Abstract
Methylphenidate (Ritalin, MPH) is the most commonly prescribed psychoactive drug for children. Used to treat attention-deficit/hyperactivity disorder (ADHD) and for cognitive enhancement in healthy individuals, its cellular mechanisms of action and potential long-term effects are poorly understood. We recently reported that a clinically relevant (1 mg/kg i.p., single injection) dose of MPH significantly decreased neuronal excitability in the juvenile rat prefrontal cortical neurons. Here we further explore the actions of acute treatment with MPH on the level of NMDA receptor subunits and NMDA receptor-mediated short- and long-term synaptic plasticity in the juvenile rat prefrontal cortical neurons. We found that a single dose of MPH treatment (1 mg/kg, intraperitoneal) significantly decreased the surface and total protein levels of NMDA receptor subunits NR1 and NR2B, but not NR2A, in the juvenile prefrontal cortex. In addition, the amplitude, decay time and charge transfer of NMDA receptor-mediated EPSCs were significantly decreased whereas the amplitude and short-term depression of AMPA receptor-mediated EPSCs were significantly increased in the prefrontal neurons. Furthermore, MPH treatment also significantly increased the probability and magnitude of LTP induction, but had only a small effect on LTD induction in juvenile rat prefrontal cortical neurons. Our data thus present a novel mechanism of action of MPH, i.e., changes in glutamatergic receptor-mediated synaptic plasticity following early-life treatment. Furthermore, since a single dosage resulted in significant changes in NMDA receptors, off-label usage by healthy individuals, especially children and adolescents, may result in altered potential for plastic learning.
Keywords: ADHD, methylphenidate, psychostimulant, excitatory synaptic transmission, synaptic plasticity, juvenile, prefrontal cortex
INTRODUCTION
Attention deficit/hyperactivity disorder, or ADHD, is an increasingly common childhood disorder, and is now recognized in adults as well. ADHD is thought to affect between 3 and 5% of school-aged children, although some estimates go as high as 8% (Faraone, Sergeant, Gillberg, and Biederman, 2003; Polanczyk, de Lima, Horta, Biederman, and Rohde, 2007). Methylphenidate (Ritalin®, MPH) is the most commonly prescribed agent for treating ADHD (Challman and Lipsky, 2000). It has been determined that MPH acts primarily on the dopaminergic and noradrenergic systems through blockade of the dopamine and norepinephrine reuptake transporters, thereby increasing the concentrations of these neurotransmitters in the brain to correct the attention deficits and hyperactivity (Arnsten and Pliszka, 2011; Berridge, Devilbiss, Andrzejewski, Arnsten, Kelley, Schmeichel, Hamilton, and Spencer, 2006; Greenhill, Kollins, Abikoff, McCracken, Riddle, Swanson, McGough, Wigal, Wigal, Vitiello, Skrobala, Posner, Ghuman, Cunningham, Davies, Chuang, and Cooper, 2006; Kuczenski and Segal, 2001; 2002).
Although MPH acts primarily on dopamine and norepinephrine, it likely has effects on other neurotransmitter systems, especially the glutamatergic system, to regulate attention, learning, and memory functions. In fact, the dopamine and glutamatergic systems are intricately interlinked, via intracellular signaling pathways as well as their physical interactions (Cepeda and Levine, 2006). During development, NR2B-containing NMDA receptors are replaced by NR2A-containing ones (Cull-Candy, Brickley, and Farrant, 2001), raising the threshold for long-term potentiation (LTP) and lowering the threshold for long-term depression (LTD), making learning more selective (Kopp, Longordo, and Luthi, 2007; Yashiro and Philpot, 2008). However, the prefrontal cortex (PFC) is unique among cortical regions in that it does not experience a significant alteration in the NR2A/NR2B ratio during development; thus, the potential for plasticity remains high throughout adulthood (Wang, Stradtman, Wang, and Gao, 2008). NMDA receptors, due to their relationship with neural plasticity and learning/memory, are likely to be affected by stimulant drugs, including MPH. Indeed, the levels of NR2B have been shown to be decreased by treatment with amphetamine, a psychostimulant closely related to MPH (Mao, Wang, Chu, Zhang, Liu, Yang, Haines, Papasian, Fibuch, Buch, Chen, and Wang, 2009).
Despite the decades of research on MPH effects, little is known about its potential developmental effects on the PFC circuitry. Furthermore, development of the PFC is not complete until young adulthood (Arnsten, 1999; Arnsten and Shansky, 2004; Gonzalez-Burgos, Kroener, Zaitsev, Povysheva, Krimer, Barrionuevo, and Lewis, 2008; Hashimoto, Nguyen, Rotaru, Keenan, Arion, Beneyto, Gonzalez-Burgos, and Lewis, 2009; Lewis, 1997; Tseng and O'Donnell, 2007; Woo, Pucak, Kye, Matus, and Lewis, 1997). We have previously reported striking differences in the response of PFC neurons to MPH in adult versus juvenile systems; a clinically-relevant 1mg/kg i.p. dose resulted in enhancement of PFC function in adults, but caused a relatively hyperdopamine state in the juveniles (Urban, Gao, and Waterhouse, 2012). It has been shown that hyperdopamine significantly attenuates NMDA receptor-mediated currents in the PFC pyramidal neurons (Li and Gao, 2011; Li, Xi, Roman, Huang, and Gao, 2009). Furthermore, excessive stimulation of the ventral tegmental area that results in dopamine overflow in the PFC has been shown to enhance the magnitude of hippocampal-PFC LTP (Jay, Rocher, Hotte, Naudon, Gurden, and Spedding, 2004). Therefore, it is important to understand how treatment with MPH affects NMDA receptors and synaptic plasticity in prefrontal cortical neurons of juvenile brain. We hypothesize that treatment with a clinically-relevant dose of MPH, as we reported recently (Urban et al., 2012), will result in decreased expression of NMDA receptors in the juvenile rat PFC, which in turn, will translate functionally to a decrease in NMDA current as well as an altered induction of LTP/LTD in the PFC cortical pyramidal neurons. We tested this hypothesis with a combination of biochemical and electrophysiological techniques and we confirmed an altered NMDA receptor composition and an altered synaptic transmission and plasticity in response to acute MPH treatment in the juvenile rat PFC.
MATERIALS AND METHODS
Animal Care and Preparation
Male and female Sprague-Dawley rats at age of postnatal day (PD) 12 were purchased from Charles River. Upon arrival, animals were allowed 3–5 days to acclimate before treatment began. Rats aged PD15–25 received a single, 1 mg/kg i.p. injection of MPH, as we reported previously (Urban et al., 2012), and were sacrificed one hour later for physiological recording. Control animals received an equivalent amount of physiological saline. Both male and female pups were included in this study because our previous work found no significant differences between juvenile males and females in regards to responses to MPH treatment at this age group (Urban et al., 2012).
Prefrontal Cortical Slice Preparation
Rats were anesthetized with sodium pentobarbital (Euthasol, Virbac Animal Health) and level of anesthesia was determined with rear toe pinch. When the animal gave no response to a toe pinch, it was quickly decapitated and the brain was removed and placed in ice-cold sucrose-rich artificial cerebrospinal fluid (aCSF) containing (87 mM NaCl, 75 mM Sucrose, 25 mM NaHCO3, 25 mM glucose, 2.5 mM KCl, 1.25 mM NaH2PO4, 0.5 mM CaCl2, 7 mM MgSO4) and bubbled with 95% O2/5% CO2. Brain region containing PFC was trimmed and coronal slices were made 300 µm thick using a Leica VT1000S vibratome (Leica Microsystems, Bannockburn, IL). Slices were collected and incubated in 35.5°C sucrose-rich aCSF bubbled with 95% O2/5% CO2 for one hour, then stored at room temperature until recording.
