Abstract
Chronic exposure to Mn results in the development of a neurological disorder known as manganism characterized by neurological deficits resembling that seen in Parkinsonism. Although dopaminergic neurons within the nigrostriatal pathway appear intact, Mn-induced irregularities in DA transmission have been observed including decreased amphetamine-induced DA release and loss of the dopamine transporter (DAT). Results of studies to evaluate the effect of Mn and DA on cell viability in control and DAT-transfected HEK cells reveal that Mn is equally toxic to both cell lines whereas DA was only toxic to cells containing DAT. DA toxicity was saturable suggesting that transport may be rate limiting. When Mn and DA were added simultaneously to the media, cell toxicity was similar to that produced by Mn alone suggesting that Mn may suppress DA uptake in the DAT containing cells. Preincubation of DA prior to the addition of Mn resulted in cell death which was essentially additive with that produced independently by the two agents. Mn was also shown to decrease DA uptake and amphetamine-induced DA efflux in DAT containing cells. Time-lapsed confocal microscopy indicates that Mn can promote trafficking of cell surface DAT into intracellular compartments which may account for the decrease in DA uptake and DA efflux in these cells. Mn-induced internalization of DAT may provide an explanation for disruption in DA transmission previously reported in the striatum.
Keywords: manganese, manganism, dopamine transporter, DAT, HEK cells, dopamine
1. Introduction
Chronic exposure to Mn results in the development of a severe, irreversible neurological disorder known as manganism characterized by a diverse assortment of behavioral, intellectual and neurological deficits(Aschner et al., 2009; Roth, 2006). Upon protracted exposure, the disorder can progress to more prominent and irreversible extrapyramidal dysfunction resembling those associated with Parkinson’s disease (Guilarte, 2011; Roth, 2009). In general, manganism is initially linked to disruption of neurotransmission in the globus pallidus, whereas Parkinson’s disease is preferentially associated with loss of dopaminergic neurons in the substantia nigra pars compacta. Despite this difference, the symptoms of manganism are often misidentified by physicians for Parkinsonism. This probably reflects the fact that the basal ganglia is both structurally and functionally very complex being an amalgamation of a variety of integrated inhibitory and excitatory neurochemical systems which when disturbed, whether it initially occurs in the globus pallidus or nigrostriatal release of DA, will manifest in potentially overlapping expressed neurological dysfunction. Although distinct anatomical features present in the initial stages of the two disorders, there is increasing evidence that chronic exposure to elevated levels of Mn correlates with increased susceptibility to develop Parkinsonism, implying there may also be common neurochemical systems that are perturbed (Gorell et al., 1999; Hudnell, 1999; Kim et al., 2002).
Upon chronic exposure to Mn, modest levels accumulate in the substantia nigra yet in comparison to the globus pallidus, dopaminergic neurons within the nigrostriatal pathway appear to remain intact (Hauser et al., 1994; Park et al., 2007). Nevertheless, Mn-induced irregularities in DA transmission have been observed which potentially can contribute to the overall neurological symptoms seen in manganism (Guilarte et al., 2008; Peneder et al., 2011; Sriram et al., 2010). Recent studies have reported that amphetamine-induced (AMPH) DA release is markedly impaired in the striatum of Mn-exposed non-human primates in the absence of changes in markers of DA terminal integrity (Guilarte et al., 2008). DAT levels were also shown to decrease but only partially accounted for the diminished DA release. This suggests that Mn may block amphetamine-induced efflux of cytosolic DA through the presynaptic Na+/Cl−-dependent DA transporter (DAT). Recent studies further indicate that high concentrations of Mn can, in fact, directly inhibit the DAT transporter in isolated rat striatal synaptosomes in a non-competitive manner having an IC50 value of approximately 11 mM (Chen et al., 2006). Since disruption in DA transmission by Mn generates a pathological condition comparable to that seen in patients with Parkinson’s disease, it is not unreasonable to expect that symptoms observed between the two disorders may appear similar. The degree to which Mn-induced inhibition of DA efflux progresses further implicates this process in the subsequent development of a Parkinson-like disorder.
DAT activity is critical for DA homeostasis in the brain as it contributes to DA efflux in an action potential independent mechanism via reverse transport (Leviel, 2011). Studies in DAT knockouts demonstrate that DAT regulates the duration and dimension of dopaminergic signaling in the brain (Giros et al., 1996; Miller et al., 2001). The magnitude and direction of DAT currents are representative of the amount and direction of DA translocation; e.g. forward transport of DA (uptake), or reverse transport of DA (efflux) (Sitte et al., 1998; Sonders et al., 1997). DA uptake, DA efflux (reverse transport), sub-cellular distribution of DAT, and DAT interaction with binding proteins are affected by a wide variety of substrates, intracellular binding partners, second messengers, and ions. Based on prior studies examining the effect of Mn on DAT activity, it is intriguing to examine the consequence this interaction on cell toxicity as well as to assess the mechanism Mn-induced regulating of DAT function either via its modulation of the single transporter molecule or by influencing the trafficking of DAT and surface DAT levels.
2. Materials and methods
2.1 Materials
HEK-293 human embryonic kidney cells were purchased from American Type Culture Collection (A.T.C.C.). HEK-YFP-DAT and vector only control cells (Khoshbouei et al., 2002) were obtained from Dr. Habibeh Khoshbouei’s lab. Dulbecco’s Modified Eagle Medium (DMEM), Opti-MEM I reduced serum media, penicillin/streptomycin, puromycin and fetal bovine serum were from Invitrogen (Gibco BRL, Grand Island, NY). The selective DAT inhibitor, GBR12935, was obtained from Sigma Aldrich, CA.
2.2 Cell culture and viability assay
HEK-293, vector only and HEK-YFP DAT cells were maintained in DMEM containing 10% fetal bovine serum and penicillin (50 U/ml) and streptomycin (50 μg/ml). Addition of the N-terminal YFP tag to DAT did not significantly alter DA uptake and did not disrupt the function of the transporter to produce substrate-induced currents (Kahlig et al., 2004). Both cell lines were grown at 37°C in humidified atmosphere containing 5% CO2 and passaged every 3–4 days at approximately 80% confluency. The concentration of Mn used in these experiments are similar to that used previously reported in the literature (Marreilha dos Santos et al., 2008; Roth et al., 2000; Sidoryk-Wegrzynowicz et al., 2010; Walowitz and Roth, 1999).
To measure cell viability, MTT assays were performed on control, vector only and DAT- HEK cells treated with varying concentrations of Mn and/or DA. MnCl2 was used throughout as the sources of the divalent metal as this is readily soluble and the most common Mn compound used to treat cells in culture. For these experiments, cells were trypsinized and centrifuged to obtain cell pellets which were re-suspended in DMEM culture media and approximately 75,000 cells were plated per well in a 6 well plate. The cells were subsequently incubated overnight at 37°C in humidified atmosphere containing 5% CO2 at which time Mn and/or DA was added. Cells were incubated for varying lengths of time as indicated. Prior to the assay, DMEM was removed from each well and the cells washed with 500 μl OptiMem. MTT was subsequently added to each well and the cells were incubated for an additional 2 hour at 37°C at which time the media was removed and isopropanol added. Aliquots from each well were centrifuged and the absorbance was monitored at 560 nm in a plate reader to obtain the percentage of viable cells. The overall percentage of cell viability was calculated by dividing the treated sample by the controls, which did not receive Mn or DA for each cell line.
2.3 Confocal microscopy
For these experiments we used HEK-YFP-DAT cells that were grown overnight on chambered dishes (Mattek chambered dish) using DMEM, 10% FBS and 1% penicillin/streptomycin. Prior to imaging the media was changed to OPTIMEM without phenol red. A single position on the dishes was selected using the confocal software and baseline (t = 0) images were obtained. Cells were treated with vehicle (water) or 0.3 mM Mn and incubated on the stage for 60 min. before imaging at 10 min. intervals. Images were taken using a 63X objective and 2X zoom using a Zeiss LSM-510 Meta NLO laser scanning confocal microscope.
