensing mechanisms
Testosterone induces a number of physiological adaptations, such as increased hemoglobin, hematocrit, and erythropoietin, and increased tissue capillarity that are similar to those induced by exposure to high altitude or hypoxia. Since hypoxia-inducible factors have been implicated in transcriptional control of hepcidin expression (Gordeuk et al., 2011; Mastrogiannaki et al., 2011; Volke M, 2009), we determined whether down regulation of hepcidin by testosterone is related to activation of the hypoxia-sensing mechanism. Red blood cell 2,3-biphosphoglycerate (2,3-BPG) levels were higher in testosterone-treated than in vehicle-treated mice (supplemental Fig. s4A); which would be expected to shift the oxygen:hemoglobin dissociation curve to favor oxygen dissociation. The hepatic expression of other markers of hypoxia-sensing was either not affected (e.g., miR-21, supplemental Fig. s4B) or down-regulated by testosterone (e.g., miR-210, hypoxia-inducible factor-1α and their target genes VEGF and DMT1, supplemental Fig. s4C). Additionally, hypoxyprobe (pimonidazole, staining revealed reduced levels of hypoxia in the kidney in response to testosterone (supplemental Fig. s4D), indicative of a better state of tissue oxygenation. To ensure that the system was capable of responding to known hypoxic stimuli, we treated female mice with vehicle, CoCl2, or testosterone and measured nuclear HIF1α expression. As expected, CoCl2 was found to increase nuclear HIF1α whereas the opposite was found in animals treated with testosterone (supplemental Fig. s4C). Hence, these data do not support the notion that testosterone induces hypoxia-sensing mechanisms in liver and kidney; instead, it suggests that adaptations induced by testosterone – increased hemoglobin, hematocrit, and 2, 3-BPG - increase tissue oxygen delivery.
6. Androgen receptor (AR) associates with Smad1 and 4, interferes with BMP-Smad signaling, and inhibits hepcidin gene transcription
Bone morphogenetic proteins (BMP) and their downstream targets, Smad1/5/8 and Smad4, are important regulators of hepcidin transcription (Andriopoulos B Jr, 2009; Casanovas G, 2009). In response to BMP-activation, Smad1/5/8 translocates into the nucleus to bind Smad4 and recruits CBP/p300 and other co-factors to form a transcription factor complex, which binds to the conserved BMP-responsive elements located in both proximal (BMP-RE1) and distal (BMP-RE2) sites of the hepcidin gene promoter to facilitate hepcidin transcription (Casanovas G, 2009). Inhibitors of Smad1 are known to down-regulate hepcidin expression (Theurl I, 2011; Yu PB, 2008). Accordingly, we assessed whether testosterone interacts with the BMP/Smad pathway.
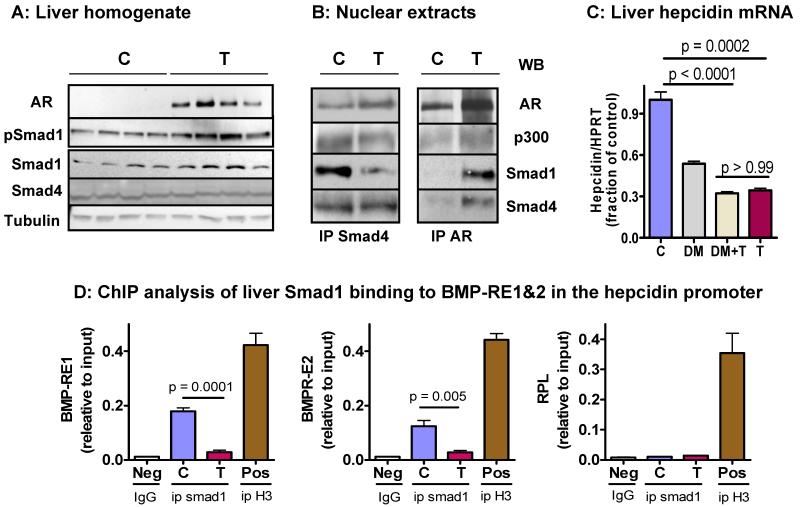
Testosterone administration up-regulated liver AR expression (Fig. 5A). However, testosterone treatment increased, rather than decreased, phospho-Smad1 in the liver. Expression of Smad4, the nuclear binding partner of phospho-Smad1, was not affected (Fig. 5A). The effect of testosterone on Smad1 appears to be post-transcriptional since it did not affect liver mRNA level of Smad1 or related Smad5/8 (supplementary Fig. s5). Testosterone also did not affect liver expression of BMP6, BMP co-receptor hemojuvelin or TMPRSS6 (supplementary Fig. s5). TMPRSS6 is an inactivating serine protease for hemojuvelin (Silvestri et al., 2008). Interestingly, testosterone reduced the liver expression of Smad7 mRNA, a downstream target of Smad1/Smad4 transcription factor complex (Kautz L, 2008). Others have reported that activated AR can disrupt the association between Smad3 and Smad4 via direct binding with each (Kang HY, 2002). We hypothesized that testosterone may facilitate similar interactions between AR and Smad1 or Smad4 in the liver, thereby impairing hepcidin transcription. We performed co-immunoprecipitation of liver nuclear proteins using antibodies against Smad4 and AR. When co-immunoprecipitation was performed using an anti-AR antibody, Western analysis of the immune-complex revealed the presence of Smad1, Smad4, and CBP/p300 as AR-associated proteins. With the increase in AR expression after testosterone injection, the association of AR with Smad 1 and Smad 4 was enhanced (Fig. 5B, right panel). When co-immunoprecipitation was performed using an anti-Smad4 antibody, Western analysis of the immune-complex confirmed the association of Smad 4 with AR, Smad1, and CBP/p300 but the amount of Smad4-associated Smad1 was reduced in the liver of testosterone-treated mice (Fig. 5B, left panel). These results indicate that AR associates with Smad1 and Smad4.