Electrophysiological Recordings
For recording, brain slices were bathed in heated (~35°C) aerated (95%O2/5%CO2) Ringer’s solution (125 mM NaCl, 25 mM NaHCO3, 25 mM glucose, 2.5 mM KCl, 1.25 mM NaH2PO4, 2 mM CaCl2, 1 mM MgCl2) on a recording chamber placed under an upright Zeiss Axioskop 1 microscope (Zeiss Microsystems). Layer 5 PFC pyramidal neurons were identified by their cell soma shape and dendrite morphology using an infrared differential interference contrast video system (Hamamatsu Cop. USA, Bridgewater, NJ). Electrode pipettes were pulled using a Sutter P-97 horizontal puller (Sutter Instruments, USA) and filled with a cesium-containing intracellular solution (110 mM D-gluconic acid, 110 mM CsOH, 10 mM CsCl2, 1 mM EGTA, 1 mM CaCl2, 10 mM HEPES, 1 mM ATP-Mg). Clampex 9.2 software (Molecular Devices) was used for data recordings and a 700A Multi-Clamp Commander was used for data amplification and neuronal recording manipulations such as bridge-balance and current/voltage injection. A bipolar stimulus electrode (CBBRC75, FHC Inc., Bowdoin, ME) was placed in layer 2/3, about 200 to 300 µm from the recording layer 5 pyramidal neurons of the PFC. Only cells that formed a gigaohm seal were kept and recorded from. The highly potent and specific chloride channel blocker 4,4′-dinitro-stilbene-2,2′-disulphonic acid (DNDS, 0.5mM, Sigma-Aldrich) was added into the recording pipette to block GABAA receptor-mediated current (Dudek and Friedlander, 1996; Volgyi, Xin, and Bloomfield, 2002) in the recording of NMDA receptor-mediated current. For short-term plasticity experiments, cells were placed in voltage-clamp mode, and held at −60 mV. A 20-Hz train of 10 pulses was given, with stimulus strength being adjusted so that the first AMPA-EPSC peak was approximately 50 pA in amplitude. Next, cells were held at +60 mV to record NMDA receptor-mediated current and summation in response to the pulse train in the presence of AMPA receptor antagonist DNQX (20 µM). To determine the NR2B component of NMDA receptor-mediated current, a potent and selective NR2B antagonist Ro-256981 (0.5 µM, Tocris) was added to the bath solution for 5 min to block NR2B-containing receptors in the NMDAR-mediated currents recorded at +60 mV in the presence of GABAA receptor antagonist picrotoxin (50 µM) and AMPA receptor antagonist DNQX (20 µM). Ro-256981 was then washed out and the NMDA-EPSCs were recorded for an additional 10 minutes for recovery. For all recordings, the input resistance of the neuron was constantly monitored with an injected negative current (−100 pA, 200 ms) and was adjusted during recording. For reversal potential, a separate set of neurons was used. Cells were held in different voltages from −80 mV to +60 mV with 20 mV increments, using the same cesium-intracellular solution used to measure AMPAR- and NMDAR- mediated currents, and resulting EPSCs were recorded for 5 minutes at each holding potential.
For LTP recordings, the stimulating electrode was placed in layer 2/3 and a low-resistance (0.5–1 MOhm) recording glass pipette containing Ringer’s solution was placed in layer 5 of the medial PFC. Field potential was recorded in current clamp, with a sweep interval of 15 seconds. Stimulus intensity was adjusted to bring the initial EPSP amplitude to about 0.5 mV. Baseline was recorded for 15 minutes with a stimulus every 15 seconds, and then LTP was induced with 4 trains of 100 pulses at 100 Hz, with an inter-train interval of 30 seconds. Following LTP protocol, fEPSPs were recorded every 15 seconds for an additional 45 minutes. Four fEPSPs were averaged to result in one data point per minute, and graphed. For LTD studies, baseline was recorded with a sweep interval of 15 seconds for 15 minutes, followed by a low-frequency stimulation protocol (900 pulses at 1 Hz) for LTD induction. Field potential EPSPs were recorded for an addition 45 minutes, and averaged to one data point per minute, and graphed.
Data Analysis for Electrophysiological Recording
The amplitudes of the evoked EPSCs were measured by averaging 30 traces from the onset to peak of EPSCs using Clampfit 9.2 software (Molecular Devices). A stable baseline, which was determined by no clear change in EPSC amplitudes and no alteration in input resistance (<20%), was a prerequisite for continuous recording and further data analysis. Specifically, to determine whether the recordings were stable and reliable, we exported the baseline EPSC amplitudes of each recording (or EPSPs for LTP/LTD recordings) to excel datasheet to make a scatter graph and examined the linear relationship by adding a trend line for each recording. If the EPSC/EPSP amplitudes increased or decreased during the recording, the trend line would not be horizontal and the R2 would increase. The stable baseline recording was thus determined by a recording showing no progressive changes (increase or decrease) in EPSC/EPSP amplitudes, including a flat/horizontal trend line and a small R2 value. All recordings without stable baseline for 5 min for EPSCs or 15 min for EPSPs in LTP/LTD data or with input resistance increased more than 20% were discarded for further analysis. The peak amplitudes of EPSCs recorded at −60 mV were directly measured to represent AMPA receptor-mediated current because the opening of NMDA receptor channel at this potential was negligible. The ratios of the 2nd divided by the 1st EPSC, as well as the ratios of nth/1st, were calculated to show the dynamic of synaptic transmission at 20 Hz train. The decay time course of EPSC was fitted with a single exponential using standard exponential formula in Clampfit 9.2. Amplitude of the NMDA receptor-mediated currents were measured 50 ms after the peak of the EPSCs recorded at +60 mV to avoid the contamination of AMPA current amplitude because AMPA current usually returns to baseline within 50 ms, allowing us to determine pure NMDA receptor-mediated EPSC component. The integrated EPSC areas (charge transfer) were measured and expressed as pico-Coulomb (pC) and a slope was obtained by fitting the points to linear regression analysis. Slopes for the amplitude curves of AMPA-EPSCs and charge transfer of NMDA-EPSCs were obtained by fitting the linear regression lines with slope-intercept equation y = mx + b, where y is the value of the point along the y-axis, x is the value along the x-axis, m is the slope of the line, and b is the y-intercept. Student’s t-test assuming non-equal variance was run on control versus MPH-treated for each parameter. Statistical significance was held at p<0.05. For LTP/LTD recordings, EPSPs were recorded every 15 seconds, then EPSP slope was measured and data points for each minute were averaged. ANOVA analysis was run on the averaged traces to determine the difference between neurons from saline- and MPH-treated rats. In addition, because the change in LTD was of small magnitude, the data in the post-trough area (5 min after the low-frequency induction protocol was applied) were plotted in a scatter graph to show the number of occurrences of EPSP slopes of MPH-treated cells vs. saline-treated cells over the unity line versus under the line. Further, a single-factor ANOVA analysis was performed to confirm the statistical differences in the EPSP slopes between saline- and MPH-treated neurons. In addition, a z-test was run to examine the distribution of EPSP slopes. To account for reversal potential shifts that might be caused by the cesium-containing intracellular solution, liquid junction potential of the solution was calculated with Clampex and subtracted from the command voltage to yield a Vm. EPSC amplitudes were plotted against Vm, and linear regression was performed to determine reversal potential. Two-way ANOVA was run to determine the significance.
BS3-crosslinking Assay
Following drug treatment, rats were anesthetized and decapitated one hour after MPH or saline injection. Prefrontal cortical tissue was removed, and 400-µm-thick slices generated. Slices were incubated for 45 minutes at 35.5°C in Ringer’s solution bubbled with 95% O2/5% CO2. Slices were then removed and incubated in Ringer’s solution containing Bis (sulfosuccinimidyl) suberate (BS3) (1 mg/ml; Pierce Biotechnology, Rockford, IL, USA) in aerated ACSF (95% O2/5% CO2) with gentle agitation for 45 minutes (Li et al., 2009). BS3 is a membrane-impermeable agent, which selectively cross-links cell surface proteins to form high-molecular-weight aggregates. Intracellular proteins are not crosslinked; thus, they retain normal molecular weight. BS3-crosslinking allows for the separation of surface-expressed and intracellular forms of proteins by SDS-PAGE and Western blotting. The slices were then washed three times with ice-cold ACSF containing 20 mM Tris (pH 7.6) to quench the remaining BS3 and halt the reaction. Surface expression was determined using Western blot analysis.
Western Blotting
Prefrontal cortical slices from BS3-crosslinking assay were homogenized in lysis buffer (20 mM Tris-HCl with pH 7.4, 200 mM NaCl, 1 mM Na3VO4, 10 mM NaF, and protease inhibitor cocktail). Homogenates were then centrifuged at 13,000g for 5 minutes; the supernatant was transferred to new tubes. Protein concentrations were measured and sample tubes created with lysis buffer, Laemli buffer and β-mercaptoethanol to a total volume of 30 µg protein. The protein samples were boiled at 95°C for 5 min, subjected to SDS–PAGE gel electrophoresis, and then transferred to PVDF membranes (Bio-Rad) overnight at 5V, 4°C. The membranes were blocked with 5% nonfat dry milk in TBST (0.05% Tween-20 in 1 Tris-buffered saline) for 1 h and were incubated in the following dilutions of primary antibodies for 1 h: monoclonal mouse anti-NR2B and anti-NR1(1:4000, Zymed/Invitrogen), or monoclonal rabbit anti-NR2A (1:4000, Zymed/Invitrogen). All blots used monoclonal mouse anti-actin (1:20,000, Sigma-Aldrich) to generate control bands. After several rinses with TBST, the membranes were incubated in horseradish peroxidase- conjugated goat anti-mouse or rabbit IgG (Jackson ImmunoResearch Laboratories) at 1:8000 for 2 h. Immunopositive protein bands were detected with ECL Western Blotting System (Amersham Bioscience). After exposure of the membranes to the film, the band densities were measured with Image J (NIH). Final data were normalized to the levels of β-actin and then to the control levels with control as 1. To minimize inter-blot variability, each treatment group included 4–5 rats and each sample from an animal was analyzed at least three times. The total protein level of a NMDA receptor subunit was calculated as sum of the surface protein (500 KD) and intracellular pool of protein level. The mean value for each sample was calculated from all of the replicates in the different animals, and the results were presented as mean ± standard error. Significance was determined with the Student t test or ANOVA.
