Abstract
The prevalence of obesity has increased dramatically in recent decades, reaching epidemic proportions. It is becoming clear that obesity is associated not only with type 2 diabetes mellitus and cardiovascular disease, but also with multiple types of cancer.
Obesity is characterized by impaired adipose tissue function, leading to adipocyte hypertrophy, inflammation, hypoxia and induced angiogenesis, extracellular matrix remodeling and fibrosis as well as additional stress responses. While epidemiological data indicate that obesity is a well-established risk factor for certain malignancies, the molecular mechanisms underlying the link between obesity and cancer are still poorly understood.
Recent data implicates systemic and paracrine factors secreted from adipose tissue during the obese state, promoting cancer development and progression. Here, we focus on the obesity-associated adipose tissue remodeling that may not only lead to metabolic complications, but also to a permissive pro-tumorigenic environment. Particular attention is given to the local pro-tumorigenic effects derived from adipocytes that present an important part of the tumor microenvironment of at least some cancers, in an attempt to describe the nature of the major players of the adipocyte-cancer cell crosstalk that dictates to a large extent tumor progression.
Keywords: Obesity, Adipocyte, Stromal Compartment, Cancer Metabolism
1. Introduction
Overweight and obesity constitute a worldwide problem, reaching epidemic proportions. Elevated body mass indices (BMIs) are not only associated with an increased risk of type 2 diabetes mellitus and cardiovascular disease, but also with various types of cancers. There is mounting evidence that excess body weight constitutes an established risk factor for cancer incidence as well as cancer-related mortality. Several pathophysiological mechanisms linking obesity to cancer have been suggested that are related to systemic and local effects of dysregulated adiposity. These include: 1) sex hormones; 2) altered insulin signaling and IGF-1 axis; 3) alterations in adipokines profiles; 4) obesity-induced chronic inflammation; 5) adipose tissue remodeling; and 6) lipid metabolites (Gallagher & LeRoith, 2011; Park, Euhus, & Scherer, 2011; Tan, Buache, Chenard, Dali-Youcef, & Rio, 2011; van Kruijsdijk, van der Wall, & Visseren, 2009).
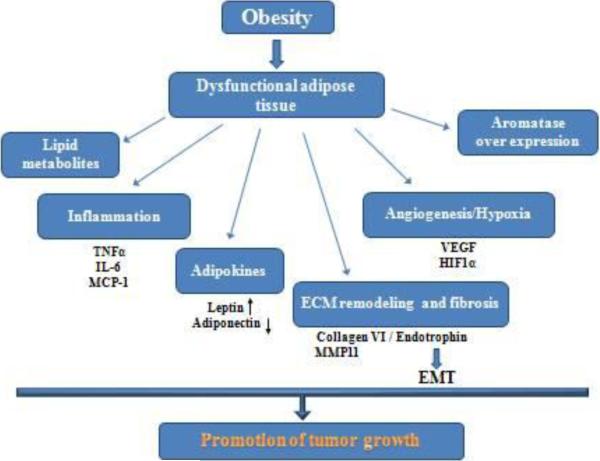
Under conditions of positive energy balance, adipose tissue expands. White adipocytes become hypertrophic and subsequently hyperplastic (Box 1) (Cinti, 2009). In this context, an important distinction needs to be made between healthy fat pad expansion and pathological fat pad expansion. Healthy adipose tissue expansion consists of an enlargement of adipose tissue through effective recruitment of adipose progenitors, along with an adequate angiogenic response, appropriate induction of ECM remodeling as well as minimal inflammation. In contrast, pathological expansion of adipose tissue consists of massive enlargement of existing adipocytes, limited angiogenesis followed by hypoxia, massive inflammation and fibrosis (Sun, Kusminski, & Scherer, 2011). Such pathological expansion of adipose tissue, facing further insults including oxidative and endoplasmic reticulum (ER) stress, leads to dysfunctional adipose tissue (Sun, et al., 2011). Dysfunctional adipocytes are less efficient at fulfilling their primary functions—lipid storage and secretion of a complement of beneficial secretory products, fulfilling their important endocrine role (J. Park, et al., 2011). Dysfunctional adipose tissue has been widely appreciated as a major cause of obesity-related metabolic disorders, including insulin resistance, type 2 diabetes and cardiovascular disease and it plays a pivotal role in obesity-related carcinogenesis due to inappropriate release of mitogenic and proinflammatory factors (Figure 1) (J. Park, et al., 2011; van Kruijsdijk, et al., 2009).
Figure 1. Putative mechanisms of dysfunctional adipose tissue in promoting tumor growth.
Obesity leads to dysfunctional adipose tissue, which is characterized by chronic inflammation, massive ECM remodeling, hypoxia followed byangiogenesis, increased availability of lipids- all together along with aberrantadipokine and hormonal production generate a permissive environment for tumor growth.
One of the unresolved questions is not only how dysfunctional adipocytes promote tumorigenesis, but also if and how cancer cells affect adipocytes, changing their basic properties and thereby shaping a more permissive pro-tumorigenic microenvironment.
To date, the molecular mechanisms responsible for this adipocyte-cancer cell heterotypic crosstalk have remained largely unknown. However, it is likely that this interplay between adipocytes and tumor cells may lead to adipose tissue remodeling, including acquisition of the adipocytes of a fibroblast-like phenotype, altered adipokine production, thereby affecting the remodeling of the local ECM as well as local and systemic inflammation, altered metabolism as well as enhanced angiogenesis. Here, we focus only on the pro-tumorigenic changes introduced by the adipocyte.
2. Epidemiological evidence linking obesity with cancer incidence
Epidemiological studies indicate that adiposity contributes to the increased incidence and/or mortality from various cancers. More than 12 million Americans are cancer survivors in the United States, and the numbers are rapidly increasing (Parekh, Chandran, & Bandera, 2012). Given the current obesity epidemic and an aging population that is more susceptible to cancer, there is mounting concern about the role that obesity plays not only in the initial tumor development, but also in the increased recurrence rate and overall reduced survival prognosis. In April 2012, the National Cancer Policy Forum of the US Institute of Medicine released a welcome report entitled The Role of Obesity in Cancer Survival and Recurrence, in which experts presented the latest clinical evidence on the obesity–cancer link and highlighted the (limited known) molecular mechanisms that might explain that link (Forum, Services, & Medicine, 2012). Growing evidence from both clinical and animal studies shows that obesity increases the risk of cancer incidence, recurrence after treatment, progression, and cancer death for many different organ sites (Parekh, et al., 2012; J. Park, et al., 2011).
In a prospective study conducted over the course of 16 years, starting in the early 80's, it has been estimated that overweight and obesity in the United states could account for up to 14% of all deaths from cancer in men and 20% of those in women (Eugenia E. Calle, Rodriguez, Walker-Thurmond, & Thun, 2003). A comprehensive systemic review of the evidence gathered by the International Agency for Research on Cancer (IARC) (Vainio, Kaaks, & Bianchini, 2002) and, more recently, by the World Cancer Research Fund (WCRF) (McMichael, 2008) concluded that an increased BMI is an established risk factor for several different types of cancers. Specifically, this association is established for esophageal adenocarcinoma, and cancers of the pancreas, colorectal, as well as post-menopausal breast, endometrial and kidney cancers. Furthermore, there is also evidence for a likely association of obesity with gallbladder cancer (Osorio-Costa, Rocha, Dias, & Carvalheira, 2009). These results were further confirmed by Reevs and colleagues in a study in which they examined the relationship between BMI and cancer incidence / mortality in more than a million British women aged 50–64. Increased BMI was associated with significantly increased incidence of postmenopausal breast cancer, endometrial cancer, kidney cancer and adenocarcinoma of the esophagus. Higher BMI was also significantly related to risk of leukemia, multiple myeloma, non-Hodgkin's lymphoma, pancreatic cancer and ovarian cancer (Reeves, et al., 2007). Renehan and colleagues presented a systemic review and a meta-analysis in which they quantified the risk of cancer associated with increased BMI based on data from 141 articles dealing with a total of 282137 incident cases. In men, BMI was strongly associated with esophageal adenocarcinoma, thyroid, colon and renal cancers, whereas in women increased BMI recorded strong associations with endometrial, esophageal adenocarcinoma and renal cancers. Weaker positive associations were noted between increased BMI and rectal cancer and malignant melanoma in men; postmenopausal breast, pancreatic, thyroid, and colon cancer in women, as well as leukemia, multiple myeloma and non-Hodgkin lymphoma in both sexes (Renehan, Tyson, Egger, Heller, & Zwahlen) (Table 1). Accordingly, obesity does not appear to have a uniform effect on all types of cancers, or to affect cancer risk the same in men and women. Despite the strong evidence supporting a link between overweight, obesity, cancer incidence and mortality, clinical studies in cancer patients have not yet firmly established how much weight loss is necessary to reduce cancer risk, what the impact of adipose distribution is on cancer risk and which underlying mechanisms may be responsible for the obesity-cancer link. Several recent studies report a significant reduction in total cancer incidence and mortality after weight loss induced by gastric bypass surgery compared with severely obese controls (Adams, et al., 2009; Sjostrom, et al., 2007).
Table 1.
Body mass index and the relative risk of different cancer types
| Cancer type | Men (95% Cl) | Women (95% Cl) |
|---|---|---|
| Breast postmenopausal premenopausal |
ND | 1.12 (1.08–1.16)a 0.92 (0.88–0.97)b |
| Colon | 1.24 (1.20–1.28)a | 1.09 (1.05–1.13)a |
| Endometrial | NA | 1.59 (1.50–1.68)a |
| Oesophageal adenocarcinoma | 1.52 (1.33–1.74)a | 1.51 (1.31–1.74)a |
| Renal | 1.24 (1.15–1.34)a | 1.34 (1.25–1.43)a |
| Leukemia | 1.08 (1.02–1.14)b | 1.17 (1.04–1.32)c |
| Malignant Melanoma | 1.17 (1.05–1.30)b | 0.96 (0.92–1.01)c |
| Multiple myeloma | 1.11 (1.05–1.18)a | 1.11 (1.07–1.15)a |
| Non-Hodgkin's lymphoma | 1.06 (1.03–1.09)a | 1.07 (1.00–1.14)c |
| Pancreas | 1.07 (0.93–1.23) | 1.12 (1.02–1.22)c |
| Prostate | 1.03 (1.00–1.07) | NA |
| Rectum | 1.09 (1.06–1.12)a | 1.02 (1.00–1.05) |
| Thyroid | 1.33 (1.04–1.70)c | 1.14 (1.06–1.23)b |
| Liver | 1.24 (0.95–1.62) | 1.07 (0.55–2.08) |
Relative risks are taken from a meta-analysis of data as reported in Renehan et al.(Renehan, Tyson et al.). The relative risk per 5 kg per m2 increase in body mass index is reported for each cancer type and sex.
p < .0001.
p < .01.
p ≤ .05.
Abbreviations: CI, confidence interval; NA, not applicable; ND, not determined.
3. Resolving the adipocyte-cancer cross-talk
Cancer cells do not exist as pure homogeneous populations in vivo. Instead, they are embedded in “cancer cell nests” that are surrounded by stromal cells. In the past decade, the tumor microenvironment and its constituent “stromal” cells have collectively risen in prominence, now embracing a broad field of investigation (Hanahan & Coussens, 2012). While much attention was invested in studying stromal components that directly relate to angiogenesis and inflammation, adipocytes represent an important and dynamic cell population in the microenvironment of a number of tumors, but their contribution has until recently been completely neglected. Adipocytes affect tumor characteristics through heterotypic signaling processes, a cross-talk that can only occur when both cell types are physically in close proximity during tumor progression and metastasis formation (Tan, et al., 2011).
3.1. Cancer-associated adipocytes (CAA)
Adipocytes surrounding tumors exhibit profound phenotypic changes that include both morphological and functional alternations. These phenotypically altered adipocytes mostly locate close to invasive cancer cells and are referred to as cancer-associated adipocytes (CAAs). Histological images of human mammary tumor biopsies contain few adipocytes, or often they are totally devoid of adipocytes. This depends however on the specific site the biopsy was taken from. The tumor invasive front exhibits a high ratio of adipocytes to fibroblasts, but may include reduced size adipocytes. In contrast, the stromal environment near the center of the tumor displays an extremely high fibroblast-like cell to adipocyte ratio (Tan, et al., 2011). These histological observations suggest that during tumor invasion, the direct interaction between adipocytes and epithelial cancer cells promote phenotypic changes of CAAs, which lead to “adipocyte dedifferentiation” and ultimately to an accumulation of fibroblast-like cells (Tan, et al., 2011; Wang, et al., 2012). The contributions of tumor surrounding fibroblasts in turn that are known as “cancer-associated fibroblasts (CAFs), is also important for tumor progression and metastasis, and these effects have been well established (Cirri & Chiarugi, 2011; Hanahan & Coussens, 2012). To date, it is still unknown which factors dictate or promote this transition of adipocyte to a more “fibroblastic” phenotype.