Figure 5. Androgen receptor associates with Smad1 and Smad4, blocks BMP/Smad signaling, and reduces Smad1 binding to the BMP-response elements (BMP-RE1 and BMP-RE2) in the hepcidin promoter.
A: Testosterone administration for 48h upregulated the expression of androgen receptor (AR) and phospho-Smad1, but had no effect on the expression of total Smad1 and Smad4. The results are representative of 3 experiments. Each lane represents the result from one animal.
B: Liver nuclear extracts were immunoprecipitated with goat-anti-Smad4 (left panel) or rabbit-anti-AR (right panel) antibodies. Immune complexes were separated by gel electrophoresis and detected using anti-Smad1, anti-Smad4, anti-CBP/p300, and anti-AR antibodies. Immune complexes immunoprecipitated with anti-Smad4 antibody contained AR, Smad1 and p300 proteins (left panel). Similarly, Immune complexes immunoprecipitated with anti-AR antibody contained Smad4, Smad1, and p300 (right panel). All samples were pre-cleared with agarose gel conjugated with normal goat IgG (for ip with Smad4) or rabbit IgG (for ip with AR) before first antibody was added to the reaction. Equal inputs were confirmed by Western blot for LaminA/C (not shown). Results are representative of 4 experiments. These data provide evidence of association of AR with Smad1, Smad4, and CBP/p300.
C: Liver hepcidin mRNA expression in mice treated with vehicle (C), dorsomorphin (DM) (an inhibitor of BMP/Smad1 signaling), DM plus testosterone (DM+T), or T alone. Administration of DM and T each down-regulated liver hepcidin mRNA expression. However, combination of both did not decrease it any more than T alone, suggesting that T and DM likely share overlapping pathways for hepcidin regulation. Results are mean± SEM, N=4 for each group.
D: Liver tissue isolated from mice treated with vehicle (C) or testosterone (T) was subjected to ChIP analysis. Immuno-precipitation of Smad1 protein-DNA complexes was performed using anti-Smad1 (Cell Signaling#6944). Negative controls (Neg) were immuno-precipitated with rabbit IgG and positive controls (Pos) were immuno-precipitated with rabbit anti-histone 3 (H3). Real-time PCR was performed using primer sets flanking BMP-RE1 and BMP-RE2 of mouse hepcidin promoter. Results were normalized to the corresponding inputs (sonicated chromatin before immuno-precipitation). Treatment with testosterone for 48h reduced the association between Smad1 and the BMP-RE1 (left panel) and BMP-RE2 (middle panel) on the hepcidin promoter. The assay specificity was validated by primers designed for RPL3 intron 2 (Cell Signaling, #7015) which strongly binds to H3 but not Smad1 or rabbit IgG (right panel). Results are mean±SEM, N=3 for each group.
To further investigate the involvement of BMP signaling, we treated mice with testosterone with and without dorsomorphin, an inhibitor of BMP/Smad1 signaling that is known to reduce liver hepcidin expression (Yu PB, 2008). We reasoned that if testosterone disrupts BMP signaling, then combined administration of testosterone and dorsomorphin will not induce additional suppression of hepcidin than that induced by testosterone alone. Indeed, treatment with dorsormorphin and testosterone each decreased liver hepcidin mRNA expression (Fig. 5C); but a combination of dorsormorphin and testosterone did not decrease hepcidin expression more than testosterone alone, suggesting that testosterone and dorsomorphin pathways overlap for hepcidin regulation.
To test this hypothesis further, we performed chromatin immuno-precipitation (ChIP) assay in liver tissue isolated from testosterone- and vehicle-treated female mice, using anti-Smad1 and anti-Smad4 antibodies. The DNA fragments immuno-precipitated using anti-Smad1 or anti-Smad4 antibodies were subjected to real-time PCR using primer sets that flanked the BMP/Smad-response elements (BMP-RE1, −84/−79 bp; and BMP-RE2, −2,255/−2,250 bp) of mouse hepcidin promoter (Casanovas G, 2009). As expected, Smad1-associated DNA fragments containing either BMP-RE1 or BMP-RE2 were amplified to a much greater extent in the control than in testosterone-treated mice (Fig. 6D), whereas no difference was found between the two groups when a DNA fragment containing non-specific ribosomal protein 3 intron 2 was amplified. The positive control, anti-histone 3, was found to robustly pull down all three DNA fragments tested (Fig. 6D), as would be expected. Similar results were found when the ChIP analysis was performed using anti-Smad4 antibody (Supplemental Fig. s6). Thus, testosterone treatment reduced the protein-DNA binding between Smad1, as well as Smad4, and the BMP/Smad-response elements in the hepcidin promoter.
