Abstract
Methylene blue USP (MB) at low doses has metabolic-enhancing and antioxidant properties and exhibits experimental neurotherapeutic benefits, but little is known about its in vivo effects on cerebral blood flow (CBF), functional evoked responses, and the associated changes in cerebral metabolic rate of oxygen consumption (CMRO2). This study used magnetic resonance imaging (MRI) to evaluate the in vivo effects of a single intravenous MB therapeutic dose (0.5 mg/kg) on basal CBF, blood oxygenation level-dependent (BOLD) and CBF responses to hypercapnic (5% CO2 in air) inhalation, as well as changes in BOLD, CBF, and CMRO2 during forepaw stimulation in the rat brain. We found that this MB therapeutic dose did not have significant effects on arterial oxygen saturation, heart rate and fMRI responses to hypercapnia. However, MB significantly potentiated forepaw-evoked BOLD and CBF changes under normoxia. To further evaluate in vivo effects of MB under metabolic stress conditions, MRI measurements were also made under mild hypoxia (15% O2). Hypoxia per se increased evoked functional MRI responses. MB under hypoxia further potentiated forepaw-evoked BOLD, CBF and oxygen consumption responses relative to normoxia. These findings provide insights into MB’s effects on cerebral hemodynamics in vivo and could help to optimize treatments in neurological diseases with mitochondrial dysfunction and oxidative stress.
Keywords: fMRI, BOLD, CBF, perfusion, oxidative metabolism, arterial spin labeling, cerebral metabolic rate of oxygen, forepaw stimulation
INTRODUCTION
Methylene blue USP (MB) is a unique auto-oxidizing pharmaceutical drug that has a hormetic dose-response with opposite effects at low and high doses (Rojas et al., 2012). At its therapeutic intravenous low doses (0.5–2 mg/kg), MB is an antidote for methemoglobinemia and cyanide poisoning and has potent antioxidant effects (Rojas et al., 2012; Scheindlin, 2008). However, at high intravenous doses (>10 mg/kg) MB produces methemoglobinemia and oxidative stress (Bruchey and Gonzalez-Lima, 2008). Low doses of MB improve brain cytochrome c oxidase activity and behavioral memory functions (Gonzalez-Lima and Bruchey, 2004; Martinez Jr. et al., 1978; Riha et al., 2005; Wrubel et al., 2007), whereas high doses are detrimental (Martinez Jr. et al., 1978; Riha et al., 2005). MB has recently been shown to have multiple experimental therapeutic benefits to reduce neurobehavioral impairment in animal models of Parkinson’s Disease (Ishiwata et al., 2006; Rojas et al., 2012), cognitive decline in Alzheimer’s animal models (Medina et al., 2010; O’Leary et al., 2010; Oz et al., 2009), and reperfusion injury in cerebral ischemia in rodent stroke models (Wen et al., 2011) (see reviews (Rojas et al., 2012; Scheindlin, 2008)).
Low-dose MB readily crosses the blood-brain barrier, accumulates in brain mitochondria, and has metabolic-enhancing and antioxidant properties because it forms a reversible reduction-oxidation system with auto-oxidizing capacity (Bruchey and Gonzalez-Lima, 2008). Low-dose MB has redox recycling properties in that it acts as an electron cycler and facilitates electron transfer in the mitochondrial electron transport chain by accepting electrons from NADH and transferring them to cytochrome c, bypassing complex I–III (Clifton and Leikin, 2003). MB thus enhances or sustains ATP production in cells (Lindahl and Oberg, 1961; Scott and Hunter, 1966; Zhang et al., 2006) and brain oxygen consumption in vitro (Riha et al., 2005). In bypassing complex I–III to generate ATP, MB also minimizes free radical production in the mitochondrial electron transport chain. This could have positive effects under metabolically stressed conditions (i.e., ischemic brain injury) where excess free radicals may lead to cellular damage and cell death. While MB mechanisms of action are well studied in vitro, the effects of MB on basal cerebral blood flow (CBF), neurovascular coupling, functional activations and oxygen consumption changes due to evoked responses in the in vivo brain remain unknown.
