Abstract
Background
Innate immune gene expression is regulated in part through high mobility group box 1(HMGB1), an endogenous proinflammatory cytokine, that activates multiple members of the interleukin-1/Toll-like receptor (IL-1/TLR) family associated with danger signaling. We investigated expression of HMGB1, TLR2, TLR3 and TLR4 in chronic ethanol treated mouse brain, post-mortem human alcoholic brain, and rat brain slice culture to test the hypothesis that neuroimmune activation in alcoholic brain involves ethanol activation of HMGB1/TLR danger signaling.
Methods
Protein levels were assessed using Western blot, ELISA, immunohistochemical immunoreactivity (+IR), and mRNA levels were measured by real time PCR in ethanol-treated mice (5 g/kg/day, i.g., 10 days + 24 hr), rat brain slice culture, and post-mortem human alcoholic brain.
Results
Ethanol treatment of mice increased brain mRNA and +IR protein expression of HMGB1, TLR2, TLR3, and TLR4. Post-mortem human alcoholic brain also showed increased HMGB1, TLR2, TLR3, and TLR4+IR cells that correlated with lifetime alcohol consumption as well as each other. Ethanol treatment of brain slice culture released HMGB1 into the media and induced the proinflammatory cytokine, IL-1β. Neutralizing antibodies to HMGB1 and small inhibitory mRNA to HMGB1 or TLR4 blunted ethanol induction of IL-1β.
Conclusions
Ethanol-induced HMGB1/TLR signaling contributes to induction of the proinflammatory cytokine, IL-1β. Increased expression of HMGB1, TLR2, TLR3, and TLR4 in alcoholic brain and in mice treated with ethanol suggests that chronic alcohol-induced brain neuroimmune activation occurs through HMGB1/TLR signaling.
Keywords: alcoholism, Toll-like receptors, ethanol, cytokines, interleukin-1, cerebral cortex
Background
Neuroinflammation is implicated in the etiology of many brain diseases. Recent discoveries indicate that endogenous danger-associated molecular pattern agonists and Toll-like receptors (TLRs) contribute to neuroinflammation [1–3]. High-mobility group box 1 (HMGB1) is an endogenous danger signaling cytokine that acts on multiple interleukin-1/Toll-like receptors (IL-1/TLRs) [2, 4, 5]. The family of IL-1/TLRs signal through kinases that activate the proinflammatory transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) increasing expression of cytokines, oxidases, and other genes associated with innate immune responses [6]. Although there are multiple TLRs in the brains of mice and humans, TLR2, TLR3, and TLR4 are the most commonly studied. HMGB1/TLR danger signaling is associated with persistent chronic inflammatory diseases [7], and our studies find that once induced, neuroimmune activation in brain persists for long periods. For example, ethanol [8] and endotoxin [9] treatment of mice increases proinflammatory gene expression in brain that persist for weeks to months after treatment. Thus, we hypothesized that HMGB1/TLR danger signaling might contribute to ethanol-induced neuroinflammation and the neurobiology of alcoholism.
We have previously found that ethanol increases expression of brain proinflammatory genes through activation of the transcription factor, NF-κB [6, 10]. Chronic ethanol-induced expression of brain proinflammatory cytokines and oxidases are linked to neurodegeneration [8, 11] and inhibition of neurogenesis [12]. In post-mortem human alcoholic brain we found increased expression of the proinflammatory chemokine, monocyte chemoattractant protein, MCP1 and markers of microglial activation [13], and increased expression of IL-1β and inflammasome proteins [14] as well as increased expression of proinflammatory NADPH oxidase [15], indicating significant neuroimmune activation in alcoholic human brain. Further, transgenic mice lacking TLR4 are protected against ethanol-induced proinflammatory gene induction, glial activation, and neurotoxicity [16]. HMGB1/TLR danger signals may also impact responses to ethanol since knockdown of amygdala TLR4 with small inhibitory RNA (siRNA) blunts ethanol-dependent rat operant responding for ethanol [17] and acute behavioral responses to ethanol are altered in transgenic mice lacking TLR2 and/or TLR4 [18]. Studies in mice have found that neuroimmune activation increases ethanol drinking [19], whereas transgenic mice lacking innate immune genes drink less than their wild-type controls [20]. These studies suggest that the actions of ethanol in brain include neuroimmune activation consistent with danger signaling through TLR4 and perhaps other TLRs. Thus, alcoholic human brain shows neuroimmune activation and ethanol-induced neuroimmune activation is linked to drinking behavior, alcohol responses, TLR upregulation, and neurodegeneration.
We report here that ethanol treatment of mice or rat brain sections in vitro increase expression of HMGB1/TLRs. Post-mortem human alcoholic brain was found to have significantly more HMGB1, TLR2, TLR3, and TLR4 which correlated with lifetime alcohol consumption. Furthermore, ethanol was found to release HMGB1 from brain slice culture activating proinflammatory IL-β synthesis through TLR4. These findings support the hypothesis that ethanol activation of HMGB1/TLR danger signaling contributes to neuroimmune activation in alcoholic brain and to the neurobiology of alcoholism.
Methods and Materials
Animals
Eight-week old male (20–22 g) C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, Maine). After at least 1 week of acclimation to the animal colony, mice were divided into control and ethanol groups. Mice were treated with water (control) or ethanol (5 g/kg, i.g., 25% ethanol w/v), with volumes matched, daily for 10 days and sacrificed 24 hr later. The average blood ethanol concentration (BEC) at 1 hr after the first ethanol treatment and the last ethanol treatment was 291 ± 16 mg/dl (w/v, n=10) and 301 ± 19 mg/dl (w/v, n=10), respectively. The BEC is high modeling alcoholic binge drinking [21]. Brain slice culture was prepared from P7 pups from timed pregnant Sprague-Dawley rats.
All protocols and procedures in this study were approved by the Institutional Animal Care and Use Committee and were conducted in accordance with the National Institute of Health regulations for the care and use of animals in research. Details on reagents and reagents sources and equipment are listed within the supplemental methods and materials section.
Brain Slice Culture
Organotypic hippocampal-entorhinal cortex (HEC) brain slice culture was prepared from P7 Sprague-Dawley rat pups as described previously [6, 22]. After 10 days in culture, slices were used for experimental treatments as described below.
Human tissue
Human post-mortem brain tissue was obtained from the New South Wales Tissue Resource Center in Australia [ethics committee approval number: X11-0107]. Paraffin sections of orbitofrontal cortex (OFC) were used in this study. The detailed patients’ medical history is presented in Table1. Human alcoholic patients, which averaged over 10 drinks per day with lifetime consumption of over 500 kg of alcohol, were compared to moderate drinkers who averaged less than 1 drink per day with lifetime consumption of less than 100 kg of alcohol. Total lifetime alcohol consumption provides an index of alcoholic neurodegeneration [23, 24], whereas there is no relationship among moderate drinkers [24, 25]. Only individuals with alcohol dependence not complicated by liver cirrhosis or nutritional deficiencies were included in this study. The most common cause of death was cardiovascular disease for both groups. Post-mortem interval (PMI) causes of death and alcohol consumption are documented. All psychiatric and alcohol use disorder diagnoses were confirmed using the Diagnostic Instrument for Brain Studies which complies with the Diagnostic Statistical Manual of Mental Disorders and has demonstrated reliability [26].
Table 1.
