Abstract
Purpose
To determine whether the genomic changes in hepatitis B virus (HBV) affect the clinical outcomes of hepatocellular carcinoma (HCC) in patients with HBV-associated HCC treated with curative surgical resection.
Methods
A total of 247 patients with HBV-associated HCC were treated with curative surgical resection. They were followed regularly for a median of 30 months. The whole X, S, basal core promoter (BCP), and precore regions of HBV were sequenced.
Results
The genomic changes such as the G1896A at precore, the A1762T/G1764A at BCP, the C1653T and the T1753V at X gene, and pre-S2 deletion were not significantly associated with postoperative recurrence of HCC or survival of patients after curative resection. However, in univariate analysis, younger age, elevated serum α-fetoprotein level, elevated serum alanine aminotransferase level, larger tumor size, microvascular invasion, and advanced Cancer of the Liver Italian Program stage were closely associated with shorter survival after surgical resection. In multivariate analysis, only microvascular invasion revealed to be an independent risk factor of postoperative recurrence (relative risk [RR] 5.406; P < 0.001); the independent risk factors of shorter survival appeared to be infiltrative type (RR 5.110; P = 0.032), larger tumor size (RR 1.976; P = 0.047), and microvascular invasion (RR 6.118; P < 0.001).
Conclusions
The postoperative recurrence or survival period may not be affected by the genomic changes at the precore, BCP, X, and pre-S2 regions in HBV of genotype C2 in patients with HBV-associated HCC treated with curative surgical resection. Rather, it may be closely associated with tumor characteristics, such as the size and type of HCC or presence of microvascular invasion.
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide, and it ranks third for cancer mortality.1 About 80 % of HCC cases are estimated to be associated with hepatitis B virus (HBV) infection, making it one of the strongest risk factors for HCC development. Recently, genomic changes in HBV have been an area of active interest because of its correlation with HCC development.2 The basal core promoter (BCP) gene is a gene frequently changed in HBV as a double mutation (A1762T/G1764T) and has been found to be a codeterminant of HCC development and rapid disease progression in several populations.3 The BCP double mutation is hypothesized to promote carcinogenesis by enhancing viral replication, increasing host immune response, and/or affecting the overlapping X gene sequence.4 The precore G1896A mutation results in a premature stop codon during transcription, thus preventing hepatitis B e antigen (HBeAg) synthesis.2 However, the relationship of the precore mutation and HCC development has not been consistent. The X gene mutations (two of the most common being C1653T and T1753V) and pre-S2 gene deletions have been associated with increased incidence of HCC.1,5,6
Surgical hepatic resection is the treatment of choice for HCC as a curative measure. Although the therapeutic efficacies of surgical resection for HCC have progressively improved, the survival periods of HCC patients who underwent this treatment modality are still disappointing, mainly as a result of frequent postoperative recurrences.7
Previous reports have suggested that certain host genetic alterations could possibly promote hepatocarcinogenesis by up-regulating oncogenes or disrupting tumor suppressor function.8,9 However, relatively little is known about the association between genomic changes in HBV and the clinical outcome of patients with HBV-associated HCC after surgical resection. Because certain HBV genomic changes have been established as risk factors for HCC development and progression of underlying chronic liver diseases, it is quite possible that they could affect the outcomes of these patients.
In this study, we sought to determine whether the common genomic changes in HBV DNA are associated with the clinical outcomes of HBV-associated HCC in patients infected with HBV of genotype C2.
METHODS
Subjects
A total of 247 consecutive patients treated with curative surgical resection for HCC were studied. Curative surgical resection was indicated in those patients who had no evidence of extrahepatic metastasis, macrovascular invasion, or portal vein thrombosis. All the subjects were confirmed as having HCC by histologic examination. Before surgery, the patient was evaluated radiologically for tumor size, number, and type. Each patient was scored by the Child-Pugh, Model for End-Stage Liver Disease (MELD), and Cancer of the Liver Italian Program (CLIP) staging systems. They were regularly followed postoperatively by serum biochemistry, serum α-fetoprotein (AFP) levels, and imaging studies such as ultrasonography or computed tomographic scan at 3- to 6-month intervals for a median of 30 months (range 1–66 months). The study was approved by the institutional review board at Asan Medical Center, Seoul, Korea.