Magnetic resonance imaging (MRI) has been widely used to study quantitative basal CBF, neurovascular coupling and evoked responses under normal conditions and in neurological diseases in vivo. CBF can be measured non-invasively using the continuous arterial spin labeling technique (Detre et al., 1992; Duong et al., 2000) that yields quantitative classical units of ml/gram/min and allows longitudinal cross-subject comparisons. Neural activity is intricately coupled to CBF (Roy and Sherrington, 1890). When a task is performed, regional neural activity increases resulting in regional CBF increase. Such increase is disproportional in that it overcompensates the needed oxygen supply, resulting in increased regional oxygen tension. Thus, blood oxygen level-dependent (BOLD) functional MRI (fMRI), which is sensitive to changes in magnetic field inhomogeneity induced by paramagnetic deoxyhemoglobin in red blood cells (Ogawa et al., 1990), can be used to map brain function in a non-invasive manner (Bandettini et al., 1992; Kwong et al., 1992; Ogawa et al., 1992). Moreover, since the BOLD fMRI signal is dependent on CBF and oxygen metabolism, BOLD fMRI data can be used to estimate the cerebral metabolic rate of oxygen consumption (CMRO2) (Davis et al., 1998; Liu et al., 2004; Sicard and Duong, 2005).
The goal of this study is to use MRI to evaluate the effects of a single intravenous MB therapeutic dose on basal CBF, BOLD and CBF responses to hypercapnic (5% CO2) challenge, and BOLD, CBF, and CMRO2 responses to forepaw stimulation in the in vivo rat brain. To further evaluate the effects of MB under stress conditions, similar measurements were also made under mild hypoxia (15% O2). Knowledge of MB’s effects on the underlying hemodynamic and evoked fMRI responses in vivo could improve understanding of MB’s neurotherapeutic effects and optimize treatments.
METHODS
Animal preparation
Animal experiments were performed in accordance with the ARRIVE guidelines on ethics and were approved by the Institutional Animal Care and Use Committee (IACUC). Male Sprague-Dawley rats (200–300 g, n = 10 under normoxia (21% O2), and n = 6 under hypoxia (15% O2)) were initially anesthetized with 2% isoflurane, intubated, mechanically ventilated, and paralyzed with pancuronium bromide (3 mg/kg first dose, 1 mg/kg/hr, ip). A femoral artery was catheterized with PE-50 tubing. Needle electrodes were inserted under the skin of the forepaws. Rats were secured in a MR-compatible rat stereotaxic headset. Anesthesia was reduced to 1.1–1.2% isoflurane. Rectal temperature was monitored and maintained at 37.0 ± 0.5° C throughout. Heart rate (HR) and oximetry were recorded continuously.
The experimental design is outlined in Figure 1. MRI measurements were made with vehicle and USP pharmaceutical grade MB (American Regent Inc, Shirley, NY) (intravenous 0.5 mg/kg, over 5 mins) injection in the same animals. The effect of MB is expected to last a few hours (Peter et al., 2000). Hypercapnic challenge was 2 mins air, 3 mins 5% CO2 (in air) inhalation followed by 5 mins air (one repetition). 5% CO2 was chosen because it is widely used in the animal literature for similar studies (Liu et al., 2004; Mandeville et al., 1998; Sicard and Duong, 2005). Bilateral forepaw stimulation used 4 epochs (96 s OFF and 30 s ON) of 2 mA, 8 Hz and 1 ms pulse (Liu et al., 2004). Four repeated trials were made for each condition on each animal. Breaks of ~15 mins were given between trials. The two forepaws were connected in series. Hypercapnic challenge was not studied in the hypoxia group.
Figure 1.

Schematic of the experimental design. The measurements are basal CBF, fMRI associated with CO2 challenge, and fMRI of forepaw stimulation. Vehicle was injected first followed by MB in the same animals. The reverse was not possible because MB has sustained effects.
MR experiments
MRI experiments were performed on a 7-T/30-cm magnet with a Biospec Bruker console (Billerica, MA), and a 40-G/cm gradient insert (ID = 12 cm, 120-μs rise time). A surface coil (2.3-cm ID) was used for brain imaging and a neck coil (Duong et al., 2000; Shen et al., 2005; Silva et al., 1999) for perfusion labeling. Coil-to-coil electromagnetic interaction was actively decoupled.
Combined CBF and BOLD measurements were made using the continuous arterial spin-labeling technique (Duong et al., 2000; Shen et al., 2003) with single-shot, gradient-echo, echo-planar-imaging (EPI) acquisition. Paired images were acquired alternately – one with arterial spin labeling and the other without (control). MRI parameters were: TR = 3 s, TE = 20 ms, matrix = 96×96, and FOV = 25.6×25.6 mm.