Patient characteristics of human post-mortem brains
| Group | Age at Death | Sex | PMI | Clinical Cause of Death | Lifetime Ethanol (gm) |
|---|---|---|---|---|---|
| Control | 60 | Male | 28 | Ischemic heart disease | 0 |
| Control | 62 | Male | 46 | Ischemic heart disease | 5000 |
| Control | 50 | Male | 30 | Coronary artery disease | 5500 |
| Control | 50 | Male | 40 | Haemopericardium | 9000 |
| Control | 43 | Male | 66 | Aspiration pneumonia | 13000 |
| Control | 46 | Male | 29 | Acute myocardial infarction | 17300 |
| Control | 24 | Male | 43 | Undetermined (but consistent with idiopathic cardiac arrhythmia) | 22000 |
| Control | 48 | Male | 24 | Ischemic heart disease | 59000 |
| Control | 44 | Male | 50 | Ischemic heart disease | 69000 |
| Control | 53 | Male | 16 | Dilated cardiomyopathy | 102000 |
|
| |||||
| Carbon monoxide intoxication/Alcohol | |||||
| Alcoholic | 43.5 | Male | 43.5 | Intoxication | 506000 |
| Alcoholic | 44 | Male | 15 | Ischemic heart disease | 639000 |
| Alcoholic | 42 | Male | 41 | Combined bromoxynil and alcohol toxicity | 1052000 |
| Alcoholic | 49 | Male | 44 | Ischemic heart disease | 1181000 |
| Alcoholic | 45 | Male | 7.5 | Drowning | 1271000 |
| Alcoholic | 49 | Male | 16 | Coronary artery thrombosis | 1278000 |
| Alcoholic | 51 | Male | 27 | Gastrointestinal hemorrhage | 1863000 |
| Alcoholic | 50 | Male | 17 | Ischaemic heart disease | 1958000 |
| Alcoholic | 61 | Male | 23.5 | Atherosclerotic cardiovascular disease | 3158000 |
| Alcoholic | 61 | Male | 59 | Myocarditis | 5811000 |
Brains were collected by the New South Wales Tissue Resource Center brain donor program at the University of Sydney. Alcohol consumption rates varied with time, such that the reporting of alcohol consumption also varied. The alcohol consumption rate was recorded in grams of ethanol/day, and calculated from the reported number of standard drinks consumed per day. A range of maximum and minimum consumption rates was obtained from hospital records and questionnaires to family members. A mean of the maximum and minimum rates was used for classification (alcoholics consumed > 80 g/day, controls consumed little or no alcohol). The age of commencement of drinking was assumed to be 25 years of age in all cases, except where other ages were recorded. The 25 year age minimum allowed for increasing consumption at earlier ages until a regular drinking pattern was established. Any period(s) of known abstinence was subtracted from the potential drinking years. Only recorded periods of abstinence greater than six months were considered in our calculations. The duration of alcohol consumption was calculated for each case. Lifetime alcohol consumption (expressed in kilograms of 100% alcohol) was calculated by multiplying the duration and the mean consumption rate.
Real-time PCR analysis
Total RNA was extracted from mouse brain or rat brain slice culture. Real-time PCR was performed as described previously [27] and is in supplemental methods and materials section. The primer sequences used in this study were as follows:
Immunohistochemistry
Mouse brains were fixed with 4% paraformaldehyde in phosphate buffered saline (PBS) and processed for immunostaining as described previously [27]. Table 3 describes the antibodies used for immunostaining of TLR2, TLR3, TLR4 and HMGB1. Immunolabeling was visualized by using nickel-enhanced 3,3′-diaminobenzidine (DAB).
Table 3.
Summary of antibodies used in the present study
| Antibody | Manufacturer | Host | Species detected | Dilution | Application |
|---|---|---|---|---|---|
| TLR2 (M-15) | Santa Cruz | Goat | Mouse | 1:200 | IHC |
| TLR2 (N17) | Santa Cruz | Goat | Human | 1:200 | IHC/WB |
| TLR3 (N-14) | Santa Cruz | Goat | Mouse, Human | 1:200 | IHC |
| TLR3 | Abcam | Rabbit | Human | 1:500 | WB |
| TLR4 | Abcam | Rabbit | Mouse, Human | 1:500 | IHC |
| TLR4 (C-18) | Santa Cruz | Goat | Human | 1:200 | WB |
| HMGB-1 (K-12) | Abcam | Rabbit | Rat, Human | 1:400 | IHC/DFL/WB |
| HMGB-1 | Santa Cruz | Goat | Mouse, Human | 1:200 | IHC |
| HMGB-1 | Abcam | Rabbit | Human | 1:500 | WB |
| β-actin | Cell Signaling Inc. | Rabbit | Human | 1:500 | WB |
| NeuN | Chemicon | Mouse | Rat, Human | 1:100 | DFL |
IHC, immunohistochemistry; DFL, double fluorescent staining; WB, Western blot
Stereological cell counts
Immunoreactive (+IR) cells were quantified using unbiased stereological cell counts in six random regions of interest from each brain sample and were counted at 40X. Cell density (Nv) was determined following the optical dissector method [28, 29], as described previously [8, 27]. More details on the cell counting methods are described in the supplement.
HMGB1 ELISA
At the conclusion of ethanol treatment, HEC slices were removed for real-time PCR or immunohistochemistry and culture media were used for ELISA detection of released HMGB1. HMGB1 ELISA was performed according to the manufacturer’s protocol of ELISA kits (IBL, Germany).
TLR4 or HMGB1 knockdown with siRNA
Rat TLR4 or HMGB1 siRNA (Ambion, Grand Island, NY) was used to knockdown TLR4 or HMGB1 expression. Preparation of transfection reagents and transfection was performed as previously described [14]. Briefly, the transfection mixture was added to serum-free N2 medium at a final concentration of 20 nM siRNA + 6 μl Lipofectamine 2000 to a total volume of 1.2 ml (500 μl on top of slices and 700 μl at bottom of the culture). Vehicle controls were treated with the same N2 medium containing negative control siRNA (Ambion, Grand Island, NY). After 24 hr transfection, siRNA-containing medium was replaced with regular serum-free N2-supplemented medium and the slices were cultured for 4 days in the absence or presence of ethanol (100 mM). At the end of experiments, the slices were removed for real-time PCR analysis.
Western blotting
For human Western blots, 30 mg of frozen OFC tissue was homogenized in 0.2 ml RIPA lysis buffer with protease inhibitor (1ml/10ml buffer) and centrifuged at 14000 rpm at 4°C for 1 hr. Protein in the supernatant was measured using Pierce BCA protein assay kit (Thermo Scientific, Rockford, IL) and 25 μg of protein from each denatured sample was subjected to SDS-PAGE on Bio-Rad Precast polyacrylamide mini-gel (4–15%) and blotted onto immunoblot PVDF membrane. Antibodies were used to identify TLR2, TLR3, TLR4, and HMGB1 bands (table 3). Bands were scanned with Odyssey Infrared imager from LI-COR Bioscience (Lincoln, NE). Band intensity was quantitated using Bioquant Imaging software and normalized to β-actin band intensity. To support antibody specificity, TLR2, TLR3, TLR4 and HMGB1 antibody preabsorption was performed using 0.5 μg/ml of the corresponding blocking peptides.
Western blot analysis of HEC slices used lysis buffer (10 mM HEPES, 1.5 mM MgCl2, 10 mM KCl, pH 7.9) plus protease inhibitor cocktail (Sigma) for 15 min and then disrupted with sonication (3 time, 2 min apart on ice). After centrifugation, the supernatant were collected, and protein determined using a Bio-Rad Bradford reagent kit. For Western blotting, an equal amount of protein (50 μg) or culture medium (40 μl) was mixed with 10/μl 5× loading buffer, and separated using a 4–15% Tris mini-gel (Bio-Rad) and transferred onto PVDF membrane. After blocking with LI-COR blocking buffer overnight, the membrane was probed with anti-HMGB1 antibody (1:400, Abcam) at 4°C overnight. After washing, membrane was incubated with second antibodies coupled with fluorescence from LI-COR Bioscience (Lincoln, NE).
