Abstract
The molecular basis of allosteric regulation remains a subject of intense interest. Staphylococcus aureus CzrA is a member of the ubiquitous arsenic repressor (ArsR) family of bacterial homodimeric metal sensing proteins, and has emerged as a model system for understanding allosteric regulation of operator DNA binding by transition metal ions. Using unnatural amino acid substitution and a standard linkage analysis, we show that a His97’ NHε2•••O=C-His67 quaternary structural hydrogen bond is an energetically significant contributor to the magnitude of the allosteric coupling free energy, ΔGc. A “cavity” introduced just beneath this hydrogen bond in V66A/L68V CzrA results in a dramatic loss of regulation by Zn(II) despite adopting a wild-type global structure and Zn(II) binding and DNA binding affinities only minimally affected from wild-type. The energetics of Zn(II) binding and heterotropic coupling free energies (ΔHc, −TΔSc) of the double mutant are also radically altered and suggest that increased internal dynamics leads to poorer allosteric negative regulation in V66A/L68V CzrA. A statistical coupling analysis of 3000 ArsR proteins reveals a sector that links the DNA-binding determinants and the α5 Zn(II) sensing sites through V66/L68 in CzrA. We propose that distinct regulatory sites uniquely characteristic of individual ArsR proteins results from evolution of distinct connectivities to this sector, each capable of driving the same biological outcome, transcriptional derepression.
Keywords: allostery, zinc sensor, metal homeostasis, statistical coupling analysis, ArsR
INTRODUCTION
Allosteric regulation is ubiquitous in biology and governs processes as diverse as signal transduction, enzymatic activity and metabolic flux, transcriptional regulation and protein degradation. Allostery is a thermodynamic phenomenon defined by the free energy differences of various allosteric states. Allosteric coupling is a quantitative measure of the degree to which two ligand binding sites, e.g., an allosteric effector vs. a substrate site in an enzyme, functionally interact, and thus fundamentally defines biological regulation by small molecules or protein ligands in the cell. Although allostery is clearly widespread and impacts virtually every cellular process,3 the underlying mechanisms remain elusive.
Allostery is strongly historically rooted in the static structures of oligomeric or multisubunit proteins, from which evolved concerted4 and sequential5 models of homotropic (same ligand) and heterotropic (different ligand) cooperativity. This simple picture of allostery has changed with our ability to measure residue-specific backbone and side chain internal dynamics over a wide range of amplitudes and timescales.6 These thermal motions are an intrinsic property of a protein that collectively define the conformational ensemble, and thus can be harnessed by an allosteric ligand to shift the populations of states within the ensemble, i.e., “remodel the energy landscape.”3 This may be particularly true in “dynamically-driven” allostery, where homotropic allostery is controlled by thermal fluctuations that occur in the absence of a large change in the average structure of the protein. This perspective of allostery is analogous to folding funnels that describe the energy landscape of protein folding. In fact, folding and allostery might be considered “two sides of the same coin,”10 since allosteric coupling is often propagated through the protein interior, through the hydrophobic core of the molecule perhaps via distinct burial modes,11 or via folding-unfolding equilibria present in the native state ensemble.12
We have employed bacterial metalloregulatory proteins to elucidate the rules by which a specific metal ion(s) governs allosteric activation or inhibition of operator DNA binding.13–17 The arsenic repressor (ArsR) family of transcriptional repressors is the largest family of metallosensors and conservatively numbers over 3000 members20 with nearly every bacterial genome encoding at least one.21 The actinomycytes Mycobacterium tuberculosis and Streptomyces ssp. encode more than ten, each of which must properly function in a common cytoplasm. In this family of proteins, metal sites with distinct coordination chemistries and metal specificities have evolved in different places on what is essentially an unchanging, single domain, winged helical N-(α0)-α1-α2-α3-αR-β1-β2-α5-C scaffold, with each site designated by the secondary structural element from which metal coordinating residues derive, e.g., α5 or α3N.23 This same structural scaffold is now also known to accommodate reversible thiol-disulfide exchange as an allosteric modulator of DNA binding activity.
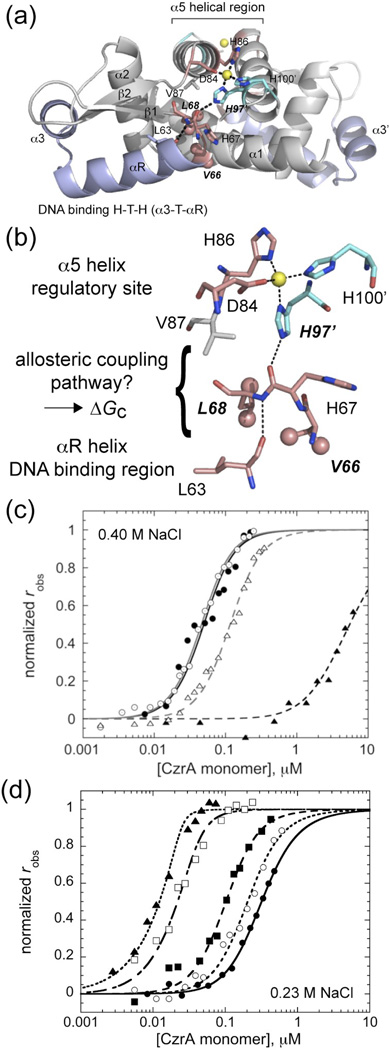
The zinc-sensing repressor Staphylococcus aureus CzrA regulates the expression of zinc efflux transporter26 and has served, with cyanobacterial SmtB, as the prototypical α5-subgroup ArsR family repressor (Fig. 1a). We have previously shown that Zn(II)-mediated quenching of the conformational dynamics of CzrA is a key feature of negative heterotropic allosteric coupling. In this work, we show that the integrity of an interprotomer side chain-main hydrogen bond originating with its nonligating Nε2 face of a histidine ligand to the Zn(II) ion (Fig. 1a,b) is an energetically important contributor to allostery in physically connecting the zinc binding sites to the winged helical DNA binding domain. The magnitude of ΔGc is strongly modulated by introduction of a methyl substituent on the Nε2 face of His97, or a “cavity” in side chain packing32 in the vicinity of this hydrogen bond, with only minor effects on zinc binding or apoprotein-DNA binding affinities. A statistical coupling analysis of ArsR family proteins is consistent with the idea that a common feature of allostery in this family of proteins is governed by concerted movement of the winged helical domain that pivots upon metal binding to distinct sites on the dimer that collectively stabilize the low affinity DNA-binding state.
Fig. 1.
Methylhistidine-substituted (H97MeH) CzrA and cavity mutant CzrAs show poor allosteric inhibition of DNA binding upon Zn(II) binding. (a) Ribbon representation of the CzrA dimer illustrating the relative disposition of the allosteric Zn(II) sites (Zn(II) ion, yellow spheres) and the DNA-binding helix-turn-helix (H-T-H) domain shaded in light blue. Residues of interest in this study are shown in stick representation. (b) Proposed allosteric coupling pathway that links each of the two ligand binding sites. (c) Normalized fluorescence anisotropy-based DNA binding isotherms of H96C (closed symbols) and H97MeH (open symbols) CzrAs acquired in the absence (circles) and presence (triangles) of 10 µM Zn(II). Curves represent the best fit using a 1:1 dimer:DNA binding model with the binding parameters compiled in Table 1. (d) Representative normalized fluorescence anisotropy-based DNA binding curves for Zn(II)-saturated wild-type (closed circles), L68V (open circles), L68A (closed squares), V66A (open squares) and V66A/L68V (triangles) CzrAs. Parameter values are compiled in Table 2.. Conditions: 10 mM Hepes, 0.23 M NaCl, 2.0 µM ZnSO4, pH 7.0, 25.0 °C.
RESULTS
Native chemical ligation and atom substitution in CzrA
Previous computational and structural studies of CzrA are consistent with a model in which an intersubunit His97 NHε2•••O=C His67’ hydrogen bond (see Fig. 1a,b) plays an important role in linking the zinc binding and DNA binding sites in CzrA. To determine the magnitude of the degree to which this hydrogen bond contributes to allostery, we used an approach previously used in the Cu(I) sensor, M. tuberculosis CsoR,36 to uniquely introduce a 1-methyl His residue (MeH) in place of His97. We did this using a semi-synthetic intein-fusion based strategy in which residues 1–95 of CzrA were expressed as a self-cleaving intein fusion as uniformly 15N-labeled, with residues 96–106 prepared as a synthetic peptide via solid phase peptide synthesis (SPPS). This peptide incorporated a non-native N-terminal H96C substitution to facilitate ligation to the 1–95 fragment,37 and a 1-Me-His residue at position 97. Ligation in the presence of 100 mM MENSA and stepwise refolding yielded H96C/His97MeH CzrA, denoted simply as H97MeH CzrA (Supplementary Fig. 1). Functional properties of H97MeH CzrA were then compared to the parent H96C CzrA prepared by conventional site-directed mutagenesis. Examination of 1H-15N HSQC spectra of H96C and H97MeH CzrAs in the absence and presence of saturating Zn(II) reveals that both proteins adopt a wild-type fold and that the small chemical shift differences observed between the parent H96C and H97MeH CzrAs in the apo-state (Δppm≤0.2 ppm) persist in the Zn2 state (Supplementary Fig. 2a,b). Critically, Zn(II) induces the same large chemical shift perturbations upon binding to H97MeH CzrA as found in the parent H96C CzrA, and thus is capable of undergoing wild-type-like Zn(II)-dependent conformational switching in the absence of DNA (Supplementary Fig. 2c–f). There is no evidence of recruitment of the non-native Cys96 into the first coordination shell as revealed by inspection of the absorption spectrum of Co2-H96C CzrA (Supplementary Fig. 2g). The Zn(II) binding affinity as measured in a chelator competition experiment for H97MeH CzrA is wild-type like as well, with KZn≈1012 M−1 (Table 1 and Supplemental Fig. 3).
Table 1.
Summary of fitting parameters for H96C and H97MeH CzrAs in comparison with wild-type CzrAa
| CzrA | KZn1 (M−1) | KZn2 (M−1) | Kapo (M−1) | KZn (M−1) | ΔGc (kcal/mol) |
|---|---|---|---|---|---|
| Wild- typea |
2.5 (±0.3) × 1012 | 3.4 (±0.6) × 1010 | 2.7 (±0.5) × 1010 | 5.7 (±1.2) × 105 | 6.3 (±0.2) |
| H96C | ≥109 ≥10 9 |
≥109 ≥109 |
2.9 (±0.5) × 109 | 7.4 (±0.1) × 105 | 4.9 (±0.1) |
| H97MeHb |
8.0 (±6.0) × 1012 |
3.0 (±1.0) × 1012 |
3.4 (±0.3) × 109 | 5.0 (±0.7) × 108 | 1.1 (±0.1) |
Determined by UV-vis and fluorescence spectroscopies adapted from reference.
