Abstract
Mediated learning is a unique cognitive phenomenon in which mental representations of physically absent stimuli enter into associations with directly-activated representations of physically present stimuli. Three experiments investigated the functional physiology of mediated learning involving the use of odor-taste associations. In Experiments 1a and 1b, basolateral amygdala lesions failed to attenuate mediated taste aversion learning. In Experiment 2, dorsal hippocampus inactivation impaired mediated learning, but left direct learning intact. Considered with past studies, the results implicate the dorsal hippocampus in mediated learning generally, and suggest a limit on the importance of the basolateral amygdala.
Keywords: mediated learning, dorsal hippocampus, basolateral amygdala, taste-aversion, olfactory learning
1. Introduction
Broadly, studies of associative learning and memory tend to focus on situations in which an innocuous stimulus is directly associated with a harmful or rewarding stimulus (i.e., direct learning). However, learning situations for humans often do not involve direct experience with a biologically-significant unconditioned stimulus (US; e.g., shock, food, etc…). Instead, symbols and surrogates are used to evoke and sometimes fabricate mental representations of real-world biologically significant stimuli. Understanding this cognitive process is important because it is critical for abstract learning, and a potential source of cognitive dysfunction in mental illness (McDannald et al., 2011; McDannald & Schoenbaum, 2009). As such, nonhuman animal models of representation-mediated learning are valuable for exploring the functional neurophysiology that underlies this unique class of learning.
Nonhuman (or nonverbal) animal models of mediated learning require subjects to learn about a stimulus in its absence, based on the activation of a robust mental representation of that stimulus (e.g., Cuevas, Rovee-Collier, & Learmonth, 2006; Dwyer, 1999; Holland, 1981, Holland, 1990). In a typical mediated-learning paradigm, a stimulus is paired with an unconditioned stimulus (US) serially or in a simultaneous compound. According to associative-learning theories the subsequent presentation of the CS alone elicits a robust representation of the US, which is the foundation of certain goal-directed forms of conditioned responding. If, during a presentation of the CS alone, a new US is presented that is likely to enter into an association with the absent US, but not the present cue, (i.e., selective associability; Garcia & Koelling, 1966), the absent US can form an association with the new US. This phenomenon has been observed by Holland (1981) as well as Dwyer (1999, 2001) in situations involving taste aversion. In their experiments, auditory and visual stimuli (Holland) or contexts (Dwyer) were initially paired with rewarding tastes. When the CSs were later paired with LiCl-induced illness, they served as surrogates for their associated taste stimuli. These CSs did not form any appreciable first-order association with an illness, but the associatively-activated taste representations did support taste-aversion learning.
The current studies were designed to investigate the brain structures involved in mediated learning of taste aversions. Based on its critical role in the representation of sensory aspects of gustatory rewards in behavioral paradigms (see Holland & Gallagher, 2004) such as responding to reinforcer devaluation (e.g., Hatfield, Han, Conley, Gallagher, & Holland, 1996), the effect of reinforcer-selective Pavlovian cues on instrumental performance (e.g., Corbit & Balleine, 2005), and the differential outcomes effect (e.g., Blundell, Hall, & Killcross, 2001), Dwyer and Killcross (2006) investigated the role of the basolateral amygdala (BLA) in mediated learning. They found that permanent lesions of the BLA attenuated mediated taste-aversion learning. Despite these precedents for involvement of the BLA in representational function in learning, we found no effects of BLA lesions in the following experiments. Thus, we examined the role of another region that has been implicated in mediated learning, the dorsal hippocampus (DH). Iordanova, Good, and Honey (2011) found that DH inactivation eliminated mediated fear learning, presumably because the task required hippocampal-dependant episodic-like memory (e.g., Fortin, Wright, & Eichenbaum, 2004). Because of the role of the DH in mnemonic function broadly, and in the formation of associations between discontiguous events specifically (e.g., Wallenstein, Eichenbaum, & Hasselmo, 1998), it is possible that the area is also important for mediated-learning processes that do not necessarily rely on episodic memory.
In our designs, the rats were first trained to drink two odor-taste compounds. The parameters for this treatment closely followed the methods of Saddoris, Holland, and Gallagher (2009) who found that this sort of training allows the odor to produce neural activity in the gustatory cortex that is similar to that produced by the taste itself, which might suggest a robust associatively-activated representation. After the odor-taste pairings, one of the odors was paired with an illness-inducing agent (LiCl). Finally, the animals were given consumption tests with the two tastes to determine whether the associatively-activated representation of the taste associate of that odor was associated with the illness. The basic designs of the experiments are depicted on Table 1.
Table 1.
Experimental Designs
| Learning | Phase 1 | Phase 2 | Taste Test | Odor Test | |
|---|---|---|---|---|---|
| Experiments 1a and 1b | Mediated | O1T1/O2T2 | O1→LiCl/O2- | T1/T2 | O1/O2 |
|
| |||||
| Experiment 2 | Direct | O1T1/O2T2 | Mus→T1→LiCl/Sal→T2→LiCl | T1/T2 | O1/O2 |
|
| |||||
| Mediated | O1T1 O2T2 |
Mus→O1→LiCl/Sal→O2→LiCl | T1/T2 | O1/O2 | |
Note. O1 and O2 = odors; T1 and T2 = nutritive tastes; LiCl = 20-ml/Kg i.p. injection of 0.15 M LiCl; Mus and Sal = muscimol and saline infusions, respectively.