Drugs
MPH was purchased from Sigma-Aldrich, and diluted to a 5 mM stock solution in physiological saline. Prepared solution was stored at −20°C in 1 ml aliquots. Ro-256981 was purchased from Tocris Bioscience and diluted to a 5 mM stock, and stored in 1 ml aliquots at −20°C. 4,4′-dinitro-stilbene-2,2′-disulphonic acid (DNDS) was purchased from Sigma-Aldrich, diluted to a 5 mM stock and stored at −20°C in 1 ml aliquots.
RESULTS
MPH treatment selectively reduces levels of NR2B and NR1, but not NR2A subunits
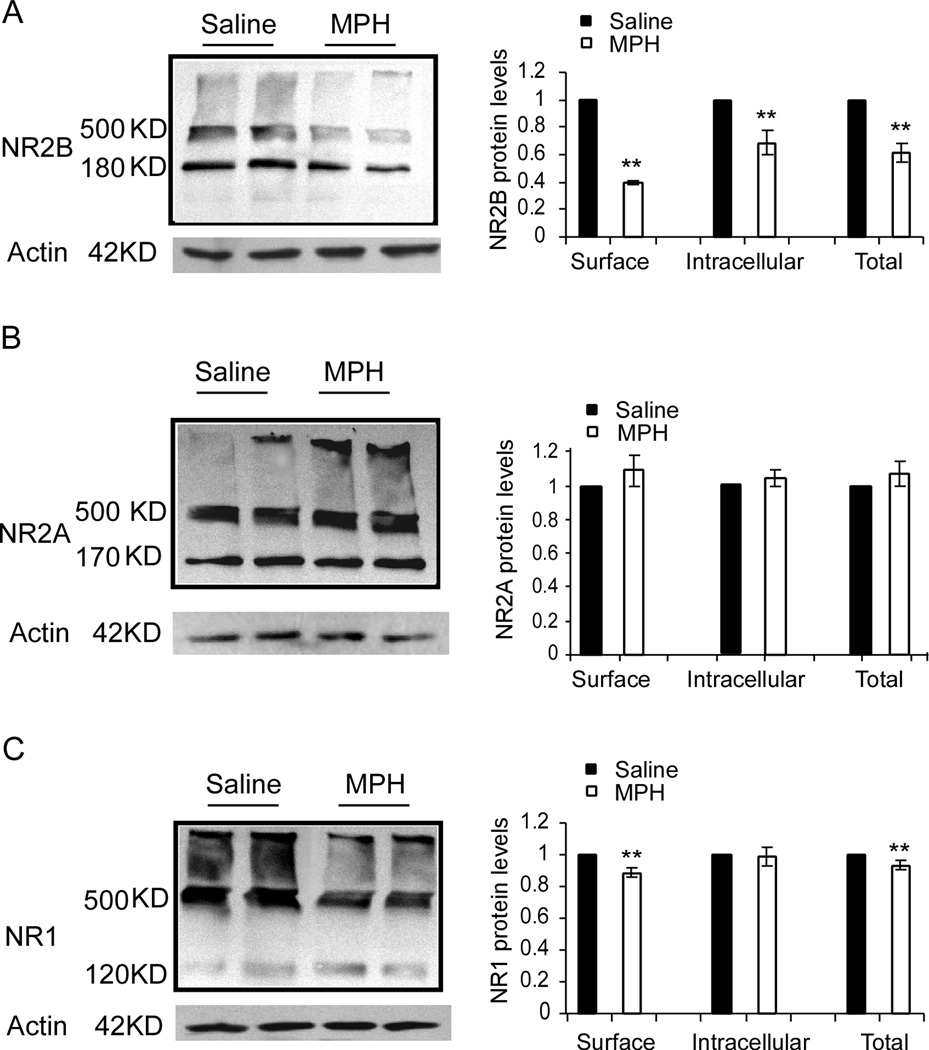
MPH is known to significantly increase extracellular level of dopamine and norepinephrine in the brain. Our previous studies have indicated that the PFC contains a relatively high ratio of NR2B subunits (Wang et al., 2008) and that a hyperdopaminergic state induces internalization of the NMDA receptor subunit NR2B (Li et al., 2009). We therefore examined whether MPH has effects on the surface expression of NMDA receptors subunits in the PFC tissue with BS3-crosslinking and western blotting. As shown in Figure 1, we found that a single dose (1 mg/kg, i.p.) of MPH treatment induced a significant decrease in the levels of NR2B protein, both surface-expressed and intracellular. Surface expressed protein was reduced to 39% of the saline vehicle control (Student’s t-test, p= 0.0007), while intracellular was reduced to 68.5% of the saline control (Student’s t-test, p= 0.01), and total protein levels were reduced to 61% of the saline control (n = 4 rats for saline, n = 5 rats for MPH, p= 0.0005; Fig. 1A).
Figure 1.
Treatment with a single dose of 1 mg/kg MPH significantly reduced levels of NR2B and NR1, but not NR2A subunits, in the juvenile rat PFC. Images on the left columns in A, B and C are raw data samples showing the BS3 crosslinked protein expressions of NR2B, NR2A and NR1 subunits exhibited with western blotting. (A) Levels of NR2B total protein, which was calculated as a sum of surface and intracellular protein level, were significantly reduced in MPH-treated tissue as compared to saline-treated control (p < 0.01), along with intracellular pools of NR2B (180 kD, p < 0.01) and surface-expressed NR2B (500 kD, p < 0.01). B, Levels of NR2A, including surface (500 kD), intracellular (170 kD) and total proteins, were slightly increased by MPH treatment but the changes were not statistically significant. C, Surface-expressed NR1 levels (120 kD, p < 0.01), as well as total protein levels (p < 0.05), but not intracellular pools (500 kD, p > 0.05), were significantly reduced. This result suggests a targeted suppressive effect of MPH on NR2B-, but not NR2A-containing NMDARs in the juvenile PFC neurons (n = 4 rats for saline and n = 5 rats for MPH, sample from each animal repeated 3 times). Note: * = p < 0.05, ** = p < 0.01
NR2A protein levels, however, were not significantly changed, neither for surface-expressed protein (n = 4 rats for saline, n = 5 rats for MPH p = 0.37), intracellular (p= 0.495), or total protein levels (p= 0.384; Fig. 1B), as examined by Student’s t-test. There was a slight increase in levels of NR2A, although non-significant, suggesting a possible compensatory response to the lack of NR2B that may become more evident following extended MPH treatment. NR1 is an obligatory subunit; every NMDA receptor must contain 2 NR1 subunits. Therefore, a change in NR1 protein levels would suggest an overall alteration in the number of NMDARs. We found that total protein levels of NR1 showed a small but significant decrease following MPH treatment (n = 4 rats for saline, n = 5 rats for MPH, 93.2% of the saline control, p= 0.0383). Surface expression was likewise reduced (88.6% of the saline control, p= 0.011) whereas the intracellular levels of NR1 remained unchanged (p= 0.84; Fig. 1C). These results suggest a selective trafficking of NR2B-containing NMDA receptors and/or a reduced transcription of the NR2B and NR1 subunits in response to acute MPH treatment in the juvenile rat PFC.
MPH treatment significantly decreases NMDA-mediated current but enhances AMPA-mediated current and alters short-term plasticity in prefrontal cortical neurons
It has been reported that NR2B subunits play a critical role in working memory and the extremely plastic property of the PFC (Li et al., 2009; Wang et al., 2008; Zhao, Toyoda, Lee, Wu, Ko, Zhang, Jia, Shum, Xu, Li, Kaang, and Zhuo, 2005) due to their slower activation kinetics and highly dynamic properties compared to the relatively stable NR2A subunits (Cull-Candy et al., 2001; Hallett, Spoelgen, Hyman, Standaert, and Dunah, 2006; Li et al., 2009; Paoletti and Neyton, 2007; Wang et al., 2008). The PFC is known to contain a high percentage of NR2B-containing NMDA receptors relative to other cortical regions; thus, there is greater activation of signaling cascades following stimulation and greater facilitation in response to physiological (20–40 Hz) inputs (Wang et al., 2008). We have therefore first confirmed the changes of NMDA receptors subunits with electrophysiology. We recorded AMPA- and NMDA-mediated currents and examined short-term plasticity in response to physiologically-relevant stimulus trains. In agreement with the significantly decreased levels of NR1 and NR2B subunits, a single dose of MPH resulted in significant depression of the NMDA receptor-mediated current.
