Abstract
Objectives
Loss of epithelial cells is one of the key factors that lead to airway fibrosis. Loss of epithelial cells may decrease the barrier to host cell infiltration into the lumen, allowing deposition of extracellular matrix, with subsequent obliteration of the airway. The objective of this study was to determine if injection of epithelial cells/progenitor cells from the recipient into the lumen of the donor trachea could prevent bronchiolitis obliterans (BO) in a mouse heterotopic tracheal transplantation (HTT) model.
Methods
An MHC class I- and class II-mismatch of mouse HTT model of BO was used. Epithelial cells from recipient mice were isolated and re-injected into the lumen of the allografts on day 3 post-transplantation. Rag-1 knock-out and isografts were also performed as controls. The grafts were analyzed by immunohistochemistry and densitometric analysis.
Results
The results demonstrated that tracheal epithelium was lost by day 3, and regenerated between 3 to 7 days, and lost again in all allografts, but not in the isografts or in Rag-1 knock-out groups by day 12. The reconstituted epithelium was donor originated on day 7 based on green fluorescent protein staining. Furthermore, with the injection of recipient cells into the tracheal lumen, loss of the epithelium was not observed and the luminal obliteration was significantly less in the allografts.
Conclusions
Injection of recipient epithelial cells prevents the second phase of epithelial loss and significantly decreases BO development in a HTT model. Clinically the use of injected recipient epithelial cells could be a novel treatment for BO.
INTRODUCTION
Lung transplantation is currently recognized as the preferred treatment for selected patients with end-stage pulmonary diseases. Despite notable advances since its inception, long term mortality of lung recipients is still highest among all solid organs transplanted. The Achilles’ heel of lung transplantation remains chronic allograft rejection (1, 2). Chronic lung allograft rejection is also called bronchiolitis obliterans (BO) and histologically is a temporal process with loss of luminal epithelium, peribronchiolar leukocyte infiltration, and subsequent fibro-obliteration of the airways (2, 3). Once BO develops progressive decline in pulmonary function is typical. Unfortunately BO responds poorly to standard immunosuppression, and most patients die of respiratory failure within 5 years of onset.
We and others have used a preclinical well-described mouse heterotopic tracheal transplant (HTT) model to better understand the mechanisms involved in BO (4–6). It is known from these studies that once the epithelial cells are lost, the basement membrane is exposed and fibroblasts/myofibroblasts infiltrate into lumen and secrete extracellular matrix, with the subsequent development of fibrosis. Mukaida et al reported that the regenerated epithelial cells were of donor phenotype on day 10, but changed to recipient phenotype after day 50 in a cryopreserved tracheal allotransplantation dog model (7). Similarly, Ikonen and Adams demonstrated that regenerated epithelial cells were of donor phenotype on day 7, but switched to recipient phenotype on day 30 and 60 in (8, 9) in orthotopic tracheal allografts in a non-immunosuppressed rat model. The origin of regenerated epithelial cells has not been reported in the mouse tracheal transplantation model. Some have proposed that circulating recipient epithelial cells may also contribute to repair (10, 11). Published data have shown that stem cells are involved in airway epithelium repair (12–14). We hypothesized that a chimeric epithelium (of donor and recipient origin) would be less immunogenic and potentially be protective against the secondary loss of epithelium. The purpose of this study was to test our hypothesis that the prevention of the secondary loss of epithelial cells could prevent BO development, and in order to prevent this secondary loss we injected recipient epithelial cells into the donor tracheas after the initial loss and during the regenerative phase with the goal of creating a chimeric epithelium that would be more tolerant. In this study, we tried to explore whether injection of epithelial progenitor cells from recipients into the donor trachea would prevent BO development by preserving the second loss of epithelium cell.
Materials and Methods
Animals
For tracheal transplant experiments, Balb/c male mice, C57BL6 male mice were purchased from Jackson Laboratory, Bar Harbor, ME. GFP (C57BL6 background) male mice were from Linden’s Laboratory (28–35 grams). Rag-1 knock out (KO, C57BL6 background) male mice were kindly donated from the laboratory of Dr. Victor Laubach. According to the published results, Rag1-KO mice have no functional T and B cells (15). However, they have normal levels of functional natural killer (NK) cells. All the experimental mice received humane care in accordance with “Principles of Laboratory Animal Care,” formulated by the National Society for Medical Research and The Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Science and published by NIH. The study protocol was approved by the Animal Care and Use Committee at the University of Virginia before experimentation.
Mouse model of heterotopic tracheal transplant
We used a heterotopic subcutaneous tracheal transplant model of BO as described in our previously publications (4, 5). Briefly, an MHC class I- and class II-mismatch was produced by tracheal transplanting between Balb/c (H-2d) tracheas and C57BL/6 or the other way around.
Experimental group design
Experimental mice were divided into four groups: (1) Balb/c tracheas transplanted to C57BL/6, GFP and Rag-1 KO; (2) GFP tracheas transplanted to Balb/c mice; (3) Balb/c tracheas transplanted to GFP mice treated with GFP-epithelial cells; (4) C57BL/6 tracheas transplanted to C57BL/6 as isograft controls. In all groups, 4 donor tracheas were transplanted into one recipient, 3 recipients were used in each group and each timepoint (day 3, 7, 12, 21 and 28). Total of 60 donors and 15 recipients were used in each group. The mice were sacrificed and the isograft and allografts were collected on days 3, 7, 12, 21, or 28 for histology and immunhistochemical staining.
