Summary
Defects in telomere maintenance genes cause pathological telomere shortening, and manifest in syndromes which have prominent phenotypes in tissues of high turnover: the skin and bone marrow. Because the gastrointestinal (GI) epithelium has rapid turnover, we sought to determine whether telomere syndromes cause GI disease, and to define its prevalence, spectrum and natural history. We queried subjects in the Johns Hopkins Telomere Syndrome Registry for evidence of luminal GI disease. In sixteen percent of Registry subjects (6 of 38), there was a history of significant GI pathology, and 43 additional cases were identified in the literature. Esophageal stenosis, enteropathy and enterocolitis were the recurrent findings. In the intestinal mucosa, there was striking villous atrophy, extensive apoptosis, and anaphase bridging pointing to regenerative defects in the epithelial compartment. GI disease was often the first and most severe manifestation of telomere disease in young children. These findings indicate that telomere dysfunction disrupts the epithelial integrity in the human GI tract manifesting in recognizable disease processes. A high index of suspicion should facilitate diagnosis and management.
Keywords: telomere, telomerase, enterocolitis, immunodeficiency
Gastrointestinal (GI) epithelium is highly proliferative and its integrity relies on the regenerative capacity of local stem cells(Barker et al., 2010). Telomeres are DNA-protein structures that protect chromosome ends. With cell division, telomeres shorten and short dysfunctional telomeres provoke apoptosis and/or senescence(Armanios and Blackburn, 2012). Telomerase synthesizes new telomere repeats to offset the loss that occurs during DNA replication(Greider and Blackburn, 1985, 1987). Mice that are null for telomerase show progressive telomere shortening and provide a model for understanding the consequences of telomere dysfunction(Armanios and Blackburn, 2012). In these mice, highly proliferative tissues, the skin and bone marrow, display degenerative phenotypes due to stem cell failure(Hao et al., 2005; Lee et al., 1998; Rossi et al., 2007; Rudolph et al., 1999). They also develop intestinal villous atrophy and enterocolitis, which contribute to their limited lifespan(Armanios et al., 2009; Hao et al., 2005; Lee et al., 1998; Rudolph et al., 1999). The question of how short telomeres affect the human GI tract has not been systematically examined.
Syndromes caused by mutant telomerase and telomere genes span the age spectrum, and their severity depends on the extent of telomere shortening(Armanios and Blackburn, 2012). Mutations in one of 4 genes account for a major subset(Armanios and Blackburn, 2012). Mutant telomerase core enzyme genes TERT, the telomerase reverse transcriptase, and TR, its RNA component, cause autosomal dominant disease. Mutations in DKC1, which encodes the telomerase-associated dyskerin protein, cause X-linked disease, and mutations in TINF2, which encodes the telomere protein TIN2, primarily cause de novo disease. In infancy, telomere-mediated disease is recognized as Hoyeraal-Hreidarsson (HH) syndrome, a rare disorder characterized by developmental delay, immunodeficiency and cerebellar hypoplasia. In children, it is recognized as dyskeratosis congenita (DC) defined by a triad of oral leukoplakia, nail dystrophy and skin hyperpigmentation(Savage and Alter, 2009). Adult-onset telomere disease is heterogeneous and manifests as isolated or syndromic clustering of bone marrow failure, pulmonary fibrosis, and liver cirrhosis(Armanios, 2009). Because the clinical presentation of telomere syndromes is diverse, lymphocyte telomere length measurement is used diagnostically to identify affected individuals(Armanios and Blackburn, 2012). We sought to determine whether telomere syndromes cause GI disease. We show here they manifest in discrete patterns and report their prevalence, spectrum and natural history.
