Abstract
The blood feeding behavior of disease-transmitting arthropods creates a unique intersection between vertebrate and invertebrate physiology. Here, we review host blood-derived factors that persist through blood digestion to affect the lifespan, reproduction, and immune responses of some of the most common arthropod vectors of human disease.
Keywords: tick, mosquito, Plasmodium, insulin, transforming growth factor-beta (TGF-β), macrophage migration inhibitory factor (MIF)
1. Introduction
The blood feeding behavior of disease-transmitting arthropods creates a unique intersection between vertebrate and invertebrate physiology. Human blood contains a myriad of nutrients, growth factors, cytokines/chemokines, pathogens, and pathogen-associated molecules that can interact with arthropod vectors. Many of these blood-derived factors persist during blood digestion to signal in the arthropod vector. This review summarizes the conserved signal transduction pathways that are activated by these vertebrate host-derived factors, and describes their downstream effects on lifespan, reproduction, and innate immune responses of the most common arthropod vectors of human disease agents. Expanding our understanding of these complex interactions can guide novel approaches for the control of a variety of vector-borne diseases.
2. Insulin and insulin-like growth factor-1
Normal circulating levels of insulin in the blood of healthy humans can range between 17 pM at fasting to 590 pM without fasting [1]. Infection with malaria parasites can induce a rise in blood insulin levels above that of a normal healthy adult by as much as 10- to 35-fold [1, 2]. Given that the occurrence of type 2 diabetes is rising in malaria-endemic countries [3], compounded hyperinsulinemia in individuals affected by both diseases could become prevalent. In particular, a hallmark of type 2 diabetes is insulin resistance, which can often result in increased insulin secretion to compensate for the inability of the body to respond to insulin. If female mosquitoes feed on co-morbid hosts, they would ingest higher than average levels of human insulin in an infectious blood meal.
The mosquito midgut is a critical site for Plasmodium development [4], and a tissue that is exquisitely responsive to ingested human insulin [5-7]. Recent experiments using radiolabeled human insulin spiked into artificial blood meals fed to Anopheles stephensi revealed that ingested, intact insulin persisted in the midgut for up to 24 h and in the head and thorax for up to 18 h post-blood feeding [8]. These data indicate that insulin persists long enough to signal in the mosquito midgut, and can cross the midgut epithelium to persist in the hemocoel to activate signaling in other tissues.
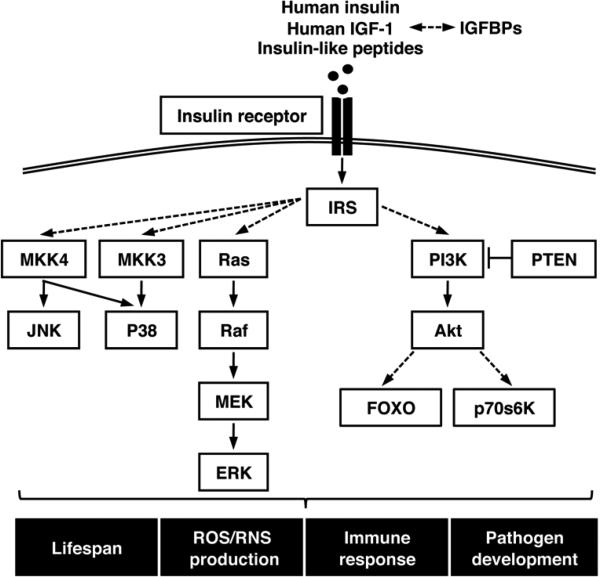
The insulin/insulin-like growth factor signaling (IIS) pathway is highly conserved and consists of two main signaling branches: a mitogen-activated protein kinase (MAPK)-dependent branch and a phosphatidylinositol 3-kinase (PI3K)/Akt-dependent branch. Both branches of the IIS pathway play integral roles in the regulation of growth, longevity, reproduction, and immunity in vertebrates and invertebrates [9]. Although these pathways can differ in their downstream physiological effects, most of the IIS proteins and their interactions appear to be conserved in mosquitoes [5-7, 10-16] (Fig. 1). Orthologs of IIS proteins exist in ticks, lice, tsetse flies and sand flies (Table 1). Expression of endogenous insulin-like peptides (ILPs) has been confirmed in Anopheles gambiae, A. stephensi, Aedes aegypti, and Culex pipiens; partial ILP sequences have been identified in the Reduviidae ‘kissing’ bug Rhodnius prolixus, the human body louse Pediculus humanus humanus, and in the tick species Ixodes scapularis and Dermacentor variabilis [17]. Therefore, it is not surprising that exogenous insulin can activate the IIS pathway in arthropods. Bovine insulin can activate the neurosecretory cells of D. variabilis, and can regulate glycogen accumulation, via PI3K signaling, in cell lines derived from the hard tick Rhipicephalus (Boophilus) microplus [18, 19].
Figure 1.
Model of insulin/IGF-1 signaling pathway in vector arthropods. Solid lines indicate proven direct interactions between proteins, and dashed lines indicate indirect interactions that may involve other signaling proteins.
Table 1.
Putative homologs of insulin/IGF-1 and TGF-β signaling pathway proteins present in ticks, body lice, tsetse flies, and sand flies.
Solid lines indicate proven direct interactions between proteins, and dashed lines indicate indirect interactions that may involve other signaling proteins.
Among vector species, the mosquito response to ingested insulin has been the most well-studied. Beier et al. [5] were the first to demonstrate that ingestion of human insulin – at levels vastly exceeding those reported to occur in human blood – could significantly increase oocyst densities of P. falciparum in anopheline mosquitoes. We have since shown that physiological doses (170 pM) of human insulin in A. stephensi can activate both the PI3K/Akt and MAPK branches of the IIS pathway in mosquitoes [6, 7, 12, 14, 16] and that at least two IIS proteins – Akt/PKB and ERK – are critical for the control of malaria parasite infection [16, 20]. Moreover, we have shown that the expression levels of A. stephensi ILPs change in response to human insulin and to P. falciparum infection, suggesting that ILP expression is finely tuned to IIS activation [11, 12].
One of the best-known effects of IIS is the control of lifespan. The free radical hypothesis of aging posits that the accumulated damage caused by reactive oxygen species (ROS), such as superoxide and hydrogen peroxide (H2O2), potentiates aging [21]. Among invertebrate model organisms, the importance of oxidative stress in aging has been demonstrated in studies with Drosophila melanogaster and Caenorhabditis elegans [9]. We have shown that stimulation by human insulin increases H2O2 synthesis by mosquito cells, perhaps in part via a reduction in the activity of antioxidants such as the mitochondrial manganese superoxide dismutase (MnSOD) [7]. This increase in damaging ROS is most likely responsible for the decreased lifespan of insulin-fed A. stephensi, as provision of a cell-permeable SOD mimetic agent to insulin-fed mosquitoes returned survivorship to control levels [7]. The positive effects of insulin-induced ROS on P. falciparum development appear to be a consequence of ROS-dependent signaling in Anopheles mosquitoes, and not due to ROS-induced damage to the midgut epithelium [6]. These data suggest that early regulation of IIS-induced ROS primes an anti-inflammatory state in which parasite development is favored [6].
We have also shown that ingested human insulin and Akt over-expression lead to increased nitric oxide (NO) production [12, 22], which likely contributes to increased midgut damage and decreased survivorship of mosquitoes. The induction of NO by insulin has also been observed in mammals [9], suggesting that this is a highly conserved response. Induction of NO production in A. stephensi midguts can limit malaria parasite development through the formation of inflammatory levels of toxic reactive nitrogen oxides [22-24]. In insulin-fed A. stephensi induction of nitric oxide synthase (NOS) expression – the enzyme that catalyzes the synthesis of NO – did not exceed control values until 36 h after feeding [12], a point at which P. falciparum ookinete invasion is largely completed [25]. In contrast, in transgenic Akt over-expressing mosquitoes NOS is induced very early after ingestion of an infected blood meal and inhibits parasite development in the midgut lumen [22]. Thus, differences in the kinetics of IIS can have distinct and dramatic impacts on malaria parasite development. Further, the beneficial effect of insulin on parasite development contrasts with the dramatic, detrimental effects of Akt over-expression on P. falciparum oocyst development in A. stephensi [16], suggesting that the effects of insulin activation of IIS are distinct from those mediated by over-expression of an individual IIS protein.
Insulin-induced signaling has been shown to regulate immunity in a variety of organisms, and research in human cell lines suggests that insulin exerts its effects on the immune response through the regulation of NF-κB transcription factors [26]. The regulation of mosquito immunity during Plasmodium infection occurs in part through the activation of the mosquito NF-κB transcription [27, 28]. NF-κB proteins comprise a family of highly conserved transcription factors whose primary role is immune regulation [29]. Mosquitoes and other vector arthropods possess sophisticated innate immune systems, including the NF-κB transcription factors that protect them from bacterial, fungal, and parasitic pathogens (Table 1, reviewed in [30]). Our most recent work suggests that activation of the mosquito IIS pathway by human insulin inhibits the NF-κB-dependent immune response of A. stephensi [15]. This inhibition appears to be a consequence of sustained activation of the PI3K/Akt, but not the MAPK, branch of the mosquito IIS pathway. Thus, ingested human insulin can significantly influence the transmission of malaria parasites through its effects on mosquito biology and immunity.