Serum HBV Markers
Patients’ sera were analyzed for hepatitis B surface antigen with a radioimmunoassay kit (North Institute of Biological Technology; Beijing, China); serum HBeAg and anti-HBe were detected by a radioimmunoassay kit (Abbott Laboratories, Abbott Park, IL, USA); and serum HBV DNA levels were determined by chemoluminescence molecular hybridization assay (Greencross Reference Laboratory, Seoul, Korea).
HBV Genotyping
HBV DNA was extracted from stored patient’s serum with the Gentra Puregene blood kit (Qiagen, Hilden, Germany). The HBV genotype of each participant was determined by polymerase chain reaction (PCR) with primers that yielded bands specific to each genotype. PCR-restriction fragment length polymorphism (PCR-RFLP) was performed on the HBV surface gene to genotype DNA as well. The HBV DNA fragment of interest for genotyping (between nucleotides 256 and 756) was amplified by GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA, USA). These PCR products were analyzed by RFLP using restriction endonuclease AvaII and DpnII (New England Biolabs, Beverly, MA, USA) to determine the HBV genotype. The HBV genotypes were identified by the polymorphism patterns, seen under ultraviolet light by the RFLP product sizes.
Amplification and Direct Sequencing of Genes to Detect HBV Mutations
The sequence was analyzed by first amplifying the HBV genes of entire X, core promoter, precore/core, and S regions of the genome. Nested PCR was performed for the amplification of these genes. For the first round, 25 μl of the reaction mixture contained 2 μl of the DNA sample, PCR buffer (1×), 0.1 mM of each dNTP, 0.5 μM of each outer primer, and 1 U of Taq DNA polymerase was amplified in a thermal cycler for 35 cycles. Each cycle included a denaturation at 95 °C for 60 s, primer annealing at varying degrees (52 °C for X, 55 °C for precore/core, 50 °C and 57 °C for pre-S2) for 30 s, and extension at 72 °C for 45 s with additional extension step at 72 °C for 7 min. The second round of PCR required a reamplification of the PCR product for another 35 cycles with 0.5 μM of each inner primer.
The PCR products were sequenced twice in forward and reverse directions using the inner primer of the X gene, the precore/core genes, and the S gene. The nucleotide sequences of all amplified products were found by using fluorescence-labeled primers with 3700 Automatic Sequencer (ABI, Foster City, CA, USA). The sequencing conditions including BCP double mutation (A1762T/G1764A) in the core promoter region, G1896A in the PC region, C1653T or T1753V in the region encoding HBx, or pre-S2 deletion were specified in the protocol for the Taq DyeDeoxy Terminator Cycle Sequencing Kit (ABI; Applied Biosystems, Foster City, CA, USA).
Statistical Analysis
We compared the presence of genomic changes (at the precore, BCP, pre-S2, and X genes) in HBV DNA as well as the clinical characteristics with the clinical outcomes of these patients after curative surgical resection. Differences were considered significant for p-values that were less than 0.05. Hazard ratios (HR) and 95 % confidence intervals (CI) were calculated in cases in which the Chi-square test or Fisher’s exact test was significant. Predisposing factors were analyzed by univariate (Kaplan–Meier method and log-rank test) and multivariate (Cox proportional hazard model) tests. All statistical tests were analyzed by SPSS for Windows (SPSS, Chicago, IL, USA).
RESULTS
Baseline Characteristics
The baseline characteristics of 247 patients with HBV-associated HCC treated with curative surgical resection are summarized in Table 1. The median age of patients with HCC was 55 years, and 80 % of the subjects were male. All of the enrolled patients had genotype C2 HBV infection, and about 26 % of the patients were positive for HBeAg. About half of them were associated with cirrhosis; most were Child-Pugh class A or B. Nearly all the cases were of nodular type HCC, and over 70 % of the patients had a single HCC. Out of 247 patients, 30 (14 %) and 4 (2 %) were revealed to have microvascular and biliary invasion on microscopic examination, respectively.