Data analysis
Data analysis employed codes written in Matlab (MathWorks Inc, Natick, MA) and the STIMULATE software (University of Minnesota). Repeated CBF measurements of the same condition in each animal were averaged. BOLD images were obtained from non-labeled images of the CBF measurements. CBF images (SCBF) with intensities in ml/g/min were calculated at each time point (Duong et al., 2000; Shen et al., 2003). Cross-correlation analysis was performed on the BOLD and CBF data sets to obtain percent-change activation maps.
For quantitative analysis, percent changes were evaluated using region-of-interest (ROI) analysis. ROIs enclosing the primary (6×6 pixels) somatosensory cortices were drawn on the averaged cross-correlation BOLD and CBF activation maps, with reference to anatomy and brain atlas (cross-correlation coefficients of the BOLD and CBF activation maps were similar). CMRO2 changes due to forepaw stimulation from the ROI data were calculated using the biophysical BOLD model (Davis et al., 1998) as described elsewhere (Liu et al., 2004) with α of 0.38 (Grubb et al., 1974; Mandeville et al., 1999) and β of 1.5 (Boxerman et al., 1995; Davis et al., 1998) used, which were taken to be constants that reflect the effect of blood volume and deoxyhemoglobin concentration to the BOLD signals, respectively. Potential errors in β and α values scaled proportionally the CMRO2 changes of both pre and post MB but did not affect the relative differences between pre and post MB. M value, the proportionality constant that reflects the maximum BOLD responses in the biophysical BOLD model, was calculated from the hypercapnic experiments.
Values in text and in graphs are mean ± SEM. Statistical tests were performed using paired t-test for between pre- and post-MB (same animals) and unpaired t-test for between normoxic and hypoxic conditions (different animals) with P < 0.05 indicating statistical significance.
RESULTS
Arterial oxygen saturation and heart rate were not statistically different (P > 0.05) between pre- and post-MB under both normoxia and hypoxia. Arterial oxygen saturation and heart rate were statistically different between normoxia and hypoxia (P < 0.05) (see Table 1).
TABLE 1.
| O2 saturation (%) | Heart rate (bpm) | |||
|---|---|---|---|---|
| Pre-MB | Post-MB | Pre-MB | Post-MB | |
|
| ||||
| Normoxia | 94.4±0.8 | 94.6±0.7 | 332±9 | 337±10 |
| Hypoxia | 87.6±1.6* | 87.5±1.9* | 313±14* | 311±18* |
P < 0.05, O2 saturation and heart rate were significantly different between normoxia and hypoxia.
O2 saturation and heart rate were not statistically different between pre- and post-MB (P > 0.05).
Figure 2 shows the group-averaged maps of basal CBF, CBF and BOLD fMRI responses to 5% CO2 challenge and to forepaw stimulation before and after MB. CBF images showed heterogeneous blood flow contrast with high CBF in gray matter relative to white matter. fMRI responses to 5% CO2 challenge were relatively uniform with higher changes in the neocortical regions and smaller changes in most subcortical structures and the corpus callosum. fMRI responses to bilateral forepaw stimulation were localized to forepaw primary somatosensory cortex (S1) as expected.
Figure 2.
Group-averaged basal CBF, BOLD and CBF fMRI responses to 5% CO2 and forepaw stimulation for before and after methylene blue (MB). fMRI data were overlaid on anatomical MRI. CBF grayscale bar ranges from 0 to 1.5 ml/gram/min. Color bars are cross-correlation coefficients.
The group-averaged CBF and BOLD fMRI responses to 5% CO2 challenge from the S1 ROIs were not statistically different (P > 0.05) between before and after MB under normoxia (Figure 3). The calculated M values from the S1 ROIs were not statistically different before and after MB (7.1 ± 0.7 % versus 7.2 ± 0.4%, P > 0.05).
Figure 3.

Group-averaged BOLD and CBF fMRI responses to 5% CO2 challenge before and after methylene blue (MB) injection in the same animals (normoxia). Two ROI’s (~6×6 pixels each) which were determined from group data of the forepaw stimulation and used in the quantitative analysis are shown. Values are in mean ± SEM (N = 8). * indicates P < 0.05 with paired t-test.
Figure 4 shows the group-averaged results of basal CBF, CBF, BOLD and CMRO2 fMRI responses to forepaw stimulation before and after MB under normoxia. These data, including basal CBF, were obtained from the S1 ROIs. MB increased basal CBF slightly but not significantly (P > 0.05). Forepaw-evoked CBF and BOLD responses were statistically different between pre- and post-MB (P < 0.05). The CMRO2 increases due to forepaw stimulation were not statistically different between pre- and post-MB under normoxia (P > 0.05).
