Abstract
Dietary supplementation with (ω)-3 long chain fatty acids including docosahexaenoic acid (DHA) has increased in popularity in recent years and adequate DHA supplementation during pregnancy and early childhood is of clinical importance. Some evidence has been built for the neuro-cognitive benefits of supplementation with long chain polyunsaturated fatty acids (LCPUFA) such as DHA during pregnancy; however, recent data indicate that the anti-inflammatory properties may be of at least equal significance. Adequate DHA availability in the fetus/infant optimizes brain and retinal maturation in part by influencing neurotransmitter pathways. The anti-inflammatory properties of LCPUFA are largely mediated through modulation of signaling either directly through binding to receptors or through changes in lipid raft formation and receptor presentation. Our goal is to review the current findings on DHA supplementation, specifically in pregnancy and infant neurodevelopment, as a pharmacologic agent with both preventative and therapeutic value. Given the overall benefits of DHA, maternal and infant supplementation may improve neurological outcomes especially in vulernable populations. However, optimal composition of the supplement and dosing and treatment strategies still need to be determined to lend support for routine supplementation.
Keywords: DHA, long chain fatty acids, natural products, lipids, omega-3
Introduction
The therapeutic value of long chain polyunsaturated fatty acids (LCPUFA) was appreciated by the early 1980’s when epidemiologic data indicated that populations consuming large quantities of cold water fish had lower incidences of inflammatory diseases [1–3]. Furthermore, cold water fish such as salmon, tuna, mackerel, and sardines contain substantial quantities of omega (ω)-3 LCPUFA. In the average Western diet, intake of fish containing high levels of (ω)-3 PUFA is limited and estimates are that only approximately 100 mg DHA+EPA/day are ingested by average adults in the United States [4].
Anthropologic data indicate that the evolution of modern man coincides with the migration of Homo sapiens to the waterways and the inclusion of marine foods in the diet [5]. These findings further coincide with the presence of DHA in neuronal tissues specifically at neural synapses and in the retina. The agricultural and industrial revolutions and the domestication of livestock and poultry have shifted mankind away from a diet rich in seafood and toward a diet high in saturated fats. This shift led to a relative disproportion in ω-6 to ω-3 fatty acids (originally 1–2:1; currently 10–20:1) and the predominance of ω-6 fatty acids [6].
Much of the US population (40% to 60%) has turned to complementary or alternative medicine to treat inflammation-based disease as well as promote health and well-being [7]. Of these alternative therapies, ω-3 LCPUFAs have received the attention of both the scientific and medical communities. Much information is available on the mechanistic actions of DHA as well as therapeutic value of DHA in improving pregnancy outcomes and enhancing infant neurodevelopment, especially in the context of prematurity (reviewed in [8–10]). However, significance and thus clinical implementation of the collective findings related to DHA therapy to improve neurodevelopmental outcomes and to attenuate inflammation in newborn infants and children have been hindered by the lack of standardization. Specifically the use of DHA alone or with other lipids and a broad range of doses have been reported, as well as varying measures of the outcomes of interest [11,12].
Mechanisms of Action
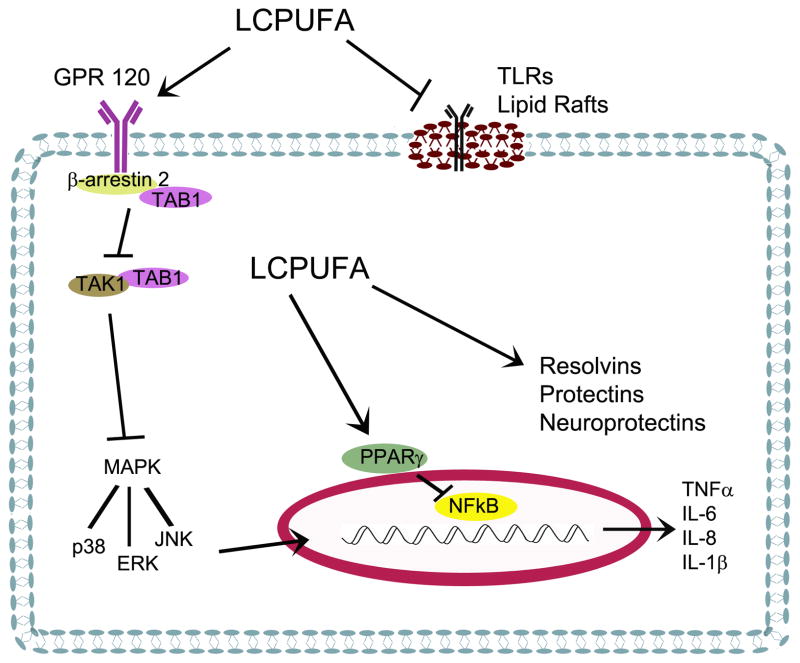
DHA is metabolically active and has been the focus of numerous studies in nutrition, neurodevelopment, and immunology [8,10,13–16]. Although the mechanisms involved are not completely understood, the active properties of DHA are thought to include effects on neuronal development and plasticity, receptor-mediated signaling, changes in membrane fluidity, the formation of second messengers, and/or enhancement of the production of anti-inflammatory lipid mediators due to the availability of DHA as substrate [8,14,16] (Figure 1).
Figure 1.
LCPUFAs can modulate inflammation through several pathways. These pathways include agonism or antagonism of receptors such as TLRs, GPR120, and PPARγ as well as providing substrate for the production of pro-resolution lipid metabolites.
Receptors
DHA has been shown to interact with several receptors, functioning as either an agonist or an antagonist in signaling responses. These receptors include plasma membrane bound Toll-like receptors (TLR)[17], the G-coupled protein receptor (GPR)120 [18], and the nuclear receptor peroxisome proliferator activated receptor gamma (PPARγ) [19]. In addition, animals studies have linked the need for LCPUFAs, specifically DHA, to dopamine and serotonin production, the activity of the respective receptors, and the presence of second messengers to facilitate neurotransmission [20–22]. Human studies have found decreased dopaminergic responses in infants that are LCPUFA deficit [23].
TLRs are key pattern recognition receptors that play a significant role in innate and adaptive immune responses [24]. Ligand binding to TLR2 and 4 activates downstream pathways that include the mitogen activated protein kinases (MAPK) and NFkB resulting in the promotion of inflammatory responses [17]. TLRs are highly expressed on microglia and mediate the expression of cytokines in the developing brain specifically in the context of inflammation (reviewed in [25]). In addition, endogenous compounds such as saturated fatty acids can mediate sterile inflammation through activating TLRs, one explanation for the chronic inflammation observed in obesity [26]. LCPUFAs have been shown to interfere with TLR activation directly inhibiting dimerization and activation of both TLR-2 and -4, thus blocking inflammatory signaling cascades [26].
