Abstract
IL-6 is an inflammatory cytokine known to be elevated in chronic diseases and following insults such as trauma and infection. While necessary for the development of B cells and Th17 cells, IL-6, at elevated levels, can also cause tissue damage and lead to a rise in inflammation. Previous work in our laboratory has shown that IL-6 is increased both systemically as well as in multiple organ systems including the ileum following ethanol exposure and burn injury. As this combined insult causes elevated intestinal morphological damage, tight junction protein localization alterations, and phospho myosin light chain (pMLC) levels, we sought to determine the role of IL-6 in these intestinal responses using a model of binge ethanol exposure and burn injury. IL-6 antibody treatment after the combined insult reduced morphological changes in the ileum, bacterial translocation, and pMLC levels relative to either injury alone. ZO-1 and occludin localization was also re-established in wild type mice given IL-6 antibody after ethanol and burn. IL-6 knockout mice given ethanol and burn injury also had reduced intestinal damage; however, no changes in bacterial translocation or tight junction protein localization were observed as compared to similarly treated wild type mice. These data suggest that IL-6 may have a role in intestinal tissue damage observed following the combined insult of binge ethanol exposure and burn injury although complete loss of IL-6 does not appear to be beneficial in this model. Modulation of IL-6 may present a new option for preventing intestinal damage and associated inflammation following a combined insult of ethanol exposure and burn injury.
Keywords: binge ethanol, burn injury, IL-6, intestine, tight junction
Introduction
Dysfunction of the intestinal epithelial barrier occurs following numerous insults including infection, trauma, and disease (1–3). Our laboratory and others have demonstrated that the combined insult of ethanol exposure and burn injury causes elevated intestinal inflammation and neutrophil influx (4). Furthermore, this combined insult is also associated with elevations in intestinal permeability and bacterial translocation, decreased ZO-1 and occludin localization to tight junctions, and increased phospho myosin light chain (pMLC) (5, 6). A common molecule found in the serum as well as many tissues of mice exposed to ethanol and burn injury is the inflammatory cytokine, interleukin (IL) 6 (5).
Important for a variety of cellular responses, IL-6 has a predominant role in the inflammatory response. Signaling through its receptor, IL-6 receptor-α (IL-6Rα) and gp130, IL-6 helps mediate the transition from acute to sustained inflammation, induces fever and acute phase responses following infection, and may contribute to tissue damage in states of elevated inflammation (7, 8). Tumor necrosis factor-α (TNFα), IL-1β, lipopolysaccharide (LPS), and viral infections can all induce IL-6 indicating its importance in the immune response. Many immune cells are known to express IL-6Rα (hepatocytes, neutrophils, macrophages, T and B cells) (9); however, with the discovery of a soluble form of the IL-6 receptor (10), all cells expressing gp130 are able to respond to IL-6. As most cells express gp130 on their surfaces, the effect of elevated IL-6 becomes global and allows for the possibility of tissue injury or damage in various organs of the body.
IL-6 has long been known as an important component of the immune response. Interestingly, recent work also indicates that IL-6 can also act as a causative or prolonging agent in disease and other cellular processes. Obesity and insulin resistance, rheumatoid arthritis, aging and cancer (8) all have symptoms or outcomes associated with elevated systemic or local levels of IL-6. With relation to the gut, IL-6 provides an anti-apoptotic signal to CD4+ T cells that aggregate in inflammatory bowel disease allowing for further inflammation and tissue damage (11). Along with transforming growth factor β (TGF-β), IL-6 aids in the induction of Th17 cell differentiation (7, 8).
Following acute insults, such as injury or infectious challenge, serum levels of IL-6 are elevated (1, 19). In particular, burn injury-induced mortality often correlates with increased IL-6 levels (12). Mouse models of burn injury cause elevated levels of IL-6 in the ileum (2) and when mice are exposed to a combined insult of binge ethanol and burn injury, IL-6 levels in the ileum are further increased (5, 13). Knockout or inhibition of IL-6 had previously been described as effective in the prevention of intestinal morphological damage and permeability in animal models of splanchnic arterial occlusion and reperfusion, sepsis, and hemorrhagic shock and resuscitation (14–16). These data suggest that IL-6 has a role in causing or perpetuating intestinal responses following injury. With the knowledge that IL-6 is elevated both systemically and at the tissue level following exposure to either ethanol or burn injury alone as well as the combined insult (13, 17), we sought to determine if IL-6 promotes the intestinal inflammation and barrier dysfunction observed after the combined insult of ethanol exposure and burn injury.
Materials & Methods
Mice
Wild type (C57BL/6) and IL-6 knockout (B6.129S2-7 IL6(tm1Kopf)/J) mice (6–7 week old,) were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were housed in sterile microisolator cages in the Loyola University Health Sciences Division Comparative Medicine facility until 8–10 weeks of age (23–25 grams). All experiments were conducted in accordance to National Institutes of Health guidelines and were approved by the Loyola Institutional Animal Care and Use Committee.
Murine Model of Ethanol and Burn Injury
A murine model of a binge ethanol exposure and burn injury was performed as described previously (18) with minor modifications (19). Briefly, mice were given a single dose of 1.11 g/kg of 20% (v/v) ethanol solution intraperitoneally that resulted in a blood ethanol level of 150–180 mg/dl at 30 minutes. The mice were then anesthetized with 100 mg/kg of Ketamine and 10 mg/kg of Xylazine (Webster Veterinary, Sterling, MA), their dorsum shaved, and placed in a plastic template exposing 15% of the total body surface area (TBSA) and subjected to a scald injury in 90–92° C water bath or a sham injury in room-temperature water. The scald injury resulted in an insensate, full-thickness burn injury (20). Mice received 1 mL of saline resuscitation and their cages were placed on warming pads until they recovered from anesthesia. At 30 minutes after injury, mice were given either rat IgG or rat anti mouse IL-6 antibody (eBioscience, 5 ug/mouse), a dose previously determined to relieve IL-6 mediated immunosuppression (21). As no differences were found between IgG and anti-IL-6 treatment in the sham groups and burn vehicle group, only mice given ethanol and burn injury plus anti-IL-6 are shown in the results section. All graphs displaying the burn ethanol + anti IL-6 group refer to mice receiving the 5 ug/mouse dose of antibody after the combined insult, with the exception of Figure 1A where the two IL-6 antibody doses are clearly defined. All other groups are treatment plus IgG. Mice were sacrificed by CO2 narcosis followed by cervical dislocation at 3 and 24 hours following injury.
Figure 1.
