Abstract
To image implant-surrounding activated macrophages, a macrophage-specific PET probe was prepared by conjugating folic acid (FA) and 2,2′,2″,2‴-(1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrayl) tetracetic acid (DOTA) to polyethylene glycol (PEG) and then labeling the conjugate with Ga-68. In vivo PET imaging evaluations demonstrate that the probe is able to detect foreign body reactions, and more importantly, quantify the degree of inflammatory responses to an implanted medical device. These results were further validated by histological analysis.
Keywords: Folic acid, Biomaterial implant, Inflammation, Biocompatibility, PET imaging
Implantable medical devices play an important role in the practice of contemporary medicine. Unfortunately, medical device-associated complications, such as bleeding, inflammation, infection and fibrosis, may cause implant failure. 1 In fact, many medical implants are surrounded by a substantial number of phagocytes, including polymorphonuclear leukocytes and macrophages [MΦ]/ monocytes.1-3 In addition, inflammatory responses and products have been shown to cause the degradation and failure of many implantable devices and the dissolution of surrounding tissue, including surgical mesh, degradable implants/scaffolds, soft tissue filler, encapsulated cell implants, implantable sensors, temporomandibular, and other joint implants.4-11 It is well established that the accumulation of macrophages serves as a good assessment for the extent of foreign body reactions to medical implants.1,12 Although conventional methods like tissue biopsy and histological evaluation have been used for assessing implant-associated MΦ responses, they are invasive, semi-quantitative, time-consuming and not applicable for continuous real time monitoring. Therefore, there is a need for the development of new tools and methods for detecting and monitoring MΦ responses at the implant site in the clinical setting.
Recent development of in vivo imaging systems has attracted great interest in different research fields, including cancer, neurological disorders, and inflammatory diseases. Most recently, research efforts have been expended on the development of optical imaging probes for non-invasive and real-time monitoring of macrophage-based inflammatory responses in vivo. For instance, antibody-based probes (FITC-mAb F4/80) have been tested for their ability to detect MΦ accumulation surrounding biomaterial implants, although they are limited by sensitivity.13 To improve sensitivity, near-infrared imaging probes were developed to target activated MΦ via folate receptors.14 However, limited penetration depth of light, as well as attenuation and scattering of light by tissues, hinder the optical imaging probes from eventual clinical applications.
To address these issues, in this study, we synthesized a macrophage-specific PET probe to assess device-mediated inflammatory responses. PET imaging was chosen since it is a quantitative, highly sensitive, tomographic clinical imaging modality without limitation of penetration depth.15 In addition, 18-fluoro-2-deoxyglucose (FDG) PET probes have been used to detect persistent foreign body reactions induced by mesh prostheses.16 Given the fact that activated MΦs possess a large number of folic acid (FA) receptors,14 we chose FA as the targeting ligand for the PET imaging probe design. To improve the probe’s water solubility and prolong its circulation time in the blood, polyethylene glycol (PEG) was introduced into the probe as described in previous studies.17,18 Basically, the probe is composed of three structural components: MΦ-targeting FA, PEG spacer, and radioisotope Ga-68 moiety. To the best of our knowledge, the application of macrophage-specific PET imaging in detection and assessment of foreign body reactions in vivo has not been reported before.
The MΦ -specific PET probe (68Ga-DOTA-PEG-FA) was prepared according to a modified procedure (Fig.1).19,20 It should be noted that although α- and γ-carboxylic acid groups of folic acid could be activated, the reactivity of the γ-carboxylic acid group to an amine group is much higher.21 Thus, conjugation of folic acid into PEG should be generated predominantly via the γ-carboxylic acid. The conjugate (DOTA-PEG-FA) was first synthesized. FTIR (Fourier transform infrared spectroscopy) spectrum of the conjugate shows characteristic IR absorption peaks of FA at 1600, 1695, and 1498 cm−1, indicating conjugation of FA moieties into PEGs.22 Average molecular weight of the conjugate was measured to be around 6,000 by using MALDI-TOF MS (Matrix assisted laser desorption ionization time-of-flight mass spectrometry). The PET probe was then obtained by radio-labeling the conjugate with radioisotope (68Ga). The radiochemical yield was 78% as determined by using radio-HPLC. After purification, the radiochemical purity was higher than 99% and the specific activity was 63 GBq/μmol. 68Ga-DOTA-PEG-FA had the same retention time as its respective conjugate at 20.5 min (Fig. 2).
