Abstract
RelB and NF-κB2 are the main effectors of NF-κB non-canonical signaling and play critical roles in many physiological processes. However, their role in hematopoietic stem/progenitor cell (HSPC) maintenance has not been characterized. To investigate this, we generated RelB/NF-κB2 double-knockout (dKO) mice and found that dKO HSPCs have profoundly impaired engraftment and self-renewal activity after transplantation into wild-type recipients. Transplantation of wild-type bone marrow cells into dKO mice to assess the role of the dKO microenvironment showed that wild-type HSPCs cycled more rapidly, were more abundant, and had developmental aberrancies: increased myeloid and decreased lymphoid lineages, similar to dKO HSPCs. Notably, when these wild-type cells were returned to normal hosts, these phenotypic changes were reversed, indicating a potent but transient phenotype conferred by the dKO microenvironment. However, dKO bone marrow stromal cell numbers were reduced, and bone-lining niche cells supported less HSPC expansion than controls. Further, increased dKO HSPC proliferation was associated with impaired expression of niche adhesion molecules by bone-lining cells and increased inflammatory cytokine expression by bone marrow cells. Thus, RelB/NF-κB2 signaling positively and intrinsically regulates HSPC self-renewal and maintains stromal/osteoblastic niches and negatively and extrinsically regulates HSPC expansion and lineage commitment through the marrow microenvironment.
Keywords: Non-canonical NF-κB, RelB, hematopoietic stem cell, osteoblast, niche
Introduction
Hematopoietic stem/progenitor cells (HSPCs) give rise to all of the hematopoietic cells within the bone marrow, blood and immune system. HSPCs include long-term (LT)-stem cells, which have the unique capacity of self-renewal, and short-term (ST)-stem cells, derived from LT-stem cells, that differentiate further to produce all the progenitor and mature blood cells [1-3]. Although LT-stem cells self-renew, they are predominantly quiescent and undergo slow cell-cycle progression to sustain sufficient numbers of them throughout life and to protect the stem cell pool from premature exhaustion in response to a variety of pathologic stresses [4, 5].
The mechanisms regulating HSPC self-renewal remain incompletely understood, but involve extrinsic and intrinsic signals [3, 6]. Extrinsic signals are derived from the bone marrow microenvironment where HSPCs undertake cell fate decisions: self-renewal or differentiation. These fate decisions occur in osteoblastic, vascular and stromal niches [7-11]. The osteoblastic niche has been investigated more extensively than other niches, and studies to date indicate that HSPCs reside preferentially along trabecular bone surfaces where osteoblastic cells maintain normal HSPC function and hematopoietic homeostasis [9-13]. However, HSPCs also reside in stromal niches and adjacent to sinusoid endothelial cells in the bone marrow [8, 14]. The relationships among different types of niches are not well understood.
HSPCs self-renew in response to cytokines, including c-kit ligand, thrombopoietin and stem cell factor (SCF) [6, 15]. They can be enriched in the KLS (c-Kit+, lineage−, Sca1+) population, while the c-kit+lineage−Sca1−population has more committed progenitors. Several intrinsic signals or molecules regulate HSPC self-renewal, including Wnts, Hedgehog, oxidative stress, cell cycle regulators, Bmi, DNA damage, Notch, and NF-κB [2, 3, 6, 15-18]. However, the mechanisms whereby these signals regulate HSPCs remain incompletely understood. The NF-κB transcription factor family includes p105 and p100 (precursor proteins, which can act as inhibitory κB proteins by binding to other NF-κB proteins), RelA (p65), RelB and c-Rel. In response to receptor ligation, p105 is processed constitutively to p50, which forms dimers typically with RelA in the canonical pathway. p100 typically is bound to RelB and undergoes proteasomal processing to p52 through receptor-mediated activation of NF-κB-inducing kinase (NIK) and IKKα in the non-canonical pathway, thus releasing p52/RelB complexes to translocate to nuclei. Canonical and non-canonical signaling plays critical roles in many physiological processes, including innate and adaptive immune responses, cell proliferation, apoptosis and bone homeostasis [19-21].
Previous studies from others and us using RelA−/−, RelB−/−, or p50/NF-κB2 double knockout (dKO) mice have clearly demonstrated that both canonical and non-canonical pathways are important for B and T cell, dendritic cell, osteoclast and myeloid differentiation [22-27]. Specifically, B cell development in bone marrow is significantly impaired in RelA−/− mice, and this defect is more severe in RelA/p50 dKO mice [22, 26]. In RelB−/− mice, T and B cell development is normal, but dendritic cell development is impaired, and there is bone marrow myeloid hyperplasia with a relative B cell population reduction [23, 24]. In p50/NF-κB2 dKO mice, bone marrow myeloid development is normal, but the mice have severe osteopetrosis due to failure of osteoclast formation, and B cell numbers are decreased significantly in the spleen, where there is extramedullary hematopoiesis [25].
In these early studies, mature bone marrow cells, peripheral blood, and spleens were analyzed extensively. Despite clear defects in hematopoiesis, the functions of these NF-κB components in adult HSPCs, myeloid and lymphoid progenitors, were not characterized. Although an important role for canonical NF-κB signaling in HSPC self-renewal has been implicated using c-rel/RelA and p50/RelA dKO fetal liver cells [26, 28], the role of the canonical NF-κB pathway in adult hematopoiesis remains unclear, since fetal liver stem cells may behave differently from adult HSPCs in their gene regulation and self-renewal ability [29, 30]. Furthermore, because canonical and non-canonical NF-κB signaling can be activated in different circumstances with different roles, we generated RelB/NF-κB2 dKO mice and investigated the role of non-canonical signaling in HSPCs. We show that non-canonical signaling plays an intrinsic role to positively regulate HSPC self-renewal, maintains the bone marrow stromal pool and the supportive functions of osteoblastic niche cells. It also regulates HSPC expansion and lineage commitment by negatively regulating the expression of HSPC supporting inflammatory cytokines.
Materials and Methods
Mice
NFκB2−/− mice and mice with a mutation-disrupted RelB locus by random integration of transgene sequences have been described previously [23, 25]. We crossed NFkB2+/−/RelB+/− mice with NFkB2−/−/RelB+/− mice to generate RelB−/−/NFkB2−/− mice. dKO and littermate control mice are in a mixed 129/C57BL6 background and were 6-8-week-old in all of our experiments. Transplant recipients (C57BL/Ka CD45.1) were 8-10 weeks old. All animal experiments were performed using URMC Institutional Animal Care and Use Committee-approved protocols.
