Abstract
OBJECTIVES
To examine receipt of mammography screening by life expectancy (LE) among women ≥75 years.
DESIGN/SETTING/PARTICIPANTS
Cross-sectional survey of community dwelling US women ≥75 years that participated in the 2008 or 2010 National Health Interview Survey.
MEASUREMENTS
Using an index we previously developed and validated, we categorized women according to life expectancy (>9-years, 5–9 years, <5-years). We examined receipt of mammography screening in the past 2 years by life expectancy adjusting for sociodemographics, access to care, preventive orientation (e.g., receipt of flu vaccination), and receipt of a clinician recommendation for screening.
RESULTS
Of 2,266 respondents, 27.1% had >9-years LE, 53.4% had 5–9 years LE, and 19.5% had <5-years LE. Overall, 55.7% reported receiving mammography screening in the past 2 years. Life expectancy was strongly associated with receipt of screening (p<0.001); yet, 36.1% of women with <5-years LE were screened while 29.2% of women with >9-years LE were not screened. A clinician recommendation for screening was the strongest predictor of screening independent of life expectancy. Higher educational attainment, age, receipt of flu vaccination, and history of benign breast biopsy, were also independently associated with being screened.
CONCLUSION
Despite uncertainty of benefit, many women >75 years are screened with mammography. Life expectancy is strongly associated with receipt of screening which may reflect clinicians and patients appropriately considering LE in screening decisions. However, 36% of women with short LEs are still screened suggesting new interventions are needed to further improve targeting of screening by LE. Patient decision aids and guidelines encouraging clinicians to consider patient LE in screening decisions may improve care.
Keywords: mammography screening, older women, life expectancy
INTRODUCTION
The incidence of breast cancer peaks between ages 75–79 with ~442 cases/100,000 women.(1) Based on population projections, the number of women ≥75 years diagnosed with breast cancer will nearly double by 2030.(2) Yet, none of the randomized trials evaluating mammography screening included women ≥75 years and it is not certain that mammography helps these women live longer.(3) Three observational studies found a mortality benefit for mammography screening among women ≥75 years in good health but not among those with substantial comorbidity.(4–6) Among younger women mammography screening is thought to reduce breast cancer mortality by 15%.(7,8) The number needed to screen (NNS) very healthy women ≥75 years to prevent one breast cancer related death may be similar to the NNS 50 year old women (~225).(3) Even if screening does not reduce mortality for older women, screening may benefit some by detecting breast cancers early that otherwise would have resulted in major morbidity had treatment been delayed. However, screening also has potential for harm, including: discomfort; anxiety; complications from biopsies following false positive tests; false reassurance from false negative tests; and overdiagnosis (diagnosis of tumors that are of no threat).(3) Overdiagnosis increases with age due to competing mortality risks, shorter life expectancies, and slower growing tumors.(3) Complications from treatment also increase with age.(9,10)
Major guideline organizations, such as the United States Preventive Services Task Force (USPSTF), state that there is insufficient evidence on whether or not to screen women ≥75 years with mammography (11) and experts report that women need 5 to 10 years life expectancy to potentially benefit.(12,13) Despite these recommendations, Medicare has covered biennial screening mammograms for women ≥65 years regardless of health since 1991 and annual mammograms since 1998. Subsequently, screening increased among older women, including those in poor health.(14–19) In general, 24–43% of women in poor health are screened compared to 60–70% of older women in excellent health.(14–19) Despite the importance of life expectancy in determining older women’s likelihood of benefiting from screening, few studies have examined receipt of mammography screening by life expectancy,(15,19) and none have used a validated measure that predicts life expectancy beyond 4-years nor examined data since 2004.
In this context, we examined mammography screening among women ≥75 years in 2008 and 2010 by life expectancy using a validated measure that predicts up to 9-years mortality and we examined whether other factors are associated with screening these women.(20,21) These data are needed to design interventions to improve targeting of mammography screening to older women by life expectancy.
METHODS
We used data from 2008 and 2010 National Health Interview Surveys (NHIS – an in-person household survey of civilian, non-institutionalized US population).(22) One randomly selected adult from each responding family was asked to complete the sample adult module, which included detailed questions about illness and health behaviors and in 2008 included a limited set of questions about cancer screening. In 2010, sample adult respondents also completed a cancer module, which included detailed questions related to cancer screening. Response rates for sample adults were 62.6% in 2008 and 60.8% in 2010.