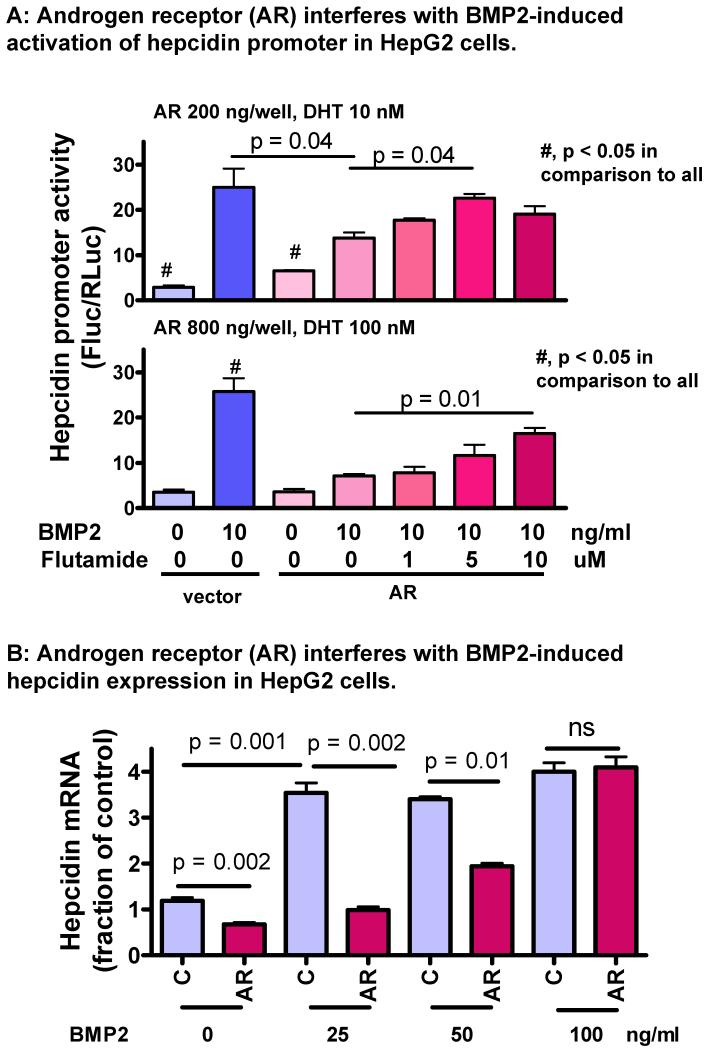
Figure 6. Androgen receptor attenuates BMP2-induced activation of hepcidin promoter activity and BMP2-induced hepcidin mRNA expression in HepG2 cells.
A: HepG2 cells were transfected with control vector, low dose (200 ng/well), and high dose (800 ng/well) of AR encoding plasmid, all in combination with a pGL4 luciferase reporter driven by a 3kb wild-type hepcidin promoter (300 ng/well). A CMV-driven Renilla luciferase vector (30 ng/well) was used as transfection control. 12h after transfection, cells were switched to 1% FBS in low glucose DMEM containing graded dose of DHT and flutamide. After 12h, BMP2 (10 ng/ml) was added to selected wells and incubation was extended for 12h. Results were normalized to Renilla luciferase activity. Experiments were repeated 3 times. Data are mean±SEM.
B: HepG2 cells were transfected with control vector or AR-encoding plasmid (800 ng/well). The AR-transfected cells were treated with DHT (100nM). BMP2 was added at 0, 25, 50, and 100ng/ml and incubation was extended for 4h. Hepcidin mRNA was analyzed by real-time PCR and normalized to HPRT. Results are mean±SEM, N=4 for each treatment.
To determine the role of androgen receptor (AR) in regulation of BMP/Smad-induced hepcidin promoter activity, we performed reporter assay in HepG2 cells. Because endogenous AR is expressed in low abundance in HepG2 cells (Jie et al., 2007), the cells were transfected with different doses of AR-encoding plasmid in combination with graded concentrations of AR ligand dihydrotestosterone (DHT) without and with AR antagonist flutamide. Flutamide alone had no effect on basal or BMP2-induced hepcidin promoter activity (supplemental Fig. s7). As expected, AR/DHT suppressed BMP2-induced hepcidin promoter activity. The suppression by low dose AR/DHT was largely reversed by flutamide at 5 uM, while the effect of a high dose AR/DHT was partially reversed by flutamide at 10 uM (Fig. 6A). Thus, AR/DHT down-regulated BMP-induced hepcidin transcriptional activity.
We further tested the effect of AR/DHT on BMP2-induced hepcidin mRNA expression. In HepG2 cells treated with high dose AR/DHT, basal hepcidin mRNA expression was reduced (Fig. 6B). In the absence AR/DHT, BMP2 at 25 ng/ml induced a maximal increase of hepcidin mRNA, which did not increase further when BMP2 was increased to 50 and 100 ng/ml. Co-treatment with AR/DHT completely abolished the increase of hepcidin transcription induced by BMP2 at 25 ng/ml, but this inhibitory effect of AR/DHT was attenuated at 50 ng/ml BMP2 and completely lost at 100 ng/ml BMP2. Therefore, our data collectively demonstrate that liganded AR suppresses hepcidin transcription by attenuating BMP/Smad signaling.