Statistical analysis
The data are expressed as mean ± SEM and statistical significance was assessed with a ANOVA followed by Bonferroni’s t-test using the StatView program (Abacus Concepts, Berkeley, CA). A value of p < 0.05 was considered statistically significant. The association between HMGB1, TLR, and lifetime alcohol consumption was analyzed with the Pearson product-moment correlation coefficient (Pearson correlations (r)) to determine meaningful relationships.
Results
Increased expression of danger signaling HMGB1 and TLR2, TLR3, and TLR4 in ethanol-treated mouse brain
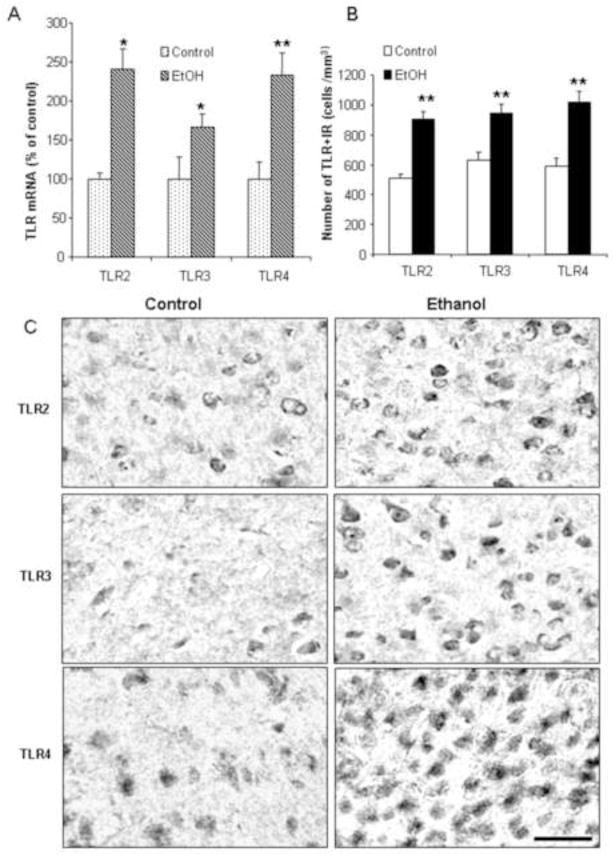
To investigate the effects of chronic binge-like ethanol treatment on danger signal expression, C57BL/6 mice were treated for 10 days with a binge drinking dose of ethanol. Ethanol treatment of mice increased brain TLR2 mRNA 241±26% (p<0.05), TLR3 mRNA 167±17% (p<0.05), and TLR4 mRNA 233±29% (p<0.01) (Figure 1). To determine protein expression in orbital frontal cortex (OFC) and entorhinal cortex (ENT), we determined immunoreactivity (+IR) for each of these proteins. The controls in both OFC (Figure 1C) and ENT (images not shown) showed low levels of diffuse staining of small punctate spots with a few cellular silhouettes for all three TLRs. Ethanol treatment increased staining, particularly cellular staining (Figure 1C). In OFC, ethanol treatment increased TLR2+IR cells 176±10% (p<0.01), TLR3+IR cells 151±10% (p<0.01) and TLR4+IR cells 174±12% (p<0.01). In ENT, ethanol treatment increased TLR2+IR cells 165±9% (p<0.01), increased TLR3+IR cells 183±9% (p<0.01), and increased TLR4+IR cells 221±13% (p<0.01). Increases in TLR2, TLR3, and TLR4 mRNA, and cell staining +IR are consistent with ethanol treatment of mice inducing expression of all three TLRs in cortex.
Figure 1. Chronic ethanol treatment of C57BL/6 mice increases mRNA and protein expression (+IR) of TLR2, TLR3, and TLR4.
(A) Levels of brain TLR2, TLR3, and TLR4 mRNA were measured by real-time PCR as described in the methods. Ethanol treatment significantly increased brain mRNA for TLR2, TLR3, and TLR4. (B) Immunoreactive (+IR) cells were quantified using unbiased stereological cell counts as described in the methods and supplement. TLR2, TLR3, and TLR4+IR cells were counted in mouse orbital frontal cortex (OFC). Ethanol significantly increased the number of TLR2, TLR3, and TLR4+IR positive cells. (C) Representative images of immunohistochemical staining for TLR2, TLR3 and TLR4 in the OFC region of control and ethanol-treated mice. Note staining for TLR in ethanol treated animal labels membranes of fairly large cells as well as smaller cells. * p < 0.05, ** p < 0.01, compared with water control group. Scale bar=50μm.
HMGB1 is a commonly expressed cellular protein [4]. In mice treated with chronic ethanol, brain HMGB1 mRNA increased to 219±20% (p<0.01) of control brain mRNA (Figure 3A). Immunohistochemical staining of protein +IR found OFC increased 181±9% (p<0.01) and ENT 195±5% (p<0.01) (Figure 3B). In controls, there were at least two types of cellular staining: (1) large cells with strong membrane staining silhouettes, and (2) smaller cells with more homogenous staining. Both forms of cellular staining appeared to increase with ethanol treatment. Thus, chronic ethanol treatment of mice increases brain HMGB1 mRNA and the number of HMGB1+IR cells in OFC and ENT. These findings suggest that chronic ethanol treatment of mice increases danger signaling agonist HMGB1 as well as danger signaling receptors TLR2, TLR3, and TLR4 in cortex.
Figure 3. Ethanol increases HMGB1 expression in mouse brain.
Chronic ethanol treatment of C57BL/6 mice (5 g/kg, i.g., daily for 10 days) increased mRNA and protein expression (+IR) of brain HMGB1. (A) HMGB1 gene expression was measured by real-time PCR. Chronic ethanol increased brain HMGB1 mRNA by approximately 2-fold. (B) Quantitative evaluation of HMGB1+IR in the OFC and ENT of control and ethanol-treated mice. Immunoreactive cells were quantified using unbiased stereological cell counts as described in the methods and supplement. The number of HMGB1+IR cells was increased 1.8 fold in OFC and 2-fold in ENT. (C) Representative images of immunostaining for HMGB1 in the OFC and ENT regions of water and ethanol-treated mice. ** p < 0.01, compared with water control group. Scale bar=50μm.