HSPC Isolation and Analysis
We used the following antibodies from e-Bioscience and BD PharMingen: Ter119, CD3, CD4, CD8, B220, Gr-1, Mac-1, CD11c, c-kit, Sca1, IL7Rα, FcγII/III, CD34, Flk-2, CD150, CD48, CD45.1, CD45.2. For isolation of c-kit+lineage−Sca-1+ (KLS) cells, whole bone marrow cells were incubated with a cocktail of lineage antibodies from BD Biosciences (biotinylated anti-mouse antibodies directed against CD3e, CD11b, CD45R/B220, Gr-1, Ter119) followed by lineage-depletion using BD IMag streptavidin particles Plus-DM, then stained with Sca-1 PE-Cy5.5, c-kit PE-Cy5 and streptavidin PE-Texas-Red. For long-term HSPC, cell cycle or BrdU incorporation analyses, lineage depletion was omitted and Hoechst 33342 and Pyronin Y or the following antibodies were added when appropriate: CD34-FITC, Flk2-PE or CD150-APC, CD48-PECy7 or CD150-APC, Annexin V-FITC, Brdu-FITC. For common lymphoid progenitor analyses, IL7Rα-FITC and Biotin-conjugated Thy1 followed by steptavidin-APC were added, and for common myeloid progenitor analyses, CD34-FITC and FcγII/III-APC were added with KLS staining. For some bone marrow analyses, PE-conjugated monoclonal antibodies to lineage markers, including CD3, CD4, CD5, CD8, B220, Gr-1, Mac-1 and Ter119 in addition to Sca-1 PECy5.5 and c-kit-PECy5 were used in combination with CD34-FITC, Flk2-APC or CD150-APC and CD48-PECy7. LSRII was used for all the analyses, and FACSAria was used for cell sorting.
Bone Marrow Transplant Experiments
For competitive repopulation unit (CRU) assays [18], we used 10, 50, 100 or 500 sorted KLS cells from control or dKO mice (both expressing CD45.2) mixed with 5×105 competing bone marrow cells derived from non-irradiated recipient mice and injected them into the retro-orbital venous sinuses of lethally irradiated CD45.1 recipients. Multilineage repopulation was assessed 16 weeks after transplantation. Calculation of CRUs was conducted using L-Cal software (Stem Cell Technologies). For competitive bone marrow (BM) transplantation, either sorted KLS cells (5000 cells/recipient) or whole BM cells (2×105 or 5×105, or 1.5-4×107) along with 2×105 competing BM cells were injected into retro-orbital venous sinuses of lethally-irradiated CD45.1 recipients. Host mice were lethally irradiated with two doses of 5 Gy each 4-24 h prior to transplantation and subsequently maintained on water containing antibiotics (sulfamethoxazole and trimethoprim). To test the role of the null BM microenvironment, wild-type congenic B6.SJL (CD45.1+) BM cells (3×107/recipient) were transplanted into 5-FU-treated (150mg/kg, 2 doses given 5d apart) control or dKO mice (CD45.2+). Beginning 4wks after transplantation and continuing for at least 16wks, blood was obtained from recipient mouse tail veins, subjected to ammonium-chloride potassium red cell lysis, and stained with CD45.2 FITC and CD45.1 APC along with B220 PECy5 and CD3, CD4, CD5 PE or Mac-1 PE and Gr1 PECy5 for monitoring donor engraftment and donor-derived lymphoid or myeloid cells, respectively. Successful engraftment was defined as the presence of a distinct CD45.2+CD45.1− population above a background set by parallel analyses of animals transplanted with only competitor cells. To assess homing in vivo, 2×106 bone marrow cells from control or dKO mice were transplanted into lethally irradiated 45.1 recipients. The recipient mice were sacrificed 20 hours post-transplantation and the presence of donor-derived cells was analyzed by FACS.
Bone-lining cell isolation analysis and cell culture experiments
Bone-lining cells were isolated from the metaphyses of marrow-depleted tibiae and femora by mechanical disruption (mortar and pestle crushing) and enzymatic digestion in 0.2% collagenase for 1h and expanded in vitro. They were characterized by staining for Ter119, CD45 and N-Cadherin (ab18203) with anti mouse IgG FITC as the secondary antibody. Third passage cells were used for osteoblast differentiation experiments. For co-culture experiments, lineage negative BM cells were isolated from wild-type mice after staining with a cocktail of PE-conjugated lineage antibodies (CD3, CD4, CD5, CD8, B220, Ter119, Gr-1, and Mac-1) by FACS. 100,000 lineage-cells were cultured with 2000 freshly FACS-sorted digested bone-lining cells for 40h and analyzed for lineage, c-Kit and Sca1 staining. For CFU-F assays using whole BM cells, 1×106 BM cells/well were placed in 6-well plates and cultured for 10d. For CFU-F assays using FACS-sorted stromal progenitors (CD45−Ter119− CD31−Sca1+PDGFRα+), 1200 cells were plated into 10-cm culture dishes and cultured for 20d and adherent cell clusters containing >50 cells were counted as colonies as previously described [31].
Real-Time RT-PCR analysis
RNA was isolated using RNeasy Micro Kit (Qiagen) and converted to cDNA using Superscript III (Invitrogen). Quantitative real-time PCR was performed using an iCycler (BioRad). Transcript levels were normalized to actin. Primer sequences are available upon request.
BrdU and Cell-Cycle Status Analysis of HSPCs
In vivo BrdU labeling was performed by intraperitoneally injecting control and dKO mice with 200 μl (10 mg/ml) of BrdU/20g body weight, then giving water containing 1 mg/ml BrdU for 20h. Bone marrow cells were stained first with surface markers, followed by BrdU staining using a BrdU-FITC staining kit (BD Biosciences).
For cell-cycle analyses, bone marrow cells were first stained with surface markers to define the stem cell population, followed by fixation and permeabilization with Cytofix/Cytoperm (BD Biosciences). Intracellular staining with Hoechst 33342 (10 μg/ml) was done in Permwash (BD Biosciences) for 30min, followed by Pyronin Y (1 mg/ml) staining for 15min. G0 phase was defined as Pyronin Y low, Hoechst low population.
Statistical Analysis
Student’s t test was used for all statistical analyses and significance was set at p < 0.05.