2.4 In Vitro electrophysiology
Before recording from parental or stably expressing DAT cells (Goodwin et al., 2009; Khoshbouei et al., 2002), cells were plated at 105 per 35 mm culture dish. Attached cells were washed three times with external solution at room temperature which contained 130 mM NaCl, 10 mM HEPES, 34 mM dextrose, 1.5 mM CaCl2, 0.5 mM MgSO4, and 1.3 mM KH2PO4 which was adjusted to pH 7.35 at a final osmolarity of 290 mOsm. The pipette solution for the patch clamp in whole-cell configuration contained the following: 120 mM CsCl, 0.1 mM CaCl2, 2 mM MgCl2, 1.1 mM EGTA, 10 mM Hepes, and 30 mM dextrose plus 2 mM DA and either 0, 10, 100 μM Mn, as specified in the text. The pH was adjusted to 7.35 and the final osmolarity was 270 mOsm.
Patch electrodes were pulled from quartz pipettes on a P-2000 puller (Sutter Instruments, Novato, CA) and filled with the pipette solution. Data were recorded and analyzed off-line using pCLAMP 9 software (Molecular Devices). The cells were voltage clamped in whole-cell configuration that allows dialysis of DA and Mn into the cell. Our previous findings (Goodwin et al., 2009; Kahlig et al., 2005; Khoshbouei et al., 2004; Khoshbouei et al., 2003) suggest that 5–8 min after the patch assumed whole-cell configuration, the intracellular ionic environment and pipette solution reached equilibrium. Accordingly, the amperometric recordings were performed 10 min. after the patch pipette assumed whole-cell configuration.
2.5 Amperometry
We monitored DA efflux using an amperometric electrode. The amperometric carbon fiber electrode (ProCFE, Dagan Corporation) is connected to an amplifier (Axopatch 200B, Molecular Devices, Sunnyvale, CA), which was placed close to the plasma membrane of the cell and held at +700 mV, a potential greater than the redox potential of DA. The diameter of the carbon fiber electrode is 5 μm. An oxidative (amperometric) current-voltage relationship was generated at membrane potential of −100 to +60 mV. Unlike the usual amperometric calibration, which requires conversion to concentration, we report the current directly without considering the effective volume. Thus, our requirements are a defined baseline, and our data represent a lower limit to the DA efflux because some transmitter is lost to the bulk solution as described previously (Goodwin et al., 2009; Kahlig et al., 2004; Khoshbouei et al., 2002).
To convert amperometric current measured to DA concentration, we assume an electrode-gathering volume of 0.1 μm3, that within this volume DA levels are constant for 1 msec. and that one DA ion converts to one electron. Then 1 pA implies 0.625 × 104 DA molecules flow into the assumed volume in 1 msec. Under these assumptions, 1 pA converts to 100 μM DA and 0.01 pA converts to 1 μM DA. The amperometric currents were low pass filtered at 100 Hz. An upward deflection in the amperometric currents corresponds to an outward flux of DA. At the “on” of the voltage step, for voltages more positive than −40 mV, the amperometric electrode recorded an oxidation current (positive) and moving the carbon fiber away from the cell caused the oxidative response to become smaller and slower. Furthermore, as expected for DA oxidation, the oxidative response diminished when we reduced the carbon fiber voltage to +300 mV and disappeared completely on further reduction.
For the amperometry recordings, the DAT-mediated DA efflux was isolated by subtracting the current produced in the presence of the selective DA uptake inhibitor, GBR12935, from the baseline current (current produced in the absence of AMPH). The AMPH-induced DA efflux was defined as the current recorded in the presence of the AMPH, minus the current recorded after addition of GBR12935 to the bath with substrate still present. The steady-state current at a particular voltage was calculated as the average current during the final 100 msec. of each potential tested. Plotting the steady-state current against the test voltage generated a current-voltage relationship.
3.0. Results
3.1 Mn and DA toxicity in control and DAT containing cells
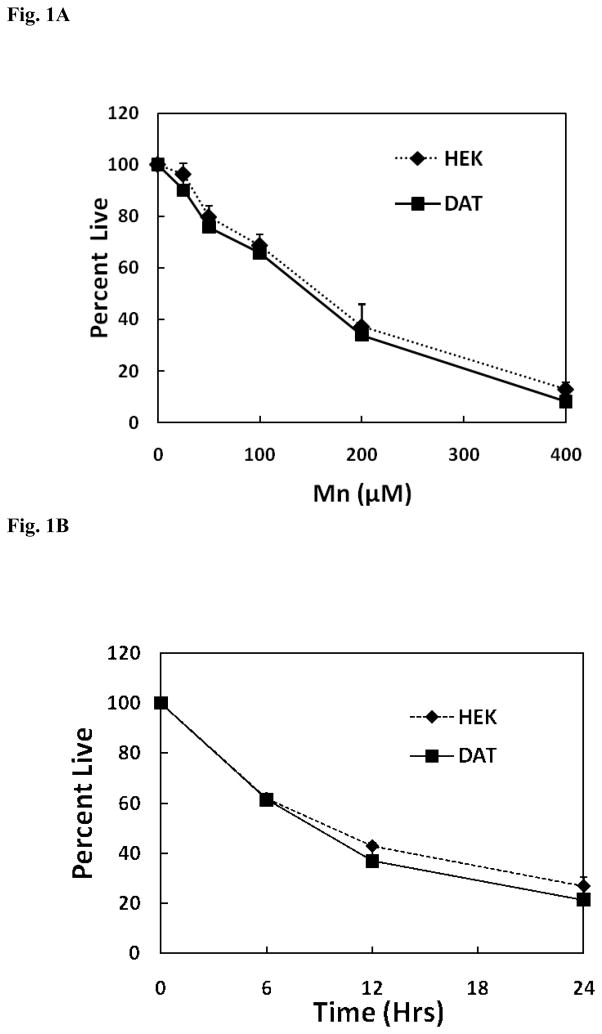
Initial experiments were performed to determine whether the presence of DAT in HEK cells can influence Mn toxicity as a function of both dose and time. Since DA is not transported into control normal HEK cells, a comparison of the response with that observed in DAT-HEK cells enables us to determine whether DA can selectively augment Mn toxicity. Thus, the non-transfected HEK cells serve as the ideal control for these experiments. As illustrated by the data presented in Fig. 1A, there is no differences in toxicity over the concentration range of Mn examined between the vector only control cells and those transfected with DAT. Consistent with this finding are the results presented Fig. 1B demonstrating that cell death induced by Mn in the two cell lines was also similar with respect to varying exposure times. These findings suggest that the presence of DAT in our cell culture model does not influence the toxic response to Mn although whether a similar situation occurs in vivo is not known.
Fig. 1.
(A) Concentration dependence for Mn toxicity in HEK-293 and HEK YFP-DAT cells. HEK-293 and HEK YFP-DAT cells were treated with 25 – 400 μM of Mn for 24 hrs. Toxicity was measured by the MTT assay and compared to the control samples treated with no Mn. Data are presented as the mean ± S.E.M for three independent experiments. There was no significant difference (p=0.997, Two way ANOVA) in cellular viability between HEK-293 and HEK YFP-DAT cells. (B) Time course for Mn toxicity in HEK-293, and HEK YFP-DAT cells. HEK-293 and HEK YFP-DAT cells were treated with 200 μM of Mn for 6, 12, and 24hrs. Toxicity was measured by MTT assay and compared to control samples treated with Mn for few seconds. Data are presented as the mean ± S.E.M for three independent experiments. There was no significant difference (p=0.533, Two Way ANOVA) in cellular viability between HEK-293 and HEK YFP-DAT cells.