Recently, investigations have identified GPRs that are activated by fatty acids. Specifically, GPR120 is highly expressed on pro-inflammatory macrophages, interacts with DHA, and can modulate anti-inflammatory pathways [18]. Oh et al. demonstrated that activation of GPR120 in macrophages and adipocytes by DHA did not involve Gαq proteins but rather required the recruitment of β-arrestin 2 into a complex that prevented the phosphorylation of transforming growth factor-β activated kinase 1 [18]. This mechanism is responsible for the inhibition of both TLR and tumor necrosis factor α (TNF-α)-mediated signaling pathways. Through the use of GPR120 knockout mice, Oh was able to show that the insulin sensitizing effects of ω-3 LCPUFAs were largely mediated through activation of GPR120 (reviewed in [27]). The expression and role of GPR120 in the brain and neurological tissues are not currently known.
LCPUFAs are natural ligands for PPARγ and retinoic acid X receptor (RXR) [28–30]. Once bound by ligand, PPARγ heterodimerizes with RXR and induces genes which control numerous cellular activities including glucose and xenobiotic metabolism. This activation requires relatively high concentrations (micromolar) of LCPUFAs [31]; however, the presence of increased levels of DHA subsequently increases the transcription of PPARγ. In addition, receptor-ligand interactions between PPARγ and LCPUFAs have been shown to modulate NFkB-mediated responses to LPS [19,32] and activation of AKT with subsequent suppression of apoptotic pathways [33].
Membrane Changes
Cellular membranes are complex in structure and play an essential role in receptor-mediated signaling. One mechanism is through the formation of lipid rafts and the localization and presentation of membrane-spanning receptors. Lipid rafts are a collection of lipid membrane microdomains characterized by detergent insolubility [34]. These lipid rafts are rich in cholesterol, sphingomyelin, and glycolipids, containing predominantly saturated fatty acyl residues [35]. Alternatively, DHA-containing phospholipids are loosely packed, are more fluid and more compressible, and create a more permeable membrane. Additionally, the presence of DHA repels cholesterol, promoting the formation of LCPUFA-containing domains within the membrane that reside away from cholesterol. The incorporation of DHA displaces saturated fatty acids within the membranes and thus disrupts lipid raft formation.
Conversely, membranes with a larger content of unsaturated phospholipids are thinner, more permeable and contain more water facilitating hydrophobic interactions [36]. Dietary supplementation with α-linolenic acid alone does not alter the membrane phospholipid distribution; however, supplementation with DHA can enrich cell phospholipid levels 2 fold or greater [37,38]. Although DHA is rapidly incorporated into phospholipids, the distribution between phospholipid classes is not equal. DHA is preferentially incorporated into the sn-2 position of phosphatidylethanolamine (PE) and to a lesser extent phosphatidylcholine (PC) or phophatidylserine (PS) which is incorporated into membranes of specific tissues such as the rod photoreceptors of the retina or grey matter in the brain, [35,39–43]. Maximal incorporation into phospholipids is reached at 4 weeks and highly correlated with the absolute amounts of LCPUFAs consumed [35].
Suppression of apoptotic activity by DHA can be partially attributed to changes in membrane composition. In the brain, incorporation of DHA into phosphatidylserine phospholipids increases AKT translocation to the nucleus to regulate the transcription of apoptotic genes [33]. Alternatively, increased membrane rigidity associated with the incorporation of saturated fatty acids, results in increased macrophage adhesion and decreased phagocytosis of apoptotic cells, suggesting that membrane composition is important for these activities [37,44].
Alternative Lipid Products
Serhan, et al. described a novel group of lipid-based anti-inflammatory mediators derived from the activity of cyclooxygenase [45,46]. The products formed are collectively termed the resolvin D (RvD) series. Additionally, oxygenated products of DHA possessing conjugated triene double-bonds are denoted protectins [47,48]. A specialized form of protectins deemed neuroprotectins, specifically neuroprotectin D1 (NPD1), are formed in neurological tissues. NPD1 prevents apoptosis, inflammation, and oxidative stress through promoting pro-survival and repair activity specifically by activating Bcl-2-related molecules and inhibiting BAD, BAX, and Bid pathways [49].
Increased levels of DHA provide additional substrate for the activities of lipoxygenase and cyclooxygenase enzymes, thus preventing the formation of pro-inflammatory arachidonic acid products. Alternatively, DHA can form other prostaglandin products [10] which are less inflammatory than the arachidonic acid-derived products. In a model of ovalbumin-induced lung injury, LCPUFA supplementation caused a decrease in F2 and an increase in F3 and F4-isoprostanes [50]. In a subsequent study, a direct correlation was observed in the concentrations of the 4 series prostaglandins and increasing or decreasing LCPUFAs, in a dose dependent manner [51]. Secondly, resolvins and protectins can serve as ligands for receptors responsible for anti-inflammatory activities. At present, the only receptors currently identified for resolvins are ChemR23 and BLT1; however, others are likely to exist [52,53]. Exogenous administration of preformed resolvins has proven efficacious in a variety of inflammatory diseases [54,55].
DHA in Pregnancy and Early Childhood
In the context of pregnancy and child development, LCPUFAs, specifically DHA, have a vital role in neurological development as well as inflammatory responses [10,14,15]. DHA is found in very high levels in the central nervous system and retina, especially in gray matter and photoreceptors and thought to be essential for optimal development of these regions [5,39,56].
Inflammation is reported to be a leading cause in complications of pregnancy, subsequent preterm birth, and neonatal neurological morbidities associated with these events [57–62]. The cytokines TNFα and IL-1β are implicated in most inflammatory conditions associated with pregnancy, birth, and childhood [57,59,60]. However, these molecules also play essential signaling roles in neurogenesis [57]. Consequently, cytokine levels in the brain as markers of inflammation must be interpreted carefully. Assessment of cytokines, as surrogates for systemic inflammatory responses, revealed dramatic decreases (77–81%) in their production by mononuclear cells after 8 weeks of fish oil supplementation [63] however studies assessing the role of fish oil on neurological inflammation have been few.
Pregnancy
The essential role for DHA in fetal neurological development in mammalian species is well established [16,23,64]. DHA is preferentially transported to the infant during the last trimester of gestation in humans and coincides with the later stages of brain and retinal maturation [9,65,66]. It is currently estimated that 67 – 75 mg/day of DHA are accumulated in utero during the last trimester of gestation [66–68]. Whether this selective transport relies completely on the circulating maternal DHA stores or whether the placenta itself is involved in DHA synthesis is not known. Dhobale et al., have shown an association between placental weight and DHA concentration, and a correlation between placental weight and infant weight and length in preterm deliveries indicating that the DHA levels within the placenta effect fetal growth patterns [69]. More recent studies in animals have suggested that early DHA exposure influences neural differentiation, neurotransmitter target finding and synaptogenesis during gestation [20,70]. Specifically, DHA is critically important for optimal development of dopaminergic signaling and once the window for development is past deficits are not later reversible [9,20].