Increased expression of danger signaling HMGB1 and TLR2, TLR3, and TLR4 in post-mortem human alcoholic brain
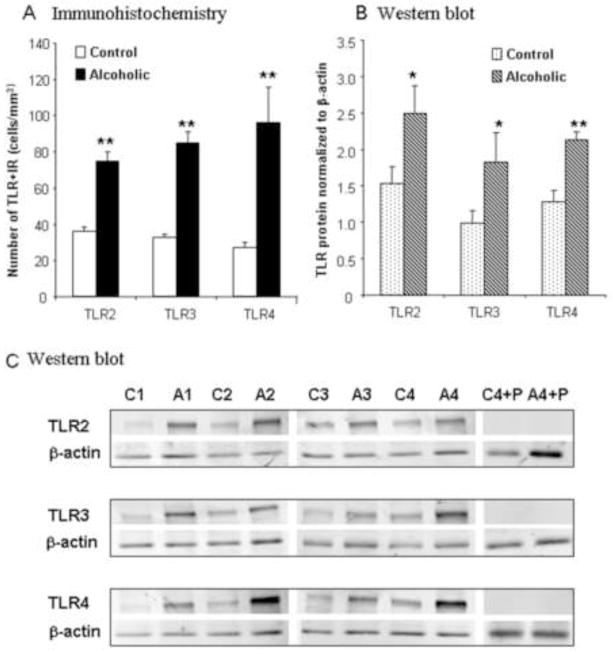
In previous studies, we found that post-mortem human alcoholic brain has increased expression of the proinflammatory chemokine MCP1 and microglial markers [13], increased expression of IL-1β and inflammasome proteins [14] and increased expression of proinflammatory NADPH oxidase (NOX) [15]. We studied post-mortem human OFC to determine if HMGB1/TLR danger signaling molecules were increased in alcoholic brain (Table 1, Figure 4 and 5). Moderate drinking controls showed little TLR2+IR staining. TLR3+IR controls showed a few cells and stained processes, but overall low levels of staining. TLR4+IR in controls showed large and small cellular silhouettes (images not shown). Alcoholic brains showed increased TLR2+IR cells 208±15% (p<0.01), TLR3+IR cells 259±18% (p<0.01), and TLR4+IR cells 356±73% (p<0.01), compared to moderate drinking controls. Western blot analysis of protein expression levels also indicated increased levels of TLR2 (147±14%, p<0.05), TLR3 (180±27, p<0.05) and TLR4 (184±24%, p<0.01) (Figure 4). Thus, both methods of quantification found significant increases in post-mortem human alcoholic OFC. Similar to that found in ethanol-treated mouse brain, post-mortem alcoholic brain OFC showed increased expression of HMGB1+IR cells (213±21%, p<0.05, Figure 5) and Western blot HMGB1 protein (151±18%, p<0.01, Figure 5). Surprisingly, most labeled cells appeared to have neuronal morphology promoting further analysis in alcoholic brain where staining was prominent. Double confocal immunohistochemistry using NeuN to identify neuronal cells indicated that NeuN+IR colocalization with 90±2.4% of TLR2+IR cells, 89.4±1.8% of TLR3+IR cells, 80.6±1.5% of TLR4+IR cells, and 82.7 ± 4.3% of HMGB1+IR cells (Supplemental Figure S1). Increased expression of the cytokine HMGB1, as well as TLR2, TLR3, and TLR4, suggest increased HMGB1/TLR signaling in alcoholic brain.
Figure 4. Increased expression of TLR2, TLR3, and TLR4 in post-mortem human alcoholic brain.
See Table 1 for human subject details. (A) Quantification of TLR2, TLR3, and TLR4+IR cells. Immunoreactive (+IR) cells were quantified using unbiased stereological cell counts as described in the methods and supplement. The OFC of human post-mortem alcoholic brain had significantly more TLR2, TLR3, and TLR4 + IR cells, compared to the OFC of human moderate drinking controls (n=10 per group) (B) Quantification of Western blot analysis relative to β-actin. (n=8 per group). Although IHC and Western blots are from different subregions of OFC and different methodologies, Pearson’s correlation of +IR vs. Western blot intensity was significant across subjects for TLR4 (r=0.61; p<0.05) and TLR3 (r=0.56, p<0.05), but TLR2 did not reach significance. (C) Images of Western blot. C is control and A is alcoholic with the # being individual patient. (C4+P = control patient 4 with peptide preabsorption; A4+P = alcoholic patient 4 with peptide preabsorption) Note that peptide preabsorption blocks antibody supporting specificity of binding. Alcoholic patients have more TLR2, TLR3, and TLR4 protein expression.
Figure 5. HMGB1 expression is increased in the post-mortem human alcoholic brain.
(A) Quantification of HMGB1 immunoreactivity. Immunoreactive (+IR) cells were quantified using unbiased stereological cell counts as described in the methods and supplement. The number of HMGB1+IR cells was significantly increased in alcoholic brain (n=10), compared to human moderate drinking controls. (B) Quantification of Western blot analysis relative to β-actin. (n=8 per group). * p < 0.05,** p < 0.01, relative to moderate drinking controls. (C) Images of Western blot. C is control and A is alcoholic with the # being individual patient. (C4+P = control patient 4 with peptide preabsorption; A4+P = alcoholic patient 4 with peptide preabsorption) Note that peptide preabsorption blocks antibody supporting specificity of binding. Alcoholic patients have more HMGB1 protein expression.
Our findings of increased HMGB1, TLR2, TLR3, and TLR4 in chronic ethanol-treated mouse brain as well as human alcoholic brain prompted a comparison of human lifetime alcohol consumption with expression of HMGB1/TLR danger signaling molecules. HMGB1, TLR2, TLR3, and TLR4 all showed significant correlations with lifetime alcohol consumption (Table 4, Figure 6). Moderate drinking control subjects consumed much less lifetime alcohol with the heaviest drinking control consuming approximately 100 kg of alcohol. Alcoholics in our study consumed between 500 and 5,000 kg of alcohol providing a broad range of consumption that shows a progressive increase in HMGB1 expression with increased lifetime drinking (Figure 6). HMGB1 and TLR3 showed the strongest correlations with lifetime alcohol consumption, although TLR2 and TLR4 were significantly correlated. The correlation of HMGB1 as well as TLR2, TLR3, and TLR4 with lifetime ethanol consumption is consistent with ethanol induction of these proteins in brain.
Table 4.
Correlations of lifetime alcohol consumption and danger signals HMGB1, and TLR2, TLR3, and TLR4 in post-mortem human OFC.
| DANGER SIGNAL | Lifetime Alcohol Consumption (kg) | TLR2 | TLR3 | TLR4 |
|---|---|---|---|---|
| TLR2 | 0.66 p=0.002 |
---- | ---- | ---- |
| TLR3 | 0.83 p<0.001 |
0.89 p<0.0001 |
---- | ---- |
| TLR4 | 0.62 p<0.005 |
0.90 p<0.0001 |
0.85 p<0.0001 |
---- |
| HMGB1 | 0.83 p<0.001 |
0.83 p<0.0001 |
0.94 p<0.0001 |
0.80 p<0.0001 |
Lifetime alcohol consumption of moderate drinking controls and alcoholics is shown in Table 1. Correlations between lifetime alcohol consumption with HMGB1, TLR2, TLR3, and TLR4 +IR cells within the orbital frontal cortex. Drinking history from family interviews and a follow back technique was used to assess drinks per year and years drinking. Lifetime alcohol consumption (expressed in kilograms of 100% alcohol) was calculated by multiplying the duration and the mean consumption rate (See methods for more details). Correlations were determined using Pearson Correlation coefficients and 2 tailed significance. The correlation coefficient is above the p value in each applicable table cell.
Figure 6. HMGB1+IR cells in OFC correlate with lifetime alcohol consumption.
Shown are individual HMGB1+IR cell counts vs. lifetime alcohol consumption. Note that control subjects are clustered along the y axis due to low lifetime alcohol consumption values and similar levels of HMGB1+IR. Alcoholic subjects show several fold variation in HMGB1+IR and over 10 fold variation in lifetime alcohol consumption. TLR2, TLR3, and TLR4+IR also correlate with lifetime alcohol consumption (Table 4). The derived straight line is shown (r = 0.82; p<0.001). In previous studies, we have shown that neuroimmune activation persists for long periods once activated [8, 9]. The persistence of neuroimmune activation likely contributes to progressive amplification of HMGB1/TLR induction with repeated drinking that leads to cumulative increases [10] that result in correlations with lifetime alcohol consumption.
HMGB1 and TLRs are required for ethanol induction of proinflammatory interleukin-1β (IL-1β)
To determine if the increased in expression of HMGB1/TLR danger signaling proteins actually increased neuroimmune gene expression, we assessed ethanol effects on danger signaling in slice culture. We have previously found that ethanol treatment of brain slice culture increases NF-κB-DNA binding and transcription of multiple neuroimmune cytokines, oxidases, and proteases [11, 30]. We first determined whether ethanol treatment induced HMGB1 in rat brain slice cultures. Ethanol treatment increased HMGB1 mRNA about 4.5-fold (Figure 7). We had limited hippocampal sections from human post-mortem brain, but staining of individual samples from moderate drinking controls and alcoholics suggest similar increases in HMGB1+IR in hippocampus (Figure 7) to those found in cortex (Figure 5). Ethanol-treated rat brain slice culture showed that expression of HMGB1 colocalizes with neurons (NeuN+IR) using confocal microscopy (i.e. red-HMGB1 and green-NeuN coincide forming yellow, Figure 7). Thus, ethanol treatment of slice culture increases HMGB1 mRNA that histochemically appears to be in neurons.