Human insulin and IGF-1 are highly similar in structure, utilize the same receptors, and activate IIS, but their downstream effects in humans are quite different. In contrast to insulin, IGF-1 is very abundant in human blood – normal circulating levels range from 0.006-0.093 M – and IGF-1 bioavailability is tightly regulated by IGF binding proteins (IGFBPs; reviewed in [31]). During malaria infection, serum levels of IGF-1 fall dramatically, and this decrease correlates to an increase in parasitemia [8]. Interestingly, human IGF-1 has very different effects on mosquito biology compared to insulin. Both insulin and IGF-1 can activate the PI3K/Akt branch of the mosquito IIS pathway [8]. However, ingested human IGF-1, in contrast to insulin, extends lifespan and enhances resistance of A. stephensi to P. falciparum [8]. In mammals, IGFBPs protect circulating IGF-1 in addition to activating IGF receptor signaling both directly and indirectly [31]. In D. melanogaster IGFBP-related proteins bind unprocessed D. melanogaster ILP-2 and ILP-5 and regulate their signaling [32, 33], while in the tick Amblyomma americanum IGFBP-related proteins regulate blood feeding behavior [34]. To date, no IGFBP-related proteins have been identified in mosquitoes. However, Ae. aegypti ovary ecdysteroidogenic hormone does share sequence similarity to mammalian IGFBPs [35], and the armyworm IGFBP-related protein can bind human insulin and IGF-1 in vitro [36]. Together, these findings suggest that the regulation of IIS by IGFBP-related proteins is quite ancient, and that arthropod-produced IGFBP-related proteins may interact with and alter the effects of ingested mammalian insulin and IGF-1.
3. TGF-β1
The mammalian transforming growth factor (TGF)-β family of cytokines consists of 33 structurally similar members [37]. TGF-β1 is present in circulating blood, is pleiotropic in its effects, and plays a critical role in regulating the immunological balance during infections, being pro-inflammatory at low concentrations and anti-inflammatory at high concentrations [38]. During early malaria infection in mammalian hosts, TGF-β1 promotes responses that control parasite growth. Later in infection, TGF-β1 down-regulates immune responses to limit inflammation-associated pathology [38]. There is also evidence to suggest that both Trypanosoma (genus of the causative agent of Chagas disease) and Leishmania (genus of the causative agent of leishmaniasis) both induce, and benefit from, increased levels of circulating TGF-β1 [38].
TGF-β1 is produced by most cell types in a latent form that is activated following dissociation of inhibitory proteins. The bioactive form of TGF-β1 exerts its cellular effects by activating type I and type II serine/threonine kinase receptors, which in turn activate the mothers against the decapentaplegic homolog (Smad) family of downstream effectors. Once activated, Smads translocate to the nucleus, where they regulate gene expression ([39]; Fig. 2). TGF-β1 can also activate non-Smad, MAPK- and PI3K-mediated signaling pathways.
Figure 2.
Model of TGF-β1 signaling pathway in vector arthropods.
In mosquitoes, ingested latent human TGF-β1 is rapidly activated in the midgut by factors commonly released during blood digestion, such as NO and heme [40]. The latent form of TGF-β1 is detected in the circulation of healthy adults at concentrations as high as 5 ng/ml and is, therefore, ingested at levels that can be biologically active for arthropod cells and tissues [41]. In addition, extensive cross-talk between mammalian and invertebrate TGF-β superfamily proteins and their corresponding Smad signaling pathways has been described in D. melanogaster [42-44], suggesting that blood feeding insects possess the signaling architecture to also respond directly to ingested human TGF-β1.
Conserved TGF-β signaling pathway proteins are found in a variety of blood feeding arthropods (Fig. 2, Table 1), suggesting that exogenous human TGF-β1 can activate these endogenous signaling pathways, in turn altering the susceptibility of blood feeding arthropods to pathogen infection. In anopheline mosquitoes, the TGF-β signaling pathway is highly conserved and includes multiple endogenous TGF-β ligands, five predicted type I and II receptors, and at least three Smad proteins and a number of inhibitors of Smad signaling in addition to MAPK and PI3K signaling pathway proteins (Fig. 2, reviewed in [41]). Putative orthologs of TGF-β signaling pathway proteins have also been described in ticks as well as the human body louse (Table 1), and studies with Ornithodoros moubata suggest that these soft ticks can respond to ingested mammalian TGF-β ligands and other growth factors [45]. Like insulin, ingested human TGF-β1 can activate mosquito MEK-ERK signaling dose-dependently [20].
Studies have shown that TGF-β1 is perhaps one of the most potent physiological regulators of NO, and is used by both mammalian and mosquito hosts to control the growth of Plasmodium parasites [46]. In particular, ingested human TGF-β1 appears to regulate NO synthesis and malaria parasite development in mosquitoes. At the highest treatment doses of human TGF-β1, inhibition of A. stephensi ERK phosphorylation increased expression of NOS, suggesting that ERK activation negatively regulates NOS expression [20]. Importantly, at infection levels similar to those found in nature, human TGF-β1 enhanced control of P. falciparum development in A. stephensi [20]. Low levels of human TGF-β1 (≤ 200 pg/ml) provided in an infectious blood meal moderately increased NOS activity and inhibited parasite growth in A. stephensi. High doses of TGF-β1 (2,000 pg/ml), however, did not affect parasite growth, instead inducing even higher levels of NOS expression that in turn negatively regulated NO synthesis. These findings are consistent with numerous observations from mammalian cells that TGF-β1 regulates NOS activity at transcriptional, post-transcriptional, and post-translational levels [47].
4. Other cytokines
Cytokines are small cell signaling proteins that regulate the immune responses of vertebrates. More recently, invertebrates have also been shown to possess cytokine-like factors that regulate immune responses to infection and wounding. One of the first cytokines to be described in mammals was macrophage migration inhibitory factor (MIF). In humans, MIF is a multifunctional, pro-inflammatory cytokine that regulates both innate and acquired immune responses to infection with vector-borne pathogens that cause a variety of diseases, including malaria, leishmaniasis, and trypanosomiasis [48]. MIF orthologs have been identified in nematodes, plants, and protozoan parasites, as well as in some arthropods. Tick orthologs of MIF have been identified in A. americanum [49], Haemaphysalis longicornis [50], D. variabilis [51], I. scapularis, and Rhipicephalus sanguineus [52], although to date none have been identified in mosquitoes.
The highest expression of MIF in ticks occurs in the midgut epithelium following blood feeding, Tick MIF is comparable to recombinant human MIF in its ability to inhibit the migration of human macrophages [52]. Amblyomma americanum can also inject MIF into the host during feeding, where MIF most likely acts to enhance inflammation at the feeding site and thereby alters the rate of blood meal acquisition [53]. Although regulation of arthropod physiology by MIF has not yet been described, these findings demonstrate the functionality of endogenous MIFs in regulating tick vector competence. Further, if ticks possess the signaling architecture to respond to the MIF produced in the gut [52], then they may have the capacity to respond to circulating mammalian MIF ingested during blood feeding as well.
Numerous studies have demonstrated that mammalian MIF is present in circulating blood, and that these levels can change during infection with arthropod-borne pathogens [48]. For example, peripheral blood lymphocytes of calves infected with tick-borne Theileria annulata produced abundant MIF [54]. Other studies have highlighted a pathological association between circulating MIF levels and infection with P. falciparum. Specifically, increased circulating MIF has been associated with increased parasitemia, increased anemia, and elevated mortality during malaria infection [48]. Increased MIF levels are also associated with Leishmania tropica [55] and Trypanosoma brucei [56] infections. Adding to the complexity of MIF signaling, orthologs of this cytokine have been identified in the vector-borne parasites Brugia malayi [57], L. major [58], and a number of Plasmodium species [59]. Plasmodium MIF is found in the serum of malaria-infected patients and can directly modulate the mammalian immune response [59]. Similarly, both Leishmania- and Brugia-derived MIF can alter the function of macrophages in infected mammalian hosts [57, 58]. Therefore, parasite-derived MIFs can alter vector-parasite interactions indirectly, by changing the cytokine milieu to which blood-feeding arthropods are exposed. Additionally, MIF signals in mammals through pathways dependent on MEK/ERK, p38 MAPK, PI3K, and NF-κB [60], and these signaling proteins are present in both mosquitoes and ticks (Fig. 1 and 2). Thus, parasite-derived MIFs have the potential to directly affect signaling in arthropod vectors.
Given the extreme conservation from arthropods to mammals of critical signaling pathways activated by cytokines and growth factors such as MIF, insulin, and TGF-β1, it is reasonable to suspect that other, as yet unidentified, host blood-derived cytokines and growth factors could impact the physiology of blood feeding arthropods. For example, IFNγ and other cytokines signal via Janus kinase/signal transducers and activators of transcription (JAK/STAT). Humans possess four JAK and seven STAT family proteins, all of which have been shown to be activated by distinct cytokines [61]. Upon interaction of IFNγ with its membrane receptors, JAK is activated, which in turn phosphorylates STAT1, allowing it to translocate to the nucleus to induce the expression of specific immune genes. This pathway is regulated in part by suppressor of cytokine signaling-1 (SOCS-1), a negative feedback regulator that is induced by STAT-1 [61].