DHA availability to the fetus is dependent on maternal diet and phospholipid composition (reviewed in [71]). Currently, clinical recommendations for supplementation with ω-3 fatty acids during pregnancy are common; however, the form of supplementation (tuna, fish oil, algal oil) and the doses are variable [72]. Several studies have evaluated the efficacy of maternal supplementation on infant neurodevelopment [73–75] or immune responses [76,77]. A recent meta-analysis has indicated an increase in mean gestational age and birth weight, and a decrease in the number of infants born prior to 37 weeks’ gestation in mothers receiving w-3 LCPUFA supplementation during pregnancy [78]. Interestingly, the studies demonstrating the greatest efficacy have used doses in the range of 1–2 g/day, much higher than studies reporting negative results and higher than most informal recommendations in the US. Consequently, a better understanding of the influence of the maternal diet and the need for DHA supplementation specifically among women at-risk of delivering a preterm infant or providing milk for preterm infants is warranted.
Preterm Birth
Since such a large proportion of the fetal DHA accumulation occurs during the last trimester of pregnancy [79], preterm infants are especially vulnerable to deficiency during critical windows of neurodevelopment [80]. In fact, Szajewska et al. [81] reported lower levels of LCPUFAs in the red blood cells of preterm infants compared to term controls. Furthermore, preterm infants can often remain on parenteral nutrition for periods of time while other immediate medical needs are addressed. Most parental nutrition used in the United States contains little or no preformed DHA, further exacerbating the deficiency in these tiny infants [80]. Many neonatal intensive care units are stressing the importance of human milk as a source of nutrition once the premature infant is able to receive enteral feeding. However, the decreased consumption of fish by women eating Western diets has affected human milk concentrations of DHA [80,82]. In fact, nursing women have been documented to have as little as 23 mg of DHA per day in their diet which in turn is reflected as 15 mg/100 mL of milk or 0.1–0.2% of the total fatty acid contents [83,84]. Specific DHA supplementation in both the infant and potentially the nursing mother may be especially important for premature infants who miss the accretion of DHA during the third trimester.
Preterm birth is associated with developmental delays and evidence of white matter injury [16,85]. The identification of white matter lesions and subsequent diagnosis of periventricular leukomylasia (PVL) is highly associated with maternal chorioamnionitis or funisitis. These infections likely influence the immune responses in the developing brain while simultaneously contributing to early parturition [58,86]. Whether DHA deficiencies in the neurological tissues of preterm infants contribute to the susceptibility to white matter injury or influence the consequences of maternal inflammation on infant neurodevelopment is unknown at this time but is a matter of consideration.
Infant Development and Growth
Given that 50–60% of the dry adult brain weight is fatty acids [87], and of these a large proportion are LCPUFAs, the availability of specific fatty acids during development is likely to be important in neurocognitive function (reviewed in [88] [89,90]). High levels of LCPUFAs have been found in the basal ganglia, pre- and post-central cortices, hippocampus, and thalamus in neonatal baboons and rats suggesting that they affect sensorimotor integration and memory [91–93]. Although synthetic capacities are functional in fetal and early neonatal life, most data indicate that the fetus or infant depends primarily on maternal sources or external supplies of LCPUFAs [94,95].
Many studies have been conducted assessing the cognitive outcomes of infants comparing breast feeding or various formula products with and without added DHA (reviewed in [89,90,96–100]). Overall the data demonstrate a subtle correlation between post-natal DHA supplementation and improved neurodevelopmental outcomes for preterm infants, but not for term infants [101–103]. A recent meta-analysis of previous trials concluded that preterm infants fed a LCPUFA-supplemented formula and tested using the 2nd edition of the Bayley Scales of Infant Development Mental Development Index displayed a 3.44-point advantage (95% CI: 0.57, 6.31) compared to infants fed a control formula [101]. This is a small difference on the individual level, less than one-quarter of one standard deviation, but may be meaningful at the population level. The same meta-analysis found no benefit to term infants (mean difference=−0.19, CI: −2.97, 2.59) [101]. \
The trials focused on neurodevelopment in children suffer from the same inconsistencies seen in other trials with DHA supplementation in that the doses and sources of the supplemented fatty acids differ broadly as well as the timing of supplementation, the outcome measures used, and the frequencies of various neonatal co-morbidities in the samples [100]. External validity is also a concern in that randomized infants were not breastfed, while most U.S. infants are breastfed for at least some period of time [104] because most studies have been conducted in developed countries. Longer follow-up of children will be important to determine if any benefits of DHA supplementation are meaningful to long-term academic performance and behavior.
Additional large trials in Western countries will be needed to determine whether better neurodevelopmental outcomes are possible with DHA supplementation prior to birth rather than after birth. Another unstudied window for intervention is between 12 months and 2–3 years of age. Particularly in regions like the prefrontal cortex which is responsible for executive function and synapse formation remains very active until at least age 2 [105]. However, intake of key LCPUFAs like DHA drops dramatically once an infant transitions from breast milk or formula to cow’s milk and typical table foods [Keim and Branum, 2012, in review]. Whether DHA supplementation would benefit children beyond infancy is another open question.
Because physical growth is a critical indicator of infant health, several trials have compared the weight, length and head circumference measures from DHA-supplemented infants to unsupplemented infants. Overall, DHA supplementation does not appear to affect the growth of infants, although a handful of studies have shown benefits for preterm infants [102,106]. Studies involving small-for-gestational age infants would be informative in this area.
In summary, the developmental benefits of DHA supplementation combined with the anti-inflammatory/pro-resolution properties of DHA has the potential provide improved outcomes through supplementation to mothers and at-risk infants.
Harmful Effects
In spite of the plethora of data on the beneficial effects of ω-3 LCPUFA supplementation, their use is not totally without risk. The antithrombotic actions of LCPUFAs could be of concern in high-risk populations (reviewed in [107]). The inhibition of FA oxidation and thus platelet aggregation is in part responsible for the efficacious effects of LCPUFA in cardiovascular disease [108,109]. These same effects could contribute to stroke or bleeding in high risk populations such as women with complicated pregnancies; however, there are no current reports of LCPUFAs and adverse birth outcomes. A second potentially harmful effect is that of immune-depression. The anti-inflammatory benefits of DHA could further decrease immune responses in an already immune-compromised person, preventing appropriate inflammatory responses. However, detrimental effects to immune compromised persons have not been reported thus far. A third effect of LCPUFA supplementation is gastrointestinal intolerance. The trial by Rice et al. [110] demonstrated no positive effect of supplementation on ventilator-free days and observed an increase in gastrointestinal morbidity. However, this trial did not single out DHA as a supplement but was combined with other antioxidant and micronutrient ingredients which may have caused the observed morbidities.