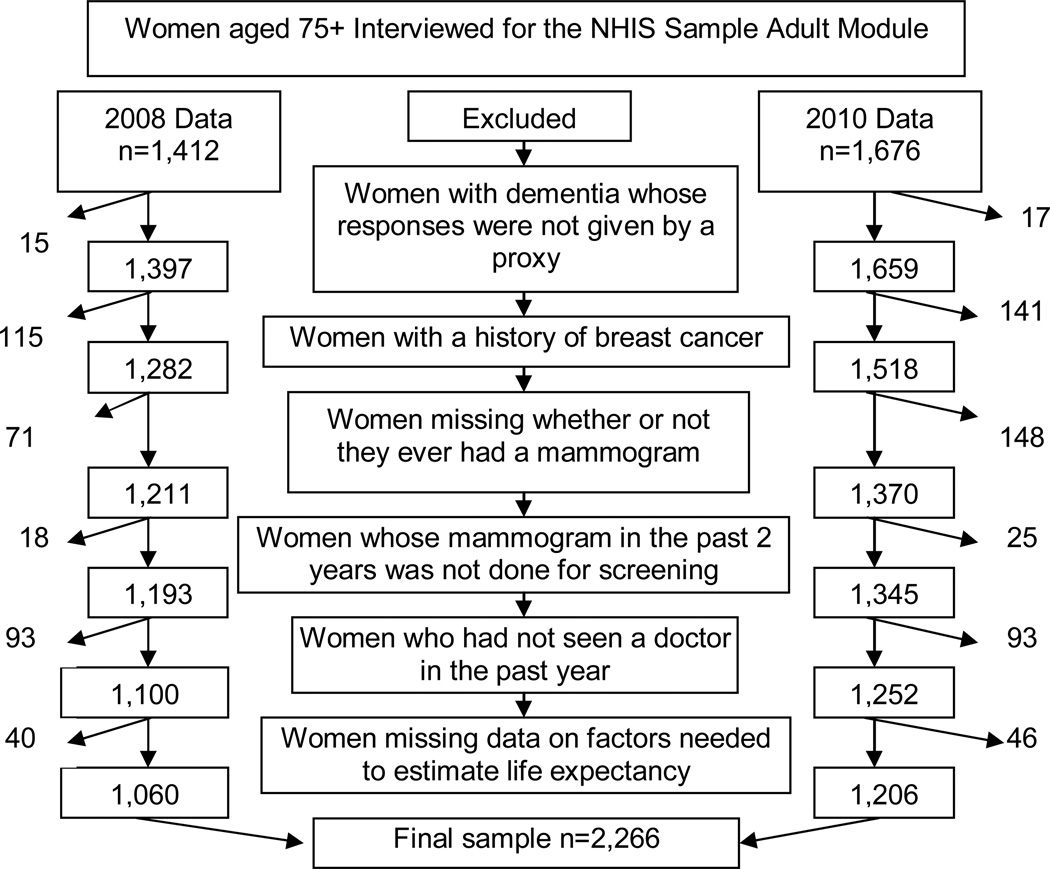
Sample (Figure 1)
Figure 1.
Study Sample
Overall, 3,088 women ≥75 years responded to the sample adult modules in 2008 and 2010. We excluded women: 1) that reported having dementia but did not have a proxy complete their interview since surveys are based on self-report (n=32); 2) with a history of breast cancer (n=256); 3) that did not answer whether or not they ever had a mammogram (n=219); 4) whose most recent mammogram was not done for screening purposes (n=43); 5) that had not seen a doctor in the past year (n=186); and 6) missing data necessary to calculate life expectancy (n=86). Our final sample consisted of 2,266 women, representing an estimated 7.7 million US community-dwelling women ≥75 years.
Outcome
Our primary outcome was receipt of mammography screening in the past two years.(10)
Life Expectancy
We used a mortality index that we previously developed and validated that predicts up to 9-year mortality.(20,21) The index includes 11 factors (age, sex, body mass index [BMI], perceived health, functional dependency, mobility, history of chronic obstructive pulmonary disease [COPD]/emphysema, cancer, diabetes, tobacco use, and hospitalizations in the past year). Based on the presence or absence of these factors, we calculated a mortality risk score for each participant. Participants at low mortality risk (0–7 points) have an 8% (95% CI 7–8%) risk of 5-year mortality and a 19% (17–20%) risk of 9-year mortality. Participants at medium risk (8–13 points) have a 27% (25–28%) risk of 5-year mortality and a 50% (48–52%) risk of 9-year mortality. Participants at high risk (≥14 points) have a 57% (55–59%) risk of 5-year mortality and an 84% (80–87%) risk of 9-year mortality. Adults with >50% mortality risk over a given time frame are generally expected to have a life expectancy less than the given time frame.(23) Therefore, women categorized as low risk have >9-year life expectancy while women categorized as high risk have <5-year life expectancy.
Covariates
We also considered sociodemographic factors (race/ethnicity, education, insurance, geographical region, marital status, and annual family income [imputed by NHIS]), indicators of access to care (usual source of medical care and number of doctor visits in the past year) and orientation towards preventive healthcare (receipt of flu vaccine in the past year and pneumonia vaccine ever).(12–17) Although only assessed in 2010, we also considered a history of a benign breast biopsy, family history (parent/sibling/child) and perceived risk of breast cancer on receipt of screening.
Clinician Recommendation
We further considered clinician recommendations for mammography. All women interviewed in 2008 and only women who had not been screened in the past two years in 2010 were asked whether their clinician recommended a mammogram in the past 12 months. In 2010, women who were screened in the past two years were asked if their most recent mammogram was clinician recommended.
Reasons for Not Being Screened
In 2010, women who reported not being screened in the past two years (n=534) were asked the most important reason that they were not screened. We categorized responses as doctor driven (“doctor did not say I needed it” or “did not know I needed it” or “never thought about it”), patient driven (“have not had any problems”, “put it off”, “did not get around too it”, “too expensive”, “no insurance”, “too painful” or “embarrassing”), or other.