Figure 7. Ethanol induction of HMGB1 in rat brain slice culture.
Brain slice culture was prepared and used after 2 weeks maturation in culture. Control or ethanol (100mM) treated slices were collected following treatment and assessed for protein or mRNA. (A) HMGB1 mRNA expression determined by real-time PCR as described in the methods. Note ethanol increased HMGB1 mRNA about 4.5 fold (p<0.05). (B-top images) Immunohistochemical double labeling of brain slice culture of control (left) and ethanol (ETOH-100mM, right) treated slices for HMGB1 (red) and neuronal marker NeuN (green). Note ethanol treatment of brain slices culture increases HMGB1+IR (red). Green is the neuronal markers NeuN that combined with HMGB1 should appear yellow (red-green merged) that represent HMGB1 expressing neurons (upper right image). (C – bottom images) Immunohistochemistry of human hippocampus. A human moderate drinking control (bottom-left) and alcoholic (bottom-right) brain section after immunohistochemistry for HMGB1 (red) and neuronal marker NeuN (green). Note alcoholics show increased HMGB1 (red) that is almost entirely expressed within NeuN (green) expressing neurons such that all appear yellow. Arrows note HMGB1+IR in the cytoplasm consistent with cellular release of nuclear HMGB1.
Our previous studies found that ethanol-induced neuroimmune activation is amplified by signals that converge upon NF-κB transcription of proinflammatory genes that form positive loops of activation [6] increasing transcription of multiple cytokines, cytokine receptors and TLRs, oxidases and proteases [8, 11, 15, 30]. To investigate the role of HMGB1/TLR signaling, we followed IL-1β which we have previously found to be induced by ethanol in brain slice culture and in mice treated with ethanol [11, 14]. Ethanol increased HMGB1 protein within the brain slices as well as the media as determined using Western blots and ELISA (Figure 8). Ethanol treatment of brain slice culture also increased expression of IL-1β mRNA, similar to previous studies [11]. Interestingly, ethanol increased IL-1β expression was blocked by the addition of a neutralizing HMGB1 antibody (Figure 8). Neutralizing antibody binds HMGB1 preventing its interaction with receptors suggesting that ethanol released HMGB1 contributes to ethanol induction of IL-1β. We used small inhibitory mRNA to knockdown expression of HMGB1 or TLR4, a key TLR in HMGB1 proinflammatory responses. We found that knockdown of HMGB1 or TLR4 blocked ethanol induction of IL-1β. These findings suggest that ethanol releases HMGB1 that stimulates TLR4 leading to induction of IL-1β.
Figure 8. Ethanol releases HMGB1 into media and activates IL-1β synthesis through TLR4.
(A) Increased HMGB1 protein level in ethanol-treated HEC slice culture cell lysate and medium. Left: HMGB1 released into culture medium, as assessed by ELISA, was increased by approximately 3-fold in ethanol group (100 mM, 4 days). *p<0.001 compared with control; n=4. This experiment was replicated 5 times and produced similar results. Right: Representative Western blots of HMGB1 protein from HEC brain slice culture cell lysates and medium. As depicted in right immunoblot, ethanol exposure increased HMGB1 protein in both cell lysates and release into the culture medium. (B) Ethanol induction of IL-1β is blocked by siRNA and neutralizing antibodies. Left: mRNA levels of the proinflammatory cytokine IL-1β, relative to negative siRNA-treated controls. Ethanol induction of IL-1β was blocked by siRNAs that knockdown either TLR4 or HMGB1 (*p<0.001 compared with control, n=4). Shown on the right are mean ± SEM of proinflammatory cytokine IL-1β mRNA level, relative to control that was treated with control antibody chicken IgY (10 μg/ml) for HMGB1 neutralization (IBL, Germany). Ethanol induction of IL-1β mRNA is blocked by HMGB1 neutralizing antibody (*p<0.0001 compared to Control; # p<0.0001 compared with EtOH).
Coincident induction of danger signaling molecules by ethanol
Our previous studies have found that ethanol induces brain neuroinflammatory responses through increased NF-κB transcription of proinflammatory genes including TLRs, cytokines such as TNFα, IL-6, IL-1β, and the chemokine MCP1, as well as oxidases COX and NOX, and other innate immune genes that contribute to persistence and amplification of brain neuroimmune responses [6, 10]. The finding that all of the HMGB1/TLR danger signaling molecules investigated in this study were increased by ethanol and correlated with lifetime ethanol consumption prompted assessment of correlations among the four HMGB1/TLR danger signaling molecules. Interestingly, HMGB1+IR cells in post-mortem human brain were correlated with TLR2, TLR3, and TLR4+IR cell expression (r=0.85, p<0.0001; Figure 9) and to each other (Table 4). The correlated increases in HMGB1 expression across all 3 TLRs is consistent with other findings of proinflammatory responses involving overlapping amplification of signals that contribute to persistent neuroimmune signaling [6]. Our finding that TLR2, TLR3, and TLR4 all correlate with increased HMGB1 and each other suggest that all are upregulated together as neuroimmune activation is amplified across cells and innate immune genes. This is consistent with ethanol exposure increasing neuroimmune activation through positive loops of innate immune gene induction that amplify multiple HMGB1/TLR danger signals and other neuroimmune genes in parallel (Figure 10).
Figure 9. HMGB1+IR cells in OFC correlate with TLR2, TLR3 and TLR4+IR cells.
Shown are individual subjects HMGB1+IR cells vs. TLR2, TLR3 or TLR4+IR cells. Across subjects, HMGB1 correlated with TLR expression (r = 0.85; p<0.0001). Note that HMGB1+IR varies several fold across subjects. Each subject’s TLR levels (e.g., TLR2, TLR3, and TLR4 arrange in vertical points over each subject’s HMGB1 level. Although all correlate with HMGB1, the TLRs showing the greatest induction varies across subjects. For example, on the right are 3 subjects with the highest HMGB1 expression (alcoholics), two showing TLR3>TLR4≥TLR2 and one with TLR4>TLR3>TLR2. Thus, among the TLRs examined, all are increased without any apparent priority once activation has occurred. The correlation of HMGB1 with TLR is consistent with neuroimmune loops of amplification coordinating induction of all TLR. Activation of innate immune proinflammatory signaling through multiple pathways is known to converge on signals leading to transcription of multiple TLRs, proinflammatory cytokines, and their receptors that spread across cells through paracrine and autocrine activation of proinflammatory signaling. The parallel induction of TLR is a well-known mechanism of amplification of innate immune signals. Our discovery of primarily neuronal localization of these signaling molecules suggest that neuroimmune signaling amplification of multiple TLRs also occurs among neurons in brain and is not unique to monocytes or microglia.
Figure 10. Schematic of ethanol-induced release of HMGB1 and activation of TLR4 on neurons and microglia IL-1β creating positive loops of amplification of innate immune cascades.