Finally, the most significant potential effect could be related to the chemical oxidation products formed from LCPUFAs. Although these oxidation products have not yet been identified in vivo, their actions in vitro indicate that they produce mutagenic and carcinogenic responses [111–113]. Oxidation products are of greatest concern in foods that have been artificially enhanced with ω-3 LCPUFAs because they lack the naturally occurring antioxidants that are available in foods which have endogenous LCPUFA, such as fish (reviewed in [114]). Correct storage and cooking of supplemented foods are a source of concern for exposure to oxidation products such as α β–unsaturated aldehydes which have defined mutagenic properties.
Conclusion
In conclusion, the essential role of DHA in neurological development during the pre- and postnatal periods has been defined. Overall, evidence for the therapeutic advantages of LCPUFAs supplementation is strong. Of primary concern are the low levels of dietary DHA consumption in women of child bearing age from westernized countries and that sufficient quantities of DHA may not be available for optimal neurological development and/or immunological support to the developing infant. This is especially important in the preterm or small for gestational age infant. Therapeutic recommendations exist for pregnant and lactating women, however, the data supporting these recommendations are not standardized and the U.S. Institute of Medicine has not taken the step to issue official dietary recommendations for Americans of any age [72]. Furthermore, no recommendations currently exist for infants and toddlers in which neurodevelopment is continuing well past birth. Future studies should be focused on optimizing supplementation strategies to provide optimal outcomes for all children.
Abbreviations
- DHA
docosahexaenoic acid
- LCPUFA
long chain polyunsaturated fatty acids
- TLR
Toll like receptor
- GPR
G-coupled protein receptor
- PPARγ
peroxisome proliferator activated receptor- γ
- MAPK
mitogen activated protein kinase
- NFkB
nuclear factor kappaB
- NPD1
neuroprotectin D1
- RvE
resolvin E
- RvD
resolvin D
- IL
interleukin
- TNFα
tumor necrosis factor α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kromann N, Green A. Epidemiological studies in the upernavik district, greenland. Incidence of some chronic diseases 1950–1974. Acta Med Scand. 1980;208:401–406. [PubMed] [Google Scholar]
- 2.Kromhout D, Bosschieter EB, de Lezenne Coulander C. The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N Engl J Med. 1985;312:1205–1209. doi: 10.1056/NEJM198505093121901. [DOI] [PubMed] [Google Scholar]
- 3.Beare-Rogers JL, Gray L, Nera EA, Levin OL. Nutritional properties of poppyseed oil relative to some other oils. Nutr Metab. 1979;23:335–346. doi: 10.1159/000176272. [DOI] [PubMed] [Google Scholar]
- 4.Ervin RB, Wang CY, Wright JD, Kennedy-Stephenson J. Dietary intake of selected minerals for the united states population: 1999–2000. Adv Data. 2004:1–5. [PubMed] [Google Scholar]
- 5.Bradbury J. Docosahexaenoic acid (dha): An ancient nutrient for the modern human brain. Nutrients. 2011;3:529–554. doi: 10.3390/nu3050529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simopoulos AP. Evolutionary aspects of diet: The omega-6/omega-3 ratio and the brain. Mol Neurobiol. 2011;44:203–215. doi: 10.1007/s12035-010-8162-0. [DOI] [PubMed] [Google Scholar]
- 7.Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United states, 2002. Adv Data. 2004:1–19. [PubMed] [Google Scholar]
- 8.Calder PC. Immunomodulation by omega-3 fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2007;77:327–335. doi: 10.1016/j.plefa.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Carlson SE. Docosahexaenoic acid supplementation in pregnancy and lactation. Am J Clin Nutr. 2009;89:678S–684S. doi: 10.3945/ajcn.2008.26811E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002;21:495–505. doi: 10.1080/07315724.2002.10719248. [DOI] [PubMed] [Google Scholar]
- 11.Beyerlein A, Hadders-Algra M, Kennedy K, Fewtrell M, Singhal A, Rosenfeld E, Lucas A, Bouwstra H, Koletzko B, von Kries R. Infant formula supplementation with long-chain polyunsaturated fatty acids has no effect on bayley developmental scores at 18 months of age--ipd meta-analysis of 4 large clinical trials. J Pediatr Gastroenterol Nutr. 2010;50:79–84. doi: 10.1097/MPG.0b013e3181acae7d. [DOI] [PubMed] [Google Scholar]
- 12.Delgado-Noguera MF, Calvache JA, Bonfill Cosp X. Supplementation with long chain polyunsaturated fatty acids (lcpufa) to breastfeeding mothers for improving child growth and development. Cochrane Database Syst Rev. 2010:CD007901. doi: 10.1002/14651858.CD007901.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Yavin E. Versatile roles of docosahexaenoic acid in the prenatal brain: From pro- and anti-oxidant features to regulation of gene expression. Prostaglandins Leukot Essent Fatty Acids. 2006;75:203–211. doi: 10.1016/j.plefa.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Calder PC. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukot Essent Fatty Acids. 2008;79:101–108. doi: 10.1016/j.plefa.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Himmelfarb J, Phinney S, Ikizler TA, Kane J, McMonagle E, Miller G. Gamma-tocopherol and docosahexaenoic acid decrease inflammation in dialysis patients. J Ren Nutr. 2007;17:296–304. doi: 10.1053/j.jrn.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 16.McCann JC, Ames BN. Is docosahexaenoic acid, an n-3 long-chain polyunsaturated fatty acid, required for development of normal brain function? An overview of evidence from cognitive and behavioral tests in humans and animals. Am J Clin Nutr. 2005;82:281–295. doi: 10.1093/ajcn.82.2.281. [DOI] [PubMed] [Google Scholar]