Results
RelB/NFκB2 signaling regulates long-term and short-term HSPC numbers
We first analyzed NF-κB mRNA expression levels in HSPC-enriched KLS (c-Kit+, lineage−, Sca1+) cells and in committed progenitors (c-kit+lineage−Sca1−) from WT mice. relb was significantly higher in the KLS population than in committed progenitors and compared to levels of rela, p50 and p52. The p52 level was higher in KLS cells, although the difference was not significant (Supporting information Fig. 1). These findings and a previous report of increased relb expression in human primitive HSPCs [32] suggest that non-canonical NF-κB signaling may play an important role in HSPC regulation.
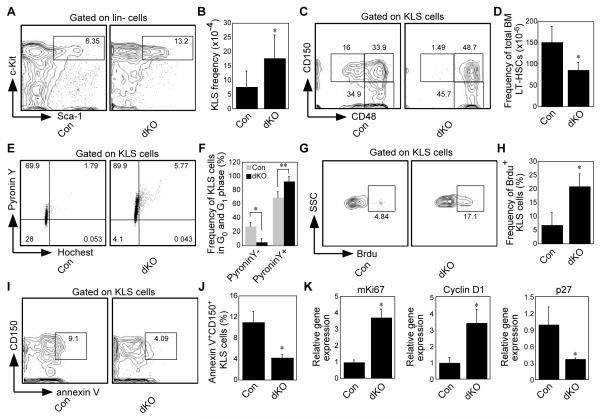
To more definitively explore the role of non-canonical signaling in HSPCs, we generated RelB/NF-κB2 double knockout (dKO) mice by crossing NF-κB2+/−;RelB+/− mice with NF-κB2+/−;RelB+/− mice. The major phenotypic features of our dKO mice on a mixed 129/C57BL6 background are largely similar to those described previously in RelB−/− mice on a C57BL6 background, including myeloid hyperplasia, extramedullary hematopoiesis and multi-organ inflammatory cell infiltration [23, 24]. However, our RelB−/− mice on a mixed 129/C57BL6 background become moribund later than RelB−/− mice on the original C57BL6 background: they begin to die ~1 month, rather than 1week after birth, and most of them are dead after 2 months. In contrast, the dKO mice do not start to die until ~2 months and many of them live longer than 3 months, some up to 10 months, which gave us the opportunity to analyze the role of the dKO microenvironment in more mature 6-8-week-old mice. These dKO mice also differ from recently-described RelB/p100 dKO mice, which lack RelB and p100, but still produce a functional p52 protein [33]. HSPCs were not described in that publication. Deletion of relb and p52 in HSPCs was confirmed by real-time PCR analysis (Supporting information Fig. 1). The dKO mice had similar numbers of total bone marrow (BM) cells as control, NF-κB2−/− or RelB−/− mice (Supporting information Fig. 2A), but like RelB−/− mice they had increased BM and spleen myeloid and decreased B cell numbers (Supporting information Fig. 2B and data not shown) [23, 24]. Further, compared to controls, the dKO mice had normal common myeloid, but significantly decreased common lymphoid progenitors (Supporting information Fig. 2 C and D). Consistent with this observation, dKO mice have decreased mature B cells and a smaller thymus (Supporting information Fig. 2B and data not shown). The frequency of the HSPC-enriched KLS population was significantly increased (around 2-fold >control; Fig. 1A and B), while the LT-HSPC frequency, defined as either CD150+CD48− KLS cells [8] (Fig. 1C and D), or CD34−CD135−KLS cells [34, 35] (Supporting information Fig. 2E) was significantly reduced (around 5-fold <control) in the KLS population. However, the total BM LT-HSPC frequency, calculated as the percentage of Lin− × KLS × CD150+CD48−, was only slightly decreased. This increase in the KLS population was likely due to increased proliferation of dKO progenitor cells, as evidenced from both cell cycle studies using Hoechst and Pyronin Y staining (Fig. 1E and F) and BrdU labeling (Fig. 1G and H). A small decrease in Annexin V+ cells suggests that reduced apoptosis may also affect the size of the KLS population in dKO mice (Fig. 1I and J). Consistent with the faster proliferation, Ki67 and cyclin D1 expression levels were increased, and those of the cell cycle inhibitor p27 were decreased in the KLS population (Fig. 1K). Along with the increased proliferation, extramedullary hematopoiesis was increased significantly in dKO mice and was already present by day 10 post-partum (Supporting information Fig. 3 and data not shown), suggesting that many dKO stem and progenitor cells were mobilized to peripheral organs. Overall, these data indicate a significant role for RelB/NF-κB2 signaling to limit proliferation and differentiation of primitive hematopoietic cells.
Figure 1. Characterization of hematopoiesis in RelB/NF-κB2 dKO mice.
(A, B) Frequency of KLS cells in control and dKO mice (n=8, *p<0.01), assessed by flow cytometry. (C, D) Frequency of long-term stem cells (CD150+CD48−KLS) in control and dKO mice (n=8, *p=0.04). (E, F) Cell cycle analysis using Hoechst 33342 and Pyronin Y staining. G0 phase defined as PyroninlowHoechstlow and G1 phase defined as PyroninhighHoechstlow. (n=3, *p<0.01 **p=0.012). (G, H) Analysis of proliferation of KLS cells using BrdU labeling. (n=4, *p<0.01). (I, J) Apoptosis analysis of the stem cell population (CD150+KLS cells). (n=3, *p<0.01), assessed by annexin V positivity. (K) Real time PCR analysis of the expression of Ki67, cyclin D1, and the cell cycle inhibitor p27. (n=3, *p<0.01 for Ki67 and cyclin D1 and p=0.025 for p27). Dot plots in (A, C, E, G and I) are shown for one representative control (left) and dKO (right). Values are means +/− SD.