Adipocytes at the invasive front of human breast tumors are prompted by cancer cells to express Matrix metalloproteinase-11 (MMP11)/stromelysin-3, which is found to act as a potent negative regulator of adipogenesis by reducing pre-adipocyte differentiation and reversing mature adipocyte differentiation (Andarawewa, et al., 2005; Motrescu & Rio, 2008). In addition pro-inflammatory cytokines such as IL-6 and IL-1β were also shown to be associated with a modified adipocytes phenotype, in terms of delipidation and decreased adipocyte markers (Dirat, et al., 2011). It was reported that malignant epithelial cells secrete large quantities of TNFα and IL-11, which have anti-adipogenic effect by inhibiting the differentiation of fibroblasts to mature adipocytes, primarily via suppression of the adipogenic transcription factors C/EBPα and peroxisome proliferator-activated receptor-γ (PPARγ) (Meng, et al., 2001).
Several clinical studies evaluated the prognostic value of local adipose tissue invasion by cancer cells at the tumor margin. It was shown that scattered invasion into fat tissues (Kimijima, Ohtake, Sagara, Watanabe, & Takenoshita, 2000) and the invasive length of fat invasion (Hasebe, Imoto, Sasaki, & Mukai, 1998), (Hasebe, Mukai, Tsuda, & Ochiai, 2000) were related to a poor prognosis of ductal breast carcinoma. These observations were recently confirmed by Yamaguchi et al. showing that adipose tissue invasion of cancer cells at the margin of the tumor mass is indeed one of the biologic indicators of tumor aggressiveness and poor prognosis (Yamaguchi, Ohtani, Nakamura, Shimokawa, & Kanematsu, 2008). This positive correlation between adipose tissue invasion by cancer cells and poor disease outcome was found not only for breast cancer, but also for prostate, pancreas, kidney and colon cancers (M.-C. Rio, 2011). Experimental data based on both in vitro and in vivo studies support the important contribution of adipocytes to cancer progression. It was reported that adipocytes, promote the growth of breast carcinoma cells as well as colon cancer cells (Amemori, et al., 2007; Manabe, Toda, Miyazaki, & Sugihara, 2003). In line with the co-culture studies, high fat diet has been shown to increase tumor growth and metastases in different models of subcutaneous cancer cell transplantation (E. J. Kim, et al., 2011; H. Park, et al., 2011). We have applied an in vivo co-injection system using 3T3-L1 adipocytes and SUM159PT cancer cells, recapitulating host-tumor interactions in primary breast tumors, demonstrating that adipocytes favor tumorigenesis. Interestingly, systemic molecular analysis indicated that adipokines specifically induce several distinct transcriptional programs involved in promoting tumorigenesis, including increased cell proliferation, invasiveness, survival and angiogenesis (Iyengar, et al., 2003). Taken together, these data implicate the paracrine function between adipocytes and cancer, however, very little clear data directly point out of the primarily mechanisms that are involved in adipocyte-cancer cell interplay in vivo.
3.2. Adipokines
Adipose tissue is not only a long-term energy storage organ, but it is known as a major endocrine gland. Adipose tissue is responsible for biosynthesis and secretion of a large number of hormones and cytokines, referred to as adipokines (MacDougald & Burant, 2007) that serve as critical mediators of the metabolic functions of the tissue. Adipose tissue dysfunction results in altered serum levels of adipokines, and these changes may be directly involved in obesity-related tumorigenesis (Table 2). Here, we limit our discussion on two key adipokines, adiponectin and leptin, both of which play an important role in tumor development and progression.
Table 2.
Summary of adipokines and their potential role in cancer
| Adipokine | Primary source(s) | Cancer-related effects | Cancer outcome |
|---|---|---|---|
| Adiponectin | Adipocytes | Pro-angiogenic effect, reduced ceramide/S1 P ratio - in vivo Circulating adiponectin is inversely associated with obesity and incidence of obesity-related cancers |
Controversial |
| Leptin | Adipocytes | Pro-mitogenic, pro-survival, and pro-angiogenic effects. Increased glycolytic capacity |
Promoted |
| TNFα | Inflammatory cells, adipocytes, Cancer cells | Associated with adipose tissue inflammation Inhibited adipocyte differentiation |
Promoted |
| IL-6 | Adipocytes, inflammatory cells, liver, muscle | Associated with adipose tissue inflammation. Acquisition of pro-tumorigenic adipocytes phenotype |
Promoted |
| Collagen 6/Endotrophin | Adipocytes | Fibrosis, angiogenesis, induced proliferation and pro-survival signals Induced epithelial-mesenchymal transition (EMT) |
Promoted |
| MMP11 | Adipocytes | Inhibited adipogenesis Involvementin collagen VI folding |
Promoted |
See relevant references within the text
I) Adiponectin
Adiponectin is an established adipokine, with its plasma levels inversely correlated with obesity and type II diabetes (Trujillo & Scherer, 2005), exhibiting profound insulin-sensitizing, anti-inflammatory and anti-atherogenic properties (Berg, Combs, Du, Brownlee, & Scherer, 2001; Turer & Scherer, 2012). Adiponectin's actions are likely mediated through its two cognate receptors, adiponectin receptors 1 and 2 (AdipoR1 and AdipoR2) (Yamauchi, et al., 2003). An additional cell surface molecule with significant affinity for adiponectin, T-cadherin, has also been identified (Hug, et al., 2004). T-cadherin binds adiponectin, but lacks an intracellular signaling domain, and hence serves as a likely co-receptor for a yet to be defined cell surface adiponectin receptor.
Clinical studies have reported that circulating adiponectin levels are inversely proportional with the incidence rate of several tumors that are associated with obesity and insulin resistance, such as endometrial cancer, postmenopausal breast cancer, leukemia, colon, gastric and renal cancers (Kelesidis, Kelesidis, & Mantzoros, 2006). However, murine breast cancer models showed that adiponectin and its receptors exert potent pro-angiogenic effects that promote tumor growth (Denzel, et al., 2009; Hebbard, et al., 2008; Landskroner-Eiger, et al., 2009). Indeed, we and others found that in the absence of adiponectin, adiponectin null mice display a significant reduction of mammary tumor growth at early stages. Moreover, the reduced tumorigenic phenotype of adiponectin null mice was associated with reduced angiogenesis, indicating that adiponectin has potent angio-mimetic properties in tumor vascularization (Denzel, et al., 2009; Landskroner-Eiger, et al., 2009). Consistent with these observations, Hebbard et al reported that T cadherin deficiency limits mammary tumor vascularization and reduces tumor growth (Hebbard, et al., 2008). These data highlight the difference between epidemiological association (in which adiponectin reflects an inverse correlation with BMI) and adiponectin's molecular effects on tumor growth, as was demonstrated by manipulating of both adiponectin and T-cadherin levels in vivo. Interestingly, it has been observed that a number of human cancers express both adiponectin receptors (AdipoR1 and AdipoR2) at very high levels (Dalamaga, et al., 2009; Korner, et al., 2007; Petridou, et al., 2007; Takahata, et al., 2007; Takemura, et al., 2006; Yoneda, et al., 2008), raising the possibility that these receptors play a major role in cancer progression.
We have recently proposed a novel mechanism explaining adiponectin's systemic effects, in which adiponectin potently stimulates a ceramidase activity associated with its two receptors, AdipoR1 and AdipoR2, and enhances ceramide catabolism and formation of its anti-apoptotic metabolite sphingosine-1-phosphate (S1P), independently of AMPK (Holland, et al., 2011). According to this model, adiponectin mediates its effects through the action of its cognate receptors, thereby exerting systemic metabolic effects through the lowering of cellular ceramide levels and altering the ratio of ceramide to S1P. While ceramides mediate anti-proliferative responses, such as growth inhibition, apoptosis, differentiation, modulation of telomerase activity and senescence, S1P induces proliferation, transformation, angiogenesis and cell motility (Ogretmen & Hannun, 2004; Spiegel & Milstien, 2003; Visentin, et al., 2006). Since ceramide and S1P have opposite functions in regulating cell fate, the balance between this ceramide/S1P “rheostat” becomes a potent therapeutic target for cancer cells. Thus, it is tempting to speculate that adiponectin may act at least under some circumstances as a pro-tumorigenic factor through its effects on enhancing ceramidase activity, leading to a net increase in the S1P levels, which in turn may promote tumor growth and chemo-resistance.
II) Leptin
Leptin plays a pivotal role in regulating systemic energy balance by decreasing appetite and increasing metabolic rates (Friedman & Halaas, 1998). In contrast to adiponectin, leptin levels are directly proportional to the amount of body fat mass. In obese subjects, there is an overproduction of leptin leading to a reduced level of leptin responsiveness or “leptin resistance” in the brain (Munzberg & Myers, 2005). The synthesis of leptin in adipocytes is regulated by different humoral factors, most notably insulin, TNF-α, glucocorticoids, reproductive hormones and prostaglandins. Importantly, many of these very same factors critically involved in the regulation of leptin have also been associated with neoplastic processes (Garofalo & Surmacz, 2006). Moreover, in the context of adipose tissue expansion and cancer, leptin expression can also be induced under hypoxic conditions (Cascio, et al., 2008; Garofalo, et al., 2006).
In addition to its primary neuroendocrine functions, leptin modulates several processes in peripheral organs, manipulating the immune response, fertility and hematopoiesis, by acting as a pro-mitogenic factor, pro-survival factor, regulator of cellular metabolism or pro-angiogenic component, depending on the target tissue (Cirillo, Rachiglio, la Montagna, Giordano, & Normanno, 2008; Wauters, Considine, & Van Gaal, 2000). Six isoforms of leptin receptor (LEPR) have been identified, with the physiological relevance of the individual leptin receptor isoforms still currently under study (Gorska, et al., 2010).
The suggestion that leptin may play a role in cancer is based on the observation that LEPR is highly expressed in many tumor cells, including those derived from mammary, pancreatic and gastrointestinal tracts, lungs and leukemia cells (Hino, et al., 2000; Howard, Pidgeon, & Reynolds, 2010; Ishikawa, Kitayama, & Nagawa, 2004; Mix, et al., 2000; Tsuchiya, Shimizu, Horie, & Mori, 1999). In vitro studies on different human cancer cells have demonstrated the mitogenic, anti-apoptotic, pro-angiogenic and prometastatic properties of leptin (Carino, et al., 2008; Hoda, et al., 2007; Hoda & Popken, 2008; McMurtry, Simeone, Nieves-Alicea, & Tari, 2009; Rene Gonzalez, et al., 2009; Somasundar, Yu, Vona-Davis, & McFadden, 2003). However, correlations linking serum leptin concentrations with cancer incidence or progression have not been seen so far, regardless of the tumor type studied (Garofalo & Surmacz, 2006).
Pre-clinical studies utilizing obese rodent models (such as ob/ob and db/db mice as well as fa/fa Zucker rats), in which leptin or its receptors are not functional due to a genetic deficiency, have not managed to differentiate between the systemic metabolic changes (due to the increased adiposity), and the direct local effects of leptin action on tumor development (Cleary, et al., 2004; Cleary, et al., 2003; Hakkak, et al., 2005; J. Park, et al., 2011). More importantly, when using a mammary tumor model based on a MMTV-driven TGF-α transgene bred into the ob/ob or db/db backgrounds, no tumors development can be observed (Cleary, et al., 2004; Cleary, et al., 2003). This is due to the fact that in the absence of leptin or its receptors, the ductal epithelium in the mammary gland does not develop, and hence, no tumor lesions can arise.
In order to test leptin action in mammary tumor microenvironment more directly, we recently used a model in which leptin receptor levels were reconstituted in the brains of db/db mice through ectopic overexpression of the leptin receptor through a transgene. This results in a complete rescue of the metabolic phenotype seen in db/db mice (de Luca, et al., 2005). Surprisingly, these mice also develop a fully mature ductal epithelium, a structure not seen in db/db mice. When bred into a pro-tumorigenic MMTV-PyMT mammary tumor background, the lack of peripheral leptin receptors attenuated tumor progression and metastasis through a reduction of the ERK1/2 and Jak2/STAT3 pathways as well as reducing aerobic glycolytic capacity (Park, Kusminski, Chua, & Scherer, 2010). In line with this data, Zheng and colleges provide additional evidence for leptin's role in promoting mammary tumor burden, focusing on the effects of leptin on cancer stem cell populations (Park & Scherer, 2011; Zheng, et al., 2011).
In summary, in light of the somewhat contradictory results between measurements of circulating adipokine levels and results from pre-clinical studies that assess the effects of local adipokine action in the tumor microenvironment, it seems likely that adipokines, such as leptin and adiponectin, may exert direct local, pro-tumorigenic effects that are not directly related to obesity per se. Correlations obtained from the measurements of circulating adipokine concentrations may simply reflect the fact that the adipokine levels serve as surrogate biomarkers for obesity, and we should exercise caution interpreting results that are based purely on epidemiological studies as to whether a given adipokine serves either a protective or pro-tumorigenic role.
3.3. Obesity-associated inflammation
Adipose tissue is composed of adipocytes as well as stromal-vascular cells. Numerous stress factors including adipocyte hyperthrophy, hypoxia and fibrosis can induce inflammation in adipose tissue. Obesity is associated with a chronic state of low-grade inflammation that is due to increased concentrations of fatty acids, inflammatory cytokine production and an influx of immune cells that also add to the local inflammatory milieu in adipose tissue (Kanneganti & Dixit, 2012). Over the course of the development of obesity, adipose tissue becomes increasingly infiltrated with macrophages, neutrophils, T- and B-cells as well as mast cells (Kanneganti & Dixit, 2012; Nishimura, et al., 2009; Osborn & Olefsky, 2012; Talukdar, et al., 2012; Weisberg, et al., 2003; Xu, et al., 2003). Furthermore, enlarged adipocytes produce more inflammatory cytokines, such as TNFα, IL-6, IL-1β and monocyte chemoattractant protein (MCP)-1 compared to smaller adipocytes (Harvey, Lashinger, & Hursting, 2011). Among several inflammatory cytokines secreted by white adipose tissue (WAT), TNFα has received most of the focus and was the first adipose derived factor suggested to represent a link between obesity, inflammation and diabetes (Hotamisligil, Shargill, & Spiegelman, 1993). Indeed, TNFα expression is induced both in animal models of obesity and diabetes (Hotamisligil, et al., 1993) as well as in human obesity and in the context of insulin resistance (Hotamisligil, Arner, Caro, Atkinson, & Spiegelman, 1995).