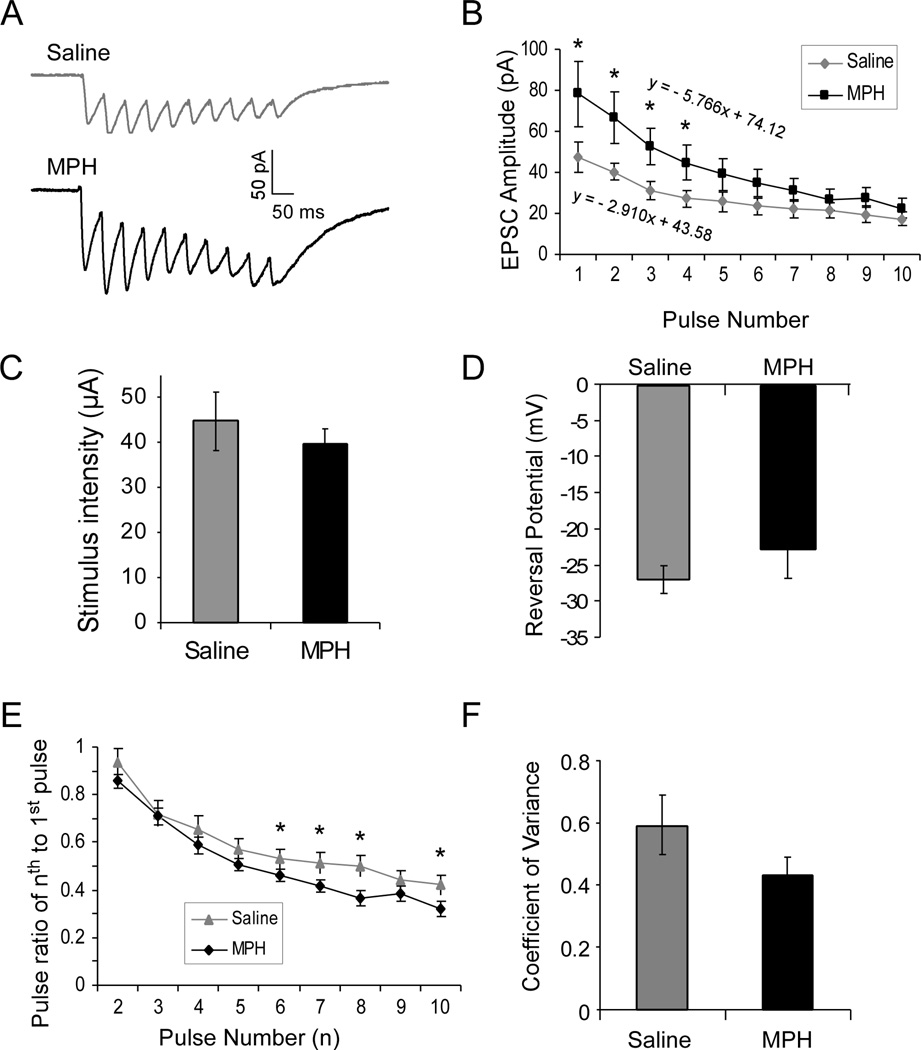
We first applied a protocol with 10-pulse train to the patch-clamped layer 5 pyramidal PFC neurons to examine AMPA receptor-mediated short-term plasticity. We found that AMPA receptor-mediated current (measured at −60 mV) exhibited a clear short-term depression in both saline- and MPH-treated neurons, i.e., the EPSCs become smaller and smaller in the train (Fig. 2A). However, the slope of the linear regression line of the EPSC amplitudes was significantly increased following MPH treatment compared with that of saline control (slope of the curve = −2.910 in saline vs −5.766 in MPH, Fig. 2B). The change in short-term depression was mainly attributable to the significant increase of EPSC amplitudes, especially the amplitudes of the first several EPSCs, which were significantly larger than those in saline controls [n = 7 cells for each group; 4 rats for saline and 5 rats for MPH; p < 0.05; one-way ANOVA, F(1, 12) = 5.108, p = 0.0364; Fig. 2B]. Despite the differences in the EPSC amplitude and summation, the stimulus intensity applied to evoke EPSCs was not significantly different between saline- and MPH-treated neurons (Student’s t-test, 44.8 ± 6.42 µA for saline vs. 39.8 ± 3.13 µA for MPH, n = 7 cells for each group, p = 0.25; Fig. 2C). Reversal potential of AMPA receptor-mediated EPSCs (n = 9 cells, 4 rats for saline, 8 cells, 5 rats for MPH) was not altered by MPH treatment [−26.78 ± 1.91 mV for saline vs. −22.7 ± 3.88 mV for MPH, F(1, 15) = 0.891, p = 0.361; Fig. 2D]. Therefore, any differences observed in the EPSCs can be attributed to the drug treatment. Consistently, the paired-pulse ratios of the successive EPSCs to the first EPSC were altered with the last several ratios showing significant decreases in MPH-treated neurons compared to those in saline controls (n = 7 neurons each group, 4 rats for saline and 5 rats for MPH; t-test, p = 0.000075; Fig.2E). However, the amplitude of the first AMPA-mediated EPSC was significantly increased by MPH-treatment ( Student’s t-test, p < 0.05; Fig. 2A, B), which indicates a possible compensatory response to the decreased postsynaptic NMDA receptors and/or presynaptic glutamate release due to the decreased neuronal excitability (Urban et al, 2012). Indeed, analysis of coefficient of variance (CV) supports the presence of a postsynaptic mechanism, as the CV was not significantly altered by MPH treatment despite a trend of change (Student’s t-test, n = 7 cells, 4 rats for saline and 7 cells, 5 rats for MPH; p = 0.096; Fig. 2F). AMPA receptor-mediated EPSC decay time was also not significantly affected by MPH treatment but a trend of change exists (p = 0.093). These data suggest that acute MPH treatment enhances AMPA receptor-mediated current and short-term plasticity in the juvenile rat PFC.
Figure 2.
The evoked AMPA receptor-mediated current was significantly increased by MPH treatment. A, Representative traces from saline controls and MPH-treated neurons. B, MPH treatment significantly changed the pattern of short-term depression of AMPA receptor-mediated currents (n = 7 for saline and 7 for MPH, ANOVA F = 4.41, p < 0.05) with the first several EPSCs significantly greater than those in saline controls (* p < 0.05). C, Stimulus intensities applied to evoked EPSCs were not different between saline-and MPH-treated neurons (44.8 ± 6.42 µA for saline vs. 39.84 ± 3.13 µA for MPH; n = 7 cells for each group, p > 0.05). D, The reversal potentials of AMPA receptor-mediated EPSCs in saline- and MPH-treated neurons were also similar, without statistical significance (n = 9 cells, 4 rats for saline, 8 cells, 4 rats for MPH, p > 0.05). E, MPH treatment decreased paired-pulse ratio of successive AMPA receptor-mediated EPSCs in a train overall (ANOVA F = 4.413, p < 0.01) but ad hoc t-test exhibited statistical significances only in the last several EPSCs (* p < 0.05), indicating an altered short-term synaptic plasticity. F, Coefficient of variance was not significantly altered by MPH treatment (p = 0.096).
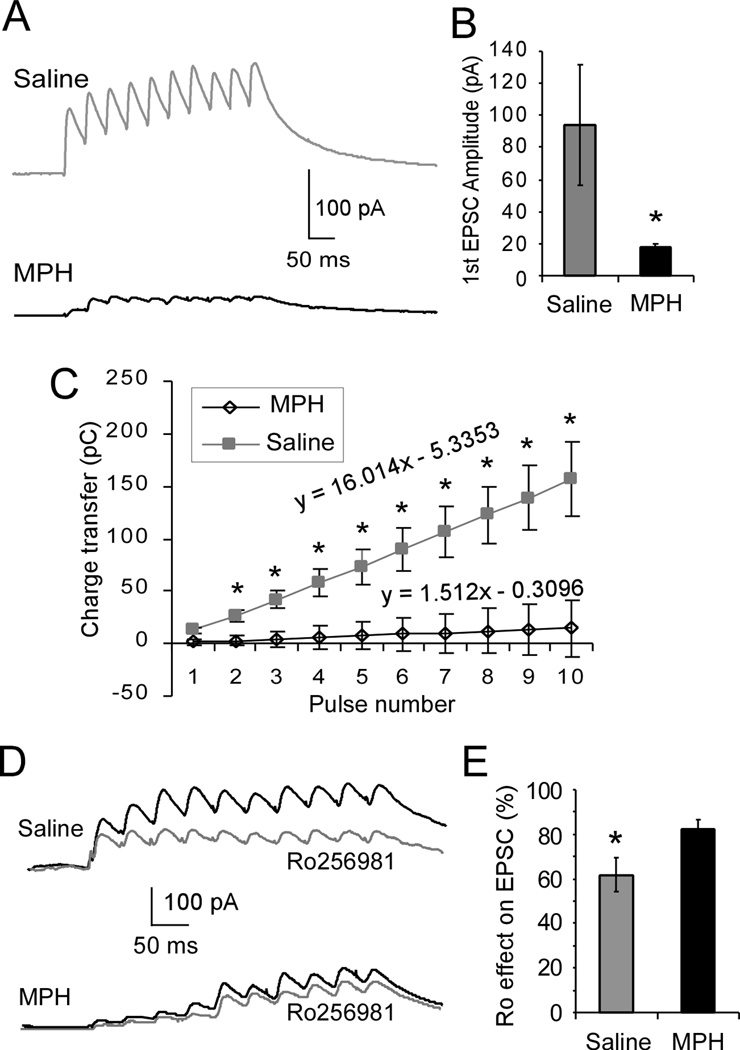
Once AMPA-mediated current was recorded, the holding potential of each recording neuron was switched to +60mV to record NMDA-mediated current. The same 10-pulse protocol was applied, and current amplitude was measured at 50ms after the peak to exclude AMPAR-mediated component. As shown in Figure 3, the amplitude of each subsequent NMDA-mediated EPSC within a 10-pulse 20-Hz train summated well to exhibit a clear and significant progressive increase of integrated areas under the EPSC train in the saline-treated controls (upper panel, Fig. 3A). In contrast, NMDA receptor-mediated current was significantly decreased with less summation in the MPH-treated neurons versus the saline-treated control neurons (lower panel, Fig. 3A). The amplitude of the first EPSC in MPH-treated neurons was significantly smaller than that in saline controls (Student’s t-test, 17.0 ± 2.3 pA in MPH vs. 99.5 ± 17.38 pA in saline control; n = 7 cells each group, 4 rats for saline and 5 rats for MPH, p= 0.0017; Fig, 3B). The decrease in NMDA-mediated EPSC amplitude and summation was further evidenced by measuring the integrated areas under the current as charge transfer. As expected, there was a strong linear increase in current areas with increased pulse number in the saline-treated controls (slope of the line = 16.014; Fig. 3C); however, in MPH-treated neurons, there was almost no increase in charge area despite increasing stimulation number (slope of the line = 1.512; Fig. 3C) and there was a significant difference between the saline-and MPH-treated neurons [F (1, 12) = 4.413, p = 0.00013). To further confirm the NR2B change, a selective NR2B antagonist Ro-256981 (0.5 µM) was applied to bath solution to examine the relative contribution of NR2B to total NMDA receptor-mediated current, and the change in current amplitude was recorded. As shown in Figure 3D and 3E, Ro-256981 caused a significantly greater decrease in NMDA receptor-mediated current in saline-treated neurons than in MPH-treated neurons, suggesting that NR2B-containing receptors comprise a lesser percentage of the total pool of NMDARs in MPH-treated brains (Student’s t-test, n = 7 cells, 4 rats for saline and 7 cells, 5 rats for MPH, p = 0.028; Fig. 3E). These data confirm the significant functional decrease of NMDA receptors in MPH-treated neurons as a consequence of the decrease in protein levels seen in our Western blotting experiments.