Statistical Analyses
Since the absolute difference of sample characteristics between 2008 and 2010 was <5% in all cases, we combined these samples to increase the precision of our estimates. We used the Mantel-Haenszel test of trend (TOT) to examine sample characteristics and receipt of a clinician recommendation for mammography by life expectancy. We then used chi-square statistics to examine receipt of mammography by age, life expectancy, sociodemographics, access to care, receipt of a clinician recommendation, and preventive orientation (covariates). We performed multivariable logistic regression to examine receipt of screening by life expectancy adjusting for covariates. Although age is a component of our life expectancy measure, we included age as an independent variable in our multivariable model since prior studies suggest that age is a strong predictor of screening.(14–19) We repeated our analyses limiting our sample to the 2010 data to determine whether factors assessed only in 2010 were associated with screening. Finally, we used the Mantel-Haenszel TOT to compare reasons for not being screened by life expectancy among the 2010 respondents.
All analyses used SAS-callable SUDAAN software, version 10.0 (Research Triangle Institute, Research Triangle Park, North Carolina). Results are weighted to reflect US population estimates and to adjust for non-response; we present sample sizes whenever possible.
RESULTS
Sample (Table 1)
Table 1.
Sample Characteristics Overall and by Life Expectancy*
| Overall | Low mortality risk |
Medium mortality risk |
High mortality risk |
P value |
|
|---|---|---|---|---|---|
| n (%) # of US women represented | 2,266 7.7 million |
583 (27.1%) 2.1 million |
1,241 (53.4%) 4.1 million |
442 (19.5%) 1.5 million |
|
| Sociodemographics | |||||
| Age† | |||||
| 75–79 % (n) | 41.0 (885) | 72.3 | 36.2 | 10.2 | <0.001 |
| 80–84 % (n) | 31.9 (735) | 25.4 | 37.4 | 25.7 | |
| 85+ % (n) | 27.1 (646) | 2.3 | 26.4 | 63.7 | |
| Insurance‡ | |||||
| No insurance/Part A only % (n) | 1.1 (18) | 2.0 | 0.8 | 0.4 | <0.001 |
| Medicare: Part A & B or Part B only %(n) | 19.1 (438) | 19.0 | 18.5 | 21.0 | |
| Medicaid % (n) | 8.3 (250) | 4.3 | 9.4 | 10.7 | |
| Private (includes: Private alone, Medicare plus a private supplemental policy, Medicare HMO, and Medicare plus choice/Medicare Advantage% (n) | 71.5 (1,554) | 74.6 | 71.2 | 67.9 | |
| Education‡ | |||||
| <high-school % (n) | 27.2 (686) | 18.4 | 27.7 | 38.4 | <0.001 |
| High-school % (n) | 37.1 (792) | 39.7 | 39.4 | 27.2 | |
| Some college % (n) | 21.3 (478) | 22.4 | 21.1 | 20.4 | |
| College or beyond % (n) | 14.3 (291) | 19.5 | 11.8 | 14.1 | |
| Race/Ethnicity | |||||
| Hispanic % (n) | 5.9 (182) | 6.3 | 6.3 | 4.2 | 0.03 |
| Non-Hispanic White % (n) | 82.3 (1,674) | 84.6 | 80.7 | 83.4 | |
| Non-Hispanic Black % (n) | 8.4 (298) | 6.7 | 8.8 | 9.7 | |
| Non-Hispanic Other % (n) | 3.5 (112) | 2.5 | 4.2 | 2.8 | |
| Marital Status | |||||
| Married or living with partner % (n) | 31.6 (447) | 42.5 | 30.8 | 18.7 | <0.001 |
| Income | |||||
| <20K | 16.4 (374) | 17.3 | 15.5 | 17.9 | 0.84 |
| 20–<35K | 16.9 (375) | 14.5 | 18.0 | 17.3 | |
| 35K–<55K | 19.6 (452) | 19.0 | 23.0 | 26.2 | |
| 55–<90K | 22.4 (495) | 19.8 | 22.8 | 20.6 | |
| 90K+ | 24.6 (570) | 26.2 | 24.0 | 24.2 | |
| Region | |||||
| Northeast % (n) | 21.7 (464) | 21.9 | 20.6 | 24.5 | 0.48 |
| Midwest % (n) | 24.7 (583) | 27.4 | 23.9 | 23.3 | |
| South % (n) | 33.0 (754) | 29.7 | 35.0 | 32.1 | |
| West % (n) | 20.6 (465) | 21.0 | 20.6 | 20.1 | |
| Access to Care | |||||
