Abstract
Immune cells expressing both NK and T cell markers include CD1d-dependent NKT cells and - independent NKT-like cells. We now describe the presence of NK1.1+CD8+ T cells in the liver, but not other tissues (spleen, bone marrow, thymus or peripheral blood) in mice receiving allogeneic hematopoietic cell transplantation (allo-HCT). These cells are CD1d-independent TCRαβ+ T cells with an effector/memory CD44hiCD62L− phenotype, and do not express Ly49 receptors. Furthermore, these cells were derived from donor splenocytes, but not bone marrow cells. Depletion of CD8+, but not NK1.1+, cells from donor splenocytes prior to transplantation prevented the generation of NK1.1+CD8+ T cells, indicating that these cells arose from donor NK1.1−CD8+ splenic T cells. These results provide direct evidence that donor CD8+ T cells can acquire NK1.1 expression upon activation in allo-HCT recipients and that these NK1.1+CD8+ NKT-like cells maintain an effector/memory phenotype and persist in the recipients with preferential localization in the liver.
Keywords: CD8 T cells, NK1.1+CD8+ T cells, allogeneic hematopoietic cell transplantation, liver
1. Introduction
Natural killer T (NKT) cells are a small population of thymus-derived T cells that are restricted by the non-classical MHC class I molecule CD1d [1]. CD1d-dependent T cells are classified into type I NKT cells that express the invariant TCRα-chain (Vα14-Jα18 in mice and Vα24-Jα18 in humans) (also known as invariant NKT cells), and type II NKT cells that express a more diverse TCR repertoire [1,2]. Mouse CD1d-dependent NKT cells are either CD4−CD8− or CD4+ [1,2].
CD1d-indepepndent T cells that express a NK phenocype have been referred to be NKT cells or NKT-like cells [2]. Although NKT-like cells consist of CD4+, CD4−CD8− and CD8+ cells, the majority of these cells are CD8+ [3,4]. NKT-like cells also differ from CD1d-dependent NKT cells in their tissue distribution, in which NKT-like cells are predominantly in the spleen and bone marrow while the liver contains primarily CD1d-dependent NKT cells [4]. Although NKT-like cells are rare in normal naïve mice, a significant proportion of viral antigen-specific CD4+ and CD8+ T cells were found to express NK cell markers in virally-infected mice [5,6]. However, it remains unclear whether the observed increase in NKT-like T cells in virally-infected mice [5,6] was a consequence of direct expansion of the pre-existing NKT-like cells or conversion of NK marker-negative T cells to NKT-like cells. In the present study, we show that CD1d-independent NK1.1+CD8+ donor cells were significantly increased in the liver, but not other tissues in mice following allogeneic hematopoietic cell transplantation (allo-HCT). The generation of these NKT-like cells is a consequence of alloresponses, and can be abrogated by depletion of CD8+, but not NK1.1+, cells from the donor HCT inoculum. These results provide direct in vivo evidence for acquisition of NK marker expression by CD8 T cells (i.e., conversion of NK marker-negative T cells into NKT-like cells) upon activation.
2. Materials and Methods
2.1 Animals
Female C57BL/6 (B6, H-2b) and B6D2F1 (B6×DBA2 F1) mice were purchased from the Frederick Cancer Research Facility (Frederick, MD). Female C57BL/6-Tg (ACTB-EGFP; GFP-Tg B6) mice were purchased from The Jackson Laboratory (Bar Harbor, Maine). All mice were housed in a specific pathogen-free microisolator environment, and used at ages of 8–12 weeks. Protocols involving animals were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care.
2.2 Allogeneic hematopoietic cell transplantation (allo-HCT)
B6D2F1 mice were sublethally irradiated (6 Gy, 137Cs source) and reconstituted the next day with B6 bone marrow cells (BMCs; 5×106) plus untreated or NK1.1+ cell- and/or CD8+ cell-depleted splenocytes (2×107). NK1.1+ and CD8+ cell depletion was performed using the magnetic-activated cell sorter separation system (Miltenyi Biotec, Auburn, CA), and completeness of depletion (<0.4% cells of the depleted phenotype remaining) was verified by flow cytometric analysis. In selected experiments, CD45.1 congeneic or GFP-Tg B6 mice were used as donors for identifying the origin of NK1.1+CD8+ T cells in allo-HCT recipients.