In D. melanogaster, three leptin-like cytokines of the IL-6 family (Upd, Upd2, and Upd3) signal through an IL-6-like receptor (Domeless) to activate a JAK kinase (Hopscotch), which in turn activates the transcription factor STAT (Stat92E) [62]. Functional members of the STAT family have been identified in A. gambiae, A. aquasalis, Ae. aegypti, Ae. albopictus, and Culex tritaeniorhynchus. In addition, orthologs of JAK and SOCS have been identified in both A. gambiae and Ae. aegypti [62]. Activation of the JAK/STAT pathway in A. gambiae mediates killing of Plasmodium parasites [63]. Furthermore, suppression of the JAK/STAT specific receptor, an ortholog of Domeless, increased the susceptibility of Ae. aegypti to dengue virus [64]. Similarly, RNAi-mediated knockdown of STAT in A. aquasalis led to increased malaria parasite development compared to controls [65]. The JAK/STAT pathway also regulates the expression of antimicrobial peptides in I. scapularis thereby limiting Anaplasma phagocytophilum, the agent of human granulocytic anaplasmosis [66]. Despite the presence of clear signaling architecture and the identification of endogenous cytokine-like ligands in D. melanogaster, no cytokine-like ligands have been identified to date in arthropod vectors. However, significantly elevated levels of IL-6 have been observed in serum of humans infected with malaria parasites [67], dengue virus [68], and Borrelia burgdorferi (causative agent of Lyme disease) [69]. In addition, putative orthologs of an IL-6 like receptor are known for A. gambiae (XP_319240.5), Ae. aegypti (XP_001662596.1), and Culex quinquefasciatus (XP_001867486.1), I. scapularis (XP_002407139.1), and Pediculus humanus corporis (XP_002428294.1), raising the possibility that exogenous human cytokines could signal through arthropod JAK/STAT pathways to alter the immune responses of these arthropod vectors to infection.
5. Antibodies
Mammalian antibodies specific for pathogen epitopes have been used successfully to block pathogen transmission in a variety of blood feeding arthropods in the laboratory [70, 71]. Therefore, one approach to controlling vector-borne diseases is the development of vaccines that induce antibodies that are carried into the blood meal to block pathogen replication or development in the arthropod vector [70-74]. This approach has been used successfully in the tick I. scapularis, the sand fly Phlebotomus papatasi, as well as mosquitoes [70, 71]. For example, the colonization of I. scapularis by B. burgdorferi requires the binding of OspA to the tick receptor of OspA (TROSPA) [71]. Antisera against OspA and TROSPA prevent the efficient colonization of ticks and the subsequent transmission of spirochetes to the mammalian host.
Several studies have demonstrated that ‘anti-midgut’ antibodies that interfere with normal blood digestion processes can also alter the ability of arthropod vectors to transmit pathogens [70]. For example, the development of the rodent parasite Plasmodium berghei was significantly reduced in mosquitoes fed on infected mice that had been immunized with A. gambiae recombinant carboxypeptidase B (CPB), an enzyme involved in blood meal digestion [70]. Antibodies against A. gambiae midgut specific protein aminopeptidase N (AgAPN1) strongly inhibited both P. berghei and P. falciparum development in mosquitoes [75]. Ae. aegypti showed significant reduction in susceptibility to infection with Ross River virus and Murray Valley encephalitis virus when fed midgut-specific antibodies [76]. Similarly, immunization of mice and cattle against the tick protein subolesin, which is involved in the modulation of tick feeding and reproduction, prevented the infection of ticks by Anaplasma marginale, Babesia bigemina, B. burgdorferi and A. phagocytophilum [77-80]. Other blood feeding arthropods are also susceptible to this approach, as antibodies against P. papatasi galectin midgut surface protein (PpGalec) have also been shown to significantly decrease L. major development in sand flies [81]. These studies demonstrate that ingested mammalian antibodies can alter the course of infection in a wide variety of blood feeding arthropods and provide a valuable tool in the generation of transmission blocking vaccines.
6. Complement
Components of mammalian complement ingested by blood feeding arthropods can affect pathogen development in the arthropod host. This has been reported for mosquitoes and ticks, suggesting that these phenomena could occur in other vector arthropods as well. In mosquitoes, a number of studies have revealed that ingested mammalian complement persists in the midgut and can reduce malaria parasite development significantly [82]. This response is mediated in part by parasite-specific antibodies in the host serum that induce parasite lysis in the mosquito midgut via activation of the classical complement cascade [83]. The classical complement cascade is initiated by the binding of the C1 complex to antibodies, including IgG subclasses IgG1 and/or IgG3, that are attached to the surface of pathogens [84]. Antibody-mediated complement lysis in the mosquito midgut appears to target parasites as they transition from gametocytes to gametes/zygotes and from gametes/zygotes to ookinetes [82]. Additionally, the alternative complement cascade, which is functionally independent of antibody action in mammals, may be active against developing midgut-stage malaria parasites in mosquitoes [85]. The alternative complement pathway is initiated by microbial surface-induced hydrolysis of the thioester bond of the protein C3, which is present in blood plasma [84]. Tsuboi et al. demonstrated that murine C3 binds to the surface of parasite zygotes – which form in the mosquito midgut within minutes after ingestion – and inhibits their transition into mobile ookinetes [86].
Ingested mammalian complement components also function to control pathogen development in ticks. Borrelia burgdorferi can activate and be lysed by complement factor C3b in the absence of Borrelia-specific antibodies [87]. However, spirochetes can also resist the bactericidal effects of activated complement by binding to mammalian host proteins that inactivate C3b, such as factor H [88]. Interestingly, patterns of resistance or sensitivity to host complement are consistent with patterns of B. burgdorferi transmission, and may function to define the tick host range for this pathogen. For example, spirochetes that are sensitive to lysis by the complement system of a particular mammalian host species are lysed early and eliminated in the midgut of the feeding tick. These findings led to the hypothesis that the tick host range of a particular spirochete strain is restricted by its ability to bind complement inhibitors [89], thereby enabling bacterial survival in a particular tick host. This example provides one of very few mechanistic explanations for host specialization by a zoonotic pathogen, and reaffirms that a complex interplay of arthropod and mammalian host factors regulates pathogen development in the arthropod host.
7. Chitinase
With the exception of ticks, most blood feeding arthropods synthesize a peritrophic matrix (PM) around the ingested blood meal. The PM is primarily composed of proteins and chitin, and like the mucus layer found in the intestines of mammals, the PM functions to protect the arthropod gut from both mechanical damage and infectious pathogens [90]. Thus, the PM forms a physical barrier that most pathogens must traverse in order to establish infection.
Chitinases are highly conserved enzymes and have been identified in a wide variety of organisms, including Plasmodium [91] and Leishmania [92] parasites. Leishmania chitinase can alter the PM of sand flies rendering them more susceptible to infection [92]. In unnatural Leishmania-sand fly combinations, the PM does not break down during blood digestion, and the parasites are excreted [93]. Together, these data suggest that transmission of Leishmania parasites is dependent on co-evolved parasite enzyme-host substrate combinations. While Plasmodium ookinetes also utilize chitinase to traverse the PM [91], no chitinase genes have been identified in Trypansoma. Rather, trypanosomes seem to benefit from the endochitinase activity induced in the Rickettsia-like symbiotic organisms found in tsetse fly midguts following blood feeding [94]. Indeed, the presence of these midgut-specific endosymbionts correlates with susceptibility of tsetse flies to Trypansoma infection [94], indicating that gut microbiota also interact with arthropod and mammalian host factors to influence transmission.
The enzyme chitotriosidase (CHIT) is the human ortholog of invertebrate chitinase and similarly catalyzes the hydrolysis of chitin [95]. During P. falciparum infection in humans, IFNγ and TNFα can up-regulate CHIT gene expression by activated macrophages and plasma CHIT activity is elevated relative to uninfected levels [96]. Interestingly, some studies have suggested that accumulation of erythrocyte membrane degradation products in macrophages trigger the overproduction of CHIT in malaria patients [96]. Human CHIT was also recently shown to alter the structure of A. stephensi PM, reducing PM thickness in midguts from A. stephensi fed on blood supplemented with malaria patient plasma containing elevated CHIT [97]. Elevated CHIT activity has also been observed in patients with leishmaniasis [98], which could alter the PM of sand flies and, therefore, the transmission of Leishmania parasites as well.
8. Hemoglobin
Most blood feeding arthropods have evolved the ability to ingest large amounts of blood in a single feeding. For example, mosquitoes, sand flies and reduviid bugs can ingest 3-10 times their body weight in blood, while ticks can ingest as much as 100 times their initial weight [99]. Hemoglobin (Hb) is the most abundant protein in mammalian blood and can make up as much of 60% of blood protein content. In addition to release of heme, the degradation of Hb results in the release of bioactive peptides, some of which exhibit antibiotic activity both in humans and arthropods [100].