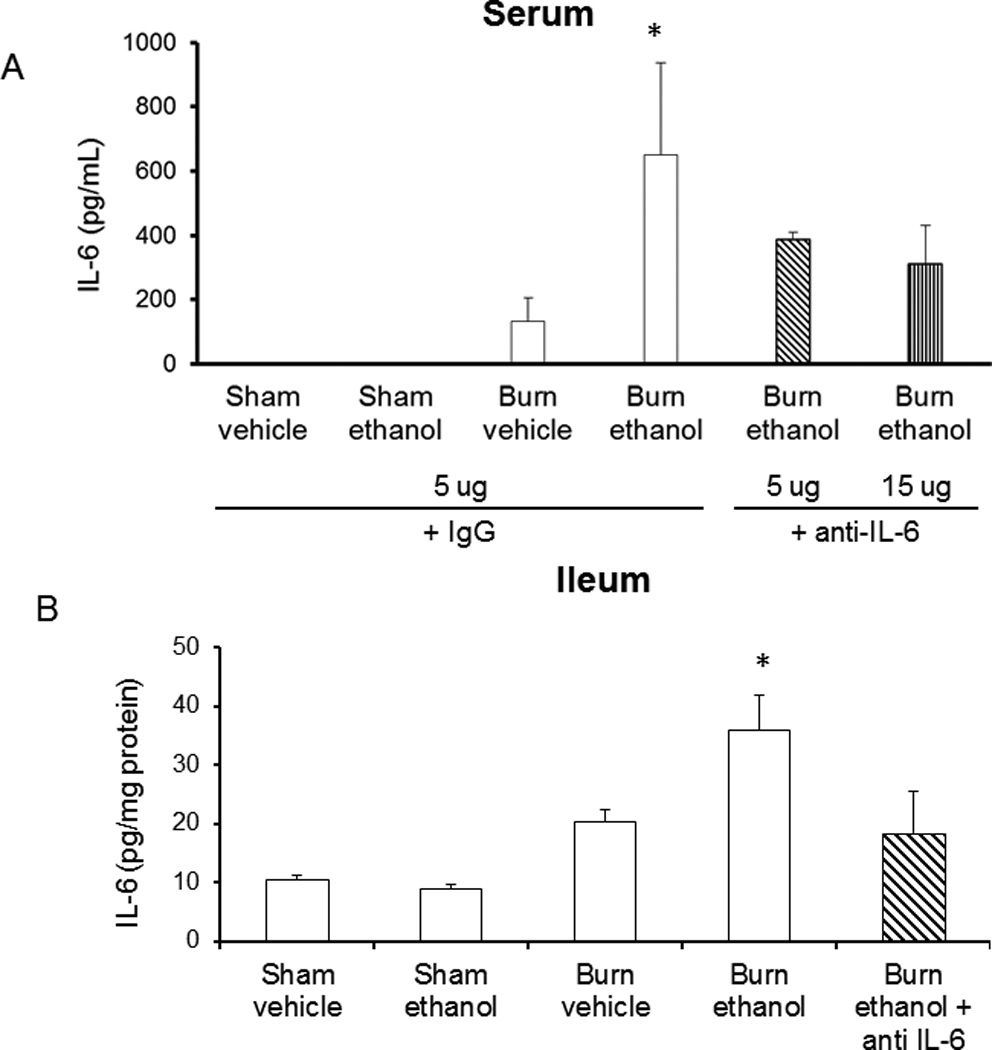
IL-6 antibody treatment reduces IL-6 in serum (A) and ileum (B) 24 hours following ethanol and burn. Levels of IL-6 were quantified by ELISA. Cytokine concentrations in ileum were normalized to total protein in the sample as determined by BioRad protein assay and all levels are presented as concentration ± SEM. *p<0.05 versus all groups except antibody treated. n = 4–6 per group.
Histopathologic and immunofluorescent examination of the ileum
At the time of sacrifice, the ileum was harvested and fixed overnight in 10% formalin. Samples were then embedded in paraffin, sectioned at 5 microns, and stained with hematoxylin and eosin (H&E). Images were taken at 200×. Immunofluorescent staining was done as previously described (3) with minor modifications. Briefly, a small section of ileum (5 mm) was embedded in OCT and frozen for immunofluorescent staining. The ileum was sectioned (8 microns) and stained with either rabbit anti-ZO-1 or rabbit anti-occludin (Invitrogen, Carlsbad, CA) followed by goat anti-rabbit Alexa 488 (Invitrogen, Carlsbad, CA). Sections were further stained with fluorescent-conjugated phalloidin (actin) and Hoechst nuclear stain (Invitrogen, Carlsbad, CA). Using Zeiss software (Zeiss LSM 510 Version 4.2 SP1), a 20 epithelial cell section (crypt or villus) was outlined and only within this outlined section were the number of co-localized (both red and green fluorescence) pixels determined. The number of co-localized pixels was divided by the total number of pixels in that section and expressed as a percentage. This process was repeated an additional 4 epithelial cell sections per animal leading to 100 total epithelial cells examined for each animal. Results were averaged for each animal and this average was then used to determine the group average.
Bacterial translocation
Bacterial translocation was assessed as previously described with minor modifications (6). Briefly, 5–6 mesenteric lymph nodes (MLN) per mouse were removed, placed in cold RPMI (with 5% fetal bovine serum) and kept on ice. Nodes were separated from connective tissue and homogenized in RPMI using frosted glass slides. Homogenates were plated in triplicate on tryptic soy agar (TSA) plates and placed in a 37°C incubator overnight. Colonies were counted the following day, averaged, and divided by the total number of lymph nodes harvested.
Intestinal epithelial cell isolation and western blotting
Intestinal epithelial cells were isolated as previously described (3) with minor modifications. Briefly, ileum sections were opened lengthwise, washed with calcium and magnesium free Hanks Basic Salt solution (HBSS), and placed in tubes containing 10 mM DTT and phosphatase inhibitor cocktail in HBSS. Samples were placed at 4°C for 30 minutes, after which tubes were shaken briefly and ileum sections moved to tubes containing 1 mM EDTA and protease inhibitor cocktail in HBSS for 1 hour at 4°C. After incubation, tubes were shaken robustly and large pieces of tissue removed. Samples were centrifuged at 1000 g for 10 minutes and the supernatant discarded. Pelleted cells were lysed in 100 uL of Cell Lysis Buffer according to manufacturer’s protocol (BioRad, Hercules, CA). Twenty micrograms of whole cell lysate protein was boiled for 5 minutes, separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene fluoride (PVDF) membranes and blotted with primary antibodies specific for phosphorylated myosin light chain (pMLC Ser19, Cell Signaling Technology, Danvers, MA), total MLCK (Abcam, Cambridge, MA), and villin-1 (Cell Signaling Technology).