Average values of two independent determinations using different concentrations of H97MeH CzrA and magfura-2 (top entry) or quin-2 (bottom entry) as described. Conditions: 10 mM Hepes, 0.40 M NaCl, pH 7.0.
The allosteric coupling free energy (ΔGc) is a quantitative measure of the degree to which the binding of one ligand influences (positively or negatively) the binding of another ligand.1 ΔGc can be measured here by comparing the DNA binding affinities in the apo (Kapo) and Zn(II)-bound states (KZn) from ΔGc=−RTln(KZn/Kapo). Application of a dimer linkage model to extract ΔGc for wild-type CzrA reveals a value of ≈5−6 kcal mol−1 at pH 7.0, 0.4 M NaCl, 25.0 °C assuming a Kdimer of ≈105 M−1 estimated by sedimentation equilibrium ultracentrifugation. To determine ΔGc for H97MeH CzrA, we measured DNA binding affinities of H96C and H97MeH CzrAs in the presence and absence of Zn(II) (Fig. 1c and Table 1). These data reveal that apo-H96C and apo-H97MeH CzrAs bind with the same affinity to a 28-bp DNA harboring a single czr operator (CzrO), although with somewhat lower affinity than wild-type CzrA (Table 1). More importantly, the Zn2 forms have vastly different affinities, with H96C CzrA strongly allosterically inhibited as expected (ΔGc=4.9 kcal mol−1), while the binding affinity of Zn2 H97MeH CzrA is only reduced ≈7-fold relative the apo-H97MeH CzrA, corresponding to a ΔGc of only 1.1 kcal mol−1 (Fig. 1c). Thus, although the methyl substituent has little or no effect on conformational switching in the absence of DNA, this substitution reduces the free energy of allosteric negative regulation of DNA binding to just ≈20% of the total.
The Zn2-CzrA-CzrO ternary complex adopts a hybrid conformation
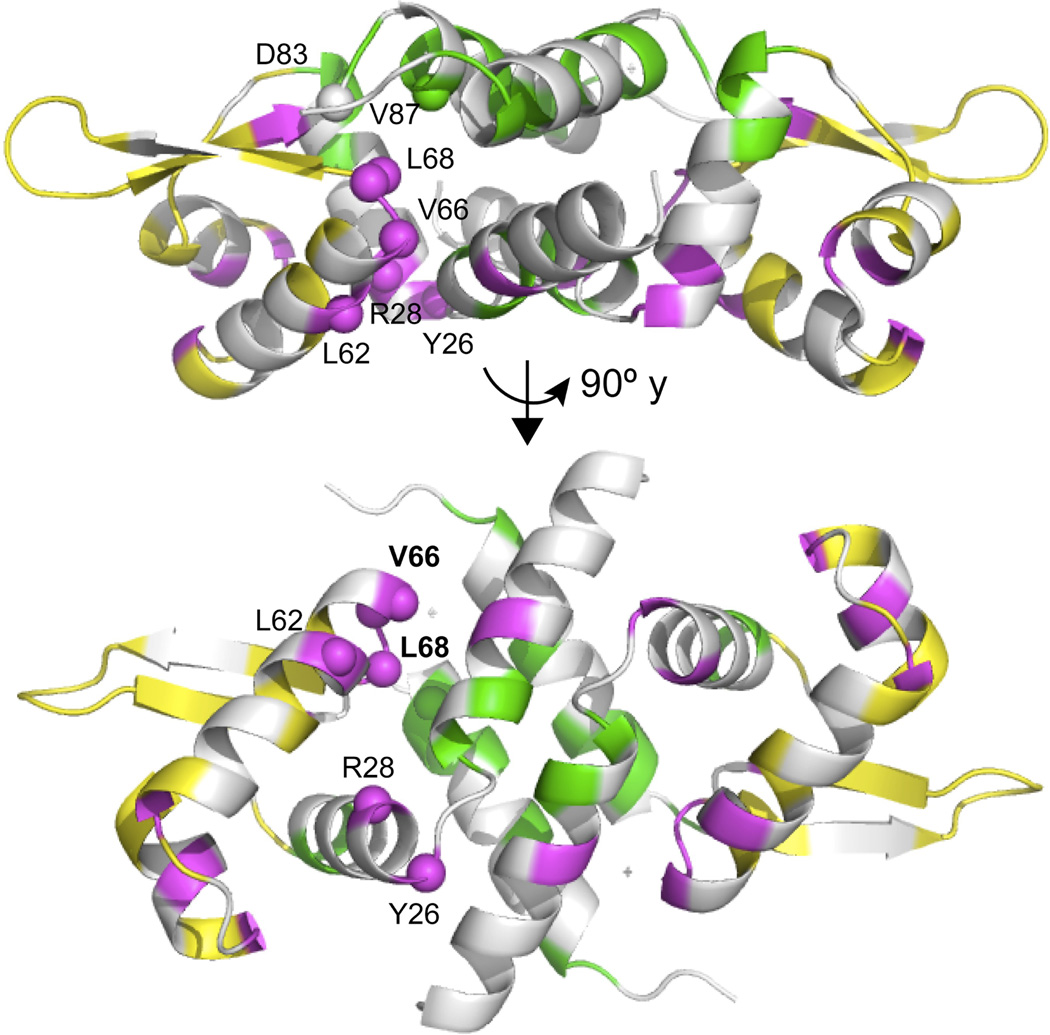
In order to identify additional residues of CzrA important for allostery, we compared 1H-15N TROSY spectra of CzrA in the Zn(II)- and czr operator (CzrO) DNA-bound allosteric end states with that obtained for a ternary complex formed with both negatively competing ligands bound (Supplementary Fig. 4a). Although the resonance linewidths are broad as might be expected for what is essentially a transiently formed intermediate in transcriptional derepression, the spectrum of the ternary complex appears to show three sets of resonances when compared to the component singly ligated states (Fig. 2). These include residues with crosspeaks most similar to the CzrO-bound state (localized to the α3 and DNA recognition (αR) helices and the β-wing; shaded yellow in Fig. 2), those most similar to the Zn(II)-bound state (primarily confined to the α1 and α5 region; shaded green in Fig. 2), and a set of resonances that appear to reside in distinct chemical environments in each of the three allosteric states (shaded magenta in Fig. 2). Residues in this latter set include L62, V66-L68 in the αR-β1 loop region, as well as additional residues that are disproportionally localized in the more peripheral winged helical regions of the CzrA. The methyl 13C and 1H resonances of V66 are also most different in the component singly ligated states (Supplementary Fig. 4b). We hypothesized that residues within this latter group might play an important role in energetically linking the two ligand binding sites40 and therefore targeted a subset of these residues for mutational analysis (Y26, R28, L62, V66, L68 highlighted in Fig. 2) compared to two additional control residues not clearly in this group (D83, V87).
Fig. 2.
Two ribbon representation views of CzrA in which the backbone amide resonances derived from an 1H-15N TROSY spectrum (see Supplementary Fig. 4a) of ternary CzrA•Zn2•DNA complex mimic those found in the apo-CzrA-DNA state (shaded yellow) or in Zn2 CzrA (shaded green) or are found in a magnetically unique environment in each of the three states (shaded magenta). Backbone resonance assignments of the CzrA•Zn2•DNA complex were obtained by inspection and included ≈50% coverage of the molecule (unassigned resonances shaded gray). Residues subjected to mutagenesis are indicated with Cα atoms represented as spheres.
Coupling free energy analysis of CzrA mutants identifies an allosteric pathway in CzrA
We used the general approach outlined above and carried out DNA binding experiments for the apo- and Zn(II)-bound mutants (Fig. 1c) and determined KZn and ΔGc for each (Table 2). Mutant CzrAs with perturbed communication between the two ligand binding sites will show increased DNA-binding affinity in the presence of Zn(II) compared to wild-type CzrA (Fig. 1d). Y26F CzrA exhibits wild-type ΔGc, while R28Q and L62V CzrAs could not be characterized as a result of a misfolding (R28Q) or weak DNA binding activity in the apo-state (L62V) (Supplementary Table 1). In contrast, Zn2 V66A CzrA shows a very large coupling defect, binding 460-fold more tightly than Zn2 wild-type CzrA to the CzrO DNA at 0.23 M NaCl, pH 7.0, while an L68V mutation is only modestly perturbed (Fig. 1d and Table 2). The V66/L68V double mutant CzrA has a dramatic influence on the magnitude of ΔGc, binding DNA ≈12,000-fold more tightly than Zn(II)-bound wild-type CzrA, which corresponds to a ΔGc of +1.1 kcal mol−1, some ≈6.5 kcal mol−1 less than wild-type CzrA (Fig. 1c and Table 2). All mutants are dimeric on the basis of gel filtration chromatography , bind Zn(II) with at or near wild-type binding affinity and retain a binding stoichiometry of two (see below) characterized by modest negative cooperativity of zinc binding (Table 2). The double mutant binds DNA with a similar [NaCl]-dependence, SKobs (Supplementary Table 2; Supplementary Figure 7) indicative of little or no change in the DNA binding interfacial region.41 This value of SKobs allowed us to obtain the binding affinity of all apoproteins at 0.23 M NaCl via linear extrapolation from conditions under which Kobs could be measured (Supplementary Figure 7), thus allowing resolution of ΔGc under these conditions.
Table 2.
Binding parameters and coupling free energies for wild-type and selected mutant CzrAs
| CzrA |
KZn1 (M−1) × 1011 |
KZn2 (M−1) × 1011 |
Kapo (M−1) × 1011,a |
KZn (M−1) × 107, b | ΔGc (kcal mol−1)c |
|---|---|---|---|---|---|
| Wild-type | 5.8 (±2.1)d | 0.48 (±0.02)d | 1.7 (±0.6) | 3.5 (±0.6) | +7.6 (±0.6) |
| V66A | 2.6 (±0.4)d | 0.42 (±0.02)d | 0.31 (±0.05) | 1600 (±300) | +3.7 (±0.6) |
| L68V | 3.0 (±0.3)d | 0.32 (±0.01)d | 0.14 (±0.03) | 7.8 (±1.6) | +6.8 (±0.5) |
| L68A | ≥0.01e | ≥0.01e | ND | 30 (±8) | +6.0 (±0.6) |
| V66A/L68V | 2.5 (±0.3)d | 0.30 (±0.04)d | 0.34 (±0.07) | 42000 (±23000) | +1.1 (±0.6) |
| V87A | ≥0.01e | ≥0.01e | ND | 6.5 (±0.9) | +6.9 (±0.5) |
Determined using DNA-binding fluorescence anisotropy at 0.40 MNaCl, 25.0 °C.
Determined using DNA-binding fluorescence anisotropy at 0.23 M NaCl, 25.0 °C. The numbers in parentheses reflect the standard error of the fitted parameters for a representative experiment of n independent experiments (n≥2).