Antimicrobial peptides can limit the growth of or kill bacteria, fungi, and parasites and are important components of innate immunity [101]. In arthropods, antimicrobial Hb peptides constitutively generated during blood digestion create a hostile environment for invading organisms in the gut. The existence of Hb fragments with antimicrobial activity was first reported in the cattle tick, R. (B.) microplus [102]. Since then, antimicrobial Hb fragments have been detected in D. variabilis [103], and O. moubata [104]. In addition, Hb peptides with activity against T. cruzi have been isolated from the reduviids Triatoma infestans [105] and R. prolixus [106]. Following the discovery of antimicrobial Hb peptides in arthropods, Hb peptides with antimicrobial activity were identified in humans [107], indicating that this physiology predates the separation of arthropods and chordates. It is intriguing to speculate that arthropods may have first used oxygen-binding globins from bacteria, yeast, or protozoa to the same effect [108], then adapted to the use of ingested Hb after the evolution of blood feeding.
The process of Hb digestion also generates ROS, which at high concentrations can participate directly in pathogen killing [109]. As Hb is digested, heme is released, which can catalyze the synthesis of toxic levels of ROS [99]. The blood stream forms of Trypanosoma and Plasmodium are sensitive to lysis by heme-induced ROS [110, 111]. In mosquitoes, the presence of malaria parasites exacerbates the oxidative stress associated with blood digestion [24], suggesting that an infected blood meal is a biochemically challenging mix of damaging ROS. In response, arthropod vectors have evolved inducible antioxidants and mechanisms to inactivate heme [99]. However these responses are not immediate or saturating thus, heme-induced ROS are likely present at some level throughout the course of blood digestion.
At low concentrations ROS can function as signaling molecules in pathways involved in growth, differentiation, and immunity [112]. Although ROS-dependent signaling has been shown to broadly regulate the innate immune responses of a variety of animals [113], the only analogous arthropod vector data available thus far are from mosquitoes. In particular, ROS-dependent activation of the Toll pathway is critical for the control of dengue virus in Wolbachia-infected Ae. aegypti [114]. Further, IIS-induced ROS can prime an early anti-inflammatory state in A. stephensi in which malaria parasite development is favored [12, 115]. Studies with mammalian cells provide context for speculation on the underlying mechanisms. Notably, ROS can directly alter the activity of PTEN, a known inhibitor of the IIS pathway [116]. Alternatively, ROS can stimulate protein kinase C (PKC) activity through the oxidative modification of PKC regulatory domains [117]; heme-induced ROS have been shown to activate PKCs in Ae. aegpyti [118]. Mosquitoes possess PTEN and a variety of PKC orthologs ([119], Pakpour et al. unpublished results, Fig. 1), suggesting that ROS-dependent IIS regulation is transduced by these signaling proteins. Given the conservation of IIS architecture (see Section 1) and the universality of ROS- and reactive nitrogen species-dependent [22] physiology in arthropods, these signaling responses are likely to be the rule rather than the exception in vector arthropods.
9. Pathogen-derived factors
Vector-borne pathogens must manipulate their hosts for survival. Over time, natural selection has favored those organisms that can subvert, inactivate or exhaust the most effective mammalian and invertebrate host immune responses. One example of a pathogen-derived factor that alters mammalian host and arthropod vector biology, and is shared by a number of parasites, is glycosylphosphatidylinositols (GPIs) and GPI-anchored proteins.
GPIs are ubiquitous in eukaryotic cells and function to anchor proteins to cell surfaces and constitute the major carbohydrate modification in Plasmodium, Leishmania, and Trypansoma parasites [120, 121]. P. falciparum GPIs (PfGPIs) contribute to pro-inflammatory responses and malaria pathogenesis in humans [122]. Similarly, T. cruzi and L. major GPIs modulate the activation and pro-inflammatory cytokine production of human macrophages [121]. Therefore, parasite-derived GPIs can alter the cytokine milieu that vector arthropods are exposed to upon blood feeding.
Parasite-derived GPIs can also act as important inflammatory mediators in invertebrate hosts. For example, PfGPIs – signaling through Akt and ERK – can also induce NOS expression in A. stephensi [12] and the secretion of anti-microbial peptides in A. gambiae [123]. In Leishmania, GPI-anchored cell surface lipophosphoglycans (LPGs) play a crucial role in the survival and infectivity of parasites in sand flies. LPG is necessary for the binding of Leishmania to the sand fly midgut and polymorphisms in LPG are thought to be the underlying cause of the specificity of Leishmania-sand fly vector relationships [120]. Similarly, GPI-anchored proteins are required for the colonization of tsetse flies by T. brucei [124]. Taken together, these studies demonstrate that the GPIs and GPI-anchored proteins of vector-borne parasites can signal and alter the course of infection in both mammalian and vector arthropod hosts.
10. Conclusions and Future Directions
In this review, we have highlighted a number of blood-derived factors that remain active or become immunologically active in vector arthropods. The conservation of response pathways, such as IIS and TGF-β signaling, argues for the universality of these phenomena. Indeed, the breadth of cross-talk that has been identified between mammalian hosts and blood feeding arthropods suggests that other connections remain to be identified. Even among known interactions, however, many show strong dose-dependency, are networked by shared proteins, and co-occur or occur within a narrow window of time. In addition, most of the factors discussed in this review are likely to present in a single blood meal. Therefore, it will also be necessary to examine how the signaling pathways activated by mammalian blood-derived factors network with each other to elucidate their ultimate downstream affects on pathogen transmission by vector arthropods. For example, ingested human insulin has been shown to shorten the lifespan of mosquitoes [7], while human IGF-1 extends lifespan [8], and these growth factors are likely to be present in a blood meal concurrently, albeit at different concentrations. Depending on the affinity of these factors for mosquito ILP receptors, and the kinetics of the signaling response, very different effects on lifespan could be observed. Thus, the interface of blood feeding represents a complex system that juxtaposes – and involves information transfer among and within – multiple organisms.
The availability of genome sequence data for blood feeding vectors, their mammalian hosts, and pathogens has improved our capacity to understand and predict the architecture for these responses, but the task of assigning and comparing functions amongst the myriad of encoded proteins remains a formidable challenge. One strategy for unraveling this daunting complexity leverages systems and computational biology approaches to extract patterns from data, create models from these patterns, generate predictions from these models, and, ultimately, to develop strategies to modulate vector biology based on these predictions to reduce parasite transmission [125]. In this approach, large experimental datasets are used to generate initially data-driven, quasi-mechanistic models and then more explicitly mechanistic computational models, which are then assessed by comparing model predictions with further experimental data. Such an approach can provide a framework of interpretation of emergent phenomena and high level manifestations that could not have been inferred from isolated datasets and is, therefore, uniquely suited for studies involving host-vector-pathogen interactions [126]. Thus far, systems biology approaches have been applied to mammalian host-pathogen interactions [127], which necessarily omit the most important interactions for successful transmission of vector-borne pathogens. A systems biology approach that incorporates the mammalian host response to infection – in the context of the vector response to an infectious blood meal – could be used to predict how the emergence of asymptomatic infection in the absence of complete cure, for example, could impact pathogen transmission [128]. Further, the capacity to model vector host interactions in this scenario, or in the context of co-infection [129], might be useful in defining more robust transmission-blocking strategies. Hence, systems analyses of the biology highlighted here can support novel interventions that are not constrained by a reductionist view or an experimental capacity that is inherently insufficient for the complexity of vector-borne pathogen transmission.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Darby SM, Miller ML, Allen RO, LeBeau M. A mass spectrometric method for quantitation of intact insulin in blood samples. J Anal Toxicol. 2001;25:8–14. doi: 10.1093/jat/25.1.8. [DOI] [PubMed] [Google Scholar]