Fig. 1.
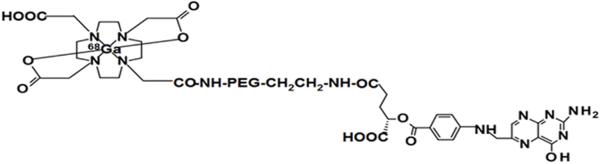
Chemical structure of the probe (68Ga-DOTA-PEG-FA).
Fig. 2.

HPLC analysis of 68Ga-DOTA-PEG-FA (upper, radio peak) and DOTA-PEG-FA (lower, UV peak at 254 nm).
The freshly synthesized 68Ga-DOTA-PEG-FA probe was then evaluated in two animal models of medical implant-triggered inflammation.23 Poly-lactic acid (PLA) and poly (N-isopropylacrylamide) (PNIPAM) particles were prepared as previously described,14 and used as model implantable devices. One day after subcutaneous implantation, 68Ga-DOTA-NH-PEG-NH-FA was injected intravenously for PET imaging. Figure 3(a) shows the fused transaxial and coronal imaging for the material-implanted mice. The PET signals were higher from the PLA sites than from the PNIPAM sites. Quantitative imaging analysis shows that the standardized uptake value (SUV) of the PLA site was 1.8 times higher than that of the PNIPAM site (Fig.3(b)), indicating that the PET probe can detect implant-triggered acute inflammation. More importantly, the degree of implant-mediated acute inflammatory responses can be quantitatively assessed by the PET probe enabled noninvasive imaging method. Histological analyses (H&E stain: hematoxylin and eosin stain) of the implant-surrounding tissues demonstrate that the PLA implant indeed triggered more inflammatory cells than PNIPAM (Fig.3(c)).
Fig. 3.
In the subcutaneous implantation model, mice were implanted with either PLA or PNIPAM particles at 24 hours prior to MΦ probe administration. After probe injection for 3 hours, the animals’ whole body images were taken. (a) Fused transaxial and coronal slices for PLA and PNIPAM implants. (b) The SUV values were calculated for PLA and PNIPAM implants. (c) Optical images of H&E stained tissue slices containing PLA- and PNIPAM-implants.
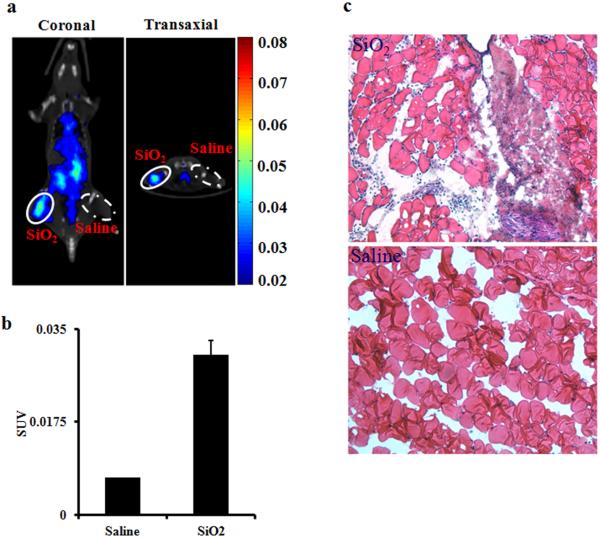
Since many medical devices, such as joint implants, are implanted in the submuscular space, the validation of the PET probe was further assessed using the SiO2 particles intramuscular implantation model. The micro CT (Computed tomography) and PET images were captured 3 hour after probe injection. As shown in Fig.4(a), the SiO2 implant site was clearly visualized by the probe, while the control site was barely visible. Quantitative imaging analysis indicated a 4-fold uptake increase at the SiO2 implant site compared to the control site (Fig.4 (b)). H&E staining revealed that more inflammatory cells were recruited to the SiO2 (Fig. 4 (c)).