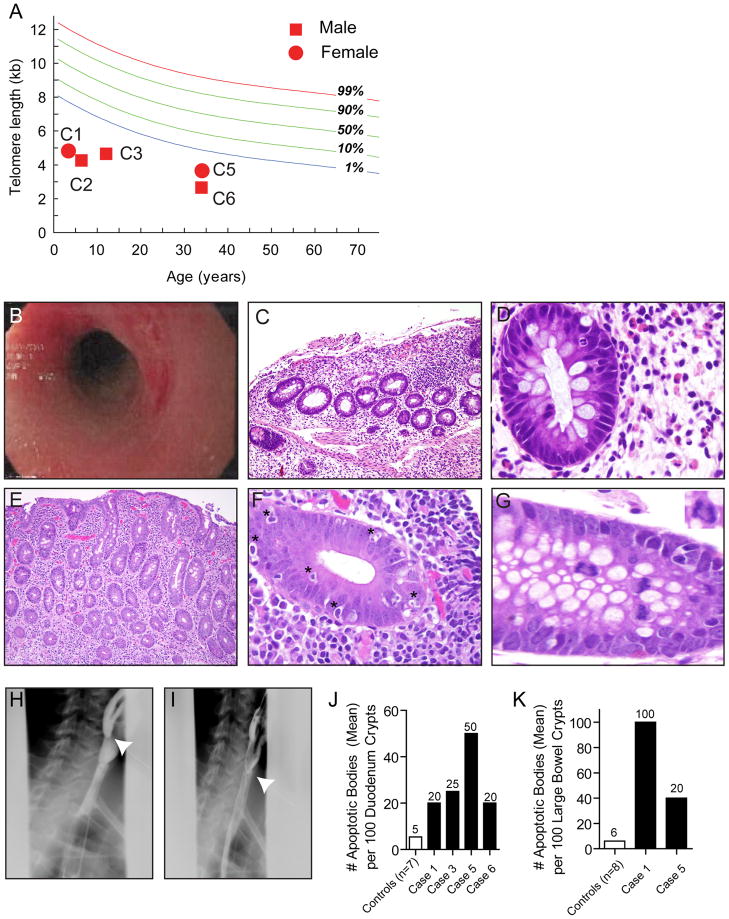
The Johns Hopkins Telomere Syndrome Registry had 38 individuals from 20 families. Of these, six (16%) required evaluation by a gastroenterologist and endoscopy. Clinical findings are summarized in Table 1 and Figure 1, and the detailed histories are in the Supplement. DKC1, TERT and TR mutations have been reported or shown to compromise telomerase activity(Knight et al., 2001; Marrone and Mason, 2003; Parry et al., 2011). In two cases, no mutations were identified. Lymphocyte telomere length was below the age-adjusted 1st percentile in 5 of 6 assessable cases (Figure 1A). Cases were on average 15 years (15 months-34 years), and the GI disease was most severe in the youngest cases. A 15 month old presented with bloody diarrhea, was diagnosed with enterocolitis and had severe B cell lymphopenia (Figure 1B–1D). The enterocolitis was refractory to immunosuppression, and she required colectomy and parenteral nutrition support. The GI symptoms persisted after bone marrow transplantation, and led to premature death within a year of diagnosis. A 3 year old boy with severe swallowing difficulties was found to have a nearly obstructing proximal esophageal web. Three additional cases presented with difficulty gaining weight and abdominal pain, and two were diagnosed with celiac enteropathy based on profound villous atrophy and intraepithelial lymphocytosis (Figures 1E–1G). Case 6 presented with chronic dysphagia and had a proximal esophageal web (Figure 1H–1I).
Table 1.
Characteristics of Johns Hopkins Telomere Syndrome Registry subjects with GI disease
| Case ID | Age | Gender | Clinical/ Genetic Diagnosis |
Telomere Phenotypes |
GI History | Esophageal and Gastric Findings |
Small and Large Bowel Findings |
|---|---|---|---|---|---|---|---|
| 1 | 15 m | F | Hoyeraal-Hreidarsson | IUGR Developmental Delay Immunodeficiency |
Failure to Thrive Diarrhea-Bloody Colectomy, TPN |
Gastric lamina propria-mild fibrosis |
Pancolitis, Epithelial sloughing ↑Apoptosis Gland dropout/ No plasma cells |
| 2 | 3 y | M | Hoyeraal-Hreidarsson DKC1 c.472C>T Arg158Trp |
IUGR Developmental Delay Thrombocytopenia |
Dysphagia-solids | Esophageal Stenosis- Cricopharynx |
No data available |
| 3 | 7 y | M | Dyskeratosis Congenita | Developmental Delay Short Stature |
Failure to Thrive Abdominal Pain- post-prandial |
Esophageal IEL | Villous Blunting, ↑Apoptosis Duodenal Neutrophil infiltrates Duodenal IEL |
| 4 | 16 y | F | Telomere Syndrome hTERT c.3075G>T Val1025Phe | Aplastic Anemia Immunodeficiency |
Failure to Thrive Diarrhea-Watery TPN |
Esophagus- inflammatory changes |
Normal duodenal biopsy* Colonic biopsy not performed |
| 5 | 34 y | F | Telomere Syndrome hTR 204C→G |
Pancytopenia | Abdominal Pain Nausea Early Satiety |
Schatzki’s ring Atrophic Gastritis Esophageal IEL Parietal Cell Dropout |
Villous Blunting ↑Apoptosis Duodenal and Colonic IEL |
| 6 | 34 y | M | Dyskeratosis Congenita DKC1 c.949C>T Leu317Phe |
Pancytopenia Lacrimal duct stenosis Urethral stenosis |
Dysphagia-solids Abdominal Pain- post-prandial |
Esophageal stenosis- Cervicothoracic junction |
↑Apoptosis |
Abbreviations: IUGR, intrauterine growth restriction; TPN, total parenteral nutrition; IEL, refers to intraepithelial lymphocytosis
This biopsy was not centrally reviewed
Figure 1. Clinicopathologic findings in individuals with telomere-mediated gastrointestinal disease.