Finally, we tested the effect of AR/DHT on hepcidin mRNA stability in HepG2 cells as it might also contribute to the overall mRNA abundance. After mRNA synthesis was blocked by actinomycin D, hepcidin mRNA levels decreased rapidly in both control cells and AR/DHT-treated cells (Supplemental Fig. s8), but the slopes of decay were not significantly different. Hence, testosterone-mediated regulation of hepcidin expression occurred primarily at the level of gene transcription.
Discussion
We have identified hepcidin, the iron regulating hormone, as a downstream target of AR. Testosterone administration suppresses hepcidin expression in both male and female mice (this work), and circulating hepcidin levels in men (Bachman E, 2010). The suppression of hepcidin and the associated up-regulation of ferroportin increase iron export from the spleen thus increasing iron availability for heme/hemoglobin synthesis in erythroid cells. Several lines of evidence presented here indicate that testosterone stimulates erythropoiesis and affects iron flux in the body. Testosterone administration increases reticulocyte count, a marker of erythropoiesis. Testosterone increases serum iron and transferrin saturation; furthermore, reticulocyte hemoglobin ratio, a marker of iron available for hemoglobin synthesis, also is increased by testosterone administration. Testosterone increases iron recycling and export from the spleen, as indicated by increased expression of ferroportin in the spleen, reduced splenic ferric iron stores, and increased serum iron and increased incorporation of transferrin-bound 58Fe into the red blood cells of testosterone-treated mice compared to controls. Testosterone-mediated suppression of hepcidin was associated with increased ferroportin expression in the spleen, consistent with a previous report that hepcidin selectively regulates ferroportin expression in splenic macrophages (Chaston T, 2008). Furthermore, sera from testosterone-treated mice induced significantly greater hemoglobin accumulation in K562 cells induced towards erythroid differentiation than sera from vehicle-treated mice.
Our data provide novel insights into the mechanisms by which testosterone suppresses hepcidin expression. We show that testosterone blocks BMP/Smad signaling to inhibit hepcidin gene transcription. When administered in combination with the BMP/Smad inhibitor dorsomorphin, testosterone did not induce greater suppression of hepcidin expression than that associated with testosterone alone. We provide evidence that testosterone injection promotes the association between AR and Smad1 as well as AR and Smad4 but reduces the association between Smad1 and Smad4. We further show that testosterone administration is associated with a substantial reduction in the protein-DNA binding between Smad1/4 and the two conserved BMR-response elements in the hepcidin promoter in the liver. Using reporter assay, we show that activated AR dose-dependently inhibits BMP2-induced hepcidin promoter activity, an effect that was blocked by AR antagonist flutamide in a dose-dependent manner. Finally, AR-mediated suppression of hepcidin expression in HepG2 cells was dose-dependently reversed by co-treatment with BMP2, providing direct evidence that AR interacts with BMP signaling pathway to regulate hepcidin expression. Taken together, these data provide strong evidence that testosterone directly regulates hepatic hepcidin transcription through direct interactions between liganded AR and BMP/Smad pathways.
Although EPO has been shown to be an important regulator of hepcidin (Lainé F, 2011; Pak M, 2006) and is transiently stimulated by testosterone, testosterone suppresses hepcidin even when EPO action is blocked effectively by administration of an anti-EPO neutralizing antibody. Similarly, we show that hypoxia-sensing mechanisms are not the likely mediators of testosterone’s actions; testosterone increases net oxygen delivery as indicated by increased hemoglobin, hematocrit, and 2,3-DPG, and the observed suppression of several hypoxia-sensing markers. Another mechanism known to regulate hepcidin expression is inflammation-related IL6 and activation of Stat3 (Ganz et al., 2009). Since our animals were young and healthy, and showed no detectable change in liver IL6 levels with testosterone administration (data not shown), the possible involvement of this pathways was not investigated further.
Testosterone administration did not increase hemoglobin in hepcidin transgenic mice. Pharmacological inhibition of hepcidin expression reverses anemia of chronic inflammation in mice (Sasu et al., 2010). However, hepcidin deficiency in mice and in humans does not cause polycythemia, suggesting that suppression of hepcidin alone cannot fully explain the increase in hemoglobin and hematocrit observed in response to testosterone administration in mice and humans; additional mechanisms are likely operative. It is remarkable that in spite of increased hemoglobin and hematocrit, EPO levels were not suppressed. Therefore, it is possible that increased EPO levels even in the face of increased hematocrit and hemoglobin, increased EPO sensitivity, or yet additional mechanisms that were not investigated may play an important role in mediating testosterone’s effects on erythropoiesis. Similarly, we recognize that hypoxia-sensing mechanisms are important and dynamic regulators of erythropoiesis, hepcidin and erythropoietin expression; their role cannot be fully excluded and requires further investigation.
These data have important clinical implications. Erythrocytosis is the most frequent adverse event associated with testosterone administration in clinical trials as well as in clinical practice. An understanding of the underlying mechanisms by which testosterone stimulates hemoglobin might provide novel strategies to prevent excessive increase in hemoglobin and hematocrit during testosterone administration. Testosterone or its analogs, or the signaling pathway unveiled here that mediate testosterone’s effects, should also be explored as potential therapeutic targets for the treatment of anemia of chronic disease or aging.