ΔGc=−RTln(KZn/Kapo), determined at 0.23 M NaCl. See Methods for details on error propagation to obtain the standard error on ΔGc.
Determined using ITC at 0.40 M NaCl, pH 7.0 from multiple experiments. The number is parentheses represents the standard deviation (s.d.) of the mean values determined from three experiments.
Lower limit obtained from a mag-fura-2 competition experiment.
Although a number of other single-site mutant CzrAs were characterized (Supplementary Table 1), the V66A substitution was found to be the single most detrimental substitution. For example, V66Q and V66Q/H67G CzrAs, the latter designed to mimic the Cd(II)/Pb(II) sensor CadC,42 have physical properties indistinguishable from that of wild-type CzrA. This “cavity” defect is also specific for V66 since substitution of another Val with Ala in the same region (V87A CzrA; see Fig. 1a,b) shows a near wild-type-like ΔGc (Table 2 and Supplementary Table 1). Val66 and Leu68 may also function cooperatively since ΔΔGc for V66A/L68V CzrA is ≈1.8 kcal mol−1 larger than the sum of the component single-site V66A and L68V mutations, although this difference may be just inside statistical significance (ΔΔGc=1.8±1.1 kcal mol−1). Additionally, an L68A mutation decreases the coupling energy further than L68V, consistent with the “cavity” defect hypothesis. V66 and L68 are found in the loop between αR helix and the β-wing, physically interact, and point toward the protomer core directly beneath the H97’-H67/L68-L63 hydrogen bonding network (vide infra). Thus, perturbation of the protein core near the hydrogen-bond network results in a substantial disruption of communication between the two ligand binding sites, in much the same way as introduction of 1-methyl substitution on the Nε2 face of His97 (Fig. 1c).
WT and V66A/L68V CzrAs have identical Zn(II)-bound crystal structures
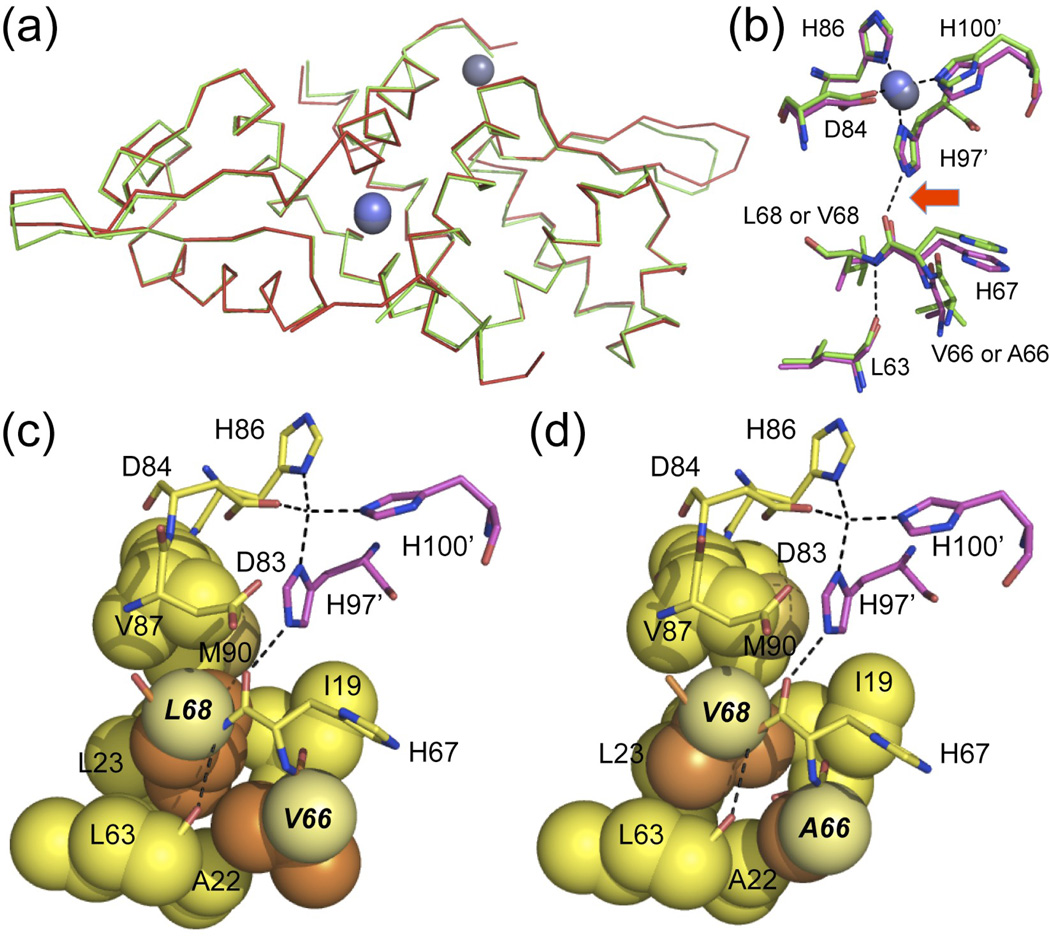
In order to determine the structural origin of this compromised allosteric linkage in V66A/L68V CzrA, we solved the crystal structure of Zn2 V66A/L68V CzrA to 2.0 Å resolution. The global structures of wild-type28 and V66A/L68V CzrAs are essentially identical, with an r.m.s.d. of 0.38 Å over 185 Cα atoms (Fig. 3a and Supplementary Table 3 for structure statistics); in addition, the first coordination shell around the Zn(II) ion and the integrity of the hydrogen-bonding pathway is intact and nearly indistinguishable in the double mutant (Fig. 3b). Likewise, examination of an 1H,15N-HSQC spectrum of the Zn(II)-bound double mutant reveals largely local perturbations of the structure immediately around the site of the substitution relative to Zn2 wild-type CzrA (Supplementary Fig. 5). These structural studies are fully consistent with very similar zinc and apoprotein DNA binding affinities of this mutant relative to wild-type CzrA (Table 2). However, closer inspection reveals the presence of a significant cavity indicative of poorer packing in the protein core for V66A/L68V CzrA relative to wild-type CzrA (Fig. 3c–d). We hypothesize that this poorer packing directly controls the magnitude of ΔGc.
Fig. 3.
The structures of Zn(II)-bound wild-type and V66A/L68V CzrAs are globally identical. (a) Global Cα wireframe superposition of wild-type (green) and V66A/L68V (red) CzrAs, with the positions of the zinc atoms shown in slate or darker slate. (b) Detailed superposition of the first and selected second coordination shell region of wild-type (green) and V66A/L68V (magenta) CzrAs. The interprotomer H97’-H67 (highlighted by the red arrow) and L68-Leu63 hydrogen bonds are indicated by the dashed lines. (c) and (d) Spacefilling representations of the van der Waals packing region in the vicinity of these hydrogen bonds in Zn2 wild-type CzrA (panel c) and V66A/L68V CzrA (panel d). Side chains of residues 66 and 68 and Cα atoms are shaded orange and pale yellow, respectively.
Energetics of Zn(II) binding to wild-type vs. V66A/L68V CzrAs and other cavity mutants
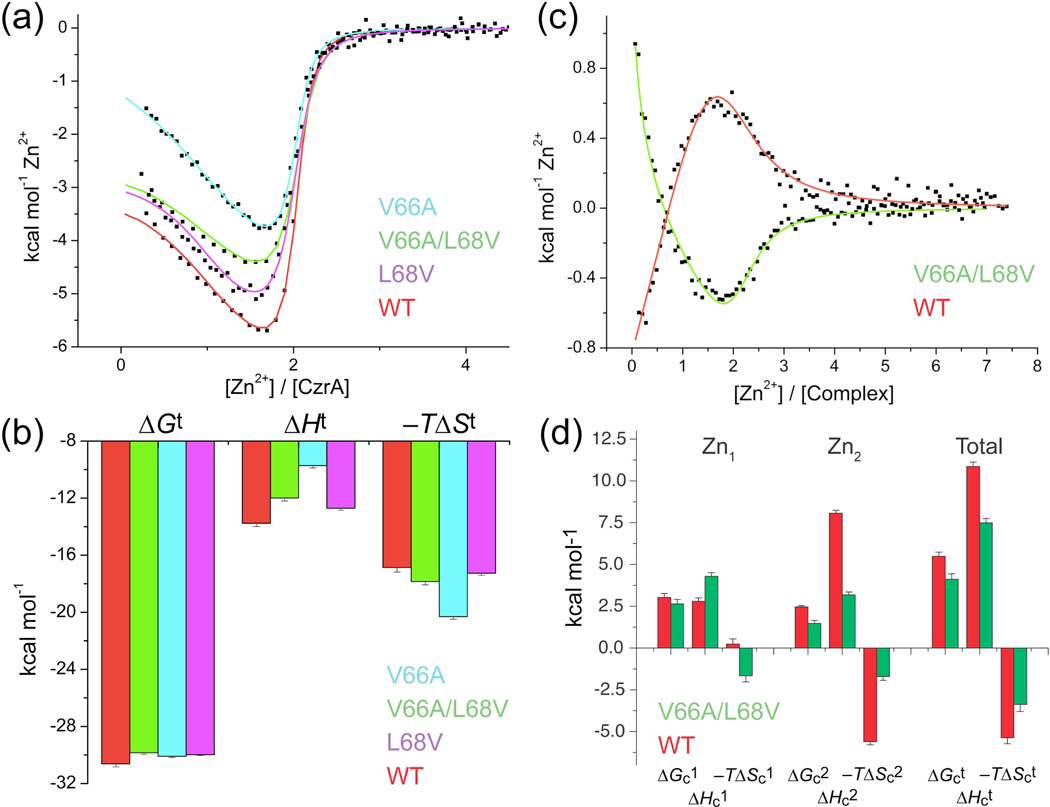
We next carried out a series of isothermal titration calorimetric (ITC) experiments in order to determine if the poorer packing of the double mutant becomes manifest in the underlying energetics of Zn(II) binding to the dimer.30 Here, we took advantage of the fact that the zinc binding affinity and structure of the first coordination sphere in the mutant are identical to that of wild-type CzrA (Fig. 3). Zn(II)-binding experiments with wild-type CzrA gives thermodynamic values comparable to those previously reported, although not corrected here for linkage to ligand deprotonation upon metal binding since this contribution will be identical in all cases (Fig. 4a–b and Supplementary Table 4).30 Comparison of V66A, L68V, and V66A/L68V mutant CzrAs show that these proteins bind two equivalents of Zn(II) per dimer with high affinity and measurable negative homotropic cooperativity, resulting in nearly identical free energies of Zn(II) binding (ΔGt) (Fig. 4a–b and Table 2). This is consistent with the fact that all mutants are known or expected to have substantially identical first coordination shells (Fig. 3) and the effect of solvent release from the metal will be identical in each case.
Fig. 4.