- 17.Lee JY, Plakidas A, Lee WH, Heikkinen A, Chanmugam P, Bray G, Hwang DH. Differential modulation of toll-like receptors by fatty acids: Preferential inhibition by n-3 polyunsaturated fatty acids. J Lipid Res. 2003;44:479–486. doi: 10.1194/jlr.M200361-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. Gpr120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Ruan XZ, Powis SH, Fernando R, Mon WY, Wheeler DC, Moorhead JF, Varghese Z. Epa and dha reduce lps-induced inflammation responses in hk-2 cells: Evidence for a ppar-gamma-dependent mechanism. Kidney Int. 2005;67:867–874. doi: 10.1111/j.1523-1755.2005.00151.x. [DOI] [PubMed] [Google Scholar]
- 20.Innis SM, de La Presa Owens S. Dietary fatty acid composition in pregnancy alters neurite membrane fatty acids and dopamine in newborn rat brain. J Nutr. 2001;131:118–122. doi: 10.1093/jn/131.1.118. [DOI] [PubMed] [Google Scholar]
- 21.Bhatia HS, Agrawal R, Sharma S, Huo YX, Ying Z, Gomez-Pinilla F. Omega-3 fatty acid deficiency during brain maturation reduces neuronal and behavioral plasticity in adulthood. PLoS One. 2011;6:e28451. doi: 10.1371/journal.pone.0028451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delion S, Chalon S, Herault J, Guilloteau D, Besnard JC, Durand G. Chronic dietary alpha-linolenic acid deficiency alters dopaminergic and serotoninergic neurotransmission in rats. J Nutr. 1994;124:2466–2476. doi: 10.1093/jn/124.12.466. [DOI] [PubMed] [Google Scholar]
- 23.Wainwright PE. Dietary essential fatty acids and brain function: A developmental perspective on mechanisms. Proc Nutr Soc. 2002;61:61–69. doi: 10.1079/pns2001130. [DOI] [PubMed] [Google Scholar]
- 24.Wong SW, Kwon MJ, Choi AM, Kim HP, Nakahira K, Hwang DH. Fatty acids modulate toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem. 2009;284:27384–27392. doi: 10.1074/jbc.M109.044065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malaeb S, Dammann O. Fetal inflammatory response and brain injury in the preterm newborn. J Child Neurol. 2009;24:1119–1126. doi: 10.1177/0883073809338066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JY, Ye J, Gao Z, Youn HS, Lee WH, Zhao L, Sizemore N, Hwang DH. Reciprocal modulation of toll-like receptor-4 signaling pathways involving myd88 and phosphatidylinositol 3-kinase/akt by saturated and polyunsaturated fatty acids. J Biol Chem. 2003;278:37041–37051. doi: 10.1074/jbc.M305213200. [DOI] [PubMed] [Google Scholar]
- 27.Oh DY, Olefsky JM. Omega 3 fatty acids and gpr120. Cell Metab. 2012;15:564–565. doi: 10.1016/j.cmet.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chapkin RS, Kim W, Lupton JR, McMurray DN. Dietary docosahexaenoic and eicosapentaenoic acid: Emerging mediators of inflammation. Prostaglandins Leukot Essent Fatty Acids. 2009;81:187–191. doi: 10.1016/j.plefa.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lengqvist J, Mata De Urquiza A, Bergman AC, Willson TM, Sjovall J, Perlmann T, Griffiths WJ. Polyunsaturated fatty acids including docosahexaenoic and arachidonic acid bind to the retinoid x receptor alpha ligand-binding domain. Mol Cell Proteomics. 2004;3:692–703. doi: 10.1074/mcp.M400003-MCP200. [DOI] [PubMed] [Google Scholar]
- 30.Tiesset H, Pierre M, Desseyn JL, Guery B, Beermann C, Galabert C, Gottrand F, Husson MO. Dietary (n-3) polyunsaturated fatty acids affect the kinetics of pro- and antiinflammatory responses in mice with pseudomonas aeruginosa lung infection. J Nutr. 2009;139:82–89. doi: 10.3945/jn.108.096115. [DOI] [PubMed] [Google Scholar]
- 31.Xu HE, Lambert MH, Montana VG, Parks DJ, Blanchard SG, Brown PJ, Sternbach DD, Lehmann JM, Wisely GB, Willson TM, Kliewer SA, Milburn MV. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol Cell. 1999;3:397–403. doi: 10.1016/s1097-2765(00)80467-0. [DOI] [PubMed] [Google Scholar]
- 32.Bradley RL, Fisher FF, Maratos-Flier E. Dietary fatty acids differentially regulate production of tnf-alpha and il-10 by murine 3t3-l1 adipocytes. Obesity (Silver Spring) 2008;16:938–944. doi: 10.1038/oby.2008.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: A positive modulator of akt signaling in neuronal survival. Proc Natl Acad Sci U S A. 2005;102:10858–10863. doi: 10.1073/pnas.0502903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 35.Wassall SR, Stillwell W. Polyunsaturated fatty acid-cholesterol interactions: Domain formation in membranes. Biochim Biophys Acta. 2009;1788:24–32. doi: 10.1016/j.bbamem.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 36.Huber T, Rajamoorthi K, Kurze VF, Beyer K, Brown MF. Structure of docosahexaenoic acid-containing phospholipid bilayers as studied by (2)h nmr and molecular dynamics simulations. J Am Chem Soc. 2002;124:298–309. doi: 10.1021/ja011383j. [DOI] [PubMed] [Google Scholar]
- 37.Kew S, Banerjee T, Minihane AM, Finnegan YE, Williams CM, Calder PC. Relation between the fatty acid composition of peripheral blood mononuclear cells and measures of immune cell function in healthy, free-living subjects aged 25–72 y. Am J Clin Nutr. 2003;77:1278–1286. doi: 10.1093/ajcn/77.5.1278. [DOI] [PubMed] [Google Scholar]
- 38.Healy DA, Wallace FA, Miles EA, Calder PC, Newsholm P. Effect of low-to-moderate amounts of dietary fish oil on neutrophil lipid composition and function. Lipids. 2000;35:763–768. doi: 10.1007/s11745-000-0583-1. [DOI] [PubMed] [Google Scholar]
- 39.Innis SM. Perinatal biochemistry and physiology of long-chain polyunsaturated fatty acids. J Pediatr. 2003;143:S1–8. doi: 10.1067/s0022-3476(03)00396-2. [DOI] [PubMed] [Google Scholar]
- 40.van Kuijk FJ, Buck P. Fatty acid composition of the human macula and peripheral retina. Invest Ophthalmol Vis Sci. 1992;33:3493–3496. [PubMed] [Google Scholar]
- 41.Kishimoto Y, Agranoff BW, Radin NS, Burton RM. Comparison of the fatty acids of lipids of subcellular brain fractions. J Neurochem. 1969;16:397–404. doi: 10.1111/j.1471-4159.1969.tb10380.x. [DOI] [PubMed] [Google Scholar]
- 42.Anderson RE. Lipids of ocular tissues. Iv. A comparison of the phospholipids from the retina of six mammalian species. Exp Eye Res. 1970;10:339–344. doi: 10.1016/s0014-4835(70)80046-x. [DOI] [PubMed] [Google Scholar]
- 43.Sun GY, Sun Y. Phospholipids and acyl groups of synaptosomal and myelin membranes isolated from the cerebral cortex of squirrel monkey (saimiri sciureus) Biochim Biophys Acta. 1972;280:306–315. doi: 10.1016/0005-2760(72)90098-7. [DOI] [PubMed] [Google Scholar]