Methods
1. Animals
Male and female mice (3-month old) were purchased from Jackson Laboratories (Bar Harbor, ME). Hepcidin transgenic mice (3 month old) (Roy CN, 2007) were backcrossed 10 generations onto C57BL/6 background. Liver-specific expression of tetracycline-responsive hepcidin transgene [TgN(TRE.mhepcidin1)] is driven by tetracycline-transactivator [TgN(tTALAP)5Uh, Tet-Off] which is restricted to hepatocytes by expression through CCAAT/enhancer binding protein-β promoter. Control mice (Tg-) were littermates that did not carry the Tet-off transgene. The mice were provided a standard diet containing 400-ppm iron. Boston University’s IACUC approved the protocol.
2. Testosterone administration
Testosterone was administered either by subcutaneous injections (1mg in 100uL sesame oil, daily for short-term and twice weekly for long-term treatments) or in some experiments by subcutaneous implantation of 2-mm silicone tubing containing testosterone (Dow Corning, #508-006). 12 mg testosterone was included in 1-cm of tubing. The control animals received injections of 100-uL sesame oil or implants of empty tubing.
3. Measurement of 58Fe incorporation into red cells
Female mice were injected with testosterone or placebo twice weekly for 2 weeks. 58Fe was obtained from Oakridge National Laboratory and complexed with apotransfferin (www.sigma-Aldrich.com) (Ponka et al., 1985). 58Fe/transferrin complex was injected into tail vein at 10 ng/g body weight. Blood was drawn from cheek vein after 8h, washed with saline, and red cells were digested by HNO3/H2O2 (2:1 v/v), dried, and reconstituted in 5% HNO3 for MC-ICPMASS measurement of 58Fe/56Fe ratio. The amount of iron incorporated into red cells was calculated using specific activity of 58Fe in serum iron pool size.
4. Hemoglobin accumulation in K562 cells
K562 erythroleukemia cells were incubated in RPMI 1640 with 10% fetal bovine serum and induced to differentiate by sodium butyrate (0.5 mM) for 48 h (Kawasaki et al., 1996). Cells were washed with serum-free medium, seeded at 2.5x105 cells/ml, and incubated with serum from female mice treated with vehicle or testosterone or in serum-free medium. Cells were harvested after 24h and stained with benzidine. 4x106 cells were lysed with 0.2% triton and centrifuged, and supernatant was incubated with benzidine and quantified by OD600.
4. Experiments Using anti-EPO antibody
Adult female mice were randomized to 1 of 4 groups: vehicle-treated controls, testosterone alone, anti-EPO antibody alone, and testosterone plus anti-EPO antibody. Rat anti-mouse EPO monoclonal antibody (R&D Systems#MAB959), was injected subcutaneously at 2 ug/g body weight/day. This dose was effective in blocking phlebotomy-induced erythropoiesis in mice (not shown). Mice were euthanized after either 72h or 2-weeks. Liver and kidney were analyzed for hepcidin and EPO mRNA expression.
5. Experiment with dorsomorphin
We treated mice with testosterone or vehicle injections for 48h. Dorsomorphin (20 ug/kg, Santa Cruz Biotechnology), an inhibitor of BMP/Smad signaling (Yu PB, 2008), or vehicle was injected intraperitoneally 3h before euthanasia. Liver was harvested for analysis of hepcidin mRNA.
6. Blood analysis
Complete blood counts were performed using an ADVIA 120 analyzer (Bayer, Tarrytown, NY). Erythrocyte 2,3-biphosphoglycerate was measured using commercial reagents (Roche #10148334001). Erythropoietin (EPO) level was measured by an immunoassay (R&D systems#MEP00).
Serum iron and total iron binding capacity (TIBC) were determined using Iron/TIBC kit (ThermoDMA, Arlington, TX). Tissue nonheme iron concentration was measured as described (Rebouche et al., 2004).
7. Real-time PCR
Real time PCR was conducted on the ABI 7500 PCR system (Applied Biosystems) using SYBR Green qPCR master mix (Promega#A6001) and mRNA expression levels were normalized to HPRT expression level
8. Measurement of MicroRNA
Total RNA was extracted using miRNeasy Mini Kit (Qiagen #217004). Expression of target miRNA was analyzed by real-time PCR (Baggish et al., 2011).
9. Tissue iron staining
Spleen was fixed in PBS-buffered formalin, cut into 5um sections, and stained in 5% potassium ferrocyanide freshly prepared in 10% HCl. The sections were counterstained with 5% hematoxylin. Ferric iron staining was quantified using NIH Image J Program.
10. Hypoxyprobe staining
24h after testosterone/vehicle injection, hypoxyprobe (pimonidazole HCl) solution was injected intraperitoneally (60 mg/kg body weight) 1h before euthanasia. Cardiac perfusion of 10% formalin was performed before tissue harvesting. Sections of 5uM were deparaffinized and stained with pimonidazole.