| Usual Care | |||||
| Usual source of care gen med doctor/clinic % (n) | 95.8 (2,175) | 97.2 | 94.8 | 96.5 | 0.06 |
| Usual source of care gynecologist/specialist % (n) | 4.0 (86) | 2.7 | 4.8 | 3.5 | |
| No usual source of care % (n) | 0.3 (5) | 0.1 | 0.4 | 0 | |
| Clinic Visits | |||||
| 1–3 visits % (n) | 33.3 (757) | 45.8 | 31.5 | 20.7 | <0.001 |
| 4–7 visits % (n) | 36.8 (824) | 38.6 | 35.1 | 39.1 | |
| 8 or more visits % (n) | 29.9 (685) | 15.6 | 33.4 | 40.2 | |
| Preventive Behavior | |||||
| Flu vaccine in past year | 57.3 (1,254) | 56.5 | 56.5 | 60.7 | 0.02 |
| No flu vaccine in the past year | 22.1 (530) | 22.3 | 24.2 | 16.1 | |
| Not assessed | 20.6 (482) | 21.2 | 19.3 | 23.2 | |
| Pneumonia vaccine ever | 67.1 (1,464) | 65.8 | 66.6 | 70.5 | 0.41 |
| Clinician Screening Recommendation | |||||
| Reported receiving recommendation for mammography screening | 52.5 (1,145) | 61.0 | 54.0 | 36.7 | <0.001 |
| Factors in Life Expectancy Index† | |||||
| Illness Burden | |||||
| History of cancer % (n) † | 10.6 (224) | 3.5 | 9.4 | 23.6 | <0.001 |
| Diabetes (including borderline) % (n) † | 19.2 (462) | 6.8 | 20.4 | 32.9 | <0.001 |
| History of COPD % (n) †§ | 10.3 (217) | 1.9 | 8.5 | 26.8 | <0.001 |
| IADL dependency % (n) †§ | 21.3 (510) | 1.8 | 18.7 | 55.6 | <0.001 |
| Hospitalizations† | |||||
| 0 % (n) | 80.2 (1,825) | 94.7 | 81.2 | 57.1 | <0.001 |
| 1 % (n) | 13.1 (298) | 4.8 | 13.5 | 23.7 | |
| 2+ % (n) | 6.7 (143) | 0.5 | 5.3 | 19.3 | |
| At least some difficulty walking % (n) † | 59.5 (1,361) | 10.7 | 70.8 | 96.4 | <0.001 |
| Perceived Health† | |||||
| Excellent/Very good % (n) | 37.7 (856) | 69.4 | 32.1 | 8.9 | <0.001 |
| Good % (n) | 36.2 (805) | 27.6 | 41.4 | 34.0 | |
| Poor/fair % (n) | 26.1 (605) | 3.1 | 26.5 | 57.0 | |
| Health Behaviors | |||||
| Tobacco Use† | |||||
| Current smoker % (n) | 5.0 (115) | 0.6 | 4.9 | 11.8 | <0.001 |
| Former smoker % (n) | 30.6 (662) | 22.8 | 30.7 | 41.4 | |
| Never smoked % (n) | 64.4 (1,489) | 76.7 | 64.5 | 46.9 | |
| BMI <25 % (n) †§ | 48.5 (1,099) | 36.8 | 48.0 | 66.0 | <0.001 |
| Breast Disease History-Only Assessed in 2010 | n=1,206 | ||||
| History of benign breast biopsy % (n) | 18.9 (219) | 21.1 | 18.6 | 17.0 | 0.60 |
| Perceived Breast Cancer risk | |||||
| Above average % (n) | 5.5 (55) | 6.3 | 5.0 | 5.7 | 0.12 |
| Below average % (n) | 51.6 (621) | 53.2 | 54.7 | 41.9 | |
| Average % (n) | 35.1 (423) | 32.1 | 33.0 | 44.0 | |
| Refused/Don’t know | 7.8 (107) | 8.4 | 7.3 | 8.5 | |
| Family history of breast cancer % (n) | 14.9 (184) | 12.7 | 17.8 | 10.8 | 0.02 |
Participants at low risk of mortality have an 8% risk of 5-year mortality and a 19% risk of 9-year mortality. Participants at medium risk have a 27% risk of 5-year mortality and a 50% risk of 9-year mortality. Participants at high risk of mortality and have a 57% risk of 5-year mortality and an 84% risk of 9-year mortality.
Sample does not add to n=2,266 due to a few missing data or answered did not know.
Abbreviations: COPD: Chronic Obstructive Pulmonary Disease, IADL: Instrumental Activity of Daily Living, ADL: Activity of Daily Living, BMI: Body Mass Index
Of the 2,266 women, 27.1% (n=583) were at low mortality risk, 53.4% (n=1,241) were at medium risk, and 19.5% (n=442) were at high mortality risk. Table 1 presents sample characteristics by life expectancy. Women at low mortality risk were more likely to report receiving a clinician recommendation for screening (70.8%) than women at high mortality risk (36.1%, p<0.001).
Screening (Table 2)
Table 2.