RelB/NF-κB2 signaling intrinsically regulates HSPC self-renewal, but not lineage commitment
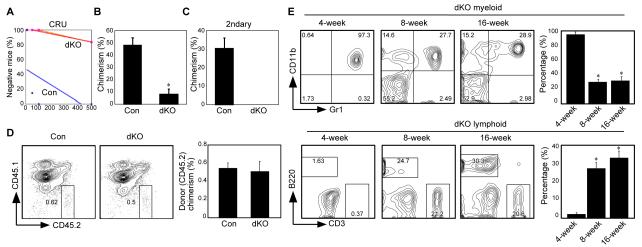
To determine whether RelB/NF-κB2 signaling activity is intrinsic to hematopoietic stem/progenitors or is extrinsically modulated via the BM microenvironment, we first carried out competitive limiting dilution experiments using a range of 10-500 KLS cells per recipient with 5 to 8 recipients per dosage, and found an approximately 110-fold reduction in the frequency of functional HSCs (1 in 34 KLS cells for control mice versus 1 in 3774 KLS cells for dKO mice) as determined by CRU software analysis (Fig. 2A). These results suggest that the function of dKO stem cells was markedly impaired. We can consistently obtain 10,000 to 15,000 KLS cells from one control mouse, and because 5000 dKO KLS cells still were unable to efficiently reconstitute lethally irradiated recipients (data not shown), we decided not to use sorted KLS cells. To determine if dKO cells can reconstitute lethally irradiated recipients, we tested different doses of whole bone marrow cells, and eventually when we increased the dKO donor cells to 1.5-4×107 per recipient, we observed chimerism in three recipients (Fig. 2B). However, no engraftment was observed after secondary BM transplantation into WT mice using dKO cells from these three recipients (Fig. 2C). In addition, using a traditional method with total marrow cells, we found that the decreased engraftment was not due to a homing defect to the bone marrow (Fig. 2D), although we cannot rule out a difference at the stem cell level. Overall, these transplantation results suggest that dKO HSPCs have an intrinsic functional defect in their self-renewal capability. However, kinetic changes in the 3 successful chimeric recipients showed that after transplantation the dKO donor cells contributed almost exclusively to myeloid cells at 4wk, to both myeloid and lymphoid cells at 8wk, and at 16wk the contributions to myeloid and lymphoid lineages were similar to control cells (Fig. 2E and data not shown). These findings suggest that differentiation is not significantly altered as a consequence of RelB/NF-κB2 inactivation.
Figure 2. RelB/NF-κB2 intrinsically regulate HSPC self-renewal.
(A) Limiting-dilution competitive repopulation analysis of control (Con) and dKO mice. Five to eight mice were transplanted at each dose (10, 50, 100 and 500 KLS cells/recipient for both Con and dKO groups). Peripheral blood cells of the recipients were analyzed 16 weeks after transplantation and recipient mice containing less than 1% donor cells in any one lineage were scored as negative. (B) Engraftment of control and dKO (3 × 107 whole bone marrow cells/recipient). (n=7, *p<0.01). (C) Secondary bone marrow transplantation using whole bone marrow cells isolated from primary recipients transplanted with control or dKO cells as in (B). (n=6, *p<0.01). (D) dKO and control bone marrow cells have equivalent homing ability. 2×106 whole bone marrow cells from control or dKO mice were transplanted into lethally irradiated recipients. Data are chimerism after 20 hours (n=3 for control and 4 for dKO). (E) Kinetic lineage contribution of dKO cells (gated on CD45.2+CD45.1−) after transplantation (n=3 at 4 weeks and n=2 at 8 and 16 weeks). Values are means +/− sem.
The RelB/NF-κB2 null bone marrow microenvironment instructs lineage differentiation
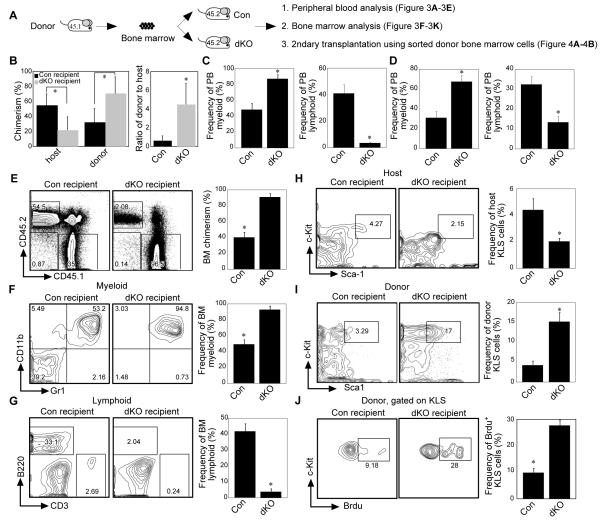
To examine the regulatory roles of the BM microenvironment in hematopoiesis and HSPC functions in the dKO mice, wild-type BM cells (CD45.1+) were transplanted into 5-FU-treated control or dKO mice (both CD45.2+) (Fig. 3A). 5-FU was chosen for these experiments because the dKO mice were able to better tolerate the less severe injury induced by this myelo-ablative strategy compared to lethal irradiation. Furthermore, 5-FU treatment is less damaging to endogenous stem cells and thus competition between donor and host stem cells can be observed. Analysis of peripheral blood at 16wk post-transplantation showed that wild-type donor cells transplanted into the dKO mouse microenvironment contributed more circulating blood and BM cells than those transplanted into the control microenvironment (Fig. 3B and E), indicating that the WT HSPCs had a selective advantage over the dKO host cells, which were out-competed by the wild-type donor cells in the dKO microenvironment (Fig. 3B). One possibility is that this may be because dKO HSPCs are more sensitive to 5-FU treatment. This would leave more empty niches in dKO recipients, provide more opportunities for donor WT cells to occupy these niches, and thus give rise to more donor-derived cells. Therefore, we analyzed 5-FU-treated dKO recipients. Surprisingly, although dKO HSPCs proliferate faster than WT HSPCs, the dKO HSPCs were phenotypically more resistant to 5-FU treatment than controls. Indeed, more HSPCs were observed in dKO mice after 5-FU treatment than in controls. Importantly, however, these dKO HSPCs were still functionally defective (Supporting information Fig. 4). Thus, although more HSPCs remained in the dKO mice, the dKO KLS cells were out-competed by WT donor KLS cells (Fig. 3H and I).
Figure 3. dKO bone marrow microenvironment controls donor cell fate.
(A) Scheme for analysis of bone marrow microenvironment. (B) Average donor and host chimerism (left) and the ratio of donor to host cells (right) after transplantation. (n=6, *p < 0.01). (C) Donor cell differentiation in the peripheral blood (PB). Bar graphs shown are donor-derived myeloid and lymphoid cells at 3 weeks after transplantation into control or dKO mice. (D) Normal peripheral blood lineage differentiation from control and dKO mice. (E) Donor chimerism in the bone marrow (BM) after transplantation. (F) Frequency of donor-derived myeloid cells in the bone marrow after transplantation. (G) Frequency of donor-derived lymphoid cells in the bone marrow after transplantation. (H, I) Analysis of the frequency of host-(H) and donor-(I) derived KLS cells after transplantation. (J) BrdU labeling of donor-derived KLS cells. (n=6 for D, F, H and G. n=3 for E, I, J, and K). Except for D and E, data were obtained at 16 weeks after transplantation.