Figure 3.
Treatment with 1 mg/kg MPH significantly decreases NMDA receptor-mediated current via depression of NR2B subunits. A, Representative traces of EPSCs recorded at +60 mV in the presence of picrotoxin (50 µM) and DNQX (20 µM) in response to a 10-pulse 20-Hz train of stimuli from saline-treated controls (n = 7) and MPH-treated neurons (n = 7). B, The amplitude of the first EPSC was significantly reduced in MPH-treated neurons compared to saline controls (*p < 0.05). C, Charge transfer, which was measured as an integrated area of the EPSCs, MPH-treated neurons exhibited a significant reduction in summation and linear relationship compared to saline-treated control neurons (F = 4.413, * p <0.05). D, Representative traces of NMDA current trains before and after applications of selective NR2B antagonist Ro-256981 are shown for both saline and MPH-treated neurons. E, Bath application of Ro-256981 resulted in a significantly greater decrease in NMDA current amplitude in saline-treated neurons vs. MPH-treated neurons (decrease to 61.7% of baseline for saline, and decrease to 82.1% of baseline for MPH, p < 0.05).
Treatment with MPH significantly increased the tendency for LTP but had a small inhibitory effect on LTD in prefrontal cortical neurons
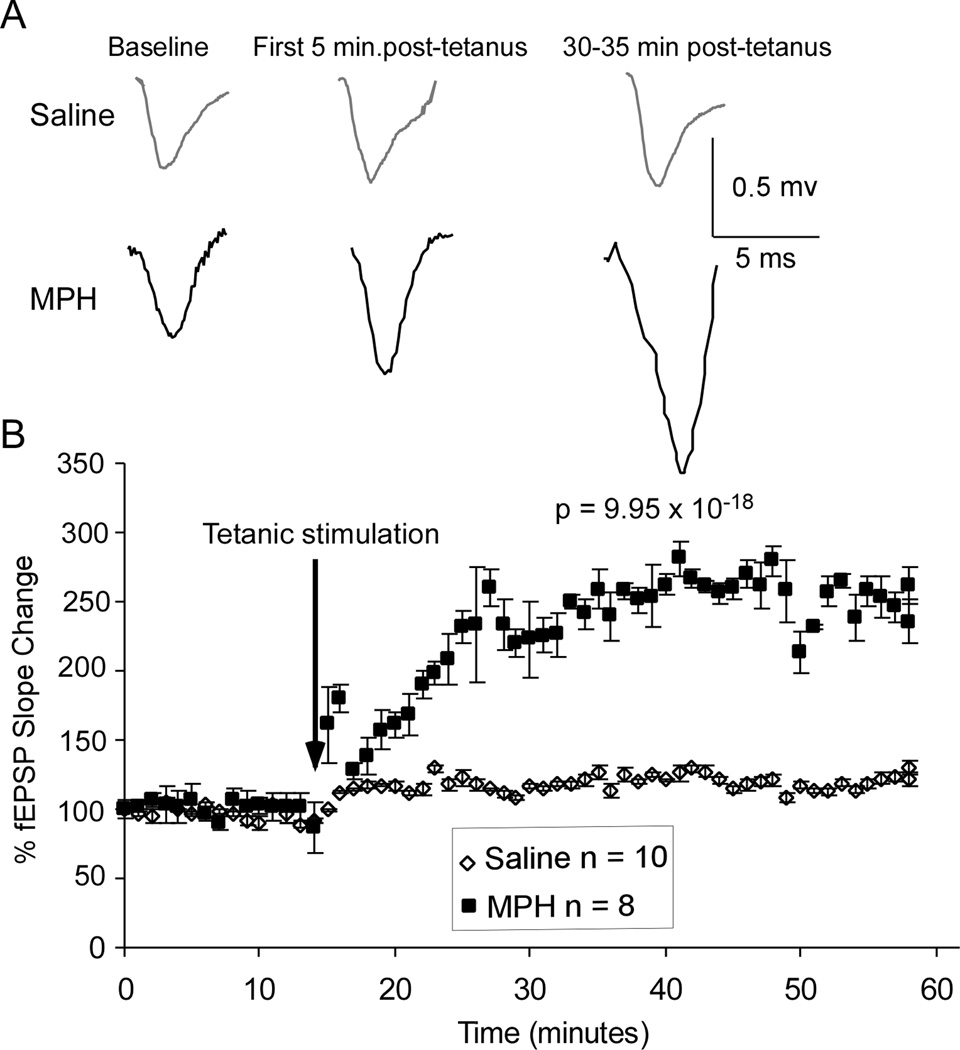
While short-term plasticity is a good measure of cortical plasticity and flexibility, it is best paired with examinations of LTP as well. Both NMDA and AMPA receptors are intricately involved in generation of LTP and LTD in response to strong or weak external stimulation. Although there is still some dissentions in the literature about the specific roles of NR2A and NR2B subunits, it is generally believed that higher levels of NR2B result in more long-term depression, whereas higher levels of NR2A result in more long-term potentiation, but this is also dependent on the relative ratios of the two receptor subtypes (Liu, Wong, Pozza, Lingenhoehl, Wang, Sheng, Auberson, and Wang, 2004; Massey, 2004; Xu, Chen, Gu, Yan, Wang, Liu, and Lu, 2009; Yashiro and Philpot, 2008). Therefore, with our Western blots revealing a specific decrease in NR2B-containing receptors following MPH treatment, we expected to see an increased LTP and/or attenuated LTD. Indeed, as shown in the representative fEPSP traces in Figure 4A, the tetanus stimulation protocol for LTP induced no change in the fEPSP amplitude in saline-treated neurons but caused a clear increase of fEPSP amplitude in MPH-treated neurons and the effect was long-lasting. Repeated-measures ANOVA of the fEPSPs taken from each minute revealed a significantly greater fEPSP slope following a single dose of 1 mg/kg i.p. MPH (n = 8 cells from 5 rats, 60 data points in final trace average) than in saline-treated controls [n = 10 neurons from 6 rats, 60 data points in final trace average; F (1, 118) = 101.99, p = 9.95×10−18; Fig. 4B].
Figure 4.
LTP induction was significantly increased by 1 mg/kg acute MPH treatment. A, Representative fEPSP traces from saline-treated control neurons (n = 10) and MPH-treated neurons (n = 8) in the PFC. B, Graphical representation showing normalized fEPSP slope over one hour. Repeated-measures ANOVA revealed a significant 302.3% increase in LTP in MPH-treated neurons (ANOVA p < 0.01).