Experiment 1a was designed to determine whether permanent excitotoxic lesions of the BLA would attenuate mediated learning. Experiment 1b investigated the same question under conditions in which the taste stimuli were more motivationally significant, which might be more likely to engage the BLA (e.g., Blundell, Hall, & Killcross, 2003). Toward this end, food access was restricted as well as water access, and the concentrations of rewarding solutions were increased. Experiment 2 tested the effect of temporarily inactivating the DH during the acquisition of direct and mediated taste aversion learning. Permanent lesions were not used in Experiment 2 because such lesions can augment latent inhibition of taste-aversion learning (e.g., Purves, Bonardi, & Hall, 1995; Reilly, Harley, & Revusky, 1993), which would be a potential problem for our designs because they inevitably involve preexposure of the to-be-conditioned taste cue. This was not a concern in Experiments 1a and 1b because permanent BLA lesions have not disrupted simple taste-aversion learning in outcome-devaluation studies from our laboratory, likely because the BLA lesions are more likely to hinder novel rather than familiar taste-aversion learning (St. Andre & Reilly, 2007). Furthermore, Iordanova et al. (2011) used a similar temporary inactivation manipulation in their study of mediated learning, strengthening the validity of any comparisons between the two findings.
2. Materials and methods
2.1. Subjects
The subjects were male Long-Evans rats (Charles River Laboratories, Raleigh, North Carolina), which weighed 352–493 g immediately before the onset of the experiments. In all experiments, the rats’ water access was restricted to a 10-min period in the morning, and a 1-hr access period in the evening (separated by 5–6 hrs). In Experiment 1b, food access was restricted to 2.5 hrs in the afternoon. These deprivation schedules were maintained for the duration of the experiments, although experimental manipulations replaced the morning access period. All procedures were approved by Johns Hopkins University Animal Care and Use Committee and the facility is accredited by the Association for the assessment and Accreditation of Laboratory Animal Care.
Some of the animals in Experiment 1a and 1b had previously participated in a Pavlovian conditioning experiment that involved exposure to an operant chamber, audiovisual stimuli, and food deprivation (Chang, McDannald, Wheeler, & Holland, 2012). However, they received no exposure to the contexts, odors, tastes, or LiCl used in this experiment.
2.2. Surgical procedures
All surgical procedures were performed with the subjects under isoflurane (Isovet; Mallinckrodt, Mundelein, IL) anesthesia. In Experiments 1a and 1b, BLA lesions were made using NMDA (Sigma-Aldrich, St. Louis, MO) at a concentration of 10.0 mg/ml in phosphate-buffered saline. NMDA was infused at a rate of 0.1 μl/min. Injections were made in two injection sites in each hemisphere, 2.8 mm posterior to bregma, 5.1 mm from the midline, and at 8.7 mm (0.16 μl) and 8.4 mm (0.08 μl) ventral from the skull surface at bregma. These microinjections were made using a 2.0-μl syringe (Hamilton, Reno, NV). During sham surgeries, a needle was placed in the BLA, but no injections were made. Thirteen of the subjects in Experiment 1a and 14 of the subjects in Experiment 1b received BLA lesions. Nine subjects in Experiment 1a and 8 subjects in Experiment 1b received sham lesions.
In Experiment 2, 18 subjects were implanted with guide cannulae (Plastics One, Roanoke, VA) that terminated immediately above the dorsal hippocampus. The cannulae were targeted 3.6 mm posterior to bregma and 2.5 mm lateral to the midline.
2.3. Materials
The subjects remained in their homecages for the experiments, but these cages were transferred to two separate rooms during odor exposure and test to prevent cross-contamination of odor exposure. The cages were placed on a table in both rooms, which were illuminated with fluorescent light. No effort was made to distinguish the rooms from each other, as the intention was that the subjects would associate the tastes with the odors more than the contexts. Sucrose and maltodextrin (M040, Grain Processing Corporation, Muscatine, IA) solutions served as T1 and T2, counterbalanced. In Experiments 1a and 2, both solutions were 8% (w/v), but this concentration was increased to 16% in Experiment 1b. Iso-amyl butyrate (International Flavors & Fragrances Inc., NY, NY) and hexenol, b, gamma (International Flavors & Fragrances Inc., NY, NY) were mixed in water (both 1.25% v/v) and used as O1 and O2, counterbalanced. The odors were presented on saturated filter paper (Pall Corp., Ann Arbor, MI) placed around the base of the drinking spouts, approximately 4 cm from the subjects’ snouts when drinking. A 20-ml/Kg injection of 0.15-M LiCl (Sigma-Aldrich, St. Louis, MO) was administered i.p. in order to induce illness. In Experiment 2, the GABAA agonist muscimol (1 mg/ml; Sigma-Aldrich, St. Louis, MO) dissolved in phosphate buffered saline was used to inactivate the DH.
2.4 Behavioral procedures for Experiments 1a and 1b
Before conditioning, the rats were acclimated to the rooms in which training occurred. All subjects received 10-min access to water in each room on two consecutive days. Over the following 6 days, odor-taste compound training occurred (Phase 1). On Days 3, 5, and 7, the subjects received 10-min access to T1 with O1 or T2 with O2. On Days 4, 6, and 8, subjects received 10-min access to the odor-taste compound they did not receive on the odd days. After Phase 1, subjects received discrimination training (Phase 2). On the first day of Phase 2, approximately half of the subjects from each group received 10-min access to O1 and a bottle filled with water, followed by an injection of LiCl 5 min after the odor was removed. The other half of the subjects received access to O2 with water, followed by nothing. On the next day, the subjects received the reverse treatment, so that all subjects experienced one O1-illness pairing. After Phase 2, subjects were given a recovery day in which they only received water in the colony room. This was followed by the first taste consumption test in which, all of the subjects received 10-min access to T1 or T2 in the colony room. On the subsequent day, subjects were tested with the other taste. In the final consumption test, they received an odor test that was identical to the Phase-2 training, except no LiCl was administered.