Unadjusted Proportions and Adjusted Odds Ratios of Receiving Screening Mammography among Women Aged 75 and Older (n=2,266).*
| N | Screened in past 2 years % (standard error) |
p-value | Both Survey Years Adjusted OR (95% CI) § |
|
|---|---|---|---|---|
| Sociodemographics | ||||
| Age† | ||||
| 75–79 | 885 | 65.8 (1.8) | <0.001 | 1.0 |
| 80–84 | 735 | 58.1 (2.2) | 0.8 (0.6–1.2) | |
| 85+ | 646 | 37.5 (2.3) | 0.7 (0.5–0.9) | |
| Insurance‡ | ||||
| Medicare: Part A & B or Part B only | 438 | 45.6 (2.9) | <0.001 | 1.0 |
| Medicaid | 250 | 38.6 (4.0) | 0.6 (0.4–1.1) | |
| Private (includes: Private alone, Medicare plus a private supplemental policy, Medicare HMO, and Medicare plus choice/Medicare Advantage) | 1,554 | 60.4 (1.5) | 1.2 (0.9–1.7) | |
| Education | ||||
| high-school or less | 1,478 | 51.8 (1.6) | <0.001 | 0.7 (0.5–0.9) |
| Some college or beyond | 769 | 63.5 (2.1) | 1.0 | |
| Race/Ethnicity | ||||
| Hispanic | 182 | 55.1 (4.9) | 1.2 (0.8–1.8) | |
| Non-Hispanic White | 1,674 | 56.5 (1.6) | 0.25 | 1.0 |
| Non-Hispanic Black | 298 | 51.5 (3.7) | 0.9 (0.6–1.3) | |
| Non-Hispanic Other | 112 | 46.5 (5.5) | 0.9 (0.6–1.3) | |
| Marital Status | ||||
| Married or living with partner | 447 | 66.8 (2.5) | <0.001 | 1.0 (0.8–1.4) |
| Not currently married | 1,819 | 50.5 (1.5) | 1.0 | |
| Income | ||||
| <20K | 374 | 58.2 (3.4) | 0.76 | 1.0 (0.7–1.5) |
| 20K–<35K | 375 | 55.8 (3.4) | 0.9 (0.6–1.4) | |
| 35K–<55K | 452 | 55.4 (3.1) | 1.0 (0.7–1.5) | |
| 55K–<90K | 495 | 52.6 (3.0) | 1.0 (0.6–1.4) | |
| 90K+ | 570 | 56.9 (3.0) | 1.0 | |
| Region | ||||
| Northeast | 464 | 53.9 (2.5) | 0.46 | 1.0 |
| Midwest | 583 | 56.7 (2.9) | 1.3 (0.9–1.9) | |
| South | 754 | 53.9 (2.2) | 1.4 (1.0–2.0) | |
| West | 465 | 59.2 (3.0) | 1.2 (0.8–1.8) | |
| Access to Care | ||||
| Usual Care | ||||
| Usual source of care gen med doctor/clinic | 2,175 | 56.0 (1.3) | 0.12 | 1.0 |
| Usual source of care gynecologist/specialist | 86 | 51.8 (6.2) | 0.8 (0.4–1.6) | |
| Clinic Visits | ||||
| 1–3 visits | 757 | 55.0 (2.3) | 0.18 | 1.0 |
| 4–7 visits | 824 | 53.6 (2.0) | 1.0 (0.7–1.3) | |
| 8 or more visits | 685 | 59.0 (2.3) | 1.2 (0.9–1.7) | |
| Preventive Behavior | ||||
| Flu vaccine in past year | 1,254 | 62.2 (1.6) | <0.001 | 1.9 (1.4–2.6) |
| No flu vaccine in past year | 530 | 41.3 (2.5) | 1.0 | |
| Not assessed | 482 | 53.1 (3.0) | 1.7 (1.1–2.6) | |
| Pneumonia vaccine ever | 1,464 | 62.7 (1.6) | <0.001 | 1.2 (0.9–1.6) |
| No pneumonia vaccine ever | 802 | 41.3 (2.2) | 1.0 | |
| Clinician Recommendation for Screening Mammogram | ||||
| Yes | 1,145 | 87.0 (1.3) | <0.001 | 8.5 (6.4–11.2) |
| No | 1,100 | 21.8 (1.5) | 1.0 | |
| Life Expectancy‡ | ||||
| Low | 583 | 70.8 (2.0) | <0.001 | 1.0 |
| Medium | 1,241 | 55.2 (1.8) | 1.4 (1.0–1.9) | |
| High | 442 | 36.1 (2.5) | 2.4 (1.6–3.7) | |
| Breast Disease History-Only Assessed in 2010□(n=1,206) | ||||
| History of benign breast biopsy | 219 | 71.5 (3.5) | <0.001 | 1.8 (1.2–2.7) |
| No history of benign biopsy | 987 | 50.5 (2.0) | 1.0 | |
| 0.07 | ||||
| Perceived Breast Cancer risk | ||||
| Above average | 55 | 65.5 (7.6) | 0.22 | 1.4 (0.7–3.1) |
| Below average | 621 | 51.7 (2.5) | 1.0 (0.6–1.5) | |
| Average | 423 | 56.3 (2.7) | 1.0 | |
| Refused/Don’t know | 107 | 56.6 (5.5) | 1.0 (0.5–2.0) | |
| Family history of breast cancer | 184 | 61.7 (4.1) | 0.07 | 1.1 (0.6–1.9) |
| No family history of breast cancer | 1,022 | 53.2 (2.0) | 1.0 | |
| Components of life expectancy† | ||||
| History of cancer | 224 | 61.1 (3.7) | 0.19 | |
| No history of cancer | 2,042 | 55.0 (1.3) | ||
| Diabetes (including borderline) | 462 | 53.5 (3.0) | 0.41 | |
| No diabetes | 1,804 | 56.2 (1.4) | ||
| History of COPD § | 217 | 56.5 (4.4) | 0.83 | |
| No history of COPD | 2,049 | 55.6 (1.3) | ||
| IADL dependency§ | 510 | 36.3 (2.6) | <0.001 | |
| No IADL dependency | 1,756 | 60.9 (1.4) | ||
| Hospitalizations | ||||
| 0 | 1,825 | 56.0 (1.4) | 0.55 | |
| 1 | 298 | 52.5 (3.2) | ||
| 2+ | 143 | 58.4 (5.4) | ||
| At least some difficulty walking | 1,361 | 48.9 (1.7) | <0.001 | |
| No difficulty walking | 905 | 65.6 (1.8) | ||
| Perceived Health | ||||
| Excellent/Very good | 856 | 61.6 (2.0) | <0.001 | |
| Good | 805 | 56.0 (2.2) | ||
| Poor/fair | 605 | 46.6 (2.4) | ||
| Tobacco Use† | 0.005 | |||
| Current smoker | 115 | 40.5 (5.3) | ||
| Former smoker | 662 | 59.3 (2.2) | ||
| Never smoked | 1,489 | 55.1 (1.6) | ||
| BMI <25 § | 1,099 | 51.8 (1.8) | 0.002 | |
| BMI 25+ | 1167 | 59.4 (1.8) |
The multivariable model included 2,198 women or 96.8% of the sample since 68 women missing data on their insurance, education, whether or not they received a clinician recommendation for mammography and women without any insurance were excluded.