Analysis by isothermal titration calorimetry of Zn(II) binding to various apo-CzrAs and the apo-CzrA-DNA complex. (a) Representative titrations of Zn(II) binding to wild-type (WT), V66A, L68V and V66A/L68V CzrA dimers. (b) Thermodynamic parameters obtained for Zn(II) binding to CzrAs obtained from experiments like those shown in panel A. t, total, obtained by summing parameters obtained for the first (Zn1) and second (Zn2) Zn(II) binding steps. (c) Representative titrations of Zn(II) into CzrO complexes formed by wild-type (WT) and V66A/L68V CzrAs. These titrations correspond to the “top” and “bottom” of the heterotropic coupling equilibrium that defines this system.30 (d) Graphical illustration of the coupling energetics of wild-type vs. V66A/L68V CzrAs derived from the first (Zn1) and second (Zn2) binding steps. t, total. Conditions: 38 µM dimer, 3 mM NTA as a zinc competitor, or 38 µM dimer-DNA complex, with 1 mM NTA as competitor in 10 mM Hepes, 0.4 M NaCl, pH 7.0, 25.0 °C.
Strikingly, the underlying energetics reveal that V66A/L68V CzrA has a significantly smaller enthalpy of Zn(II) binding, ΔHt, than wild-type CzrA (Fig. 4a,b). This smaller enthalpy change is nearly precisely compensated by a more favorable entropy term for Zn(II) binding to V66A/L68V CzrA (−TΔSt). This result is as anticipated for a cavity mutant CzrA containing fewer van der Waals contacts in the protein core (Fig. 3d) resulting in increased internal dynamics (Fig. 4b and Supplementary Table 4). The same trend is observed for each of the two component single mutants, with the effect of the single V66A substitution larger than that of the L68V substitution (Fig. 4b and Supplementary Table 4).
We next examined the energetics of Zn(II) binding to the CzrA dimer-DNA complex formed by wild-type vs. V66A/L68V CzrAs (Fig. 4c and Supplementary Table 4). Strikingly, the ΔH contribution to Zn(II)-binding to the V66A/L68V complex is easily distinguished from that of the wild-type CzrA-DNA complex, with the two isotherms of nearly opposite sign (Fig. 4c). Propagating these energetics of Zn(II)-binding to obtain ΔHci and −TΔSc1 for each ith zinc binding step reveals a less positive ΔHc and a less positive ΔSc, manifested largely in the second zinc binding step, i.e., in ΔHc2 and −TΔSc2, as expected if the distinct energetics of the Zn(II)-binding isotherms (Fig. 4a–c) propagate to the energetics of heterotropic coupling (Fig. 4d). These findings reveal that poorer side chain packing observed crystallographically (Fig. 3d) and implied by the underlying energetics of Zn(II) binding to V66A/L68V CzrA relative to wild-type CzrA (Fig. 4) directly impact the magnitude and underlying energetics (ΔHc, ΔSc) of ΔGc (Table 2). Since a major contributor to zinc-dependent allosteric inhibition of DNA binding is global “stiffening” of the dimer, much of which also occurs on the second Zn(II) binding step, decreased quenching of the conformational dynamics associated with the allosterically inhibited Zn(II)-bound state functions to reduce the effectiveness of negative allosteric regulation by Zn(II) on V66A/L68V CzrA function.
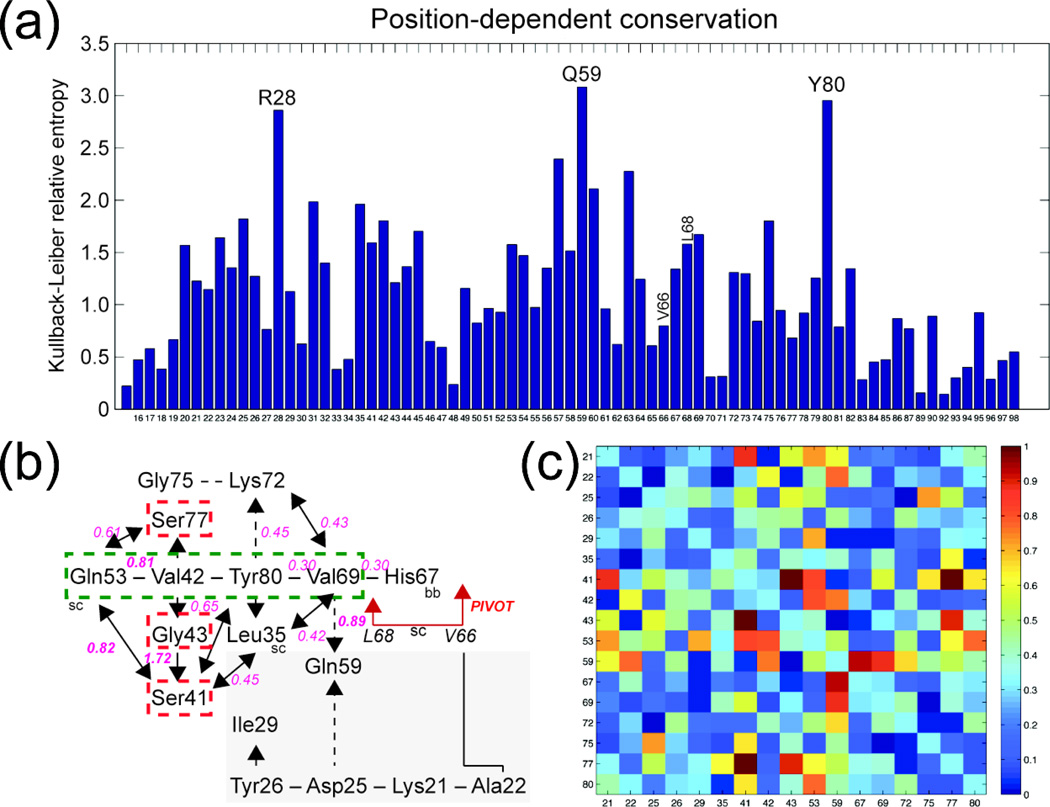
Statistical coupling analysis of ArsR family repressors
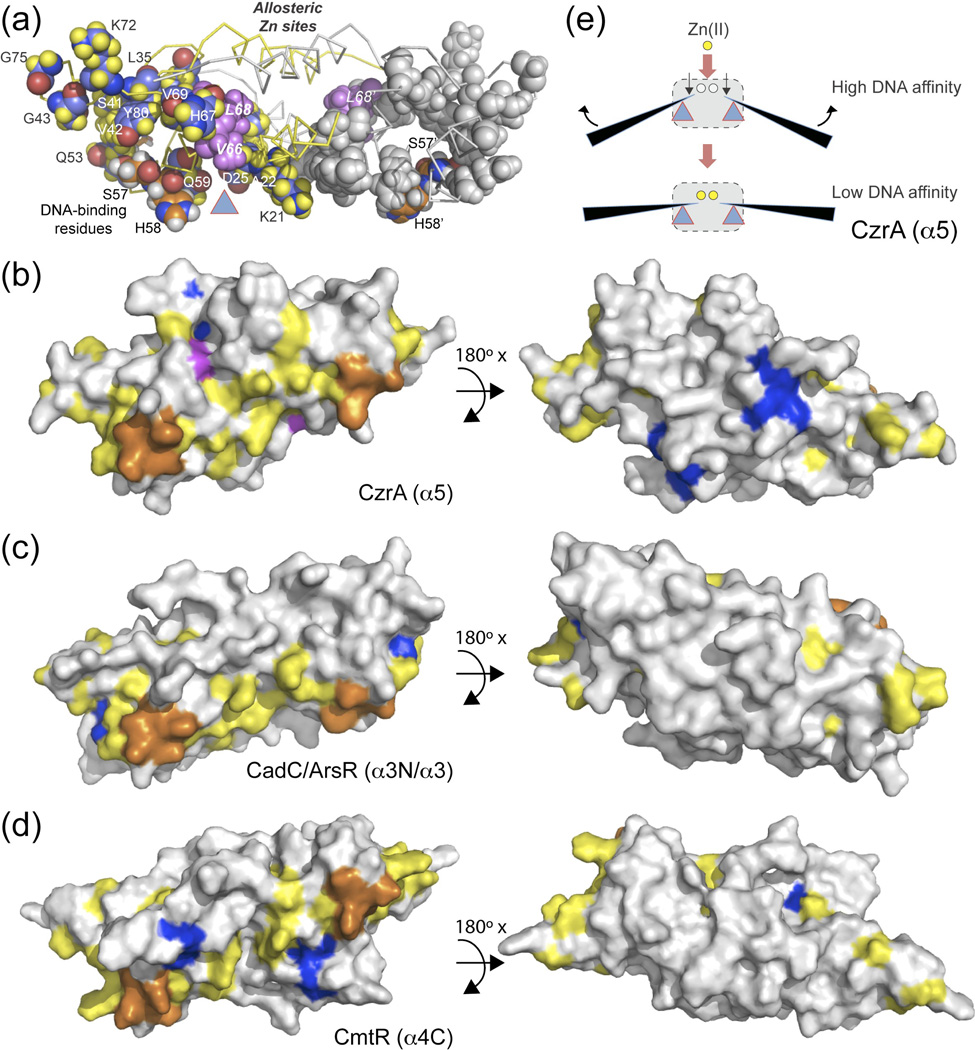
Having determined that V66 and L68 may function cooperatively in controlling the magnitude of ΔGc in CzrA, we then asked if these two residues are evolutionarily pairwise coupled in ArsR family repressors. To address this, we carried out a multiple sequence alignment-based statistical coupling analysis (SCA) of 3000 ArsR family repressors (Fig. 5) and mapped the results of this analysis onto the structure of Zn(II)-bound CzrA (Fig. 6a). We find that V66 and L68 are not strongly conserved, nor are they strongly evolutionarily coupled (see Fig. 5). Despite this, V66 and L68 appear to physically connect the α5 allosteric sites with a contiguous network of coupled residues, or sector, that extends from the α1 helical region to the α3-αR-β-wing region, largely along the DNA-binding interface, that collectively encircles key DNA residues on the αR helix (S57, H58),15 which themselves do not co-vary (Fig. 5a). Residues in the α5 helix do not strongly covary either, as expected for a family of repressors that respond to a range of metal and non-metal effectors that bind to distinct sites. Indeed, analysis of the α5 helical region reveals a near complete absence of interacting residues (Fig. 6a,b). This sequence based identification of a sector residues bears strong similarity to the subset of “hybrid-state” and “DNA-binding-state” residues determined by analysis of the experimental 1H-15N TROSY spectrum of Zn2-CzrA•CzrO (Fig. 2).
Fig. 5.