2.3 Flow cytometric analysis
Single cell suspensions, including peripheral blood mononuclear cells (PBMCs), splenocytes, BMCs, thymocytes, and ficolled liver cells, were stained by various combinations of the following fluorescence-conjugated anti-mouse mAbs: CD4, CD8, CD44, CD62L, TCRαβ, CD49b, Ly-49c, Ly-49D, Ly-49G2 (Becton Dickinson, San Jose, CA), and CD1d tetramer loaded with an analog of the αGalCer ligand, PBS57 (National Institute of Allergy and Infectious Diseases Tetramer Facility, Germantown, MD). Living cells were collected and analyzed by a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA).
2.4 Statistical analysis
Significant differences between groups were determined by student’s t test using GraphPad Prism 5.0 (San Diego, CA). A P value of less than 0.05 was considered statistically significant.
3. Results
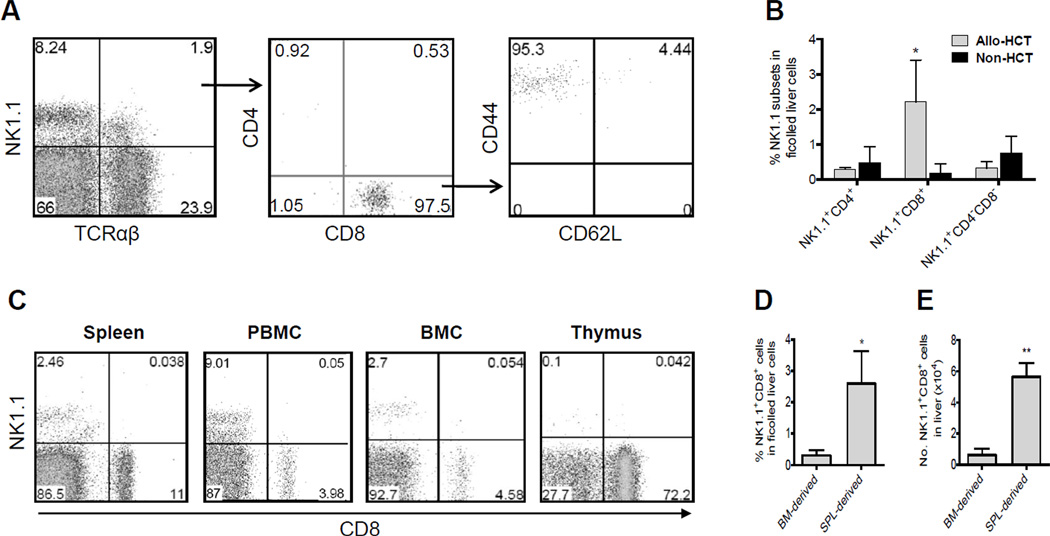
We first analyzed NK1.1+TCRαβ+ cells in the liver of allo-HCT recipients. Ficolled liver cells were prepared from B6D2F1 recipients 56 days after allo-HCT from B6 donors, when all hematopoietic cells in the recipients were of donor origin as confirmed by staining with anti-recipient MHC class I mAb [7] (data not shown). Unlike liver NK1.1+TCRαβ+ T cells in normal naïve mice that are primarily CD4+ or CD4−CD8− CD1d-dependent NKT cells [4], almost all NK1.1+TCRαβ+ in the liver of allo-HCT recipients were found to express CD8 and an effector/memory phenotype (i.e., CD44hi and CD62L−; Figure 1A). Although it is a minor fraction of cells in the ficolled liver cells (2.56±0.6814%), the percentage of NK1.1+CD8+ T cells in the liver was significantly higher in allo-HCT than in non-HCT controls (P<0.05), while the ratios of CD4+ and CD4−CD8− NKT cells in the liver were similar between the two groups (Figure 1B). Interestingly, NK1.1+CD8+ cells were undetectable or extremely low in other tissues examined, including spleen, PBMCs, bone marrow and thymus (Figure 1C). Similar results were also seen in CB6F1 (BALB/c × B6) mice receiving allo-HCT from BALB/c donors (data not shown). These data demonstrate a preferential localization of NK1.1+CD8+ effector/memory T cells in the liver of allo-HCT recipients.