(A) Lymphocyte telomere length is plotted relative to distribution of length in 400 controls. C1, C2....etc. refer to cases in Table 1. (B) Rectal endoscopy shows marked erythema with linear ulcerations and narrowing at the rectosigmoid junction (Case 1). (C) Colonic mucosa from Case 1 shows an expanded lamina propria with lymphoid cells and several crypts are completely absent or damaged resulting in mucosal atrophy. The mucosal surface itself is damaged and is partially sloughed off (original magnification, 20X). (D) Higher magnification of the colonic mucosa from Case 1 showing that although the lamina propria on the right side of the field is expanded with lymphoid cells, plasma cells are wholly absent, a finding typically associated with congenital immunodeficiency. The colonic crypt at the left side of the field shows an intraepithleial neutrophil infiltrate (focal acute colitis) at the 2:00 position in the crypt (original magnification 100X). (E) Although the mucosa in this image has the appearance of colonic mucosa, this biopsy was from the duodenum and shows villous atrophy and an expanded lamina propria containing lymphoplasmacytic cells (Case 5, original magnification, 20X). (F) High magnification of colonic mucosa displaying striking crypt apoptosis with significant intraepithelial lymphocytosis (Case 5, original magnification 100X). Examples of apoptotic bodies are indicated by *. (G) High magnification of colon demonstrating an anaphase bridge at the center left portion of the field (Case 5, original magnification, 125X). Inset of the anaphase bridging is shown in the right upper corner. (H) & (I) Still images from cineesophagopharyngogram demonstrating a tapered luminal defect in the cervical esophagus (Case 6). (J) & (K) Number of apoptotic bodies per 100 crypts in the duodenum and colon, respectively is plotted relative to controls.
GI mucosal biopsies in symptomatic cases revealed severe epithelial defects. In the case with enterocolitis, there was profound mucosal sloughing, crypt dropout, increased apoptosis and absent plasma cells. In milder enteropathy cases, there was villous atrophy, intraepithelial lymphocytosis, and increased mitotic errors such as rings and anaphase bridges (Figure 1G). There was also an increase in epithelial apoptotic bodies (Figure 1F). We compared epithelial apoptotic bodies in cases and controls and found a significant increase in the duodenum [28.7 ± 7.2 SEM/100 crypts (n=4) vs. 5.4 ± 1.3 SEM/100 crypts (n=7), P<0.01, unpaired t-test] and colon [70.0 ± 30.0 SEM (n=2) vs. 6.0 ± 1.0 SEM/100 crypts (n=8), P<0.01, Figures 1J–1K]. Gastric epithelial apoptosis was seen in one case with parietal cell dropout.