- 2.White NJ, Warrell DA, Chanthavanich P, Looareesuwan S, Warrell MJ, Krishna S, Williamson DH, Turner RC. Severe hypoglycemia and hyperinsulinemia in falciparum malaria. N Engl J Med. 1983;309:61–66. doi: 10.1056/NEJM198307143090201. [DOI] [PubMed] [Google Scholar]
- 3.Danquah I, Bedu-Addo G, Mockenhaupt FP. Type 2 diabetes mellitus and increased risk for malaria infection. Emerg Infect Dis. 2010;16:1601–1604. doi: 10.3201/eid1610.100399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitten MM, Shiao SH, Levashina EA. Mosquito midguts and malaria: cell biology, compartmentalization and immunology. Parasite Immunol. 2006;28:121–130. doi: 10.1111/j.1365-3024.2006.00804.x. [DOI] [PubMed] [Google Scholar]
- 5.Beier MS, Pumpuni CB, Beier JC, Davis JR. Effects of para-aminobenzoic acid, insulin, and gentamicin on Plasmodium falciparum development in anopheline mosquitoes (Diptera: Culicidae) J Med Entomol. 1994;31:561–565. doi: 10.1093/jmedent/31.4.561. [DOI] [PubMed] [Google Scholar]
- 6.Surachetpong W, Pakpour N, Cheung KW, Luckhart S. Reactive oxygen species-dependent cell signaling regulates the mosquito immune response to Plasmodium falciparum. Antioxid Redox Signal. 2011;14:943–955. doi: 10.1089/ars.2010.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang MA, Mott TM, Tapley EC, Lewis EE, Luckhart S. Insulin regulates aging and oxidative stress in Anopheles stephensi. J Exp Biol. 2008;211:741–748. doi: 10.1242/jeb.012955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drexler A, Nuss A, Hauck E, Glennon E, Cheung K, Brown M, Luckhart S. Human IGF1 extends lifespan and enhances resistance to Plasmodium falciparum infection in the malaria vector Anopheles stephensi. J Exp Biol. 2013;216:208–217. doi: 10.1242/jeb.078873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luckhart S, Riehle MA. The insulin signaling cascade from nematodes to mammals: insights into innate immunity of Anopheles mosquitoes to malaria parasite infection. Dev Comp Immunol. 2007;31:647–656. doi: 10.1016/j.dci.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drexler A, Nuss A, Hauck E, Glennon E, Brown MR, Luckhart S. Human IGF1 extends lifespan and enhances resistance to Plasmodium falciparum infection in the malaria vector Anopheles stephensi under review at. J Exp Bio. 2011 doi: 10.1242/jeb.078873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marquez AG, Pietri JE, Smithers HM, Nuss A, Antonova Y, Drexler AL, Riehle MA, Brown MR, Luckhart S. Insulin-like peptides in the mosquito Anopheles stephensi: Identification and expression in response to diet and infection with Plasmodium falciparum. Gen Comp Endocrinol. 2011;173:303–312. doi: 10.1016/j.ygcen.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim J, Gowda DC, Krishnegowda G, Luckhart S. Induction of nitric oxide synthase in Anopheles stephensi by Plasmodium falciparum: mechanism of signaling and the role of parasite glycosylphosphatidylinositols. Infect Immun. 2005;73:2778–2789. doi: 10.1128/IAI.73.5.2778-2789.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akman-Anderson L, Olivier M, Luckhart S. Induction of nitric oxide synthase and activation of signaling proteins in Anopheles mosquitoes by the malaria pigment, hemozoin. Infect Immun. 2007;75:4012–4019. doi: 10.1128/IAI.00645-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horton AA, Wang B, Camp L, Price MS, Arshi A, Nagy M, Nadler SA, Faeder JR, Luckhart S. The mitogen-activated protein kinome from Anopheles gambiae: identification, phylogeny and functional characterization of the ERK, JNK and p38 MAP kinases. BMC Genomics. 2011;12:574. doi: 10.1186/1471-2164-12-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pakpour N, Corby-Harris V, Green GP, Smithers HM, Cheung KW, Riehle MA, Luckhart S. Ingested human insulin inhibits the mosquito NF-kappaB-dependent immune response to Plasmodium falciparum. Infect Immun. 2012;80:2141–2149. doi: 10.1128/IAI.00024-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corby-Harris V, Drexler A, Watkins de Jong L, Antonova Y, Pakpour N, Ziegler R, Ramberg F, Lewis EE, Brown JM, Luckhart S, Riehle MA. Activation of Akt signaling reduces the prevalence and intensity of malaria parasite infection and lifespan in Anopheles stephensi mosquitoes. PLoS Pathog. 2010;6:e1001003. doi: 10.1371/journal.ppat.1001003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonova Y, Arik AJ, Moore W, Riehle MR, Brown MR. Insulin-like peptides: structure, signaling, and function. In: Gilbert LI, editor. Insect endocrinology. Elsevier/Academic Press; Waltham, MA: 2012. pp. 63–92. [Google Scholar]
- 18.Davis HH, Dotson EM, Oliver JH., Jr. Localization of insulin-like immunoreactivity in the synganglion of nymphal and adult Dermacentor variabilis (Acari: Ixodidae) Exp Appl Acarol. 1994;18:111–122. doi: 10.1007/BF00055035. [DOI] [PubMed] [Google Scholar]
- 19.de Abreu LA, Fabres A, Esteves E, Masuda A, da Silva Vaz I, Jr., Daffre S, Logullo C. Exogenous insulin stimulates glycogen accumulation in Rhipicephalus (Boophilus) microplus embryo cell line BME26 via PI3K/AKT pathway. Comp Biochem Physiol B Biochem Mol Biol. 2009;153:185–190. doi: 10.1016/j.cbpb.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 20.Surachetpong W, Singh N, Cheung KW, Luckhart S. MAPK ERK signaling regulates the TGF-beta1-dependent mosquito response to Plasmodium falciparum. PLoS Pathog. 2009;5:e1000366. doi: 10.1371/journal.ppat.1000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Humphries KM, Szweda PA, Szweda LI. Aging: a shift from redox regulation to oxidative damage. Free Radic Res. 2006;40:1239–1243. doi: 10.1080/10715760600913184. [DOI] [PubMed] [Google Scholar]
- 22.Luckhart S, Giulivi C, Drexler AL, Antonova-Koch Y, Sakaguchi D, Napoli E, Wong S, Price MS, Eigenheer R, Phinney BS, Pakpour N, Pietri JE, Cheung K, Georgis M, Riehle M. Sustained activation of Akt elicits mitochondrial dysfunction to block Plasmodium falciparum Infection in the mosquito host. PLoS Pathog. doi: 10.1371/journal.ppat.1003180. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luckhart S, Vodovotz Y, Cui L, Rosenberg R. The mosquito Anopheles stephensi limits malaria parasite development with inducible synthesis of nitric oxide. Proc Natl Acad Sci U S A. 1998;95:5700–5705. doi: 10.1073/pnas.95.10.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterson TM, Gow AJ, Luckhart S. Nitric oxide metabolites induced in Anopheles stephensi control malaria parasite infection. Free Radic Biol Med. 2007;42:132–142. doi: 10.1016/j.freeradbiomed.2006.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sinden RE, Dawes EJ, Alavi Y, Waldock J, Finney O, Mendoza J, Butcher GA, Andrews L, Hill AV, Gilbert SC, Basanez MG. Progression of Plasmodium berghei through Anopheles stephensi is density-dependent. PLoS Pathog. 2007;3:e195. doi: 10.1371/journal.ppat.0030195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dandona P, Aljada A, Mohanty P, Ghanim H, Hamouda W, Assian E, Ahmad S. Insulin inhibits intranuclear nuclear factor kappaB and stimulates IkappaB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J Clin Endocrinol Metab. 2001;86:3257–3265. doi: 10.1210/jcem.86.7.7623. [DOI] [PubMed] [Google Scholar]
- 27.Frolet C, Thoma M, Blandin S, Hoffmann JA, Levashina EA. Boosting NF-kappaB-dependent basal immunity of Anopheles gambiae aborts development of Plasmodium berghei. Immunity. 2006;25:677–685. doi: 10.1016/j.immuni.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 28.Meredith JM, Munks RJ, Grail W, Hurd H, Eggleston P, Lehane MJ. A novel association between clustered NF-kappaB and C/EBP binding sites is required for immune regulation of mosquito Defensin genes. Insect Mol Biol. 2006;15:393–401. doi: 10.1111/j.1365-2583.2006.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 30.Cirimotich CM, Dong Y, Garver LS, Sim S, Dimopoulos G. Mosquito immune defenses against Plasmodium infection. Dev Comp Immunol. 2010;34:387–395. doi: 10.1016/j.dci.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohan S, Baylink DJ. IGF-binding proteins are multifunctional and act via IGF-dependent and -independent mechanisms. J Endocrinol. 2002;175:19–31. doi: 10.1677/joe.0.1750019. [DOI] [PubMed] [Google Scholar]