Figure 1.
NK1.1+CD8+ T cells with an effector/memory phenotype, which were derived from donor splenocytes, preferentially located in the liver of allo-HCT recipients. Sublethally-irradiated B6D2F1 mice were administered 5×106 BMCs and 2×107 splenocytes from allogeneic B6 donors. Ficolled liver cells, splenocytes, PBMCs, BMCs, and thymocytes were prepared 56 days after HCT for analyses. A) Representative FACS profiles showing staining of ficolled liver cells with anti-mouse TCRαβ and NK1.1 (left); NK1.1+TCRαβ+ cells (indicated as arrow pointed cells) were then analyzed for CD4 and CD8 expression (middle); and CD8+NK1.1+TCRαβ+ cells (indicated as arrow pointed cells) were further analyzed for CD44 and CD62L expression (right). B) Percentages (mean±SEMs) of NK1.1+CD4+, NK1.1+CD8+ and NK1.1+CD4−CD8− cells in the liver (ficolled liver cells) of allo-HCT recipients. * p<0.05 for allo-HCT versus non-HCT groups in the indicated cell population. C) Representative FACS profiles showing the lack of NK1.1+CD8+ cells in splenocytes, PBMCs, BMCs, and thymocytes of allo-HCT recipients. B6D2F1 (CD45.2+) mice were sublethally (6 Gy)-irradiated and injected next day with 5×106 CD45.1+ B6 BMCs and 2×107 CD45.2+ B6 splenocytes. Ficolled liver cells were prepared from allo-HCT recipients 56 days after HCT and analyzed for the percentages (D) and numbers (E) of CD45.1+ BM-derived and CD45.2+ splenocytes-derived donor NK1.1+CD8+ cells. Data are presented as mean±SEMs. * p<0.05; ** p<0.01 for CD45.2+ splenocyte (SPL)- versus CD45.1+ BM-derived NK1.1+CD8+ cells..
To further determine whether the NK1.1+CD8+ cells were derived from donor BMCs and/or splenocytes, we performed allo-HCT using BMCs and splenocytes from CD45 congeneic B6 donors. Briefly, B6D2F1 (CD45.2+) mice were sublethally-irradiated and injected with 5×106 CD45.1+ B6 BMCs and 2×107 CD45.2+ B6 splenocytes. Ficolled liver cells were prepared from recipients 56 days after HCT and analyzed by flow cytometry. As shown in Figure 1D & 1E, the majority of NK1.1+CD8+ T cells were derived from CD45.2+ donor splenocytes.
We next determined whether NK1.1+CD8+ T cells were derived from injected donor splenic NK1.1+CD8−, NK1.1−CD8+ or NK1.1+CD8+ cells. B6D2F1 recipients were sublethally-irradiated, and either received no further treatment (Non-HCT controls; Group E), or injected with GFP− B6 BMCs and splenocytes (1×107), plus GFP+ B6 splenocytes (1×107) that were unmanipulated (Group A), CD8+ cell-depleted (Group B), NK1.1+ cell-depleted (Group C), or CD8+/NK1.1+ cell-depleted (Group D). As expected, NK1.1+CD8+ T cells in the liver of Group A mice consisted of equal proportions of GFP+ and GFP− NK1.1+CD8+ T cells (Figure 2A and2B). The lack of GFP+ NK1.1+CD8+ T cells in Group B and Group D, and the comparable frequencies of GFP+ vs. GFP− NK1.1+CD8+ T cells in Group C indicate that the NK1.1+CD8+ T cells detected in the liver of allo-HCT recipients were derived from donor NK1.1−CD8+ splenic T cells. Consistent with this observation, NK1.1+CD8+TCRαβ+ T cells in the liver of allo-HCT recipients were stained negative with CD1d-αGalCer tetramers (Figure 2C). Furthermore, the NK1.1+CD8+ cells in the liver of allo-HCT recipients were negative for CD49b, Ly-49C/I, Ly-49G2, and Ly-49D (Figure 2D).
Figure 2.