To extend our findings, we reviewed 591 Pubmed entries which fulfilled prespecified criteria (Supplement), and identified 43 additional HH and DC cases. Supplementary Table 1 summarizes the 24 upper GI cases(Addison and Rice, 1965; Arca et al., 2003; Baselga et al., 1998; Berezin et al., 1996; de Roux-Serratrice et al., 2000; Demirgunes et al., 2008; Dokal et al., 1992; Elliott et al., 1999; Ghavamzadeh et al., 1999; Handley and Ogden, 2006; Herman et al., 1997; Kanegane et al., 2005; Knight et al., 1999; Krishnan et al., 1997; Paul et al., 1992; Robledo Aguilar et al., 1974; Russo et al., 1990; Sasa et al., 2011; Sawant et al., 1994; Sznajer et al., 2003; Utz et al., 2005; Yaghmai et al., 2000), and Supplementary Table 2 the 23 lower GI cases(Arca et al., 2003; Berezin et al., 1996; Berthet et al., 1994; Borggraefe et al., 2009; Cossu et al., 2002; Jyonouchi et al., 2011; Kehrer et al., 1992; Knight et al., 1999; Paul et al., 1992; Sasa et al., 2011; Steier et al., 1972; Sznajer et al., 2003; Touzot et al., 2010). Dysphagia was the most common upper GI complaint, and esophageal stenosis the most prevalent diagnosis (23 of 24 cases, 96%). Mean age at was 11.4 years (1 month-27 years). Strictures localized to the proximal esophagus (8 of 9, 89%), and symptoms improved after dilatation but repeat procedure was at times required(Addison and Rice, 1965; Baselga et al., 1998; Paul et al., 1992). Stenosis was congenital in 4 cases with poor feeding since birth(Knight et al., 1999; Russo et al., 1990; Sznajer et al., 2003; Yaghmai et al., 2000), while older children had long-standing swallowing difficulties. In most cases (21 of 24 cases, 88%), DC was the underlying telomere disorder indicating this complication may occur at higher frequency in this population.
Diarrhea due to severe enteropathy was the most common lower GI diagnosis (n=14, 61%) presenting at a mean of 6.7 years (1 month-21 years). HH syndrome was the predominant underlying telomere disorder (17 of 23, 71%). Findings included pancolitis and atrophic mucosa, and pathology showed gland dropout, lamina propria fibrosis, intraepithelial lymphocytosis, and apoptosis. Esophageal stenosis preceded or followed lower GI disease at times(Arca et al., 2003; Berezin et al., 1996; Knight et al., 1999; Sasa et al., 2011; Sznajer et al., 2003). Intestinal disease presented earlier in HH than DC (mean 1.4 vs. 17 years, respectively). In all HH cases with intestinal disease, there was a concurrent B cell lymphopenia and/or hypogammaglobulinemia (16 of 16, 100%). Intestinal disease caused significant morbidity in children requiring colectomy or parenteral nutrition support (Berthet et al., 1994; Cossu et al., 2002; Knight et al., 1999; Paul et al., 1992; Sznajer et al., 2003). Therefore, telomere-mediated intestinal disease can be life threatening, especially in HH patients.
We show here that short telomere length disrupts GI mucosal integrity in telomere syndromes. Disease affected 16% of our Registry subjects and was at times the first and most severe presentation. The collective experience we report indicates non-malignant telomere-mediated GI disease manifests in three discrete categories: esophageal stricture, enteropathy and enterocolitis. Of these, enterocolitis is most severe and occurs in young children with telomere-related B cell immunodeficiency. Enteropathy has a milder course and is associated with villous atrophy. Esophageal stenosis represents one of several luminal stenotic defects that occur in DC, such as lacrimal duct and urethral stenosis, and likely reflects developmental defects.
The convergence of findings in human telomere syndromes with those seen in telomerase null mice suggests the GI pathology we see is telomere-dependent. Villous atrophy in mice represents a telomere-mediated stem cell failure(Rudolph et al., 1999), while the enterocolitis is thought to represent a compound defect in the epithelium-immune barrier(Armanios et al., 2009). Mice with short telomeres also develop intestinal microadenoma, and DC patients have an increased incidence of esophageal, rectal and possibly gastric cancer although the overall incidence is relatively low(Alter et al., 2009).
Our findings have clinical implications. A heightened index of suspicion for the GI processes described here in affected cases, and conversely of telomere disorders in individuals with GI pathology can facilitate early diagnosis, prevent un-necessary work-up and anticipate/avert complications. Telomere length testing in these cases can be a critical diagnostic tool. We note the GI disease patterns we describe share features of poorly understood processes such as celiac and inflammatory bowel disease. It is possible that telomere length may be a relevant genetic modifier of disease severity in these disorders where the GI mucosa is disrupted. Because short telomere length is acquired with age, the processes we describe may also point to yet-unrecognized age-dependent disease patterns in the GI tract.