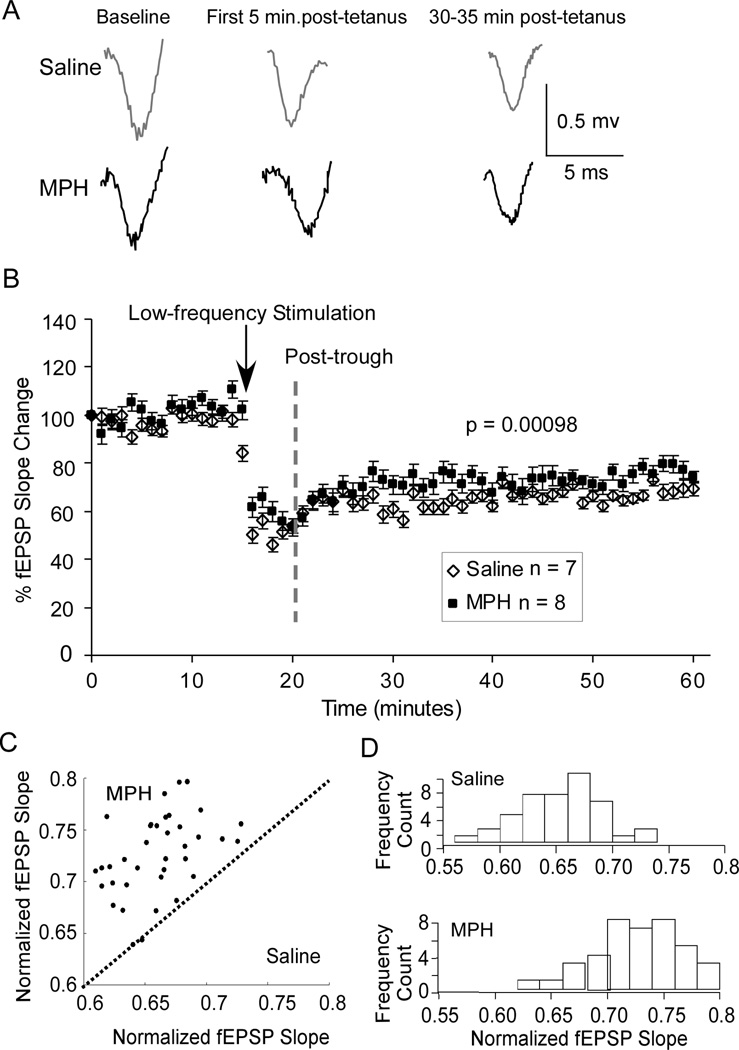
In contrast, LTD was slightly decreased, although significantly, by MPH treatment. As in the representative traces shown in Figure 5A, the amplitude of fEPSPs taken from each minute was slightly (8.14% reduction at 30-min after tetanus vs. saline control) but significantly decreased by the low-frequency stimulation [repeated-measures ANOVA; F (1, 118) = 5.57, p = 0.0098; n = 7 neurons from 5 rats, 60 data points in final trace average for saline, n = 8 neurons from 5 rats, 60 data points in final trace average for MPH; Figure 5B]. When EPSP slope values for MPH-treated cells were plotted against those from saline-treated controls, the data showed a skewed scatterplot, with 38 post-trough points above the unity line, but only two on the line (p = 2×10−13, Fig. 5C). Furthermore, histograms of EPSP frequency count revealed different distributions, with a mean for saline at 6.6 and a mean for MPH at 7.25 (Fig. 5D). A z-test revealed statistically significant distributions (z = 5.53, p = 1.6×10−8).
Figure 5.
Treatment with 1 mg/kg MPH exhibited a small effect on the LTD induction. A, Representative fEPSP traces from saline-treated controls (n = 7) and MPH-treated neurons (n = 8) in the PFC. B, Summary graph shows that the change in LTD, which was measured as the normalized fEPSP slopes, following MPH treatment was minimal (decreased by only 8.14% at 30-min after tetanus) but repeated-measures ANOVA revealed a significant decrease in magnitude of LTD (p < 0.01). C, Scatter graph of EPSP slope values in MPH-treated neurons plotted against those in saline controls reveals a biased distribution of the points, indicating a significant effect of MPH treatment (p < 0.01). D, Distribution histograms of the normalized fEPSP slope values (MPH vs. Saline), binned by 2 minutes, y-axis is number of slopes falling at that value. Z-test revealed significant effect of drug treatment (z = 5.53, p < 0.01).
DISCUSSION
We have shown in this study that treatment of juvenile rats with 1 mg/kg i.p. MPH, a dose thought to create blood serum levels equivalent to clinically effective doses, results in a significant decrease in the total protein levels of NR2B subunit, as well as a significant decrease in surface expression and intracellular pools of this protein. In addition, total and surface expressed NR1 protein levels also decreased whereas the expression of NR2A was unaltered. Furthermore, we showed that the changes in NR1 and NR2B levels resulted in decrease in NMDA-EPSC that was accompanied with an altered AMPA receptor-mediated short-term plasticity and an enhanced potential for LTP induction. Our data thus provide novel evidence for a role of NMDA receptors in the effects of MPH on brain function, and identify changes to prefrontal cortical plasticity following a dose of MPH generally accepted as therapeutic, particularly for juveniles.
MPH is widely used to treat ADHD in children and adolescents as well as in adults. The behavioral and cognitive actions of MPH also occur in both normal human subjects and animal models; thus, comparative studies can be performed on normal, healthy rats (Arnsten and Dudley, 2005; Berridge et al., 2006; Kuczenski and Segal, 2002; Mehta, Owen, Sahakian, Mavaddat, Pickard, and Robbins, 2000). Most evidence supports the continued use of MPH as the best available pharmacotherapy for the treatment of children with ADHD, but little is known about the consequences of psychostimulant exposure early in life (Grund, Lehmann, Bock, Rothenberger, and Teuchert-Noodt, 2006; Yano and Steiner, 2007).
Previous studies have indicated that low, therapeutic doses of MPH enhance levels of dopamine and norepinephrine only in the PFC (Arnsten, 1999; Berridge, 2006; Casey, 2008; Giedd, 2004). Because the PFC continues to undergo development until young adulthood (Lewis, 1997; Woo et al., 1997), it is interesting to know whether early life perturbation of the dopamine and norepinephrine systems with MPH treatment would alter the developmental course of the PFC. We have previously reported that treatment with 1 mg/kg i.p. MPH resulted in a dose-dependent depression of pyramidal neuron excitability and spontaneous release of glutamate in the PFC, an effect opposite to that seen at the same dosage in adult rats, and that these effects were not fully reversible at dosages above 1mg/kg (Urban et al., 2012). However, how this cellular depression occurs, and what effects it may have on circuitry level function remained unclear.
In order to understand the potential effects on cortical plasticity of MPH in the juvenile brain, one must look beyond the dopamine/norepinephrine systems, to the glutamate system, which is involved in regulating plasticity via AMPA and NMDA receptors (Lee and Kirkwood, 2011; Malinow and Malenka, 2002; Nicoll and Malenka, 1999). The dopamine and glutamatergic systems are intricately interlinked via signaling pathways as well as physical interactions (Cepeda and Levine, 2006). Indeed, we found that a single injection of MPH induced a significant decrease in protein expressions of both NR1 and NR2B subunits in the juvenile rat PFC. The findings of this study are certainly intriguing but the question remains: how does acute MPH treatment induce these changes? The NMDA receptor is composed of two obligatory NRI subunits, and two other subunits - NR2A, NR2B, NR3A, or NR3B. It is generally believed that the NMDA receptor subunit is targeted for transport to the cellular membrane through phosphorylation by PKA, which is increased through the adenylate cyclase cascade by D1-like receptor activation but decreased by D2-like receptor activation (Cepeda and Levine, 1998; Greengard, 2001; Neve, Seamans, and Trantham-Davidson, 2004; Seamans, Durstewitz, Christie, Stevens, and Sejnowski, 2001; Yang and Chen, 2005; Zheng, Zhang, Bunney, and Shi, 1999). Indeed, our recent study indicated that excessive levels of dopamine indiscriminately activate the D2-like receptors, which result in a reduction in NMDAR trafficking (Li et al., 2009). It is likely that MPH at 1 mg/kg dosage induces a hyperdopaminergic state in the juvenile rat PFC that has been shown to result in an attenuated NMDA current (Li and Gao, 2011; Li et al., 2009). This presumption is in agreement with a previous study indicating that the NR2A subunit was not associated with response to psychostimulant drugs or to plasticity, while NR2B was dramatically decreased by amphetamine exposure (Mao et al., 2009). Since MPH is a derivative of amphetamine with closely related chemical structures, it is possible that it results in similar reduction in NR2B expression but does not affect levels of NR2A, as observed in this study.
The decreased protein levels in NR1 and NR2B subunits were further confirmed by the electrophysiological findings. However, a downregulation of NR1 surface level seemingly cannot explain the profound reduction in EPSC amplitude measure at the + 60mV holding potential. However, there was almost 60% reduction in NR2B levels. In the juvenile PFC, a much larger proportion of NMDAR channels are derived from NR1-NR2B combination (Wang et al., 2008); thus, reducing NR2B will have a profound effect on the NMDAR-mediated current. Indeed, we have determined a subunit-specific mechanism for the decreased NMDA-EPSC. It is known that NR2B-containing NMDA receptors have a two- to three-fold longer decay time than NR2A-containing receptors (Cull-Candy et al., 2001; Wang et al., 2008), which allows for the summation of responses to incoming physiological stimuli, usually in the range of 10–40 Hz (Wang et al., 2008).
The behavioral and functional consequences of such a specific decrease in NR1 and NR2B levels are striking and potentially devastating to proper prefrontal cortical function. The PFC is unique among cortical regions in that it does not experience a significant alteration in the NR2A/B ratio during development (Wang et al., 2008). Thus, selectively decreasing the levels of NR2B-containing NMDA receptors in the PFC would remove the unique plasticity, rendering the region similar to other cortical regions and potentially alter executive function. Indeed, following our treatment with a single dose of 1 mg/kg MPH, we saw a significantly decreased NMDA-EPSC in response to 20-Hz train stimulation, suggesting the MPH may affect the integration of incoming signals and thus impair working memory function in the juvenile brain.