Fig. 4.
In the intramuscular implantation model, mice were administered with either SiO2 particles or saline (as control) at 24 hours prior to MΦ probe injection. After probe injection for 3 hours, whole body images of animals were taken. (a) Fused transaxial and coronal slices for SiO2 implant and saline. (b) The SUV values were calculated for SiO2 implant and saline. (c) Optical images of H&E stained tissue slices of SiO2- and saline implantation sites.
In summary, the PET imaging studies have shown that 68Ga-DOTA-PEG-FA is an effective PET probe for detection of implant-associated MΦ and associated foreign body reactions. These results suggest that the imaging probe can potentially be translated for detecting immune responses to various medical implants, including surgical mesh, degradable implants/scaffolds, soft tissue encapsulated cell implants, implantable sensors, and temporomandibular and other joint implants.
Acknowledgments
The research was supported by NIH Grants EB007271, EB014404 (L.T.) and U24 CA126608 (X.S.). The authors acknowledge the generous support of a private donor that allowed the purchase of the Siemens Inveon PET-CT Multi-modality System.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Tang L, Hu W. Expet. Rev. Med. Dev. 2005;2:493. doi: 10.1586/17434440.2.4.493. [DOI] [PubMed] [Google Scholar]
- 2.Weng H, Zhou J, Tang L, Hu Z. J. Biomater. Sci., Polym. Ed. 2004;15:1167. doi: 10.1163/1568562041753106. [DOI] [PubMed] [Google Scholar]
- 3.Thevenot P, Hu W, Tang L. Curr. Top. Med. Chem. 2008;8:270. doi: 10.2174/156802608783790901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang WW, Su SH, Eberhart RC, Tang L. J. Biomed. Mater. Res. A. 2007;82:492. doi: 10.1002/jbm.a.31175. [DOI] [PubMed] [Google Scholar]
- 5.Sailes FC, Walls J, Guelig D, Mirzabeigi M, Long WD, Crawford A, Moore JH, Jr., Copit SE, Tuma GA, Fox J. Ann. Plast. Surg. 2010;64:696. doi: 10.1097/SAP.0b013e3181dc8409. [DOI] [PubMed] [Google Scholar]
- 6.Requena L, Requena C, Christensen L, Zimmermann US, Kutzner H, Cerroni L. J. Am. Acad. Dermatol. 2011;64:1. doi: 10.1016/j.jaad.2010.02.064. [DOI] [PubMed] [Google Scholar]
- 7.Piemonti L, Guidotti LG, Battaglia M. Adv. Exp. Med. Biol. 2010;654:725. doi: 10.1007/978-90-481-3271-3_32. [DOI] [PubMed] [Google Scholar]
- 8.Santos E, Zarate J, Orive G, Hernandez R, MPedraz JL. Adv. Exp. Med. Biol. 2010;670:5. doi: 10.1007/978-1-4419-5786-3_2. [DOI] [PubMed] [Google Scholar]
- 9.Morais JM, Papadimitrakopoulos F, Burgess D. J. AAPS J. 2010;12:188. doi: 10.1208/s12248-010-9175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouloux GF. J. Oral Maxillofac. Surg. 2009;67:2497. doi: 10.1016/j.joms.2009.04.103. [DOI] [PubMed] [Google Scholar]
- 11.Lidgren L. J. Bone Joint. Surg. Br. 2008;90:7. doi: 10.1302/0301-620X.90B1.19823. [DOI] [PubMed] [Google Scholar]
- 12.Tang L, Eaton JW. Mol. Med. 1999;5:351. [PMC free article] [PubMed] [Google Scholar]
- 13.Bratlie KM, Dang TT, Lyle S, Nahrendorf M, Weissleder R, Langer R, Anderson DG. PLoS One. 2010;5:e10032. doi: 10.1371/journal.pone.0010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou J, Tsai YT, Weng H, Baker DW, Tang L. Biomaterials. 2011;32:9383. doi: 10.1016/j.biomaterials.2011.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herschman HR. Curr. Opin. Immunol. 2003;15:378. doi: 10.1016/s0952-7915(03)00066-9. [DOI] [PubMed] [Google Scholar]