- 32.Honegger B, Galic M, Kohler K, Wittwer F, Brogiolo W, Hafen E, Stocker H. Imp-L2, a putative homolog of vertebrate IGF-binding protein 7, counteracts insulin signaling in Drosophila and is essential for starvation resistance. J Biol. 2008;7:10. doi: 10.1186/jbiol72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sajid W, Kulahin N, Schluckebier G, Ribel U, Henderson HR, Tatar M, Hansen BF, Svendsen AM, Kiselyov VV, Norgaard P, Wahlund PO, Brandt J, Kohanski RA, Andersen AS, De Meyts P. Structural and biological properties of the Drosophila insulin-like peptide 5 show evolutionary conservation. J Biol Chem. 2011;286:661–673. doi: 10.1074/jbc.M110.156018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mulenga A, Khumthong R. Silencing of three Amblyomma americanum (L.) insulin-like growth factor binding protein-related proteins prevents ticks from feeding to repletion. J Exp Biol. 2010;213:1153–1161. doi: 10.1242/jeb.035204. [DOI] [PubMed] [Google Scholar]
- 35.Brown MR, Clark KD, Gulia M, Zhao Z, Garczynski SF, Crim JW, Suderman RJ, Strand MR. An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti. Proc Natl Acad Sci U S A. 2008;105:5716–5721. doi: 10.1073/pnas.0800478105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersen L, Jorgensen PN, Jensen LB, Walsh D. A new insulin immunoassay specific for the rapid-acting insulin analog, insulin aspart, suitable for bioavailability, bioequivalence, and pharmacokinetic studies. Clin Biochem. 2000;33:627–633. doi: 10.1016/s0009-9120(00)00183-1. [DOI] [PubMed] [Google Scholar]
- 37.Attisano L, Wrana JL. Signal transduction by the TGF-beta superfamily. Science. 2002;296:1646–1647. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- 38.Omer FM, Kurtzhals JA, Riley EM. Maintaining the immunological balance in parasitic infections: a role for TGF-beta? Parasitol Today. 2000;16:18–23. doi: 10.1016/s0169-4758(99)01562-8. [DOI] [PubMed] [Google Scholar]
- 39.Moustakas A, Heldin CH. Non-Smad TGF-beta signals. J Cell Sci. 2005;118:3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- 40.Luckhart S, Crampton AL, Zamora R, Lieber MJ, Dos Santos PC, Peterson TM, Emmith N, Lim J, Wink DA, Vodovotz Y. Mammalian transforming growth factor beta1 activated after ingestion by Anopheles stephensi modulates mosquito immunity. Infect Immun. 2003;71:3000–3009. doi: 10.1128/IAI.71.6.3000-3009.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lieber MJ, Luckhart S. Transforming growth factor-betas and related gene products in mosquito vectors of human malaria parasites: signaling architecture for immunological crosstalk. Mol Immunol. 2004;41:965–977. doi: 10.1016/j.molimm.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 42.Padgett RW, Wozney JM, Gelbart WM. Human BMP sequences can confer normal dorsal-ventral patterning in the Drosophila embryo. Proc Natl Acad Sci U S A. 1993;90:2905–2909. doi: 10.1073/pnas.90.7.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newfeld SJ, Mehra A, Singer MA, Wrana JL, Attisano L, Gelbart WM. Mothers against dpp participates in a DDP/TGF-beta responsive serine-threonine kinase signal transduction cascade. Development. 1997;124:3167–3176. doi: 10.1242/dev.124.16.3167. [DOI] [PubMed] [Google Scholar]
- 44.Brummel T, Abdollah S, Haerry TE, Shimell MJ, Merriam J, Raftery L, Wrana JL, O'Connor MB. The Drosophila activin receptor baboon signals through dSmad2 and controls cell proliferation but not patterning during larval development. Genes Dev. 1999;13:98–111. doi: 10.1101/gad.13.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsuo T, Cerruto Noya CA, Taylor D, Fujisaki K. Immunohistochemical examination of PDGF-AB, TGF-beta and their receptors in the hemocytes of a tick, Ornithodoros moubata (Acari: Argasidae) J Vet Med Sci. 2007;69:317–320. doi: 10.1292/jvms.69.317. [DOI] [PubMed] [Google Scholar]
- 46.Vodovotz Y, Zamora R, Lieber MJ, Luckhart S. Cross-talk between nitric oxide and transforming growth factor-beta1 in malaria. Curr Mol Med. 2004;4:787–797. doi: 10.2174/1566524043359999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vodovotz Y. Control of nitric oxide production by transforming growth factor-beta1: mechanistic insights and potential relevance to human disease. Nitric Oxide. 1997;1:3–17. doi: 10.1006/niox.1996.0105. [DOI] [PubMed] [Google Scholar]
- 48.Bozza MT, Martins YC, Carneiro LA, Paiva CN. Macrophage migration inhibitory factor in protozoan infections. J Parasitol Res. 2012;2012:413052. doi: 10.1155/2012/413052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jaworski DC, Jasinskas A, Metz CN, Bucala R, Barbour AG. Identification and characterization of a homologue of the pro-inflammatory cytokine Macrophage Migration Inhibitory Factor in the tick, Amblyomma americanum. Insect Mol Biol. 2001;10:323–331. doi: 10.1046/j.0962-1075.2001.00271.x. [DOI] [PubMed] [Google Scholar]
- 50.Umemiya R, Hatta T, Liao M, Tanaka M, Zhou J, Inoue N, Fujisaki K. Haemaphysalis longicornis: molecular characterization of a homologue of the macrophage migration inhibitory factor from the partially fed ticks. Exp Parasitol. 2007;115:135–142. doi: 10.1016/j.exppara.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 51.Wasala NB, Jaworski DC. Dermacentor variabilis: characterization and modeling of macrophage migration inhibitory factor with phylogenetic comparisons to other ticks, insects and parasitic nematodes. Exp Parasitol. 2012;130:232–238. doi: 10.1016/j.exppara.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 52.Bowen CJ, Jaworski DC, Wasala NB, Coons LB. Macrophage migration inhibitory factor expression and protein localization in Amblyomma americanum (Ixodidae) Exp Appl Acarol. 2010;50:343–352. doi: 10.1007/s10493-009-9324-5. [DOI] [PubMed] [Google Scholar]
- 53.Jaworski DC, Bowen CJ, Wasala NB. Amblyomma americanum (L): tick macrophage migration inhibitory factor peptide immunization lengthens lone star tick feeding intervals in vivo. Exp Parasitol. 2009;121:384–387. doi: 10.1016/j.exppara.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Rehbein G, Ahmed JS, Schein E, Horchner F, Zweygarth E. Immunological aspects of Theileria annulata infection calves. 2. Production of macrophage migration inhibition factor (MIF) by sensitized lymphocytes from Theileria annulata-infected calves. Tropenmed Parasitol. 1981;32:154–156. [PubMed] [Google Scholar]
- 55.Kozaci DL, Ertug S, Kavak T, Okyay P, Chikanza IC, Ertabaklar H. [Investigation of Serum Macrophage Migration Inhibitor Factor (MIF) levels in patients with cutaneous leishmaniasis.] Turkiye Parazitol Derg. 2005;29:145–148. [PubMed] [Google Scholar]
- 56.Nishimura K, Nakaya H, Nakagawa H, Matsuo S, Ohnishi Y, Yamasaki S. Differential effects of Trypanosoma brucei gambiense and Trypanosoma brucei brucei on rat macrophages. J Parasitol. 2011;97:48–54. doi: 10.1645/GE-2466.1. [DOI] [PubMed] [Google Scholar]
- 57.Prieto-Lafuente L, Gregory WF, Allen JE, Maizels RM. MIF homologues from a filarial nematode parasite synergize with IL-4 to induce alternative activation of host macrophages. J Leukoc Biol. 2009;85:844–854. doi: 10.1189/jlb.0808459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kamir D, Zierow S, Leng L, Cho Y, Diaz Y, Griffith J, McDonald C, Merk M, Mitchell RA, Trent J, Chen Y, Kwong YK, Xiong H, Vermeire J, Cappello M, McMahon-Pratt D, Walker J, Bernhagen J, Lolis E, Bucala R. A Leishmania ortholog of macrophage migration inhibitory factor modulates host macrophage responses. J Immunol. 2008;180:8250–8261. doi: 10.4049/jimmunol.180.12.8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Han C, Lin Y, Shan G, Zhang Z, Sun X, Wang Z, Wei C, Deng Y, Zhang L, Bu L, Shao D, Wang H. Plasma concentration of malaria parasite-derived macrophage migration inhibitory factor in uncomplicated malaria patients correlates with parasitemia and disease severity. Clin Vaccine Immunol. 2010;17:1524–1532. doi: 10.1128/CVI.00149-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lue H, Kleemann R, Calandra T, Roger T, Bernhagen J. Macrophage migration inhibitory factor (MIF): mechanisms of action and role in disease. Microbes Infect. 2002;4:449–460. doi: 10.1016/s1286-4579(02)01560-5. [DOI] [PubMed] [Google Scholar]
- 61.Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007;282:20059–20063. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- 62.Zhou F, Agaisse H. In: JAK/STAT Signaling and Invertebrate Immune Responses Jak-Stat Signaling : From Basics to Disease. Decker T, Müller M, editors. Springer Vienna; 2012. pp. 133–151. [Google Scholar]