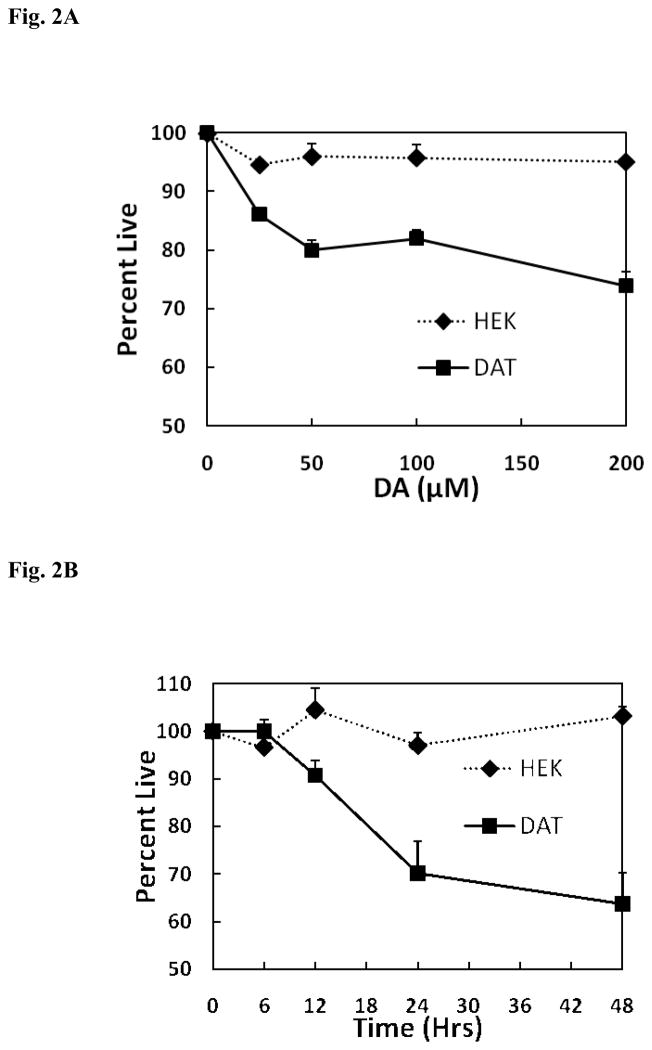
While it might be anticipated that Mn toxicity is unaffected by the presence or the absence of DAT, DA toxicity is expected to be influenced by the transporter as it will selectively facilitate internalization of DA and the subsequent formation of oxidative products leading to apoptosis. HEK293 cells devoid of DAT serve as ideal controls, as these cells will not to take up DA and therefore, enables evaluation of the effects of external DA on cell viability. Initial studies shown in Fig. 2A focused on examining the effect of DA concentration at 24 hrs. on cell survival in both the control and DAT containing HEK293 cells. As anticipated DA was not toxic in cells devoid of DAT but produced a significant increase in DA-induced toxicity in the DAT containing cells. The effect of DA on cell death was concentration dependent with the maximal response occurring at around 50 μM. As illustrated by the data presented in Fig. 2B, a similar and significant difference in Mn-induced toxicity was only observed in the DAT containing cells as a function of time. These findings clearly demonstrate that DA toxicity is significantly greater in the HEK YFP-DAT cells reflecting their ability to internalize DA. In addition, the lack of DA toxicity in the HEK cells devoid of DAT suggests that internalization of DA is required to produce toxicity.
Fig. 2.
(A) Concentration dependence for DA toxicity in HEK-293 and HEK YFP-DAT cells. HEK-293 and HEK YFP-DAT cells were treated with 25 – 200 μM of DA for 24 hrs. Toxicity was measured by the MTT assay and compared to the control samples treated with no DA. Data are presented as the mean ± S.E.M for three independent experiments. There was significant difference (p < 0.001) in cellular viability between HEK-293 and HEK YFP-DAT cells. (B) Time course for DA toxicity on HEK-293, and HEK YFP-DAT cells. HEK-293 and HEK YFP-DAT cells were treated with 100 μM dopamine for 6, 12, 24 and 48 hrs. Toxicity was measured by the MTT assay and compared to the control samples treated with no DA. Data are presented as the mean ± S.E.M for three independent experiments. There was significant difference (p < 0.01) in cellular viability between HEK-293 and HEK YFP-DAT cells.
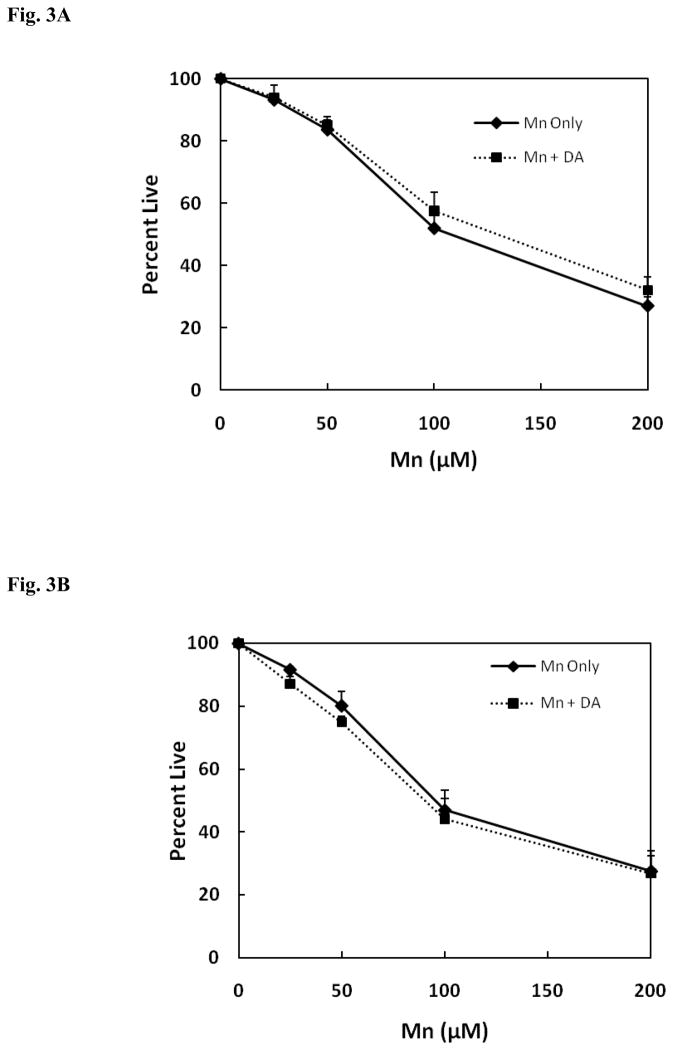
Because exposure to excess Mn has been suggested to promote premature symptoms that resemble Parkinsonism, studies were also performed to determine whether DA would stimulate the toxic response of Mn when the two agents were added simultaneously to media of both the HEK and DAT-HEK cells. As shown by the data in Fig. 3A, as expected the toxic response produced by Mn in the presence of 50 μM DA in HEK cells devoid of DAT, relative to the vehicle only control, is independent of presence of DA, as cell death was essentially equivalent to that produced by Mn alone. As presented by the data in Fig. 3B, similar results were obtained for the toxic response with the DAT containing HEK cells when simultaneously treated with both Mn and 50 μM DA. In this case, Mn, unexpectedly, attenuated the toxic response produced by 50 μM DA in these cells at 24 hrs. as indicated by the fact that when the two substances were combined, overall toxicity was essentially equivalent to that produced by Mn alone (compare to Fig. 1 above). Although not shown, similar results were obtained using 100 μM DA and with control HEK cells. These data suggests that when Mn is added to the media at the same time as DA, it can either directly or indirectly interfere with DAT activity (such as DAT-mediated DA uptake, DA efflux via DAT, trafficking of surface DAT) or act as an antioxidant preventing formation of the reactive oxidized products of the catechol.