2.5. Behavioral procedures for Experiment 2
Experiment 2 differed from Experiments 1a and 1b in that it used a mixed design with temporary inactivation rather than permanent lesions. In order to assess the potentially divergent effects of DH inactivation on direct- and mediated-learning, half of the rats were assigned to a direct-learning group, whereas the other half were assigned to a mediated-learning group. In Phase 2, both odors were paired once with LiCl in the mediated-learning group, and both tastes were paired with LiCl in the direct-learning group. In both cases, fluid access was limited to 5 ml and exposure was extended to 15 min in order to ensure relatively equal consumption between the two groups (otherwise, rats in the direct-learning group would presumably consume more, because the palatable, nutritive tastes were preferred to the odor-water solutions). Additionally, subjects were given a recovery day between the two pairings. Approximately 15–30 min before one of the odor (O1) or taste (T1) pairings, rats were infused bilaterally with 0.5 μl of muscimol (1 mg/ml) over 2 min. This procedure has been shown in the past to effectively disrupt DH-dependent tasks such as context-dependent fear conditioning (Holt & Maren, 1999). Before the other pairing, vehicle was infused in the same fashion. To deliver these microinfusions, an internal cannula (Plastics One, Roanoke, VA) was attached to a 2-μl syringe and inserted so that it protruded 1 mm below the end of the guide cannula.
2.6. Histology
The subjects were perfused transcardially with 200 ml of 0.9% saline followed by 400 ml of 4% formaldehyde (Fisher Scientific, Fair Lawn, NJ). The brains were then removed and post-fixed for at least 48 hrs in a 4%-formaldehyde and 20%-sucrose solution. They were then sliced into 40-micron thick sections, mounted, and stained with a thionin solution.
3. Results
3.1. Experiment 1a
3.1.1. Histology
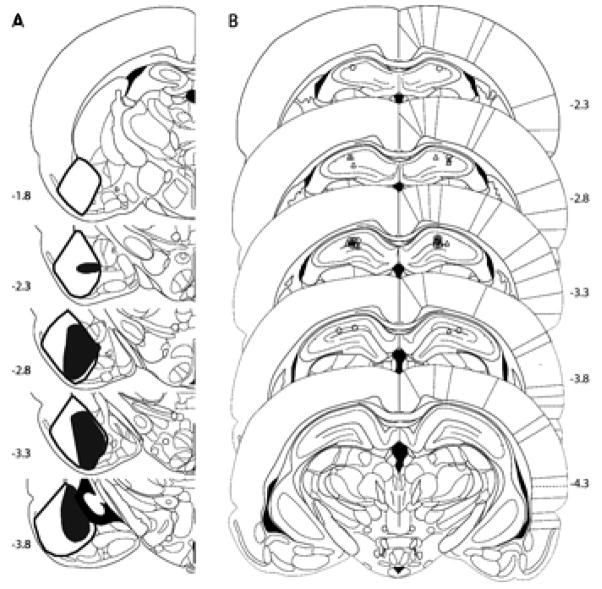
Each lesion was quantified by measuring the area of a hand-drawn depiction of the lesion with a dot-matrix. Drawings were made of the 4 sections that best represented Figures 26, 29, 31, and 33 in Paxinos and Watson’s (1998) rat brain atlas. For Experiments 1a and 1b, animals that received less than 70% damage to the BLA in each hemisphere were not included in the analyses. As a result, 7 BLA-lesioned (BLAx) subjects were excluded. This left 6 BLAx subjects and 9 sham-lesioned subjects. Figure 1 depicts the size of the largest and smallest lesions in the two lesion experiments presented here. In Experiment 1a, the included lesions ranged from 75% to 100% damage, with an average of 87%. The BLA lesions were accompanied by significant (78% average) lateral amygdala (LA) damage. Some subjects with larger lesions also sustained cortical damage, but there was otherwise little damage outside of the BLA/LA complex.
Figure 1.
A) Depicts the largest (denoted by the white area with black outline) and smallest (denoted by the black-filled area) lesions included in Experiments 1a and 1b. B) Depicts cannula placements in Experiment 2 for the direct- (triangles) and mediated- (circles) learning groups. Figures were derived from Paxinos and Watson (1998).
3.1.2. Behavior
BLAx and Sham subjects consumed similar amounts of the fluids throughout training. Table 2 shows the mean consumption rates of the solutions in Phases 1 and 2. As expected, consumption of the two odor-taste compounds in Phase 1 was similar in the two groups. In Phase 2, there was a tendency for greater consumption of water in the presence of O1 than O2. The results of the taste and odor tests are presented on Figure 2. Both groups consumed more T2 than T1, suggesting an aversion to T1. In contrast, there was no difference in the consumption of water in the presence of O1 and O2 at test. A series of mixed Analyses of Variance (ANOVAs) tested the reliability of these observations. An analysis of the Phase-1 consumption data averaged across trials revealed no effects or interactions, Fs < 1.00, ps > .55. An analysis of the Phase 2 data showed greater consumption of water in the presence of O1 relative to O2, F (1, 13) = 15.44, p = .002, but no lesion effect or interaction, Fs < 1.00, ps > .36. This difference was not due to an inherent preference for water in the presence of O1 (the physical identities of the odors were counterbalanced), but to a combination of novelty and generalized bait-shyness effects. First, rats that were tested first with O2 drank less water (mean = 9.41 ml, SEM = 1.04 ml) on that first, novel test (the first presentation of an odor without a taste) than they did on the second, O1 test (mean = 11.15 ml, SEM = 11.15ml). Second, rats that were tested first with O1 (mean = 8.53 ml, sem = 0.85 ml) received LiCl on that trial, which induced general bait shyness on the following day and hence lower consumption of water on the subsequent O2 trial (mean = 5.14 ml, sem = 0.95 ml). Thus, the mean consumption of water in the presence of O1 and O2 did not differ on the first day of Phase 2, but diverged in opposite directions.