TABLE 1.
Baseline characteristics of patients with HBV-associated HCC treated with curative surgical resection (n = 247)
| Variable | Valuea |
|---|---|
| Clinical characteristics | |
| Age (y) | 55 (30–74) |
| Sex, M:F | 197:50 (80 %:20 %) |
| Serum AFP (ng/ml) | 43 (1–334,000) |
| Serum ALT (IU/L) | 35 (9–774) |
| Serum HBV DNA (copies/mL) | 3600 (0–2.3 × 109) |
| HBeAg positivity | 63 (26 %) |
| HBV genotype C2 | 100 (100 %) |
| Anti-HCV positivity | 0 (0 %) |
| Association with cirrhosis | 135 (54 %) |
| Serum creatinine (mg/dL) | 0.9 (0–9) |
| Total bilirubin (mg/dL) | 1.0 (0.3–14) |
| MELD score | 6 (–2 to 31) |
| Child-Pugh class | |
| A | 201 (81 %) |
| B | 41 (17 %) |
| C | 5 (2 %) |
| Radiological characteristics | |
| Tumor number | |
| Single | 178 (72 %) |
| Multiple | 69 (28 %) |
| Tumor type | |
| Nodular | 241 (98 %) |
| Infiltrative | 6 (2 %) |
| Tumor size (cm) | 3.3 (1–21) |
| Histological characteristics | |
| Differentiation (Edmondson-Steiner grade) | |
| Grade 1 | 3 (1 %) |
| Grade 2 | 87 (35 %) |
| Grade 3 | 131 (53 %) |
| Grade 4 | 26 (11 %) |
| Microvascular invasion | 30 (14 %) |
| Bile duct invasion | 4 (2 %) |
| Classification system | |
| CLIP stage | |
| Early | 110 (45 %) |
| Intermediate | 137 (55 %) |
| Advanced | 0 (0 %) |
HBV hepatitis B virus, HCC hepatocellular carcinoma, AFP α-fetoprotein, ALT alanine aminotransferase, HBeAg hepatitis B e antigen, HCV hepatitis C virus, MELD Model for End-Stage Liver Disease, CLIP Cancer of the Liver Italian Program
Data are presented as median (range) or n (%)
Prevalence of HBV Genomic Mutations in Patients with HBV-associated HCC
Figure 1 shows the prevalence of each HBV genomic mutations in patients with HBV-associated HCC treated with curative surgical resection. Presence of BCP double mutations at A1762T/G1764A was found in 80 % of the subjects. Point mutation of C1653T and T1753V in the X region was found in 24 % and 16 % of the patients, respectively. The G1896A mutation in the PC region was detected in 51 % of patients with HBV-associated HCC. The pre-S2 deletion was also noted in 23 % of the cases.
FIG. 1.