It should be pointed out that in our recent study, we reported a dose-dependent depression of pyramidal neuron excitability and a decreased spontaneous release of glutamate in the presynaptic site following treatment with 1 mg/kg MPH in the PFC (Urban et al., 2012) whereas the amplitude of AMPA-EPSC was significantly increased by MPH treatment in this study. These results are seemingly contradictory but the change in AMPA-EPSC observed here is likely attributable to a compensatory increase of AMPA receptors in the postsynaptic site because the paired-pulse ratio in the first several EPSCs and the CV were not altered. In fact, NMDA receptors control acute plasticity by regulating trafficking of AMPARs to the postsynaptic sites (Barry and Ziff, 2002; Bredt and Nicoll, 2003; Keifer and Zheng, 2010) and reduced presynaptic release of glutamate could potentially initiate a compensatory increase of AMPA receptor trafficking or transcription as well (Adesnik, Li, During, Pleasure, and Nicoll, 2008). Nevertheless, the increase of AMPA receptor-mediated current is consistent with the increase in LTP induction. The AMPA receptor has long been associated with synaptic plasticity and we found that the MPH effects on long-term plasticity were indeed significant.
One may ask how a reduced NMDA-mediated transmission affects LTP induction. In fact, the mechanism associated with NR2 subunits and long-term synaptic plasticity remains unclear. There is dissention in the field as to the exact roles of NR2B and NR2A in regulating the balance of LTP to LTD (Berberich, 2005 ; Liu et al., 2004; Massey, 2004; Weitlauf, 2005; Xu et al., 2009) but the NR2A/NR2B ratio is proposed to control the LTP/LTD threshold (Yashiro and Philpot, 2008). We examined both LTP and LTD and we found that treatment with a single dose of 1 mg/kg MPH increased the magnitude and potential of LTP induction but had a small depressive effect on LTD. While hippocampal plasticity has been very well characterized, prefrontal plasticity and its dependence on NMDA receptor composition is not as well understood. Prefrontal cortical LTP has been shown to require a balance of contributions from both NR2A and NR2B receptors; however, blocking NR2A had a stronger inhibitory effect on LTP than blocking NR2B (Zhao et al., 2005). Furthermore, a recent study by Liu et al. suggested that selectively blocking NMDARs that contain the NR2B subunit abolishes the induction of LTD but not LTP. In contrast, preferential inhibition of NR2A-containing NMDARs prevents the induction of LTP without affecting LTD production (Liu et al., 2004). It is not as clear how this relates to PFC plasticity; however, due to the interconnectedness of the two brain regions, these results demonstrate that distinct NMDAR subunits are critical factors that determine the polarity of synaptic plasticity (Liu et al., 2004). Thus, by selectively inhibiting NR2B-containing NMDA receptors without affecting NR2A-containing receptors, the plasticity may be skewed in favor of depression. Furthermore, the dopamine levels in the PFC have been found to tightly regulate cortical potentiation and depression. MPH treatment is known to result in a blockade of the dopamine and norepinephrine reuptake transporters, creating higher synaptic levels of the neurotransmitters that would impair the synaptic transmission and plasticity. Otani recently found that LTP in prefrontal cortical slices is more reliably generated when dopamine is added to the bath prior to the induction protocol: a priming effect (Blond, Crepel, and Otani, 2002). In addition, Matsuda et al demonstrated that background dopamine signal in the prefrontal cortical slices can shift the result of a tetanic stimulation from depression to potentiation (Matsuda, Marzo, and Otani, 2006). Thus, we believe that the switch from depression to potentiation in our MPH-treated slices is likely a result of both selective removal of NR2B-containing receptors and a higher synaptic level of dopamine in the brain slices creating a dopamine-priming effect similar to that reported by Otani (Blond et al., 2002).
Accumulating evidence suggests that prefrontal cortical neurons possess the cellular machinery of synaptic plasticity and exhibit lasting changes of neural activity associated with various cognitive processes. Alterations or deficits in the mechanisms of synaptic plasticity induction in the PFC might be involved in the pathophysiology of psychiatric disorders (Goto, Yang, and Otani, 2010). Indeed, our results suggest that even relatively low dose of MPH could result in hyperdopaminergic states in the healthy juvenile PFC, and a single dose could be sufficient to alter synaptic function and plasticity. While we did not examine adult rats in this study, the decreased NR2B levels and NMDA-mediated current that we believe to be evidence of hyperdopamine-related pathology in the juvenile rats should not be evident in adult rats given the same dosage of 1mg/kg i.p. MPH, as previous studies in adult animals suggested (Arnsten, 1999; Berridge, 2006; Casey, 2008; Giedd, 2004).
In conclusion, this study presents several striking findings on the effects of MPH treatment on the juvenile PFC. These results should be cause for reevaluation of the safety of MPH and other psychostimulants in the treatment of children and adolescents. While it is currently unclear whether the selective decrease in NR2B protein levels indicates a deleterious side effect of treatment, or suggests that ADHD pathology may involve an alteration (presumably an increase) in NR2B levels, we need to develop stronger diagnostic and treatment measures. Currently the diagnosis based on Diagnostic and Statistical Manual, Version IV (DSM-IV, 1999) is subjective, and leaves room for erroneous diagnosis and treatment. Findings such as those presented in this study stress the need for the development of more stringent, effective diagnostic and treatment measures that will help to minimize the number of persons needing psychostimulant treatment. Furthermore, our results suggest a potential role of glutamatergic system dysfunction in the pathology of ADHD. Future studies will be needed to examine the effects of related psychostimulants on NR2B levels and plasticity, as well as to determine if selective enhancement of NR2B levels can result in some of the symptoms associated with ADHD pathology.
Methylphenidate (MPH) is the most prescribed psychoactive drug for children Widely used for ADHD and cognition, its cellular mechanisms are poorly understood
MPH decreased NR1 and NR2B, but not NR2A subunits, in juvenile prefrontal cortex
MPH also decreased NMDAR-EPSCs but increased AMPAR-mediated short-term plasticity
MPH increased the probability of LTP induction, but had a small effect on LTD
Acknowledgements
The authors thank Dr. Corey Hart for his assistance in the statistical analysis of the LTD data and Dr. Melissa A. Snyder for her thoughtful comments on the manuscript. This work is supported by NIH R01MH085666 to W.J. Gao.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
We affirm that the content of this manuscript is not under consideration for publication elsewhere nor has the information been previously published. All of the authors have fulfilled all conditions required for authorship and have approved the submission. Institutional review board approval is not required for this project. The authors declare no financial disclosure and conflict of interests.
Author Contributions
W.J.G. conceived the study, supervised the project, and wrote the manuscript. K.R.U. designed and carried out all experiments and data analysis of western blots and electrophysiological recordings and she wrote the paper. Y.C.L. supervised and conducted the western blot experiments.