Table 2.
Fluid Intake
| Exp. | Group | Phase 1 | Phase 2 | ||
|---|---|---|---|---|---|
|
| |||||
| O1T1 | O2T2 | O1 | O2 | ||
| 1a | Sham | 14.56 (± 0.83) | 14.97 (± 0.87) | 9.33 (± 0.69) | 7.33 (± 0.96) |
| BLAx | 15.81 (± 1.57) | 15.24 (± 1.39) | 10.82 (± 1.26) | 7.55 (± 1.76) | |
|
| |||||
| 1b | Sham | 11.80 (± 1.51) | 12.22 (± 0.95) | 5.63 (± 0.92) | 2.61 (± 0.69) |
| BLAx | 10.18 (± 0.77) | 10.91 (± 0.97) | 3.81 (± 0.74) | 2.69 (± 0.76) | |
|
| |||||
| 2 | Mediated | 11.90 (±0.68) | 12.78 (±0.93) | --- | --- |
| Direct | 11.61 (±1.38) | 11.39 (±0.89) | |||
Note. Table 2 shows the means (± standard error) from Phases 1 and 2 in all experiments. Phase 1 means are averaged across trials.
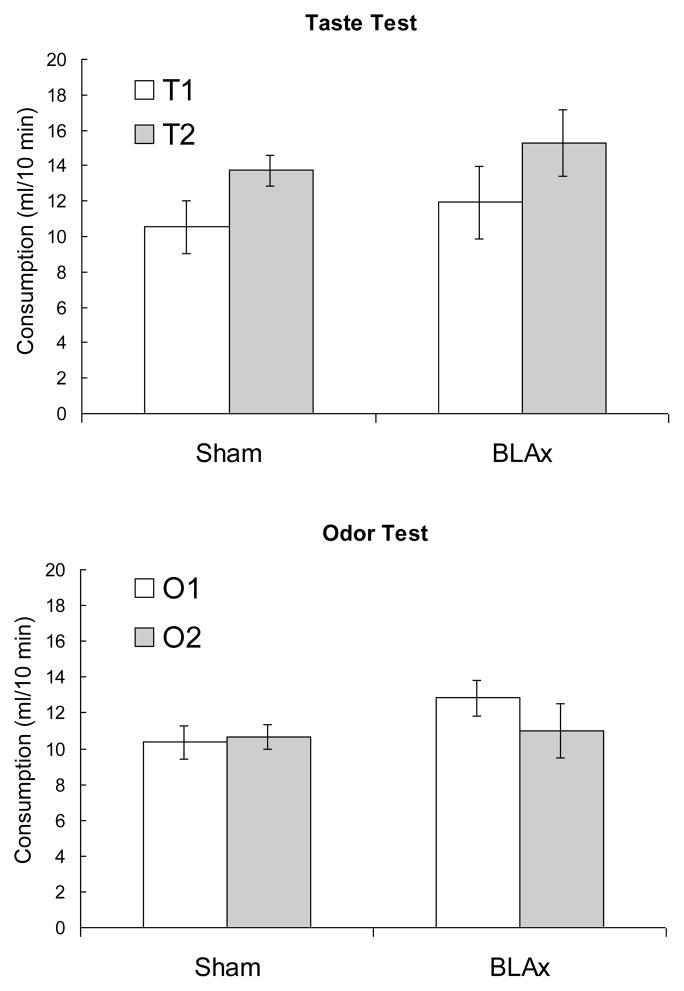
Figure 2.
Depicts consumption in the taste (A) and odor (B) tests. Consumption was measured in a counterbalanced series of 10-min single-bottle tests.
Most important, in the taste test, both groups consumed more T2 than T1, F (1, 13) = 10.59, p = .006, but there was no other effect or interaction, Fs < 1.00, ps > .47. A similar analysis of the odor test indicated no difference in consumption of water in the presence of the two odors, regardless of group, Fs < 1.77, ps > .20.
The fact that Phase-2 training resulted in less consumption of T1, but not of water in the presence of O1, suggests that O1 formed no direct association with illness, but only served to activate the representation of T1 during Phase 2. That is, O1 mediated the conditioning of T1 in Phase 2, rather than mediating the aversive response to T1 at test, as likely occurs in sensory-preconditioning procedures (e.g., Rizley & Rescorla, 1972). Further, the results clearly indicate that the BLA was not necessary for the acquisition and expression of the mediated-taste aversion. This result is inconsistent with that observed by Dwyer and Killcross (2006), but according to contemporary theories of BLA function (e.g., Balleine & Killcross, 2006), that region is specifically engaged in learning about motivationally significant stimuli. It is possible that the 8% solutions here were not remarkably motivationally significant to water-deprived rats, because the subjects were more interested in the hydrating properties of the fluids rather than the nutritive or gustatory properties. This possibility was explored in Experiment 1b by altering the subjects’ deprivation state and the caloric content of the solutions.
3.2. Experiment 1b
3.2.1. Histology
Seven BLAx rats were eliminated from the analyses because of insufficient bilateral damage. The remaining 7 rats had BLA damage that ranged from 71% to 100%. The mean damage was 82% and 83% to the BLA and LA, respectively.
3.2.2. Behavior
Table 2 shows the consumption in Phases 1 and 2. In Phase 1, both groups consumed comparable amounts of the two odor-taste compounds. As in Experiment 1a, subjects tended to drink more water in the presence of O1 than O2 in Phase 2. Most important, the taste test revealed a similar mediated aversion in both groups, with rats tending to consume more T2 than T1 (see Figure 3). In the odor test, there was no difference in consumption of water in the presence of O1 and O2. Thus, the results of Experiment 1b complemented those of Experiment 1a.