Cytokine determination in the ileum
Two one-inch sections of ileum were removed, luminal contents removed, and homogenized in 1 mL of Cell Lysis Buffer according to manufacturer’s protocol (BioRad, Hercules, CA). Homogenates were then filtered and analyzed for IL-6 levels using ELISA (BD Biosciences, San Diego CA). The results were normalized to total protein present in the homogenate using the BioRad protein assay based on the methods of Bradford (5) (BioRad, Hercules, CA).
Statistical Analysis
Statistical comparisons (GraphPad Instat and Prism) were made between the sham vehicle, sham ethanol, burn vehicle, and burn ethanol treatment groups, resulting in 4 total groups analyzed. One-way analysis of variance was used to determine differences between treatment responses, and Tukey’s post-hoc test once significance was achieved (p < 0.05). Statistical comparisons made between the burn ethanol and burn ethanol plus anti-IL-6 treatment groups were done using student’s t-test and Tukey’s post-hoc test (p<0.05). Comparisons made between wild type and knockout in the same treatment group (i.e. burn ethanol) were done using two-way ANOVA and Bonferroni’s post-hoc test (p<0.05). All statistical analyses were done within the individual time point.
Results
Lower IL-6 levels following anti-IL-6 antibody treatment
Previous work from our laboratory indicates that IL-6 is increased both systemically and in the lung and ileum following ethanol exposure and burn injury (5, 13, 17, 22). Consistent with these observations, we found higher IL-6 levels in both the serum and ileum of mice exposed to the combined insult (650 ± 150 pg/mL, 35 ± 5 pg/mg protein, Figure 1A, B). However, following IL-6 neutralizing antibody treatment, IL-6 was not significantly decreased in the serum or ileum (400 ± 20 pg/mL, 20 ± 7 pg/mg protein), but this was not expected as antibody treatment was only administered once following insult. A 15 ug/mouse antibody dose was also utilized in our combined injury model, but it did not reduce serum IL-6 levels any further than the 5 ug/mouse dose (Figure 1A) and had no reparative effect in our model of ethanol exposure and burn injury. For these reasons, the higher antibody dose was not used in any further experiments. IL-6 knockout animals had no measureable levels of IL-6 in serum, ileum, lung or liver (data not shown). Our laboratory has recently observed that these models reduce levels of phospho-signal transducer and activator of transcription 3 (pSTAT3) levels indicating that IL-6 signaling has been reduced (data submitted for publication). Furthermore, as previously described (5), IL-1β and TNF-α were not elevated in the ileum following the combined insult of ethanol and burn injury, and IL-6 antibody treatment did not affect these levels (data not shown). This suggests that antibody treatment is not causing non-specific effects or a compensatory inflammatory response.
IL-6 antibody treatment reduces pMLC levels in intestinal epithelial cells
Our laboratory has recently described a role for myosin light chain kinase activity in intestinal responses following ethanol exposure and burn injury (5). To determine if inhibition of IL-6 affects MLCK activity, we measured phosphorylation (Ser 19) of myosin light chain (pMLC) in isolated intestinal epithelial cells. Treatment with an IL-6 neutralizing antibody reduced pMLC levels by nearly 70% 3 hours after exposure to the combined insult as compared to mice not receiving antibody treatment after exposure to ethanol and burn injury (Figure 2A). At 24 hours post antibody treatment, pMLC was still reduced in mice exposed to the combined insult as compared to mice not receiving antibody; however, this difference was no longer significant (data not shown). Interestingly, at 24 hours post insult mice deficient in IL-6 had no change in pMLC following any of the treatments (Figure 2 E, F). As these samples were not run on the same gels, no statistical analyses between wild type and IL-6 knockout mice were done. Due to this result and subsequent outcomes to be described in upcoming sections, westerns were not completed at the 3 hour time point in IL-6 deficient mice.
Figure 2.
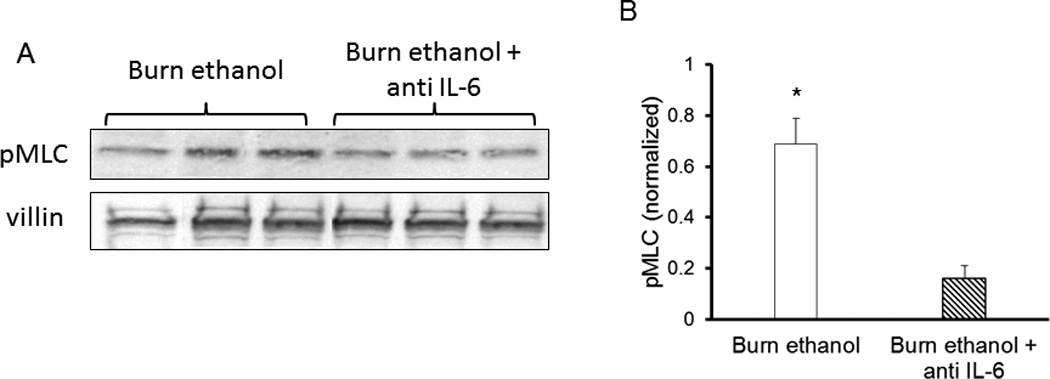
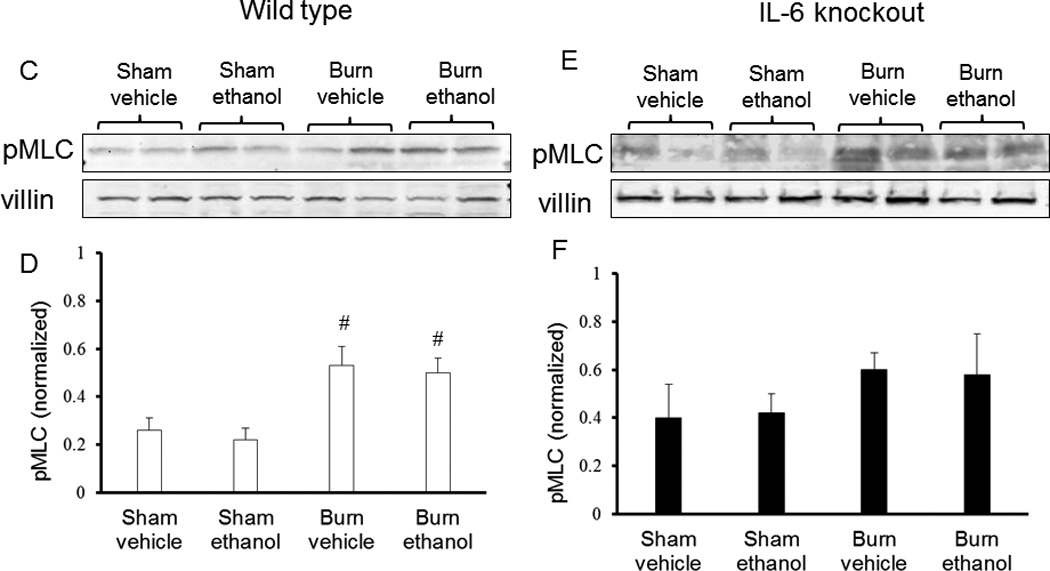
Anti-IL-6 antibody treatment reduces pMLC (Ser19) in intestinal epithelial cells 3 hours following ethanol exposure and burn injury. Isolated intestinal epithelial cells were lysed and analyzed by western blot for levels of pMLC (A) 3 hours and 24 hours (C, D) wild type (A–D) and IL-6 knockout mice (E–F). Quantification (B, D, F) of pMLC levels was normalized to villin-1 levels and done using BioRad Image Lab software. Images are representative of 3 experiments, *p<0.05 versus antibody treated group. #p<0.05 versus wild type sham groups. Quantification is of n = 4–6 per group.