Interestingly, once the transplanted wild-type hematopoietic cells were “educated” in the RelB/NF-κB2 null microenvironment they mimicked the behavior of RelB/NF-κB2 null hematopoietic cells in both the peripheral blood and bone marrow. Specifically, wild-type cells in dKO mice had increased donor-derived myeloid lineage and reduced donor-derived B lineage development (Fig. 3C, D, F and G). These changes were seen as early as 3wks post-transplantation (Fig. 3C) and continued for at least 16wks (Fig. 3F and G) and also were observed in the spleen (Supporting information Fig. 5). Furthermore, WT donor KLS cells “educated” in the dKO mice were more resistant to apoptosis (data not shown) and incorporated more BrdU than “educated” donor cells from the control microenvironment (Fig. 3J). Thus, the wild-type donor cells behaved similarly to native dKO cells after they were put into the dKO microenvironment.
The instructive effect of RelB/NF-κB2 null bone marrow microenvironment on WT marrow cells is reversible
Intriguingly, when wild-type donor cells “educated” in the dKO microenvironment were removed and transplanted back into normal wild-type mice, all the lineage changes were reversed, with a slight transient increase in myeloid and a decrease in B cell lineage cells that was observed at 4wks post-transplantation (Fig. 4A). In addition, whole BM cell numbers were increased after wild-type donor cells were transplanted into the dKO mice compared to transplantation of wild-type donor cells into the control microenvironment (Supporting information Fig. 6). More importantly, after exposure to the dKO microenvironment, the WT donor stem cells were functionally intact in contrast to dKO stem cells (Fig. 2B) in that after transplantation back to wild-type recipients they had similar or even slightly higher chimerism (Fig. 4A and B). Collectively and importantly, the findings of intact wild-type donor stem cell functions along with increased BM cell numbers in the transplanted dKO mice indicate that the dKO microenvironment is more supportive of hematopoietic proliferation than the control microenvironment.
Figure 4. The instructive effect of dKO microenvironment is reversible.
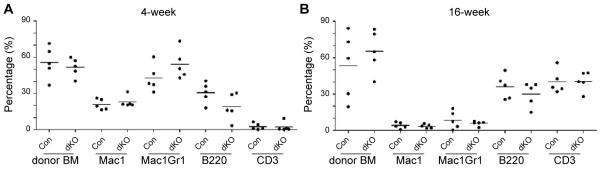
Chimerism and lineage commitment after “educated” donor cells were transplanted back to a wild-type microenvironment. 1×106 FACS-sorted “educated” donor cells from control or dKO mice were transplanted back to lethally irradiated wild-type recipients (n=5). Donor chimerism and lineage differentiation at 4-weeks (A) and 16-weeks (B) are shown. Each dot represents an individual mouse. Values are means +/− SD.
RelB/NF-κB2 positively regulates the size of bone marrow stromal pool and osteoblastic niches
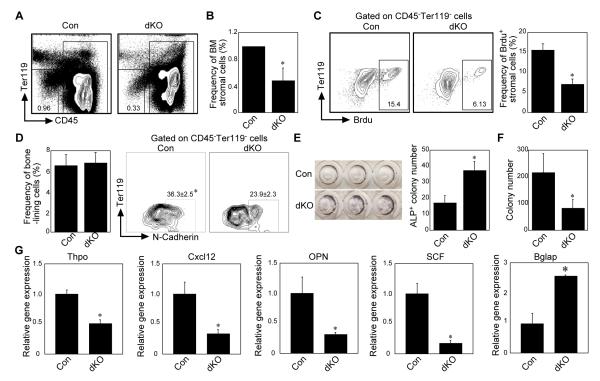
The aforementioned observations that 1) the RelB/NF-κB2 null BM microenvironment reversibly instructs lineage differentiation, and 2) the dKO microenvironment is more supportive of hematopoiesis, led us to further examine the components of the dKO microenvironment. First, we focused on the stromal and osteoblastic niches, the former having been established recently as a new BM stem cell niche [7]. Analysis of the BM stromal compartment showed that the frequency of BM stromal cells was significantly decreased in dKO mice (Fig. 5A and B), and they proliferated slower than control stromal cells, which is different from their hematopoietic counterpart (Fig. 5C and Fig. 1E-H). Consistent with the decreased stromal cell numbers, the dKO BM cells had significantly decreased colony forming and osteoblast differentiation ability (Supporting information Fig. 7&8). In addition, we further tested the dKO stromal function by plating equal numbers of flow cytometer-sorted immature stromal progenitors (CD45−Ter119−CD31−Sca1+PDGFRα+), which have been reported to behave like mesenchymal stem cells [31] and confirmed the CFU-F functional defect of dKO stromal progenitors (Supporting information Fig. 9).
Figure 5. RelB/NF-κB2 positively regulate bone marrow stromal and bone-lining cell functions.
(A, B) Analysis of the frequency of bone marrow stromal cells (CD45−Ter119−CD31−). (n=6, *p < 0.01). (C) Assessment of proliferation of bone marrow stromal cells using BrdU labeling. (D) Analysis of the frequency of bone-lining cells from collagenase-digested control or dKO bone fragments (left) and the expression of N-Cadherin (right). (E) Alkaline phosphate (ALP) staining of bone-lining cells after 5 days culture with osteogenic medium (1×105/96-well). (F) Co-culture of collagenase-digested bone-lining cells from control or dKO mice with sorted lineage− wild-type bone marrow cells. 40 hours later, bone marrow cells were collected for colony forming assay. (G) Real time PCR analysis of the expression of Tpho, Cxcl12, osteopontin (OPN), SCF and Osteocalcin (Bglap) mRNA in control and dKO bone-lining cells. (n=3, *p< 0.01).