Fig. 3.
(A) Mn and DA-induced toxicity when co-incubated in HEK cells. A. Mn and DA co-incubation with HEK-293 cells. DA (50 μM) was preincubated for 24 hrs. prior to the addition of Mn. Toxicity was measured by the MTT assay and compared to the control samples treated with no DA. Data presents the mean ± S.E.M for three independent experiments. There was no significant difference in cellular viability between Mn treated and Mn/DA treated in HEK-293 cells. (B) Mn and DA-induced toxicity when co-incubated in HEK YFP-DAT cells. DA (50 μM) was preincubated for 24 hrs. prior to the addition of Mn. Toxicity was measured by the MTT assay and compared to the control samples treated with no DA. Data presents the mean ± S.E.M for three independent experiments. There was no significant difference in cellular viability between Mn treated and Mn/DA treated in HEK YFP-DAT.
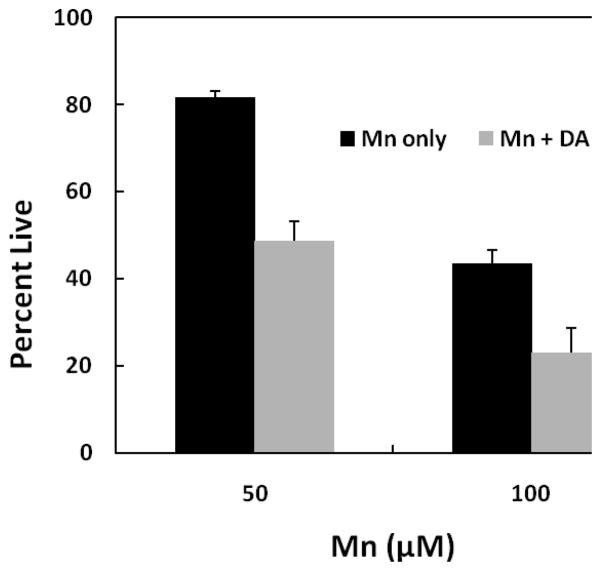
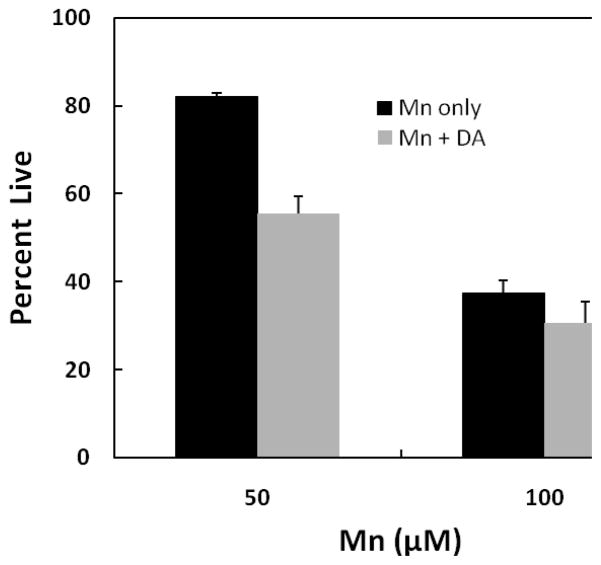
To ascertain which of these mechanisms may account for the observed response, studies were performed to examine the combine actions of the two agents when DA is first preincubated prior to the addition of Mn in the DAT-HEK cells. We hypothesized that if Mn was indeed inhibiting uptake, we would observe an increase in overall toxicity under these conditions as DA would have access to the cells prior to the addition of Mn. For these studies, DA was initially preincubated for 12 hrs. to permit uptake of DA before addition of Mn to the media. As shown by the data presented in Fig. 4, when DAT-HEK cells were treated with 50 and 100 μM Mn for an additional 24 hrs. after the initial DA treatment, total toxicity (grey bars) exceeded that with Mn treatment alone (black bars). For these experiments, DA treatment (100 μM) alone produced 15 and 35% toxicity at the 12 and 36 hrs. time points, respectively. As indicated, the contribution of DA toxicity in the presence of Mn to the overall value, as indicated by the difference between the grey and black bars, was close to the total 36 hr. time point seen upon DA treatment alone. The critical value here is the difference between 50 μM Mn only (black bar) vs. Mn + DA (grey bar) treatment which resulted in a decrease of approximately 30%. This value is close to the expected difference (35% of 81) between the two treatments if they were additive. Similarly, the difference at 100 μM Mn is approximately 20% which is close to the expected value (35% of 43) if an additive response occurred. The differences between Mn and Mn + DA treatment are statistically different at both the 50 and 100 μM Mn concentrations (p ≤ 0.001 at 50 μM Mn and p ≤ 0.001 at 100 μM). This implies that once DA was internalized within the cells, it was capable of augmenting toxicity produced by Mn independent of external DA.
Fig. 4.
Mn-induced toxicity in HEK YFP-DAT cells pre-incubation with DA. Cells were pre-incubated with 100 μM DA for 12 hrs. and then with 50 and 100 μM of Mn for an additional 24 hrs. Toxicity was measured by MTT assay and compared to the control samples treated with no Mn and no DA. Data are presented as the mean ± S.E.M of three independent experiments. For all these experiments, there was significant difference in cellular viability between manganese treated and manganese/dopamine treated HEK YFP-DAT cells (p-value for Mn and Mn + DA 50 or 100 μM respectively: p < 0.001, p < 0.001). There was a significant difference between cell viability for Mn only treatment in comparison to the no Mn control (50 μM Mn, p < 0.0005; 100 μM Mn, p < 0.001). The cell viability for DA only for 36 hr. exposure was 65 percent.
It was also of interest to determine the combined effects of Mn and DA in which DA was initially preincubated with the DAT-HEK cells for 12 hrs. but then removed prior to the addition of Mn. Results of these studies presented in Fig. 5 reveal that the inhibition pattern observed is similar to that presented above in Fig. 5 although the overall inhibition pattern is slightly less for the cells exposed to both DA and Mn. This resulted in the observation that a significant difference in cell viability between Mn only and Mn + DA treatment was only observed for the 50 μM Mn treatment.
Fig. 5.
Mn-induced toxicity in HEK YFP-DAT cells initially pre-incubation with DA for 12 hrs. Cells were pre-incubated with 100 μM DA for 12 hrs., then the DA was removed and incubation continued with 50 and 100 μM of Mn for another 24 hrs. Toxicity was measured by MTT assay and compared to the control samples treated with no Mn and no DA. Data are presented as the mean ± S.E.M of three independent experiments. For the 50 μM experiment, there was significant difference in cellular viability between manganese treated and manganese/dopamine treated HEK YFP-DAT samples. However, for the 100 μM experiment there was no significant difference in cell viability between Mn treated and Mn/dopamine treated HEK YFP-DAT. (p-value for Mn and Mn + DA 50 and 100 μM respectively: (p < 0.001, p = 0.2). There was a significant difference between cell viability for Mn only treatment in comparison to the no Mn controls (50 μM Mn, p < 0.001, 100 μM Mn, p < 0.001). The cell viability for DA only control for 12 hr. washed exposure is 66 percent.