Ethanol (alcohol) is shown releasing HMGB1 from the neuronal nucleus that stimulates TLR4 on glia and neurons. Microglia contribute to the initiation of neuroimmune cascades [59], and under basal conditions, are the only brain cells that express TLR4 in mice [37, 39]. TLR4 are critical for HMGB1 activation of innate immune cells [38], although the effect on neurons is poorly understood. Ethanol activation of innate immune gene induction is blocked by minocycline, an inhibitor of microglial activation [15], inhibitors of NF-κB [10], inhibitors of IL-1β [14], naltrexone [15], and in microglial culture for transgenic mice lacking TLR4 [40], as well as in ethanol-treated mouse brain of transgenic mice lacking TLR4 [16]. We found HMGB1 is expressed primarily in neurons, consistent with studies finding that neuronal hyperexcitability releases HMGB1 [46]. HMGB1/TLR4 activation releases IL-1β, which activates its own receptors linked to proinflammatory transcription cascades as well as releasing additional HMGB1 contributing to positive loops of amplification of innate immune gene induction. In previous studies, we found ethanol-induced NF-κB transcription of IL-1β, TNFα, MCP1, and IL-6 as well as oxidases, proteases, and other innate immune genes [11]. Illustrated are our findings reported here that ethanol releases HMGB1, and neutralizing antibody to HMGB1 and siRNA knockdown of HMGB1 block ethanol induction of IL-1β. Also, TLR4 siRNA knockdown blocked ethanol induction of IL-1β. This schematic illustrates ethanol initiation of positive loops of activation between neurons and microglia. Astrocytes are activated as well and contribute to altered neurotransmission although not illustrated for simplicity. Neuroimmune activation increases TLR expression, which is primarily neuronal. HMGB1 is an agonist at multiple TLRs and other brain neuroimmune receptors such as the receptor for glycation signals (RAGE) [60] and (MAC-1) [61]. We found that chronic ethanol and post-mortem human alcoholic brain had increased levels of TLR2, TLR3, and TLR4 as well as HMGB1 consistent with chronic neuroimmune activation that includes astrocytes, microglia, and neurons. Activation of microglia increases neuronal excitatory synaptic potentials [62] as well as initiating glial glutamate release [63] and neuronal extrasynaptic NMDA 2B receptors are sensitized by HMGB1/TLR4 increased phosphorylation [46]. Further, cytokines are known to inhibit glial glutamate transporters [22]. These findings support the schematic illustration of increased HMGB1/TLR signaling leading to hyper-excitability due to increased glutamate from glia and sensitized neuronal glutamate receptors. These findings support hypotheses linking alcohol dependence to a hyperglutamate state [64, 65].
Discussion
We report here that chronic ethanol treatment of mice increases HMGB1 mRNA and cellular expression of protein in OFC (i.e., HMGB1+IR). We also found HMGB1+IR increased in the human post-mortem OFC. HMGB1 is a common nuclear protein that is released by active and passive processes and has cytokine-like properties expressed through activation of IL-1/TLRs that stimulate NF-κB transcription of innate immune genes [2, 4]. Using cultured brain slices, we found that ethanol increased HMGB1 in medium, consistent with ethanol both inducing and releasing HMGB1. We have previously found that chronic ethanol treatment increases expression of brain NF-κB proinflammatory target genes including COX [31], NOX [15], multiple cytokines, proteases, and oxidases [8, 11, 15] as well as increasing NF-κB-DNA binding in vivo [10] and in brain slice culture [30]. Although HMGB1 can bind to and activate multiple TLRs, signaling is complex due to neuroimmune amplification within and across cells that release additional HMGB1, other cytokines, and molecules that contribute to the response [2, 4]. In previous studies, we found ethanol pretreatment of mice potentiated TLR3 agonist Poly I:C [32] and TLR4 agonist LPS [8] induced proinflammatory gene induction. Although HMGB1 can activate multiple receptors and HMGB1 injected into brain causes cognitive deficits through multiple receptors [33], proinflammatory activation of innate immune cells by HMGB1 requires TLR4 [34, 35]. Many studies have suggested that brain neuroimmune activation requires microglia activation through TLR4 [36, 37]. Microglial TLR4 is also critical for activation of astrocyte proinflammatory responses [38] perhaps due to basal expression of TLR4 on microglia, but not other brain cells [39]. Ethanol activation of NF-κB proinflammatory transcription in isolated microglia [40] and mouse brain [16] is dependent upon TLR4. Since microglia are known to express high basal levels of TLR4 and to rapidly release IL-1β in response to danger signals [4, 41], it is possible that the pivotal role of TLR4 for ethanol responses is due to the critical role of TLR4 in HMGB1 microglial activation and the resulting amplification of responses by astrocytes and neurons (Figure 10). Although HMGB1 signaling is complex, our findings are consistent with ethanol releasing HMGB1 that activates TLR4 receptors that increase NF-κB transcription of IL-1β and other proinflammatory genes that lead to neuroimmune activation (Figure 10).
We found increased expression of HMGB1, TLR2, TLR3, and TLR4 in OFC of human post-mortem alcoholic brain and chronic ethanol-treated mouse brain. Similar to other reports, we found low levels of TLR expression in non-diseased human brain [42]. Other human postmortem studies report low levels of expression in healthy brain and broad brain regional increases in the expression of multiple TLRs across various brain diseases [42]. Proinflammatory gene induction is linked to neurodegeneration and the brains of transgenic mice lacking TLRs are protected against brain insults including ethanol [16, 43–45]. For instance, in models of stroke, mice lacking TLR2 or TLR4 have reduced infarct size and neuroimmune activation [43, 44], whereas in normal mice stroke models increase brain TLR2 and TLR4 expression [34, 44] and brain injection of HMGB1 increases infarct size [34]. Increased HMGB1 expression has been reported in hippocampal tissue from patients with temporal lobe epilepsy [46]. These studies found that glutamate hyperexcitability increases release of neuronal HMGB1 that activates TLR4 receptors releasing the proconvulsant IL-1β, increasing seizure severity, and a long-lasting decrease in seizure threshold contributing to persistent risk of additional seizures [4, 41, 46]. Chronic alcohol treatment is also known to reduce seizure threshold, perhaps due to HMGB1/TLR induction. Similarly, neuroimmune microglial activation is associated with increased spinal cord hyperexcitability in models of chronic pain [47] that also finds persistent endogenous release of HMGB1 associated with the hyperexcitability [48]. Chronic ethanol also impacts chronic pain [49]. We studied human and mouse frontal cortex, the brain region most associated with alcoholic neurodegeneration [24, 50] and found increased expression of HMGB1/TLRs. We have previously established ethanol induced neuroimmune activation is a key component of neurodegeneration associated with ethanol exposure [6, 12]. Our studies of post-mortem human alcoholic brain find increased microglial markers and proinflammatory MCP-1 in multiple brain regions [13], increased IL-1β and inflammasome proteins [14], and increased NADPH oxidase gp91, an innate immune oxidase linked to neurodegeneration [15]. In addition to neurodegeneration, HMGB1/TLR signaling links neuroimmune activation to components of addiction [6, 51]. Genetic studies have identified NF-κB and proinflammatory genes as contributing to a genetic preference for drinking alcohol [52] and human genetic studies have identified alleles of NF-κB1, a gene encoding a subunit of NF-κB, are associated with increased risk of alcoholism [53] and other studies have linked innate immune genes to alcoholism [54]. Transgenic animals lacking innate immune genes show reduced alcohol drinking [6, 19] and altered acute ethanol motor and sedative responses [18]. Further, endotoxin produces persistent neuroimmune activation [9] and increased ethanol drinking in mice [1, 19]. Lever pressing for ethanol by ethanol-dependent rats is reduced by viral vector siRNA knockdown of TLR4 in brain [3, 17] and naltrexone, a drug that reduces alcohol drinking in rodents and is used to treat alcoholism, that also blunts ethanol induced neuroimmune activation [15], likely through a recently discovered antagonist action on TLR4 receptors [55]. These findings suggest increased HMGB1/TLR signaling contributes to alcoholic pathology.