- 16.Aide N, Deux JF, Peretti I, Mabille L, Mandet J, Callard P, Talbot JN. AJR Am. J. Roentgenol. 2005;184:1172. doi: 10.2214/ajr.184.4.01841172. [DOI] [PubMed] [Google Scholar]
- 17.Blom E, Velikyan I, Estrada S, Hall H, Muhammad T, Ding C, Nair M, Långström B. Int. J. Clin. Exp. Med. 2012;5:165. [PMC free article] [PubMed] [Google Scholar]
- 18.Eder M, Krivoshein AV, Backer M, Backer JM, Haberkorn U, Eisenhut M. Nucl. Med. Biol. 2010;37:405. doi: 10.1016/j.nucmedbio.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Singh P, Gupta U, Asthana A, Jain NK. Bioconjugate Chem. 2008;19:2239. doi: 10.1021/bc800125u. [DOI] [PubMed] [Google Scholar]
- 20.Carboxyl acid of FA was activated with 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and N-Hydroxysuccinimide (NHS) (molar ratio of FA/EDC/NHS:1:2:2. The activated FA-NHS was incubated with heterobifunctional t-butoxycarbonyl-protected amine-PEG-amine (t-Boc-PEG-NH2) (Mw: 5000. JenKem Technology USA Inc., Allen, TX). After cleavage of the protected t-Boc group from FA-PEG-t-Boc by treating with trifluoroacetic acid (TFA), 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid 1-(2,5-dioxo-1-pyrrolidinyl) ester (DOTA-NHS. Macrocyclics, Inc., Dallas, TX) was chemically attached to the other end of the FA-PEG-NH2 to obtain DOTA-PEG-FA. DOTA-PEG-FA was purified by dialysis and further by HPLC (XTerra RP18 column, 250×10 mm) with a mobile phase gradient from 90% Solvent A (0.1% TFA in water) and 10% Solvent B (0.1% TFA in acetonitrile) to 100% Solvent B at 45 min at a flow rate of 4 mL/min. Purity of the DOTA-PEG-FA conjugates was more than 99%. The average molecular weight of FA-PEG-DOTA was determined by using MALDI-TOF MS (Matrix assisted laser desorption ionization time-of-flight mass spectrometry). The radiolabeling was carried out by the addition of 0.44 GBq of freshly eluted 68GaCl3 in 400μL of 0.6 M HCl solution to 3.79 μM of DOTA-PEG-FA in 880μL of 1 M 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) solution and then incubated at 75°C for 30 min. The radiochemical yield was determined using radio-HPLC. The purification of 68Ga-DOTA-PEG-FA was performed by a pre-activated Sep-Pak C-18 plus cartridge.
- 21.Wang S, Lee RJ, Mathias CJ, Green MA, Low PS. Bioconjugate Chem. 1996;7:56. doi: 10.1021/bc9500709. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Zhang J. J. Colloid Interface Sci. 2005;283:352. doi: 10.1016/j.jcis.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 23.In subcutaneous implantation model, both PLA and PNIPAM (100μL, 5% wt) were subcutaneously injected on the back of normal BALB/c mice. In the intramuscular implantation model, SiO2 particles (10 nm in diameter) were injected into the right thighs of mice, while the left thighs were treated with saline as a control. One day after implantation, 3.7 MBq of 68Ga-DOTA-PEG-FA was injected via the tail vein for PET imaging. The imaging studies were carried out in a Siemens Inveon Multimodality PET/CT system (Siemens Medical Solutions Inc., Knoxville, TN, USA) 3 hours after probe injection. Micro CT and PET images were reconstructed using Cobra Reconstruction Software, following Fourier Rebinning and Ordered Subsets Expectation Maximization 3D (OSEM3D) algorithms respectively. The microCT imaging was used to position the region of interest (ROI). Reconstructed CT and PET images were fused and analyzed using MATLAB software. Tracer SUVs were defined as the ratio of the total concentration of radiotracer in ROI to the injected dose normalized by the weight of the animal.