Visceral fat accumulation rather than subcutaneous fat has been shown to cause impaired glucose metabolism, lipid disorders, and hypertension, and therefore it is considered to be a key player in the metabolic syndrome (Matsuzawa, 2008). Interestingly, major local concentration differences have been established between these two fat depots. For instance, the levels of plasma IL-6 are higher in the portal vein than in peripheral arterial blood in obese individuals, highlighting that visceral fat (draining into the portal vein) is an important site for IL-6 secretion and provides a potential mechanistic link between visceral adipose dysfunction and systemic inflammation in individuals with abdominal obesity (Fontana, Eagon, Trujillo, Scherer, & Klein, 2007). In line with these data, it was reported that infiltration of macrophages in obesity seems to be greater in visceral than in subcutaneous adipose tissue (Cancello, et al., 2006). More recently, it has been observed in obese subjects that endothelial cells isolated from visceral fat show a higher expression of genes related to angiogenesis and inflammation than endothelial cells from subcutaneous adipose tissue (Villaret, et al., 2010). All together suggest that visceral fat is more clinically relevant than the subcutaneous fat with regard to chronic inflammation and metabolic complication in general.
Both TNFα and IL-6 contribute to obesity-associated insulin resistance (Borst, 2004; T. H. Kim, et al., 2011) and much of the local production of inflammatory cytokines within adipose tissue is actually derived from infiltrating macrophages (Weisberg, et al., 2003) and other immune cells that can be found at higher abundance in adipose tissue of obese individuals (Ruan, Zarnowski, Cushman, & Lodish, 2003). Much attention has been directed towards the study of adipose tissue macrophages, a cell population that has a key role in systemic insulin resistance, glucose tolerance and the development of the metabolic syndrome and type 2 diabetes (Osborn & Olefsky, 2012). Adipose tissue from obese mice and humans is infiltrated with a large number of macrophages, which can ultimately comprise up to 40% of the cells in obese adipose tissue (Weisberg, et al., 2003; Xu, et al., 2003). Excess caloric intake causes adipocytes to secrete chemokines such as MCP-1 (Christiansen, Richelsen, & Bruun, 2005; Gerhardt, Romero, Cancello, Camoin, & Strosberg, 2001; Weisberg, et al., 2006), and additional critical factors that attract monocytes into adipose tissue, where they become activated and function as adipose tissue macrophages. In addition to the absolute number of macrophages recruited to adipose tissue, the polarization status of macrophages also influences the pathogenesis of obesity (Kanneganti & Dixit, 2012). “M2 macrophages” which are characterized by the production of anti-inflammatory molecules, are more frequently found in states of metabolic homeostasis and lean body mass, whereas M1 macrophages, in contrast, secrete higher concentrations of pro-inflammatory cytokines, and play a central role in promoting obesity-associated inflammation (Kanneganti & Dixit, 2012; Patsouris, et al., 2008). Several studies has suggested that the phenotypic switch from M2 to M1 is associated with the development of insulin resistance (Yehuda-Shnaidman & Schwartz, 2012). Depletion of CD11c-positive cells, a subset of pro-inflammatory adipose tissue macrophages, in obese insulin resistant mice leads to a rapid normalization of insulin sensitivity in adipose tissue as well as in muscle and liver (Patsouris, et al., 2008).
More than 150 years ago, Rudolph Virchow was the first to recognize that inflammation is a pre-disposing factor for tumorigenesis (Schmidt & Weber, 2006). Now, Inflammation is a recognized hallmark of cancer and pre-existing pro-inflammatory microenvironments are associated with increased cancer risk (Coussens & Werb, 2002; Hanahan & Weinberg, 2011). Inflammatory responses play decisive roles at different stages of tumor development, including initiation, promotion, malignant conversion, invasion and metastasis. They also affect immune surveillance and responses to therapy (Grivennikov, Greten, & Karin, 2010). In fact, up to 20% of cancers are linked to chronic infections, 30% can be attributed to tobacco smoking and inhaled pollutants, and 35% can be attributed to dietary factors (20% of cancer burden is linked to obesity) (Grivennikov, et al., 2010). Therefore, both the unique inflammatory milieu with many inflammatory cytokines and the increased immune cell infiltration into adipose tissue in the obese state are thought to be important links between obesity and cancer. Tumor-associated macrophages (TAMs), for instance, have a key role in the tumor microenvironment (Pollard, 2004), partially by promoting tissue invasion (Coussens, Tinkle, Hanahan, & Werb, 2000), angiogenesis (Murdoch, Muthana, Coffelt, & Lewis, 2008), and metastasis (Qian, et al., 2011). Moreover, inflammatory cytokines, such as TNFα and IL-6, that are highly expressed in chronically inflame individuals during obesity, have a well established pro-tumorigenic function, and act as regulators of tumor-associated inflammation (Grivennikov & Karin, 2011). Obesity-induced hepatocarcinogenesis was dependent on enhanced production of IL-6 and TNFα which cause hepatic inflammation and activation of the oncogenic transcription factor STAT3 (E. J. Park, et al., 2010). Changes in lipid metabolism in cancer cachexia, which result in marked fat mass reduction and increased lipolysis, were proposed to be induced by TNFα, along with other pro-inflammatory cytokines (Batista, et al., 2012).
Interestingly, the gene expression profile of human adipose tissue macrophages resembles human tumor-associated macrophages, suggesting that adipose tissue macrophages may very well contribute to cancer development in obese subjects, further strengthening the link between obesity and cancer (Mayi, et al., 2012).
3.4. Adipose tissue angiogenesis and hypoxia
Angiogenesis - the process of new blood vessel formation - plays a critical role in tumor expansion. The tumor microenvironment is composed of a variety of cell types that influence the angiogenic response to a tumor. Once a tumor exceeds a few millimeters in diameter, hypoxia and nutrient deprivation triggers an “angiogenic switch” to allow the tumor to progress (Folkman, 1974; Folkman, Watson, Ingber, & Hanahan, 1989). At that point, tumor cells release growth factors, chemokines, and cytokines, which initiate the sprouting and proliferation of quiescent endothelial cells on nearby blood vessels and lymphatics. These signals also recruit fibroblasts that deposit a repertoire of ECM proteins and other factors in an attempt to remodel and repair the site. Moreover, infiltrating myeloid cells into the tumor microenvironment, as part of the inflammatory response, release additional pro-angiogenic factors (Weis & Cheresh, 2011). Therefore, tumor angiogenesis is not exclusively regulated by cancer cells alone triggering expression of pro-angiogenic factors, but stromal cells in the tumor microenvironment are also instrumental in switching on and sustaining chronic angiogenesis in many tumor types (Hanahan & Coussens, 2012).
Many studies have pointed to the importance of microvasculature and angiogenesis within adipose tissue as a critical player in adipose tissue health and expansion. Remarkably, the expansion of adipose tissue is not unlike the propagation of a solid tumor: rapid growth induces hypoxia that in turn induces angiogenesis, which fuels more growth (Rutkowski, Davis, & Scherer, 2009). Like in tumor growth, in which both epithelial and stromal cells cooperate to induce a proper angiogenic response, adipose tissue expansion requires a tight cooperation between adipocytes, inflammatory and stromal cells, altogether contributing to the enhanced overall pro-angiogenic environment. Furthermore, as with tumor growth, the expansion of adipose tissue results in hypoxia. Hypoxia occurs in solid tumors when the consumption of oxygen exceeds its delivery by the vascular system (Vaupel & Mayer, 2007). In most large clinical studies, the prognosis is worse for patients with hypoxic tumors (Vaupel & Mayer, 2007). Furthermore, hypoxia leads to resistance to radiotherapy and anticancer chemotherapy, as well as predisposing for increased tumor metastasis (Brizel, et al., 1996; Brown, 2002; Hockel, et al., 1996; Semenza, 2012).
Several anti-angiogenic therapies are currently approved by the FDA for cancer. However, it is still questionable whether starving a tumor of its blood supply should be considered as an effective approach suppressing cancer progression. In both preclinical and clinical settings, resistance mechanisms limit the long-term benefit of VEGF-targeted strategies. Furthermore, recent evidence showed how VEGF-targeted therapies can change how tumor cells interact with their environment by converting them to a more aggressive and metastatic phenotype (Weis & Cheresh, 2011). Therefore, understanding how anti-angiogenic drugs function in vivo and what types of drugs could be combined to produce better results in different cancer diseases are key steps to improving the success of anti-angiogenic strategies.
Adipose tissue displays has high plasticity, and retains the potential to grow throughout the entire life (Box 1). The expansion of adipose tissue under conditions of nutritional excess is however highly dependent upon angiogenesis. Different fat pads vary with respect to their degree of angiogenic potential (Sun, et al., 2011). For instance, the vascular density and abundance of endothelial cells is higher in visceral adipose tissue compared to subcutaneous fat pads (Villaret, et al., 2010). A reciprocal interplay between endothelial cells and adipocytes suggests that dysfunction of either compartment would have a substantial impact on the other system (Cao, 2010). It is well established that endothelial dysfunction in obese individuals contributes to the development and progression of type 2 diabetes (Jansson, 2007). Consistent with this idea, we have recently demonstrated that up-regulation of VEGF-A specifically in adipocytes improves vascularization of the fat pads and causes “browning” (i.e. an increased number of brown adipocytes) in white adipose tissue, associated with increased energy expenditure and resistance to high fat diet mediated insults. In contrast, inhibition of VEGF-A leads to induced weight gain and systemic insulin resistance (Sun, et al., 2012). Adipocytes, adipose stromal cells and inflammatory cells produce multiple angiogenic factors (as VEGFA and FGF2), adipokines (as leptin, resistin and adiponectin) and cytokines (as IL-6) all of which stimulate angiogenesis and contribute to an overall pro-angiogenic microenvironment (Cao, 2010).
Hypoxia is a well-established characteristic feature of obese adipose tissue, due to the inability of the vasculature to keep pace with adipose tissue growth (Trayhurn, Wang, & Wood, 2008). Hypoxia stimulates both adipose-related inflammatory responses (Trayhurn, et al., 2008), as well as adipose tissue fibrosis (Halberg, et al., 2009; Khan, et al., 2009) leading to further adipose dysfunction. Using a transgenic model of overexpression of a constitutively active form of HIF-1α, we determined that HIF-1α initiates adipose tissue fibrosis, with an associated increase in local inflammation, rather than expected pro-angiogenic response (Halberg, et al., 2009). This suggests that HIF1α alone is unable to unleash the full pro-angiogenic potential of adipose tissue, and additional factors are critically involved in this process.
3.5. Remodeling ECM factors
The adipose tissue extracellular matrix (ECM) is an important component of the adipose tissue microenvironment that is constantly subjected to active modulation depending on the nutritional status of the individual (J. Park, et al., 2011). The ECM of adipose tissue not only provides mechanical support for a fat pad, but also regulates the physiological and pathological events of adipose tissue remodeling through variety of signaling pathways (Sun, et al., 2011). Remodeling of ECM by matrix metalloproteinases, such as MT1-MMP, contributes to the three-dimensional development of white adipose tissue in mice (Chun, et al., 2006). During adipose tissue expansion, the ECM requires constant remodeling to accommodate adipocyte growth. We have demonstrated a general upregulation of several ECM components in adipose tissue in the diabetic state, leading to significant tissue fibrosis with a negative impact on the metabolic performance of adipose tissue. In support of this model, the absence of collagen VI an important ECM component predominantly expressed in adipose tissue, is associated with substantial improvements in whole-body energy homeostasis (Khan, et al., 2009) due to the fact that less fibrosis persists and the tissue is more at ease to expand.