The decreased stromal cell number suggests that the stromal niche size and/or number are reduced. However, because our transplantation results showed that hematopoiesis is supported more by the dKO than the wild-type microenvironment, we hypothesized that other cells in the dKO osteoblastic/stromal lineage compensated for the reduction of the stromal niche and thus should be more able to support and maintain immature hematopoietic cells than WT cells. Thus, we analyzed collagenase-digested bone-lining cells from metaphyseal trabecular and cortical bone fragments from dKO and control mice since these cells display HSPC supportive functions [12]. In contrast to the reduced frequency of BM stromal cells in dKO mice, the dKO bone-lining cell frequency was similar to that of control cells (Fig. 5D left). However, the dKO bone-lining cells expressed less N-Cadherin, which is important for regulating HSPC function [11], than control cells (Fig. 5D right). Further, these bone-lining cells had increased capacity to differentiate into osteoblasts (Fig. 5E). To analyze their hematopoietic supportive ability, we co-cultured harvested osteoblastic bone-lining cells with lin-control BM cells. At 40h post-culture, the co-cultured cells were harvested for colony forming assays. Compared to controls, dKO osteoblastic bone-lining cells did not efficiently promote BM immature cell proliferation (Fig. 5F). Gene expression profile analysis of osteoblastic bone-lining cells showed that expression of Cxcl12, thrombopoietin (Thpo), stem cell factor (SCF), and osteopontin (OPN) was significantly lower in the dKO than controls cells (Figure 5G). The expression of osteocalcin was increased, consistent with increased osteoblast differentiation of dKO stromal cells (Fig. 5G and 5E).
Cytokines from the dKO microenvironment stimulate HSPC expansion
The above gene expression profile of reduced expression of adhesion molecules by osteoblastic stromal cells suggests that the absence of non-canonical pathway signaling in them is likely to reduce the ability of HSPCs to attach to these cells in their niches. This could explain why the osteoblastic niche cells do not support HSPC expansion, but it does not explain why wild-type BM cells expanded more when they were transplanted into the dKO microenvironment (Fig. 3). Since cytokines such as G-CSF, GM-CSF can stimulate HSPC proliferation and have been used clinically prior to BM transplantation to enhance HSPC recovery [36], we examined cytokine levels in WT and dKO BM and stromal cells. We found that the dKO BM cells had significantly higher levels of G-CSF, GM-CSF and IL-6 mRNA (Fig. 6A, 6B and 6C) than controls, but similar expression levels of TNFα, IL-3 and TGFβ (data not shown). It should be noted that IL-6, G-CSF and GM-CSF were expressed at minimal levels or were undetectable in both WT and dKO stromal cells (data not shown), indicating that the increased cytokine expression was from dKO hematopoietic cells. Consistent with the increased IL-6, G-CSF and GM-CSF expression levels, the serum from dKO mice induced BM cell differentiation more efficiently toward myeloid cells (Fig. 6D) and stimulated more HSPC expansion than control serum in vitro (Fig. 6E).
Figure 6. Increased expression of inflammatory cytokines in dKO marrow cells induces myeloid differentiation and HSPC expansion.
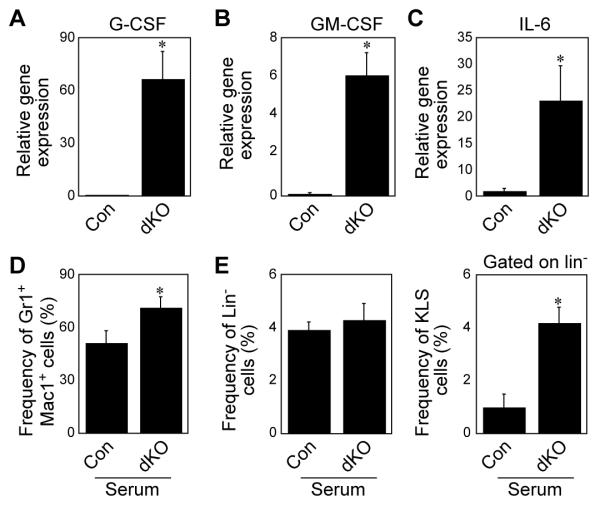
(A, B and C) Real time PCR analysis of the expression of G-CSF, GM-CSF and IL-6 mRNA levels in control and dKO whole bone marrow cells. (n=3, *p< 0.01). (D) Myeloid differentiation after WT whole bone marrow cells were cultured with 20% control or dKO mouse serum. (E) Analysis of the frequency of lineage (left panel) and KLS cells (right panel) after WT whole bone marrow cells cultured with 20% of control or dKO mouse serum.
Discussion
Steady production of red and white blood cells from hematopoietic stem/precursor cells is required for normal homeostasis and immune system functions. Increased hematopoiesis is necessary in numerous pathologic settings, including following hemorrhage, microbial infections, and marrow ablation as part of the treatment for some leukemias before bone marrow transplantation [1, 3, 6]. NF-κB signaling is activated in a variety of cell types in many of these settings and is required for immune cell formation and function [19, 21]. Thus, it is important to fully understand the mechanisms that regulate HSPCs in normal and disease states and the role of NF-κB in these states in particular. Previous studies have reported NF-κB canonical pathway involvement in HSPC functions, but it has been difficult to study this definitively because RelA−/− mice die during embryogenesis [22], making it impossible to generate mice deficient in both RelA and p50, the major signaling proteins in the canonical pathway. Here, we generated RelB/NF-κB2 dKO mice to examine the role of non-canonical NF-κB signaling in the regulation of HSPC functions and have identified intrinsic roles for RelB and NF-κB2 in HSPCs and new and important extrinsic roles for them in cells in the marrow microenvironment.
RelB/NF-κB2 dKO mice have an overall ~2-fold increase in KLS marrow progenitor cells due to increased proliferation and decreased apoptosis, with only a slight, but significant decrease in LT-HSCs. This suggests an intrinsic role for the non-canonical pathway to maintain long-term stem cells or limit their differentiation or an extrinsic role or both. Importantly, however, dKO HSPCs were unable to functionally reconstitute the marrow of lethally irradiated WT mice in standard competitive, non-competitive, and serial transplantation experiments, and were out-competed by control HSPCs in chimeric mice. Thus, RelB/NF-κB2 intrinsically and positively regulate long-term stem cell self-renewal. Unlike the canonical pathway, which is activated quickly following ligand/receptor interaction, the non-canonical pathway is activated after several hours and following RelA/p50 induction of NF-κB p100 expression [19, 21]. Further study will be required to determine the factors that stimulate non-canonical signaling in HSPCs and the genes that are activated in them by RelB/NF-κB2.