3.2 Mn-induced alterations in DAT distribution
The above findings suggest that Mn may preferentially be acting by inhibiting uptake of DA and thereby prevent the ensuing signaling events leading to cell death. This may possible explain why Mn suppressed DA transport and toxicity when the two were added simultaneously to the DAT-HEK cells. Prior studies have demonstrated that Mn can directly inhibit DA transport in isolated rat synaptosomes, though the concentration of Mn need to accomplish this was considerably higher (IC50 = 11 mM) than that used in our experiments. Another plausible mechanism which may account for the results we observed in HEK cells may relate to the trafficking of surface DAT to internal compartments as Mn has previously been reported to alter the distribution of other plasma membrane proteins. Accordingly, studies were performed to examine whether Mn can correspondingly affect the distribution of DAT in HEK cells. For these studies, DAT-HEK cells were grown overnight on chambered dishes in DMEM before being changed to OPTIMEM prior to imaging. A single position on the dishes was selected using the confocal software and baseline images were taken prior to addition of Mn. Cells were subsequently treated with vehicle or 0.3 mM Mn and incubated on the stage for 60 min. before imaging at 10 min. intervals for a total of 2 hrs. Results of these studies, shown in Fig. 6, reveal that Mn within this time framed caused approximately a 60% increase in the redistribution of DAT from the surface to the internal compartment of the cell.
Fig. 6.
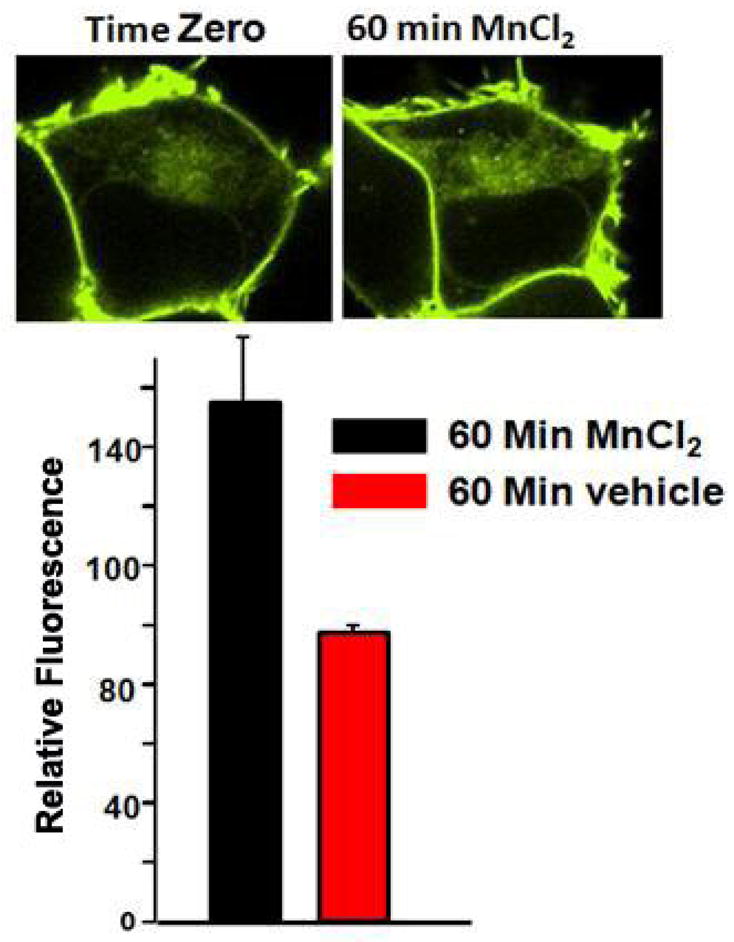
Effect of Mn on the redistribution of DAT in HEK YFP-DAT cells. DAT-HEK cells were grown overnight on chambered dishes in DMEM before being changed to OPTIMEM prior to imaging. A single position on the dishes was selected using the confocal software and baseline images were taken prior to addition of Mn. Cells were treated with vehicle or 0.3 mM Mn and incubated on the stage for 60 min. prior to imaging on a Zeiss LSM-510 Meta NLO laser scanning confocal microscope. The data is average of 15–20 cells from five independent experiments.
3.3 Mn effect on amphetamine-induced DA efflux
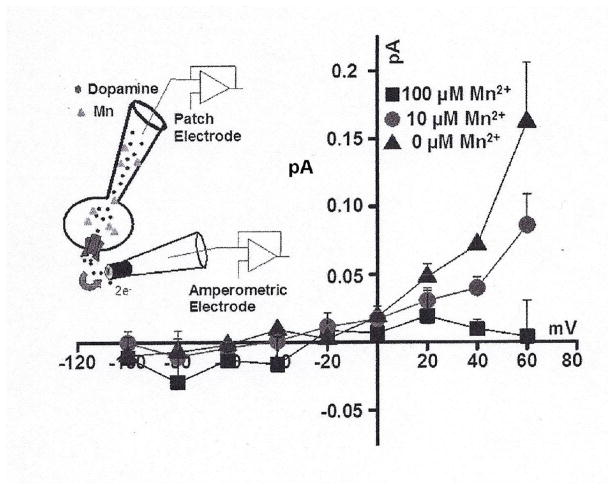
Based on these findings, it was also of interest to determine whether exposure to Mn can potentially influence DAT-mediated reverse transport of DA (DA efflux). To accomplish this, we used simultaneous whole-cell patch clamp and amperometry. This approach helps to resolve the mechanistic relationship between the overall “activity” of the transporter and the regulatory role of this activity on the DA efflux via DAT. We determined the amphetamine-induced DAT-mediated DA efflux in the presence and absence of 10 and 100 μM intracellular Mn. The DAT-mediated DA efflux was measured after dialysis of Mn into the cell via the patch pipette at +60 to −100 mV membrane potentials. Consistent with our previous reports, amphetamine increases DAT-mediated DA efflux in a GBR12935 sensitive fashion (Fig. 7). In the presence of intracellular Mn, however, there was a concentration-dependent decreases in DAT-mediated DA efflux (the current blocked by GBR12935) (Goodwin et al., 2009; Swant et al., 2011). For control experiments, we also examined whether Mn, via an unknown mechanism, diffuses out of the cells and influences the measured oxidative current. To examine this possibility, we repeated the experiment, with no Mn in the recording electrode, when either 50 μl (electrode volume) of a 100 μM Mn solution or physiological-like mixture is added to the bath solution. The DAT-mediated DA efflux, at the membrane potentials examined, was not different as compared to control condition when 50 μl of physiological-like bath solution was added (data not shown).
Fig. 7.
Changes is current-voltage (I(V)) relationships produced by Mn on DAT-mediated DA efflux. DAT mediated DA efflux was measured at +60 to −100 mV membrane potentials when 0, 10 and 100 μM after Mn was dialyzed into the cell. Addition of 50 μl (pipette volume) of a 100 μM Mn solution to the bath solution did not affect DAT-mediated DA efflux at these membrane potentials (data not shown). DAT-mediated DA efflux was isolated by subtracting the current produced in the presence of the selective DA uptake inhibitor, GBR12935, from the baseline current (current produced in the absence of AMPH). The AMPH-induced DA efflux was defined as the current recorded in the presence of the AMPH, minus the current recorded after addition of GBR12935 to the bath with substrate still present.
4.0. Discussion
Although the globus pallidus is the initial site of injury in manganism, dopaminergic transmission in the striatum is also compromised partially explaining the overlapping symptoms with that of Parkinson’s disease (Guilarte et al., 2008; Peneder et al., 2011; Sriram et al., 2010). This is supported by recent studies reporting that amphetamine-induced DA release is markedly impaired in the striatum of Mn-exposed non-human primates in the absence of changes in markers of DA terminal integrity (Guilarte et al., 2008). DAT levels were also shown to decrease which, in part, may accounted for the diminished DA release.