We found that HMGB1+IR cells in OFC correlated with lifetime alcohol consumption and with TLR2, TLR3, and TLR4+IR cells. Alcoholic subject’s lifetime alcohol consumption varied by over 10 fold and was far greater than the highest control. The correlation of HMGB1/TLR expression with lifetime alcohol consumption is consistent with alcohol induction of persistent neuroimmune activation. Neuroimmune activation by ethanol [8] or endotoxin [9] persists for long periods. HMGB1 and the TLRs all correlated with each other consistent with proinflammatory amplification of neuroimmune signals through positive loops of activation (Figure 10). Although TLR4 is critical for initiation of microglial activation, amplification through positive loops likely involves multiple proinflammatory cytokines and HMGB1/TLR danger signals that are upregulated in multiple brain cell types as neuroimmune activation spreads across the brain. Neuroimmune activation is associated with drug reward and addiction [6, 51, 56], depression-negative affect [57] and neurodegeneration [45, 58] consistent with HMGB1/TLR signaling contributing to the alcoholic pathologies.
Supplementary Material
Figure 2. Chronic ethanol treatment of C57BL/6 mice (5 g/kg, i.g., daily for 10 days) increases TLR2, TLR3, and TLR4+IR cells in entorhinal cortex.

Immunoreactive (+IR) cells were quantified in the entorhinal cortex (ENT) using unbiased stereological cell counts as described in the methods and supplement. Ethanol increased the number of TLR2, TLR3 and TLR4 +IR cells. ** p < 0.01, compared with water control group.
Table 2.
Real-time PCR primers
| Primer pairs | |
|---|---|
| Mouse TLR2 | 5′-TGC TTT CCT GCT GAA GAT TT-3′ 5′-TGT ACC GCA ACA GCT TCA GG-3′ |
| Mouse TLR3 | 5′-TTG TCT TCT GCA CGA ACC TG-3′ 5′-GGC AAC GCA AGG ATT TTA TT-3′ |
| Mouse TLR4 | 5′-ACC TGG CTG GTT TAC ACG TC-3′ 5′-GTG CCA GAG ACA TTG CAG AA-3′ |
| Mouse HMGB-1 | 5′-CCA TTG GTG ATG TTG CAA AG-3′ 5′-CTT TTT CGC TGC ATC AGG TT-3′ |
| Mouse β-actin | 5′-GTA TGA CTC CAC TCA CGG CAA A-3′ 5′-GGT CTC GCT CCT GGA AGA TG-3′ |
| Rat HMGB1 | 5′-ATGGGCAAAGGAGATCCTA-3′ 5′-ATTCTCATCATCTCTTCT-3′ |
| Rat IL-1β | 5′-GAAACAGCAATGGTCGGGAC-3′ 5′-AAGACACGGGTTCCATGGTG-3′ |
| Rat β-actin | 5′-CTACAATGAGCTGCGTGTGGC-3′ 5′-CAGGTCCAGACGCAGGATGGC-3′ |
Acknowledgments
This work was supported in part by the National Institutes of Health, National Institute on Alcoholism and Alcohol Abuse [AA020023, AA020024, AA020022, AA019767, AA11605 and AA007573] and the Bowles Center for Alcohol Studies. Tissues were received from the New South Wales Tissue Resource Centre at the University of Sydney supported by the National Health and Medical Research Council of Australia, Schizophrenia Research Institute and the National Institute of Alcohol Abuse and Alcoholism [AA012725]. The authors thank Diana Lotito for help with the preparation of the manuscript.
Footnotes
Financial Disclosures:
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Trendelenburg G. Acute neurodegeneration and the inflammasome: central processor for danger signals and the inflammatory response? Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2008;28:867–881. doi: 10.1038/sj.jcbfm.9600609. [DOI] [PubMed] [Google Scholar]
- 2.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annual review of immunology. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 3.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. Journal of leukocyte biology. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 4.Vezzani A, Maroso M, Balosso S, Sanchez MA, Bartfai T. IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain, behavior, and immunity. 2011;25:1281–1289. doi: 10.1016/j.bbi.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P. Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochim Biophys Acta. 2010;1805:53–71. doi: 10.1016/j.bbcan.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Crews FT, Zou J, Qin L. Induction of innate immune genes in brain create the neurobiology of addiction. Brain, behavior, and immunity. 2011;25 (Suppl 1):S4–S12. doi: 10.1016/j.bbi.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signalling. Mediators of inflammation. 2010 doi: 10.1155/2010/672395. [DOI] [PMC free article] [PubMed]
- 8.Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. Journal of neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crews F, Nixon K, Kim D, Joseph J, Shukitt-Hale B, Qin L, Zou J. BHT blocks NF-kappaB activation and ethanol-induced brain damage. Alcoholism, clinical and experimental research. 2006;30:1938–1949. doi: 10.1111/j.1530-0277.2006.00239.x. [DOI] [PubMed] [Google Scholar]
- 11.Zou J, Crews F. Induction of innate immune gene expression cascades in brain slice cultures by ethanol: key role of NF-kappaB and proinflammatory cytokines. Alcoholism, clinical and experimental research. 2010;34:777–789. doi: 10.1111/j.1530-0277.2010.01150.x. [DOI] [PubMed] [Google Scholar]
- 12.Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol and alcoholism. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Experimental neurology. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zou J, Vetreno RP, Crews FT. ATP-P2X7 receptor signaling controls basal and TNFalpha-stimulated glial cell proliferation. Glia. 2012;60:661–673. doi: 10.1002/glia.22302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin L, Crews FT. NADPH oxidase and reactive oxygen species contribute to alcohol-induced microglial activation and neurodegeneration. Journal of neuroinflammation. 2012;9:5. doi: 10.1186/1742-2094-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:8285–8295. doi: 10.1523/JNEUROSCI.0976-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Yang AR, Kelly T, Puche A, Esoga C, June HL, Jr, Elnabawi A, Merchenthaler I, Sieghart W, June HL, Sr, Aurelian L. Binge alcohol drinking is associated with GABAA alpha2-regulated Toll-like receptor 4 (TLR4) expression in the central amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4465–4470. doi: 10.1073/pnas.1019020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Y, Lousberg EL, Moldenhauer LM, Hayball JD, Robertson SA, Coller JK, Watkins LR, Somogyi AA, Hutchinson MR. Attenuation of microglial and IL-1 signaling protects mice from acute alcohol-induced sedation and/or motor impairment. Brain, behavior, and immunity. 2011;25(Suppl 1):S155–164. doi: 10.1016/j.bbi.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Blednov YA, Benavidez JM, Geil C, Perra S, Morikawa H, Harris RA. Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain, behavior, and immunity. 2011;25 (Suppl 1):S92–S105. doi: 10.1016/j.bbi.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blednov YA, Ponomarev I, Geil C, Bergeson S, Koob GF, Harris RA. Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addiction biology. 2012;17:108–120. doi: 10.1111/j.1369-1600.2010.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carson EJ, Pruett SB. Development and characterization of a binge drinking model in mice for evaluation of the immunological effects of ethanol. Alcoholism, clinical and experimental research. 1996;20:132–138. doi: 10.1111/j.1530-0277.1996.tb01055.x. [DOI] [PubMed] [Google Scholar]
- 22.Zou JY, Crews FT. TNF alpha potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: neuroprotection by NF kappa B inhibition. Brain research. 2005;1034:11–24. doi: 10.1016/j.brainres.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Ding J, Eigenbrodt ML, Mosley TH, Jr, Hutchinson RG, Folsom AR, Harris TB, Nieto FJ. Alcohol intake and cerebral abnormalities on magnetic resonance imaging in a community-based population of middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) study. Stroke; a journal of cerebral circulation. 2004;35:16–21. doi: 10.1161/01.STR.0000105929.88691.8E. [DOI] [PubMed] [Google Scholar]
- 24.Harper C. The neuropathology of alcohol-related brain damage. Alcohol and alcoholism. 2009;44:136–140. doi: 10.1093/alcalc/agn102. [DOI] [PubMed] [Google Scholar]