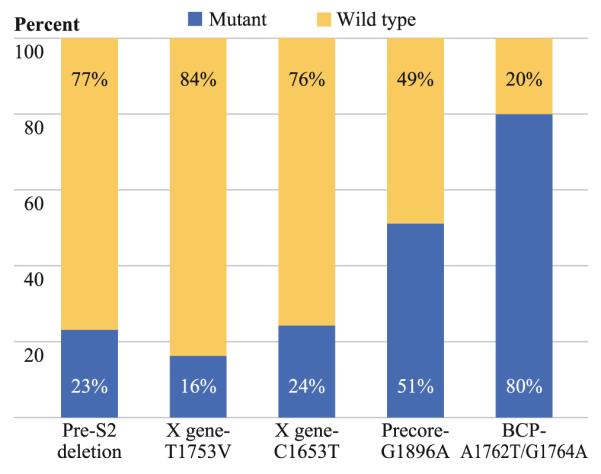
Genomic changes in HBV DNA in patients with HBV-associated HCC treated with curative surgical resection (n = 247). BCP basal core promoter
Overall Postoperative Recurrence and Survival after Curative Surgical Resection in Patients with HBV-associated HCC
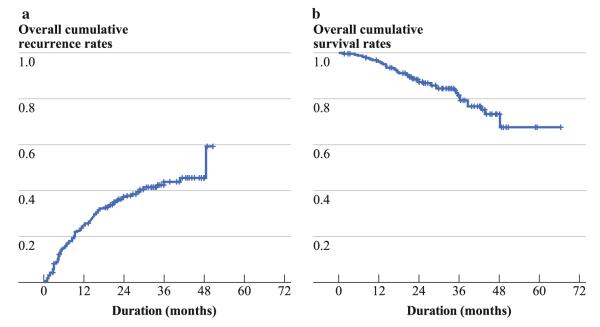
Of 247 HBV-associated HCC patients treated with curative surgical resection, 99 were diagnosed to have recurrent HCC, and 44 patients died during follow-up. The overall cumulative recurrence rates of HCC were 25 % and 44 % at 1 year and 3 years, respectively (Fig. 2a). The overall cumulative survival rates were 96 % and 82 % at 1 year and 3 years, respectively (Fig. 2b).
FIG. 2.
Overall cumulative recurrence (a) and survival (b) rates in patients with HBV-associated HCC treated with curative surgical resection
Postoperative Recurrence of HCC in Relation to Genomic Changes in HBV
The presence of mutants such as G1896A in the precore region, A1762T/G1764A in the BCP region, and C1653T and T1753V in the X gene of HBV were not associated with postoperative recurrence of HCC in patients with HBV-associated HCC treated with curative surgical resection. Also, pre-S2 deletion in HBV DNA did not affect the frequency of postoperative recurrence in these patients (Table 2).
TABLE 2.
Comparison of recurrence rates in relation to genomic changes in HBV and clinical characteristics
| Variable | Univariate analysis P |
Multivariate analysis P |
HR (95 % CI) |
|---|---|---|---|
| Characteristic | |||
| Genomic changes | |||
| BCP, wild type/mutant | 0.139 | – | – |
| Precore, wild type/mutant | 0.944 | – | – |
| X C1653T, wild type/mutant | 0.368 | – | – |
| X T1753V, wild type/mutant | 0.558 | – | – |
| Pre-S2, wild type/deletion | 0.657 | – | – |
| Clinical characteristics | |||
| Age ≤40 y | 0.031 | 0.601 | 0.601 (0.214–1.687) |
| Male | 0.002 | 0.595 | 0.595 (0.223–1.587) |
| Serum HBV DNA > 105 copies/ml | 0.089 | 0.984 | 1.007 (0.502–2.019) |
| Serum AFP > 200 ng/mL | 0.001 | 0.354 | 1.462 (0.655–3.261) |
| Serum ALT > 80 IU/L | 0.136 | 0.461 | 1.511 (0.504–4.530) |
| Advanced MELD score | 0.041 | 0.075 | 4.076 (0.869–19.110) |
| Tumor characteristics | |||
| Size >5 cm | <0.001 | 0.135 | 1.768 (0.838–3.731) |
| Differentiation, E-S grade 3–4 | 0.035 | 0.532 | 0.798 (0.392–1.622) |
| Microvascular invasion | <0.001 | <0.001 | 5.406 (2.437–11.991) |
| Advanced CLIP stage | 0.107 | 0.930 | 1.036 (0.470–2.284) |
HBV hepatitis B virus, HR hazard ratio, CI confidence interval, BCP basal core promoter, AFP α-fetoprotein, ALT alanine aminotransferase, MELD Model for End-Stage Liver Disease, E-S grade Edmondson–Steiner grade, CLIP Cancer of the Liver Italian Program
Predisposing Factors of Postoperative Recurrence of HCC
Age less than 40 years (P = 0.031), male gender (P = 0.002), serum AFP level greater than 200 ng/ml (P = 0.001), tumor size greater than 5 cm (P < 0.001), and microvascular invasion (P < 0.001) were significant predisposing factors of postoperative recurrence of HCC after curative surgical resection in patients with HBV-associated HCC by univariate analysis. However, other baseline characteristics, such as HBeAg positivity, Child-Pugh class, and number and type of tumor, did not affect HCC recurrence. In the multivariate analysis, only microvascular invasion was demonstrated to be an independent risk factor of postoperative recurrence of HCC in these patients (HR 5.406; 95 % CI 2.437–11.991; P < 0.001) (Table 2).