To characterize the effect of Mn on DAT and DA transport, we utilized HEK293 (human embryonic kidney) cells and HEK293 cells transfected with the DA transporter (DAT) which allowed us to regulate and compare the influx and effects of internal and external DA on Mn toxicity in these cell. Over the past decade this model system has been routinely and extensively used to study DAT trafficking (Cremona et al., 2011; Kahlig and Galli, 2003; Saunders et al., 2000) and DAT-mediated DA efflux (Kahlig et al., 2004; Khoshbouei et al., 2004; Khoshbouei et al., 2003). The results reported in the literature, using this model system, are replicate in neuronal system expressing DAT (Fog et al., 2006; Kahlig et al., 2006). Therefore, the consensus in the literature is that HEK-YFP-DAT cells represent a useful model for studying DAT function. Since HEK293 cells express the neurofilament subunits, NF-L, NF-M, NF-H, and α-internexin as well as many other proteins which are typically found in neurons, they represent an appropriate model to examine the effect of DAT on both DA and Mn toxicity (Shaw et al., 2002). In this regard, HEK293 cells have previously been used as a model to study Parkinsonism (Carballo-Carbajal et al., 2010; Ren et al., 2003) and to assess the actions of Mn (Garrick et al., 2006; Yin et al., 2010). Thus, we used this model system to examine the Mn-regulation of DAT activity (trafficking and reverse transport of dopamine).
Initial studies were performed to evaluate the effect of Mn on cell viability in the presence and absence of DA in vector only control HEK cells and those transfected with DAT. The concentration and exposures times for Mn used in this paper are comparable to that employed in other publications examining the actions of Mn in cultured cells (Marreilha dos Santos et al., 2008; Roth et al., 2000; Sidoryk-Wegrzynowicz et al., 2010; Walowitz and Roth, 1999). Results of these experiments reveal that Mn was equally toxic to both the DAT transfected as well as the vector only control cells indicating that the presence of DAT does not influence Mn-induced cell death. In contrast, results indicate that DA was only toxic to cells containing DAT suggesting that external DA in control cells was not sufficiently internalized to generate toxic oxidative products. DA toxicity in DAT containing cells appeared to be saturable suggesting that transport may be the rate limiting process regulating cell death. This data is in contrast to the effect of DA on the viability of PC12 cells which contain endogenous levels of DA (Offen et al., 1997; Offen et al., 1996). Consistent with transport being the limiting factor in our studies are other findings demonstrating that DA-induced apoptosis was decreased when the DA transporter was inhibited by cocaine and by antisense to DAT (Simantov et al., 1996).
One of the major goals of this paper was to examine the combined pathophysiological activities of Mn with that of DA as chronic exposure to Mn has been reported to lead to early onset of a neurological disorder resembling that of Parkinsonism (Racette et al., 2005). Results of these studies, surprisingly, revealed that when both Mn and DA were added simultaneously to the media, cell toxicity was remarkably similar to that produced by Mn alone. Although there was a very slight increase in toxicity observed in the DAT containing cell, this difference failed to reach statistical significance. The concentration of Mn needed to inhibit HEK cell death was approximately 100 times lower than that reported previously to directly inhibit the DAT transporter in rat synaptosome preparations (Chen et al., 2006). The direct inhibition of DAT by Mn was shown to be non-competitive with no change in Km but a decrease in the Vmax value observed. These data are consistent with our findings suggesting that Mn may suppress toxicity though the mechanisms by which they achieve this outcome may be different. One possible explanation for the inhibition of DA transport observed in isolated rat synaptosomes was possibly caused by the high concentrations of Mn used which may have disrupted membrane integrity altering transport function without affecting the affinity of DA for the transporter which is consistent with the non-competitive inhibition pattern observed. The data in the current paper suggest that Mn suppression of DA transport may result from the loss and redistribution of cell surface DAT or by its prevention of oxidative stress and subsequent formation of the downstream apoptotic signals. To distinguish which of these processes is responsible for the observed protective actions of Mn, we examined the combine actions of the two agents by first preincubating DAT cells with DA prior to addition of Mn. When DA was exposed to the cells prior to the addition of Mn to permit its uptake, the observed cell death was essentially additive with the toxic response produced by Mn after an additional 24 hr. incubation period. This implies that Mn may be inhibiting DA transport when the two agents were simultaneously added to the media. Additional studies were also performed to examine toxicity when DA was washed-out of the media prior to the addition of Mn in the DAT containing HEK cells. Results of these studies suggest the overall toxicity was slightly less when DA was removed from the media prior to the addition when compared to a similar condition when DA was not washed out of the media. The reason for this apparent discrepancy in toxicity when DA was present for only the initial 12 hr. period is not readily apparent though it infers that the exposure to DA for the entire 36 hr. treatment period possibly results in more DA entering the cell which is sufficient to promote greater toxicity. It is also feasible that in experiments in which DA was washed out of the media, mitochondrial insufficiencies which are emphasized in the MTT assay dissipate as mitochondria recover from initial 12 hr. exposure period to DA. Although Mn by itself can cause mitochondrial dysregulation, it is equally feasible that Mn can act as an antioxidant and attenuate the actions of the oxidative products produced by DA which were generated within the cells.
Our rationale to use live cell confocal microscopy to determine the influence of Mn on DAT trafficking in the present manuscript is based on several studies (Kahlig et al., 2004; Kahlig et al., 2006; Saunders et al., 2000) using this technique to establish the time-course of YFP-DAT trafficking in HEK cells. Based on these prior findings, the data obtained herein support the conclusion that Mn can suppress DA toxicity by promoting trafficking of surface DAT to internal compartments of the cell. Although we cannot rule out the feasibility that Mn can also induced changes in protein synthesis which accounts for the observed increase in intracellular DAT levels, we believe this is less likely as we correspondingly measured a concurrent decrease in surface DAT. Therefore, Mn-induced increases in intracellular DAT can be due to: 1) Mn-induced internalization rate of DAT, 2) Mn-induced increase DAT synthesis which fails to traffic to the membrane, or 3) possibly both mechanisms. Even if the second possibility is true, then the newly synthesized DAT protein which accumulates within the cell cannot be delivered to the cell surface thus, supporting our overall hypothesis that Mn alters DAT redistribution. This observation is consistent with previous reports demonstrating that Mn can alter the distribution of other membrane proteins (Mukhopadhyay et al., 2010; Wang et al., 2008). Once internalized, DAT can undergo ubiquitination and proteasomal degradation via a PKC-dependent pathway (Boudanova et al., 2008; Miranda et al., 2007). Relevant to this is the fact that Mn has similarly been reported to promote ubiquitination of the glutamine transporter in a PKC-dependent process (Sidoryk-Wegrzynowicz et al., 2011; Sidoryk-Wegrzynowicz et al., 2010). Interestingly, proteasomal degradation of both transporters also requires NEDD4 ligase for ubiquitination. The consequence of DAT internalization may also help explain the observation reported herein that Mn causes a decrease in DA efflux in DAT containing HEK cells as well as a decrease in amphetamine-induced release of DA in the striatum of primate brains acutely treated with Mn (Guilarte et al., 2006).
The uptake of the released DA is one of the main mechanisms for recycling and replenishment of intracellular DA. Therefore, long-term inhibition or elimination of uptake mechanism can reduce the available synaptic DA (Giros et al., 1996). In all likelihood, Mn induced disruption in DA transmission generates a condition which potentially can resemble the pathology observed in patients with Parkinson’s disease and therefore, is expected to contribute to the symptoms seen in manganism. Results of this paper demonstrate that Mn can alter DA transport and DA-stimulated cell toxicity by promoting internalization of DAT. As demonstrated, this process results in a reduction of DA release and thus, presents a plausible explanation as to why exposure to high levels of Mn can suppress DA flux from dopaminergic neurons in the striatum. The magnitude and progression of Mn-induced inhibition of DA release may also be implicated in the characteristics and severity of manganism and the subsequent development of idiopathic Parkinson’s disease.
Highlights.