Statistical coupling analysis of ArsR family repressors. (a) Residue-specific conservation plotted as relative entropy of residue positions 15–35, 41–87, 89–90 and 92–98 which correspond to CzrA residue positions. Gaps correspond to gaps in the multiple sequence alignment. Selected residues with the highest conservation are highlighted with CzrA residue type and number. (b) Schematic illustration of residues in physical contact arranged as two parts of the same network of 17 coupled residues (upper left; lower right, shaded gray), with selected pairwise statistical coupling energies shown in pink (see Methods). Three key residues that are strongly pairwise coupled to one another are shown boxed in red. The green box represents four residues in direct physical contact that connect β-wing and more peripheral regions of the DNA-binding site to the pivot point defined by V66/L68. (c) Normalized heat-map of all (17×17) significantly pairwise coupled interactions scaled from 0 (no coupling) to 1 (strong coupling). The diagonal is false-colored light blue and represents the mean value of the correlation map. Residues pairs 41/43 and 41/77 exhibit the strongest pairwise couplings. It is interesting to note that the CXC Cys pair in Pb(II)/Cd(II)-sensing CadCs (which correspond to residue positions 41/43 in CzrA) are ligands to the Cd/Pb ion.42
Fig. 6.
Structural representation of a statistical coupling analysis of ArsR family repressor reveals a coupled network connected to an allosteric “hot-spot”. (a) Network of coupled residues shown in spacefill on both protomers (CPK coloring shown in left protomer with protons shaded yellow and carbons slate; gray on right protomer). Key DNA binding residues (S57, H58)15 shaded CPK with protons white and carbon atoms shaded orange; V66 and L68 define an allosteric “hot-spot” with all atoms shaded magenta. CzrA is shown in its open or “flat” allosterically inhibited Zn(II)-bound low DNA binding affinity state. (b)-(d) Surface representations of (b) CzrA (1R1V); (c) CadC (1U2W) and (d) CmtR (2JSC) highlighting a sector of coevolving residues (shaded yellow), the allosteric metal site chelates (shaded blue), major energetic determinants of the DNA binding domain (shaded orange; corresponding to residues 54–58 in CzrA)15 and the V66/L68 in CzrA (shaded magenta). View from the DNA binding interface is shown on the left, with a view from the top of the molecule shown on the right. (e) Schematic rendering of the conformational transition from a high to a low DNA binding conformation with the sector schematized as a lever, and the V66/L68 “pivot” point indicated by the triangle with the α5 metal sites shown as circles.
This sector may allow the more peripheral winged-helical region to move in a concerted fashion with respect to the α1-α5 core in response to inducer recognition to distinct sites on the ArsR scaffold that “moves” and/or “stiffens” the DNA-binding interface which ultimately inhibits DNA binding (Fig. 6c,d). Consistent with this, a number of residues in CzrA in or near this sector exhibit dynamical quenching upon Zn(II) binding.15 Previous studies of other ArsR family sensors are also consistent with this model. Pb(II)/Cd(II)-sensing CadCs and canonical As(III)/Sb(III) sensing ArsRs employ a pair of metal-binding Cys residues arranged as a CXC motif in the α3 helix, the C-terminal of which aligns with strongly coupled residue G43 (Fig. 5c) and is a known allosteric ligand.42 This metal site is surrounded by sector residues (Fig. 6c). The α4C Cd(II)/Pb(II) sensor M. tuberculosis CmtR binds metal ions to a pair of cysteines in a CXXXC motif, the C-terminal of which aligns precisely with V66 in CzrA. This metal site is also in physical contact with sector residues (Fig. 6d), and Cd(II) binding to CmtR has also been reported to quench the internal dynamics of CmtR particularly across the dimer interface.45 Finally, in the related nickel sensor, M. tuberculosis NmtR, Ni(II) binding to the α5 sites induces conformational exchange broadening to distal residues corresponding to Q53, H67 and G75 each of which are part of the deduced sector (Fig. 5b,c).17 These data suggest that a region defined by allosteric residues Val66 and Leu68 in ArsR family homodimers has evolved as an allosteric “hot-spot” on which the DNA-binding domain is capable of pivoting or damping in response to the binding of an allosteric metal ion (Fig. 6e).
DISCUSSION
In this work, we deconstruct the heterotropic allosteric linkage free energy into component thermodynamic driving forces in the paradigm ArsR-family zinc sensing repressor S. aureus CzrA. A comprehensive analysis of heterotropic allostery involves structural, dynamical and thermodynamic interrogation of all four allosteric states of a two-ligand system, which in this case includes the ligand-free (apo) state, the two singly-ligated states (Zn2-CzrA, CzrA•DNA) and the doubly ligated (Zn2•CzrA)•DNA state and coupled equilibria between states as a function of ligand activity.40 High resolution crystallographic structures of apo- and Zn2 CzrAs are available, as are solution structural models of Zn2 and DNA-bound CzrAs. A major conclusion from the previous work is that zinc binding narrows the conformational ensemble and “freezes out” a low affinity DNA conformation that is unable to undergo the large quaternary structural change required to form a high affinity complex with the DNA operator.15 Similar conclusions have been reached for the Cd(II)/Pb(II)-selective ArsR family sensor, M. tuberculosis CmtR.45 Here we use the NMR fingerprint region of the doubly-ligated ternary complex to identify candidate residues that control the magnitude of the heterotropic allosteric coupling free energy, ΔGc, and in so doing uncover an allosteric hot-spot in CzrA.
As in other studies of protein-ligand interactions, e.g., CAP•cAMP2•DNA46 and calmodulin–peptide complexes,47 we find that Zn(II) binds to allosteric mutants of CzrA with similar free energies, but very different component enthalpies and entropies of binding, and thus represent clear examples of enthalpy-entropy compensation in biomolecular interactions.48 The striking departure reported here is that enthalpy-entropy compensation of ligand Zn(II) binding leads to an uncoupling of Zn(II) and DNA binding to varying degrees, resulting in a loss in biological regulation. This is true for the two cases discussed here (methyl substitution and cavity mutants), in which the functional defect lies squarely in the magnitude of ΔGc with little or no effect on the binding affinity of Zn(II) or operator DNA, thus allowing resolution of underlying entropic and enthalpic driving forces to the coupling free energy (ΔHc, −TΔSc) (Fig. 4). Since unfavorable entropic driving force is a major contributor to negative allosteric regulation of DNA binding, we propose that this contribution is reduced by introduction of poor side chain packing (as reported by decreased ΔHcal) and concomitant increased mobility (increased −TΔScal) in the immediate vicinity of a key hydrogen bonding interaction, which itself plays a major role in controlling the magnitude of ΔGc (Fig. 4).
It is formally possible that some of the CzrA mutants characterized here perturb the dimer stability (Kdimer) of CzrA. V66 is not part of the dimer interface, but packs against the α1 helix of the same protomer, which forms the α1-α1’-α5-α5’ helical dimer core (Fig. 2). If so, this will have an impact on our determination of ΔGc, since the DNA binding constants Kapo and KZn are determined under conditions of strong linkage to the monomer-dimer equilibrium using fluorescence anisotropy methods (see Supplementary Figure 6). Although the zinc dependence of Kdimer is known for wild-type CzrA29 (see Methods) and we did not determine this for mutant CzrAs. We consider significant perturbation of Kdimer unlikely for several reasons. First, the affinity of the apo-CzrAs for DNA are all within a factor of 10 of wild-type CzrA, and even the most strongly allosterically perturbed double mutant, V66A/L68V, is within a factor of ≈5 of wild-type and is characterized by a very similar salt-dependence of Kapo (Supplementary Figure 7). If there were a large change in dimer stability this would result in a far more greatly perturbed Kapo and the mutants would not chromatograph as dimers by gel filtration chromatography (see Methods). To significantly impact our resolution of ΔGc then, zinc binding would have to stabilize the mutant dimers far more strongly than the wild-type dimer (simulations suggest by >105-fold), which would then lead to tighter DNA binding and weaker apparent allosteric negative regulation by zinc. This possibility seems remote given that the dimer interface is basically unchanged in V66A/L68V CzrA (Fig. 3) and a smaller rather than larger ΔH component is associated with zinc binding to the mutants relative to wild-type CzrA, with similar KZn (Table 2). In any case, even some perturbation of the monomer-dimer equilibrium would formally remain an allosteric effect and naturally derives from the fact that cooperativity and folding are intimately interconnected.10
Two limiting models have been put forth in an effort to explain the physicochemical linkage of two ligand binding sites in classical heterotropic allostery in structural or dynamical terms. These models differ on the presence49–51 or absence of a preferred or dominant pathway54 of allosteric connectivity between ligand binding sites. In CzrA, we show here that allostery in CzrA hinges on the integrity of a key interprotomer side chain-main chain hydrogen bond. The coordinate covalent nature of transition metal-ligand bonds may well establish strong directionality into this allosteric network. Blocking formation of this hydrogen bond chemically or introduction of a cavity (but not a larger side chain), just below this hydrogen bond quantitatively and specifically reduces the magnitude of ΔGc. This latter effect is selective and focused on V66. However, it is important to recognize that ΔGc is not zero in any CzrA mutant, consistent with the idea that otherwise non-dominant pathways can make a contribution when a major one is disrupted.54
Our elucidation of what would appear to be a compact pathway of allosteric communication between Zn(II) and DNA binding sites in CzrA stabilized by a cooperative network of van der Waals interactions involving V66 and L68 does not become manifest in a standard pairwise covariation or statistical coupling analysis (SCA) carried out on a large family of ArsR family sensors (Figs. 5–6), as had been found in previous systems examined by SCA. This is perhaps not so surprising given the involvement of both main chain and side atoms in allosteric hydrogen bonding in CzrA (Fig. 1b), the small subset of interactions that control much of the magnitude of ΔGc, and the breadth of distinct subfamilies of sensors with different regulatory ligand binding sites and specificities.23 The major finding from this analysis is the identification of a sector of interconnected residues which immediately suggests a way in which the more peripheral elements of the ArsR fold that control DNA binding affinity, i.e., the recognition helices and β-wing, move in a concerted fashion (Fig. 6a). The allosteric “hot-spot” identified here centered on V66 and L68 then simply defines a pivot point on which the entire DNA-binding interface is remodeled upon metal binding (Fig. 6b–d), rather than an allosteric pathway.56 Rapid evolution of new inducer specificity on this simple scaffold would then become a matter of evolving distinct connectivities to this sector of co-evolving residues (Fig. 6b–d), the end result of which is to reposition the DNA-binding levers and/or quench the dynamics in a way that ultimately stabilizes a low-affinity “open” DNA binding conformation. The recent structures of two ArsR family proteins that are known or projected to exploit thiol-disulfide chemistry to stabilize “closed” and “open” conformations, e.g., B. subtilis HypR24 and Xylella fastidiosa BigR,25 are generally consistent with this model as a common feature that underlies ArsR family protein allosteric function.