We also found that RelB/NF-κB2 regulate hematopoiesis extrinsically through effects on the bone marrow microenvironment. An important role for osteoblasts in the marrow microenvironment to maintain hematopoiesis was recognized several years ago [10, 11], and since then roles for endothelial cells in hematopoiesis have been identified in a vascular niche [5, 8, 14]. More recently, nestin-expressing bone marrow mesenchymal stem cells have been identified as another type of regulatory niche cell [7]. We found that the frequency of bone marrow stromal cells, including that of immature stromal progenitor cells, is decreased in the dKO mice, indicating that the non-canonical pathway also intrinsically and positively regulates the bone marrow stromal population. In addition, dKO bone-lining cells displayed enhanced osteoblast differentiation, and dKO mice have increased bone volume (to be described in another publication), suggesting that this osteoblastic niche may have enhanced functions to compensate for the reduced number or activity of the stromal niche. However, our data showed that these bone-lining cells were unable to support hematopoietic expansion and differentiation as efficiently as controls. Molecularly, the dKO bone-lining cells express less Cxcl12, Thpo, osteopontin, and SCF (c-Kit ligand) than control cells. Thpo and Cxcl12, along with their receptors MPL and CXCR4, play essential roles in maintaining quiescence of the HSPC pool [37-39]. Osteopontin maintains HSPC functions and keeps them attached to and in the osteoblastic niche [40, 41], and SCF stimulates HSPC proliferation [6, 15, 42]. Thus, the decreased expression of these key players by dKO bone-lining cells could leave HSPCs in a state where they are freer to proliferate and differentiate because they have looser than normal contact to their niches, although these dKO bone-lining cells themselves do not support HSPC expansion. These data suggest that non-canonical signaling functions in the osteoblastic niche primarily to maintain HSPC-niche interactions and thus limit their differentiation into myeloid cells, which are increased in the dKO mice. These findings could also explain the results of transplantation of WT bone marrow cells into the dKO microenvironment: the WT HSPCs attain a relatively active status because they have less contact with the RelB/NF-κB2-null osteoblastic niche and they can expand because of the increased expression of other stimulatory factors (see below). Thus, non-canonical signaling appears to have an important role in bone marrow homeostasis coordinating the functions of both the stromal and osteoblastic niches to keep HSPCs quiescent. A key question needing further exploration is whether non-canonical signaling regulates the expression of these niche-interacting molecules by direct or indirect mechanisms.
Our data that wild-type donor hematopoietic cells proliferate faster in the dKO microenvironment and maintain their functional self-renewal capability when transplanted back to a control microenvironment, suggest that factors other than the less supportive HSPC function of dKO osteoblastic bone-lining cells are responsible for the HSPC expansion. To this end, we found that the expression levels of inflammatory cytokines capable of stimulating HSPC expansion and myeloid proliferation, including IL-6, G-CSF, and GM-CSF, were significantly increased in dKO bone marrow cells. Our in vitro culture experiments with dKO serum support this conclusion. Further support for an instructive role for these cytokines from the microenvironment comes from the results of 5-FU treatment and transplantation of WT bone marrow cells into the dKO microenvironment. For example, when either dKO bone marrow cells or wild-type donor cells were exposed to the dKO microenvironment, they became more myeloid and had less B lymphoid differentiation. In addition, after dKO bone marrow cells engrafted successfully in wild-type recipients, or wild-type donor cells “educated” in the dKO microenvironment were transplanted back into wild-type recipients, lineage commitment reverted almost completely to normal. Furthermore, although dKO KLS cells proliferated faster, they were phenotypically resistant to 5-FU treatment, and when WT cells were in the dKO microenvironment, the WT cells became 5-FU-resistant. However, we realize that after transplantation of WT bone marrow cells into dKO mice, and even when dKO recipients were stable and had higher chimerism, the donor cells were still skewed to the myeloid lineage and proliferated faster. One explanation is that even after 16 weeks, an inflammatory cell infiltrate could still be identified in the organs we examined, including lung and liver, although the severity was greatly reduced compared to dKO mice without transplantation (Supporting information Fig. 10). These data suggest that local and systemic inflammatory cytokines are responsible for the enhanced hematopoiesis.
The increased myelopoiesis in our dKO mice is also seen in IκBα−/− mice, which was attributed to a hepatocyte/bone marrow stroma-mediated effect on myeloid lineage cells [43]. This provides further support for the possibility that inflammatory cytokines released from other organs in our dKO mice may also be responsible for the enhanced hematopoiesis. However, we cannot exclude the possibility of the existence of other key as yet unidentified cytokines. Interestingly, earlier studies have reported increased absolute and relative HSPC numbers in chronic infection [44-47]. Currently, whether and how infection affects non-canonical and canonical signaling in HSPCs and their niches is unknown. Nevertheless, our data at least partially explain the myeloid proliferative phenotype and the increased HSPCs in the dKO bone marrow microenvironment.
Conclusion
Our observations uncover important roles for non-canonical signaling in regulating hematopoiesis both intrinsically and extrinsically (Supporting information Fig. 11). In fact, a recent report shows that RelB deficiency delayed the onset of leukemia in the TEL-JAK2 transgenic mouse model of human T acute lymphoblastic leukemia [48]. This finding is consistent with our data that dKO stromal/osteoblastic cells have defective support of hematopoietic cell proliferation and differentiation and supports our conclusion that RelB/NF-κB2 positively regulate stromal cell functions. Further dissection of the roles of non-canonical signaling in the regulation of stromal/osteoblastic niches and inflammatory cytokines using conditional tissue-specific RelB knockout mice is necessary. Overall, our findings raise the possibility that stimulating non-canonical NF-κB signaling may enhance the function of both HSPCs and the stromal/osteoblastic niche.
Supplementary Material
Acknowledgements
We thank Tannishtha, Reya, Leah Dimascio, Jordan Blum for reviewing the manuscript; Mitchele Au and Sarah Neering for cell sorting, Yanyun Li, and Zhenqiang Yao for experimental help. This work is supported by the NIH grant AR43510 to BFB.
This work is supported by the NIH grant AR43510 to BFB.
Footnotes
Author contribution summary:
Chen Zhao: Conception and design, data analysis and interpretation, manuscript writing
Yan Xiu: Conception and design, data analysis and interpretation
John Ashton: Provision of study material or patients, data analysis and interpretation
Lianping Xing: Data analysis and interpretation, final approval of manuscript
Yoshikazu Morita: Provision of study material or patients, data analysis and interpretation
Craig T. Jordan: Provision of study material or patients, data analysis and interpretation, final approval of manuscript
Brendan F. Boyce: Data analysis and interpretation, manuscript writing, final approval of manuscript
Disclosure of Potential Conflicts of Interest: The authors have no competing financial interests.