Isolation of murine tracheal epithelial cells
Mice (C57BL/6 or GFP, the same strain of mice as recipients) were euthanized and total of 12 tracheas were isolated and washed with HEPES-DMEM supplemented with 1% penicillin– streptomycin (Invitrogen, Grand Island, NY). The isolated trachea was placed into 60 mm cell culture dish. 50 µL of digestion media, which contain DMEM with 0.01% collagenase A (Roche Diagnostics, Mannheim, Germany) and 1% penicillin–streptomycin, was injected into the lumen of the isolated trachea and incubated for 1 hour in 5% CO2 at 37 °C, the tracheas were turned over every 15 minutes. Then the lumen were flushed with 1XPBS contain 1% penicillin–streptomycin. The collected epithelial rich solution was centrifuged at 600 rpm for 5 minutes. The cells were confirmed by staining with keratins (K14 and K5) antibodies and cell numbers were counted using hemacytometer under microscopy. Then the cells from each trachea were resuspended in PBS (30 µL), which is supplied with 1% penicillin–streptomycin. The digested tracheas were immediately fixed in 4% Zinc-formalin. After 24 hours, they were embedded in paraffin, sectioned and stained for histological examination.
Injection of the isolated epithelial cells into the lumen of allograft
On the third day after tracheal transplantation, the recipient mice were anesthetized. An incision was made to expose the transplanted trachea followed by the injection of the isolated epithelial cells directly into the lumen of the allografts. The isolated epithelial cells (30 µL) from each trachea were injected into one allograft only with a syringe. Same volume (30 µL) of 1XPBS supplied with 1% penicillin–streptomycin was also injected into each allograft in the control group.
Histology
Transplanted tracheal tissues were collected and immediately fixed in 4% Zinc-formalin, After 24 hours they were embedded in paraffin, sectioned and stained with hematoxylin & eosin (H&E) or with anti-Mac-2, anti- neutrophil, anti-CD3, and anti-GFP antibodies. Collagen deposition was detected by Direct Red 80 collagen staining.
Immunohistochemical Staining of Macrophages and Neutrophils
Macrophages and neutrophils were detected by immunohistochemical analysis as described previously (4, 5). Briefly, rat anti-mouse neutrophil (AbD Serotec, Raleigh, NC) or rat anti-mouse macrophage (Mac-2, Accurate Chem, Westbury, NY) antibodies were used as primary antibodies. Alkaline phosphatase - conjugated anti-rat IgG (Sigma, St Louis MO) were employed as secondary antibody. Fast-Red (Sigma, St Louis MO) was used as substrate. Purified normal rat IgG (eBioscience Inc, San Diego, CA) was used as a negative control. The sections were counterstained lightly with hematoxylin for viewing negatively stained cells. Macrophage and neutrophil infiltration were semi quantified using Image-Pro Plus software. The densitometric value of positive staining area of each section was blindly selected and measured.
Immunohistochemical staining of CD3+ T cells
The staining was performed according to our previous publications (4, 5, 16). Briefly, the transplanted trachea sections (5µm) were dehydrated, and incubated with 1% hydrogen peroxide. Sections were then boiled in Unmasking Solution (Vector Laboratories, Burlingame, CA) and blocked with 10% serum. Goat anti-mouse CD3ε antibody (Santa Cruz Biotechnology) is primary antibody. After incubation with an avidin-biotin complex, immunoreactivity was visualized by incubating the sections with 3, 3-diaminobenzidine tetrahydrochloride (DAKO Corp) to produce a brown precipitate, and then counterstained with hematoxylin. The number of CD3+ T cells per high power field was assessed by immunohistochemical staining of tracheal sections, and at least 5 fields were counted per trachea by blinded observers. The average cell number was used for statistical analysis.
Measurement of the luminal obliteration
The luminal obliteration was evaluated according to our previous publication (4, 5). Briefly, allografts were photographed at 4× magnification and the area of the obliterated lumen and the total area of lumen were measured using the Image-Pro Plus software. The percent of the obliteration was calculated by the area of the fibrosis divided by the total area of lumen. Eight to ten allografts were measured in each group. The data was used for statistical analysis.
Statistical analysis
Data are presented as the mean ± SEM. The macrophage, neutrophil and CD3+ T cell counts were compared using one-way ANOVA followed by the Student's t test for unpaired data with Bonferroni correction. Square roots of tissue cell counts were compared using one-way ANOVA. A P<0.05 was considered significant.