Figure 4.
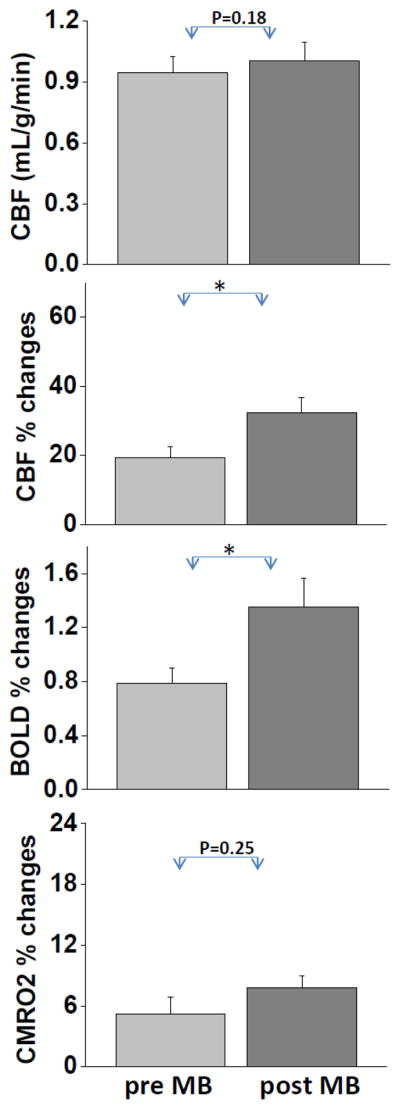
Group-averaged basal CBF, BOLD, CBF and CMRO2 fMRI responses to forepaw stimulation before and after methylene blue (MB) injection in the same animals (normoxia). Values are in mean ± SEM (N = 10). Forepaw-evoked CBF and BOLD responses were statistically different between pre- and post-MB. * indicates P < 0.05 with paired t-test.
Similar data under hypoxia (15% O2) are shown in Figure 5. These data, including basal CBF, were obtained from the S1 ROIs. Under hypoxia, post-MB basal CBF was statistically different (higher) from pre-MB CBF (P < 0.05), in contrast to normoxia. All CBF, BOLD and CMRO2 evoked responses post-MB were statistically different (higher) from pre-MB (P < 0.05).
Figure 5.

Group-averaged basal CBF, BOLD, CBF and CMRO2 fMRI responses to forepaw stimulation before and after methylene blue (MB) injection in the same animals (hypoxia). Values are in mean ± SEM (N = 6). All measures post-MB were statistically different (higher) from pre-MB. * indicates P < 0.05 with paired t-test.
By comparison, basal CBF was slightly lower during hypoxia compared to normoxia, but was not statistically different for both pre-MB (P > 0.05) and post-MB (P > 0.05). BOLD, CBF and CMRO2 fMRI responses under hypoxia were statistically different (higher) from those under normoxia for both pre-MB (P < 0.05) and post-MB (P < 0.05), except the BOLD responses post-MB (P > 0.05).
DISCUSSION
This study investigated basal cerebral blood flow, neurovascular coupling, functional activations and oxygen consumption changes in the brain in vivo following acute MB administration. A therapeutic low dose of MB does not appear to have significant effects on cerebrovascular responses to hypercapnia but markedly potentiates evoked fMRI responses and oxygen consumption changes. Hypoxia per se increases stimulus-evoked fMRI responses. MB under hypoxia further potentiates fMRI responses and oxygen consumption changes.
MB has a low toxicity profile at 0.5–1 mg/kg with no reported negative effects in both rats and humans (O’Leary et al., 2010; Oz et al., 2009; Wen et al., 2011), but showed adverse effects at high doses (> 10 mg/kg) in vivo as would be expected from its hormetic dose-response (Bruchey and Gonzalez-Lima, 2008; Rojas et al., 2012). MB reaches its maximum concentration in blood by 5 mins after intravenous administration in humans (Peter et al., 2000). Moreover, MB is trapped with concentrations 50 times higher in the brain than in the circulation one hour after intravenous administration in rats (Peter et al., 2000). The half-life of MB in blood after intravenous administration is 5.25 hrs in humans (Peter et al., 2000). Similar half-life data in rats are not yet available.