- 25.de Bruin EA, Hulshoff Pol HE, Bijl S, Schnack HG, Fluitman S, Bocker KB, Kenemans JL, Kahn RS, Verbaten MN. Associations between alcohol intake and brain volumes in male and female moderate drinkers. Alcoholism, clinical and experimental research. 2005;29:656–663. doi: 10.1097/01.alc.0000159110.17351.c0. [DOI] [PubMed] [Google Scholar]
- 26.Dedova I, Harding A, Sheedy D, Garrick T, Sundqvist N, Hunt C, Gillies J, Harper CG. The importance of brain banks for molecular neuropathological research: The New South wales tissue resource centre experience. International journal of molecular sciences. 2009;10:366–384. doi: 10.3390/ijms10010366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin L, Liu Y, Wang T, Wei SJ, Block ML, Wilson B, Liu B, Hong JS. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. The Journal of biological chemistry. 2004;279:1415–1421. doi: 10.1074/jbc.M307657200. [DOI] [PubMed] [Google Scholar]
- 28.Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- 29.West MJ, Gundersen HJ. Unbiased stereological estimation of the number of neurons in the human hippocampus. The Journal of comparative neurology. 1990;296:1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- 30.Zou J, Crews F. CREB and NF-kappaB transcription factors regulate sensitivity to excitotoxic and oxidative stress induced neuronal cell death. Cellular and molecular neurobiology. 2006;26:385–405. doi: 10.1007/s10571-006-9045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knapp DJ, Crews FT. Induction of cyclooxygenase-2 in brain during acute and chronic ethanol treatment and ethanol withdrawal. Alcohol Clin Exp Res. 1999;23:633–643. [PubMed] [Google Scholar]
- 32.Qin L, Crews FT. Chronic ethanol increases systemic TLR3 agonist-induced neuroinflammation and neurodegeneration. Journal of neuroinflammation. 2012 doi: 10.1186/1742-2094-9-130. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazarati A, Maroso M, Iori V, Vezzani A, Carli M. High-mobility group box-1 impairs memory in mice through both toll-like receptor 4 and Receptor for Advanced Glycation End Products. Experimental neurology. 2011;232:143–148. doi: 10.1016/j.expneurol.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang QW, Lu FL, Zhou Y, Wang L, Zhong Q, Lin S, Xiang J, Li JC, Fang CQ, Wang JZ. HMBG1 mediates ischemia-reperfusion injury by TRIF-adaptor independent Toll-like receptor 4 signaling. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2011;31:593–605. doi: 10.1038/jcbfm.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, Lu B, Chavan S, Rosas-Ballina M, Al-Abed Y, et al. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11942–11947. doi: 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury. Glia. 2010;58:253–263. doi: 10.1002/glia.20928. [DOI] [PubMed] [Google Scholar]
- 37.Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holm TH, Draeby D, Owens T. Microglia are required for astroglial Toll-like receptor 4 response and for optimal TLR2 and TLR3 response. Glia. 2012;60:630–638. doi: 10.1002/glia.22296. [DOI] [PubMed] [Google Scholar]
- 39.Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett PL, Jensen FE, Rosenberg PA, Volpe JJ, Vartanian T. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:2478–2486. doi: 10.1523/JNEUROSCI.22-07-02478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernandez-Lizarbe S, Pascual M, Guerri C. Critical role of TLR4 response in the activation of microglia induced by ethanol. Journal of immunology. 2009;183:4733–4744. doi: 10.4049/jimmunol.0803590. [DOI] [PubMed] [Google Scholar]
- 41.Maroso M, Balosso S, Ravizza T, Liu J, Bianchi ME, Vezzani A. Interleukin-1 type 1 receptor/Toll-like receptor signalling in epilepsy: the importance of IL-1beta and high-mobility group box 1. Journal of internal medicine. 2011;270:319–326. doi: 10.1111/j.1365-2796.2011.02431.x. [DOI] [PubMed] [Google Scholar]
- 42.Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61:1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- 43.Crack PJ, Bray PJ. Toll-like receptors in the brain and their potential roles in neuropathology. Immunology and cell biology. 2007;85:476–480. doi: 10.1038/sj.icb.7100103. [DOI] [PubMed] [Google Scholar]
- 44.Okun E, Griffioen KJ, Lathia JD, Tang SC, Mattson MP, Arumugam TV. Toll-like receptors in neurodegeneration. Brain research reviews. 2009;59:278–292. doi: 10.1016/j.brainresrev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, Rossetti C, Molteni M, Casalgrandi M, Manfredi AA, et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med. 2010;16:413–419. doi: 10.1038/nm.2127. [DOI] [PubMed] [Google Scholar]
- 47.Graeber MB. Changing face of microglia. Science. 2010;330:783–788. doi: 10.1126/science.1190929. [DOI] [PubMed] [Google Scholar]
- 48.Feldman P, Due MR, Ripsch MS, Khanna R, White FA. The persistent release of HMGB1 contributes to tactile hyperalgesia in a rodent model of neuropathic pain. Journal of neuroinflammation. 2012;9:180. doi: 10.1186/1742-2094-9-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gatch MB. Ethanol withdrawal and hyperalgesia. Current drug abuse reviews. 2009;2:41–50. doi: 10.2174/1874473710902010041. [DOI] [PubMed] [Google Scholar]
- 50.Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacology, biochemistry, and behavior. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coller JK, Hutchinson MR. Implications of central immune signaling caused by drugs of abuse: Mechanisms, mediators and new therapeutic approaches for prediction and treatment of drug dependence. Pharmacology & therapeutics. 2012;134:219–245. doi: 10.1016/j.pharmthera.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 52.Harris RA, Blednov YA. Neuroimmune genes and alcohol drinking behavior. 2012 doi: 10.1111/j.1601-183X.2012.00780.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edenberg HJ, Xuei X, Wetherill LF, Bierut L, Bucholz K, Dick DM, Hesselbrock V, Kuperman S, Porjesz B, Schuckit MA, et al. Association of NFKB1, which encodes a subunit of the transcription factor NF-kappaB, with alcohol dependence. Human molecular genetics. 2008;17:963–970. doi: 10.1093/hmg/ddm368. [DOI] [PubMed] [Google Scholar]
- 54.Crews F. Immune Function Genes, Genetics, and the Neurobiology of Addiction. Alcohol Research & Health The Journal of the National Institute on Alcohol Abuse and Alcoholism. 2011;34:355–361. doi: 10.35946/arcr.v34.3.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hutchinson MR, Shavit Y, Grace PM, Rice KC, Maier SF, Watkins LR. Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacological reviews. 2011;63:772–810. doi: 10.1124/pr.110.004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plane JM, Shen Y, Pleasure DE, Deng W. Prospects for minocycline neuroprotection. Archives of neurology. 2010;67:1442–1448. doi: 10.1001/archneurol.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eyre H, Baune BT. Neuroplastic changes in depression: A role for the immune system. Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 58.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annual review of immunology. 2011;29:139–162. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nature reviews Neuroscience. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 60.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 61.Gao HM, Zhou H, Zhang F, Wilson BC, Kam W, Hong JS. HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:1081–1092. doi: 10.1523/JNEUROSCI.3732-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pascual O, Ben Achour S, Rostaing P, Triller A, Bessis A. Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E197–205. doi: 10.1073/pnas.1111098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nature neuroscience. 2007;10:331–339. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- 64.Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, Qin L, Szabo G, Wheeler M, Zou J. Cytokines and alcohol. Alcoholism, clinical and experimental research. 2006;30:720–730. doi: 10.1111/j.1530-0277.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- 65.Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D, Soyka M, et al. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nature medicine. 2005;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.