- 44.Calder PC, Bond JA, Harvey DJ, Gordon S, Newsholme EA. Uptake and incorporation of saturated and unsaturated fatty acids into macrophage lipids and their effect upon macrophage adhesion and phagocytosis. Biochem J. 1990;269:807–814. doi: 10.1042/bj2690807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serhan CN. Novel eicosanoid and docosanoid mediators: Resolvins, docosatrienes, and neuroprotectins. Curr Opin Clin Nutr Metab Care. 2005;8:115–121. doi: 10.1097/00075197-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serhan CN. Novel chemical mediators in the resolution of inflammation: Resolvins and protectins. Anesthesiol Clin. 2006;24:341–364. doi: 10.1016/j.atc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 48.Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, Yang R, Colgan SP, Petasis NA. Anti-inflammatory actions of neuroprotectin d1/protectin d1 and its natural stereoisomers: Assignments of dihydroxy-containing docosatrienes. J Immunol. 2006;176:1848–1859. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- 49.Niemoller TD, Bazan NG. Docosahexaenoic acid neurolipidomics. Prostaglandins Other Lipid Mediat. 2010;91:85–89. doi: 10.1016/j.prostaglandins.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yin H, Liu W, Goleniewska K, Porter NA, Morrow JD, Peebles RS., Jr Dietary supplementation of omega-3 fatty acid-containing fish oil suppresses f2-isoprostanes but enhances inflammatory cytokine response in a mouse model of ovalbumin-induced allergic lung inflammation. Free Radic Biol Med. 2009;47:622–628. doi: 10.1016/j.freeradbiomed.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burdge GC, Powell J, Dadd T, Talbot D, Civil J, Calder PC. Acute consumption of fish oil improves postprandial vldl profiles in healthy men aged 50–65 years. Br J Nutr. 2009;102:160–165. doi: 10.1017/S0007114508143550. [DOI] [PubMed] [Google Scholar]
- 52.Bannenberg G, Arita M, Serhan CN. Endogenous receptor agonists: Resolving inflammation. Scientific World Journal. 2007;7:1440–1462. doi: 10.1100/tsw.2007.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin e1 selectively interacts with leukotriene b4 receptor blt1 and chemr23 to regulate inflammation. J Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 54.Ariel A, Serhan CN. Resolvins and protectins in the termination program of acute inflammation. Trends Immunol. 2007;28:176–183. doi: 10.1016/j.it.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 55.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin e1 and protectin d1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83:1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 57.von Ehrenstein OS, Neta GI, Andrews W, Goldenberg R, Goepfert A, Zhang J. Child intellectual development in relation to cytokine levels in umbilical cord blood. Am J Epidemiol. 2012;175:1191–1199. doi: 10.1093/aje/kwr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoon BH, Park CW, Chaiworapongsa T. Intrauterine infection and the development of cerebral palsy. BJOG. 2003;110 (Suppl 20):124–127. doi: 10.1016/s1470-0328(03)00063-6. [DOI] [PubMed] [Google Scholar]
- 59.Pickler R, Brown L, McGrath J, Lyon D, Rattican D, Cheng CY, Howland L, Jallo N. Integrated review of cytokines in maternal, cord, and newborn blood: Part ii-- associations with early infection and increased risk of neurologic damage in preterm infants. Biol Res Nurs. 2010;11:377–386. doi: 10.1177/1099800409344619. [DOI] [PubMed] [Google Scholar]
- 60.Pickler RH, McGrath JM, Reyna BA, McCain N, Lewis M, Cone S, Wetzel P, Best A. A model of neurodevelopmental risk and protection for preterm infants. J Perinat Neonatal Nurs. 2010;24:356–365. doi: 10.1097/JPN.0b013e3181fb1e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bland ST, Beckley JT, Young S, Tsang V, Watkins LR, Maier SF, Bilbo SD. Enduring consequences of early-life infection on glial and neural cell genesis within cognitive regions of the brain. Brain Behav Immun. 2010;24:329–338. doi: 10.1016/j.bbi.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huleihel M, Golan H, Hallak M. Intrauterine infection/inflammation during pregnancy and offspring brain damages: Possible mechanisms involved. Reprod Biol Endocrinol. 2004;2:17. doi: 10.1186/1477-7827-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am J Clin Nutr. 1996;63:116–122. doi: 10.1093/ajcn/63.1.116. [DOI] [PubMed] [Google Scholar]
- 64.Kawakita E, Hashimoto M, Shido O. Docosahexaenoic acid promotes neurogenesis in vitro and in vivo. Neuroscience. 2006;139:991–997. doi: 10.1016/j.neuroscience.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 65.Carlson SE. Docosahexaenoic acid and arachidonic acid in infant development. Semin Neonatol. 2001;6:437–449. doi: 10.1053/siny.2001.0093. [DOI] [PubMed] [Google Scholar]
- 66.Innis SM. Essential fatty acid transfer and fetal development. Placenta. 2005;26 (Suppl A):S70–75. doi: 10.1016/j.placenta.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 67.Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. Intrauterine fatty acid accretion rates in human brain: Implications for fatty acid requirements. Early Hum Dev. 1980;4:121–129. doi: 10.1016/0378-3782(80)90015-8. [DOI] [PubMed] [Google Scholar]
- 68.Henriksen C, Haugholt K, Lindgren M, Aurvag AK, Ronnestad A, Gronn M, Solberg R, Moen A, Nakstad B, Berge RK, Smith L, Iversen PO, Drevon CA. Improved cognitive development among preterm infants attributable to early supplementation of human milk with docosahexaenoic acid and arachidonic acid. Pediatrics. 2008;121:1137–1145. doi: 10.1542/peds.2007-1511. [DOI] [PubMed] [Google Scholar]