Antibody treatment prevents tissue and tight junction protein alterations
As found previously (5), mice given ethanol and burn injury had visually shorter and wider villi along with an elevation in intestinal epithelial cell damage as compared to sham treated animals (only sham ethanol shown). IL-6 knockout or wild type mice given an IL-6 neutralizing antibody had little to no intestinal morphological damage following exposure to the combined insult. Villi were taller and narrower in ethanol-exposed and burn-injured IL-6 knockout or antibody-treated wild type mice in contrast to burn ethanol wild type mice not treated with anti-IL-6 antibody (Figure 3).
Figure 3.
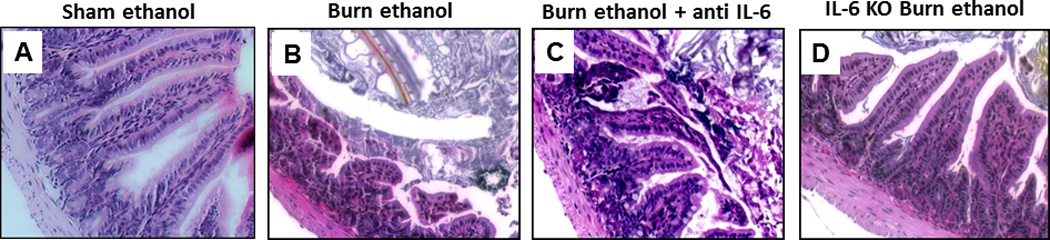
Treatment with anti-IL-6 preserves morphology 24 hours after ethanol exposure and burn injury. Ileum sections from wild type, antibody treated, and IL-6 knockout mice were stained with hematoxylin and eosin (A–C) and then examined for degree of inflammation and damage. Notice the shorter and wider villi in mice exposed to the combined insult (B) as compared to sham animals (A). Gaps between epithelial cells can be observed in mice exposed to ethanol and burn but these changes are not observed in sham wild type mice given anti IL-6 antibody following the combined insult (C) or ethanol-exposed and burn-injured IL-6 knockout mice (D). Representative H&E images taken at 200×. n = 4–6 per group.
Greater damage to intestinal epithelial cells correlated with a reduction in tight junction protein co-localization with actin. Specifically, we found that mice given ethanol and burn injury had a significantly less zonula occludens protein-1 (ZO-1) (88%, p<0.05) and occludin (83%, p<0.05) at tight junctions (Figure 4) when compared to mice exposed to sham injury (Figure 4G). Despite the resolution of intestinal morphological damage observed, IL-6 knockout mice exposed to ethanol and burn injury had similar localization patterns of ZO-1 as wild type mice given the combined insult. Interestingly, occludin localization was restored in ethanol-exposed and burn-injured knockout mice (Figure 4H), but this reestablishment was not nearly as effective as antibody treatment. Treatment of wild type mice given ethanol and burn injury with an IL-6 neutralizing antibody promotes maintenance of ZO-1 and occludin localization with actin (Figure 4B, E). These data suggest that inhibition, but not life-long deficiency, of IL-6 is protective of intact tight junctions within the intestinal epithelial cell layer of mice exposed to ethanol and burn injury.
Figure 4.
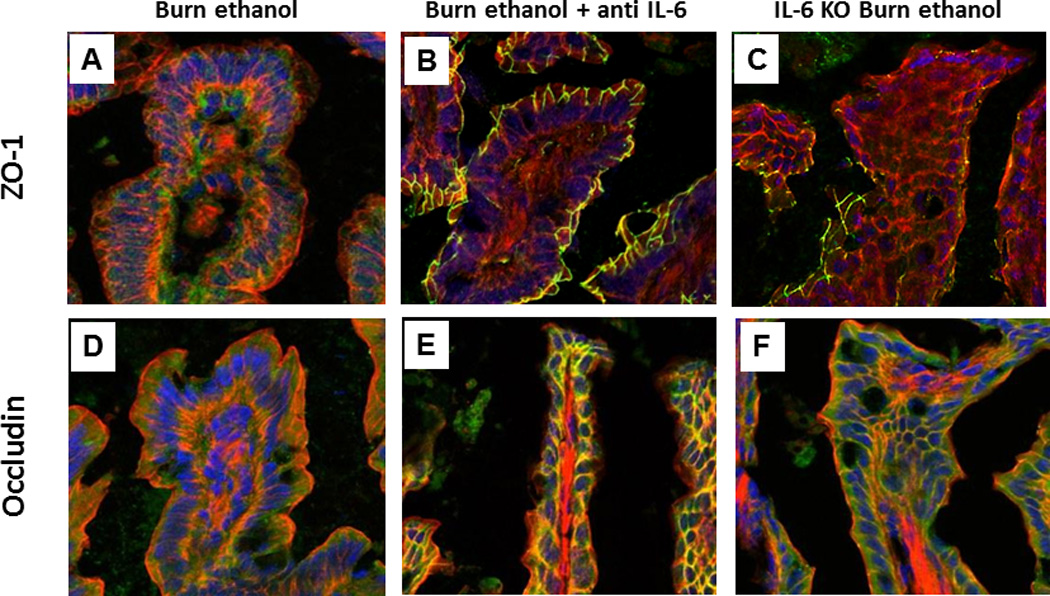
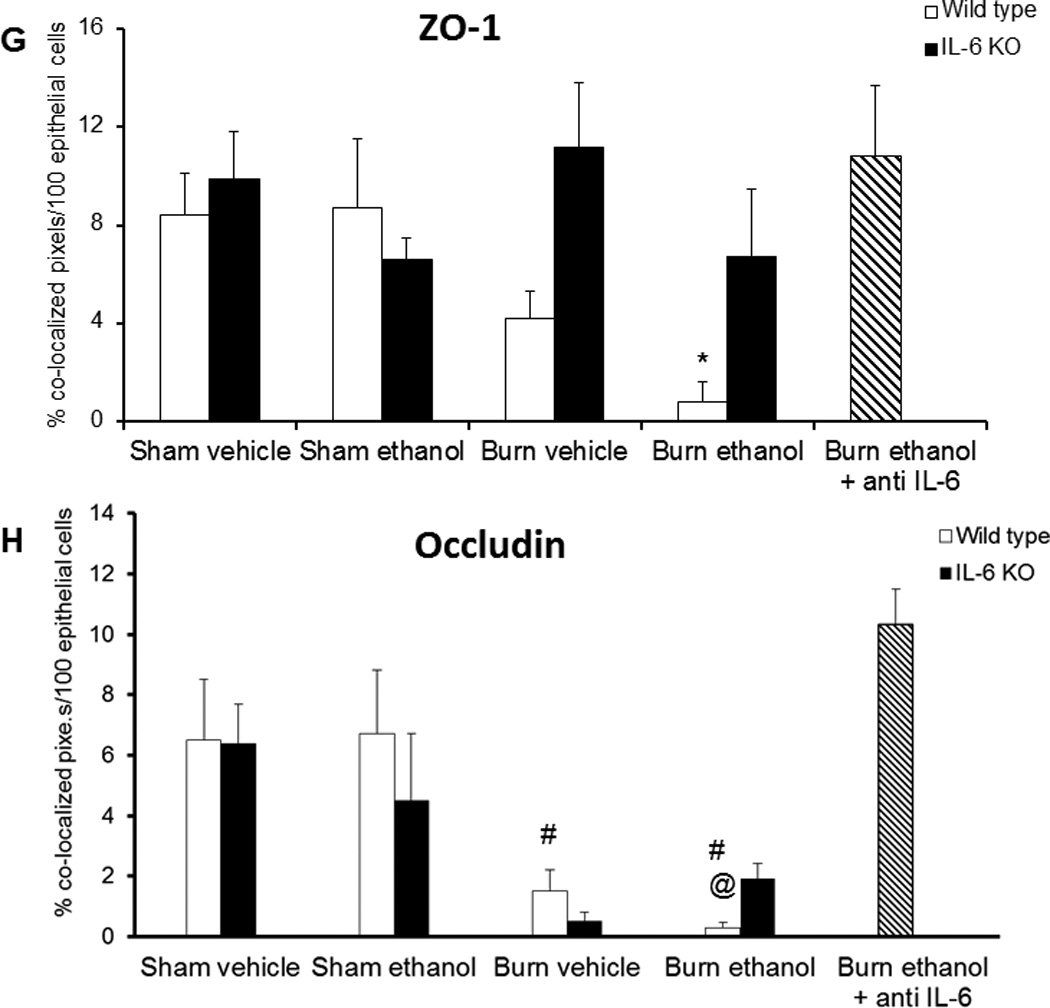