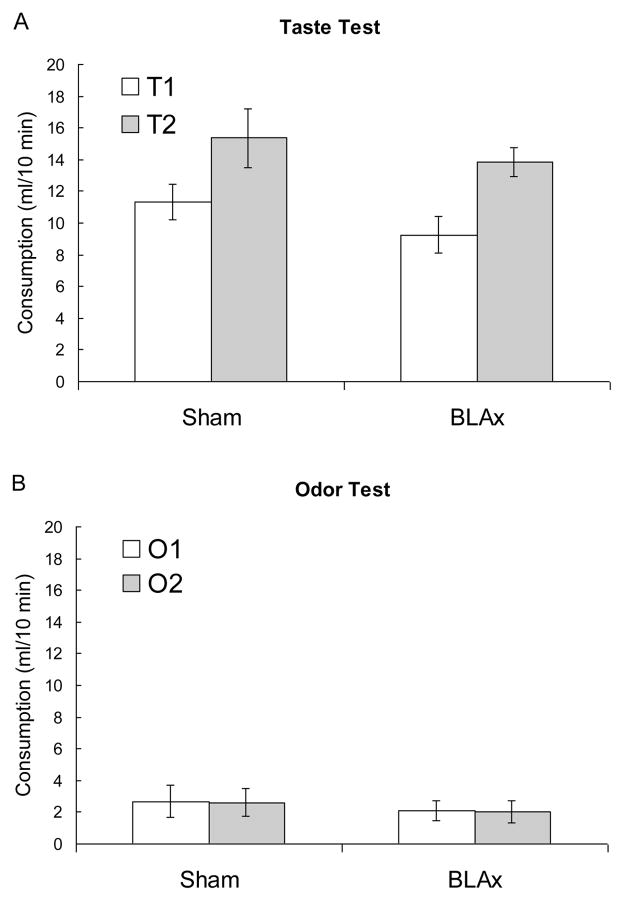
Figure 3.
Depicts consumption in the taste (A) and odor (B) tests. Consumption was measured in a counterbalanced series of 10-min single-bottle tests.
An analysis of the Phase 1 consumption data indicated no effect of lesion or preference, Fs < 1.30, ps > .27. In Phase 2, there was greater consumption of water in the presence of O1 than O2, F (1, 13) = 39.89, p < .001, but no effect of lesion, F (1, 13) = 0.61, p = .45. However, the difference in consumption of water in the presence of O1 and O2 was affected by the lesion, F (1, 13) = 8.26, p = .013, with sham subjects exhibiting a greater difference. The analysis of the taste test revealed a greater intake of T2 regardless of group, F (1, 13) = 18.02, p < .001, with no effect of lesion or taste X lesion interaction, Fs < 1.30, ps > .27. The analysis of the odor test showed no effects or interaction, Fs < 1.00, ps > .58.
The absence of an effect of BLA lesions on mediated learning in Experiments 1a and 1b provokes questions about the generality of the necessity of BLA function in representational aspects of appetitive conditioning. Specifically, it was not critical in this situation that required an odor-evoked taste representation regardless of the motivational significance of the nutrients. It is possible that olfacto-gustatory learning and retrieval differs physiologically from the sort of appetitive learning that is typically used in studies of BLA function. This issue is examined in more detail in the general discussion.
3.3. Experiment 2
3.3.1. Histology
One rat (placements not shown) from the direct-learning group was eliminated because there was evidence of appreciable unilateral DH damage. This left 10 rats in the mediated-learning group and 7 rats in the direct-learning group. Figure 1b shows the placement of the internal cannulae in the remaining rats.
3.3.2. Behavior
Table 2 shows the consumption in Phase 1. Consumption of the odor-taste compounds was similar in the two groups. In Phase 2, all of the subjects consumed the entirety of the available fluid on training day except one rat in the mediated-learning group, who only drank 4 ml of water during O2 exposure.
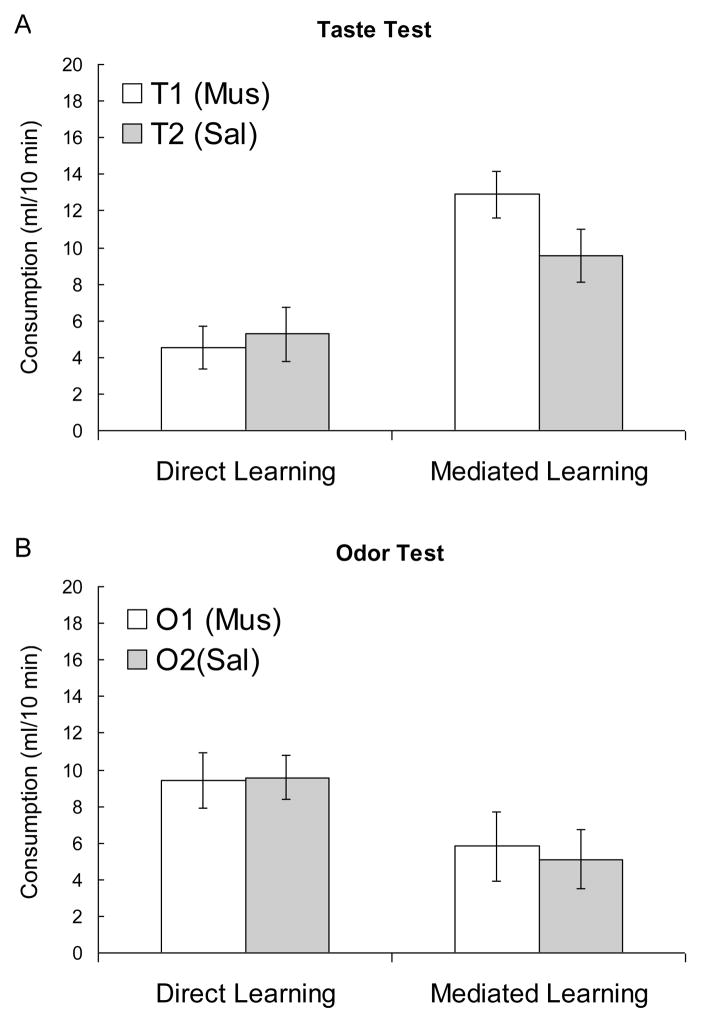
Figure 4a shows the primary data of Experiment 2, the consumption of the two taste solutions. In the mediated-learning group, there was a strong preference for T1, suggesting that T2 was more aversive than T1. Notably, T1’s odor associate (O1) had been paired with illness while the DH was inactivated, whereas T2’s odor partner (O2) had been paired with LiCl while DH function was intact. By contrast, rats in the direct-learning group showed no such preferences and tended to drink less than rats in the mediated-learning group, suggesting a directly-learned taste aversion that was unimpaired by DH inactivation. Finally, consumption of water in the presence of the odor (see Fig 4b) suggested some nonspecific aversion to the odors in the mediated-learning group (which had direct odor-illness pairings), but no effect of muscimol inactivation.