RESULTS
Loss of epithelial cells in allografts
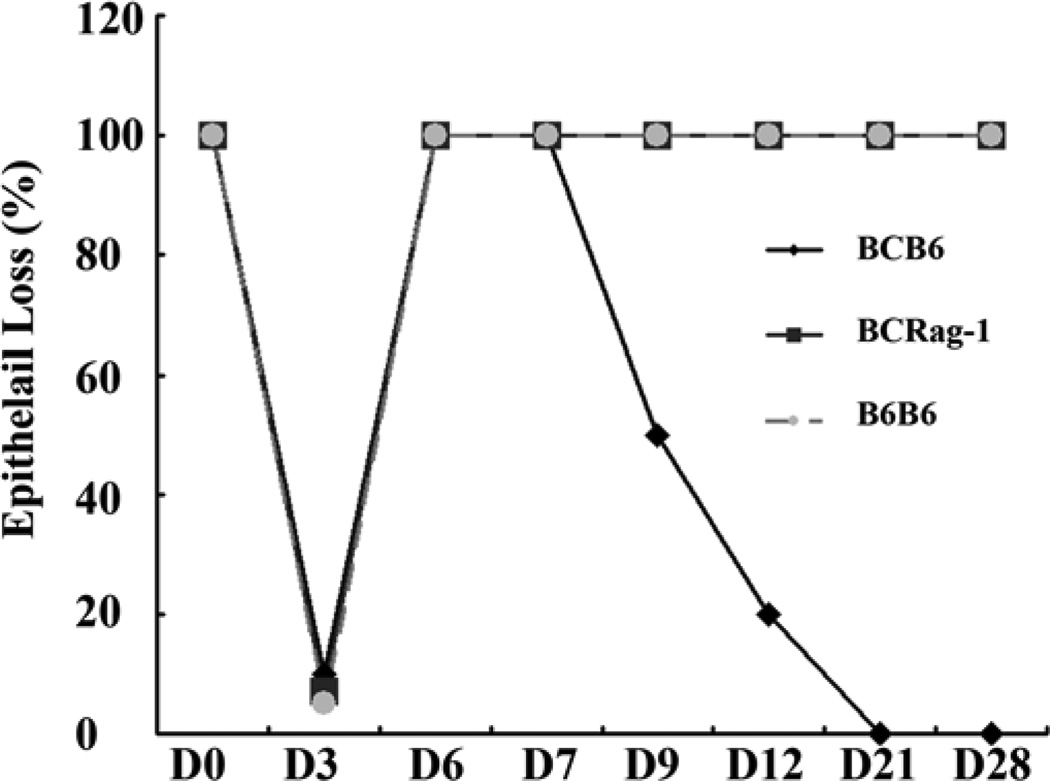
Using a well established HTT model (Balb/C trachea as donor and wild type C57BL/6), we have observed the histological kinetics of allografts post-transplantation from the different group and different time points. The results confirmed our previous finding that the first complete loss of the epithelium occurred in all transplanted trachea on day 3 post-transplantation (Figures 1 & 2). The cause of the first (phase I) epithelial loss is early ischemia-reperfusion injury, and thus this also happened in Rag-1 KO mice and isograft control group (C57BL/6 into C57BL/6, (Figures 1 & 2). There was full re-epithelialization of the basement membrane between 3 to 7 days in all of the transplants. Interestingly, the second loss of epithelial cells (phase II) were found in C57BL/6 WT mice from days 7 to day 12 (Figures 1 & 2). In contrast, there were no second loss of epithelial cells in RAG-1 KO mice and isograft controls (Figures 1 and 2). The results show that the second loss of epithelial cells on Day 12 is closely correlated with luminal obliteration on Day 21(Figures 1 & 2).
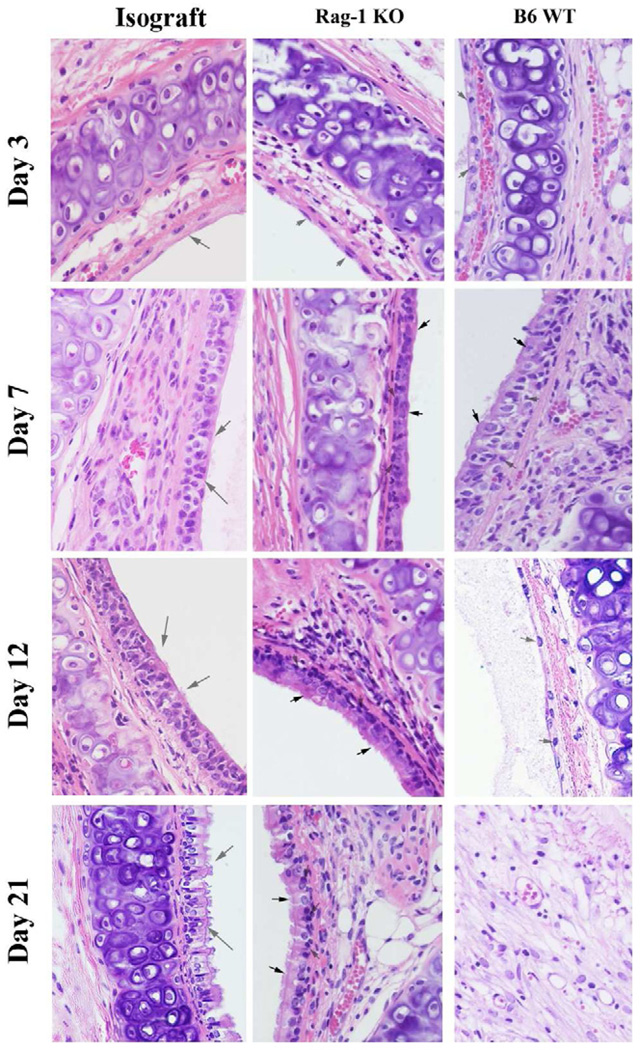
Figure 1.