Survival in Relation to Genomic Changes in HBV
Patients with any genomic change in HBV (G1896A in precore region, A1762T/G1764A in BCP region, C1653T and T1753V in X region, or pre-S2 deletion) did not exhibit different survival periods from those without such changes (Table 3). However, in the subgroup whose serum HBeAg was negative (n = 161), the pre-S2 deletion tended to be associated with shorter survival, although the difference was not statistically significant (P = 0.129) (Fig. 3).
TABLE 3.
Comparison of survival periods in relation to genomic changes in HBV and clinical characteristics
| Variable | Univariate analysis P |
Multivariate analysis P |
HR (95 % CI) |
|---|---|---|---|
| Genomic changes | |||
| BCP, wild type/mutant | 0.876 | – | – |
| Precore, wild type/mutant | 0.442 | – | – |
| X C1653T, wild type/mutant | 0.665 | – | – |
| X T1753V, wild type/mutant | 0.331 | – | – |
| Pre-S2, wild type/deletion | 0.228 | – | – |
| Clinical characteristics | |||
| Age ≤40 y | 0.015 | 0.113 | 0.467 (0.182–1.196) |
| Male | 0.209 | – | – |
| Serum AFP > 200 ng/mL | 0.012 | 0.228 | 1.549 (0.760–3.157) |
| Serum ALT > 80 IU/L | 0.027 | 0.089 | 2.165 (0.889–5.268) |
| Serum creatinine > 1.4 mg/dL | 0.003 | 0.001 | 8.711 (2.172–34.933) |
| Child-Pugh class B or C | 0.038 | 0.085 | 2.175 (0.900–5.259) |
| Tumor characteristics | |||
| Size >5 cm | 0.004 | 0.047 | 1.976 (1.010–3.866) |
| Infiltrative type | 0.083 | 0.032 | 5.110 (1.156–22.602) |
| Microvascular invasion | <0.001 | <0.001 | 6.118 (2.986–12.535) |
| Advanced CLIP stage | 0.029 | 0.528 | 0.755 (0.351–1.711) |
HBV hepatitis B virus, HR hazard ratio, CI confidence interval, BCP basal core promoter, AFP α-fetoprotein, ALT alanine aminotransferase, CLIP Cancer of the Liver Italian Program
FIG. 3.
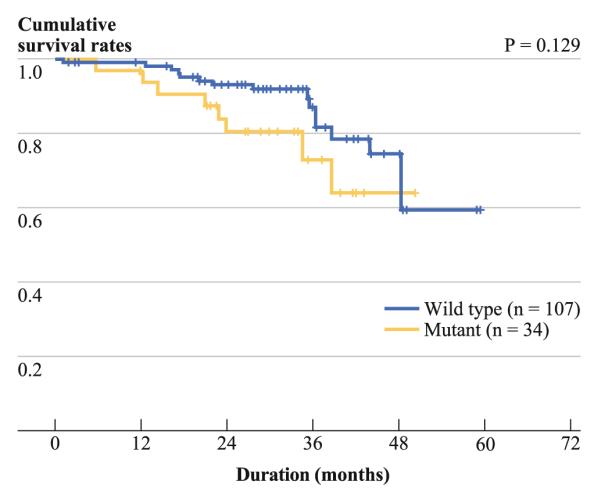
Cumulative survival rates in relation to the presence of pre-S2 deletion in HBV in patients with HBeAg-negative HBV-associated HCC (n = 161). Patients with pre-S2 deletion tended to have lower survival rates than those without pre-S2 deletion (P = 0.129)
Predisposing Factors of Shorter Survival Period
Age less than 40 years (P = 0.015), serum AFP level greater than 200 ng/ml (P = 0.012), serum alanine transaminase (ALT) level greater than 80 IU/L (P = 0.027), tumor size greater than 5 cm (P = 0.004), microvascular invasion (P < 0.001), and advanced CLIP stage (P = 0.029) were associated with shorter survival in patients with HBV-associated HCC after curative surgical resection by univariate analysis. However, the survival periods of patients were not affected by serum HBV DNA titer, HBeAg positivity, MELD score, and the number of tumor. In multivariate analysis, serum creatinine level greater than 1.4 mg/dL (HR 8.711; 95 % CI 2.172–34.933; P = 0.001), infiltrative type of tumor (HR 5.110; 95 % CI 1.156–22.602; P = 0.032), larger tumor size (HR 1.976; 95 % CI 1.010–3.866; P = 0.047), and microvascular invasion (HR 6.118; 95 % CI 2.986–12.535; P < 0.001) were independent risk factors of shorter survival in these HCC patients (Table 3).