NK1.1+CD8+ cells in the liver of allo-HCT recipients were derived from donor NK1.1− CD8+ splenocytes. Five HCT groups were prepared as detailed in Materials and methods. Briefly, B6D2F1 recipients were sublethally-irradiated, and either received no further treatment (Non-HCT controls; Group E), or injected with GFP− B6 BMCs and splenocytes (1×107), plus GFP+ B6 splenocytes (1×107) that were unmanipulated (Group A), CD8+ cell-depleted (Group B), NK1.1+ cell-depleted (Group C), or CD8+/NK1.1+ cell-depleted (Group D). Shown are ratios (mean±SDs) of GFP+NK1.1+CD8+ to GFP−NK1.1+CD8+ cells in each group at week 4 (A) and week 8 (B) after HCT. Ficolled liver cells were prepared from allo-HCT recipients 56 and 80 days after allo-HCT. C) Ficolled liver cells were stained with anti-CD8, anti-NK1.1 and CD1d-αGalCer tetramer. No NK1.1+CD1d-αGalCer+ cells were detected in the gated CD8+ cell population. D). Ficolled liver cells were stained with anti-NK1.1, anti-CD8 plus anti-CD49b, Ly-49C/I, Ly-49G2, or Ly-49D. Shown are CD49b, Ly-49C/I, Ly-49G2, and Ly-49D expression on NK1.1+CD8−, NK1.1+CD8+ and NK1.1−CD8+ cells. Data presented in Figures 2C and 2D are from a representative of 3 mice.
4. Discussion
In this study we identify a population of donor NK1.1+CD8+TCRαβ+ T cells that are derived from injected donor NK1.1−CD8+ splenocytes and have a preferential localization in the liver of allo-HCT recipients. The conversion of NK1.1−CD8+ T cells into NK1.1+CD8+ T cells is likely a consequence of alloresponses, as it occurred only in allo-HCT recipients, but not non-HCT controls, and almost all these cells express a memory T cell phenotype. In view of the fact that these NK1.1+CD8+ T cells are generated de novo upon activation, maintain a memory T cell phenotype and persist long-term in allo-HCT recipients, a better understanding of their conversion and function may provide insight into the pathogenesis of chronic GVHD.
Although mechanisms remain unidentified, a significant proportion of viral antigen-specific CD4+ and CD8+ T cells were found to express NK cell markers in virally-infected mice [5,6]. It has also been reported that CD1d-independent CD8+ NKT-like cells with lytic activity against tumors can be expanded from bone marrow, thymus and spleen cells in vitro by stimulation with anti-CD3, IFN-γ and IL-2, and that the expanded CD8+ NKT-like cells mediate strong anti-leukemia effects without GVHD after injection into allogeneic recipients [8,9]. Interestingly, CD8+ NKT-like cells expanded in cultures with anti-CD3/CD28 and IL-2 were paradoxically found to be strongly immunosuppressive [10]. In these studies, because NKT-like cells were not depleted before culture, the CD8+ NKT-like cells obtained could be expanded directly from the small number of NKT-like cells under these culture conditions. Alternatively, these cells could arise from CD8+ T cells, and this possibility is supported by a previous observation that CD8+ T cells can acquire NK cell markers in vitro after cytokine stimulation [11]. The present study, to our knowledge, provides the first direct evidence that mouse CD8+ T cells can gain NK1.1 expression upon activation in vivo. Unlike CD8+ NKT-like cells in naïve mice that express CD62L and mainly home in lymphoid organs [12], these donor NK1.1−CD8+ cell-derived NK1.1+CD8+ T cells were CD62L− and specifically allocated in the liver of allo-HCT recipients. Further studies are required to determine whether NK1.1 expression on these cells were gained prior to or after their migration into the liver and their role in GVHD vs. anti-tumor responses in allo-HCT recipients.
Highlights.
CD1d-independent NKT-like cells are rare in normal naïve mice and predominantly reside in spleen and bone marrow.
This study demonstrates conversion of donor CD8 T cells into NKT-like cells upon activation in allogeneic recipients.
These NK1.1+CD8+ donor cells are long lived, express an effector/memory phenotype and preferentially reside in the recipient liver.