Normoxia
We found MB at 0.5 mg/kg had no significant effects on hypercapnic responses, suggesting MB has no significant effects on vascular reactivity at this dosage. MB also had no significant effects on the M value, corroborating the absence of significant cerebrovascular responses to hypercapnia. At higher doses, MB can interact with nitric oxide synthase and produce cardiovascular effects (Rojas et al., 2012). For example, the vascular dose-response to MB follows a hormetic inverted-U curve, with an intermediate dose (3 mg/kg) improving systemic and pulmonary vascular resistance, compared to little vascular effects with a lower dose (1 mg/kg) or a higher dose (7 mg/kg) (Juffermans et al., 2010).
Basal CBF, which reflects basal metabolism, was higher after MB but did not reach statistical significance (P = 0.18) at this dosage and sample size, consistent with previous in vitro studies showing MB stimulates cell glucose uptake (Louters et al., 2006) and brain tissue oxygen consumption (Riha et al., 2005).
MB significantly enhanced stimulus-evoked CBF and BOLD fMRI responses, suggesting that MB potentiates evoked responses in addition to its effect on basal conditions. This novel finding in vivo is consistent with two previous studies that have documented enhancing effects of MB on evoked neural responses, mapped by quantitative cytochrome oxidase histochemistry ex vivo (Gonzalez-Lima and Bruchey, 2004; Riha et al., 2011). Such enhanced potentiation of increased neural activity could be one of the mechanisms that accounts for memory and performance enhancement widely reported in the MB literature (see review (Rojas et al., 2012; Scheindlin, 2008)) in that MB provides energy substrates to enhance neural evoked responses.
Hypoxia
Mild hypoxia per se decreased basal CBF relative to air (from 0.91 to 0.85 ml/g/min), but did not reach statistical significance. Such mild CBF reduction suggests that basal metabolism was slightly reduced. A previous report under more severe hypoxic conditions with similar experimental conditions and with isoflurane anesthesia found that 12% O2 decreased CBF relative to air (from 1.1 to 0.9 ml/g/min) (Sicard and Duong, 2005).
The effect of hypoxia on oxygen consumption changes during stimulation is of significant interest from the perspective of energy metabolism per se as well as its clinical relevance to hypoxic injury and cerebral ischemia. We found that CBF, BOLD and CMRO2 forepaw-evoked fMRI responses were significantly higher during hypoxia relative to air, suggesting that the brain uses more oxygen to perform the same task under hypoxia. Although hypoxia lowered basal oxygen saturation and thus reduced the denominator, it could not account for the substantially larger BOLD fMRI responses to forepaw stimulation. The larger increases in CBF fMRI responses under hypoxia showed that the evoked BOLD responses are driven in large part by stimulus-evoked CBF increases as a result of increased neural activities. Our findings are consistent with a previous study (Sicard and Duong, 2005) which reported higher evoked CMRO2 changes under hypoxia. Hyperoxia, by contrast, has been reported to decrease evoked oxygen consumption (Sicard and Duong, 2005).
It is conceivable that MB could have a stronger effect under stress conditions where energy substrates are limiting. We thus tested the hypothesis that MB has a stronger effect under mild hypoxic (not ischemic) conditions. During hypoxia, basal CBF was higher. Comparison between normoxia and hypoxia data shows that MB under hypoxia induced a larger potentiation of the forepaw-evoked responses and oxygen consumption increases compared to normoxia. Such enhanced potentiation during hypoxia could be one of the mechanisms that accounts for MB’s neuroprotective effects in metabolically stressed conditions reported in the literature (see review (Rojas et al., 2012)). For example, the higher the metabolic demand for oxygen consumption, the higher the respiratory chain electron flow produced by MB’s electron cycling action in mitochondria (Rojas et al., 2012). Therefore, MB’s effects during hypoxia may potentiate fMRI responses by further increasing mitochondrial electron transport. We predict that more severe (i.e., 9–12% O2) hypoxia could evoke a larger MB effect.
The novel finding of MB’s greater potentiation of evoked fMRI responses under hypoxia is in agreement with previous MB studies. For example, MB can stimulate glucose metabolism in anoxic conditions in vitro (Lee and Urban, 2002). MB can also prevent brain damage induced by hypoxia-reperfusion injury after cardiac arrest, as shown by a decrease in the plasma level of the astroglial marker of hypoxic brain injury (protein S-100Beta) (Miclescu et al., 2006).