Tumors are also characterized by ECM remodeling and stiffness of the microenvironment. Indeed, the fibrotic reaction, referred to as desmoplasia, leading to the accumulation of ECM proteins, is strongly associated with many malignancies and has also been observed at tumor-distant sites where it may facilitate the successful establishment of metastatic lesions (Egeblad, Rasch, & Weaver, 2010). Various collagens, including collagen I, II, III, V, and IX show increased deposition during tumor formation (Egeblad, et al., 2010). Elevated deposition of fibrillar collagen, has been associated with mammary tumorigenesis, correlating with increased mammographic density and greater breast cancer risk (Provenzano, et al., 2006). Increased stromal collagen in mouse mammary tissue significantly increases tumor formation and results in a significantly more invasive phenotype accompanied with increased lung metastasis (Provenzano, et al., 2008). Levental and colleagues addressed the link between stromal collagen density, matrix stiffness, and tumor progression. They demonstrated collagen crosslinking by manipulating lysyl oxidase (LOX) expression (an enzyme that cross-links collagen fibers and other ECM components thereby mediating an increase in ECM rigidity) modulates tissue fibrosis and enhances focal adhesion formation, growth factor signaling and overall malignancy of the tumor (Levental, et al., 2009). In a number of papers, we have demonstrated that adipocyte-derived collagen VI is involved in mammary tumor progression in vivo (Iyengar, et al., 2003). We employed collagen VI knockout mice in the background of MMTV-PyMT, a mammary cancer model, demonstrating a dramatically reduced rate of early hyperplasia and primary tumor growth. Interestingly, collagen VI activates the pro-survival and proliferation pathways involving Akt, β-catenin and cyclinD1 to achieve this effect (Iyengar, et al., 2005). More recently, we have shown that the cleaved C5 domain of the COLVIα3 chain (COL6A3-C5, a fragment that we referred to as “endotrophin”) augments fibrosis, angiogenesis, and inflammation, and is associated with more aggressive mammary tumor growth and metastasis in the MMTV-PyMT mouse model. More importantly, these effects are partially mediated by the TGFβ pathway, contributing tissue fibrosis and epithelialmesenchymal transition (EMT) phenomena, a process associated with the acquisition of cancer cell invasiveness and survival. Furthermore, endotrophin is a novel chemokine effectively recruiting endothelial cells into the tumor microenvironment, resulting in a dramatic increase in angiogenesis. Endotrophin is therefore a potent tumor-promoting factor in the context of tumor / stromal adipocyte interactions (Park & Scherer, 2012).
MMP11 is another example of an ECM factor that is highly expressed by cancer associated adipocytes in the proximity of invading cancer cells relative to normal resting mature adipocytes that do not express MMP-11 to any significant extent. Overexpression of MMP11 is associated with poor clinical outcome in patients in numerous carcinomas (M. C. Rio, 2005). MMP-11 plays a role in adipogenesis and the acquisition of fibroblast-like phenotypes of adipocytes (Andarawewa, et al., 2005; Tan, et al., 2011) and is required for correct collagen VI folding and therefore for fat tissue cohesion and adipocyte function (Motrescu, et al., 2008).
Taken together, dysregulated ECM components produced by adipocytes, as part of the fibrotic response, influence numerous aspects of cancer cell behavior, as well as affect the recruitment of endothelial and immune cells to the local microenvironment (Lu, Takai, Weaver, & Werb, 2011; Lu, Weaver, & Werb, 2012).
3.6. Sex hormones
Adiposity influences the synthesis and bioavailability of sex hormones through at least three mechanisms (E. E. Calle & Kaaks, 2004). First, adipose tissues express a variety of sex-steroid metabolizing enzymes, such as aromatase, which convert adrenal androgens into estrogen. Second, obesity associated with increased circulation levels of insulin and IGF-1-both inhibit the synthesis of sex hormone-binding globulin, which is the major carrier protein for testosterone and estradiol in the plasma. As a result, the amount of unbound sex-steroid availability for bioactivity is increased (E. E. Calle & Kaaks, 2004). Finally, high insulin levels can increase ovarian, and possibly also adrenal androgen synthesis, and can cause the development of the polycystic ovary syndrome in some genetically susceptible premenopausal women (E. E. Calle & Kaaks, 2004). Epidemiological evidence supports the role of estrogens produced by adipose tissue in the pathogenesis of the breast cancer. Indeed, overweight or obese postmenopausal women exhibit a threefold increased risk for developing breast cancer compared with normal-weight postmenopausal women (Bulun, Chen, Moy, Brooks, & Zhao, 2012). Estrogen, a product of the aromatase enzyme in adipose tissue, has been considered as the hormone responsible for increasing breast cancer risk in obese postmenopausal women (Bulun, et al., 2005). There are two sources of estrogen for breast cancer. First, estrogen that arises from extraovarian body sites such as subcutaneous adipose tissue and skin reaches breast cancer by way of circulation in an endocrine manner. Second, estrogen locally produced in breast cancer tissue makes an impact via paracrine or intracrine mechanisms (Bulun, et al., 2005). Work from several laboratories over the past two decades has suggested that breast adipose tissue fibroblasts are crucial site for aromatase expression and estrogen production, and has linked them to the development of breast cancer (Bulun, et al., 2012). Approximately 90% of aromatase activity and mRNA in breast adipose tissue is found in undifferentiated fibroblasts rather than mature adipocytes. Therefore, aromatase overexpression is linked to inhibition of adipogenic differentiation and a desmoplastic reaction. Obesity is known to induce inflammatory factors such as TNFα and prostaglandin E2, which are known as inducers of aromatase expression in adipose fibroblasts (Bulun, et al., 2012). Aromatase in breast adipose tissue (versus adipose tissue at other body sites) might have a substantially higher impact on carcinogenesis because of its proximity to the ductal epithelial cells. In fact, the most effective hormonal treatment of postmenopausal breast cancer has been the use of aromatase inhibitors that block aromatase activity in the breast and the periphery, thereby reducing the amount of local estrogen production - which in turn helps to suppress recurrence of the breast tumors (Bulun, et al., 2012). In addition to breast cancer, aromatase is also expressed in endometrial cancer tissue, and aromatase inhibitors have been used to treat endometrial cancer as well, however the pathologic significance of local estrogen biosynthesis via aromatase expression in endometrial cancer tissue or the therapeutic potential of aromatase inhibitors in the management of the disease are not yet clear (Bulun, et al., 2007).
3.7. Stromal-epithelial metabolic coupling to cancer growth
Multiple molecular mechanisms, both intrinsic and extrinsic, converge to alter the cellular metabolism of cancer cells and provide support to the three basic needs of dividing cells: rapid ATP generation to maintain cellular energy status; increased biosynthesis of macromolecules; and tightened maintenance of appropriate cellular redox status (Cairns, Harris, & Mak, 2011). To meet these demands, both cancer cells and stromal cells undergo metabolic changes. In addition to the genetic changes that alter tumor cell metabolism, the tumor microenvironment has a major role in determining the metabolic phenotype of tumor cells.
The best characterized and most widely appreciated metabolic phenotype observed in tumor cells is the “Warburg effect”, which is a shift of ATP generation from oxidative phosphorylation to glycolysis, even under normal oxygen concentrations. This effect was later found to be regulated by intrinsic genetic factors, such as oncogenes and tumor suppressor genes, as well as external responses to the tumor microenvironment (Cairns, et al., 2011). However, aerobic glycolysis is not a universal feature of all cancers in humans (Moreno-Sanchez, Rodriguez-Enriquez, Marin-Hernandez, & Saavedra, 2007), and both clinical FDG-PET data, as well as in vitro and in vivo experimental studies show that tumor cells are capable of using alternative fuel sources. In fact, amino acids, fatty acids and even lactate have been shown to function as fuels for tumor cells in a subset of micro-environmental settings (Sonveaux, et al., 2008; Whitaker-Menezes, et al., 2011; Yang, et al., 2009). Accordingly, the hallmarks of cancer metabolism is much more nuanced than can be explained by a single metabolic phenotype and recent evidence highlights an unexpected complexity of alterations in metabolic pathways within tumors. These recent observations also highlight the importance of “metabolic coupling” (i.e. an active exchange of substrates) between cancer and stromal cells.
Lisanti and co-workers proposed a new model to explain the Warburg effect in tumor metabolism, termed “the Reverse Warburg Effect”, in which stromal fibroblasts undergo aerobic glycolysis and provide adjacent cancer cells with energy-rich nutrients (such as pyruvate and lactate) in a paracrine fashion (Pavlides, et al., 2009), as evidenced mainly through the use of in vitro models (Bonuccelli, et al., 2010; Migneco, et al., 2010; Whitaker-Menezes, et al., 2011). In line with this concept, enhanced lipolysis in host tissues can potentially fuel tumor growth by releasing free fatty acids (FFAs) from host adipocytes to be recycled via β-oxidation in cancer cell mitochondria.
Nieman et al. showed that primary omental adipocytes promote homing, migration, and invasion of ovarian cancer cells by induced lipolysis in adipocytes, providing fatty acids for use in cancer cells, in order to support tumor metastasis (Nieman, et al., 2011). A co-culture model of adipocytes with ovarian cancer cells suggested that In the presence of ovarian cancer cells, adipocytes released significantly higher levels of FFAs and glycerol than adipocytes cultured in the absence of cancer cells. The adipocyte lipid binding protein FABP4 was identified as a key mediator of ovarian-cancer cell-adipocyte interaction in the host, mediating increased lipid availability leading to a more effective support of metastatic growth (Nieman, et al., 2011). Collectively, this observations suggests that “metabolic coupling” between adipocytes and cancer cells favors primary tumor and metastatic growth, which can be reversed by inhibiting lipid transport from adipocytes to carcinoma cells.
Das et al. present strong evidence for the critical role of adipocyte lipolysis in cancer-associated cachexia (Das, et al., 2011). Cancer-associated cachexia (CAC) is a multi-factorial wasting syndrome, accounting for about 20% of cancer deaths, characterized by the loss of adipose tissue and skeletal muscle mass (Tisdale, 2002). Patients with cancer cachexia show a progressive loss of body weight, which is mainly due to loss of fat and skeletal muscle, and directly correlates with poor prognosis and survival. Although anorexia is frequently present in cancer patients, lower food intake alone cannot explain the metabolic changes that are seen in the cachectic state, and unfortunately, nutritional supplementation and pharmacological manipulation of appetite are unable to reverse this catabolic process (Tisdale, 2002). Das and colleagues examined the role of two lipases: adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL), both of which are crucial for triglyceride lipolysis in CAC. Inhibition of ATGL, and to a lesser extent, HSL, prevented cachexia and weight loss. Moreover, total lipase activities (combined ATGL and HSL) were significantly higher in visceral WAT of cancer patients compared with individuals without cancer, and significantly higher in cancer patients with cachexia compared with cancer patients without cachexia (Das, et al., 2011). Based on this work, it is tempting to speculate that drugs targeting adipose tissue lipases may represent a therapeutic strategy to avoid the devastating conditions of CAC. Furthermore, these data provide additional support for the intense metabolic coupling between cancer and host cells (Martinez-Outschoorn, et al., 2011).
Interestingly, many tumor cells display a lipogenic phenotype. For example, elevated levels of fatty acid synthase (FASN) - a key lipogenic multi-enzyme complex catalyzing the terminal steps in the de novo biogenesis of fatty acids, correlates with poor prognosis in breast cancer patients, while inhibiting FASN results in decreased cell proliferation, loss of cell viability and decreased tumor growth in vivo (Kuhajda, et al., 2000; Menendez & Lupu, 2007; Zhou, et al., 2007). On the other hand, a lipolytic enzyme, monoacylglycerol lipase (MAGL), was found to be highly expressed in aggressive human cancer cells and primary tumors, where it regulates a fatty acid network (Nomura, et al., 2010). Although the relationship between the synthesis, storage and the use of FFAs in tumor cells is poorly understood, it becomes clear that lipid metabolites, supplied by either by exogenous sources - such as adipose tissue, or by intracellular de novo synthesis, are required for promoting tumor growth and metastasis.
4. Therapeutic implications
A better understanding of adipocyte-cancer cell interactions, as well as the molecular alterations of adipose tissue dysfunction manifest during obesity, may open up new avenues for potential therapeutic interventions. To date, the clinical data related to therapeutic strategies is very limited in this area (Table 3). However, focusing on some of the pro-tumorigenic factors discussed above, a number of factors bear promise as targets that lend themselves to effectively disrupt the cross-talk between adipocytes and cancer cells.
Table 3.
Clinically available metabolic agents and their anti-tumorigenic effects
| Therapeutic strategy | Molecular target | Agents | Cancer-related effects | Testing model | Clinical evidences |
|---|---|---|---|---|---|
| Adipocyte differentiation | PPARγ | TZDs |
|
|
|
| Insulin resistance | AMPK/mTOR axis | Metformin |
|
|
|
| Inflammation | COX2 | NSAIDs |
|
|
|
| Estrogen synthesis | Aromatase | Aromatase inhibitors |
|
Human and mouse studies on breast cancer |
|
See relevant references within the text
4.1. Targeting adipocyte differentiation- Thiazolidinediones and PPARγ agonists
Thiazolidinediones (TZDs) are anti-diabetic drugs that act as insulin sensitizers and that were used in the management of type 2 diabetes mellitus. TZDs, which are ligands for the transcription factor peroxisome proliferator-activated receptor gamma (PPARγ), have a wide spectrum of actions, including modulation of glucose and lipid homeostasis, inflammation, atherosclerosis, bone remodeling and cell proliferation (Cariou, Charbonnel, & Staels, 2012). PPARγ, a member of the PPAR subfamily of nuclear receptors, which functions as an obligate heterodimer with RXRs, plays a dominant role in adipose cell differentiation, modulates metabolism and inflammation in immune cells, and has strong anti-mitogenic properties (Tontonoz & Spiegelman, 2008). The role of PPARγ in carcinogenesis is however not well understood, and its functions may differ from tumor type to tumor type.