An alternative view is that this sector comprises a classically defined allosteric network which links the energetically important but more peripheral components of the DNA binding interface, e.g., the N-terminus of the αR helix and β-wing tips15 to the core of the dimer defined largely by the α1-α5 helical bundle, to achieve a quaternary structural conformational change. Evolution of distinct allosteric sites in the ArsR family would then be possible provided only that these sites physically connect to any point along this sector.54 In this view, the allosteric hot-spot defined here becomes more of an extension of the allosteric ligand binding site itself (Fig. 6b) that mediates a physical connectivity to a pre-existing allosteric network. This view is perhaps more consistent with the spectral characteristics of the Zn2-CzrA•CzrO complex (Fig. 2), which reveal that candidate allosteric residues are interspersed with DNA binding-like residues or alternatively, serve to physically connect the Zn(II) and DNA binding sites on the dimer. The overrepresentation of these residues at the termini of secondary structural elements (see Fig. 2) may help drive this connectivity within the sector in a concerted manner.
The compact, essentially single-domain architecture of CzrA and related ArsR proteins17 does not allow us to distinguish between these two general views. On the other hand, a survey of known, structurally defined regulatory sites on the ubiquitous ArsR repressor scaffold suggests an overrepresentation of allosteric sites positioned roughly on opposite ends of the sector defined here (Fig. 6), sometimes including determinants from both ends. Evolution of distinct metal coordination chemistries and chemical reactivities characteristic of each functional ArsR subfamily would then result in new biological specificities.
METHODS
Protein Production
Construction and purification of H97MeH CzrA using native chemical ligation
The C-terminal peptide of CzrA (residues 96–106, H96 substituted with Cys) was synthesized with incorporation of MeH (1-methylhistidine) obtained as a Boc-derivative (Bachem, CA) at residue 97 using Boc-based solid phase peptide synthesis. The DNA sequence encoding the N-terminal peptide (residue 1–95) was cloned into the pTXB1 vector (New England Biolabs) between NdeI and SpeI restriction sites in frame to a C-terminal intein fusion. The CzrA 1–95-intein fusion was expressed in E. coli BL21(DE3) and after sonication in Buffer C (25 mM Tris, 0.5 M NaCl, 2 mM TCEP, pH 8.0), was found to remain in the low speed lysis pellet. This pellet was then resuspended in Buffer C containing 7 M urea and refolded by stepwise decreasing the urea concentration in Buffer C. The resultant soluble fraction of CzrA 1–95-intein was cleaved with the addition of 100 mM -mercaptoethanesulfonate (MESNA) (Sigma, MO) with CzrA 1–95-thioester further purified on a C18 reverse phase column by running a 0–75% acetonitrile gradient in 0.1% TFA. Fractions containing CzrA 1–95 thioester were pooled and concentrated to ~1 mL and ligated to the C-terminal peptide using conditions analogous to those as previously described.36 The resultant H96C/H97MeH CzrA (denoted simply as H97MeH CzrA) was further purified on a µRP (GE Healthcare, NJ) reverse phase column under denaturing conditions and finally refolded into Buffer P by stepwise increasing pH (10 mM Hepes, 0.4 M NaCl, pH 7.0) with 1 mM TCEP.
Purification of mutant CzrAs
Overexpression plasmids encoding mutant S. aureus CzrAs were constructed by site-directed PCR-based quick-change mutagenesis using pET3a-CzrA as template35 with plasmid integrity verified using DNA sequencing. The proteins were expressed in E. coli BL21(DE3) at 37 °C on M9 minimum medium containing 100 mg/mL ampicillin supplemented with 15NH4Cl as the sole nitrogen source15 or on LB medium containing 100 mg/mL ampicillin and purified using published procedures. For H96C CzrA, 2 mM dithiothreitol was added to all the buffers used during the purification. Purified H96C CzrA was extensively dialyzed anaerobically against Buffer P (10 mM Hepes, 0.4 M NaCl, pH 7.0). The protein concentration was determined using ε280nm=4470 M−1cm−1 and the mol equiv of free reduced thiol was determined by the DTNB assay to be 0.9 (1.0 expected). All other mutant CzrAs were purified using the same purification as wild-type CzrA,29 dialyzed extensively and confirmed to contain less than 0.05 mol equivalents of Zn(II) by atomic absorption spectroscopy. All chromatographed as dimers by gel filtration chromatography.
Co(II) and Zn(II) binding to H96C and H97MeH CzrAs
All metal binding experiments were conducted on a Hewlett-Packard model 8452A spectrophotometer. CoCl2 titrations with 100 µM CzrA monomer (50 µM dimer) were carried out in Buffer P anaerobically as previously described. Two zinc chelator indicator dyes were used for zinc competition experiments: Quin-2 (KZn= 2.7 × 1011 M−1 at pH 7.0 and 25 °C59) and mag-fura-2 (KZn= 5.0 × 107 M−1).60 For mag-fura-2 Zn(II) titrations, ZnSO4 was titrated into a mixture of 2.4 µM mag-fura-2 and 1.7 µM CzrA monomer in Buffer P containing 0.1 mM TCEP. The excitation spectrum from 265–455 nm with λem=497 nm was measured after each ith addition. Fluorescence intensities at 325 and 379 nm were plotted against total Zn(II) concentration and the data were simultaneously fitted to a simple competition model using Dynafit61 as described.62 Quin-2 experiments were carried as described previously63 with ZnSO4 titrated into a mixture of 1.7 µM CzrA monomer and 1.5 µM quin-2 in Buffer P containing 0.1 mM TCEP. The presence of TCEP does not interfere with Zn(II) binding in these experiments.64
Zinc Binding Experiments for all other mutant CzrAs
Briefly, mag-fura-2 Zn(II) binding competition assays were carried out in 10 mM Hepes, 400 mM NaCl, pH 7.0, 10–15 µM protein dimer, 10–15 µM mag-fura-2, using KZn=5.0 × 107 M−1 29. Under these conditions mag-fura-2 shows no Zn(II) binding competition with CzrA, and therefore only a lower limit is reported. Co(II) was also titrated into all CzrAs until metal binding saturation and the optical spectrum recorded to confirm the presence of a tetrahedral metal coordination environment.
Fluorescence Anisotropy-based DNA Binding Experiments and ΔGc calculations
The DNA binding affinities of H96C and H97MeH were measured in 10 mM Hepes, 0.40 M NaCl, 2 mM DTT, pH 7.0 with 10 µM Zn(II) or 1 mM EDTA present in the solution. 4 nM fluorescein labeled 28 bp native CzrO DNA-operator with the 12-2-12 inverted repeat underlined was used (5’-FL-TAATATATGAACAAATATTCA GATGAAA-3’) (FL, fluorescein). For the apo-CzrA–DNA binding experiments the data were fit with Dynafit61 using a dimer linkage 1:1 dimer:DNA binding model15 with the dimerization constant fixed at Kdimer=1.7×105 M−1.29 The initial anisotropy was fixed to the measured value (ro) for the free DNA, with rcomplex, the anisotropy of the protein:DNA complex, optimized during a nonlinear least squares fit (see Supplementary Figure 6 for Dynafit61 script file). For other mutant CzrAs, the same 28-bp oligonucleotide was used at 10 nM concentration with the data fit in the same way.29 All Zn(II)-bound experiments employed protein stocks that were preloaded 1:1 with Zn(II) as titrant, with an additional 3 µM Zn(II) in the fluorescence cuvette in a buffer containing 10 mM Hepes, 0.40 M NaCl, pH 7.0 unless otherwise noted, and data were fit in the same manner with Kdimer=4.5×105 M−1.29 ΔGc was calculated from ΔGc=−RTln(KZn/Kapo) for wild-type, H96C and H97MeH CzrAs at 0.40 M NaCl (see Table 1), with the error in ΔGc propagated from the square root of the sum of the squares of the standard deviation of the mean value of Kapo and KZn obtained from two or more experiments. For all other CzrA mutants, Kapo at 0.23 M NaCl was obtained via linear extrapolation41 of a plot of log Kapo versus log [NaCl] for wild-type and V66A/L68V CzrAs with Kapo measured at various [NaCl] (Supplementary Table 2; Supplementary Figure 7). This gave Kapo values of 1.3×1013 M−1 and 2.9×1012 M−1 for wild-type and V66A/L68V CzrAs, respectively, at 0.23 M NaCl. KZn was measured by direct titration at 0.23 M NaCl (see Fig. 1d) and ΔGc was then calculated from, ΔGc=−RTln(KZn/Kapo). In order to estimate the associated error on ΔGc, minimal and maximal ΔGc values, ΔGc,min and ΔGc,min, respectively, were calculated by first determining Kapomin and Kapomax by incorporating the associated errors on the slope and y-intercept obtained from the linear fit of log Kapo versus log [NaCl] for wild-type and V66A/L68V CzrAs (see Supplementary Figure 7). KZnmin and KZnmax were determined by using the standard error of the fit giving ΔGc,min= −RTln(KZnmax/Kapomin) and ΔGc,max = −RTln(KZnmin/Kapomax). The standard error reported for ΔGc, is the average error of the difference of ΔGcmax minus ΔGc and ΔGc minus ΔGcmin for wild-type and V66A/L68V CzrAs. For all other single mutants, Kapo was calculated as the average value of the extrapolated values for Kapo for wild-type and V66A/L68V CzrAs at 0.23 M NaCl (8.0 ×1012 M−1). ΔGc and the associated error was then calculated in the same manner, except Kapomin= Kapo,V66A/L68V and Kapomax=Kapo, WT.
NMR spectroscopy
NMR spectra were acquired on a Varian VNMRS 600 MHz spectrometer equipped with a cryoprobe in the METACyt Biomolecular NMR Laboratory at Indiana University. NMR samples contained ≈0.25 mM 15N-labeled H96C CzrA or 0.07 mM 15N-labeled H97MeH CzrA in 10 mM d13-MES, 50 mM NaCl and 2 mM DTT with or without 1.1 monomer mol equiv of Zn(II) added (pH 6.0). 1H–15N HSQC or TROSY spectra were acquired at 40 °C as described previously.35 All spectra were processed and analyzed using NMRPipe and SPARKY with resonance assignments made by inspection. Uniformly 13C, 15N-labeled V66A/L68V CzrA was prepared as previously described for wild-type CzrA, and residue-specific backbone assignments of Zn2 V66A/L68V CzrA were obtained in 10 mM d13-MES, 50 mM NaCl, pH 6.0 using standard triple resonance methods.
X-ray crystallography
V66A/L68V CzrA was extensively dialyzed into 10 mM Hepes, 50 mM NaCl, pH 7.0. The calculated protein concentration after dialysis was 460 µM. The protein stock was loaded 1:1 with Zn(II), and crystallized under conditions of 100 mM CHES (pH 9.5), 200 mM NaCl, 10% PEG-8000 (Wizard I, Emerald Biosystems) by hanging drop diffusion at 20 °C. Diffraction data were collected at −160 °C on an R-AXIX IV+ detector at Indiana University. All data were processed with HKL2000,68 and phase calculations were performed using the PHENIX AutoMR module.69 The Zn(II)-bound wild-type CzrA structure was used as a molecular replacement model,28 and an initial refinement model was produced using the PHENIX AutoBuild module.69 Model building was conducted using Coot70 and subsequent refinement models in PHENIX (see Supplementary Table 3 for structure statistics).