Figure 4.
Depicts consumption in the taste (A) and odor (B) tests. Muscimol infusions preceded O1-LiCl (mediated learning) or T1-LiCl (direct learning) pairings. Consumption was measured in a counterbalanced series of 10-min single-bottle tests.
These observations are supported by the results of a series of mixed ANOVAs with a between-subjects factor of group (mediated- vs. direct-learning) and within-subjects factors comparing consumption of fluids in the different phases of the experiment. An analysis of Phase 1 consumption revealed no effects or interactions, Fs < 1.00, ps > .34. An analysis of the taste test revealed a significant effect of group, F (1, 15) = 13.46, p = .002, and a significant group X taste interaction, F (1, 15) = 4.64, p < .05, but no effect of taste, F (1, 15) = 1.93, p = .18. A planned comparison of consumption in the mediated-learning group indicated that they consumed more T1 than T2, F (1, 15) = 7.62, p = .015. A similar comparison of consumption in the direct-learning group revealed no such difference, F (1, 15) = 0.25, p = .63. A final analysis of consumption of water in the presence of the odors suggested a nonsignificant trend toward greater overall consumption in the direct-learning condition, F (1, 15) = 3.22, p = .09, but no other effects or interactions, Fs < 1.00, ps > .58.
4. Discussion
Previous research suggested that the BLA and DH are critical for the cognitive processes underlying mediated learning. Experiments 1a and 1b show that the BLA is not necessary in our mediated taste aversion paradigm. In contrast, Experiment 2 extends the results of previous experiments that demonstrated the importance of the DH in mediated learning (Iordanova et al., 2011) to a taste-aversion paradigm widely used in the study of mediated learning (Holland & Wheeler, 2008). When comparing Experiments 1a and 1b to Experiment 2, it is important to note that permanent lesions potentially differ from temporary inactivation because the former technique is more vulnerable to recovery of function through compensation. However, the individual experiments here can be compared to the wider literature, including many studies that show that permanent BLA lesions produce impairments in associative learning that should have had an impact in Experiments 1a and 1b.
Specifically, the results of Experiment 1a and 1b are at odds with the results of Dwyer and Killcross (2006). One potentially important difference between our design and theirs lies in the type of stimulus used as a surrogate for the taste in Phase 2. Dwyer and Killcross employed overtly distinct contexts that were cleaned before each session with different dilute solutions. Although they did feature an odor cue, it was likely not as salient as our relatively-stronger odor cues that were presented in close spatial contiguity with the drinking tube. Additionally, our immediate context was always the same (the plastic home cage), and no attempt was made to explicitly differentiate the rooms. Thus, the odor, rather than the context, was more likely to form an association with the taste. Because the overall experimental designs were rather similar otherwise, it seems most likely that the nature of the surrogate stimulus (odor or context) determined the involvement of the BLA. Although the BLA is important for the formation and use of sensory-specific associations between gustatory rewards and operant responses (e.g., Corbit & Balleine, 2005; Ostlund & Balleine, 2008) or auditory/visual (e.g., Hatfield et al., 1996; Johnson, Gallagher, & Holland, 2009) cues, it might not be necessary for similar sensory-specific olfacto-gustatory learning and memory. This notion is supported by Scarlet, Delamater, Campese, Fein, and Wheeler’s (2012) observation that the BLA is not critical for appropriate responding in a devaluation task that involves olfactory cues and gustatory outcomes, in contrast to operant or Pavlovian learning situations that involve auditory and visual cues. Unfortunately, our limited attempts to replicate a mediated-learning effect similar to that observed by Dwyer and Killcross (using distinct contexts) failed, so we were unable to directly assess this possibility.
Although the data from Experiments 1a and 1b are not consistent with theories of BLA function with regard to general learning processes, they are consistent with another study of odor-taste learning that used very similar parameters. By examining immediate-early gene expression, Saddoris et al. (2009) found that an odor that had been paired with a taste would produce neural activity in the gustatory cortex that was similar to that produced by the taste itself, suggesting that neurons in this area encode associatively-activated as well as directly-activated representations of taste stimuli. Interestingly, although a unilateral BLA lesion reduced overall activity in the ipsilateral gustatory cortex, it had no effect on the degree of overlap in activity produced by a taste and an odor that was associated with that taste. As noted by Saddoris et al., this result suggests that the BLA is not critical for the activity in the GC that might denote an associatively-activated taste representation. The question remains as to whether direct disruption of GC activity during Phase 2 (through lesion or inactivation) would attenuate mediated learning in this paradigm.