DISCUSSION
To improve the therapeutic efficacy of surgical resection in the treatment of HCC, it is crucial to clarify predisposing factors of postoperative recurrence of HCC and significant factors determining the survival periods of HCC patients after curative surgical resection. Several previous studies have found prognostic factors in HCC patients that are associated with the outcome after surgical resection. Age, presence of cirrhosis, virus load, and serum AFP and ALT levels have been associated with postoperative recurrence in patients with HBV-associated HCC.10 Larger tumor size and advanced tumor stage have also been associated with a higher incidence of postoperative recurrence of HCC.11 Survival periods of HBV-associated HCC patients treated with surgical resection have been associated with gender, serum ALT level, tumor size, number or stage, and vascular invasion.12 The genotype of HBV has been known to be critical because of the strong correlation between the HBV genotype and the pattern of disease progression or risk of HCC development.13 Especially in patients with HBV-associated HCC, genotype C HBV has been associated with a greater chance of recurrence of HCC after curative resection compared with other genotypes.14 In the current study, ethnically homogenous patients with HBV-associated HCC were studied. Furthermore, all of the patients were infected with HBV of genotype C2, which is consistent with other HBV studies of a Korean population.15 Such a homogenous population minimizes the influences of such confounding factors as host genetic difference and HBV genotype variation.
Previous studies have determined significant associations between the genomic changes in HBV with increased risk of HCC development, rapid progression of chronic liver disease, and poor response to antiviral therapy.16 Thus, they could presumably affect the development of new HCC in patients with HBV-associated HCC even after curative resection and therefore also determine the survival periods of these patients. In this study, we intended to evaluate the effects of genomic changes in HBV on the postoperative recurrences of HCC and the survival periods of patients with HBV-associated HCC treated with curative surgical resection.
The mutations at BCP A1762T/G1764A, precore G1896A, X gene C1653T, and X gene T1753V, as well as pre-S2 deletion in HBV were not significantly associated with the prognosis of HBV-associated HCC treated with curative surgical resection. Although several studies have shown the importance of these genomic changes in increasing the risk of HCC development, they did not affect the postoperative recurrence of HCC or the survival period in our study population. These results could be due to a true lack of correlation, or due to an insufficient follow-up duration because all of these surgeries were conducted within the past 5 years. Therefore, a longer follow-up period and a larger number of samples with readable sequencing data may uncover an association between these genetic mutations and the prognosis of patients treated with curative surgical resection.