Supplementary Material
Acknowledgments
We are grateful to the subjects and families who participated in this research and to all their referring clinicians. We acknowledge helpful discussions and critical comments from Dr. Mark Donowitz, Dr. Frank Giardiello and Dr. Maria Oliva-Hemker. The authors acknowledge support from the National Institutes of Health T32DK007632 (NL), RO1CA160433 (MA), and the Doris Duke Charitable Foundation (MA).
Footnotes
Author Contributions
Conceived the idea NLJ and MA; evaluated and analyzed clinical data NLJ, EAM, MA; provided important reagents/tools NG, JC; drafted the manuscript: NJL and MA. All the authors reviewed the manuscript.
Potential Competing Interests
Dr. Califano is the Director of Research of the Milton J. Dance Head and Neck Endowment. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies. The other authors have no relevant financial conflict of interest to declare.
References
- Addison M, Rice MS. The Association of Dyskeratosis Congenita and Fanconi’s Anaemia. Med J Aust. 1965;1:797–799. doi: 10.5694/j.1326-5377.1965.tb72218.x. [DOI] [PubMed] [Google Scholar]
- Alter BP, Giri N, Savage SA, Rosenberg PS. Cancer in dyskeratosis congenita. Blood. 2009;113:6549–6557. doi: 10.1182/blood-2008-12-192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arca E, Tuzun A, Tastan HB, Akar A, Kose O. Dyskeratosis congenita with esophageal and anal stricture. Int J Dermatol. 2003;42:555–557. doi: 10.1046/j.1365-4362.2003.01699_2.x. [DOI] [PubMed] [Google Scholar]
- Armanios M. Syndromes of telomere shortening. Annu Rev Genomics Hum Genet. 2009;10:45–61. doi: 10.1146/annurev-genom-082908-150046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armanios M, Alder JK, Parry EM, Karim B, Strong MA, Greider CW. Short telomeres are sufficient to cause the degenerative defects associated with aging. Am J Hum Genet. 2009;85:823–832. doi: 10.1016/j.ajhg.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armanios M, Blackburn EH. The telomere syndromes. Nature reviews Genetics. 2012;13:693–704. doi: 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, Bartfeld S, Clevers H. Tissue-resident adult stem cell populations of rapidly self-renewing organs. Cell Stem Cell. 2010;7:656–670. doi: 10.1016/j.stem.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Baselga E, Drolet BA, van Tuinen P, Esterly NB, Happle R. Dyskeratosis congenita with linear areas of severe cutaneous involvement. Am J Med Genet. 1998;75:492–496. [PubMed] [Google Scholar]
- Berezin S, Schwarz SM, Slim MS, Beneck D, Brudnicki AR, Medow MS. Gastrointestinal problems in a child with dyskeratosis congenita. The American journal of gastroenterology. 1996;91:1271–1272. [PubMed] [Google Scholar]
- Berthet F, Caduff R, Schaad UB, Roten H, Tuchschmid P, Boltshauser E, Seger RA. A syndrome of primary combined immunodeficiency with microcephaly, cerebellar hypoplasia, growth failure and progressive pancytopenia. Eur J Pediatr. 1994;153:333–338. doi: 10.1007/BF01956413. [DOI] [PubMed] [Google Scholar]
- Borggraefe I, Koletzko S, Arenz T, Fuehrer M, Hoffmann F, Dokal I, Vulliamy T, Weiler V, Griese M, Belohradsky BH, et al. Severe variant of x-linked dyskeratosis congenita (Hoyeraal-Hreidarsson Syndrome) causes significant enterocolitis in early infancy. Journal of Pediatric Gastroenterology and Nutrition. 2009;49:359–363. doi: 10.1097/MPG.0b013e3181a15b94. [DOI] [PubMed] [Google Scholar]
- Cossu F, Vulliamy TJ, Marrone A, Badiali M, Cao A, Dokal I. A novel DKC1 mutation, severe combined immunodeficiency (T+B-NK- SCID) and bone marrow transplantation in an infant with Hoyeraal-Hreidarsson syndrome. Br J Haematol. 2002;119:765–768. doi: 10.1046/j.1365-2141.2002.03822.x. [DOI] [PubMed] [Google Scholar]