Although age is a component of the life expectancy measure we also adjusted for age independently in our analyses. We did not further adjust for each component of the life expectancy measure.(20,21)
Participants at low risk of mortality have an 8% risk of 5-year mortality and a 19% risk of 9-year mortality. Participants at medium risk have a 27% risk of 5-year mortality and a 50% risk of 9-year mortality. Participants at high risk of mortality and have a 57% risk of 5-year mortality and an 84% risk of 9-year mortality.
Abbreviations: COPD: Chronic Obstructive Pulmonary Disease, IADL: Instrumental Activity of Daily Living, ADL: Activity of Daily Living, BMI: Body Mass Index
These factors were only assessed in 2010 so the aOR are from a multivariable model including data from only survey year 2010.
Overall, 55.7% of women reported mammography screening in the past two years. Receipt of screening did not differ significantly between 2008 (56.9%) and 2010 (54.5%, p=0.33). In bivariable analyses, women at high mortality risk (36.1%) were significantly less likely to report being screened than women at low mortality risk (70.8%, p<0.001). Younger age, private insurance, higher education, being married, receipt of a flu and/or pneumonia vaccine, were also significantly associated with receipt of mammography in bivariable analyses. Reported receipt of a clinician recommendation was strongly associated with being screened (87.0% of women that reported receiving a clinician recommendation reported being screened). Among the factors included in the mortality index, having a functional limitation, difficulty walking, poor perceived health, BMI <25, and current tobacco use were associated with being less likely to report screening. A history of benign breast biopsy was associated with being screened based on 2010 respondents. Multivariable analyses yielded similar results; however, insurance, marital status, and pneumonia vaccination were not found to be significantly associated with screening in these analyses.
Reasons for Not Being Screened
Reasons for not being screened tended to vary by life expectancy but the results did not achieve statistical significance (p=0.06). Women at low mortality risk tended to be more likely to report patient driven factors (25.7%, e.g., “put it off,” “painful”) for not being screened than women at high mortality risk (8.6%). Women at high mortality risk were more likely to report (84.2%) that “a doctor did not say I needed it” or “did not know I needed it” than women at low mortality risk (69.1%).
DISCUSSION
Despite uncertainty of benefit, 56% of community-dwelling US women >75 years reported receiving mammography screening in the past two years. In the absence of clinical trial data, experts and the American Geriatrics Society recommend that clinicians consider patient life expectancy in screening decisions.(12, 13, 24) Reassuringly, we found that life expectancy was strongly associated with receipt of mammography among older women. The majority (71%) of older women with >9 year life expectancy were screened while the majority of older women (64%) with <5 year life expectancy were not screened. A clinician recommendation was the strongest predictor of screening, suggesting that clinicians do consider patient life expectancy when deciding whether or not to recommend mammography screening. However, consistent with prior data, we found that over a third of women with <5 years life expectancy were screened. New interventions may be needed to further improve targeting of screening by life expectancy. (3,14–19) Patient decision aids on mammography screening may help inform older women’s screening decisions. Increased incorporation of life expectancy into mammography screening guidelines may also improve care.
Higher educational attainment, an orientation towards receipt of preventive healthcare, being <85 years, and history of benign breast biopsy, were associated with receipt of screening independent of life expectancy. Williams et al. previously found that high net worth predicted receipt of mammography screening among older women regardless of life expectancy.(19) Together, our findings suggest that highly educated women (correlated with high net worth), are the at high risk for being overscreened. Meanwhile, highly educated women may be the most likely to benefit from communication aids about the benefits and burdens of mammography due to higher literacy. To further improve targeting of mammography screening to older women by life expectancy, clinicians may need to teach highly educated women with short life expectancies that mammography may not benefit them. Fortunately, this is the most teachable group of older women.