Tight junction protein localization maintained following anti-IL-6 treatment in combined injury animals at 24 hours post insult. Frozen ileum sections were stained with antibodies against ZO-1 (green, A–C) or occludin (green, D–F) as well as phalloidin (red) and nuclei (blue). Representative immunofluorescent images taken at 400×. Immunofluorescent images were analyzed for co-localization of ZO-1 or occludin with actin (G,H). Co-localization is presented as % colocalized pixels/100 epithelial cells. *p<0.05 versus wild type sham vehicle, sham ethanol and antibody treated groups. #p<0.05 versus wild type sham vehicle and sham ethanol. @p<0.05 versus knockout burn ethanol and antibody treated groups. n = 4–6 per group.
Reduced bacterial translocation following antibody treatment
Alterations in tight junction protein localization affect the integrity and function of the intestinal epithelial cell barrier. As observed previously in our model (5), mice exposed to ethanol and burn injury have significantly greater bacterial translocation to the mesenteric lymph nodes (MLN) as compared to mice receiving either insult alone (5, Figure 5A). Anti-IL-6 antibody treatment decreased bacterial translocation by 50% (p<0.05) when compared to mice not receiving antibody after the combined insult (Figure 5A). In contrast to antibody treated mice, knockout of IL-6 had no effect on bacteria accumulating in the MLN and ethanol-exposed and burn-injured knockout mice actually had an increase in bacterial translocation (Figure 5B). This increase in bacteria in the MLN of knockout mice exposed to the combined insult is associated with decreases in ZO-1 and occludin localization at tight junctions as seen in Figure 4. These data indicate that while IL-6 may have a role in the intestinal epithelial damage, loss of tight junction integrity, and rise in bacterial translocation following ethanol exposure and burn injury, complete loss of IL-6 is perhaps more harmful than beneficial.
Figure 5.
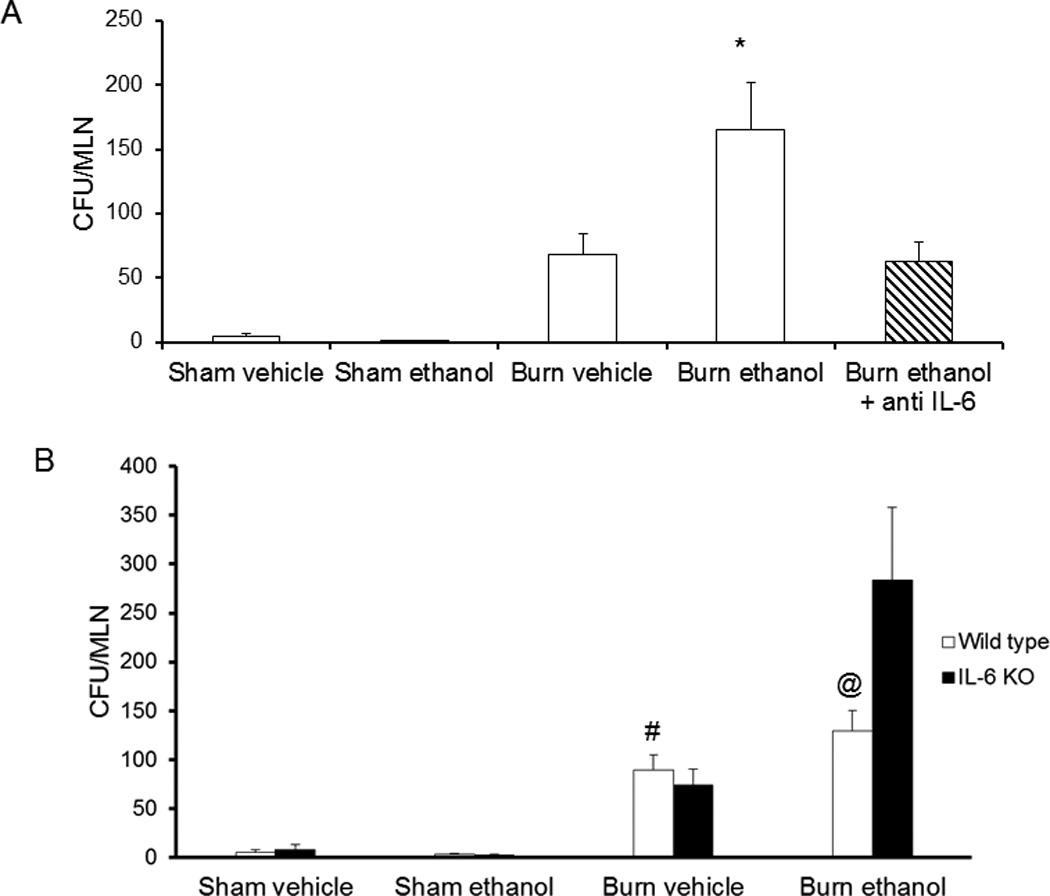
Decreased bacterial translocation to mesenteric lymph 24 hours following antibody treatment in mice exposed to ethanol and burn injury. Mesenteric lymph nodes were isolated from antibody-treated mice (A) and knockout mice (B) at 24 hours following insult. Lymph nodes were homogenized and plated on tryptic soy agar plates. Colonies were counted the next day and normalized on the total number of lymph nodes removed from each mouse. Levels presented as CFU/MLN ± SEM. *p<0.05 versus all other groups. #p<0.05 versus all other wild type groups. @p<0.05 versus all other wild type groups and knockout burn ethanol. n= 6–8 per group.