- de Roux-Serratrice C, Serratrice J, Escoffier JM, Granel B, Disdier P, Weiller PJ. Esophageal web in Zinsser-Engman-Cole-Fanconi disease. Gastrointest Endosc. 2000;52:561–562. doi: 10.1067/mge.2000.108663. [DOI] [PubMed] [Google Scholar]
- Demirgunes FE, Elcin G, Sahin S. Dyskeratosis congenita: report of two cases with distinct clinical presentations. Turk J Pediatr. 2008;50:604–608. [PubMed] [Google Scholar]
- Dokal I, Bungey J, Williamson P, Oscier D, Hows J, Luzzatto L. Dyskeratosis congenita fibroblasts are abnormal and have unbalanced chromosomal rearrangements. Blood. 1992;80:3090–3096. [PubMed] [Google Scholar]
- Elliott AM, Graham GE, Bernstein M, Mazer B, Teebi AS. Dyskeratosis congenita: an autosomal recessive variant. Am J Med Genet. 1999;83:178–182. doi: 10.1002/(sici)1096-8628(19990319)83:3<178::aid-ajmg6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Ghavamzadeh A, Alimoghadam K, Nasseri P, Jahani M, Khodabandeh A, Ghahremani G. Correction of bone marrow failure in dyskeratosis congenita by bone marrow transplantation. Bone Marrow Transplant. 1999;23:299–301. doi: 10.1038/sj.bmt.1701567. [DOI] [PubMed] [Google Scholar]
- Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- Greider CW, Blackburn EH. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987;51:887–898. doi: 10.1016/0092-8674(87)90576-9. [DOI] [PubMed] [Google Scholar]
- Handley TP, Ogden GR. Dyskeratosis congenita: oral hyperkeratosis in association with lichenoid reaction. J Oral Pathol Med. 2006;35:508–512. doi: 10.1111/j.1600-0714.2006.00434.x. [DOI] [PubMed] [Google Scholar]
- Hao LY, Armanios M, Strong MA, Karim B, Feldser DM, Huso D, Greider CW. Short telomeres, even in the presence of telomerase, limit tissue renewal capacity. Cell. 2005;123:1121–1131. doi: 10.1016/j.cell.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Herman TE, McAlister WH, Mallory SB. Dyskeratosis congenita. Pediatr Radiol. 1997;27:286. doi: 10.1007/s002470050128. [DOI] [PubMed] [Google Scholar]
- Jyonouchi S, Forbes L, Ruchelli E, Sullivan KE. Dyskeratosis congenita: a combined immunodeficiency with broad clinical spectrum--a single-center pediatric experience. Pediatric allergy and immunology: official publication of the European Society of Pediatric Allergy and Immunology. 2011;22:313–319. doi: 10.1111/j.1399-3038.2010.01136.x. [DOI] [PubMed] [Google Scholar]
- Kanegane H, Kasahara Y, Okamura J, Hongo T, Tanaka R, Nomura K, Kojima S, Miyawaki T. Identification of DKC1 gene mutations in Japanese patients with X-linked dyskeratosis congenita. Br J Haematol. 2005;129:432–434. doi: 10.1111/j.1365-2141.2005.05473.x. [DOI] [PubMed] [Google Scholar]
- Kehrer H, Krone W, Schindler D, Kaufmann R, Schrezenmeier H. Cytogenetic studies of skin fibroblast cultures from a karyotypically normal female with dyskeratosis congenita. Clin Genet. 1992;41:129–134. doi: 10.1111/j.1399-0004.1992.tb03648.x. [DOI] [PubMed] [Google Scholar]
- Knight SW, Heiss NS, Vulliamy TJ, Aalfs CM, McMahon C, Richmond P, Jones A, Hennekam RC, Poustka A, Mason PJ, et al. Unexplained aplastic anaemia, immunodeficiency, and cerebellar hypoplasia (Hoyeraal-Hreidarsson syndrome) due to mutations in the dyskeratosis congenita gene, DKC1. Br J Haematol. 1999;107:335–339. doi: 10.1046/j.1365-2141.1999.01690.x. [DOI] [PubMed] [Google Scholar]
- Knight SW, Vulliamy TJ, Morgan B, Devriendt K, Mason PJ, Dokal I. Identification of novel DKC1 mutations in patients with dyskeratosis congenita: implications for pathophysiology and diagnosis. Human genetics. 2001;108:299–303. doi: 10.1007/s004390100494. [DOI] [PubMed] [Google Scholar]