Isothermal titration calorimetry
ITC experiments were carried out using a MicroCal VP-ITC calorimeter using 1.61 mM Zn(II) as titrant in the syringe and solution conditions of 50 mM Hepes, 3.0 mM NTA (Zn(II)→CzrA) or 1.0 mM NTA (Zn(II)→CzrA•CzrO) as a Zn(II) competitor, 0.40 M NaCl, pH 7.0, 25.0 °C, 30–50 µM protein dimer, or 38 µM complex. A self-complementary 28mer DNA was synthesized (MerMade 4) based on the native czr operator sequence (5’- TAACATATGAACATATGTTCATATGTTA) annealed and purified as previously described.30 CzrA•CzrO complex was formed by mixing 38 µM CzrA dimer, and 41 µM CzrO. The Raw ITC data were integrated, concentration normalized, and plotted as heat vs. metal-protein ratio using Origin(r). All data were fit using the sequential two-site model included in the data analysis software provided by MicroCal. NTA-independent binding constants were determined by using methods previously described.30 The standard deviation (s.d.) of the mean values from multiple experiments is given for all thermodynamic parameters.
Collection of ArsR sequences and the statistical coupling analysis (SCA)
We started with sequences assigned to ArsR (PFAM accession number: PF01022) in the PFAM database (total 10519 sequences), and selected ArsR sequences of typical lengths (90–140 aa) that match to PF01022 with significant E-value (<1e-10) for the SCA analysis. Selection resulted in 3000 non-redundant ArsR sequences. MUSCLE71 was used to prepare the multiple alignment of the selected ArsR sequences, which was used as input for the SCA Toolbox 5.0 (downloaded from http://systems.swmed.edu/rr_lab). Details of the characteristics of this MSA are shown in Supplemental Figure 8. The input multiple sequence alignment was truncated to sequence positions with % gap frequency no greater than 20%, so that only largely non-gapped positions were used for the co-evolution analysis. Outputs from the SCA analysis include the conservation scores of different positions, measured as the Kullback-Leibler relative entropy, and a positional correlation matrix, which quantitatively indicates the correlated evolution of all pairs of positions in the alignment, with larger numbers indicating stronger coupling. The positional correlation matrix was further analyzed using the eigenvalue decomposition approach available in SCA toolbox. Examination of the top eigenmodes revealed a single sector72 in CzrA (106 residues) consisting 17 co-evolving, physically connected, residues: K21, A22, D25, Y26, L29, L35, S41, V42, G43, Q53, Q59, H67, V69, K72, G75, S77, Y80.
Supplementary Material
Highlights.
>The molecular basis of allosteric regulation or linkage remains a subject of intense interest. >A zinc-regulated repressor, CzrA, is used to investigate the underpinnings of allostery. >Selective excision of a quaternary structural H-bond nearly blocks linkage in CzrA. >Introduction of a “cavity” just below this H-bond also compromises linkage in the absence of a global structural change. >A statistical coupling analysis reveals a sector that links the DNA-binding and zinc-sensing sites through an allosteric “hot-spot” defined by cavity residues.
Acknowledgements
This work was supported by grants from the National Institutes of Health to D.P.G. (GM042569) and C.E.D. III (GM094472). We thank Drs. A. Arunkumar, X. Kong and D. Ma for help in acquiring some of the NMR spectra presented here and Dr. Randy Arnold for assistance in analyzing MS/MS data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession Codes
The coordinates and structure factors for Zn2 V66A/L68V CzrA have been deposited in the Protein Data Bank under accession code 4GGG.
Supplementary Data
Supplementary data to this article, including Supplementary Methods, Supplementary Tables 1–4, and Supplementary Figs. 1–8, can be found online at http://dx.doi.org/10.1016/j.jmb
References
- 1.Reinhart GD. Quantitative analysis and interpretation of allosteric behavior. Methods Enzymol. 2004;380:187–203. doi: 10.1016/S0076-6879(04)80009-0. [DOI] [PubMed] [Google Scholar]
- 2.Toncrova H, McLeish TC. Substrate-modulated thermal fluctuations affect long-range allosteric signaling in protein homodimers: exemplified in CAP. Biophys J. 2010;98:2317–2326. doi: 10.1016/j.bpj.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smock RG, Gierasch LM. Sending signals dynamically. Science. 2009;324:198–203. doi: 10.1126/science.1169377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monod J, Wyman J, Changeux JP. On the Nature of Allosteric Transitions: A Plausible Model. J Mol Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 5.Koshland DE, Jr, Nemethy G, Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966;5:365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- 6.Henzler-Wildman KA, Lei M, Thai V, Kerns SJ, Karplus M, Kern D. A hierarchy of timescales in protein dynamics is linked to enzyme catalysis. Nature. 2007;450:913–916. doi: 10.1038/nature06407. [DOI] [PubMed] [Google Scholar]
- 7.Henzler-Wildman KA, Thai V, Lei M, Ott M, Wolf-Watz M, Fenn T, et al. Intrinsic motions along an enzymatic reaction trajectory. Nature. 2007;450:838–844. doi: 10.1038/nature06410. [DOI] [PubMed] [Google Scholar]
- 8.Henzler-Wildman K, Kern D. Dynamic personalities of proteins. Nature. 2007;450:964–972. doi: 10.1038/nature06522. [DOI] [PubMed] [Google Scholar]
- 9.Popovych N, Sun S, Ebright RH, Kalodimos CG. Dynamically driven protein allostery. Nat Struct Mol Biol. 2006;13:831–838. doi: 10.1038/nsmb1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luque I, Leavitt SA, Freire E. The linkage between protein folding and functional cooperativity: two sides of the same coin? Annu Rev Biophys Biomol Struct. 2002;31:235–256. doi: 10.1146/annurev.biophys.31.082901.134215. [DOI] [PubMed] [Google Scholar]
- 11.England JL. Allostery in protein domains reflects a balance of steric and hydrophobic effects. Structure. 2011;19:967–975. doi: 10.1016/j.str.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Schrank TP, Bolen DW, Hilser VJ. Rational modulation of conformational fluctuations in adenylate kinase reveals a local unfolding mechanism for allostery and functional adaptation in proteins. Proc Natl Acad Sci U S A. 2009;106:16984–16989. doi: 10.1073/pnas.0906510106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu T, Ramesh A, Ma Z, Ward SK, Zhang L, George GN, et al. CsoR is a novel Mycobacterium tuberculosis copper-sensing transcriptional regulator. Nat Chem Biol. 2007;3:60–68. doi: 10.1038/nchembio844. [DOI] [PubMed] [Google Scholar]
- 14.Ma Z, Cowart DM, Scott RA, Giedroc DP. Molecular insights into the metal selectivity of the copper(I)-sensing repressor CsoR from Bacillus subtilis. Biochemistry. 2009;48:3325–3334. doi: 10.1021/bi900115w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arunkumar AI, Campanello GC, Giedroc DP. Solution structure of a paradigm ArsR family zinc sensor in the DNA-bound state. Proc Natl Acad Sci U S A. 2009;106:18177–18182. doi: 10.1073/pnas.0905558106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guerra AJ, Dann CE, Giedroc DP. Crystal Structure of the Zinc-Dependent MarR Family Transcriptional Regulator AdcR in the Zn(II)-Bound State. J Am Chem Soc. 2011;133:19614–19617. doi: 10.1021/ja2080532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee CW, Chakravorty DK, Chang FM, Reyes-Caballero H, Ye Y, Merz KM, Jr, et al. Solution structure of Mycobacterium tuberculosis NmtR in the apo state: insights into Ni(II)-mediated allostery. Biochemistry. 2012;51:2619–2629. doi: 10.1021/bi3001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi W, Wu J, Rosen BP. Identification of a putative metal binding site in a new family of metalloregulatory proteins. Journal of biological chemistry. 1994;269:19826–19829. [PubMed] [Google Scholar]
- 19.Morby AP, Turner JS, Huckle JW, Robinson NJ. SmtB is a metal-dependent repressor of the cyanobacterial metallothionein gene smtA: identification of a Zn inhibited DNA-protein complex. Nucleic Acids Res. 1993;21:921–925. doi: 10.1093/nar/21.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell DR, Chapman KE, Waldron KJ, Tottey S, Kendall S, Cavallaro G, et al. Mycobacterial cells have dual nickel-cobalt sensors: sequence relationships and metal sites of metal-responsive repressors are not congruent. Journal of biological chemistry. 2007;282:32298–32310. doi: 10.1074/jbc.M703451200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osman D, Cavet JS. Bacterial metal-sensing proteins exemplified by ArsR-SmtB family repressors. Nat Prod Rep. 2010;27:668–680. doi: 10.1039/b906682a. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Kendall J, Cavet JS, Giedroc DP. Elucidation of the functional metal binding profile of a Cd(II)/Pb(II) sensor CmtR(Sc) from Streptomyces coelicolor. Biochemistry. 2010;49:6617–6626. doi: 10.1021/bi100490u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma Z, Jacobsen FE, Giedroc DP. Coordination chemistry of bacterial metal transport and sensing. Chem Rev. 2009;109:4644–4681. doi: 10.1021/cr900077w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palm GJ, Khanh Chi B, Waack P, Gronau K, Becher D, Albrecht D, et al. Structural insights into the redox-switch mechanism of the MarR/DUF24-type regulator HypR. Nucleic Acids Research. 2012;40:4178–4192. doi: 10.1093/nar/gkr1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guimaraes BG, Barbosa RL, Soprano AS, Campos BM, de Souza TA, Tonoli CC, et al. Plant pathogenic bacteria utilize biofilm growth-associated repressor (BigR), a novel winged-helix redox switch, to control hydrogen sulfide detoxification under hypoxia. J Biol Chem. 2011;286:26148–26157. doi: 10.1074/jbc.M111.234039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuroda M, Hayashi H, Ohta T. Chromosome-determined zinc-responsible operon czr in Staphylococcus aureus strain 912. Microbiol Immunol. 1999;43:115–125. doi: 10.1111/j.1348-0421.1999.tb02382.x. [DOI] [PubMed] [Google Scholar]
- 27.VanZile ML, Chen X, Giedroc DP. Structural characterization of distinct alpha3N and alpha5 metal sites in the cyanobacterial zinc sensor SmtB. Biochemistry. 2002;41:9765–9775. doi: 10.1021/bi0201771. [DOI] [PubMed] [Google Scholar]