11. Cell cultures and transfections
HepG2 cells were cultured in 24-well plate with Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum and antibiotics. Full length human androgen receptor cDNA (pCMV5AR) was provided by Dr. Elizabeth Wilson and empty vector pCMV5 by Dr. David Russell. A pGL4 luciferase reporter driven by a 3kb wild-type hepcidin promoter was constructed as described (Casanovas G, 2009). Control pRL-CMV Vector was used to control for transfection efficiency. Graded doses of plasmids were transfected into HepG2 cells using GenJet™ In Vitro DNA Transfection Reagent (SigaGen#SL100489-HEPG2). 12h after transfection, the cells were switched to DMEM containing 1% FBS and DHT and flutamide. After another 12h, BMP2 was added and the incubation was extended for another 12h. Hepcidin promoter activity was measured by the promoter-driven firefly luciferase activity normalized to constitutive Renilla luciferase activity (Promega E1910). In separate experiments, HepG2 cells were transfected with AR-encoding plasmid (800 ng/well) and treated with DHT (100 nM) and BMP2 or actinomycin D (1 ug/ml) and cells were harvested at different time points after treatment to assess hepcidin mRNA expression.
12. Western analysis and co-immunoprecipitation
Spleen and liver tissue, rapidly frozen in liquid N2, were pulverized on dry ice and homogenized in RIPA buffer containing phosphatase and protease inhibitors. Antibodies were purchased from Santa Cruz (AR #sc-13062, beta-tubulin #sc9104, Smad4 #sc7966), Abcam (ferroportin #ab78066), BD Pharmingen (CBP/p300 #554215), and Cell Signaling (Smad1 #9511, Smad4 #9515, phosphor-Smad1/5/8 #9743). For co-immunoprecipitation experiments, the liver tissue was homogenized in 20-volumes of a low-salt solution containing 10mM Hepes pH7.5, 2mM MgCl2, 1mM EDTA, 1mM EGTA, 10mM KCl, 10mM NaF, 1mM Na3VO4, 1mM DTT and 1mM PMSF. The homogenate was filtered and IGEPAL was added to achieve a final concentration of 0.25%, mixed and centrifuged at 1000g for 20min at 4C. The pellet was washed with low salt buffer and re-suspended in equal volume of high salt buffer (25mM Hepes pH7.5, 500mM NaCl, 10mM NaF, 10% Glycerol, 0.2% NP40, 5mM MgCl, 1mM Na3VO4, 1mM DTT, 1mM PMSF). After incubation on ice for 15 min, the mixture was sonicated, and diluted in low salt solution to lower total salt concentration and centrifuged at 14,000g × 20min at 4°C. One milligram of supernatant was pre-cleared with goat-IgG-AC (sc-2346, for use with goat-anti-Smad4) or rabbit-IgG-AC (sc-2345, for use with rabbit-anti-AR) for one hour. Sample was incubated with goat-anti-Smad4 or rabbit-anti-AR overnight. Protein A/G agarose (sc-2003) was added to the protein-antibody mixture and incubation was continued for 4h at 4°C. Agarose beads were washed with PBS containing 0.05% IGEPAL 5 times and boiled in SDS buffer followed by Western analysis.
13. Chromatin immunoprecipitation (ChIP) assay
Liver tissue isolated from female animals 48h after testosterone injection was processed using SimpleChIP® Plus Enzymatic Chromatin IP Kit (Cell Signaling #9005) (Figure s9). The chromatin fraction equivalent to 10ug DNA was incubated with 5ul rabbit anti-Smad1 antibody (Cell Signaling #6944) or 6ug mouse anti-Smad4 antibody (Santa Cruz #sc-7966) with rotation overnight at 4°C. ChIP-grade protein G magnetic beads were added and samples rotated for another 4h at 4°C. Positive control was incubated with rabbit anti-histone 3 (H3) antibody and negative control was incubated with normal rabbit IgG. A 10% fraction of each sample was set aside as input reference that was processed in parallel but without the antibody incubations. After the reversal of cross-linking, DNA from each ip reaction or its corresponding input was eluted into 60ul of elution buffer. The antibody-associated DNA was quantified by real-time PCR using 3ul of each DNA template and primer sets designed for BMP-RE1 (F: gctggctgtaggtgacacaa, R: acccccattttgctctgact) and BMP-RE2 (F: cagtctgtcgcctcaaattgt, R: caaccatcactgtggcttct), and control primer set for RPL30 Intron 2 (Cell Signaling #7015). The sample intensity of amplified DNA fragments was normalized to the values of corresponding inputs.
14. Statistical Analyses
The results are presented as mean±SEM. For experiments that include more than two groups, comparison was conducted using one-way ANOVA followed by Tukey’s test, which acknowledges multiple comparisons. Simple comparisons between control and testosterone-treated animals were performed using Student’s T-test for indepndent samples. For assessment of modification of testosteroene effects by other factors, multiple regression analyses were employed, with statistical signficance determined according to Wald-type tests of estimated interaction effects. Where multiple measurements were obtained over time, linear trends in responses were not assumed.
Supplementary Material
Acknowledgements
Grant support: NIH grants 5RO1AG037193 and 1UO1AG14369 to SB; R21AG037859, R01DK056690, and Department of Medicine Bridge grant to WG; and R01DK082722 to CNR. We thank Dr. Elizabeth Wilson for providing mouse AR cDNA.