The ability of PPARγ to direct the program of adipocyte differentiation, characterized by complete cessation of cell growth, was a stimulus to examine the ability of this receptor to affect tumor growth (Tontonoz & Spiegelman, 2008). Since PPARγ's expression in adipocytes is the highest, its effect on liposarcoma, a malignancy of the adipose lineage, was first examined. Indeed, PPARγ is highly expressed in each of the major histologic types of human liposarcomas and by treatment with the PPARγ ligand pioglitazone, primary human liposarcoma cells are induced to undergo terminal differentiation, suggesting that PPARγ ligands may be useful therapeutic agents for the treatment of liposarcomas through triggering terminal differentiation events (Tontonoz, et al., 1997). Accordingly, in a small clinical trial, in which patients with advanced liposarcoma were treated with troglitazone (yet another TZD compound), several patients showed a dramatic differentiation of their tumors in situ, indicating that lineage-appropriate differentiation can be induced pharmacologically in a human solid tumor (Demetri, et al., 1999). In addition to adipocytes, PPARγ modulates the proliferation and apoptosis of many cancer cell types, and it is expressed in many human tumors, including lung, breast, colon, prostate and bladder (Han & Roman, 2007). Furthermore, PPARγ agonists reduce aromatase activity in cultured mammary pre-adipocytic stromal cells. This may affect local estrogen production in breast fat and may have potential therapeutic benefit in the treatment of breast cancer (Rubin, Zhao, Kalus, & Simpson, 2000).
Despite the accumulating data supporting the anti-proliferative and pro-differentiation effects of PPARγ, an increased risk of bladder cancer led to discontinuation of pioglitazone's commercialization in a number of countries (Cariou, et al., 2012). To date, a clear mechanistic explanation for the proposed increased risk of bladder cancer in pioglitazone-treated patients is however still lacking (Cariou, et al., 2012).
Interestingly, naturally occurring somatic mutations in the gene encoding PPARγ have been found in sporadic colorectal carcinomas, although the frequency of mutations is likely to be low (Koeffler, 2003). A murine model does point to the protective role of PPARγ, since loss of even one allele of PPARγ gene predisposes mice to cancer. Thus, PPARγ must formally be considered a tumor suppressor gene (Tontonoz & Spiegelman, 2008). TZDs can alter tumor growth both in a PPARγ-dependent and PPARγ-independent manner. How PPARγ ligands can act independently of PPARγ is under active study. However, some “off-target” effects may underlie the anti-tumorigenic effects of TZDs. These effects attributed to TZDs include the inhibition of Bcl-2/Bcl-xL function (leading to inhibition of anti-apoptotic functions), induction of proteasomal degradation of cell-cycle and apoptosis regulatory proteins, and transcriptional repression of androgen receptor (AR), through degradation of the transcription factor Sp1 (Wei, Yang, Lee, Kulp, & Chen, 2009).
PPARγ ligands have been utilized in a therapeutic context as a monotherapy in several advanced forms of human cancer, including prostate, breast and colon. Unfortunately, no indications of beneficial effects were observed. However, combination therapies of PPARγ agonists with other drugs should still be entertained (Tontonoz & Spiegelman, 2008). Indeed, Girnun and colleges have observed a striking synergy between rosiglitazone (another TZD compound) and platinum-based drugs in several different cancer types both in vitro and using transplantable and chemically induced “spontaneous” tumor models (Girnun, et al., 2007). In view of the above, PPARγ ligands may represent a promising, novel therapeutic approach for a subset of human malignancies.
4.2. Targeting insulin resistance- Metformin
Metformin is a widely used anti-diabetic drug prescribed for decades for the treatment of type 2 diabetes. In diabetic patients, it reduces hepatic glucose production, increases insulin sensitivity and glucose utilization by muscle and adipocytes, resulting in decreased insulinemia and amelioration of insulin resistance (Bost, Sahra, Le Marchand-Brustel, & Tanti, 2012).
Metformin activates AMP-activated kinase (AMPK), a kinase regulated by the liver kinase B1 (LKB1), a tumor suppressor gene. AMPK activation inhibits the mammalian target of rapamycin (mTOR), which controls protein synthesis and cell proliferation, is frequently activated in malignant cells and is associated with resistance to anticancer drugs (Bost, et al., 2012; Jalving, et al., 2010). The hypothesis that metformin may have anti-tumorigenic effects was confirmed by Evans and colleagues, demonstrating that metformin decreases the incidence of cancer in diabetic patients (Evans, Donnelly, Emslie-Smith, Alessi, & Morris, 2005; Libby, et al., 2009). A large prospective study performed in Taiwan, indicates that metformin treatment reduces the incidence of several gastroenterological cancers in treated diabetic patients to near or even below the incidence observed in non-diabetic patients (Lee, et al., 2011). Studies in rodent models confirmed that metformin induces AMPK activation, can inhibit tumor growth and prevent or delay tumor development (Jalving, et al., 2010). Additional reported mechanisms of action for metformin include reduced levels of insulin-like growth factor, insulin and HER2-mediated signaling, inhibition of mTOR signaling, inhibition of angiogenesis and induction of cell cycle arrest and apoptosis (Jalving, et al., 2010).
4.3. Targeting tumor metabolism
The concept of metabolic coupling between tumor cells and their hosts raises the possibility for new therapeutic avenues. As such, drugs that target glycolysis or catabolism in the surrounding tumor stroma may be beneficial in preventing tumor progression and metastasis. As discussed above, FFAs are another crucial source of metabolic fuel derived from adipocytes, supporting both energetic and anabolic requirements of tumor cells. Therefore, drugs that target lipolysis and FFAs efflux from adipocytes to cancer cells, as well as drugs that interfere with FFA oxidation in cancer cells may provide additional mechanism-based strategies to curb tumor growth.
Another aspect of tumor metabolism that deserves attention is the area of cancer cell mitochondria. Mitochondria are emerging as promising targets, since inhibition of mitochondrial activity ultimately not only leads to impaired bioenergetic function of cancer cell mitochondria but also to the induction of intrinsic apoptotic pathways (Ralph, Rodriguez-Enriquez, Neuzil, & Moreno-Sanchez, 2010). Interestingly, metformin treatment inhibits complex I of the respiratory chain in mitochondria, providing another AMPK independent mechanism contributing to its cancer protective role (Hirsch, et al., 2012). A recent study has demonstrated that the combination of metformin and 2-deoxyglucose inhibited mitochondrial respiration and glycolysis in prostate cancer cells, leading to a massive ATP depletion which affected cell viability by inducing apoptosis (Ben Sahra, Tanti, & Bost, 2010). Furthermore, most chemotherapeutic agents target the induction of apoptosis in cancer cells, emphasizing the central role of mitochondria both as regulator of cancer cell metabolism as well as a target for overcoming drug resistance. In this context, stromal-epithelial metabolic interactions have also been proposed to induce chemo-resistance (Martinez-Outschoorn, et al., 2011).
4.4 Targeting dysfunctional adiposity
Adipose tissue dysfunction associated with obesity may play a role in carcinogenesis by affecting production of adipokines, chronic inflammation, hypoxia, angiogenesis, and ECM remodeling (Figure 1). Each of these aspects of the dysregulated adipose tissue promoting tumorigenesis provides a promising mechanism-based strategy for clinical intervention. For example, increased adiposity is very much associated with chronic inflammation, ultimately leading to an increased cancer risk. Evidence for the significance of inflammation in neoplastic progression comes from study of cancer risk among long-term users of aspirin and Non-Steroidal Anti Inflammatory Drugs (NSAIDs). The use of NSAIDs for cancer prophylaxis has gained much attention in recent years. A number of epidemiological studies have indicated that long term Aspirin/NSAIDs use is associated with 40–50% reduction in the risk of colorectal cancer (Garcia Rodriguez & Huerta-Alvarez, 2000; Giovannucci, et al., 1995; Gupta & Dubois, 2001; Thun, Namboodiri, & Heath, 1991), and a meta-analysis suggested that it may be effective in other inflammation-induced cancers as well (liver, oesophagus), but is less relevant for cancers of the ovary, lung or kidney (Gonzalez-Perez, Garcia Rodriguez, & Lopez-Ridaura, 2003). Based on this idea, clinical studies of cancer incidence in the obese population treated with either aspirin or NSAIDs should further determine the therapeutic potential of modulating inflammation in obesity.
4.5. Targeting estrogen synthesis- aromatase inhibitors
Extra-ovarian tissues provide the major source of estrogen synthesis in men and in postmenopausal women through aromatization of adrenal androgens (Bulun, et al., 2005). Studies of postmenopausal women showed that the conversion of androgens to estrogen was higher in obese subjects, suggesting that adipose tissue might be the primary site of aromatization (Santen, Brodie, Simpson, Siiteri, & Brodie, 2009).
Two separate treatment strategies, available for at least the past three decades, can reduce the target organ effects of estrogen. One is designed to block estrogen action with anti-estrogens that bind to the estrogen receptor and interfere with receptor-mediated transcriptional events. The other uses aromatase inhibitors to block the rate-limiting step in synthesis of estradiol, involving the conversion of androgens to estrogens. Aromatase inhibitors lower the concentrations of estradiol in plasma and tissue and reduce the amount of estrogen available to stimulate estrogen receptor-mediated transcription. At present, aromatase inhibitors are the most effective endocrine treatment of estrogen-responsive breast cancer (Santen, 2002). The third-generation aromatase inhibitors, which were approved in the United States to treat postmenopausal breast cancer in the 1990s, were proven to be superior to tamoxifen (the direct estrogen receptor antagonist). These new inhibitors have a benign side-effect profile and suppress estrogen production in extra-ovarian tissues and within the breast cancer tissue itself. This effectively blocks estrogenic action, reduces recurrences, and prolongs disease-free survival in postmenopausal women with breast cancer. Aromatase inhibitors are also effective in the treatment of breast cancer that becomes resistant to treatment with tamoxifen (Bulun, et al., 2005; Santen, 2002; Santen, et al., 2009).
5. Conclusions
Over the past three decades, ground-breaking work has been performed on expanding our knowledge of the altered cellular signaling mechanisms responsible for the progression from healthy cells to transformed, aggressively growing tissues. Two new, interrelated areas have received more attention in the more recent past. One is the increased realization that the stromal microenvironment surrounding the tumor cells plays a critical role for tumor progression and metastasis. Growing evidence from epidemiologic and preclinical studies highlights the link between obesity and cancer. This moves adipocytes center stage as important contributors that help shape the tumor microenvironment. The second area of interest focuses on tumor metabolism, reviving the original observations related to the Warburg effect. While most of the tumor metabolic studies have been focused on the intrinsic metabolic changes occurring in tumor cells, the exchange of metabolites between stromal cells and tumor cells has been widely ignored. More recent studies highlighted the significance of the tumor microenvironment, especially the contributions of adipocytes and fibroblasts, towards supplying relevant metabolites that fuel cellular growth of the tumor cell. With this more comprehensive map of cancer-associated metabolic changes at hand, novel opportunities arise, targeted at manipulating pathways that relate to the stromal compartment and affect both growth factors as well as production of critical substrates that fuel tumor growth.
Box 1. Adipose tissue composition and plasticity.
Adipose tissue is mainly comprised of adipocytes that are crucial for both its energy storage capabilities and its endocrine activity. Other cell types, including pre-adipocytes, lymphocytes, macrophages, fibroblasts and vascular cells, are referred as the stromal vascular fraction (SVF) of adipose tissue, contribute as well to its growth and function (Galic, Oakhill, & Steinberg, 2010). The composition of the adipose organ varies in different anatomical locations, and under different physiological conditions. Acclimatization to different temperatures, pregnancy/lactation, obesity, fasting, and caloric restrictions and caloric excess are the most common physiological and pathological conditions in which the plasticity of the adipose organ is challenged (Cinti, 2009). For example, mammals have two types of adipocytes, white and brown, with a distinct anatomy and physiology. White adipocytes store lipids, brown adipocytes oxidize them to produce heat. White adipose tissue of cold-acclimatized mice is darker in color than that of animals exposed to euthermic conditions, suggesting a switch to a more brown-like phenotype, due to a reversible increase in the amount of brown adipocytes, capillaries and sympathetic neuronal in the organ (Cinti, 2009).
Box 2. Adipose progenitors and cancer.
Clinically, expansion of white adipose tissue (WAT) leading to obesity results from mature adipocyte hypertrophy, as well as hyperplasia resulting from progenitor cell proliferation. Multipotent progenitors of preadipocytes in WAT have been identified in the stromal vascular fraction (SVF) (Zhang, Bellows, & Kolonin, 2010). Preadipocytes considered as intermediary cell type along the adipocyte differentiation axis between the bone marrow derived mesenchymal cells and mature adipocytes, and they are morphologically indistinguishable from fibroblasts, although they are already committed to the adipocytic lineage (Rosen & Spiegelman, 2000). It has been proposed that WAT-derived mesenchymal stem (stromal) cells, termed adipose stem cells (ASC) contribute to tumorigenesis by increasing tumor angiogenesis, or by paracrine or endocrine signalling to malignant cells (Zhang, et al., 2010). Studies in animal models demonstrate that ASCs injected intravenously or subcutaneously engrafted into the tumor stroma and vasculature and promote tumor growth. Recently, it was shown that recruitment of endogenous ASCs into tumors in obesity is associated with increased vascularization and adipogenesis accompanied by proliferation of malignant cells (Zhang, et al., 2010; Zhang, et al., 2012). These data raise many open questions regarding the relative functional contribution of adipose tissue subpopulations in various cancers types and stages among obese versus lean subjects. It is also unknown what constitutes the critical signal that induces cellular trafficking and recruitment into the tumor microenvironment.
Acknowledgements
The authors were supported by US National Institutes of Health grants R01-DK55758 and P01-DK088761 (P.E.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest The authors do not report any conflicts of interest.