In a subgroup analysis of HBeAg-negative patients, patients with pre-S2 deletion tended to have shorter survival periods compared with patients with the wild type of the pre-S2 gene, although the difference was not statistically significant. It has been reported that early recurrence of HCC after curative surgical resection predominantly results from the remaining primary tumor, while late recurrence usually originates from newly appeared tumor.17,18 Thus, early recurrence could be affected by tumor characteristics such as microvascular invasion. On the other hand, late recurrence might be influenced by other factors associated with surrounding chronic liver,disease such as virus load or genomic change of HBV.19 Therefore, we can speculate that pre-S2 deletion in HBeAg-negative patients may affect late recurrence rather than early recurrence. In addition, the rates of especially late recurrence after curative surgical resection may be higher—and consequently the survival periods shorter—in patients with pre-S2 deletion rather than in patients without it, if the follow-up periods are longer.
Previous studies have demonstrated that HBeAg-negative chronic HBV patients with pre-S deletions have more advanced liver disease than those with the wild type of the pre-S gene.20,21 A Korean study reported a significant correlation between pre-S deletion and HCC development in HBeAg-negative patients.22 This may be explained by speculating that the mutants can better evade immunological surveillance compared with wild type. Therefore, in the future, further studies are warranted to confirm the prognostic role of pre-S2 deletion in HBV, especially in HBeAgnegative patients with HBV-associated HCC treated with curative surgical resection.
The strongest independent risk factor of poor postoperative clinical outcome in patients with HBV-associated HCC was the presence of microvascular invasion in the resected liver tissue. HCC patients who had microvascular invasion on microscopic examination had significantly higher post-operative recurrence rates and shorter survival periods than patients without microvascular invasion. Microvascular invasion has also been previously found to be an important prognostic factor for survival after hepatic surgical resection.23 Thus, clinicians need to apply additional anticancer treatment modalities even after curative surgical resection in cases that include microvascular invasion to improve the clinical outcomes of these patients.24
The statistical power of our study comes from the relatively large number of HBV-associated HCC patients who were treated with curative surgical resection. However, there are several limitations in the current study. The HBV DNA could not be sequenced in a small percentage of cases, which could influence the results. Also, because the patients included in this study were treated with surgical resection within the past 5 years, our results might be altered with longer follow-up.
In summary, none of common genomic changes in HBV (A1762T/G1764A in BCP, G1896A in precore, C1653T and T1753V in X gene, and pre-S2 deletion) was associated with clinical outcome of patients with HBV-associated HCC after curative surgical resection. However, infiltrative tumor type and large tumor size were independent risk factors for shorter survival period. In addition, microvascular invasion showed the strongest predisposing factor for both higher postoperative recurrence rate and shorter survival period in this study population. In conclusion, the postoperative recurrence or survival period may not be affected by the genomic changes at the precore, BCP, X, and pre-S2 regions in HBV of genotype C2 in patients with HBV-associated HCC treated with curative surgical resection; rather, it may be closely associated with tumor characteristics, such as size and type of HCC, or presence of microvascular invasion.
Footnotes
Presented in part at the 46th annual meeting of the European Association for the Study of the Liver (EASL) at the International Liver Congress 2011.
REFERENCES