Acknowledgements
We thank Drs. Haowei Li and Ben Sprangers for critical review of the manuscript, Ms. Shumei Wang for technical support, and Mr. Orlando Moreno for outstanding animal husbandry. This study was supported by grants from NIH (5P01CA111519), Zhejiang Province Extremely Key Subject Building Funding (2012R10042-11), and Natural Science Funding of China (81102452).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berzins SP, Smyth MJ, Baxter AG. Presumed guilty: natural killer T cell defects and human disease. Nat Rev Immunol. 2011;11:131–142. doi: 10.1038/nri2904. [DOI] [PubMed] [Google Scholar]
- 2.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van KL. NKT cells: what's in a name? Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 3.Hammond KJ, Pelikan SB, Crowe NY, Randle-Barrett E, Nakayama T, Taniguchi M, Smyth MJ, van DI, Scollay R, Baxter AG, Godfrey DI. NKT cells are phenotypically and functionally diverse. Eur J Immunol. 1999;29:3768–3781. doi: 10.1002/(SICI)1521-4141(199911)29:11<3768::AID-IMMU3768>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 4.Eberl G, Lees R, Smiley ST, Taniguchi M, Grusby MJ, MacDonald HR. Tissue-specific segregation of CD1d-dependent and CD1d-independent NK T cells. J Immunol. 1999;162:6410–6419. [PubMed] [Google Scholar]
- 5.Slifka MK, Pagarigan RR, Whitton JL. NK markers are expressed on a high percentage of virus-specific CD8+ and CD4+ T cells. J Immunol. 2000;164:2009–2015. doi: 10.4049/jimmunol.164.4.2009. [DOI] [PubMed] [Google Scholar]
- 6.Kambayashi T, Assarsson E, Michaelsson J, Berglund P, Diehl AD, Chambers BJ, Ljunggren HG. Emergence of CD8+ T cells expressing NK cell receptors in influenza A virus-infected mice. J Immunol. 2000;165:4964–4969. doi: 10.4049/jimmunol.165.9.4964. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Asavaroengchai W, Yeap BY, Wang MG, Wang S, Sykes M, Yang YG. Paradoxical effects of IFN-gamma in graft-versus-host disease reflect promotion of lymphohematopoietic graft-versus-host reactions and inhibition of epithelial tissue injury. Blood. 2009;113:3612–3619. doi: 10.1182/blood-2008-07-168419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker J, Verneris MR, Ito M, Shizuru JA, Negrin RS. Expansion of cytolytic CD8(+) natural killer T cells with limited capacity for graft-versus-host disease induction due to interferon gamma production. Blood. 2001;97:2923–2931. doi: 10.1182/blood.v97.10.2923. [DOI] [PubMed] [Google Scholar]
- 9.Verneris MR, Ito M, Baker J, Arshi A, Negrin RS, Shizuru JA. Engineering hematopoietic grafts: purified allogeneic hematopoietic stem cells plus expanded CD8+ NK-T cells in the treatment of lymphoma. Biol Blood Marrow Transplant. 2001;7:532–542. doi: 10.1016/s1083-8791(01)70014-6. [DOI] [PubMed] [Google Scholar]
- 10.Zhou L, Wang H, Zhong X, Jin Y, Mi QS, Sharma A, McIndoe R, Garge N, Podolsky R, She JX. The IL-10 and IFN-gamma pathways are essential to the potent immunosuppressive activity of cultured CD8+ NKT-like cells. Genome Biology. 2008;9:R119. doi: 10.1186/gb-2008-9-7-r119. 10.1186/gb-2008-9-7-r119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Assarsson E, Kambayashi T, Sandberg JK, Hong S, Taniguchi M, Van KL, Ljunggren HG, Chambers BJ. CD8+ T cells rapidly acquire NK1.1 and NK cell-associated molecules upon stimulation in vitro and in vivo. J Immunol. 2000;165:3673–3679. doi: 10.4049/jimmunol.165.7.3673. [DOI] [PubMed] [Google Scholar]
- 12.Anfossi N, Robbins SH, Ugolini S, Georgel P, Hoebe K, Bouneaud C+, Ronet C, Kaser A, DiCioccio CB, Tomasello E, Blumberg RS, Beutler B, Reiner SL, Alexopoulou L, Lantz O, Raulet DH, Brossay L, Vivier E. Expansion and Function of CD8+ T Cells Expressing Ly49 Inhibitory Receptors Specific for MHC Class I Molecules. The Journal of Immunology. 2004;173:3773–3782. doi: 10.4049/jimmunol.173.6.3773. [DOI] [PubMed] [Google Scholar]