6. References
- Adams TD, Stroup AM, Gress RE, Adams KF, Calle EE, Smith SC, Halverson RC, Simper SC, Hopkins PN, Hunt SC. Cancer incidence and mortality after gastric bypass surgery. Obesity. 2009;17:796–802. doi: 10.1038/oby.2008.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amemori S, Ootani A, Aoki S, Fujise T, Shimoda R, Kakimoto T, Shiraishi R, Sakata Y, Tsunada S, Iwakiri R, Fujimoto K. Adipocytes and preadipocytes promote the proliferation of colon cancer cells in vitro. American journal of physiology. Gastrointestinal and liver physiology. 2007;292:G923–929. doi: 10.1152/ajpgi.00145.2006. [DOI] [PubMed] [Google Scholar]
- Andarawewa KL, Motrescu ER, Chenard MP, Gansmuller A, Stoll I, Tomasetto C, Rio MC. Stromelysin-3 is a potent negative regulator of adipogenesis participating to cancer cell-adipocyte interaction/crosstalk at the tumor invasive front. Cancer research. 2005;65:10862–10871. doi: 10.1158/0008-5472.CAN-05-1231. [DOI] [PubMed] [Google Scholar]
- Batista ML, Jr., Peres SB, McDonald ME, Alcantara PS, Olivan M, Otoch JP, Farmer SR, Seelaender M. Adipose tissue inflammation and cancer cachexia: possible role of nuclear transcription factors. Cytokine. 2012;57:9–16. doi: 10.1016/j.cyto.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Ben Sahra I, Tanti JF, Bost F. The combination of metformin and 2 deoxyglucose inhibits autophagy and induces AMPK-dependent apoptosis in prostate cancer cells. Autophagy. 2010;6 doi: 10.4161/auto.6.5.12434. [DOI] [PubMed] [Google Scholar]
- Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nature medicine. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- Bonuccelli G, Tsirigos A, Whitaker-Menezes D, Pavlides S, Pestell RG, Chiavarina B, Frank PG, Flomenberg N, Howell A, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Ketones and lactate “fuel” tumor growth and metastasis: Evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell cycle. 2010;9:3506–3514. doi: 10.4161/cc.9.17.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst SE. The role of TNF-alpha in insulin resistance. Endocrine. 2004;23:177–182. doi: 10.1385/ENDO:23:2-3:177. [DOI] [PubMed] [Google Scholar]
- Bost F, Sahra IB, Le Marchand-Brustel Y, Tanti JF. Metformin and cancer therapy. Current opinion in oncology. 2012;24:103–108. doi: 10.1097/CCO.0b013e32834d8155. [DOI] [PubMed] [Google Scholar]
- Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Bean JM, Prosnitz LR, Dewhirst MW. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer research. 1996;56:941–943. [PubMed] [Google Scholar]
- Brown JM. Tumor microenvironment and the response to anticancer therapy. Cancer biology & therapy. 2002;1:453–458. doi: 10.4161/cbt.1.5.157. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Chen D, Lu M, Zhao H, Cheng Y, Demura M, Yilmaz B, Martin R, Utsunomiya H, Thung S, Su E, Marsh E, Hakim A, Yin P, Ishikawa H, Amin S, Imir G, Gurates B, Attar E, Reierstad S, Innes J, Lin Z. Aromatase excess in cancers of breast, endometrium and ovary. The Journal of steroid biochemistry and molecular biology. 2007;106:81–96. doi: 10.1016/j.jsbmb.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulun SE, Chen D, Moy I, Brooks DC, Zhao H. Aromatase, breast cancer and obesity: a complex interaction. Trends in endocrinology and metabolism: TEM. 2012;23:83–89. doi: 10.1016/j.tem.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulun SE, Lin Z, Imir G, Amin S, Demura M, Yilmaz B, Martin R, Utsunomiya H, Thung S, Gurates B, Tamura M, Langoi D, Deb S. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev. 2005;57:359–383. doi: 10.1124/pr.57.3.6. [DOI] [PubMed] [Google Scholar]
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nature reviews. Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nature reviews. Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. New England Journal of Medicine. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- Cancello R, Tordjman J, Poitou C, Guilhem G, Bouillot JL, Hugol D, Coussieu C, Basdevant A, Bar Hen A, Bedossa P, Guerre-Millo M, Clement K. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes. 2006;55:1554–1561. doi: 10.2337/db06-0133. [DOI] [PubMed] [Google Scholar]
- Cao Y. Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nature reviews. Drug discovery. 2010;9:107–115. doi: 10.1038/nrd3055. [DOI] [PubMed] [Google Scholar]
- Carino C, Olawaiye AB, Cherfils S, Serikawa T, Lynch MP, Rueda BR, Gonzalez RR. Leptin regulation of proangiogenic molecules in benign and cancerous endometrial cells. International journal of cancer. Journal international du cancer. 2008;123:2782–2790. doi: 10.1002/ijc.23887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariou B, Charbonnel B, Staels B. Thiazolidinediones and PPARgamma agonists: time for a reassessment. Trends in endocrinology and metabolism: TEM. 2012;23:205–215. doi: 10.1016/j.tem.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Cascio S, Bartella V, Auriemma A, Johannes GJ, Russo A, Giordano A, Surmacz E. Mechanism of leptin expression in breast cancer cells: role of hypoxia-inducible factor-1alpha. Oncogene. 2008;27:540–547. doi: 10.1038/sj.onc.1210660. [DOI] [PubMed] [Google Scholar]
- Christiansen T, Richelsen B, Bruun JM. Monocyte chemoattractant protein-1 is produced in isolated adipocytes, associated with adiposity and reduced after weight loss in morbid obese subjects. International journal of obesity. 2005;29:146–150. doi: 10.1038/sj.ijo.0802839. [DOI] [PubMed] [Google Scholar]
- Chun TH, Hotary KB, Sabeh F, Saltiel AR, Allen ED, Weiss SJ. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell. 2006;125:577–591. doi: 10.1016/j.cell.2006.02.050. [DOI] [PubMed] [Google Scholar]
- Cinti S. Transdifferentiation properties of adipocytes in the adipose organ. American journal of physiology. Endocrinology and metabolism. 2009;297:E977–986. doi: 10.1152/ajpendo.00183.2009. [DOI] [PubMed] [Google Scholar]
- Cirillo D, Rachiglio AM, la Montagna R, Giordano A, Normanno N. Leptin signaling in breast cancer: an overview. Journal of cellular biochemistry. 2008;105:956–964. doi: 10.1002/jcb.21911. [DOI] [PubMed] [Google Scholar]
- Cirri P, Chiarugi P. Cancer associated fibroblasts: the dark side of the coin. American journal of cancer research. 2011;1:482–497. [PMC free article] [PubMed] [Google Scholar]
- Cleary MP, Juneja SC, Phillips FC, Hu X, Grande JP, Maihle NJ. Leptin receptor-deficient MMTV-TGF-alpha/Lepr(db)Lepr(db) female mice do not develop oncogene-induced mammary tumors. Experimental biology and medicine. 2004;229:182–193. doi: 10.1177/153537020422900207. [DOI] [PubMed] [Google Scholar]
- Cleary MP, Phillips FC, Getzin SC, Jacobson TL, Jacobson MK, Christensen TA, Juneja SC, Grande JP, Maihle NJ. Genetically obese MMTV-TGF-alpha/Lep(ob)Lep(ob) female mice do not develop mammary tumors. Breast cancer research and treatment. 2003;77:205–215. doi: 10.1023/a:1021891825399. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalamaga M, Migdalis I, Fargnoli JL, Papadavid E, Bloom E, Mitsiades N, Karmaniolas K, Pelecanos N, Tseleni-Balafouta S, Dionyssiou-Asteriou A, Mantzoros CS. Pancreatic cancer expresses adiponectin receptors and is associated with hypoleptinemia and hyperadiponectinemia: a case-control study. Cancer causes & control : CCC. 2009;20:625–633. doi: 10.1007/s10552-008-9273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SK, Eder S, Schauer S, Diwoky C, Temmel H, Guertl B, Gorkiewicz G, Tamilarasan KP, Kumari P, Trauner M, Zimmermann R, Vesely P, Haemmerle G, Zechner R, Hoefler G. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science. 2011;333:233–238. doi: 10.1126/science.1198973. [DOI] [PubMed] [Google Scholar]
- de Luca C, Kowalski TJ, Zhang Y, Elmquist JK, Lee C, Kilimann MW, Ludwig T, Liu SM, Chua SC., Jr. Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. The Journal of clinical investigation. 2005;115:3484–3493. doi: 10.1172/JCI24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetri GD, Fletcher CD, Mueller E, Sarraf P, Naujoks R, Campbell N, Spiegelman BM, Singer S. Induction of solid tumor differentiation by the peroxisome proliferator-activated receptor-gamma ligand troglitazone in patients with liposarcoma. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:3951–3956. doi: 10.1073/pnas.96.7.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzel MS, Hebbard LW, Shostak G, Shapiro L, Cardiff RD, Ranscht B. Adiponectin deficiency limits tumor vascularization in the MMTV-PyV-mT mouse model of mammary cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:3256–3264. doi: 10.1158/1078-0432.CCR-08-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S, Garrido I, Escourrou G, Valet P, Muller C. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer research. 2011;71:2455–2465. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Rasch MG, Weaver VM. Dynamic interplay between the collagen scaffold and tumor evolution. Current opinion in cell biology. 2010;22:697–706. doi: 10.1016/j.ceb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis. Advances in cancer research. 1974;19:331–358. doi: 10.1016/s0065-230x(08)60058-5. [DOI] [PubMed] [Google Scholar]
- Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339:58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- Forum NC, Services B. o. H. C., Medicine I. o. The Role of Obesity in Cancer Survival and Recurrence: Workshop Summary. The National Academies Press; 2012. [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Molecular and cellular endocrinology. 2010;316:129–139. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Gallagher EJ, LeRoith D. Minireview: IGF, Insulin, and Cancer. Endocrinology. 2011;152:2546–2551. doi: 10.1210/en.2011-0231. [DOI] [PubMed] [Google Scholar]
- Garcia Rodriguez LA, Huerta-Alvarez C. Reduced incidence of colorectal adenoma among long-term users of nonsteroidal antiinflammatory drugs: a pooled analysis of published studies and a new population-based study. Epidemiology. 2000;11:376–381. doi: 10.1097/00001648-200007000-00003. [DOI] [PubMed] [Google Scholar]
- Garofalo C, Koda M, Cascio S, Sulkowska M, Kanczuga-Koda L, Golaszewska J, Russo A, Sulkowski S, Surmacz E. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:1447–1453. doi: 10.1158/1078-0432.CCR-05-1913. [DOI] [PubMed] [Google Scholar]
- Garofalo C, Surmacz E. Leptin and cancer. Journal of cellular physiology. 2006;207:12–22. doi: 10.1002/jcp.20472. [DOI] [PubMed] [Google Scholar]
- Gerhardt CC, Romero IA, Cancello R, Camoin L, Strosberg AD. Chemokines control fat accumulation and leptin secretion by cultured human adipocytes. Molecular and cellular endocrinology. 2001;175:81–92. doi: 10.1016/s0303-7207(01)00394-x. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Egan KM, Hunter DJ, Stampfer MJ, Colditz GA, Willett WC, Speizer FE. Aspirin and the risk of colorectal cancer in women. The New England journal of medicine. 1995;333:609–614. doi: 10.1056/NEJM199509073331001. [DOI] [PubMed] [Google Scholar]
- Girnun GD, Naseri E, Vafai SB, Qu L, Szwaya JD, Bronson R, Alberta JA, Spiegelman BM. Synergy between PPARgamma ligands and platinum-based drugs in cancer. Cancer cell. 2007;11:395–406. doi: 10.1016/j.ccr.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Perez A, Garcia Rodriguez LA, Lopez-Ridaura R. Effects of non-steroidal anti-inflammatory drugs on cancer sites other than the colon and rectum: a meta-analysis. BMC cancer. 2003;3:28. doi: 10.1186/1471-2407-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorska E, Popko K, Stelmaszczyk-Emmel A, Ciepiela O, Kucharska A, Wasik M. Leptin receptors. European journal of medical research. 2010;15(Suppl 2):50–54. doi: 10.1186/2047-783X-15-S2-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov SI, Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Annals of the rheumatic diseases. 2011;70(Suppl 1):i104–108. doi: 10.1136/ard.2010.140145. [DOI] [PubMed] [Google Scholar]
- Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nature reviews. Cancer. 2001;1:11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- Hakkak R, Holley AW, Macleod SL, Simpson PM, Fuchs GJ, Jo CH, Kieber-Emmons T, Korourian S. Obesity promotes 7,12-dimethylbenz(a)anthracene-induced mammary tumor development in female zucker rats. Breast cancer research : BCR. 2005;7:R627–633. doi: 10.1186/bcr1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, Brekken RA, Scherer PE. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Molecular and cellular biology. 2009;29:4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Roman J. Peroxisome proliferator-activated receptor gamma: a novel target for cancer therapeutics? Anti-cancer drugs. 2007;18:237–244. doi: 10.1097/CAD.0b013e328011e67d. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Harvey AE, Lashinger LM, Hursting SD. The growing challenge of obesity and cancer: an inflammatory issue. Annals of the New York Academy of Sciences. 2011;1229:45–52. doi: 10.1111/j.1749-6632.2011.06096.x. [DOI] [PubMed] [Google Scholar]