- 28.Eicken C, Pennella MA, Chen X, Koshlap KM, VanZile ML, Sacchettini JC, et al. A metal-ligand-mediated intersubunit allosteric switch in related SmtB/ArsR zinc sensor proteins. J Mol Biol. 2003;333:683–695. doi: 10.1016/j.jmb.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Pennella MA, Shokes JE, Cosper NJ, Scott RA, Giedroc DP. Structural elements of metal selectivity in metal sensor proteins. Proc Natl Acad Sci U S A. 2003;100:3713–3718. doi: 10.1073/pnas.0636943100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grossoehme NE, Giedroc DP. Energetics of allosteric negative coupling in the zinc sensor S. aureus CzrA. J Am Chem Soc. 2009;131:17860–17870. doi: 10.1021/ja906131b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chakravorty DK, Wang B, Lee CW, Giedroc DP, Merz KM., Jr Simulations of allosteric motions in the zinc sensor CzrA. J Am Chem Soc. 2012;134:3367–3376. doi: 10.1021/ja208047b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mulder FA, Hon B, Mittermaier A, Dahlquist FW, Kay LE. Slow internal dynamics in proteins: application of NMR relaxation dispersion spectroscopy to methyl groups in a cavity mutant of T4 lysozyme. J Am Chem Soc. 2002;124:1443–1451. doi: 10.1021/ja0119806. [DOI] [PubMed] [Google Scholar]
- 33.Lockless SW, Ranganathan R. Evolutionarily conserved pathways of energetic connectivity in protein families. Science. 1999;286:295–299. doi: 10.1126/science.286.5438.295. [DOI] [PubMed] [Google Scholar]
- 34.Suel GM, Lockless SW, Wall MA, Ranganathan R. Evolutionarily conserved networks of residues mediate allosteric communication in proteins. Nat Struct Biol. 2003;10:59–69. doi: 10.1038/nsb881. [DOI] [PubMed] [Google Scholar]
- 35.Pennella MA, Arunkumar AI, Giedroc DP. Individual metal ligands play distinct functional roles in the zinc sensor Staphylococcus aureus CzrA. J Mol Biol. 2006;356:1124–1136. doi: 10.1016/j.jmb.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 36.Ma Z, Cowart DM, Ward BP, Arnold RJ, DiMarchi RD, Zhang L, et al. Unnatural amino acid substitution as a probe of the allosteric coupling pathway in a mycobacterial Cu(I) sensor. J Am Chem Soc. 2009;131:18044–18045. doi: 10.1021/ja908372b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hackeng TM, Griffin JH, Dawson PE. Protein synthesis by native chemical ligation: expanded scope by using straightforward methodology. Proc Natl Acad Sci U S A. 1999;96:10068–10073. doi: 10.1073/pnas.96.18.10068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee S, Arunkumar AI, Chen X, Giedroc DP. Structural insights into homo- and heterotropic allosteric coupling in the zinc sensor S. aureus CzrA from covalently fused dimers. J Am Chem Soc. 2006;128:1937–1947. doi: 10.1021/ja0546828. [DOI] [PubMed] [Google Scholar]
- 39.Arunkumar AI, Pennella MA, Kong X, Giedroc DP. Resonance assignments of the metal sensor protein CzrA in the apo-. Zn2- and DNA-bound (42 kD) states. Biomol NMR Assign. 2007;1:99–101. doi: 10.1007/s12104-007-9027-y. [DOI] [PubMed] [Google Scholar]
- 40.Fenton AW. Allostery: an illustrated definition for the 'second secret of life'. Trends Biochem Sci. 2008;33:420–425. doi: 10.1016/j.tibs.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Record MT, Jr, Ha JH, Fisher MA. Analysis of equilibrium and kinetic measurements to determine thermodynamic origins of stability and specificity and mechanism of formation of site-specific complexes between proteins and helical DNA. Methods Enzymol. 1991;208:291–343. doi: 10.1016/0076-6879(91)08018-d. [DOI] [PubMed] [Google Scholar]
- 42.Busenlehner LS, Weng TC, Penner-Hahn JE, Giedroc DP. Elucidation of primary (a3N) and vestigial (a5) heavy metal-binding sites in Staphylococcus aureus pI258 CadC: evolutionary implications for metal ion selectivity of ArsR/SmtB metal sensor proteins. J. Mol. Biol. 2002;319:685–701. doi: 10.1016/S0022-2836(02)00299-1. [DOI] [PubMed] [Google Scholar]
- 43.Ma Z, Jacobsen FE, Giedroc DP. Coordination chemistry of bacterial metal transport and sensing. Chem Rev. 2009;109:4644–4681. doi: 10.1021/cr900077w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Hemmingsen L, Giedroc DP. Structural and functional characterization of Mycobacterium tuberculosis CmtR, a PbII/CdII-sensing SmtB/ArsR metalloregulatory repressor. Biochemistry. 2005;44:8976–8988. doi: 10.1021/bi050094v. [DOI] [PubMed] [Google Scholar]
- 45.Banci L, Bertini I, Cantini F, Ciofi-Baffoni S, Cavet JS, Dennison C, et al. NMR structural analysis of cadmium sensing by winged helix repressor CmtR. Journal of biological chemistry. 2007;282:30181–30188. doi: 10.1074/jbc.M701119200. [DOI] [PubMed] [Google Scholar]
- 46.Tzeng SR, Kalodimos CG. Dynamic activation of an allosteric regulatory protein. Nature. 2009;462:368–372. doi: 10.1038/nature08560. [DOI] [PubMed] [Google Scholar]
- 47.Frederick KK, Marlow MS, Valentine KG, Wand AJ. Conformational entropy in molecular recognition by proteins. Nature. 2007;448:325–329. doi: 10.1038/nature05959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olsson TS, Ladbury JE, Pitt WR, Williams MA. Extent of enthalpy-entropy compensation in protein-ligand interactions. Protein Sci. 2011;20:1607–1618. doi: 10.1002/pro.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diehl C, Engstrom O, Delaine T, Hakansson M, Genheden S, Modig K, et al. Protein flexibility and conformational entropy in ligand design targeting the carbohydrate recognition domain of galectin-3. J Am Chem Soc. 2010;132:14577–14589. doi: 10.1021/ja105852y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Datta D, Scheer JM, Romanowski MJ, Wells JA. An allosteric circuit in caspase-1. J Mol Biol. 2008;381:1157–1167. doi: 10.1016/j.jmb.2008.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen A, Lupardus PJ, Gersch MM, Puri AW, Albrow VE, Garcia KC, et al. Defining an allosteric circuit in the cysteine protease domain of Clostridium difficile toxins. Nat Struct Mol Biol. 2011;18:364–371. doi: 10.1038/nsmb.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wrabl JO, Gu J, Liu T, Schrank TP, Whitten ST, Hilser VJ. The role of protein conformational fluctuations in allostery, function, and evolution. Biophys Chem. 2011;159:129–141. doi: 10.1016/j.bpc.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hilser VJ, Wrabl JO, Motlagh HN. Structural and energetic basis of allostery. Annu Rev Biophys. 2012;41:585–609. doi: 10.1146/annurev-biophys-050511-102319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.del Sol A, Tsai CJ, Ma B, Nussinov R. The origin of allosteric functional modulation: multiple pre-existing pathways. Structure. 2009;17:1042–1050. doi: 10.1016/j.str.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hatley ME, Lockless SW, Gibson SK, Gilman AG, Ranganathan R. Allosteric determinants in guanine nucleotide-binding proteins. Proc Natl Acad Sci U S A. 2003;100:14445–14450. doi: 10.1073/pnas.1835919100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smock RG, Rivoire O, Russ WP, Swain JF, Leibler S, Ranganathan R, et al. An interdomain sector mediating allostery in Hsp70 molecular chaperones. Mol Syst Biol. 2010;6:414. doi: 10.1038/msb.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ye J, Kandegedara A, Martin P, Rosen BP. Crystal structure of the Staphylococcus aureus pI258 CadC Cd(II)/Pb(II)/Zn(II)-responsive repressor. J Bacteriol. 2005;187:4214–4221. doi: 10.1128/JB.187.12.4214-4221.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.VanZile ML, Chen X, Giedroc DP. Allosteric negative regulation of smt O/P binding of the zinc sensor, SmtB, by metal ions: a coupled equilibrium analysis. Biochemistry. 2002;41:9776–9786. doi: 10.1021/bi020178t. [DOI] [PubMed] [Google Scholar]
- 59.Jefferson JR, Hunt JB, Ginsburg A. Characterization of indo-1 and quin-2 as spectroscopic probes for Zn2(+)-protein interactions. Anal Biochem. 1990;187:328–336. doi: 10.1016/0003-2697(90)90465-l. [DOI] [PubMed] [Google Scholar]
- 60.Walkup GK, Imperiali B. Fluorescent chemosensors for divalent zinc based on zinc finger domains. Enhanced oxidative stability, metal binding affinity, and structural and functional characterization. J Am Chem Soc. 1997;119:3443–3450. [Google Scholar]
- 61.Kuzmic P. Program DYNAFIT for the analysis of enzyme kinetic data: application to HIV proteinase. Anal Biochem. 1996;237:260–273. doi: 10.1006/abio.1996.0238. [DOI] [PubMed] [Google Scholar]
- 62.Lee YH, Dorwart MR, Hazlett KR, Deka RK, Norgard MV, Radolf JD, et al. The crystal structure of Zn(II)-free Treponema pallidum TroA, a periplasmic metalbinding protein, reveals a closed conformation. J Bacteriol. 2002;184:2300–2304. doi: 10.1128/JB.184.8.2300-2304.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reyes-Caballero H, Guerra AJ, Jacobsen FE, Kazmierczak KM, Cowart D, Koppolu UM, et al. The metalloregulatory zinc site in Streptococcus pneumoniae AdcR, a zinc-activated MarR family repressor. J Mol Biol. 2010;403:197–216. doi: 10.1016/j.jmb.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma Z, Gabriel SE, Helmann JD. Sequential binding and sensing of Zn(II) by Bacillus subtilis Zur. Nucleic Acids Research. 2011 doi: 10.1093/nar/gkr625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 66.Goddard TD, Kneller DG. Sparky 3. San Francisco: University of California; [Google Scholar]
- 67.Lee CW, Giedroc DP. (1)H, (13)C, and (15)N resonance assignments of NmtR, a Ni(II)/Co(II) metalloregulatory protein of Mycobacterium tuberculosis. Biomol NMR Assign. 2012 doi: 10.1007/s12104-012-9397-7. [DOI] [PubMed] [Google Scholar]
- 68.Otwinowski Z. Oscillation data reduction program. In: Sawyer L, Isaacs N, Bailey S, editors. Proceedings of the CCP4 study weekend: data collection and processing. Warrington, UK: SERC Daresbury Laboratory; 1993. pp. 56–62. [Google Scholar]
- 69.Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 71.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Halabi N, Rivoire O, Leibler S, Ranganathan R. Protein sectors: evolutionary units of three-dimensional structure. Cell. 2009;138:774–786. doi: 10.1016/j.cell.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Giedroc DP, Arunkumar AI. Metal sensor proteins: nature's metalloregulated allosteric switches. Dalton Trans. 2007;29:3107–3120. doi: 10.1039/b706769k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.