These findings agree with the proposed pharmacological action of MB on brain energy metabolism (Bruchey and Gonzalez-Lima, 2008) and neuroprotection (Rojas et al., 2012). Specifically, the local distribution of MB is determined by the electrochemical gradients formed inside intracellular compartments, especially within mitochondria during the electron transport chain (Rojas et al., 2012). Within mitochondria, MB acts as an artificial electron cycler that at low MB concentrations can enter a reversible redox cycle (Rojas et al., 2012). It is well established that reduced MB can donate electrons to electron carriers in the mitochondrial electron transport chain, thereby increasing cytochrome oxidase activity and oxygen consumption and promoting neuroprotection under metabolically stressed conditions (Riha et al., 2005; Riha et al., 2011; Scott and Hunter, 1966; Zhang et al., 2006).
Brain regions that are more affected by MB would seem to depend on regions that have higher activity-dependent energy demand (i.e., localized effects). For example, memory tasks that evoke greater energy demand in the prefrontal cortex, such as extinction memory, show activation specificity after MB (Gonzalez-Lima and Bruchey, 2004). Rats with MB-enhanced extinction memory show relatively greater increases in cytochrome oxidase activity in prefrontal cortical regions thought to contribute to extinction memory than in other regions (Gonzalez-Lima and Bruchey, 2004). Similarly, after MB administration, forepaw stimulation results in an even greater fMRI activation of the somatosensory cortex.
Together these findings suggest that the effects of low-dose MB are not mediated by a global enhancement of brain metabolism but occur in a use-dependent fashion. This use-dependent specificity leads to MB preferential potentiation of regions with a higher metabolic demand although the brain bioavailability and distribution of MB has the potential to be homogeneous. Therefore, cortical regions with the largest metabolic energy demands in an activation task such as forelimb stimulation show the largest increases in evoked CBF and BOLD responses, and those activated regions benefit the most from the metabolic-enhancing effects of MB.
CONCLUSIONS
This study investigated the effects of low-dose MB on basal cerebral blood flow, neurovascular coupling, functional activations and oxygen consumption changes due to forepaw stimulation in the rat brain during normoxia and hypoxia. These findings have implications in neurological conditions with mitochondrial dysfunction and oxidative stress, such as Alzheimer’s and Parkinson’s diseases, normal aging, cerebral ischemia and ischemic reperfusion injury. Future studies will evaluate ATP synthesis rate, glucose consumption and lactate production in vivo in acute and chronic MB administration, and in association with cerebral ischemia. MB is approved for human use at its therapeutic low doses. Clinical trials for additional indications can be readily explored and MRI has the potential to offer longitudinal evaluation of MB treatment efficacy.
Highlights.
Methylene blue (MB) has metabolic-enhancing and antioxidant properties.
MB did not have significant effects on fMRI responses to hypercapnia.
MB potentiated evoked fMRI changes to forepaw stimulation under normoxia.
MB further potentiated fMRI changes under hypoxia relative to normoxia.
These findings could help optimize treatments in neurological diseases with mitochondrial dysfunction.
Acknowledgments
This work was supported in part by a Translational Technology Resource grant via the Clinical Translational Science Award (CTSA, parent grant NIH 8UL1TR000149), the NIH/NEI (R01 EY014211 and EY018855), NIH/NINDS (R01-NS45879). TQD holds the Stanley I Glickman, MD, Endowed Chair in Ophthalmic Research. We thank Dr. Shaohau Yang and Dr. Ailing Lin for helpful discussion in various phases of the project.