- Hasebe T, Imoto S, Sasaki S, Mukai K. A proposal for a new histological classification scheme for predicting short-term tumor recurrence and death in patients with invasive ductal carcinoma of the breast. Japanese journal of cancer research : Gann. 1998;89:1358–1373. doi: 10.1111/j.1349-7006.1998.tb00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasebe T, Mukai K, Tsuda H, Ochiai A. New prognostic histological parameter of invasive ductal carcinoma of the breast: clinicopathological significance of fibrotic focus. Pathology international. 2000;50:263–272. doi: 10.1046/j.1440-1827.2000.01035.x. [DOI] [PubMed] [Google Scholar]
- Hebbard LW, Garlatti M, Young LJ, Cardiff RD, Oshima RG, Ranscht B. T-cadherin supports angiogenesis and adiponectin association with the vasculature in a mouse mammary tumor model. Cancer research. 2008;68:1407–1416. doi: 10.1158/0008-5472.CAN-07-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hino M, Nakao T, Yamane T, Ohta K, Takubo T, Tatsumi N. Leptin receptor and leukemia. Leukemia & lymphoma. 2000;36:457–461. doi: 10.3109/10428190009148392. [DOI] [PubMed] [Google Scholar]
- Hirsch A, Hahn D, Kempna P, Hofer G, Nuoffer JM, Mullis PE, Fluck CE. Metformin Inhibits Human Androgen Production by Regulating Steroidogenic Enzymes HSD3B2 and CYP17A1 and Complex I Activity of the Respiratory Chain. Endocrinology. 2012;153:4354–4366. doi: 10.1210/en.2012-1145. [DOI] [PubMed] [Google Scholar]
- Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer research. 1996;56:4509–4515. [PubMed] [Google Scholar]
- Hoda MR, Keely SJ, Bertelsen LS, Junger WG, Dharmasena D, Barrett KE. Leptin acts as a mitogenic and antiapoptotic factor for colonic cancer cells. The British journal of surgery. 2007;94:346–354. doi: 10.1002/bjs.5530. [DOI] [PubMed] [Google Scholar]
- Hoda MR, Popken G. Mitogenic and anti-apoptotic actions of adipocyte-derived hormone leptin in prostate cancer cells. BJU international. 2008;102:383–388. doi: 10.1111/j.1464-410X.2008.07534.x. [DOI] [PubMed] [Google Scholar]
- Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, Wade MR, Tenorio VM, Kuo MS, Brozinick JT, Zhang BB, Birnbaum MJ, Summers SA, Scherer PE. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nature medicine. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. The Journal of clinical investigation. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Howard JM, Pidgeon GP, Reynolds JV. Leptin and gastro-intestinal malignancies. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2010;11:863–874. doi: 10.1111/j.1467-789X.2010.00718.x. [DOI] [PubMed] [Google Scholar]
- Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Kitayama J, Nagawa H. Enhanced expression of leptin and leptin receptor (OB R) in human breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10:4325–4331. doi: 10.1158/1078-0432.CCR-03-0749. [DOI] [PubMed] [Google Scholar]
- Iyengar P, Combs TP, Shah SJ, Gouon-Evans V, Pollard JW, Albanese C, Flanagan L, Tenniswood MP, Guha C, Lisanti MP, Pestell RG, Scherer PE. Adipocyte-secreted factors synergistically promote mammary tumorigenesis through induction of anti-apoptotic transcriptional programs and proto-oncogene stabilization. Oncogene. 2003;22:6408–6423. doi: 10.1038/sj.onc.1206737. [DOI] [PubMed] [Google Scholar]
- Iyengar P, Espina V, Williams TW, Lin Y, Berry D, Jelicks LA, Lee H, Temple K, Graves R, Pollard J, Chopra N, Russell RG, Sasisekharan R, Trock BJ, Lippman M, Calvert VS, Petricoin EF, Liotta L, Dadachova E, Pestell RG, Lisanti MP, Bonaldo P, Scherer PE. Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. 3rd. The Journal of clinical investigation. 2005;115:1163–1176. doi: 10.1172/JCI23424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalving M, Gietema JA, Lefrandt JD, de Jong S, Reyners AK, Gans RO, de Vries EG. Metformin: taking away the candy for cancer? European journal of cancer. 2010;46:2369–2380. doi: 10.1016/j.ejca.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Jansson PA. Endothelial dysfunction in insulin resistance and type 2 diabetes. Journal of internal medicine. 2007;262:173–183. doi: 10.1111/j.1365-2796.2007.01830.x. [DOI] [PubMed] [Google Scholar]
- Kanneganti TD, Dixit VD. Immunological complications of obesity. Nature immunology. 2012;13:707–712. doi: 10.1038/ni.2343. [DOI] [PubMed] [Google Scholar]
- Kelesidis I, Kelesidis T, Mantzoros CS. Adiponectin and cancer : a systematic review. British journal of cancer. 2006;94:1221–1225. doi: 10.1038/sj.bjc.6603051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Scherer PE. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Molecular and cellular biology. 2009;29:1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Choi MR, Park H, Kim M, Hong JE, Lee JY, Chun HS, Lee KW, Yoon Park JH. Dietary fat increases solid tumor growth and metastasis of 4T1 murine mammary carcinoma cells and mortality in obesity-resistant BALB/c mice. Breast cancer research : BCR. 2011;13:R78. doi: 10.1186/bcr2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Choi SE, Ha ES, Jung JG, Han SJ, Kim HJ, Kim DJ, Kang Y, Lee KW. IL-6 induction of TLR-4 gene expression via STAT3 has an effect on insulin resistance in human skeletal muscle. Acta diabetologica. 2011 doi: 10.1007/s00592-011-0259-z. doi 10.1007/s00592-011-0259-z. [DOI] [PubMed] [Google Scholar]
- Kimijima I, Ohtake T, Sagara H, Watanabe T, Takenoshita S. Scattered fat invasion: an indicator for poor prognosis in premenopausal, and for positive estrogen receptor in postmenopausal breast cancer patients. Oncology. 2000;59(Suppl 1):25–30. doi: 10.1159/000055284. [DOI] [PubMed] [Google Scholar]
- Koeffler HP. Peroxisome proliferator-activated receptor gamma and cancers. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9:1–9. [PubMed] [Google Scholar]
- Korner A, Pazaitou-Panayiotou K, Kelesidis T, Kelesidis I, Williams CJ, Kaprara A, Bullen J, Neuwirth A, Tseleni S, Mitsiades N, Kiess W, Mantzoros CS. Total and high-molecular-weight adiponectin in breast cancer : in vitro and in vivo studies. The Journal of clinical endocrinology and metabolism. 2007;92:1041–1048. doi: 10.1210/jc.2006-1858. [DOI] [PubMed] [Google Scholar]
- Kuhajda FP, Pizer ES, Li JN, Mani NS, Frehywot GL, Townsend CA. Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:3450–3454. doi: 10.1073/pnas.050582897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landskroner-Eiger S, Qian B, Muise ES, Nawrocki AR, Berger JP, Fine EJ, Koba W, Deng Y, Pollard JW, Scherer PE. Proangiogenic contribution of adiponectin toward mammary tumor growth in vivo. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:3265–3276. doi: 10.1158/1078-0432.CCR-08-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang YH, Huang YC. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC cancer. 2011;11:20. doi: 10.1186/1471-2407-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes care. 2009;32:1620–1625. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harbor perspectives in biology. 2011;3 doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. The Journal of cell biology. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougald OA, Burant CF. The rapidly expanding family of adipokines. Cell metabolism. 2007;6:159–161. doi: 10.1016/j.cmet.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Manabe Y, Toda S, Miyazaki K, Sugihara H. Mature adipocytes, but not preadipocytes, promote the growth of breast carcinoma cells in collagen gel matrix culture through cancer-stromal cell interactions. The Journal of pathology. 2003;201:221–228. doi: 10.1002/path.1430. [DOI] [PubMed] [Google Scholar]
- Martinez-Outschoorn UE, Pestell RG, Howell A, Tykocinski ML, Nagajyothi F, Machado FS, Tanowitz HB, Sotgia F, Lisanti MP. Energy transfer in “parasitic” cancer metabolism: mitochondria are the powerhouse and Achilles' heel of tumor cells. Cell cycle. 2011;10:4208–4216. doi: 10.4161/cc.10.24.18487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa Y. The role of fat topology in the risk of disease. International journal of obesity. 2008;32(Suppl 7):S83–92. doi: 10.1038/ijo.2008.243. [DOI] [PubMed] [Google Scholar]
- Mayi TH, Daoudi M, Derudas B, Gross B, Bories G, Wouters K, Brozek J, Caiazzo R, Raverdi V, Pigeyre M, Allavena P, Mantovani A, Pattou F, Staels B, Chinetti-Gbaguidi G. Human adipose tissue macrophages display activation of cancer-related pathways. The Journal of biological chemistry. 2012;287:21904–21913. doi: 10.1074/jbc.M111.315200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael AJ. Food, nutrition, physical activity and cancer prevention. Authoritative report from World Cancer Research Fund provides global update. Public health nutrition. 2008;11:762–763. doi: 10.1017/S1368980008002358. [DOI] [PubMed] [Google Scholar]
- McMurtry V, Simeone AM, Nieves-Alicea R, Tari AM. Leptin utilizes Jun N-terminal kinases to stimulate the invasion of MCF-7 breast cancer cells. Clinical & experimental metastasis. 2009;26:197–204. doi: 10.1007/s10585-008-9231-x. [DOI] [PubMed] [Google Scholar]
- Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nature reviews. Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- Meng L, Zhou J, Sasano H, Suzuki T, Zeitoun KM, Bulun SE. Tumor necrosis factor alpha and interleukin 11 secreted by malignant breast epithelial cells inhibit adipocyte differentiation by selectively down-regulating CCAAT/enhancer binding protein alpha and peroxisome proliferator-activated receptor gamma: mechanism of desmoplastic reaction. Cancer research. 2001;61:2250–2255. [PubMed] [Google Scholar]
- Migneco G, Whitaker-Menezes D, Chiavarina B, Castello-Cros R, Pavlides S, Pestell RG, Fatatis A, Flomenberg N, Tsirigos A, Howell A, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Glycolytic cancer associated fibroblasts promote breast cancer tumor growth, without a measurable increase in angiogenesis: evidence for stromal-epithelial metabolic coupling. Cell cycle. 2010;9:2412–2422. doi: 10.4161/cc.9.12.11989. [DOI] [PubMed] [Google Scholar]
- Mix H, Widjaja A, Jandl O, Cornberg M, Kaul A, Goke M, Beil W, Kuske M, Brabant G, Manns MP, Wagner S. Expression of leptin and leptin receptor isoforms in the human stomach. Gut. 2000;47:481–486. doi: 10.1136/gut.47.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Sanchez R, Rodriguez-Enriquez S, Marin-Hernandez A, Saavedra E. Energy metabolism in tumor cells. The FEBS journal. 2007;274:1393–1418. doi: 10.1111/j.1742-4658.2007.05686.x. [DOI] [PubMed] [Google Scholar]
- Motrescu ER, Blaise S, Etique N, Messaddeq N, Chenard MP, Stoll I, Tomasetto C, Rio MC. Matrix metalloproteinase-11/stromelysin-3 exhibits collagenolytic function against collagen VI under normal and malignant conditions. Oncogene. 2008;27:6347–6355. doi: 10.1038/onc.2008.218. [DOI] [PubMed] [Google Scholar]
- Motrescu ER, Rio MC. Cancer cells, adipocytes and matrix metalloproteinase 11: a vicious tumor progression cycle. Biological chemistry. 2008;389:1037–1041. doi: 10.1515/BC.2008.110. [DOI] [PubMed] [Google Scholar]
- Munzberg H, Myers MG., Jr. Molecular and anatomical determinants of central leptin resistance. Nature neuroscience. 2005;8:566–570. doi: 10.1038/nn1454. [DOI] [PubMed] [Google Scholar]
- Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nature reviews. Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB, Hotamisligil GS, Yamada SD, Peter ME, Gwin K, Lengyel E. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nature medicine. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nature medicine. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- Nomura DK, Long JZ, Niessen S, Hoover HS, Ng SW, Cravatt BF. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell. 2010;140:49–61. doi: 10.1016/j.cell.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nature reviews. Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nature medicine. 2012;18:363–374. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- Osorio-Costa F, Rocha GZ, Dias MM, Carvalheira JB. Epidemiological and molecular mechanisms aspects linking obesity and cancer. Arquivos brasileiros de endocrinologia e metabologia. 2009;53:213–226. doi: 10.1590/s0004-27302009000200013. [DOI] [PubMed] [Google Scholar]
- Parekh N, Chandran U, Bandera EV. Obesity in Cancer Survival. Annual review of nutrition. 2012 doi: 10.1146/annurev-nutr-071811-150713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Kim M, Kwon GT, Lim DY, Yu R, Sung MK, Lee KW, Daily JW, 3rd, Park JH. A high-fat diet increases angiogenesis, solid tumor growth, and lung metastasis of CT26 colon cancer cells in obesity-resistant BALB/c mice. Molecular carcinogenesis. 2012;51:869–880. doi: 10.1002/mc.20856. [DOI] [PubMed] [Google Scholar]