Representative pictures of HE staining of the allograft from 3, 7, 12 and 21 days post-transplantation. The magnification of all the pictures was 40×. Solid arrows indicate epithelial cells. The open arrows indicate basal cells. The donors are Balb/c mice and the recipients are Rag-1 KO, and C57BL/6 wild type mice. C57BL/6 to C57BL/6 isograft controls are also performed.
Figure 2.
Losing of epithelial cells in the transplanted trachea. Epithelial cells are disappeared rapidly due to ischemia-reperfusion injury at early stage (Days 1 to 3 post-tracheal transplantation) in all transplanted trachea. The pseudoepithelial cells are regenerated from days 4 to 7 in all allografts. The second loss of the regenerated epithelium occurred from days 7 to 14 in all of the allografts except in RaG-1 KO mice, which show similar pattern as isograft controls.
The regenerated epithelium originates from donor basal cells, not from a recipient source
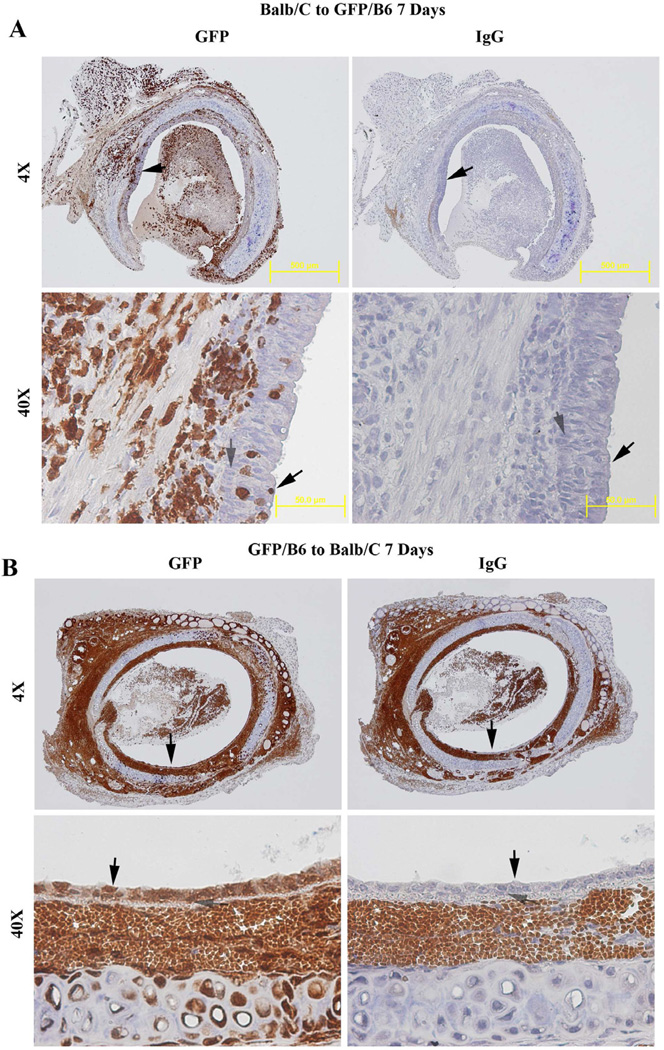
To determine the origin of the regenerated epithelial cells, genetic GFP mice (on C57BL/6 background) was employed. First, we confirmed that the cells in trachea, lung, small intestine, spleen, heart and kidney (data not shown) from GFP mice are GFP-positive cells. Then, using Balb/C as donor and GFP mice as recipients, we found that the regenerated epithelial cells in the allografts are GFP-negative (Figure 3A), evidence that these cells are of donor origin. We did note a small portion of GFP-positive cells in the epithelial layer but these were verified to be CD3+ T cells (Figure 4A). Confirming this result we found when we used GFP mice as donor and Balb/C as recipients, the regenerated epithelial cells were all GFP-positive, evidence again that they are again of donor origin (Figure 3B). These results indicate that the regenerated epithelial cells originate from the donor, not from the recipients. Of note in the isografts there were no inflammatory or T cells seen infiltrating in the epithelial layer in isograft (Figure 4B).
Figure 3.
Immunohistochemical staining of GFP in allografts on day 7 post tracheal transplantation. (A) Representative picture of GFP staining in allograft (Balb/c to GFP/C57BL/6) on day 7. (B) Representative picture of GFP staining in allograft (GFP/C57BL/6 to Balb/c) on day 7. Cells stained brown/dark brown indicate GFP positive cells. All sections were counterstained lightly with hematoxylin for viewing negatively stained cells. Normal IgG was employed as controls. Solid arrows indicate epithelial cells. The open arrows indicate basal cells. The magnifications are indicated in the pictures.