Footnotes
Disclosure/Conflict of Interest
The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bandettini PA, Wong EC, Hinks RS, Rikofsky RS, Hyde JS. Time course EPI of human brain function during task activation. Magn Reson Med. 1992;25:390–397. doi: 10.1002/mrm.1910250220. [DOI] [PubMed] [Google Scholar]
- Boxerman JL, Bandettini PA, Kwong KK, Baker JR, Davis TL, Rosen BR, Weisskoff RM. The intravascular contribution to fMRI signal change: Monte Carlo modeling and diffusion-weighted studies in vivo. Magn Reson Med. 1995;34:4–10. doi: 10.1002/mrm.1910340103. [DOI] [PubMed] [Google Scholar]
- Bruchey AK, Gonzalez-Lima F. Behavioral, physiological and biochemical hormetic responses to the autoxidizable dye methylene blue. Am J Pharmacol Toxicol. 2008;3:72–79. doi: 10.3844/ajptsp.2008.72.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton J, 2nd, Leikin JB. Methylene blue. Am J Ther. 2003;10:289–291. doi: 10.1097/00045391-200307000-00009. [DOI] [PubMed] [Google Scholar]
- Davis TL, Kwong KK, Weisskoff RM, Rosen BR. Calibrated functional MRI: Mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci USA. 1998;95:1834–1839. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med. 1992;23:37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- Duong TQ, Silva AC, Lee SP, Kim SG. Functional MRI of calcium-dependent synaptic activity: cross correlation with CBF and BOLD measurements. Magn Reson Med. 2000;43:383–392. doi: 10.1002/(sici)1522-2594(200003)43:3<383::aid-mrm10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lima F, Bruchey AK. Extinction memory improvement by the metabolic enhancer methylene blue. Learn Mem. 2004;11:633–640. doi: 10.1101/lm.82404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb RL, Raichle ME, Eichling JO, Ter-Pogossian MM. The effects of changes in PaCO2 on cerebral blood volume, blood flow, and vascular mean transit time. Stroke. 1974;5:630–639. doi: 10.1161/01.str.5.5.630. [DOI] [PubMed] [Google Scholar]
- Ishiwata A, Sakayori O, Minoshima S, Mizumura S, Kitamura S, Katayama Y. Preclinical evidence of Alzheimer changes in progressive mild cognitive impairment: a qualitative and quantitative SPECT study. Acta Neurol Scand. 2006;114:91–96. doi: 10.1111/j.1600-0404.2006.00661.x. [DOI] [PubMed] [Google Scholar]
- Juffermans NP, Vervloet MG, Daemen-Gubbels CR, Binnekade JM, de Jong M, Groeneveld AB. A dose-finding study of methylene blue to inhibit nitric oxide actions in the hemodynamics of human septic shock. Nitric Oxide. 2010;22:275–280. doi: 10.1016/j.niox.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Kwong K, Hoppel B, Weisskoff R, Kiihne S, Barrere B, Moore J, Poncelet B, Rosen B, Thulborn K. Regional cerebral tissue oxygenation studied with EPI at clinical field strengths. J Magn Reson Imag. 1992;2:44. [Google Scholar]
- Lee RB, Urban JP. Functional replacement of oxygen by other oxidants in articular cartilage. Arthritis Rheum. 2002;46:3190–3200. doi: 10.1002/art.10686. [DOI] [PubMed] [Google Scholar]
- Lindahl PE, Oberg KE. The effect of rotenone on respiration and its point of attack. Exp Cell Res. 1961;23:228–237. doi: 10.1016/0014-4827(61)90033-7. [DOI] [PubMed] [Google Scholar]
- Liu ZM, Schmidt KF, Sicard KM, Duong TQ. Imaging oxygen consumption in forepaw somatosensory stimulation in rats under isoflurane anesthesia. Magn Reson Med. 2004;52:277–285. doi: 10.1002/mrm.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louters LL, Dyste SG, Frieswyk D, Tenharmsel A, Vander Kooy TO, Walters L, Whalen T. Methylene blue stimulates 2–deoxyglucose uptake in L929 fibroblast cells. Life Sci. 2006;78:586–591. doi: 10.1016/j.lfs.2005.05.082. [DOI] [PubMed] [Google Scholar]
- Mandeville JB, Marota JJ, Ayata C, Moskowitz MA, Weisskoff RM, Rosen BR. MRI measurement of the temporal evolution of relative CMRO2 during rat forepaw stimulation. Magn Reson Med. 1999;42:944–951. doi: 10.1002/(sici)1522-2594(199911)42:5<944::aid-mrm15>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Mandeville JB, Marota JJ, Kosofsky BE, Keltner JR, Weissleder R, Rosen BR. Dynamic functional imaging of relative cerebral blood volume during rat forepaw stimulation. Magn Reson Med. 1998;39:615–624. doi: 10.1002/mrm.1910390415. [DOI] [PubMed] [Google Scholar]
- Martinez J, Jr, Jensen RA, Vasquez B, McGuiness T, McGaugh JL. Methylene blue alters retention of inhibitory avoidance responses. Physiological Psychology. 1978;6:387–390. [Google Scholar]