- 1.Liu S, Zhang H, Gu C, et al. Associations between hepatitis B virus mutations and the risk of hepatocellular carcinoma: a meta-analysis. J Natl Cancer Inst. 2009;101:1066–82. doi: 10.1093/jnci/djp180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pujol FH, Navas MC, Hainaut P, et al. Worldwide genetic diversity of HBV genotypes and risk of hepatocellular carcinoma. Cancer Lett. 2009;286:80–8. doi: 10.1016/j.canlet.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Yuan JM, Ambinder A, Fan Y, et al. Prospective evaluation of hepatitis B 1762(T)/1764(A) mutations on hepatocellular carcinoma development in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2009;18:590–4. doi: 10.1158/1055-9965.EPI-08-0966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang HI, Yeh SH, Chen PJ, et al. Associations between hepatitis B virus genotype and mutants and the risk of hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:1134–43. doi: 10.1093/jnci/djn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang ZL, Sabin CA, Dong BQ, et al. Hepatitis B virus pre-S deletion mutations are a risk factor for hepatocellular carcinoma: a matched nested case-control study. J Gen Virol. 2008;89:2882–90. doi: 10.1099/vir.0.2008/002824-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murakami S. Hepatitis B virus X protein: a multifunctional viral regulator. J Gastroenterol. 2001;36:651–60. doi: 10.1007/s005350170027. [DOI] [PubMed] [Google Scholar]
- 7.Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–24. doi: 10.1053/jhep.2002.32089. [DOI] [PubMed] [Google Scholar]
- 8.Mann CD, Neal CP, Garcea G, et al. Prognostic molecular markers in hepatocellular carcinoma: a systematic review. Eur J Cancer. 2007;43:979–92. doi: 10.1016/j.ejca.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Su H, Zhao J, Xiong Y, et al. Large-scale analysis of the genetic and epigenetic alterations in hepatocellular carcinoma from Southeast China. Mutat Res. 2008;641:27–35. doi: 10.1016/j.mrfmmm.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Qin LX, Tang ZY. The prognostic significance of clinical and pathological features in hepatocellular carcinoma. World J Gastroenterol. 2002;8:193–9. doi: 10.3748/wjg.v8.i2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pawlik TM, Delman KA, Vauthey JN, et al. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086–92. doi: 10.1002/lt.20472. [DOI] [PubMed] [Google Scholar]
- 12.Poon RT, Ng IO, Fan ST, et al. Clinicopathologic features of long-term survivors and disease-free survivors after resection of hepatocellular carcinoma: a study of a prospective cohort. J Clin Oncol. 2001;19:3037–44. doi: 10.1200/JCO.2001.19.12.3037. [DOI] [PubMed] [Google Scholar]
- 13.Chan HL, Hui AY, Wong ML, et al. Genotype C hepatitis B virus infection is associated with an increased risk of hepatocellular carcinoma. Gut. 2004;53:1494–8. doi: 10.1136/gut.2003.033324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen JD, Liu CJ, Lee PH, et al. Hepatitis B genotypes correlate with tumor recurrence after curative resection of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2004;2:64–71. doi: 10.1016/s1542-3565(03)00293-3. [DOI] [PubMed] [Google Scholar]
- 15.Ahn SH, Yuen L, Han KH, et al. Molecular and clinical characteristics of hepatitis B virus in Korea. J Med Virol. 2010;82:1126–34. doi: 10.1002/jmv.21844. [DOI] [PubMed] [Google Scholar]
- 16.Asim M, Malik A, Sarma MP, et al. Hepatitis B virus BCP, Precore/core, X gene mutations/genotypes and the risk of hepatocellular carcinoma in India. J Med Virol. 2010;82:1115–25. doi: 10.1002/jmv.21774. [DOI] [PubMed] [Google Scholar]
- 17.Poon RT, Fan ST, Ng IO, et al. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89:500–7. [PubMed] [Google Scholar]
- 18.Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–7. doi: 10.1016/s0168-8278(02)00360-4. [DOI] [PubMed] [Google Scholar]
- 19.Wu JC, Huang YH, Chau GY, et al. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol. 2009;51:890–7. doi: 10.1016/j.jhep.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Chen CH, Hung CH, Lee CM, et al. Pre-S deletion and complex mutations of hepatitis B virus related to advanced liver disease in HBeAg-negative patients. Gastroenterology. 2007;133:1466–74. doi: 10.1053/j.gastro.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335–52. doi: 10.1016/j.jhep.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Mun HS, Lee SA, Jee Y, et al. The prevalence of hepatitis B virus preS deletions occurring naturally in Korean patients infected chronically with genotype C. J Med Virol. 2008;80:1189–94. doi: 10.1002/jmv.21208. [DOI] [PubMed] [Google Scholar]
- 23.Sumie S, Kuromatsu R, Okuda K, et al. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol. 2008;15:1375–82. doi: 10.1245/s10434-008-9846-9. [DOI] [PubMed] [Google Scholar]
- 24.Cha C, Fong Y, Jarnagin WR, et al. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J Am Coll Surg. 2003;197:753–8. doi: 10.1016/j.jamcollsurg.2003.07.003. [DOI] [PubMed] [Google Scholar]