Experiment 2 showed that DH inactivation during Phase 2 impaired mediated learning. Based on the literature concerning DH function, there are at least two potential cognitive mechanisms that could have been disrupted by DH inactivation. First, it is possible that DH inactivation prevents the subject from retrieving a robust taste memory, which in turn precludes the formation of an association between the absent taste and illness. This is supported by evidence that shows that DH inactivation can impair odor-based associative retrieval. For example, Parsons and Otto (2008) found that DH inactivation impaired the acquisition and retrieval of an odor-cued fear memory, but did not affect fear memories evoked by auditory cues or olfactory perception generally. If DH inactivation eliminates retrieval of taste memories, then mediated learning would be impossible.
It is also possible that DH inactivation interferes with mediated learning because of its general role in forming associations between temporally discontiguous events (Wallenstein et al., 1998). Deleterious DH manipulations including permanent lesions (e.g., Chowdhury, Quinn, & Fanselow, 2005) and temporary inactivation (e.g., Raybuck & Lattal, 2011) attenuate trace conditioning (in which a CS and US are separated by an appreciable interval) in various behavioral preparations. Most pertinent to the present research, Koh, Wheeler, and Gallagher (2009) found that DH lesions attenuate long-delay taste aversion learning. In a way, mediated learning may be viewed as the quintessential example of associating discontiguous events because the two physical events are not even remotely contiguous. In this regard, it is not surprising that this type of learning is impaired by DH inactivation. Further, it suggests that other brain areas that are important for trace conditioning might also be important for mediated learning.
The lack of an effect of DH inactivation on direct learning is notable because data indicate that hippocampus lesions can influence direct taste-aversion learning. For example, permanent hippocampus lesions can attenuate direct taste aversion-learning in situations in which the taste is preexposed prior to learning (e.g., Purves et al., 1995; Reilly et al., 1993), which is a procedural necessity of our design. In contrast to permanent lesions, Stone, Grimes, and Katz (2005) found that temporary inactivation of the DH can enhance taste learning, provided that it occurs immediately before taste-illness pairings. However, here we found no detectable effect of DH inactivation on direct learning. The present results do not indicate why DH inactivation was effective in Stone et al.’s design, but it is clear that there are a number of procedural differences between the two experiments that might have contributed to the null result observed here.
Taken together with past studies of mediated learning, the present experiments show that the BLA has a limited role in learning about absent events, whereas the DH has a broader role, probably because different types of mediated learning require various mnemonic and associative processes, many of which are mediated by this structure. This finding constrains theories of BLA function in general learning and emphasizes the importance of the hippocampus in discontiguous associative learning. The potentially broad role of the hippocampus is of particular interest for future research because recent studies indicate that mediated learning might be a valid animal model for disordered thinking and psychosis in schizophrenia (McDannald et al., 2011), a disease that is itself correlated with physiological and functional hippocampal abnormalities (Heckers, 2001).
Highlights.
Rats were able to learn about a gustatory stimulus in its absence.
Mediated learning was not affected by pretraining basolateral amygdala lesions.
Mediated learning was attenuated by dorsal hippocampal inactivation.
The dorsal hippocampus is generally involved in representational learning.
Acknowledgments
This research was supported by National Institute of Mental Health Grant MH65879 (P.C.H.) and National Institute of Neurological Disorders and Stroke Grant NS061587 (D.S.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends in Neurosciences. 2006;29:272–279. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Blundell P, Hall G, Killcross S. Lesions of the basolateral amygdala disrupt selective aspects of reinforcer representation in rats. The Journal of Neuroscience. 2001;21:9018–9026. doi: 10.1523/JNEUROSCI.21-22-09018.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell P, Hall G, Killcross S. Preserved sensitivity to outcome value after lesions of the basolateral amygdala. The Journal of Neuroscience. 2003;23:7702–7709. doi: 10.1523/JNEUROSCI.23-20-07702.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, McDannald MA, Wheeler DS, Holland PC. The effects of basolateral amygdala lesions on unblocking. Behavioral Neuroscience. 2012;126:279–289. doi: 10.1037/a0027576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury N, Quinn JJ, Fanselow MS. Dorsal hippocampus involvement in trace fear conditioning with long, but not short, trace intervals in mice. Behavioral Neuroscience. 2005;119:1396–1402. doi: 10.1037/0735-7044.119.5.1396. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on general and outcome-specific forms of Pavlovian-instrumental transfer. The Journal of Neuroscience. 2005;25:962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas K, Rovee-Collier C, Learmonth AE. Infants form associations between memory representations of stimuli that are absent. Psychological Science. 2006;17:543–549. doi: 10.1111/j.1467-9280.2006.01741.x. [DOI] [PubMed] [Google Scholar]
- Dwyer DM. Retrospective revaluation or mediated conditioning? The effect of different reinforcers. The Quarterly Journal of Experimental Psychology B, Comparative and Physiological Psychology. 1999;52:289–306. doi: 10.1080/027249999393013. [DOI] [PubMed] [Google Scholar]
- Dwyer DM. Mediated conditioning and retrospective revaluation with LiCl then flavour pairings. The Quarterly Journal of Experimental Psychology B, Comparative and Physiological Psychology. 2001;54:145–165. doi: 10.1080/713932750. [DOI] [PubMed] [Google Scholar]