References
- Adesnik H, Li G, During MJ, Pleasure SJ, Nicoll RA. NMDA receptors inhibit synapse unsilencing during brain development. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5597–5602. doi: 10.1073/pnas.0800946105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Development of the cerebral cortex: XIV. Stress impairs prefrontal cortical function. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:220–222. doi: 10.1097/00004583-199902000-00024. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Dudley AG. Methylphenidate improves prefrontal cortical cognitive function through alpha2 adrenoceptor and dopamine D1 receptor actions: Relevance to therapeutic effects in Attention Deficit Hyperactivity Disorder. Behavior and Brain Function. 2005;1:2. doi: 10.1186/1744-9081-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Pliszka SR. Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacology and Biochemistry of Behavior. 2011;99:211–216. doi: 10.1016/j.pbb.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Shansky RM. Adolescence: vulnerable period for stressinduced prefrontal cortical function? Introduction to part IV. Annals of the New York Academy of Sciences. 2004;1021:143–147. doi: 10.1196/annals.1308.017. [DOI] [PubMed] [Google Scholar]
- Barry MF, Ziff EB. Receptor trafficking and the plasticity of excitatory synapses. Current Opinions in Neurobiology. 2002;12:279–286. doi: 10.1016/s0959-4388(02)00329-x. [DOI] [PubMed] [Google Scholar]
- Berberich SP, Jensen P, Pawlak V, Seeburg PH, Hvalby O, Kohr G. Lack of NMDA receptor subtype specificity for hippocampal long-term potentiation. Journal of Neuroscience. 2005;25:6907–6910. doi: 10.1523/JNEUROSCI.1905-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, Hamilton C, Spencer RC. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biological Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Berridge CWD, D M, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, Hamilton C, Spencer RC. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biological Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Blond O, Crepel F, Otani S. Long-term potentiation in rat prefrontal slices facilitated by phased application of dopamine. European Journal of Pharmacology. 2002;438:115–116. doi: 10.1016/s0014-2999(02)01291-8. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- Casey BJJ, R M, Hare TA. The Adolescent Brain. Annals of the New York Academy of Sciences. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Levine MS. Dopamine and N-methyl-D-aspartate receptor interactions in the neostriatum. Developmental Neuroscience. 1998;20:1–18. doi: 10.1159/000017294. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Levine MS. Where do you think you are going? The NMDA-D1 receptor trap. Science's Signal Transduction Knowledge Environment: STKE. 2006;2006:pe20–pe24. doi: 10.1126/stke.3332006pe20. [DOI] [PubMed] [Google Scholar]
- Challman TD, Lipsky JJ. Methylphenidate: its pharmacology and uses. Mayo Clinic Proceedings. 2000;75:711–721. doi: 10.4065/75.7.711. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Current Opinions in Neurobiology. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- DSM-IV. The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. The American Psychological Association; 1999. [Google Scholar]
- Dudek SM, Friedlander MJ. Intracellular blockade of inhibitory synaptic responses in visual cortical layer IV neurons. Journal of Neurophysiology. 1996;75:2167–2173. doi: 10.1152/jn.1996.75.5.2167. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Sergeant J, Gillberg C, Biederman J. The worldwide prevalence of ADHD: is it an American condition? World Psychiatry. 2003;2:104–113. [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Kroener S, Zaitsev AV, Povysheva NV, Krimer LS, Barrionuevo G, Lewis DA. Functional maturation of excitatory synapses in layer 3 pyramidal neurons during postnatal development of the primate prefrontal cortex. Cerebral Cortex. 2008;18:626–637. doi: 10.1093/cercor/bhm095. [DOI] [PubMed] [Google Scholar]
- Goto Y, Yang CR, Otani S. Functional and dysfunctional synaptic plasticity in prefrontal cortex: roles in psychiatric disorders. Biological Psychiatry. 2010;67:199–207. doi: 10.1016/j.biopsych.2009.08.026. [DOI] [PubMed] [Google Scholar]
- Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- Greenhill L, Kollins S, Abikoff H, McCracken J, Riddle M, Swanson J, McGough J, Wigal S, Wigal T, Vitiello B, Skrobala A, Posner K, Ghuman J, Cunningham C, Davies M, Chuang S, Cooper T. Efficacy and safety of immediate-release methylphenidate treatment for preschoolers with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:1284–1293. doi: 10.1097/01.chi.0000235077.32661.61. [DOI] [PubMed] [Google Scholar]
- Grund T, Lehmann K, Bock N, Rothenberger A, Teuchert-Noodt G. Influence of methylphenidate on brain development - an update of recent animal experiments. Behavior and Brain Function. 2006;2:2. doi: 10.1186/1744-9081-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett PJ, Spoelgen R, Hyman BT, Standaert DG, Dunah AW. Dopamine D1 activation potentiates striatal NMDA receptors by tyrosine phosphorylation-dependent subunit trafficking. Journal of Neuroscience. 2006;26:4690–4700. doi: 10.1523/JNEUROSCI.0792-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Nguyen QL, Rotaru D, Keenan T, Arion D, Beneyto M, Gonzalez-Burgos G, Lewis DA. Protracted developmental trajectories of GABAA receptor alpha1 and alpha2 subunit expression in primate prefrontal cortex. Biological Psychiatry. 2009;65:1015–1023. doi: 10.1016/j.biopsych.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TM, Rocher C, Hotte M, Naudon L, Gurden H, Spedding M. Plasticity at hippocampal to prefrontal cortex synapses is impaired by loss of dopamine and stress: importance for psychiatric diseases. Neurotoxicology Research. 2004;6:233–244. doi: 10.1007/BF03033225. [DOI] [PubMed] [Google Scholar]
- Keifer J, Zheng Z. AMPA receptor trafficking and learning. European Journal of Neuroscience. 2010;32:269–277. doi: 10.1111/j.1460-9568.2010.07339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp C, Longordo F, Luthi A. Experience-dependent changes in NMDA receptor composition at mature central synapses. Neuropharmacology. 2007;53:1–9. doi: 10.1016/j.neuropharm.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Locomotor effects of acute and repeated threshold doses of amphetamine and methylphenidate: relative roles of dopamine and norepinephrine. Journal of Pharmacology and Experimental Therapeutics. 2001;296:876–883. [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. Journal of Neuroscience. 2002;22:7264–7271. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Kirkwood A. AMPA receptor regulation during synaptic plasticity in hippocampus and neocortex. Seminars in Cell and Developmental Biology. 2011;22:514–520. doi: 10.1016/j.semcdb.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA. Development of the prefrontal cortex during adolescence: insights into vulnerable neural circuits in schizophrenia. Neuropsychopharmacology. 1997;16:385–398. doi: 10.1016/S0893-133X(96)00277-1. [DOI] [PubMed] [Google Scholar]
- Li YC, Gao WJ. GSK-3beta activity and hyperdopamine-dependent behaviors. Neuroscience Biobehavioral Reviews. 2011;35:645–654. doi: 10.1016/j.neubiorev.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Xi D, Roman J, Huang YQ, Gao WJ. Activation of glycogen synthase kinase-3 beta is required for hyperdopamine and D2 receptor-mediated inhibition of synaptic NMDA receptor function in the rat prefrontal cortex. Journal of Neuroscience. 2009;29:15551–15563. doi: 10.1523/JNEUROSCI.3336-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annual Review of Neuroscience. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Mao LM, Wang W, Chu XP, Zhang GC, Liu XY, Yang YJ, Haines M, Papasian CJ, Fibuch EE, Buch S, Chen JG, Wang JQ. Stability of surface NMDA receptors controls synaptic and behavioral adaptations to amphetamine. Nature Neurosci. 2009;12:602–610. doi: 10.1038/nn.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey PVJ, B E, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2Bcontaining NMDA receptors in cortical long-term potentiation and long-term depression. Journal of Neuroscience. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y, Marzo A, Otani S. The presence of background dopamine signal converts long-term synaptic depression to potentiation in rat prefrontal cortex. Journal of Neuroscience. 2006;26:4803–4810. doi: 10.1523/JNEUROSCI.5312-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. Journal of Neuroscience. 2000;20:RC65. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. Journal of Receptors and Signal Transduction Research. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC. Expression mechanisms underlying NMDA receptor-dependent long-term potentiation. Annals of the New York Academy of Sciences. 1999;868:515–525. doi: 10.1111/j.1749-6632.1999.tb11320.x. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Current Opinions in Pharmacology. 2007;7:39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. American Journal of Psychiatry. 2007;164:942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ. Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:301–306. doi: 10.1073/pnas.011518798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O'Donnell P. D2 dopamine receptors recruit a GABA component for their attenuation of excitatory synaptic transmission in the adult rat prefrontal cortex. Synapse. 2007;61:843–850. doi: 10.1002/syn.20432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban KR, Gao WJ, Waterhouse BW. Distinct age-dependent effects of methylphenidate on developing and adult prefrontal neurons. Biological Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.04.018. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgyi B, Xin D, Bloomfield SA. Feedback inhibition in the inner plexiform layer underlies the surround-mediated responses of AII amacrine cells in the mammalian retina. Journal of Physiology. 2002;539:603–614. doi: 10.1113/jphysiol.2001.013133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Stradtman GG, 3rd, Wang XJ, Gao WJ. A specialized NMDA receptor function in layer 5 recurrent microcircuitry of the adult rat prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:16791–16796. doi: 10.1073/pnas.0804318105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitlauf CHY, Auberson YP, Mishima M, Lovinger DM, Winder DG. Activation of NR2A-containing receptors is not obligatory for NMDA receptor-dependent long-term potentiation. Journal of Neuroscience. 2005;25:8386–8390. doi: 10.1523/JNEUROSCI.2388-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo TU, Pucak ML, Kye CH, Matus CV, Lewis DA. Peripubertal refinement of the intrinsic and associational circuitry in monkey prefrontal cortex. Neuroscience. 1997;80:1149–1158. doi: 10.1016/s0306-4522(97)00059-6. [DOI] [PubMed] [Google Scholar]
- Xu Z, Chen RQ, Gu QH, Yan JZ, Wang SH, Liu SY, Lu W. Metaplastic regulation of long-term potentiation/long-term depression threshold by activity-dependent changes of NR2A/NR2B ratio. Journal of Neuroscience. 2009;29:8764–8773. doi: 10.1523/JNEUROSCI.1014-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CR, Chen L. Targeting prefrontal cortical dopamine d1 and Nmethyl- d-aspartate receptor interactions in schizophrenia treatment. Neuroscientist. 2005;11:452–470. doi: 10.1177/1073858405279692. [DOI] [PubMed] [Google Scholar]
- Yano M, Steiner H. Methylphenidate and cocaine: the same effects on gene regulation? Trends in Pharmacological Sciences. 2007;28:588–596. doi: 10.1016/j.tips.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008;55:1081–1094. doi: 10.1016/j.neuropharm.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M-G, Toyoda H, Lee Y-S, Wu L-J, Ko SW, Zhang X-H, Jia Y, Shum F, Xu H, Li B-M, Kaang B-K, Zhuo M. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron. 2005;47:859–872. doi: 10.1016/j.neuron.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Zheng P, Zhang XX, Bunney BS, Shi WX. Opposite modulation of cortical N-methyl-D-aspartate receptor-mediated responses by low and high concentrations of dopamine. Neuroscience. 1999;91:527–535. doi: 10.1016/s0306-4522(98)00604-6. [DOI] [PubMed] [Google Scholar]