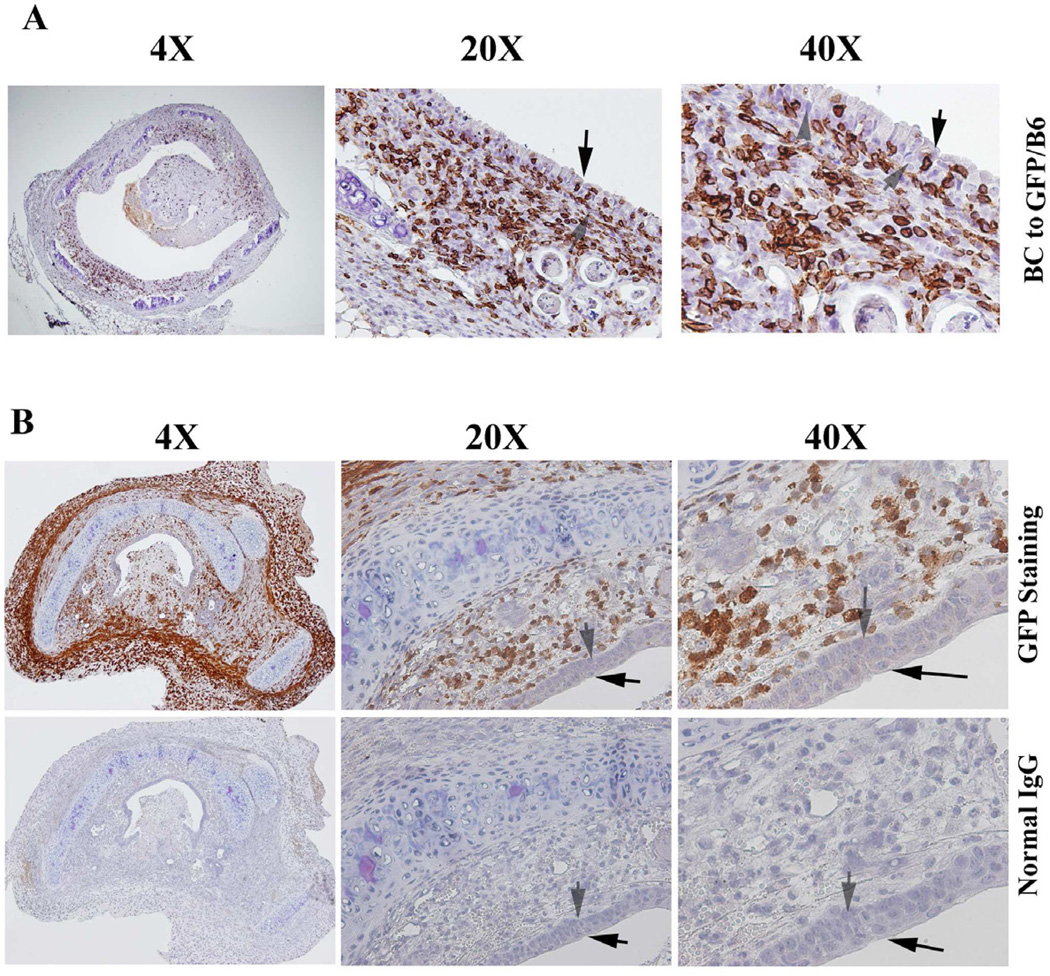
Figure 4.
Immunohistochemical staining of CD3+ T-cells in (A) the allografts of Balb/c to GFP/C57BL/6, and in (B) isograft of C57BL/6 to C57BL/6. Cells stained brown indicate CD3+ T-cell infiltration. All sections were counterstained lightly with hematoxylin for viewing negatively stained cells. The slides were stained with anti- CD3 antibody. Normal IgG was used as control. The magnifications were indicated in the pictures.
Leukocyte Infiltration
Macrophages infiltration was observed with peak on day 7 in the allografts of C57BL/6 wild type mice, while the macrophage infiltration reached peak on Day 12 in Rag-1 KO (data not shown). However, the neutrophil infiltration peak was on day 7 in all the allografts except on Day 3 in Rag-1 KO mice. Interestingly, the neutrophil infiltration was vanished after day 7 in Rag-1 KO mice. These findings indicate that the early infiltration (Days 1–3) of the macrophage and neutrophil were a reaction of acute injury caused by ischemia-reperfusion. The CD3+ T cells may play an important role in later neutrophil infiltration (Days 4–21), because Rag-1 KO mice had no neutrophil infiltration during these time period. The macrophage infiltration was delayed in Rag-1 KO mice.
To determine if there is an adaptive immune role of CD3+ T cell in development of BO, infiltration of CD3+ T cells was assessed via immunohistochemistry in the different groups. The results showed that few CD3+ T cells were detected in allografts during the first 3 days after transplantation. Increasing infiltration of CD3+ T cells was apparent on day 7 (Figure 4A) which peaked on day 7– 12 in different allografts, and significantly decreased afterwards (data not shown). However, the number of infiltrating CD3+ T cells was significantly abrogated in the epithelial layer of isografts (C57BL/6 to C57BL/6, Figure 4B). As expected, only few naïve CD3+ T cells were detected in Rag-1 KO mice (Data not shown). These results suggest that CD3+ T cells infiltrating into the epithelial layer may be a key contributor for second loss of epithelial cells in the transplanted trachea.