- Dwyer DM, Killcross S. Lesions of the basolateral amygdala disrupt conditioning based on the retrieved representations of motivationally significant events. The Journal of Neuroscience. 2006;26:8305–8309. doi: 10.1523/JNEUROSCI.1647-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin NJ, Wright SP, Eichenbaum H. Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature. 2004;431:188–191. doi: 10.1038/nature02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J, Koelling RA. Relation of cue to consequence in avoidance learning. Psychonomic Science. 1966;4:123–124. [Google Scholar]
- Hatfield T, Han J, Conley M, Gallagher M, Holland PC. Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer devaluation effects. The Journal of Neuroscience. 1996;16:5256–5265. doi: 10.1523/JNEUROSCI.16-16-05256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus. 2001;11:520–528. doi: 10.1002/hipo.1068. [DOI] [PubMed] [Google Scholar]
- Holland PC. Acquisition of Representation-Mediated Conditioned Food Aversions. Learning and Motivation. 1981;12:1–18. doi: 10.1016/j.lmot.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC. Event representations in Pavlovian conditioning: Image and action. Cognition. 1990;37:105–131. doi: 10.1016/0010-0277(90)90020-k. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala-frontal interactions and reward expectancy. Current Opinion in Neurobiology. 2004;14:148–155. doi: 10.1016/j.conb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Holland PC, Wheeler DS. Representation-mediated food aversions. In: Reilly S, Schachtman T, editors. Conditioned Taste Aversion: Behavioral and Neural Processes. Oxford: Oxford University Press; 2008. pp. 196–225. [Google Scholar]
- Holt W, Maren S. Muscimol Inactivation of the Dorsal Hippocampus Impairs Contextual Retrieval of Fear Memory. The Journal of Neuroscience. 1999;19:9054–9062. doi: 10.1523/JNEUROSCI.19-20-09054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanova MD, Good M, Honey RC. Retrieval-mediated learning involving episodes requires synaptic plasticity in the hippocampus. The Journal of Neuroscience. 2011;31:7156–7162. doi: 10.1523/JNEUROSCI.0295-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AW, Gallagher M, Holland PC. The basolateral amygdala is critical to the expression of Pavlovian and instrumental outcome-specific reinforcer devaluation effects. The Journal of Neuroscience. 2009;29:696–704. doi: 10.1523/JNEUROSCI.3758-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh MT, Wheeler DS, Gallagher M. Hippocampal lesions interfere with long-trace taste aversion conditioning. Physiology & Behavior. 2009;98:103–107. doi: 10.1016/j.physbeh.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannald M, Schoenbaum G. Toward a model of impaired reality testing in rats. Schizophrenia Bulletin. 2009;35:664–667. doi: 10.1093/schbul/sbp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannald M, Whitt JP, Calhoon GG, Piantadosi PT, Karlsson RM, O’Donnell P, Schoenbaum G. Impaired reality testing in an animal model of schizophrenia. Biological Psychiatry. 2011;70:1122–1126. doi: 10.1016/j.biopsych.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. Differential involvement of the basolateral amygdala and mediodorsal thalamus in instrumental action selection. The Journal of Neuroscience. 2008;28:4398–4405. doi: 10.1523/JNEUROSCI.5472-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons TC, Otto T. Temporary inactivation of dorsal hippocampus attenuates explicitly nonspatial, unimodal, contextual fear conditioning. Neurobiology of Learning and Memory. 2008;90:261–268. doi: 10.1016/j.nlm.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain stereotaxic coordinates. New York, NY: Academic Press; 1998. [Google Scholar]
- Purves D, Bonardi C, Hall G. Enhancement of latent inhibition in rats with electrolytic lesions of the hippocampus. Behavioral Neuroscience. 1995;109:366–370. doi: 10.1037//0735-7044.109.2.366. [DOI] [PubMed] [Google Scholar]
- Raybuck JD, Lattal KM. Double dissociation of amygdala and hippocampal contributions to trace and delay fear conditioning. PLoS ONE. 2011;6:1–6. doi: 10.1371/journal.pone.0015982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly S, Harley C, Revusky S. Ibotenate lesions of the hippocampus enhance latent inhibition in conditioned taste aversion and increase resistance to extinction in conditioned taste preference. Behavioral Neuroscience. 1993;107:996–1004. doi: 10.1037//0735-7044.107.6.996. [DOI] [PubMed] [Google Scholar]
- Rizley RC, Rescorla RA. Associations in second-order conditioning and sensory preconditioning. Journal of Comparative and Physiological Psychology. 1972;81:1–11. doi: 10.1037/h0033333. [DOI] [PubMed] [Google Scholar]
- Saddoris MP, Holland PC, Gallagher M. Associatively learned representations of taste outcomes activate taste-encoding neural ensembles in gustatory cortex. The Journal of Neuroscience. 2009;29:15386–15396. doi: 10.1523/JNEUROSCI.3233-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlet J, Delamater AR, Campese V, Fein M, Wheeler DS. Differential involvement of the basolateral amygdala and orbitofrontal cortex in the formation of sensory-specific associations in conditioned flavor preference and magazine approach paradigms. European Journal of Neuroscience. 2012;35:1799–1809. doi: 10.1111/j.1460-9568.2012.08113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Andre J, Reilly S. Effects of central and basolateral amygdala lesions on conditioned taste aversion and latent inhibition. Behavioral Neuroscience. 2007;121:90–99. doi: 10.1037/0735-7044.121.1.90. [DOI] [PubMed] [Google Scholar]
- Stone ME, Grimes BS, Katz DB. Hippocampal inactivation enhances taste learning. Learning & Memory. 2005;12:579–586. doi: 10.1101/lm.32305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenstein GV, Eichenbaum H, Hasselmo ME. The hippocampus as an associator of discontiguous events. Trends in Neurosciences. 1998;21:317–323. doi: 10.1016/s0166-2236(97)01220-4. [DOI] [PubMed] [Google Scholar]