Studies suggest that many older patients would welcome discussions about their life expectancy when making decisions about medical interventions.(25) However, clinicians report discomfort discussing patient life expectancy, are overly optimistic in their estimates, and lack confidence in available prognostic tools.(25) Since a clinician’s recommendation is the strongest driver of screening, it is essential that continuing medical education courses include training on estimating patient life expectancy and individualizing discussions about mammography screening. Studies examining physician discussions around mammography screening indicate that physicians favor benefits while rarely mentioning harms.(26) Documentation of informed decision-making around mammography screening may be an important marker of quality of care rather than receipt of screening among older women.(27)
We also found that 29% of older women with >9-year life expectancy were not screened in the past two years. Based on the 2010 data, 26% chose not to be screened based on their own preferences while 69% reported that they did not know they needed a mammogram and/or a doctor did not recommend a mammogram. As it is important to discuss stopping screening with older women with <5-years life expectancy, it may be important to discuss reasons to continue screening with older women with >9-years life expectancy; especially since recent data suggest that declines in breast cancer mortality have preferentially favored women <75 years.(28)
A history of benign breast biopsy was associated with receipt of screening independent of life expectancy. While some older women with a history of benign breast biopsy may be appropriately considering their risk of breast cancer when deciding on screening, some older women with short life expectancies may be overestimating their risk of breast cancer in their remaining life span, especially if the benign breast biopsy was years ago and no atypical cells were found.(29) In addition to estimating life expectancy, helping older women adequately assess their breast cancer risk may be important for informed decision-making around mammography screening.
This study has limitations. Information was based on self-report and is subject to recall and response bias. However, self-report is a valid method for assessing screening rates and can be highly correlated with medical record report.(30) Women with lower participation in breast cancer screening may be less likely to recall clinician recommendations for mammography. Also, the question asking women who were screened whether or not they received a clinician recommendation for screening changed between 2008 and 2010. We repeated our multivariable analyses excluding receipt of a clinician recommendation for screening and our results were similar except that having better insurance, more clinic visits, and receipt of pneumococcal vaccination were also significantly associated with receipt of screening. We excluded women missing data on mammography screening and women who had not seen a doctor in the past year (n=367 with life expectancy data); however, the predicted life expectancies of these women did not differ from those in our sample (p=0.10). We included 93 women (4.9%) with proxy responses since validation of our mortality index included proxies.(23) Although life expectancy may have declined since some of the oldest women were screened, 76.8% of those screened reported their mammogram was done in the past year. We repeated our analyses examining receipt of mammography screening in the past year and our results did not change.
Mammography screening remains common (36%) among older women with <5-year life expectancies while 29% of older women with >9-year life expectancies are not screened. Interventions such as revising guidelines to encourage clinicians to consider patient life expectancy, training clinicians how to prognosticate, and providing patients with balanced educational materials about screening, may further improve mammography screening decisions among older women.
ACKNOWLEDGMENTS
This research was conducted while Dr. Mara Schonberg was supported by a Paul B. Beeson Career Development Award in Aging supported by the National Institute on Aging K23 [K23AG028584], The John A. Hartford Foundation, The Atlantic Philanthropies, The Starr Foundation, and The American Federation for Aging Research. Dr. McCarthy was supported by the American Cancer Society [RSGT-10-080-CPHSPS].
Footnotes
Financial Disclosure Information:
none disclosed.
Presentation: This research has been submitted for presentation at the 2012 annual meeting of the Gerontological Society of America in San Diego, CA.
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions:
Dr. Schonberg: Conception and design, acquisition, analysis and interpretations of the data, drafted and approved the final article.
Dr. Breslau: Design of the study, analyzed and interpreted the data, critically revised the manuscript, and approved the final version.
Dr. McCarthy: Analyzed and interpreted the data, critically revised the manuscript, and approved the final version.
Sponsor’s Role: The sponsors played no role in the design, methods, subject recruitment, data collection, analysis and preparation of paper.
REFERENCES
- 1.American Geriatrics Society. Breast Cancer Facts & Figures 2009–2010. Atlanta: American Cancer Society, Inc; [Accessed July 19, 2012]. Availabe at: http://www.cancer.org/acs/groups/content/@nho/documents/document/f861009final90809pdf.pdf. [Google Scholar]
- 2.Table 3 - Population Projections by Race and Hispanic Origin for Persons 85 and older: 2000 to 2050. [Accessed July 19];2012 Available at: http://aoa.gov/AoARoot/Aging_Statistics/minority_aging/docs/Table%203_Population_Projections_by_Race_85+.xls.