Mn is equally toxic to control and DAT transfected HEK cell whereas dopamine is only toxic to the DAT transfected cells
Mn suppresses DA toxicity in the DAT containing cells
Mn promotes internalization of cell surface DAT
Mn inhibits amphetamine-induced DA efflux in DAT containing cells
Acknowledgments
This research supported in part by grants from the NIH, ES015762 and ES0810301 (JAR) and DA026947 and NS071122 (HK).We acknowledge the assistance of the Confocal Microscope and Flow Cytometry Facility in the School of Medicine and Biomedical Sciences, University at Buffalo.
Abbreviations used
- DAT
dopamine transporter
- DA
dopamine
- Mn
manganese
- HEK
human embryonic kidney
- NEDD-4
neural precursor cell expressed developmentally down-regulated protein 4
- AMPH
amphetamine
Footnotes
There is no conflict of interest which effects objectivity in regard to publishing this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aschner M, Erikson KM, Herrero Hernandez E, Tjalkens R. Manganese and its role in Parkinson’s disease: from transport to neuropathology. Neuromolecular Med. 2009;11:252–66. doi: 10.1007/s12017-009-8083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudanova E, Navaroli DM, Stevens Z, Melikian HE. Dopamine transporter endocytic determinants: carboxy terminal residues critical for basal and PKC-stimulated internalization. Mol Cell Neurosci. 2008;39:211–7. doi: 10.1016/j.mcn.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballo-Carbajal I, Weber-Endress S, Rovelli G, Chan D, Wolozin B, Klein CL, Patenge N, Gasser T, Kahle PJ. Leucine-rich repeat kinase 2 induces alpha-synuclein expression via the extracellular signal-regulated kinase pathway. Cell Signal. 2010;22:821–7. doi: 10.1016/j.cellsig.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MK, Lee JS, McGlothan JL, Furukawa E, Adams RJ, Alexander M, Wong DF, Guilarte TR. Acute manganese administration alters dopamine transporter levels in the non-human primate striatum. Neurotoxicology. 2006;27:229–36. doi: 10.1016/j.neuro.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Cremona ML, Matthies HJ, Pau K, Bowton E, Speed N, Lute BJ, Anderson M, Sen N, Robertson SD, Vaughan RA, Rothman JE, Galli A, Javitch JA, Yamamoto A. Flotillin-1 is essential for PKC-triggered endocytosis and membrane microdomain localization of DAT. Nat Neurosci. 2011;14:469–77. doi: 10.1038/nn.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fog JU, Khoshbouei H, Holy M, Owens WA, Vaegter CB, Sen N, Nikandrova Y, Bowton E, McMahon DG, Colbran RJ, Daws LC, Sitte HH, Javitch JA, Galli A, Gether U. Calmodulin kinase II interacts with the dopamine transporter C terminus to regulate amphetamine-induced reverse transport. Neuron. 2006;51:417–29. doi: 10.1016/j.neuron.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Garrick MD, Singleton ST, Vargas F, Kuo HC, Zhao L, Knopfel M, Davidson T, Costa M, Paradkar P, Roth JA, Garrick LM. DMTI: Which metals does it transport? Biological Research. 2006;39:79–85. doi: 10.4067/s0716-97602006000100009. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–12. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Goodwin JS, Larson GA, Swant J, Sen N, Javitch JA, Zahniser NR, De Felice LJ, Khoshbouei H. Amphetamine and methamphetamine differentially affect dopamine transporters in vitro and in vivo. J Biol Chem. 2009;284:2978–89. doi: 10.1074/jbc.M805298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Brown GG, Richardson RJ. Occupational exposure to manganese, copper, lead, iron, mercury and zinc and the risk of Parkinson’s disease. Neurotoxicology. 1999;20:239–47. [PubMed] [Google Scholar]
- Guilarte TR. Manganese and Parkinson’s disease: a critical review and new findings. Cien Saude Colet. 2011;16:4549–66. doi: 10.1590/s1413-81232011001200028. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Burton NC, McGlothan JL, Verina T, Zhou Y, Alexander M, Pham L, Griswold M, Wong DF, Syversen T, Schneider JS. Impairment of nigrostriatal dopamine neurotransmission by manganese is mediated by pre-synaptic mechanism(s): implications to manganese-induced parkinsonism. J Neurochem. 2008;107:1236–47. doi: 10.1111/j.1471-4159.2008.05695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Chen MK, McGlothan JL, Verina T, Wong DF, Zhou Y, Alexander M, Rohde CA, Syversen T, Decamp E, Koser AJ, Fritz S, Gonczi H, Anderson DW, Schneider JS. Nigrostriatal dopamine system dysfunction and subtle motor deficits in manganese-exposed non-human primates. Exp Neurol. 2006;202:381–90. doi: 10.1016/j.expneurol.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Hauser RA, Zesiewicz TA, Rosemurgy AS, Martinez C, Olanow CW. Manganese intoxication and chronic liver failure. Ann Neurol. 1994;36:871–5. doi: 10.1002/ana.410360611. [DOI] [PubMed] [Google Scholar]
- Hudnell HK. Effects from environmental Mn exposures: a review of the evidence from non- occupational exposure studies. Neurotoxicology. 1999;20:379–97. [PubMed] [Google Scholar]
- Kahlig KM, Binda F, Khoshbouei H, Blakely RD, McMahon DG, Javitch JA, Galli A. Amphetamine induces dopamine efflux through a dopamine transporter channel. Proc Natl Acad Sci U S A. 2005;102:3495–500. doi: 10.1073/pnas.0407737102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlig KM, Galli A. Regulation of dopamine transporter function and plasma membrane expression by dopamine, amphetamine, and cocaine. European Journal of Pharmacology. 2003;479:153–8. doi: 10.1016/j.ejphar.2003.08.065. [DOI] [PubMed] [Google Scholar]
- Kahlig KM, Javitch JA, Galli A. Amphetamine regulation of dopamine transport. Combined measurements of transporter currents and transporter imaging support the endocytosis of an active carrier. J Biol Chem. 2004;279:8966–75. doi: 10.1074/jbc.M303976200. [DOI] [PubMed] [Google Scholar]
- Kahlig KM, Lute BJ, Wei Y, Loland CJ, Gether U, Javitch JA, Galli A. Regulation of dopamine transporter trafficking by intracellular amphetamine. Mol Pharmacol. 2006;70:542–8. doi: 10.1124/mol.106.023952. [DOI] [PubMed] [Google Scholar]
- Khoshbouei H, Cecchi M, Dove S, Javors M, Morilak DA. Behavioral reactivity to stress: amplification of stress-induced noradrenergic activation elicits a galanin-mediated anxiolytic effect in central amygdala. Pharmacol Biochem Behav. 2002;71:407–17. doi: 10.1016/s0091-3057(01)00683-9. [DOI] [PubMed] [Google Scholar]
- Khoshbouei H, Sen N, Guptaroy B, Johnson L, Lund D, Gnegy ME, Galli A, Javitch JA. N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS Biol. 2004;2:E78. doi: 10.1371/journal.pbio.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshbouei H, Wang H, Lechleiter JD, Javitch JA, Galli A. Amphetamine-induced dopamine efflux. A voltage-sensitive and intracellular Na+-dependent mechanism. J Biol Chem. 2003;278:12070–7. doi: 10.1074/jbc.M212815200. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kim JM, Kim JW, Yoo CI, Lee CR, Lee JH, Kim HK, Yang SO, Chung HK, Lee DS, Jeon B. Dopamine transporter density is decreased in parkinsonian patients with a history of manganese exposure: what does it mean? Mov Disord. 2002;17:568–75. doi: 10.1002/mds.10089. [DOI] [PubMed] [Google Scholar]