References
- 1.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 2.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol. 2006;7:333–337. doi: 10.1038/ni1331. [DOI] [PubMed] [Google Scholar]
- 5.Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8:290–301. doi: 10.1038/nri2279. [DOI] [PubMed] [Google Scholar]
- 6.Zon LI. Intrinsic and extrinsic control of haematopoietic stem-cell self-renewal. Nature. 2008;453:306–313. doi: 10.1038/nature07038. [DOI] [PubMed] [Google Scholar]
- 7.Mendez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiel MJ, Yilmaz OH, Iwashita T, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 9.Arai F, Hirao A, Ohmura M, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 12.Chitteti BR, Cheng YH, Poteat B, et al. Impact of interactions of cellular components of the bone marrow microenvironment on hematopoietic stem and progenitor cell function. Blood. 2010;115:3239–3248. doi: 10.1182/blood-2009-09-246173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raaijmakers MH, Mukherjee S, Guo S, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464:852–857. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi H, Butler JM, O’Donnell R, et al. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat Cell Biol. 2010;12:1046–1056. doi: 10.1038/ncb2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson A, Laurenti E, Trumpp A. Balancing dormant and self-renewing hematopoietic stem cells. Curr Opin Genet Dev. 2009;19:461–468. doi: 10.1016/j.gde.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao C, Chen A, Jamieson CH, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458:776–779. doi: 10.1038/nature07737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao C, Blum J, Chen A, et al. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12:528–541. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 20.Boyce BF, Yao Z, Xing L. Functions of nuclear factor kappaB in bone. Ann N Y Acad Sci. 2010;1192:367–375. doi: 10.1111/j.1749-6632.2009.05315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghosh S, Hayden MS. New regulators of NF-kappaB in inflammation. Nat Rev Immunol. 2008;8:837–848. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- 22.Beg AA, Sha WC, Bronson RT, et al. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 23.Burkly L, Hession C, Ogata L, et al. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature. 1995;373:531–536. doi: 10.1038/373531a0. [DOI] [PubMed] [Google Scholar]
- 24.Weih F, Carrasco D, Durham SK, et al. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-kappa B/Rel family. Cell. 1995;80:331–340. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- 25.Franzoso G, Carlson L, Xing L, et al. Requirement for NF-kappaB in osteoclast and B-cell development. Genes Dev. 1997;11:3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horwitz BH, Scott ML, Cherry SR, et al. Failure of lymphopoiesis after adoptive transfer of NF-kappaB-deficient fetal liver cells. Immunity. 1997;6:765–772. doi: 10.1016/s1074-7613(00)80451-3. [DOI] [PubMed] [Google Scholar]
- 27.Iotsova V, Caamano J, Loy J, et al. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat Med. 1997;3:1285–1289. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]
- 28.Grossmann M, Metcalf D, Merryfull J, et al. The combined absence of the transcription factors Rel and RelA leads to multiple hemopoietic cell defects. Proc Natl Acad Sci U S A. 1999;96:11848–11853. doi: 10.1073/pnas.96.21.11848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mold JE, Venkatasubrahmanyam S, Burt TD, et al. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330:1695–1699. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Satyanarayana A, Gudmundsson KO, Chen X, et al. RapGEF2 is essential for embryonic hematopoiesis but dispensable for adult hematopoiesis. Blood. 116:2921–2931. doi: 10.1182/blood-2010-01-262964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morikawa S, Mabuchi Y, Kubota Y, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206:2483–2496. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panepucci RA, Oliveira LH, Zanette DL, et al. Increased Levels of NOTCH1, NF-kappaB, and Other Interconnected Transcription Factors Characterize Primitive Sets of Hematopoietic Stem Cells. Stem Cells Dev. 2009 doi: 10.1089/scd.2008.0397. [DOI] [PubMed] [Google Scholar]
- 33.Soysa NS, Alles N, Weih D, et al. The pivotal role of the alternative NF-kappaB pathway in maintenance of basal bone homeostasis and osteoclastogenesis. J Bone Miner Res. 2010;25:809–818. doi: 10.1359/jbmr.091030. [DOI] [PubMed] [Google Scholar]
- 34.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci U S A. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osawa M, Hanada K, Hamada H, et al. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 36.Cashen AF, Lazarus HM, Devine SM. Mobilizing stem cells from normal donors: is it possible to improve upon G-CSF? Bone Marrow Transplant. 2007;39:577–588. doi: 10.1038/sj.bmt.1705616. [DOI] [PubMed] [Google Scholar]
- 37.Sugiyama T, Kohara H, Noda M, et al. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 38.Qian H, Buza-Vidas N, Hyland CD, et al. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell. 2007;1:671–684. doi: 10.1016/j.stem.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Yoshihara H, Arai F, Hosokawa K, et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 2007;1:685–697. doi: 10.1016/j.stem.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 40.Stier S, Ko Y, Forkert R, et al. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med. 2005;201:1781–1791. doi: 10.1084/jem.20041992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nilsson SK, Johnston HM, Whitty GA, et al. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005;106:1232–1239. doi: 10.1182/blood-2004-11-4422. [DOI] [PubMed] [Google Scholar]
- 42.Hannum C, Culpepper J, Campbell D, et al. Ligand for FLT3/FLK2 receptor tyrosine kinase regulates growth of haematopoietic stem cells and is encoded by variant RNAs. Nature. 1994;368:643–648. doi: 10.1038/368643a0. [DOI] [PubMed] [Google Scholar]
- 43.Rupec RA, Jundt F, Rebholz B, et al. Stroma-mediated dysregulation of myelopoiesis in mice lacking I kappa B alpha. Immunity. 2005;22:479–491. doi: 10.1016/j.immuni.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 44.Baldridge MT, King KY, Boles NC, et al. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature. 2010;465:793–797. doi: 10.1038/nature09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh P, Yao Y, Weliver A, et al. Vaccinia virus infection modulates the hematopoietic cell compartments in the bone marrow. Stem Cells. 2008;26:1009–1016. doi: 10.1634/stemcells.2007-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murray PJ, Young RA, Daley GQ. Hematopoietic remodeling in interferon-gamma-deficient mice infected with mycobacteria. Blood. 1998;91:2914–2924. [PubMed] [Google Scholar]
- 47.Feng CG, Weksberg DC, Taylor GA, et al. The p47 GTPase Lrg-47 (Irgm1) links host defense and hematopoietic stem cell proliferation. Cell Stem Cell. 2008;2:83–89. doi: 10.1016/j.stem.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.dos Santos NR, Williame M, Gachet S, et al. RelB-dependent stromal cells promote T-cell leukemogenesis. PLoS One. 2008;3:e2555. doi: 10.1371/journal.pone.0002555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.