- 63.Gupta L, Molina-Cruz A, Kumar S, Rodrigues J, Dixit R, Zamora RE, Barillas-Mury C. The STAT pathway mediates late-phase immunity against Plasmodium in the mosquito Anopheles gambiae. Cell Host Microbe. 2009;5:498–507. doi: 10.1016/j.chom.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Souza-Neto JA, Sim S, Dimopoulos G. An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proc Natl Acad Sci U S A. 2009;106:17841–17846. doi: 10.1073/pnas.0905006106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bahia AC, Kubota MS, Tempone AJ, Araujo HR, Guedes BA, Orfano AS, Tadei WP, Rios-Velasquez CM, Han YS, Secundino NF, Barillas-Mury C, Pimenta PF, Traub-Cseko YM. The JAK-STAT pathway controls Plasmodium vivax load in early stages of Anopheles aquasalis infection. PLoS Negl Trop Dis. 2011;5:e1317. doi: 10.1371/journal.pntd.0001317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu L, Dai J, Zhao YO, Narasimhan S, Yang Y, Zhang L, Fikrig E. Ixodes scapularis JAK-STAT pathway regulates tick antimicrobial peptides, thereby controlling the agent of human granulocytic anaplasmosis. J Infect Dis. 2012;206:1233–1241. doi: 10.1093/infdis/jis484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lyke KE, Burges R, Cissoko Y, Sangare L, Dao M, Diarra I, Kone A, Harley R, Plowe CV, Doumbo OK, Sztein MB. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immun. 2004;72:5630–5637. doi: 10.1128/IAI.72.10.5630-5637.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang YH, Lei HY, Liu HS, Lin YS, Liu CC, Yeh TM. Dengue virus infects human endothelial cells and induces IL-6 and IL-8 production. Am J Trop Med Hyg. 2000;63:71–75. doi: 10.4269/ajtmh.2000.63.71. [DOI] [PubMed] [Google Scholar]
- 69.Sigal LH. Lyme disease: a review of aspects of its immunology and immunopathogenesis. Annu Rev Immunol. 1997;15:63–92. doi: 10.1146/annurev.immunol.15.1.63. [DOI] [PubMed] [Google Scholar]
- 70.Lavazec C, Bourgouin C. Mosquito-based transmission blocking vaccines for interrupting Plasmodium development. Microbes Infect. 2008;10:845–849. doi: 10.1016/j.micinf.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 71.de la Fuente J, Kocan KM. Strategies for development of vaccines for control of ixodid tick species. Parasite Immunol. 2006;28:275–283. doi: 10.1111/j.1365-3024.2006.00828.x. [DOI] [PubMed] [Google Scholar]
- 72.Crompton PD, Pierce SK, Miller LH. Advances and challenges in malaria vaccine development. J Clin Invest. 2010;120:4168–4178. doi: 10.1172/JCI44423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schuijt TJ, Hovius JW, van der Poll T, van Dam AP, Fikrig E. Lyme borreliosis vaccination: the facts, the challenge, the future. Trends Parasitol. 2011;27:40–47. doi: 10.1016/j.pt.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 74.Dinglasan RR, Jacobs-Lorena M. Flipping the paradigm on malaria transmission-blocking vaccines. Trends Parasitol. 2008;24:364–370. doi: 10.1016/j.pt.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dinglasan RR, Kalume DE, Kanzok SM, Ghosh AK, Muratova O, Pandey A, Jacobs-Lorena M. Disruption of Plasmodium falciparum development by antibodies against a conserved mosquito midgut antigen. Proc Natl Acad Sci U S A. 2007;104:13461–13466. doi: 10.1073/pnas.0702239104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ramasamy MS, Sands M, Kay BH, Fanning ID, Lawrence GW, Ramasamy R. Anti-mosquito antibodies reduce the susceptibility of Aedes aegypti to arbovirus infection. Med Vet Entomol. 1990;4:49–55. doi: 10.1111/j.1365-2915.1990.tb00259.x. [DOI] [PubMed] [Google Scholar]
- 77.de la Fuente J, Almazan C, Blas-Machado U, Naranjo V, Mangold AJ, Blouin EF, Gortazar C, Kocan KM. The tick protective antigen, 4D8, is a conserved protein involved in modulation of tick blood ingestion and reproduction. Vaccine. 2006;24:4082–4095. doi: 10.1016/j.vaccine.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 78.Merino O, Almazan C, Canales M, Villar M, Moreno-Cid JA, Galindo RC, de la Fuente J. Targeting the tick protective antigen subolesin reduces vector infestations and pathogen infection by Anaplasma marginale and Babesia bigemina. Vaccine. 2011;29:8575–8579. doi: 10.1016/j.vaccine.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 79.de la Fuente J, Moreno-Cid JA, Canales M, Villar M, de la Lastra JM, Kocan KM, Galindo RC, Almazan C, Blouin EF. Targeting arthropod subolesin/akirin for the development of a universal vaccine for control of vector infestations and pathogen transmission. Vet Parasitol. 2011;181:17–22. doi: 10.1016/j.vetpar.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 80.Bensaci M, Bhattacharya D, Clark R, Hu LT. Oral vaccination with vaccinia virus expressing the tick antigen subolesin inhibits tick feeding and transmission of Borrelia burgdorferi. Vaccine. 2012;30:6040–6046. doi: 10.1016/j.vaccine.2012.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kamhawi S, Ramalho-Ortigao M, Pham VM, Kumar S, Lawyer PG, Turco SJ, Barillas-Mury C, Sacks DL, Valenzuela JG. A role for insect galectins in parasite survival. Cell. 2004;119:329–341. doi: 10.1016/j.cell.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 82.Gouagna LC, Bonnet S, Gounoue R, Verhave JP, Eling W, Sauerwein R, Boudin C. Stage-specific effects of host plasma factors on the early sporogony of autologous Plasmodium falciparum isolates within Anopheles gambiae. Trop Med Int Health. 2004;9:937–948. doi: 10.1111/j.1365-3156.2004.01300.x. [DOI] [PubMed] [Google Scholar]
- 83.Healer J, McGuinness D, Hopcroft P, Haley S, Carter R, Riley E. Complement-mediated lysis of Plasmodium falciparum gametes by malaria-immune human sera is associated with antibodies to the gamete surface antigen Pfs230. Infect Immun. 1997;65:3017–3023. doi: 10.1128/iai.65.8.3017-3023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fujita T. Evolution of the lectin-complement pathway and its role in innate immunity. Nat Rev Immunol. 2002;2:346–353. doi: 10.1038/nri800. [DOI] [PubMed] [Google Scholar]
- 85.Margos G, Navarette S, Butcher G, Davies A, Willers C, Sinden RE, Lachmann PJ. Interaction between host complement and mosquito-midgut-stage Plasmodium berghei. Infect Immun. 2001;69:5064–5071. doi: 10.1128/IAI.69.8.5064-5071.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsuboi T, Cao YM, Torii M, Hitsumoto Y, Kanbara H. Murine complement reduces infectivity of Plasmodium yoelii to mosquitoes. Infect Immun. 1995;63:3702–3704. doi: 10.1128/iai.63.9.3702-3704.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Breitner-Ruddock S, Wurzner R, Schulze J, Brade V. Heterogeneity in the complement-dependent bacteriolysis within the species of Borrelia burgdorferi. Med Microbiol Immunol. 1997;185:253–260. doi: 10.1007/s004300050038. [DOI] [PubMed] [Google Scholar]
- 88.Stevenson B, El-Hage N, Hines MA, Miller JC, Babb K. Differential binding of host complement inhibitor factor H by Borrelia burgdorferi Erp surface proteins: a possible mechanism underlying the expansive host range of Lyme disease spirochetes. Infect Immun. 2002;70:491–497. doi: 10.1128/IAI.70.2.491-497.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kurtenbach K, De Michelis S, Etti S, Schafer SM, Sewell HS, Brade V, Kraiczy P. Host association of Borrelia burgdorferi sensu lato--the key role of host complement. Trends Microbiol. 2002;10:74–79. doi: 10.1016/s0966-842x(01)02298-3. [DOI] [PubMed] [Google Scholar]
- 90.Terra WR. The origin and functions of the insect peritrophic membrane and peritrophic gel. Arch Insect Biochem Physiol. 2001;47:47–61. doi: 10.1002/arch.1036. [DOI] [PubMed] [Google Scholar]
- 91.Langer RC, Vinetz JM. Plasmodium ookinete-secreted chitinase and parasite penetration of the mosquito peritrophic matrix. Trends Parasitol. 2001;17:269–272. doi: 10.1016/s1471-4922(01)01918-3. [DOI] [PubMed] [Google Scholar]
- 92.Rogers ME, Hajmova M, Joshi MB, Sadlova J, Dwyer DM, Volf P, Bates PA. Leishmania chitinase facilitates colonization of sand fly vectors and enhances transmission to mice. Cell Microbiol. 2008;10:1363–1372. doi: 10.1111/j.1462-5822.2008.01132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Walters LL, Irons KP, Guzman H, Tesh RB. Peritrophic envelopes of Lutzomyia spinicrassa (Diptera: Psychodidae) J Med Entomol. 1995;32:711–725. doi: 10.1093/jmedent/32.5.711. [DOI] [PubMed] [Google Scholar]
- 94.Welburn SC, Arnold K, Maudlin I, Gooday GW. Rickettsia-like organisms and chitinase production in relation to transmission of trypanosomes by tsetse flies. Parasitology. 1993;107(Pt 2):141–145. doi: 10.1017/s003118200006724x. [DOI] [PubMed] [Google Scholar]
- 95.Malaguarnera L. Chitotriosidase: the yin and yang. Cell Mol Life Sci. 2006;63:3018–3029. doi: 10.1007/s00018-006-6269-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barone R, Simpore J, Malaguarnera L, Pignatelli S, Musumeci S. Plasma chitotriosidase activity in acute Plasmodium falciparum malaria. Clin Chim Acta. 2003;331:79–85. doi: 10.1016/s0009-8981(03)00089-5. [DOI] [PubMed] [Google Scholar]