Discussion
IL-6 is a multi-functional cytokine capable of promoting both beneficial and detrimental outcomes depending on the level of IL-6. At elevated levels, as seen in inflammatory bowel disease and rheumatoid arthritis, IL-6 is known to perpetuate the inflammatory state and tissue destruction of these diseases partially through the induction of Th17 cells (11). Previous work indicates that increased systemic IL-6 observed following sepsis and burn injury leads to cellular immunosuppression (22) and our laboratory has demonstrated that ethanol exposure combined with burn injury causes even further cellular immunosuppression thus allowing for increased susceptibility to infection (17). A minimal amount of IL-6 is required for normal cellular functions like the development of B and Th17 cells (8); however, it is the sustained elevation in systemic and tissue levels of IL-6 that can cause tissue injury, increased inflammation, and delayed resolution of the immune response following insult (3, 7, 12). In the gut, intestinal epithelial cells have been shown to produce IL-6 in response to cytokine and TLR4 signaling induced by infection, disease, or injury (8). As seen in Figure 1, the combined insult of ethanol exposure and burn injury leads to significant increase in both serum and ileum levels of IL-6. IL-6 has been associated with permeability in models of ethanol exposure or burn injury alone (2, 23). Other studies indicate that elevations in IL-6 are linked to mucosal permeability in both rodents and humans (24, 25). Additionally, using Caco-2 cells as a model of the intestinal epithelial cell barrier, work by Suzuki and colleagues suggests that IL-6 increases tight junction permeability via an increase in expression of the leaky tight junction protein, claudin-2 (26). Combined, these studies indicate the importance of controlled IL-6 signaling in the intestine both in a healthy individual and following insult.
The role of IL-6 in tissue injury is dependent on the type of injury, the specific tissue, and the level of IL-6. IL-6 treatment was found to be protective of ischemia/reperfusion injury in a fatty liver (27) and in ethanol-induced hepatocyte oxidative stress and mitochondral dysfunction (28). Interestingly, IL-6 has also been described to up-regulate keratins in the intestinal epithelial cell barrier in a dodecyl sodium sulfate (DSS)-induced barrier dysfunction model (29). This up-regulation of keratins protected barrier function suggesting that in situations of intestinal barrier compromise, IL-6 may be necessary for maintenance of the intestinal epithelial cells. The work of Wang et al. may provide a possible explanation for why our IL-6 knockout mice exposed to ethanol and burn had patterns of localization similar to comparably treated wild type mice (Figure 2).
In contrast to the above studies, IL-6 has also been shown to be necessary for intestinal barrier dysfunction in a model of hemorrhagic shock and resuscitation (14). Knockout of IL-6 led to decreased intestinal permeability following induction of sepsis (15) and reduced ileal morphological injury, neutrophil infiltration into the intestine and serum levels of pro-inflammatory cytokines in a model of splanchnic artery occlusion (16). Interestingly, data presented in this paper suggest that knockout of IL-6 is not protective of intestinal barrier function but does prevent morphological damage induced by the combined insult of ethanol exposure and burn injury (Figures 2 & 3). IL-6 knockout mice subjected to hind limb ischemia or hemorrhage also did not have a reduction in bacterial translocation (30), further indicative that IL-6 may play different roles at different time points in tissue inflammation and injury depending on the nature of the insult. We have demonstrated that IL-6 levels begin to rise early after ethanol exposure and burn injury (5), and IL-6 levels at 6 hours post injury have been shown to predict mortality (31) suggesting that the levels of IL-6, as well as the time point at which is elevated, are important factors in the response to insult. To investigate these possibilities, animals exposed to the combined insult could be sacrificed at earlier time points and ileum could be examined for apoptosis markers or markers of mitosis or proliferation. These studies could be combined with our current model using a neutralizing IL-6 antibody to determine what level of IL-6 is necessary for proper ileum repair following the insult of ethanol and burn injury and at what time this repair may be occurring. We recognize that a limitation of our study is that IL-6 knockout studies have not been conducted at earlier time points; however, these studies may be conducted in the future.
IL-6 signaling causes numerous responses depending on the cell type. Known to signal through STAT3 and induce transcription of genes, stimulation through IL-6 also leads to activation of the phosphatidyl-inositol 3 kinase (PI3K)/Akt pathway. Signaling through the PI3K/Akt pathway is usually associated with being anti-apoptotic. Furthermore, IL-6 activation induces the mitogen activated protein kinase (MAPK) cascade, specifically ERK1/2 (extracellular signal-related kinase), a pathway associated with cell survival. While numerous stimuli activate the MAPK and PI3K/Akt pathways IL-6 deficiency may be a compounding factor in detrimental intestinal responses observed following ethanol exposure and burn injury. Grivennikov and colleagues found decreased intestinal epithelial cell survival and crypt cell proliferation in dextran sodium sulfate (DSS)-treated IL-6 knockout mice as compared to similarly treated wild type mice (32). This further supports the idea that IL-6 is important for IEC survival and maintenance at the time of injury.