- 3.Walter LC, Covinsky KE. Cancer screening in elderly patients: A framework for individualized decision making. JAMA. 2001;285:2750–2756. doi: 10.1001/jama.285.21.2750. [DOI] [PubMed] [Google Scholar]
- 4.Badgwell BD, Giordano SH, Duan ZZ, et al. Mammography before diagnosis among women age 80 years and older with breast cancer. J Clin Oncol. 2008;26:2482–2488. doi: 10.1200/JCO.2007.12.8058. [DOI] [PubMed] [Google Scholar]
- 5.McCarthy EP, Burns RB, Freund KM, et al. Mammography use, breast cancer stage at diagnosis, and survival among older women. J Am Geriatr Soc. 2000;48:1226–1233. doi: 10.1111/j.1532-5415.2000.tb02595.x. [DOI] [PubMed] [Google Scholar]
- 6.McPherson CP, Swenson KK, Lee MW. The effects of mammographic detection and comorbidity on the survival of older women with breast cancer. J Am Geriatr Soc. 2002;50:1061–1068. doi: 10.1046/j.1532-5415.2002.50261.x. [DOI] [PubMed] [Google Scholar]
- 7.Gotzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD001877.pub4. CD001877. [DOI] [PubMed] [Google Scholar]
- 8.Nelson HD, Tyne K, Naik A, et al. Screening for breast cancer: An update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151:727–737. doi: 10.1059/0003-4819-151-10-200911170-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hurria A, Rosen C, Hudis C, et al. Cognitive function of older patients receiving adjuvant chemotherapy for breast cancer: A pilot prospective longitudinal study. J Am Geriatr Soc. 2006;54:925–931. doi: 10.1111/j.1532-5415.2006.00732.x. [DOI] [PubMed] [Google Scholar]
- 10.Lopez E, Nunez MI, Guerrero MR, et al. Breast cancer acute radiotherapy morbidity evaluated by different scoring systems. Breast Cancer Res Treat. 2002;73:127–134. doi: 10.1023/a:1015296607061. [DOI] [PubMed] [Google Scholar]
- 11.Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716–26. doi: 10.7326/0003-4819-151-10-200911170-00008. W-236. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher SW, Elmore JG. Clinical practice. Mammographic screening for breast cancer. N Engl J Med. 2003;348:1672–1680. doi: 10.1056/NEJMcp021804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warner E. Clinical practice. Breast-cancer screening. N Engl J Med. 2011;365:1025–1032. doi: 10.1056/NEJMcp1101540. [DOI] [PubMed] [Google Scholar]
- 14.Bynum JP, Braunstein JB, Sharkey P, et al. The influence of health status age, race on screening mammography in elderly women. Arch Intern Med. 2005;165:2083–2088. doi: 10.1001/archinte.165.18.2083. [DOI] [PubMed] [Google Scholar]
- 15.Koya DL, Chen JG, Smith TG, et al. Screening mammography use in Medicare beneficiaries reflects 4-year mortality risk. Am J Med. 2011;124:369 e1–369 e8. doi: 10.1016/j.amjmed.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Schonberg MA, Leveille SG, Marcantonio ER. Preventive health care among older women: Missed opportunities and poor targeting. Am J Med. 2008;121:974–981. doi: 10.1016/j.amjmed.2008.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schonberg MA, McCarthy EP, Davis RB, et al. Breast cancer screening in women aged 80 and older: results from a national survey. J Am Geriatr Soc. 2004;52:1688–1695. doi: 10.1111/j.1532-5415.2004.52462.x. [DOI] [PubMed] [Google Scholar]
- 18.Walter LC, Lindquist K, Covinsky KE. Relationship between health status and use of screening mammography and Papanicolaou smears among women older than 70 years of age. Ann Intern Med. 2004;140:681–688. doi: 10.7326/0003-4819-140-9-200405040-00007. [DOI] [PubMed] [Google Scholar]
- 19.Williams BA, Lindquist K, Sudore RL, et al. Screening mammography in older women. Effect of wealth and prognosis. Arch Intern Med. 2008;168:514–520. doi: 10.1001/archinternmed.2007.103. [DOI] [PubMed] [Google Scholar]
- 20.Schonberg MA, Davis RB, McCarthy EP, et al. External validation of an index to predict up to 9-year mortality of community-dwelling adults aged 65 and older. J Am Geriatr Soc. 2011;59:1444–1451. doi: 10.1111/j.1532-5415.2011.03523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schonberg MA, Davis RB, McCarthy EP, et al. Index to Predict 5-Year Mortality of Community-Dwelling Adults Aged 65 and Older Using Data from the National Health Interview Survey. J Gen Intern Med. 2009;24:1115–1122. doi: 10.1007/s11606-009-1073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control. National Health Interview Survey. [Accessed July 19, 2012]; Available at: http://www.cdc.gov/nchs/nhis/quest_data_related_1997_forward.htm.
- 23.Centers for Disease Control. Vital and Health Statistics from the Centers from Disease Control and Prevention, Method for construction of complete annual US life tables: December 1999. [Accessed July 19, 2012]; Available at: http://www.cdc.gov/nchs/data/series/sr_02/sr02_129.pdf.
- 24.American Geriatrics Society. Breast Cancer Screening in Older Women (reviewed and updated in 2005) Available at: http://www.americangeriatrics.org/education/cp_index.shtml. [Google Scholar]
- 25.Smith AK, Williams BA, Lo B. Discussing overall prognosis with the very elderly. N Engl J Med. 2011;365:2149–2151. doi: 10.1056/NEJMp1109990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wegwarth O, Gigerenzer G. There is nothing to worry about: gynecologists' counseling on mammography. Patient Educ Couns. 2011;84:251–256. doi: 10.1016/j.pec.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 27.Walter LC. What is the right cancer screening rate for older adults? Arch Intern Med. 2011;171:2037–2039. doi: 10.1001/archinternmed.2011.556. [DOI] [PubMed] [Google Scholar]
- 28.Smith BD, Jiang J, McLaughlin SS, et al. Improvement in breast cancer outcomes over time: Are older women missing out? J Clin Oncol. 2011;29:4647–4653. doi: 10.1200/JCO.2011.35.8408. [DOI] [PubMed] [Google Scholar]
- 29.Worsham MJ, Raju U, Lu M, et al. Multiplicity of benign breast lesions is a risk factor for progression to breast cancer. Clin Cancer Res. 2007;13:5474–5479. doi: 10.1158/1078-0432.CCR-07-0928. [DOI] [PubMed] [Google Scholar]
- 30.Caplan LS, Mandelson MT, Anderson LA. Validity of self-reported mammography: Examining recall and covariates among older women in a Health Maintenance Organization. Am J Epidemiol. 2003;157:267–272. doi: 10.1093/aje/kwf202. [DOI] [PubMed] [Google Scholar]