References
- Andriopoulos B, Jr, Corradini E, Xia Y, Faasse SA, Chen S, Grgurevic L, Knutson MD, Pietrangelo A, Vukicevic S, Lin HY, Babitt JL. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009;41:482–7. doi: 10.1038/ng.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachman E, Feng R, Travison T, Li M, Olbina G, Ostland V, Ulloor J, Zhang A, Basaria S, Ganz T, Westerman M, Bhasin S. Testosterone suppresses hepcidin in men: a potential mechanism for testosterone-induced erythrocytosis. J Clin Endocrinol Metab. 2010;95:4743–4747. doi: 10.1210/jc.2010-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggish AL, Hale A, Weiner RB, Lewis GD, Systrom D, Wang F, Wang TJ, Chan SY. Dynamic regulation of circulating microRNA during acute exhaustive exercise and sustained aerobic exercise training. J Physiol. 2011;589:3983–3994. doi: 10.1113/jphysiol.2011.213363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran M, Spitzer G, Verma DS. Testosterone and synthetic androgens improve the in vitro survival of human marrow progenitor cells in serum-free suspension cultures. J Lab Clin Med. 1982;99:247–53. [PubMed] [Google Scholar]
- Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- Casanovas G, Mleczko-Sanecka K, Altamura S, Hentze MW, Muckenthaler MU. Bone morphogenetic protein (BMP)-responsive elements located in the proximal and distal hepcidin promoter are critical for its response to HJV/BMP/SMAD. J Mol Med (Berl) 2009;87:471–80. doi: 10.1007/s00109-009-0447-2. [DOI] [PubMed] [Google Scholar]
- Chaston T, Chung B, Mascarenhas M, Marks J, Patel B, Srai SK, Sharp P. Evidence for differential effects of hepcidin in macrophages and intestinal epithelial cells. Gut. 2008;57:374–82. doi: 10.1136/gut.2007.131722. [DOI] [PubMed] [Google Scholar]
- Coviello AD, Kaplan B, Lakshman KM, Chen T, Singh AB, Bhasin S. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J Clin Endocrinol. Metab. 2008;93:914–919. doi: 10.1210/jc.2007-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Maggio M, Bandinelli S, Basaria S, Lauretani F, Ble A, Valenti G, Ershler WB, Guralnik JM, Longo DL. Low testosterone levels and the risk of anemia in older men and women. Arch Intern Med. 2006;166:1380–1388. doi: 10.1001/archinte.166.13.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbane S, Galgano C, Langley RC, Jr, Canfield W, Maesaka JK. Reticulocyte hemoglobin content in the evaluation of iron status of hemodialysis patients. Kidney Int. 1997;52:217–22. doi: 10.1038/ki.1997.323. [DOI] [PubMed] [Google Scholar]
- Ganz T, Nemeth E. Iron sequestration and anemia of inflammation. Semin Hematol. 2009;46:387–393. doi: 10.1053/j.seminhematol.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011;117:4425–33. doi: 10.1182/blood-2011-01-258467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordeuk VR, Miasnikova GY, Sergueeva AI, Niu X, Nouraie M, Okhotin DJ, Polyakova LA, Ammosova T, Nekhai S, Ganz T, Prchal JT. Chuvash polycythemia VHLR200W mutation is associated with down-regulation of hepcidin expression. Blood. 2011;118:5278–5282. doi: 10.1182/blood-2011-03-345512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara N, Nishiyama T, Takizawa I, Saito T, Kitamura Y, Takahashi K. Decline of the red blood cell count in patients receiving androgen deprivation therapy for localized prostate cancer: impact of ADT on insulin-like growth factor-1 and erythropoiesis. Urology. 2010;75:1441–1445. doi: 10.1016/j.urology.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Jie X, Lang C, Jian Q, Chaoqun L, Dehua Y, Yi S, Yanping J, Luokun X, Qiuping Z, Hui W, Feili G, Boquan J, Youxin J, Jinquan T. Androgen activates PEG10 to promote carcinogenesis in hepatic cancer cells. Oncogene. 2007;26:5741–5751. doi: 10.1038/sj.onc.1210362. [DOI] [PubMed] [Google Scholar]
- Kang HY, Huang KE, Chang SY, Ma WL, Lin WJ, Chang C. Differential modulation of androgen receptor-mediated transactivation by Smad3 and tumor suppressor Smad4. J Biol Chem. 2002;277:43749–56. doi: 10.1074/jbc.M205603200. [DOI] [PubMed] [Google Scholar]
- Kautz L, Meynard D, Monnier A, Darnaud V, Bouvet R, Wang RH, Deng C, Vaulont S, Mosser J, Coppin H, Roth MP. Iron regulates phosphorylation of Smad1/5/8 and gene expression of Bmp6, Smad7, Id1, and Atoh8 in the mouse liver. Blood. 2008;112:1503–9. doi: 10.1182/blood-2008-03-143354. [DOI] [PubMed] [Google Scholar]
- Kawasaki N, Morimoto K, Tanimoto T, Hayakawa T. Control of hemoglobin synthesis in erythroid differentiating K562 cells. I. Role of iron in erythroid cell heme synthesis. Arch Biochem Biophys. 1996;328:289–294. doi: 10.1006/abbi.1996.0175. [DOI] [PubMed] [Google Scholar]