Epithelial/basal cells harvest for allograft injection
The epithelial cells from recipient mice were isolated using digestion solution with 0.01% collagenase A as described in method section. After digestion, the tracheas were processed for histological examination. As shown in supplemental Figure 1, the epithelial cells was not detached within 15 minutes, but detached after 30 minutes. Most of the epithelial cells, including the basal cells were detached by 1 hour of digestion.
Injection of the isolated epithelial cells from recipients prevent the second loss of epithelial cells and BO development
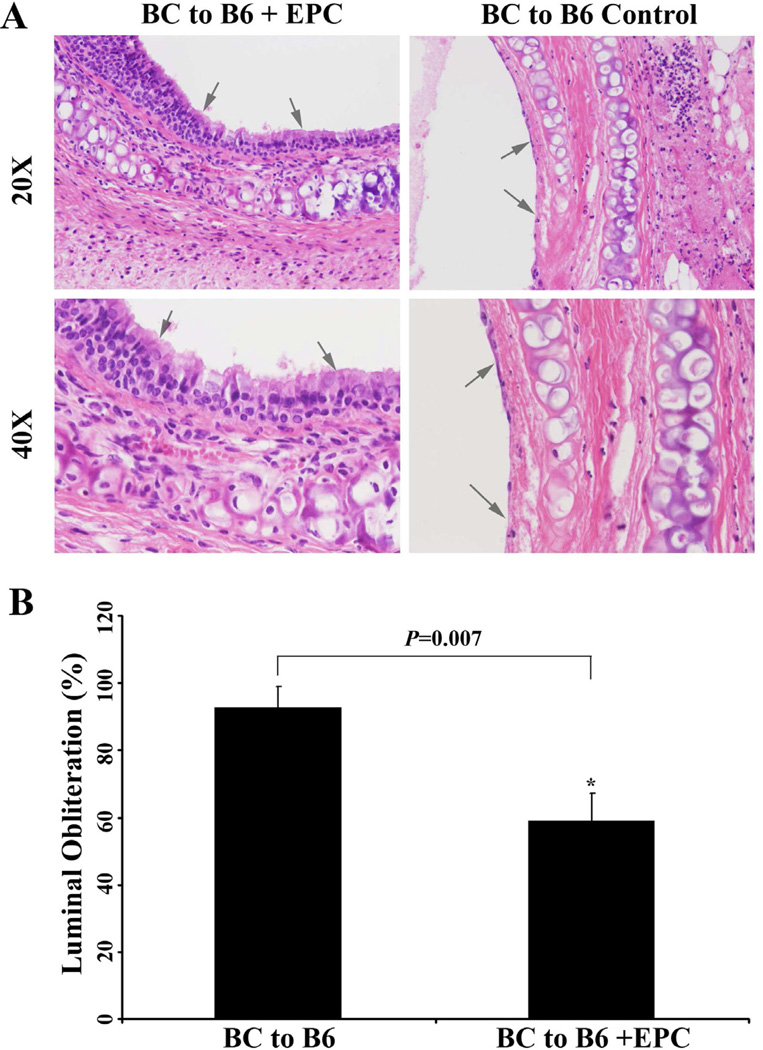
After injection of the isolated epithelial cells from recipients into the allografts on day 3 post-transplantation, the chimerized pseudoepithelial cells formed. It should be noted that the chimerized epithelial cells did not undergo disappearance when compared with the control groups on day 12 (Figure 5A). Luminal fibro-obliteration was observed from days 12–21 in all the experimental groups except isograft control and Rag-1 KO groups. The luminal obliteration of the allograft received epithelial injection were significantly less on days 21 (P=0.007) compared to the 1XPBS control group (Figure 5B). It is notable that the luminal obliteration was markedly decreased not only in isografts and Rag-1 KO mice, but in the recipient epithelial cell injected allografts as well.
Figure 5.
(A) Injection of recipients epithelial cells prevent the second epithelial loss on day 12 (top panel) when compared with non-injected mice (bottom panel). B) Comparison of luminal obliteration of the allografts with and without epithelial injection on day 21. Data shown are the mean ± S.D. *, P=0.007, n=6.
DISCUSSION
A mouse HTT model used in these studies is often referred to as obliterative airway disease (3, 6) because it displays several features that are similar to human BO. Heterotopic tracheas transplanted into HLA mismatched recipients develop injury that can be divided into an acute phase (days 1 to 3) characterized by loss of epithelial cells and inflammation resulting from ischemia-reperfusion injury and a later phase (days 4 to 12) involving the adaptive immune system with lymphocyte infiltration and eventually fibro-obliteration (days 13 to 28). We acknowledge that the mouse HTT model has significant shortcomings: Most notably, this model is neither vascularized nor aerated and is a large airway model for a small airway disease. There are other models used to study BO, including an orthotopic tracheal model (17). However, the orthotopic model is similarly a large airway model, non vascularized, technically more challenging, and unable to uniformly develop histopathologic OB. A single lung transplantation has been successfully performed in mice (18, 19), however these transplanted lungs allografts have not been shown to reliably develop histopathologic lesions of BO. On the other had, human small airways have epithelial layer, while the mouse small airways lack of epithelial layer, therefore, mouse large airway with the epithelial cells may better represent human small airway in this animal model.