- Medina DX, Caccamo A, Oddo S. Methylene blue reduces abeta levels and rescues early cognitive deficit by increasing proteasome activity. Brain Pathol. 2010;21:140–149. doi: 10.1111/j.1750-3639.2010.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miclescu A, Basu S, Wiklund L. Methylene blue added to a hypertonic-hyperoncotic solution increases short-term survival in experimental cardiac arrest. Crit Care Med. 2006;34:2806–2813. doi: 10.1097/01.CCM.0000242517.23324.27. [DOI] [PubMed] [Google Scholar]
- O’Leary JC, 3rd, Li Q, Marinec P, Blair LJ, Congdon EE, Johnson AG, Jinwal UK, Koren J, 3rd, Jones JR, Kraft C, Peters M, Abisambra JF, Duff KE, Weeber EJ, Gestwicki JE, Dickey CA. Phenothiazine-mediated rescue of cognition in tau transgenic mice requires neuroprotection and reduced soluble tau burden. Mol Neurodegener. 2010;5:45. doi: 10.1186/1750-1326-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci USA. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz M, Lorke DE, Petroianu GA. Methylene blue and Alzheimer’s disease. Biochem Pharmacol. 2009;78:927–932. doi: 10.1016/j.bcp.2009.04.034. [DOI] [PubMed] [Google Scholar]
- Peter C, Hongwan D, Kupfer A, Lauterburg BH. Pharmacokinetics and organ distribution of intravenous and oral methylene blue. Eur J Clin Pharmacol. 2000;56:247–250. doi: 10.1007/s002280000124. [DOI] [PubMed] [Google Scholar]
- Riha PD, Bruchey AK, Echevarria DJ, Gonzalez-Lima F. Memory facilitation by methylene blue: dose-dependent effect on behavior and brain oxygen consumption. Eur J Pharmacol. 2005;511:151–158. doi: 10.1016/j.ejphar.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Riha PD, Rojas JC, Gonzalez-Lima F. Beneficial network effects of methylene blue in an amnestic model. Neuroimage. 2011;54:2623–2634. doi: 10.1016/j.neuroimage.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas JC, Bruchey AK, Gonzalez-Lima F. Neurometabolic mechanisms for memory enhancement and neuroprotection of methylene blue. Progress in Neurobiology. 2012;96:32–45. doi: 10.1016/j.pneurobio.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy CS, Sherrington CS. On the regulation of blood supply of the brain. J Physiol. 1890;1:85–108. doi: 10.1113/jphysiol.1890.sp000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheindlin S. Something old... something blue. Mol Interv. 2008;8:268–273. doi: 10.1124/mi.8.6.1. [DOI] [PubMed] [Google Scholar]
- Scott A, Hunter FE., Jr Support of thyroxine-induced swelling of liver mitochondria by generation of high energy intermediates at any one of three sites in electron transport. J Biol Chem. 1966;241:1060–1066. [PubMed] [Google Scholar]
- Shen Q, Meng X, Fisher M, Sotak CH, Duong TQ. Pixel-by-pixel spatiotemporal progression of focal ischemia derived using quantitative perfusion and diffusion imaging. J Cereb Blood Flow and Metab. 2003;23:1479–1488. doi: 10.1097/01.WCB.0000100064.36077.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Ren H, Cheng H, Fisher M, Duong TQ. Functional, perfusion and diffusion MRI of acute focal ischemic brain injury. J Cereb Blood Flow and Metab. 2005;25:1265–1279. doi: 10.1038/sj.jcbfm.9600132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard KM, Duong TQ. Effects of hypoxia, hyperoxia and hypercapnia on baseline and stimulus-evoked BOLD, CBF and CMRO2 in spontaneously breathing animals. Neuroimage. 2005;25:850–858. doi: 10.1016/j.neuroimage.2004.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A, Lee SP, Yang C, Iadecola C, Kim SG. Simultaneous BOLD and perfusion functional MRI during forepaw stimulation in rats. J Cereb Blood Flow Metab. 1999;19:871–879. doi: 10.1097/00004647-199908000-00006. [DOI] [PubMed] [Google Scholar]
- Wen Y, Li W, Poteet EC, Xie L, Tan C, Yan LJ, Ju X, Liu R, Qian H, Marvin MA, Goldberg MS, She H, Mao Z, Simpkins JW, Yang SH. Alternative mitochondrial electron transfer as a novel strategy for neuroprotection. J Biol Chem. 2011;286:16504–16515. doi: 10.1074/jbc.M110.208447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrubel KM, Riha PD, Maldonado MA, McCollum D, Gonzalez-Lima F. The brain metabolic enhancer methylene blue improves discrimination learning in rats. Pharmacol Biochem Behav. 2007;86:712–717. doi: 10.1016/j.pbb.2007.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Rojas JC, Gonzalez-Lima F. Methylene blue prevents neurodegeneration caused by rotenone in the retina. Neurotox Res. 2006;9:47–57. doi: 10.1007/BF03033307. [DOI] [PubMed] [Google Scholar]