- 97.Di Luca M, Romi R, Severini F, Toma L, Musumeci M, Fausto AM, Mazzini M, Gambellini G, Musumeci S. High levels of human chitotriosidase hinder the formation of peritrophic membrane in anopheline vectors. Parasitol Res. 2007;100:1033–1039. doi: 10.1007/s00436-006-0372-z. [DOI] [PubMed] [Google Scholar]
- 98.Hollak CE, van Weely S, van Oers MH, Aerts JM. Marked elevation of plasma chitotriosidase activity. A novel hallmark of Gaucher disease. J Clin Invest. 1994;93:1288–1292. doi: 10.1172/JCI117084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Graca-Souza AV, Maya-Monteiro C, Paiva-Silva GO, Braz GR, Paes MC, Sorgine MH, Oliveira MF, Oliveira PL. Adaptations against heme toxicity in blood-feeding arthropods. Insect Biochem Mol Biol. 2006;36:322–335. doi: 10.1016/j.ibmb.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 100.Ivanov VT, Karelin AA, Philippova MM, Nazimov IV, Pletnev VZ. Hemoglobin as a source of endogenous bioactive peptides: the concept of tissue-specific peptide pool. Biopolymers. 1997;43:171–188. doi: 10.1002/(SICI)1097-0282(1997)43:2<171::AID-BIP10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 101.Bulet P, Stocklin R, Menin L. Anti-microbial peptides: from invertebrates to vertebrates. Immunol Rev. 2004;198:169–184. doi: 10.1111/j.0105-2896.2004.0124.x. [DOI] [PubMed] [Google Scholar]
- 102.Fogaca AC, da Silva PI, Jr., Miranda MT, Bianchi AG, Miranda A, Ribolla PE, Daffre S. Antimicrobial activity of a bovine hemoglobin fragment in the tick Boophilus microplus. J Biol Chem. 1999;274:25330–25334. doi: 10.1074/jbc.274.36.25330. [DOI] [PubMed] [Google Scholar]
- 103.Sonenshine DE, Hynes WL, Ceraul SM, Mitchell R, Benzine T. Host blood proteins and peptides in the midgut of the tick Dermacentor variabilis contribute to bacterial control. Exp Appl Acarol. 2005;36:207–223. doi: 10.1007/s10493-005-2564-0. [DOI] [PubMed] [Google Scholar]
- 104.Nakajima Y, Ogihara K, Taylor D, Yamakawa M. Antibacterial hemoglobin fragments from the midgut of the soft tick, Ornithodoros moubata (Acari: Argasidae) J Med Entomol. 2003;40:78–81. doi: 10.1603/0022-2585-40.1.78. [DOI] [PubMed] [Google Scholar]
- 105.Fraidenraich D, Pena C, Isola EL, Lammel EM, Coso O, Anel AD, Pongor S, Baralle F, Torres HN, Flawia MM. Stimulation of Trypanosoma cruzi adenylyl cyclase by an alpha D-globin fragment from Triatoma hindgut: effect on differentiation of epimastigote to trypomastigote forms. Proc Natl Acad Sci U S A. 1993;90:10140–10144. doi: 10.1073/pnas.90.21.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Garcia ES, Gonzalez MS, de Azambuja P, Baralle FE, Fraidenraich D, Torres HN, Flawia MM. Induction of Trypanosoma cruzi metacyclogenesis in the gut of the hematophagous insect vector, Rhodnius prolixus, by hemoglobin and peptides carrying alpha D-globin sequences. Exp Parasitol. 1995;81:255–261. doi: 10.1006/expr.1995.1116. [DOI] [PubMed] [Google Scholar]
- 107.Parish CA, Jiang H, Tokiwa Y, Berova N, Nakanishi K, McCabe D, Zuckerman W, Xia MM, Gabay JE. Broad-spectrum antimicrobial activity of hemoglobin. Bioorg Med Chem. 2001;9:377–382. doi: 10.1016/s0968-0896(00)00263-7. [DOI] [PubMed] [Google Scholar]
- 108.Poole RK, Hughes MN. New functions for the ancient globin family: bacterial responses to nitric oxide and nitrosative stress. Mol Microbiol. 2000;36:775–783. doi: 10.1046/j.1365-2958.2000.01889.x. [DOI] [PubMed] [Google Scholar]
- 109.Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 110.Meshnick SR, Chang KP, Cerami A. Heme lysis of the bloodstream forms of Trypanosoma brucei. Biochem Pharmacol. 1977;26:1923–1928. doi: 10.1016/0006-2952(77)90167-8. [DOI] [PubMed] [Google Scholar]
- 111.Orjih AU, Banyal HS, Chevli R, Fitch CD. Hemin lyses malaria parasites. Science. 1981;214:667–669. doi: 10.1126/science.7027441. [DOI] [PubMed] [Google Scholar]
- 112.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 113.Bogdan C, Rollinghoff M, Diefenbach A. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr Opin Immunol. 2000;12:64–76. doi: 10.1016/s0952-7915(99)00052-7. [DOI] [PubMed] [Google Scholar]
- 114.Pan X, Zhou G, Wu J, Bian G, Lu P, Raikhel AS, Xi Z. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc Natl Acad Sci U S A. 2012;109:E23–31. doi: 10.1073/pnas.1116932108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Baton LA, Ranford-Cartwright LC. Plasmodium falciparum ookinete invasion of the midgut epithelium of Anopheles stephensi is consistent with the Time Bomb model. Parasitology. 2004;129:663–676. doi: 10.1017/s0031182004005979. [DOI] [PubMed] [Google Scholar]
- 116.Leslie NR, Bennett D, Lindsay YE, Stewart H, Gray A, Downes CP. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003;22:5501–5510. doi: 10.1093/emboj/cdg513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gopalakrishna R, Jaken S. Protein kinase C signaling and oxidative stress. Free Radic Biol Med. 2000;28:1349–1361. doi: 10.1016/s0891-5849(00)00221-5. [DOI] [PubMed] [Google Scholar]
- 118.Oliveira JH, Goncalves RL, Lara FA, Dias FA, Gandara AC, Menna-Barreto RF, Edwards MC, Laurindo FR, Silva-Neto MA, Sorgine MH, Oliveira PL. Blood meal-derived heme decreases ROS levels in the midgut of Aedes aegypti and allows proliferation of intestinal microbiota. PLoS Pathog. 2011;7:e1001320. doi: 10.1371/journal.ppat.1001320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Riehle MA, Brown JM. Characterization of phosphatase and tensin homolog expression in the mosquito Aedes aegypti: six splice variants with developmental and tissue specificity. Insect Mol Biol. 2007;16:277–286. doi: 10.1111/j.1365-2583.2007.00724.x. [DOI] [PubMed] [Google Scholar]
- 120.de Assis RR, Ibraim IC, Nogueira PM, Soares RP, Turco SJ. Glycoconjugates in New World species of Leishmania: Polymorphisms in lipophosphoglycan and glycoinositolphospholipids and interaction with hosts. Biochim Biophys Acta. 2012;1820:1354–1365. doi: 10.1016/j.bbagen.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 121.Ropert C, Gazzinelli RT. Signaling of immune system cells by glycosylphosphatidylinositol (GPI) anchor and related structures derived from parasitic protozoa. Curr Opin Microbiol. 2000;3:395–403. doi: 10.1016/s1369-5274(00)00111-9. [DOI] [PubMed] [Google Scholar]
- 122.Krishnegowda G, Hajjar AM, Zhu J, Douglass EJ, Uematsu S, Akira S, Woods AS, Gowda DC. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: cell signaling receptors, glycosylphosphatidylinositol (GPI) structural requirement, and regulation of GPI activity. J Biol Chem. 2005;280:8606–8616. doi: 10.1074/jbc.M413541200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Arrighi RB, Debierre-Grockiego F, Schwarz RT, Faye I. The immunogenic properties of protozoan glycosylphosphatidylinositols in the mosquito Anopheles gambiae. Dev Comp Immunol. 2009;33:216–223. doi: 10.1016/j.dci.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 124.Guther ML, Lee S, Tetley L, Acosta-Serrano A, Ferguson MA. GPI-anchored proteins and free GPI glycolipids of procyclic form Trypanosoma brucei are nonessential for growth, are required for colonization of the tsetse fly, and are not the only components of the surface coat. Mol Biol Cell. 2006;17:5265–5274. doi: 10.1091/mbc.E06-08-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kitano H. Systems biology: a brief overview. Science. 2002;295:1662–1664. doi: 10.1126/science.1069492. [DOI] [PubMed] [Google Scholar]
- 126.Drexler AL, Vodovotz Y, Luckhart S. Plasmodium development in the mosquito: biology bottlenecks and opportunities for mathematical modeling. Trends Parasitol. 2008;24:333–336. doi: 10.1016/j.pt.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fruh K, Finlay B, McFadden G. On the road to systems biology of host-pathogen interactions. Future Microbiol. 2010;5:131–133. doi: 10.2217/fmb.09.130. [DOI] [PubMed] [Google Scholar]
- 128.da Silva-Nunes M, Moreno M, Conn JE, Gamboa D, Abeles S, Vinetz JM, Ferreira MU. Amazonian malaria: asymptomatic human reservoirs, diagnostic challenges, environmentally driven changes in mosquito vector populations, and the mandate for sustainable control strategies. Acta Trop. 2012;121:281–291. doi: 10.1016/j.actatropica.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Trott KA, Chau JY, Hudgens MG, Fine J, Mfalila CK, Tarara RP, Collins WE, Sullivan J, Luckhart S, Abel K. Evidence for an increased risk of transmission of simian immunodeficiency virus and malaria in a rhesus macaque coinfection model. J Virol. 2011;85:11655–11663. doi: 10.1128/JVI.05644-11. [DOI] [PMC free article] [PubMed] [Google Scholar]