Previous publications have shown the regenerated epithelial cells to be of donor origin in the early stage, but these studies were in rat and dog tracheal transplantation models (7–9). Our current study confirmed these results using a mouse HTT model. In this model there is early loss of epithelial cells (Phase I) caused by ischemia-reperfusion injury, followed by regeneration and then a second loss of epithelial cells (Phase II) which occurs only in allografts, and is believed to be immunologic in origin. Our current result revealed that Rag-1 KO mice which lack T and B-lymphocytes underwent the initial loss of epithelium, but not the secondary immunologic loss. Additionally, we used GFP recipients and donors to show the origin of the regenerated epithelial cells to be of donor origin. We also used this strain to detect the integration of injected recipient epithelial cells into the donor trachea. The novel findings of this study are injection of epithelial cells, which were isolated from recipient trachea, could block BO development by preventing the second loss of epithelial cells in the tested allografts.
Regeneration and restoration of the airway epithelium after physical, viral or bacterial injury plays a role in numerous respiratory diseases, such as chronic bronchitis, asthma and cystic fibrosis (20). Data from published animal models show epithelial regeneration to be a complex process involving migration of the basal cells adjacent to the injury; rapid restoration of tight junctions followed by squamous metaplasia, active mitosis leading to basal and mucous cell hyperplasia, and progressive redifferentiation with the emergence of ciliated and mucous cells, and ciliogenesis. This process allows for the regeneration of a functional mucociliary epithelium (20).
Evidence suggests that stem/progenitor cells play an important role in epithelial regeneration following trachea/lung injuries (13, 21–24). The idea and technique of implanting recipient epithelium into a heterotopic donor trachea prior to orthotopic transplantation was previously published as a successful case report by the Leuven group (25). Because the second loss of epithelial cells is associated with alloimmune reaction, we explored the possibility that injection of epithelial stem/progenitor cells from recipients may prevent BO development in the mouse HTT model.
Several potential sources of stem/progenitor cells for airway epithelium have been identified including endogenous stem/progenitor cells present in the respiratory tract and/or from other tissues in the body; these latter cells can be transported to the lung, where they can divide and grow (13). In this study, we focus on the potential function of local epithelial stem/progenitor cells (basal and Clara cells) after tracheal transplantation. Since both basal and secretory epithelial cells may serve as stem/progenitors during trachea/lung injuries, we investigated the use of mixed epithelial cells from recipients in our current study. The results demonstrated that injection of tracheal epithelial cells, were able to prevent the second loss of epithelial cells and as consequence, prevented the BO development. Our results provide preclinical evidence that epithelial stem/progenitor cells may be a viable treatment strategy for BO after lung transplantation.
In conclusion our results show injection of epithelial stem/progenitor cells obtained from recipients into the allografts on day 3 prevent second loss of epithelial cell in the allografts, which subsequently decreased BO development in mouse heterotopic tracheal transplantation model. These results suggest that employing epithelial stem/progenitor cells may provide a novel therapeutic strategy to prevent BO after lung transplantation.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Joel Linden who provided us with the GFP/B6 mice and Dr. Victor Laubach provided us with the Rag-1 KO mice.
GRANTS
CLL is supported by a grant sponsored by the National Heart, Lung, and Blood Institute (1K08HL094704). ILK is supported by 5RO1HL092953 from NIH. GRU is supported by 5RO1HL081629-06 from NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: There is no conflict of interest to disclose.