- Park J, Euhus DM, Scherer PE. Paracrine and endocrine effects of adipose tissue on cancer development and progression. Endocrine reviews. 2011;32:550–570. doi: 10.1210/er.2010-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Kusminski CM, Chua SC, Scherer PE. Leptin receptor signaling supports cancer cell metabolism through suppression of mitochondrial respiration in vivo. The American journal of pathology. 2010;177:3133–3144. doi: 10.2353/ajpath.2010.100595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Scherer PE. Leptin and cancer: from cancer stem cells to metastasis. Endocrine-related cancer. 2011;18:C25–29. doi: 10.1530/ERC-11-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Scherer PE. Adipocyte-derived endotrophin promotes malignant tumor progression. The Journal of clinical investigation. 2012;122:4243–56. doi: 10.1172/JCI63930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell metabolism. 2008;8:301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S, Pestell RG, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell cycle. 2009;8:3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- Petridou ET, Mitsiades N, Gialamas S, Angelopoulos M, Skalkidou A, Dessypris N, Hsi A, Lazaris N, Polyzos A, Syrigos C, Brennan AM, Tseleni-Balafouta S, Mantzoros CS. Circulating adiponectin levels and expression of adiponectin receptors in relation to lung cancer: two case-control studies. Oncology. 2007;73:261–269. doi: 10.1159/000127424. [DOI] [PubMed] [Google Scholar]
- Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nature reviews. Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC medicine. 2006;4:38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, White JG, Keely PJ. Collagen density promotes mammary tumor initiation and progression. BMC medicine. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph SJ, Rodriguez-Enriquez S, Neuzil J, Moreno-Sanchez R. Bioenergetic pathways in tumor mitochondria as targets for cancer therapy and the importance of the ROS-induced apoptotic trigger. Molecular aspects of medicine. 2010;31:29–59. doi: 10.1016/j.mam.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rene Gonzalez R, Watters A, Xu Y, Singh UP, Mann DR, Rueda BR, Penichet ML. Leptin-signaling inhibition results in efficient anti-tumor activity in estrogen receptor positive or negative breast cancer. Breast cancer research: BCR. 2009;11:R36. doi: 10.1186/bcr2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. The Lancet. 371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- Rio M-C. The Role of Cancer-Associated Adipocytes (CAA) in the Dynamic Interaction Between the Tumor and the Host. In: Mueller MM, Fusenig NE, editors. Tumor-Associated Fibroblasts and their Matrix. Vol. 4. Springer; Netherlands: 2011. pp. 111–123. [Google Scholar]
- Rio MC. From a unique cell to metastasis is a long way to go: clues to stromelysin-3 participation. Biochimie. 2005;87:299–306. doi: 10.1016/j.biochi.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol. 2000;16:145–171. doi: 10.1146/annurev.cellbio.16.1.145. [DOI] [PubMed] [Google Scholar]
- Ruan H, Zarnowski MJ, Cushman SW, Lodish HF. Standard isolation of primary adipose cells from mouse epididymal fat pads induces inflammatory mediators and down-regulates adipocyte genes. The Journal of biological chemistry. 2003;278:47585–47593. doi: 10.1074/jbc.M305257200. [DOI] [PubMed] [Google Scholar]
- Rubin GL, Zhao Y, Kalus AM, Simpson ER. Peroxisome proliferator-activated receptor gamma ligands inhibit estrogen biosynthesis in human breast adipose tissue: possible implications for breast cancer therapy. Cancer research. 2000;60:1604–1608. [PubMed] [Google Scholar]
- Rutkowski JM, Davis KE, Scherer PE. Mechanisms of obesity and related pathologies: the macro- and microcirculation of adipose tissue. The FEBS journal. 2009;276:5738–5746. doi: 10.1111/j.1742-4658.2009.07303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santen RJ. To block estrogen's synthesis or action: that is the question. The Journal of clinical endocrinology and metabolism. 2002;87:3007–3012. doi: 10.1210/jcem.87.7.8589. [DOI] [PubMed] [Google Scholar]
- Santen RJ, Brodie H, Simpson ER, Siiteri PK, Brodie A. History of aromatase: saga of an important biological mediator and therapeutic target. Endocrine reviews. 2009;30:343–375. doi: 10.1210/er.2008-0016. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Weber OF. In memoriam of Rudolf virchow: a historical retrospective including aspects of inflammation, infection and neoplasia. Contributions to microbiology. 2006;13:1–15. doi: 10.1159/000092961. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends in pharmacological sciences. 2012;33:207–214. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lonroth H, Naslund I, Olbers T, Stenlof K, Torgerson J, Agren G, Carlsson LM. Effects of bariatric surgery on mortality in Swedish obese subjects. The New England journal of medicine. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- Somasundar P, Yu AK, Vona-Davis L, McFadden DW. Differential effects of leptin on cancer in vitro. The Journal of surgical research. 2003;113:50–55. doi: 10.1016/s0022-4804(03)00166-5. [DOI] [PubMed] [Google Scholar]
- Sonveaux P, Vegran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, De Saedeleer CJ, Kennedy KM, Diepart C, Jordan BF, Kelley MJ, Gallez B, Wahl ML, Feron O, Dewhirst MW. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. The Journal of clinical investigation. 2008;118:3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nature reviews. Molecular cell biology. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. The Journal of clinical investigation. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Wernstedt Asterholm I, Kusminski CM, Bueno AC, Wang ZV, Pollard JW, Brekken RA, Scherer PE. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5874–5879. doi: 10.1073/pnas.1200447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahata C, Miyoshi Y, Irahara N, Taguchi T, Tamaki Y, Noguchi S. Demonstration of adiponectin receptors 1 and 2 mRNA expression in human breast cancer cells. Cancer letters. 2007;250:229–236. doi: 10.1016/j.canlet.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Takemura Y, Osuga Y, Yamauchi T, Kobayashi M, Harada M, Hirata T, Morimoto C, Hirota Y, Yoshino O, Koga K, Yano T, Kadowaki T, Taketani Y. Expression of adiponectin receptors and its possible implication in the human endometrium. Endocrinology. 2006;147:3203–3210. doi: 10.1210/en.2005-1510. [DOI] [PubMed] [Google Scholar]
- Talukdar S, Oh DY, Bandyopadhyay G, Li D, Xu J, McNelis J, Lu M, Li P, Yan Q, Zhu Y, Ofrecio J, Lin M, Brenner MB, Olefsky JM. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nature medicine. 2012;18:1407–1412. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Buache E, Chenard MP, Dali-Youcef N, Rio MC. Adipocyte is a non-trivial, dynamic partner of breast cancer cells. The International journal of developmental biology. 2011;55:851–859. doi: 10.1387/ijdb.113365jt. [DOI] [PubMed] [Google Scholar]
- Thun MJ, Namboodiri MM, Heath CW., Jr. Aspirin use and reduced risk of fatal colon cancer. The New England journal of medicine. 1991;325:1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- Tisdale MJ. Cachexia in cancer patients. Nature reviews. Cancer. 2002;2:862–871. doi: 10.1038/nrc927. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Singer S, Forman BM, Sarraf P, Fletcher JA, Fletcher CD, Brun RP, Mueller E, Altiok S, Oppenheim H, Evans RM, Spiegelman BM. Terminal differentiation of human liposarcoma cells induced by ligands for peroxisome proliferator-activated receptor gamma and the retinoid × receptor. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:237–241. doi: 10.1073/pnas.94.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annual review of biochemistry. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Wang B, Wood IS. Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? The British journal of nutrition. 2008;100:227–235. doi: 10.1017/S0007114508971282. [DOI] [PubMed] [Google Scholar]
- Trujillo ME, Scherer PE. Adiponectin--journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. Journal of internal medicine. 2005;257:167–175. doi: 10.1111/j.1365-2796.2004.01426.x. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T, Shimizu H, Horie T, Mori M. Expression of leptin receptor in lung: leptin as a growth factor. European journal of pharmacology. 1999;365:273–279. doi: 10.1016/s0014-2999(98)00884-x. [DOI] [PubMed] [Google Scholar]
- Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55:2319–2326. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- Vainio H, Kaaks R, Bianchini F. Weight control and physical activity in cancer prevention: international evaluation of the evidence. European journal of cancer prevention. 2002;11(Suppl 2):S94–100. [PubMed] [Google Scholar]
- van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18:2569–2578. doi: 10.1158/1055-9965.EPI-09-0372. [DOI] [PubMed] [Google Scholar]
- Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer metastasis reviews. 2007;26:225–239. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- Villaret A, Galitzky J, Decaunes P, Esteve D, Marques MA, Sengenes C, Chiotasso P, Tchkonia T, Lafontan M, Kirkland JL, Bouloumie A. Adipose tissue endothelial cells from obese human subjects: differences among depots in angiogenic, metabolic, and inflammatory gene expression and cellular senescence. Diabetes. 2010;59:2755–2763. doi: 10.2337/db10-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visentin B, Vekich JA, Sibbald BJ, Cavalli AL, Moreno KM, Matteo RG, Garland WA, Lu Y, Yu S, Hall HS, Kundra V, Mills GB, Sabbadini RA. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer cell. 2006;9:225–238. doi: 10.1016/j.ccr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Wang YY, Lehuede C, Laurent V, Dirat B, Dauvillier S, Bochet L, Le Gonidec S, Escourrou G, Valet P, Muller C. Adipose tissue and breast epithelial cells: a dangerous dynamic duo in breast cancer. Cancer letters. 2012;324:142–151. doi: 10.1016/j.canlet.2012.05.019. [DOI] [PubMed] [Google Scholar]
- Wauters M, Considine RV, Van Gaal LF. Human leptin: from an adipocyte hormone to an endocrine mediator. European journal of endocrinology / European Federation of Endocrine Societies. 2000;143:293–311. doi: 10.1530/eje.0.1430293. [DOI] [PubMed] [Google Scholar]
- Wei S, Yang J, Lee SL, Kulp SK, Chen CS. PPARgamma-independent antitumor effects of thiazolidinediones. Cancer letters. 2009;276:119–124. doi: 10.1016/j.canlet.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nature medicine. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. The Journal of clinical investigation. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. The Journal of clinical investigation. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker-Menezes D, Martinez-Outschoorn UE, Lin Z, Ertel A, Flomenberg N, Witkiewicz AK, Birbe RC, Howell A, Pavlides S, Gandara R, Pestell RG, Sotgia F, Philp NJ, Lisanti MP. Evidence for a stromal-epithelial “lactate shuttle” in human tumors: MCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell cycle. 2011;10:1772–1783. doi: 10.4161/cc.10.11.15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. The Journal of clinical investigation. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi J, Ohtani H, Nakamura K, Shimokawa I, Kanematsu T. Prognostic impact of marginal adipose tissue invasion in ductal carcinoma of the breast. American journal of clinical pathology. 2008;130:382–388. doi: 10.1309/MX6KKA1UNJ1YG8VN. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- Yang C, Sudderth J, Dang T, Bachoo RM, McDonald JG, DeBerardinis RJ. Glioblastoma cells require glutamate dehydrogenase to survive impairments of glucose metabolism or Akt signaling. Cancer research. 2009;69:7986–7993. doi: 10.1158/0008-5472.CAN-09-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda-Shnaidman E, Schwartz B. Mechanisms linking obesity, inflammation and altered metabolism to colon carcinogenesis. Obesity reviews. 2012;13:1083–1095. doi: 10.1111/j.1467-789X.2012.01024.x. [DOI] [PubMed] [Google Scholar]
- Yoneda K, Tomimoto A, Endo H, Iida H, Sugiyama M, Takahashi H, Mawatari H, Nozaki Y, Fujita K, Yoneda M, Inamori M, Nakajima N, Wada K, Nagashima Y, Nakagama H, Uozaki H, Fukayama M, Nakajima A. Expression of adiponectin receptors, AdipoR1 and AdipoR2, in normal colon epithelium and colon cancer tissue. Oncology reports. 2008;20:479–483. [PubMed] [Google Scholar]
- Zhang Y, Bellows CF, Kolonin MG. Adipose tissue-derived progenitor cells and cancer. World J Stem Cells. 2010;2:103–113. doi: 10.4252/wjsc.v2.i5.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Daquinag AC, Amaya-Manzanares F, Sirin O, Tseng C, Kolonin MG. Stromal progenitor cells from endogenous adipose tissue contribute to pericytes and adipocytes that populate the tumor microenvironment. Cancer research. 2012;72:5198–5208. doi: 10.1158/0008-5472.CAN-12-0294. [DOI] [PubMed] [Google Scholar]
- Zheng Q, Dunlap SM, Zhu J, Downs-Kelly E, Rich J, Hursting SD, Berger NA, Reizes O. Leptin deficiency suppresses MMTV-Wnt-1 mammary tumor growth in obese mice and abrogates tumor initiating cell survival. Endocrine-related cancer. 2011;18:491–503. doi: 10.1530/ERC-11-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Han WF, Landree LE, Thupari JN, Pinn ML, Bililign T, Kim EK, Vadlamudi A, Medghalchi SM, El Meskini R, Ronnett GV, Townsend CA, Kuhajda FP. Fatty acid synthase inhibition activates AMP-activated protein kinase in SKOV3 human ovarian cancer cells. Cancer research. 2007;67:2964–2971. doi: 10.1158/0008-5472.CAN-06-3439. [DOI] [PubMed] [Google Scholar]