Tight junction proteins are often disrupted in the intestinal barrier following many insults including trauma, disease, and infection (1–3). These insults also lead to elevated IL-6 levels; however, the majority of studies have examined other cytokines, such as tumor necrosis factor α (TNFα) and IL-1β, in tight junction protein regulation. IL-6 treatment of HUVECs (human umbilical vein endothelial cells) led to an elevation in monolayer permeability and alterations in ZO-1 localization. Using a PKCα (protein kinase C) and PKCβ inhibitor in conjunction with IL-6 partially restored barrier function of the HUVECs (33). This result is similar to our ethanol-exposed and burn-injured mice given an IL-6 antibody (Figures 2 & 3); however, when no IL-6 was present the therapeutic effect was lost suggesting that some IL-6 is necessary for proper tight junctions in the IEC barrier. In a model of reflux esophagitis, Li and colleagues found that IL-6 was elevated in infiltrating inflammatory cells (34). This elevation in IL-6 corresponded with a rise in claudin-1, occludin and ZO-1 in surrounding epithelial cells; however, newly synthesized tight junction proteins failed to organize at the plasma membrane. Furthermore, production of junction proteins was not enough to repair lesions induced in the model. Whether IL-6 affects tight junction protein localization directly or indirectly is not clear from these studies, but it does insinuate that IL-6 has an effect on tight junction protein production and possibly localization.
Pro-inflammatory cytokines, in particular TNFα, have been shown to induce barrier dysfunction in models of IBD as well as traumatic injury (2). TNFα, as well as LIGHT, are known enhancers of MLCK transcription and activation (35). Interestingly, we found that anti-IL-6 antibody treatment led to less pMLC (Figure 3) following ethanol exposure and burn injury. Few other studies have examined IL-6’s role in MLCK signaling, but Suzuki et al. discovered that IL-6 treatment had no effect on pMLC in a Caco-2 cell culture model (26) although barrier function of this monolayer was decreased. While this would advocate for IL-6-induced barrier function not being MLCK-dependent, this work was done in cell culture indicating that other cells may be necessary for an effect. Current research in our laboratory is examining whether IL-6 does have a direct effect on MLCK activity. Preliminary experiments will examine changes in Src kinase activation, an enzyme downstream of IL-6 signaling and known to enhance MLCK activity.
Our data indicate that an elevation in serum and ileum levels of IL-6 may have a role in intestinal epithelial cell barrier alterations following ethanol exposure and burn injury. When IL-6 was inhibited with a neutralizing antibody bacterial translocation was reduced and greater ZO-1 and occludin localized to the tight junction. These results were accompanied by reduced pMLC in intestinal epithelial cells. IL-6 knockout mice exposed to ethanol and burn had similar intestinal damage and tight junction protein alterations as observed in similarly treated wild type mice. These data suggest that complete loss of IL-6 may be as detrimental as high levels of IL-6. These results indicate that dampening IL-6, but not complete loss of it, may be important for allowing the gut to repair itself following the combined insult without causing significant damage.
Acknowledgements
The authors would like to thank Dr. Sherri Yong for thoughtful discussion and analysis of ileum pathology. This work was funded by NIH R01 AA012034 (EJK), T32 AA013527 (EJK), F31 AA019913 (AZ), F32 AA018068 (MDB), P30 AA019373 (EJK), Margaret A. Baima Endowment Fund for Alcohol Research and the Ralph and Marian C. Falk Medical Research Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- 1.Samonte VA, Goto M, Ravindranath TM, Fazal N, Holloway VM, Goyal A, Gamelli RL, Sayeed MM. Exacerbation of intestinal permeability in rats after a two-hit injury: burn and Enterococcus faecalis infection. Critical Care Medicine. 2004;32(11):2267–2273. doi: 10.1097/01.ccm.0000145579.66001.05. [DOI] [PubMed] [Google Scholar]
- 2.Costantini TW, Peterson CY, Kroll L, Loomis WH, Putnam JG, Wolf P, Eliceiri BP, Baird A, Bansal V, Coimbra R. Burns, inflammation, and intestinal injury: protective effects of an anti-inflammatory resuscitation strategy. Journal of Trauma. 2009;67(6):1162–1168. doi: 10.1097/TA.0b013e3181ba3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clayburgh DR, Barrett TA, Tang Y, Meddings JB, Van Eldik LJ, Watterson DM, Clarke LL, Mrsny RJ, Turner JR. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. Journal of Clinical Investigations. 2005;115(10):2702–2715. doi: 10.1172/JCI24970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X, Schwacha MG, Chaudry IH, Choudhry MA. Acute alcohol intoxication potentiates neutrophil-mediated intestinal tissue damage after burn injury. Shock. 2008;29(3):377–383. doi: 10.1097/shk.0b013e31815abe80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zahs A, Bird MD, Ramirez L, Turner JR, Choudhry MA, Kovacs EJ. Long myosin light chain kinase promotes intestinal damage after acute ethanol exposure and burn injury. American Journal of Physiology Gastrointestinal and Liver Physiology. 2012;303(6):G705–G712. doi: 10.1152/ajpgi.00157.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kavanaugh MJ, Clark C, Goto M, Kovacs EJ, Gamelli RL, Sayeed MM, Choudhry MA. Effect of acute alcohol ingestion prior to burn injury on intestinal bacterial growth and barrier function. Burns. 2005;31(3):290–296. doi: 10.1016/j.burns.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 7.Hyams JS, Fitzgerald JE, Treem WR, Wyzga N, Kreutzer DL. Relationship of functional and antigenic interleukin 6 to disease activity in inflammatory bowel disease. Gastroenterology. 1993;104(5):1285–1292. doi: 10.1016/0016-5085(93)90336-b. [DOI] [PubMed] [Google Scholar]
- 8.Naugler WE, Kevin M. The wolf in sheep’s clothing: the role of interleulkin+6 in immunity, inflammation, and cancer. Trends in Molecular Medicine. 2008;14(3):109–119. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)-6-typecytokine signaling and its regulation. Biochemical Journal. 2003;374(Part 1):1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rose-John S, Scheller J, Elson G, Jones SA. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. Journal of Leukocyte Biology. 2006;80(2):227–236. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