- Krishnan SS, Yesudian PD, Jayaraman M, Janaki VR, Raj JB. Atypical dyskeratosis congenita. Indian J Dermatol Venereol Leprol. 1997;63:47–49. [PubMed] [Google Scholar]
- Lee HW, Blasco MA, Gottlieb GJ, Horner JW, 2nd, Greider CW, DePinho RA. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–574. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- Marrone A, Mason PJ. Dyskeratosis congenita. Cellular and molecular life sciences: CMLS. 2003;60:507–517. doi: 10.1007/s000180300042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry EM, Alder JK, Qi X, Chen JJ, Armanios M. Syndrome complex of bone marrow failure and pulmonary fibrosis predicts germline defects in telomerase. Blood. 2011;117:5607–5611. doi: 10.1182/blood-2010-11-322149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul SR, Perez-Atayde A, Williams DA. Interstitial pulmonary disease associated with dyskeratosis congenita. Am J Pediatr Hematol Oncol. 1992;14:89–92. [PubMed] [Google Scholar]
- Robledo Aguilar A, Gomez F, Tojo Sierra R, Larripa P. Dyskeratosis congenita Zinsser-Cole-Engmann form with abnormal karyotype. Dermatologica. 1974;148:98–103. doi: 10.1159/000251605. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C, DePinho RA. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96:701–712. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- Russo CL, Glader BE, Israel RJ, Galasso F. Treatment of neutropenia associated with dyskeratosis congenita with granulocyte-macrophage colony-stimulating factor. Lancet. 1990;336:751–752. doi: 10.1016/0140-6736(90)92246-e. [DOI] [PubMed] [Google Scholar]
- Sasa G, Ribes-Zamora A, Nelson N, Bertuch A. Three novel truncating TINF2 mutations causing severe dyskeratosis congenita in early childhood. Clin Genet. 2011 doi: 10.1111/j.1399-0004.2011.01658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage SA, Alter BP. Dyskeratosis congenita. Hematol Oncol Clin North Am. 2009;23:215–231. doi: 10.1016/j.hoc.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawant P, Chopda NM, Desai DC, Dave UR, Satarkar RP, Nanivadekar SA. Dyskeratosis congenita with esophageal stricture and dermatological manifestations. Endoscopy. 1994;26:711–712. doi: 10.1055/s-2007-1009076. [DOI] [PubMed] [Google Scholar]
- Steier W, Van Voolen GA, Selmanowitz VJ. Dyskeratosis congenita: relationship to Fanconi’s anemia. Blood. 1972;39:510–521. [PubMed] [Google Scholar]
- Sznajer Y, Baumann C, David A, Journel H, Lacombe D, Perel Y, Blouin P, Segura JF, Cezard JP, Peuchmaur M, et al. Further delineation of the congenital form of X-linked dyskeratosis congenita (Hoyeraal-Hreidarsson syndrome) Eur J Pediatr. 2003;162:863–867. doi: 10.1007/s00431-003-1317-5. [DOI] [PubMed] [Google Scholar]
- Touzot F, Callebaut I, Soulier J, Gaillard L, Azerrad C, Durandy A, Fischer A, de Villartay JP, Revy P. Function of Apollo (SNM1B) at telomere highlighted by a splice variant identified in a patient with Hoyeraal-Hreidarsson syndrome. Proc Natl Acad Sci U S A. 2010;107:10097–10102. doi: 10.1073/pnas.0914918107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utz JP, Ryu JH, Myers JL, Michels VV. Usual interstitial pneumonia complicating dyskeratosis congenita. Mayo Clin Proc. 2005;80:817–821. doi: 10.1016/S0025-6196(11)61538-3. [DOI] [PubMed] [Google Scholar]
- Yaghmai R, Kimyai-Asadi A, Rostamiani K, Heiss NS, Poustka A, Eyaid W, Bodurtha J, Nousari HC, Hamosh A, Metzenberg A. Overlap of dyskeratosis congenita with the Hoyeraal-Hreidarsson syndrome. J Pediatr. 2000;136:390–393. doi: 10.1067/mpd.2000.104295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.