- 69.Dhobale MV, Wadhwani N, Mehendale SS, Pisal HR, Joshi SR. Reduced levels of placental long chain polyunsaturated fatty acids in preterm deliveries. Prostaglandins, leukotrienes, and essential fatty acids. 2011;85:149–153. doi: 10.1016/j.plefa.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 70.McNamara RK, Carlson SE. Role of omega-3 fatty acids in brain development and function: Potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fatty Acids. 2006;75:329–349. doi: 10.1016/j.plefa.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 71.Lauritzen L, Carlson SE. Maternal fatty acid status during pregnancy and lactation and relation to newborn and infant status. Matern Child Nutr. 2011;7 (Suppl 2):41–58. doi: 10.1111/j.1740-8709.2011.00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jordan RG. Prenatal omega-3 fatty acids: Review and recommendations. J Midwifery Womens Health. 2010;55:520–528. doi: 10.1016/j.jmwh.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 73.Dunstan JA, Mitoulas LR, Dixon G, Doherty DA, Hartmann PE, Simmer K, Prescott SL. The effects of fish oil supplementation in pregnancy on breast milk fatty acid composition over the course of lactation: A randomized controlled trial. Pediatr Res. 2007;62:689–694. doi: 10.1203/PDR.0b013e318159a93a. [DOI] [PubMed] [Google Scholar]
- 74.Lauritzen L, Jorgensen MH, Mikkelsen TB, Skovgaard M, Straarup EM, Olsen SF, Hoy CE, Michaelsen KF. Maternal fish oil supplementation in lactation: Effect on visual acuity and n-3 fatty acid content of infant erythrocytes. Lipids. 2004;39:195–206. doi: 10.1007/s11745-004-1220-8. [DOI] [PubMed] [Google Scholar]
- 75.Meldrum SJ, D’Vaz N, Simmer K, Dunstan JA, Hird K, Prescott SL. Effects of high-dose fish oil supplementation during early infancy on neurodevelopment and language: A randomised controlled trial. Br J Nutr. 2012:1–12. doi: 10.1017/S0007114511006878. [DOI] [PubMed] [Google Scholar]
- 76.Furuhjelm C, Warstedt K, Fageras M, Falth-Magnusson K, Larsson J, Fredriksson M, Duchen K. Allergic disease in infants up to 2 years of age in relation to plasma omega-3 fatty acids and maternal fish oil supplementation in pregnancy and lactation. Pediatr Allergy Immunol. 2011;22:505–514. doi: 10.1111/j.1399-3038.2010.01096.x. [DOI] [PubMed] [Google Scholar]
- 77.Noakes PS, Vlachava M, Kremmyda LS, Diaper ND, Miles EA, Erlewyn-Lajeunesse M, Williams AP, Godfrey KM, Calder PC. Increased intake of oily fish in pregnancy: Effects on neonatal immune responses and on clinical outcomes in infants at 6 mo. Am J Clin Nutr. 2012;95:395–404. doi: 10.3945/ajcn.111.022954. [DOI] [PubMed] [Google Scholar]
- 78.Salvig JD, Lamont RF. Evidence regarding an effect of marine n-3 fatty acids on preterm birth: A systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2011;90:825–838. doi: 10.1111/j.1600-0412.2011.01171.x. [DOI] [PubMed] [Google Scholar]
- 79.Kuipers RS, Luxwolda MF, Offringa PJ, Boersma ER, Dijck-Brouwer DA, Muskiet FA. Fetal intrauterine whole body linoleic, arachidonic and docosahexaenoic acid contents and accretion rates. Prostaglandins, leukotrienes, and essential fatty acids. 2012;86:13–20. doi: 10.1016/j.plefa.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 80.Valentine CJ. Maternal dietary dha supplementation to improve inflammatory outcomes in the preterm infant. Adv Nutr. 2012;3:370–376. doi: 10.3945/an.111.001248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Szajewska H, Makrides M. Is early nutrition related to short-term health and long-term outcome? Ann Nutr Metab. 2011;58 (Suppl 1):38–48. doi: 10.1159/000323465. [DOI] [PubMed] [Google Scholar]
- 82.Valentine CJ, Morrow G, Fernandez S, Gulati P, Bartholomew D, Long D, Welty SE, Morrow AL, Rogers LK. Docosahexaenoic acid and amino acid content in pastuerized donor milk are low for preterm infants. Journal of Pediatrics. 2010 doi: 10.1016/j.jpeds.2010.06.017. in press. [DOI] [PubMed] [Google Scholar]
- 83.Valentine CJ, Morrow G, Pennell M, Morrow AL, Hodge A, Haban-Bartz A, Collins K, Rogers LK. Randomized controlled trial of docosahexaenoic acid supplementation in midwestern u.S. Human milk donors. Breastfeed Med. 2012 doi: 10.1089/bfm.2011.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brenna JT, Varamini B, Jensen RG, Diersen-Schade DA, Boettcher JA, Arterburn LM. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am J Clin Nutr. 2007;85:1457–1464. doi: 10.1093/ajcn/85.6.1457. [DOI] [PubMed] [Google Scholar]
- 85.Goldenberg RL, DuBard MB, Cliver SP, Nelson KG, Blankson K, Ramey SL, Herman A. Pregnancy outcome and intelligence at age five years. Am J Obstet Gynecol. 1996;175:1511–1515. doi: 10.1016/s0002-9378(96)70099-6. [DOI] [PubMed] [Google Scholar]
- 86.Wharton KN, Pinar H, Stonestreet BS, Tucker R, McLean KR, Wallach M, Vohr BR. Severe umbilical cord inflammation-a predictor of periventricular leukomalacia in very low birth weight infants. Early Hum Dev. 2004;77:77–87. doi: 10.1016/j.earlhumdev.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 87.Martinez M. Tissue levels of polyunsaturated fatty acids during early human development. J Pediatr. 1992;120:S129–138. doi: 10.1016/s0022-3476(05)81247-8. [DOI] [PubMed] [Google Scholar]
- 88.Bazan NG, Molina MF, Gordon WC. Docosahexaenoic acid signalolipidomics in nutrition: Significance in aging, neuroinflammation, macular degeneration, alzheimer’s, and other neurodegenerative diseases. Annu Rev Nutr. 2011;31:321–351. doi: 10.1146/annurev.nutr.012809.104635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hadders-Algra M. The role of long-chain polyunsaturated fatty acids (lcpufa) in growth and development. Adv Exp Med Biol. 2005;569:80–94. doi: 10.1007/1-4020-3535-7_13. [DOI] [PubMed] [Google Scholar]
- 90.Hadders-Algra M, Bouwstra H, van Goor SA, Dijck-Brouwer DA, Muskiet FA. Prenatal and early postnatal fatty acid status and neurodevelopmental outcome. J Perinat Med. 2007;35 (Suppl 1):S28–34. doi: 10.1515/JPM.2007.034. [DOI] [PubMed] [Google Scholar]