- 11.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52(1):65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gauglitz G, Finnerty C, Herndon D, Mlcak R, Jeschke M. Are serum cytokines early predictors for the outcome of burn patients with inhalation injuries who do not survive? Critical Care. 2008;12(3):R81. doi: 10.1186/cc6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bird MD, Zahs A, Deburghgraeve C, Ramirez L, Choudhry MA, Kovacs EJ. Decreased pulmonary inflammation following ethanol and burn injury in mice deficient in TLR4 but not TLR2 signaling. Alcohol Clinical Experimental Research. 2010;34(10):1733–1741. doi: 10.1111/j.1530-0277.2010.01260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang R, Han X, Uchiyama T, Watkins SK, Yaguchi A, Delude RL, Fink MP. IL-6 is essential for development of gut barrier dysfunction after hemorrhagic shock and resuscitation in mice. American Journal of Physiology Gastrointestinal and Liver Physiology. 2003;285(3):G621–G629. doi: 10.1152/ajpgi.00177.2003. [DOI] [PubMed] [Google Scholar]
- 15.Wang Q, Fang CH, Hasselgren PO. Intestinal permeability is reduced and IL-10 levels are increased in septic IL-6 knockout mice. American Journal of Physiology Regulatory, Integrative, and Comparative Physiology. 2001;281(3):R1013–R1023. doi: 10.1152/ajpregu.2001.281.3.R1013. [DOI] [PubMed] [Google Scholar]
- 16.Cuzzocrea S, De Sarro G, Costantino G, Ciliberto G, Mazzon E, De Sarro A, Caputi AP. IL-6 knock-out mice exhibit resistance to splanchnic artery occlusion shock. Journal of Leukocyte Biology. 1999;66(3):471–480. doi: 10.1002/jlb.66.3.471. [DOI] [PubMed] [Google Scholar]
- 17.Faunce DE, Gregory MS, Kovacs EJ. Glucocorticoids protect against suppression of T cell responses in a murine model of acute ethanol exposure and thermal injury by regulating IL-6. Journal of Leukocyte Biology. 1998;64(6):724–732. doi: 10.1002/jlb.64.6.724. [DOI] [PubMed] [Google Scholar]
- 18.Faunce DE, Gregory MS, Kovacs EJ. Effects of acute ethanol exposure on cellular immune responses in a murine model of thermal injury. Journal of Leukocyte Biology. 1997;62(6):733–740. doi: 10.1002/jlb.62.6.733. [DOI] [PubMed] [Google Scholar]
- 19.Murdoch EL, Brown HG, Gamelli RL, Kovacs EJ. Effects of ethanol on pulmonary inflammation in postburn intratracheal infection. Journal of Burn Care Research. 2008;29(2):323–330. doi: 10.1097/BCR.0b013e3181667599. [DOI] [PubMed] [Google Scholar]
- 20.Faunce DE, Llanas JN, Patel PJ, Gregory MS, Duffner LA, Kovacs EJ. Neutrophil chemokine production in the skin following scald injury. Burns. 1999;25(5):403–410. doi: 10.1016/s0305-4179(99)00014-5. [DOI] [PubMed] [Google Scholar]
- 21.Fontanilla CV, Faunce DE, Gregory MS, Messingham KA, Durbin EA, Duffner LA, Kovacs EJ. Anti-interleukin-6 antibody treatment restores cell-mediate immune function in mice with acute ethanol exposure before burn trauma. Alcoholism Clinical and Experimental Research. 2000;24(9):1392–1399. [PubMed] [Google Scholar]
- 22.Kowal-Vern A, Walenga JM, Hoppensteadt D, Sharp-Pucci M, Gamelli RL. Interleukin-2 and interleukin-6 in relation to burn wound size in the acute phase of thermal injury. Journal of the American College of Surgeons. 1994;178(4):357–362. [PubMed] [Google Scholar]
- 23.Amin PB, Diebel LN, Liberati DM. Dose-dependent effects of ethanol and E. coli on gut permeability and cytokine production. Journal of Surgical Research. 2009;157(2):187–192. doi: 10.1016/j.jss.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 24.Wang W, Smail N, Wang P, Chaudry IH. Increased gut permeability after hemorrhage is associated with upregulation of local and systemic IL-6. Journal of Surgical Research. 1998;79(1):39–46. doi: 10.1006/jsre.1998.5385. [DOI] [PubMed] [Google Scholar]
- 25.Janu P, Li L, Minard G, Kudsk K. Systemic interleukin-6 (IL-6) correlates with intestinal permeability. Surgery Forum. 1996;47:7–9. [Google Scholar]
- 26.Suzuki T, Yoshinaga N, Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in the intestinal epithelium. Journal of Biological Chemistry. 2011;286(36):31263–31271. doi: 10.1074/jbc.M111.238147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong F, Radaeva S, Pan H, Tian Z, Veech Z, Gao B. Interleukin 6 alleviates hepatic steatosis and ischemia/reperfusion injury in mice with fatty liver disease. Hepatology. 2004;40(4):933–941. doi: 10.1002/hep.20400. [DOI] [PubMed] [Google Scholar]
- 28.El-Assal O, Hong F, Kim WH, Radaeva S, Gao B. IL-6-deficient mice are susceptible to ethanol-induced hepatic steatosis: IL-6 protects against ethanol-induced oxidative stress and mitochondrial permeability transition in the liver. Cell Molecular Immunology. 2004;1(3):205–211. [PubMed] [Google Scholar]
- 29.Wang L, Srinivasan S, Theiss AL, Merlin D, Sitaraman SV. Interleukin-6 induces keratin expression in intestinal epithelial cells. Journal of Biological Chemistry. 2007;282(11):8219–8227. doi: 10.1074/jbc.M604068200. [DOI] [PubMed] [Google Scholar]
- 30.Gunji H, Scarth S, Carlson GL, Warhurst G, Little RA, Hopkins SJ. Variability of bacterial translocation in the absence of intestinal mucosal damage following injury and the influence of interleukin-6. Pathophysiology. 2006;13(1):39–49. doi: 10.1016/j.pathophys.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Remick DG, Bolgos GR, Siddiqui J, Shin J, Nemzek JA. Six at six: interleukin-6 measured 6h after the initiation of sepsis predicts mortality over 3 days. Shock. 2002;17(6):463–467. doi: 10.1097/00024382-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15(2):103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desai TR, Leeper NJ, Hynes KL, Gewertz BL. Interleukin-6 causes endothelial barrier dysfunction via the protein kinase C pathway. Journal of Surgical Research. 2002;104(2):118–123. doi: 10.1006/jsre.2002.6415. [DOI] [PubMed] [Google Scholar]
- 34.Li FY, Li Y. Interleukin-6, desmosome and tight junction protein expression levels in reflux esophagitis-affected mucosa. World Journal of Gastroenterology. 2009;15(29):3621–3630. doi: 10.3748/wjg.15.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwarz BT, Wang F, Shen L, Clayburgh DR, Su L, Wang Y, Fu YX, Turner JR. LIGHT signals directly to intestinal epithelia to cause barrier dysfunction via cytoskeletal and endocytic mechanisms. Gastroenterology. 2007;132(7):2383–2394. doi: 10.1053/j.gastro.2007.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]