- Leviel V. Dopamine release mediated by the dopamine transporter, facts and consequences. J Neurochem. 2011;118:475–89. doi: 10.1111/j.1471-4159.2011.07335.x. [DOI] [PubMed] [Google Scholar]
- Marreilha dos Santos AP, Santos D, Au C, Milatovic D, Aschner M, Batoreu MC. Antioxidants prevent the cytotoxicity of manganese in RBE4 cells. Brain Res. 2008;1236:200–5. doi: 10.1016/j.brainres.2008.07.125. [DOI] [PubMed] [Google Scholar]
- Miller GW, Wang YM, Gainetdinov RR, Caron MG. Dopamine transporter and vesicular monoamine transporter knockout mice: implications for Parkinson’s disease. Methods Mol Med. 2001;62:179–90. doi: 10.1385/1-59259-142-6:179. [DOI] [PubMed] [Google Scholar]
- Miranda M, Dionne KR, Sorkina T, Sorkin A. Three ubiquitin conjugation sites in the amino terminus of the dopamine transporter mediate protein kinase C-dependent endocytosis of the transporter. Mol Biol Cell. 2007;18:313–23. doi: 10.1091/mbc.E06-08-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Bachert C, Smith DR, Linstedt AD. Manganese-induced trafficking and turnover of the cis-Golgi glycoprotein GPP130. Mol Biol Cell. 2010;21:1282–92. doi: 10.1091/mbc.E09-11-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offen D, Ziv I, Panet H, Wasserman L, Stein R, Melamed E, Barzilai A. Dopamine-induced apoptosis is inhibited in PC12 cells expressing Bcl-2. Cell Mol Neurobiol. 1997;17:289–304. doi: 10.1023/A:1026390201168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offen D, Ziv I, Sternin H, Melamed E, Hochman A. Prevention of dopamine-induced cell death by thiol antioxidants: possible implications for treatment of Parkinson’s disease. Exp Neurol. 1996;141:32–9. doi: 10.1006/exnr.1996.0136. [DOI] [PubMed] [Google Scholar]
- Park JD, Chung YH, Kim CY, Ha CS, Yang SO, Khang HS, Yu IK, Cheong HK, Lee JS, Song CW, Kwon IH, Han JH, Sung JH, Heo JD, Choi BS, Im R, Jeong J, Yu IJ. Comparison of high MRI T1 signals with manganese concentration in brains of cynomolgus monkeys after 8 months of stainless steel welding-fume exposure. Inhal Toxicol. 2007;19:965–71. doi: 10.1080/08958370701516108. [DOI] [PubMed] [Google Scholar]
- Peneder TM, Scholze P, Berger ML, Reither H, Heinze G, Bertl J, Bauer J, Richfield EK, Hornykiewicz O, Pifl C. Chronic exposure to manganese decreases striatal dopamine turnover in human alpha-synuclein transgenic mice. Neuroscience. 2011;180:280–92. doi: 10.1016/j.neuroscience.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Racette BA, Antenor JA, McGee-Minnich L, Moerlein SM, Videen TO, Kotagal V, Perlmutter JS. [18F]FDOPA PET and clinical features in parkinsonism due to manganism. Mov Disord. 2005;20:492–6. doi: 10.1002/mds.20381. [DOI] [PubMed] [Google Scholar]
- Ren Y, Zhao J, Feng J. Parkin binds to alpha/beta tubulin and increases their ubiquitination and degradation. J Neurosci. 2003;23:3316–24. doi: 10.1523/JNEUROSCI.23-08-03316.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth JA. Homeostatic and toxic mechanisms regulating manganese uptake, retention, and elimination. Biol Res. 2006;39:45–57. doi: 10.4067/s0716-97602006000100006. [DOI] [PubMed] [Google Scholar]
- Roth JA. Are there common biochemical and molecular mechanisms controlling manganism and parkisonism. Neuromolecular Med. 2009;11:281–96. doi: 10.1007/s12017-009-8088-8. [DOI] [PubMed] [Google Scholar]
- Roth JA, Feng L, Walowitz J, Browne RW. Manganese-induced rat pheochromocytoma (PC12) cell death is independent of caspase activation. J Neurosci Res. 2000;61:162–71. doi: 10.1002/1097-4547(20000715)61:2<162::AID-JNR7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Saunders C, Ferrer JV, Shi L, Chen J, Merrill G, Lamb ME, Leeb-Lundberg LM, Carvelli L, Javitch JA, Galli A. Amphetamine-induced loss of human dopamine transporter activity: an internalization-dependent and cocaine-sensitive mechanism. Proc Natl Acad Sci U S A. 2000;97:6850–5. doi: 10.1073/pnas.110035297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G, Morse S, Ararat M, Graham FL. Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. FASEB J. 2002;16:869–71. doi: 10.1096/fj.01-0995fje. [DOI] [PubMed] [Google Scholar]
- Sidoryk-Wegrzynowicz M, Lee E, Mingwei N, Aschner M. Disruption of astrocytic glutamine turnover by manganese is mediated by the protein kinase C pathway. Glia. 2011;59:1732–43. doi: 10.1002/glia.21219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidoryk-Wegrzynowicz M, Lee ES, Ni M, Aschner M. Manganese-induced downregulation of astroglial glutamine transporter SNAT3 involves ubiquitin-mediated proteolytic system. Glia. 2010;58:1905–12. doi: 10.1002/glia.21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simantov R, Blinder E, Ratovitski T, Tauber M, Gabbay M, Porat S. Dopamine-induced apoptosis in human neuronal cells: inhibition by nucleic acids antisense to the dopamine transporter. Neuroscience. 1996;74:39–50. doi: 10.1016/0306-4522(96)00102-9. [DOI] [PubMed] [Google Scholar]
- Sitte HH, Huck S, Reither H, Boehm S, Singer EA, Pifl C. Carrier-mediated release, transport rates, and charge transfer induced by amphetamine, tyramine, and dopamine in mammalian cells transfected with the human dopamine transporter. J Neurochem. 1998;71:1289–97. doi: 10.1046/j.1471-4159.1998.71031289.x. [DOI] [PubMed] [Google Scholar]
- Sonders MS, Zhu SJ, Zahniser NR, Kavanaugh MP, Amara SG. Multiple ionic conductances of the human dopamine transporter: the actions of dopamine and psychostimulants. J Neurosci. 1997;17:960–74. doi: 10.1523/JNEUROSCI.17-03-00960.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram K, Lin GX, Jefferson AM, Roberts JR, Chapman RS, Chen BT, Soukup JM, Ghio AJ, Antonini JM. Dopaminergic neurotoxicity following pulmonary exposure to manganese-containing welding fumes. Arch Toxicol. 2010;84:521–40. doi: 10.1007/s00204-010-0525-9. [DOI] [PubMed] [Google Scholar]
- Swant J, Goodwin JS, North A, Ali AA, Gamble-George J, Chirwa S, Khoshbouei H. alpha-Synuclein stimulates a dopamine transporter-dependent chloride current and modulates the activity of the transporter. J Biol Chem. 2011;286:43933–43. doi: 10.1074/jbc.M111.241232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walowitz JL, Roth JA. Activation of ERK1 and ERK2 is required for manganese-induced neurite outgrowth in rat pheochromocytoma (PC12) cells. J Neurosci Res. 1999;57:847–54. [PubMed] [Google Scholar]
- Wang X, Miller DS, Zheng W. Intracellular localization and subsequent redistribution of metal transporters in a rat choroid plexus model following exposure to manganese or iron. Toxicol Appl Pharmacol. 2008;230:167–74. doi: 10.1016/j.taap.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Jiang H, Lee ES, Ni M, Erikson KM, Milatovic D, Bowman AB, Aschner M. Ferroportin is a manganese-responsive protein that decreases manganese cytotoxicity and accumulation. J Neurochem. 2010;112:1190–8. doi: 10.1111/j.1471-4159.2009.06534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]