- 91.Diau GY, Hsieh AT, Sarkadi-Nagy EA, Wijendran V, Nathanielsz PW, Brenna JT. The influence of long chain polyunsaturate supplementation on docosahexaenoic acid and arachidonic acid in baboon neonate central nervous system. BMC Med. 2005;3:11. doi: 10.1186/1741-7015-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Favreliere S, Barrier L, Durand G, Chalon S, Tallineau C. Chronic dietary n-3 polyunsaturated fatty acids deficiency affects the fatty acid composition of plasmenylethanolamine and phosphatidylethanolamine differently in rat frontal cortex, striatum, and cerebellum. Lipids. 1998;33:401–407. doi: 10.1007/s11745-998-0221-y. [DOI] [PubMed] [Google Scholar]
- 93.Bourre JM, Pascal G, Durand G, Masson M, Dumont O, Piciotti M. Alterations in the fatty acid composition of rat brain cells (neurons, astrocytes, and oligodendrocytes) and of subcellular fractions (myelin and synaptosomes) induced by a diet devoid of n-3 fatty acids. J Neurochem. 1984;43:342–348. doi: 10.1111/j.1471-4159.1984.tb00906.x. [DOI] [PubMed] [Google Scholar]
- 94.Lauritzen L, Hansen HS, Jorgensen MH, Michaelsen KF. The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog Lipid Res. 2001;40:1–94. doi: 10.1016/s0163-7827(00)00017-5. [DOI] [PubMed] [Google Scholar]
- 95.Martin JC, Bougnoux P, Fignon A, Theret V, Antoine JM, Lamisse F, Couet C. Dependence of human milk essential fatty acids on adipose stores during lactation. Am J Clin Nutr. 1993;58:653–659. doi: 10.1093/ajcn/58.5.653. [DOI] [PubMed] [Google Scholar]
- 96.Clandinin MT, Van Aerde JE, Merkel KL, Harris CL, Springer MA, Hansen JW, Diersen-Schade DA. Growth and development of preterm infants fed infant formulas containing docosahexaenoic acid and arachidonic acid. J Pediatr. 2005;146:461–468. doi: 10.1016/j.jpeds.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 97.Hadders-Algra M. Prenatal long-chain polyunsaturated fatty acid status: The importance of a balanced intake of docosahexaenoic acid and arachidonic acid. J Perinat Med. 2008;36:101–109. doi: 10.1515/JPM.2008.029. [DOI] [PubMed] [Google Scholar]
- 98.Koletzko B, Agostoni C, Carlson SE, Clandinin T, Hornstra G, Neuringer M, Uauy R, Yamashiro Y, Willatts P. Long chain polyunsaturated fatty acids (lc-pufa) and perinatal development. Acta Paediatr. 2001;90:460–464. [PubMed] [Google Scholar]
- 99.Birch EE, Birch DG, Hoffman DR, Uauy R. Dietary essential fatty acid supply and visual acuity development. Invest Ophthalmol Vis Sci. 1992;33:3242–3253. [PubMed] [Google Scholar]
- 100.Meldrum SJ, Smith MA, Prescott SL, Hird K, Simmer K. Achieving definitive results in long-chain polyunsaturated fatty acid supplementation trials of term infants: Factors for consideration. Nutr Rev. 2011;69:205–214. doi: 10.1111/j.1753-4887.2011.00381.x. [DOI] [PubMed] [Google Scholar]
- 101.Makrides M, Smithers LG, Gibson RA. Role of long-chain polyunsaturated fatty acids in neurodevelopment and growth. Nestle Nutr Workshop Ser Pediatr Program. 2010;65:123–133. doi: 10.1159/000281154. discussion 133–126. [DOI] [PubMed] [Google Scholar]
- 102.Schulzke SM, Patole SK, Simmer K. Longchain polyunsaturated fatty acid supplementation in preterm infants. Cochrane Database Syst Rev. 2011:CD000375. doi: 10.1002/14651858.CD000375.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Simmer K, Patole SK, Rao SC. Longchain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database Syst Rev. 2011:CD000376. doi: 10.1002/14651858.CD000376.pub2. [DOI] [PubMed] [Google Scholar]
- 104.National immunization survey, provisional data. U.S. Centers for DIsease Control and Prevention; 2008. [Google Scholar]
- 105.Belsky J, de Haan M. Annual research review: Parenting and children’s brain development: The end of the beginning. J Child Psychol Psychiatry. 2011;52:409–428. doi: 10.1111/j.1469-7610.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- 106.Makrides M, Gibson RA, Udell T, Ried K. Supplementation of infant formula with long-chain polyunsaturated fatty acids does not influence the growth of term infants. Am J Clin Nutr. 2005;81:1094–1101. doi: 10.1093/ajcn/81.5.1094. [DOI] [PubMed] [Google Scholar]
- 107.Lagarde M, Calzada C, Guichardant M, Vericel E. Dose-effect and metabolism of docosahexaenoic acid: Pathophysiological relevance in blood platelets. Prostaglandins Leukot Essent Fatty Acids. 2012 doi: 10.1016/j.plefa.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 108.Guillot N, Caillet E, Laville M, Calzada C, Lagarde M, Vericel E. Increasing intakes of the long-chain omega-3 docosahexaenoic acid: Effects on platelet functions and redox status in healthy men. FASEB J. 2009;23:2909–2916. doi: 10.1096/fj.09-133421. [DOI] [PubMed] [Google Scholar]
- 109.Phang M, Garg ML, Sinclair AJ. Inhibition of platelet aggregation by omega-3 polyunsaturated fatty acids is gender specific-redefining platelet response to fish oils. Prostaglandins Leukot Essent Fatty Acids. 2009;81:35–40. doi: 10.1016/j.plefa.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 110.Rice TW, Wheeler AP, Thompson BT, DeBoisblanc BP, Steingrub J, Rock P, Network NACT. Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. Jama-J Am Med Assoc. 2011;306:1574–1581. doi: 10.1001/jama.2011.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Serini S, Fasano E, Piccioni E, Cittadini AR, Calviello G. Dietary n-3 polyunsaturated fatty acids and the paradox of their health benefits and potential harmful effects. Chem Res Toxicol. 2011;24:2093–2105. doi: 10.1021/tx200314p. [DOI] [PubMed] [Google Scholar]
- 112.Grundt H, Nilsen DW, Mansoor MA, Nordoy A. Increased lipid peroxidation during long-term intervention with high doses of n-3 fatty acids (pufas) following an acute myocardial infarction. Eur J Clin Nutr. 2003;57:793–800. doi: 10.1038/sj.ejcn.1601730. [DOI] [PubMed] [Google Scholar]
- 113.Palozza P, Sgarlata E, Luberto C, Piccioni E, Anti M, Marra G, Armelao F, Franceschelli P, Bartoli GM. N-3 fatty acids induce oxidative modifications in human erythrocytes depending on dose and duration of dietary supplementation. Am J Clin Nutr. 1996;64:297–304. doi: 10.1093/ajcn/64.3.297. [DOI] [PubMed] [Google Scholar]
- 114.Taneja A, Singh H. Challenges for the delivery of long-chain n-3 fatty acids in functional foods. Annu Rev Food Sci Technol. 2012;3:105–123. doi: 10.1146/annurev-food-022811-101130. [DOI] [PubMed] [Google Scholar]