References
- 1.Granton J. Update of early respiratory failure in the lung transplant recipient. Curr Opin Crit Care. 2006;12:19–24. doi: 10.1097/01.ccx.0000198995.44943.63. [DOI] [PubMed] [Google Scholar]
- 2.Chan A, Allen R. Bronchiolitis obliterans: an update. Curr Opin Pulm Med. 2004;10(2):133–141. doi: 10.1097/00063198-200403000-00008. [DOI] [PubMed] [Google Scholar]
- 3.McDyer JF. Human and murine obliterative bronchiolitis in transplant. Proc Am Thorac Soc. 2007;4(1):37–43. doi: 10.1513/pats.200605-107JG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao Y, LaPar DJ, Steidle J, Emaminia A, Kron IL, Ailawadi G, et al. Adenosine signaling via the adenosine 2B receptor is involved in bronchiolitis obliterans development. J Heart Lung Transplant. 2010;29(12):1405–1414. doi: 10.1016/j.healun.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lau CL, Zhao Y, Kron IL, Stoler MH, Laubach VE, Ailawadi G, et al. The role of adenosine A2A receptor signaling in bronchiolitis obliterans. Ann Thorac Surg. 2009;88(4):1071–1078. doi: 10.1016/j.athoracsur.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hele DJ, Yacoub MH, Belvisi MG. The heterotopic tracheal allograft as an animal model of obliterative bronchiolitis. Respir Res. 2001;2(3):169–183. doi: 10.1186/rr55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mukaida T, Shimizu N, Aoe M, Andou A, Date H, Moriyama S. Origin of regenerated epithelium in cryopreserved tracheal allotransplantation. Ann Thorac Surg. 1998;66(1):205–208. doi: 10.1016/s0003-4975(98)00154-4. [DOI] [PubMed] [Google Scholar]
- 8.Ikonen TS, Brazelton TR, Berry GJ, Shorthouse RS, Morris RE. Epithelial re-growth is associated with inhibition of obliterative airway disease in orthotopic tracheal allografts in non-immunosuppressed rats. Transplantation. 2000;70(6):857–863. doi: 10.1097/00007890-200009270-00002. [DOI] [PubMed] [Google Scholar]
- 9.Adams BF, Brazelton T, Berry GJ, Morris RE. The role of respiratory epithelium in a rat model of obliterative airway disease. Transplantation. 2000;69(4):661–664. doi: 10.1097/00007890-200002270-00031. [DOI] [PubMed] [Google Scholar]
- 10.Gomperts BN, Belperio JA, Rao PN, Randell SH, Fishbein MC, Burdick MD, et al. Circulating progenitor epithelial cells traffic via CXCR4/CXCL12 in response to airway injury. J Immunol. 2006;176:1916–1927. doi: 10.4049/jimmunol.176.3.1916. [DOI] [PubMed] [Google Scholar]
- 11.Kleeberger W, Versmold A, Rothamel T, Glockner S, Bredt M, Haverich A, et al. Increased chimerism of bronchial and alveolar epithelium in human lung allografts undergoing chronic injury. Am J Pathol. 2003;162:1487–1494. doi: 10.1016/S0002-9440(10)64281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roomans GM. Tissue engineering and the use of stem/progenitor cells for airway epithelium repair. Eur Cell Mater. 2010;19:284–299. doi: 10.22203/ecm.v019a27. [DOI] [PubMed] [Google Scholar]
- 14.Giangreco A, Arwert EN, Rosewell IR, Snyder J, Watt FM, Stripp BR. Stem cells are dispensable for lung homeostasis but restore airways after injury. Proc Natl Acad Sci USA. 2009;106:9286–9291. doi: 10.1073/pnas.0900668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mombaerts P, Iacomino J, Johnson RS, et al. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Sharma AK, LaPar DJ, Kron IL, Ailawadi G, Liu Y, et al. Depletion of tissue plasminogen activator attenuates lung ischemia-reperfusion injury via inhibition of neutrophil extravasation. Am J Physiol Lung Cell Mol Physiol. 2011;300(5):L718–L729. doi: 10.1152/ajplung.00227.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Genden EM, Boros P, Liu J, Bromberg JS, Mayer L. Orthotopic tracheal transplantation in the murine model. Transplantation. 2002 May 15;73(9):1420–1425. doi: 10.1097/00007890-200205150-00010. [DOI] [PubMed] [Google Scholar]
- 18.Okazaki M, Krupnick AS, Kornfeld CG, Lai JM, Ritter JH, Richardson SB, et al. A mouse model of orthotopic vascularized aerated lung transplantation. Am J Transplant. 2007 Jun;7(6):1672–1679. doi: 10.1111/j.1600-6143.2007.01819.x. [DOI] [PubMed] [Google Scholar]
- 19.Jungraithmayr WM, Korom S, Hillinger S, Weder W. A mouse model of orthotopic, single-lung transplantation. J Thorac Cardiovasc Surg. 2009 Feb;137(2):486–491. doi: 10.1016/j.jtcvs.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Puchelle E, Peault B. Human airway xenograft models of epithelial cell regeneration. Respir Res. 2000;1:125–128. doi: 10.1186/rr21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inayama Y, Hook GER, Brody AR, Jetten A, Gilmore L, Gray T, et al. In vitro and in vivo growth and differentiation of clones of tracheal basal cells. Am J Pathol. 1989;134:539–549. [PMC free article] [PubMed] [Google Scholar]
- 22.Ford J, Terzaghi-Howe M. Basal cells are the progenitors of primary tracheal epithelial cell cultures. Exp Cell Res. 1992;198:69–77. doi: 10.1016/0014-4827(92)90150-7. [DOI] [PubMed] [Google Scholar]
- 23.Rawlins EL. Lung epithelial progenitor cells: lessons from development. Proc Am Thorac Soc. 2008;5(6):675–681. doi: 10.1513/pats.200801-006AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rawlins EL, Okubo T, Que J, Xue Y, Clark C, Luo X, et al. Epithelial stem/progenitor cells in lung postnatal growth,maintenance, and repair. Cold Spring Harb Symp Quant Biol. 2008;73:291–295. doi: 10.1101/sqb.2008.73.037. Epub 2008 Nov 21. [DOI] [PubMed] [Google Scholar]
- 25.Delaere P, Vranckx J, Verleden G, De Leyn P, Van Raemdonck D. Tracheal allotransplantation after withdrawal of immunosuppressive therapy. N Engl J Med. 2010 Jan 14;362(2):138–145. doi: 10.1056/NEJMoa0810653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.