- Lainé F, Laviolle B, Ropert M, Bouguen G, Morcet J, Hamon C, Massart C, Westermann M, Deugnier Y, Loréal O. Early effects of erythropoietin on serum hepcidin and serum iron bioavailability in healthy volunteers. Eur J Appl Physiol. 2011;112:1391–7. doi: 10.1007/s00421-011-2097-7. [DOI] [PubMed] [Google Scholar]
- Mastrogiannaki M, Matak P, Mathieu JR, Delga S, Mayeux P, Vaulont S, Peyssonnaux C. Hepatic hypoxia-inducible factor-2 down-regulates hepcidin expression in mice through an erythropoietin-mediated increase in erythropoiesis. Haematologica. 2011;97:827–834. doi: 10.3324/haematol.2011.056119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirand EA, Gordon AS, Wenig J. Mechanism of testosterone action in erythropoiesis. Nature. 1965;206:270–272. doi: 10.1038/206270a0. [DOI] [PubMed] [Google Scholar]
- Mirand EA, Murphy GP. Erythropoietin activity in anephric humans given prolonged androgen treatment. J Surg Oncol. 1971;3:59–65. doi: 10.1002/jso.2930030111. [DOI] [PubMed] [Google Scholar]
- Moriyama Y, Fisher JW. Effects of testosterone and erythropoietin on erythroid colony formation in human bone marrow cultures. Blood. 1975;45:665–70. [PubMed] [Google Scholar]
- Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006;108:3730–5. doi: 10.1182/blood-2006-06-028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponka P, Schulman HM. Acquisition of iron from transferrin regulates reticulocyte heme synthesis. J Biol Chem. 1985;260:14717–14721. [PubMed] [Google Scholar]
- Ponka P. Tissue-specific regulation of iron metabolism and heme synthesis: distinct control mechanisms in erythroid cells. Blood. 1997;89:1–25. [PubMed] [Google Scholar]
- Rebouche CJ, Wilcox CL, Widness JA. Microanalysis of non-heme iron in animal tissues. J Biochem Biophys Methods. 2004;58:239–251. doi: 10.1016/j.jbbm.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Roe MA, Heath AL, Oyston SL, Macrow C, Hoogewerff JA, Foxall R, Dainty JR, Majsak-Newman G, Willis G, Fairweather-Tait SJ. Iron absorption in male C282Y heterozygotes. Am J Clin Nutr. 2005;81:814–821. doi: 10.1093/ajcn/81.4.814. [DOI] [PubMed] [Google Scholar]
- Roy CN, Mak HH, Akpan I, Losyev G, Zurakowski D, Andrews NC. Hepcidin antimicrobial peptide transgenic mice exhibit features of the anemia of inflammation. Blood. 2007;109:4038–4044. doi: 10.1182/blood-2006-10-051755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasu BJ, Cooke KS, Arvedson TL, Plewa C, Ellison AR, Sheng J, Winters A, Juan T, Li H, Begley CG, Molineux G. Antihepcidin antibody treatment modulates iron metabolism and is effective in a mouse model of inflammation-induced anemia. Blood. 2010;115:3616–3624. doi: 10.1182/blood-2009-09-245977. [DOI] [PubMed] [Google Scholar]
- Shahidi NT, Diamond LK. Testosterone-induced remission in aplastic anemia. AMA J Dis Child. 1959;98:293–302. doi: 10.1001/archpedi.1959.02070020295002. [DOI] [PubMed] [Google Scholar]
- Silvestri L, Pagani A, Nai A, De Domenico I, Kaplan J, Camaschella C. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008;8:502–511. doi: 10.1016/j.cmet.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava RC, Khan S, Shankar U, Pandya KP. Distribution of Fe59 in benzene and iomex treated rats. Arch Toxicol. 1979;42:43–49. doi: 10.1007/BF00351823. [DOI] [PubMed] [Google Scholar]
- Theurl I, Schroll A, Sonnweber T, Nairz M, Theurl M, Willenbacher W, Eller K, Wolf D, Seifert M, Sun CC, Babitt JL, Hong CC, Menhall T, Gearing P, Lin HY, Weiss G. Pharmacologic inhibition of hepcidin expression reverses anemia of chronic disease in rats. Blood. 2011;118:4977–84. doi: 10.1182/blood-2011-03-345066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volke M, Gale DP, Maegdefrau U, Schley G, Klanke B, Bosserhoff AK, Maxwell PH, Eckardt KU, Warnecke C. Evidence for a lack of a direct transcriptional suppression of the iron regulatory peptide hepcidin by hypoxia-inducible factors. PLoS One. 2009;4:e7875. doi: 10.1371/journal.pone.0007875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkinson G, McMenemey WH, Evans G. Hypopituitarism, hypogonadism, and anaemia treated with testosterone. Lancet. 1947;1:631–633. [PubMed] [Google Scholar]
- Yeap BB, Beilin J, Shi Z, Knuiman MW, Olynyk JK, Bruce DG, Milward EA. Serum testosterone levels correlate with haemoglobin in middle-aged and older men. Intern Med J. 2009;39:532–538. doi: 10.1111/j.1445-5994.2008.01789.x. [DOI] [PubMed] [Google Scholar]
- Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.