Abstract
OBJECTIVES
To assess the feasibility of refining physician quality indicators of screening mammography use based on patient life expectancy.
DESIGN
Retrospective population-based cohort study
SETTING
Texas
PARTICIPANTS
3,595 usual care providers (UCPs) with at least 10 women in their patients aged 67+ on 1/1/2008 with an estimated life expectancy of ≥7 years (222,584 women) and at least 10 women with an estimated life expectancy of <7 years (90,903 women), based on age and comorbidity.
MEASUREMENTS
Screening mammography use in 2008–09 by each provider with each population.
RESULTS
The average adjusted mammography screening rates for UCPs were 31.1% and 55.2% for women with a life expectancy of <7 years and ≥7 years, respectively. For women with limited life expectancy, 3.7% of UCPs had significantly lower and 9.2% had significantly higher than average adjusted mammography screening rates. For women with longer life expectancy, 16.7% and 19.7% UCPs had significantly lower and higher than average rates, respectively. UCP adjusted screening rates were stable over time (2006–2007 vs. 2008–2009, r=0.65, p<0.001). There was a strong correlation among UCPs between screening rates of their women patients with a life expectancy of <7 years and for those with a life expectancy of ≥7 years (r=0.67, p<0.001). Most physician characteristics associated with higher screening rates (e.g., being female and foreign trained) in women with longer life expectancy were also associated with higher screening rates in women with limited life expectancy.
CONCLUSION
Providers with high mammography screening rates for women with longer life expectancy also tend to screen women with limited life expectancy. Quality indicators for screening practice can be improved by distinguishing appropriate use from overuse based on patient life expectancy.
Keywords: quality of care, screening mammography, older women, life expectancy
INTRODUCTION
Receipt of cancer screening is commonly used as an indicator of high-quality primary care.1, 2 However, cancer screening in patients with limited life expectancy is unlikely to prevent death from cancer, and is quite likely to lead to unnecessary diagnoses and treatments in patients who otherwise would have died before the cancer became clinically apparent.3–5 For example, randomized trials of mammography did not find a reduction in breast cancer mortality before seven years follow-up in women aged 65–75.5 There is an estimated four year or longer lag between mammography-detected and clinically-detected breast cancer in this age group.4 This means that many mammographically detected breast cancers in women with <7 years to live would otherwise not have been diagnosed and treated in their lifetime. The lack of benefit among women with limited life expectancy may be accompanied by physical, emotional and economic suffering imposed by over-diagnosis and over-treatment.6 Receipt of screening mammography by women whose limited life expectancy reduces the likelihood of benefit below the likelihood of harm is an indicator of over-utilization, not of high quality.
Lee and Walter7, 8 have suggested that quality indicators should include estimates of the avoidance of over-screening as well as rates of screening in appropriate populations. Previous studies on over-screening focused on patients with conditions known to result in limited life expectancy.9–12 For example, 25% women aged 70–74 with severe cognitive impairment and 12% of those aged 65–74 with advanced cancer at another site received mammography screening.9, 10 To our knowledge, there have been no attempts to distinguish appropriate screening from over-screening for the general older population at the physician-level. In this report, we assessed the feasibility of refining physician quality indicators of screening mammography use based on patient life expectancy using Medicare claims data. We evaluated screening mammography utilization at the level of the physician for women with an estimated life expectancy of <7 years (over-screening) vs. those with a life expectancy of ≥7 years (appropriate screening).
METHODS
Study Subjects
We identified a cohort of women aged 67–90 on 1/1/08 using 100% Texas Medicare data, which contain medical claims for all Texas Medicare beneficiaries. We used data from 2007 to identify the usual care provider (UCP) for these women, as well as their comorbidities. Data from 2006 and 2007 were used to assess potential indications for mammography other than screening (described in detail later). Comorbidity and age were used to estimate life expectancy as of 1/1/08.13 We then assessed the women’s screening mammography utilization in 2008–2009. The information on each woman’s demographics, Medicare entitlement, Parts A (hospital care) and B (physician and outpatient services) coverage and HMO enrollment was obtained from the Medicare enrollment files. Women’s screening status and comorbidity status were determined from Carrier File, Outpatient Statistical Analysis File (OUTSAF) and Medicare Provider Analysis and Review (MEDPAR) files. We included only those with full Medicare Parts A and B coverage and without Health Maintenance Organization (HMO) coverage during 1/1/2006 to 12/31/2009 (n=788,136). Women with HMO coverage were excluded because their claims may be incomplete. Data from 2006 and 2007 were used to assess potential indications for mammography other than screening.
Patient Characteristics
The subjects were stratified by 2-year age intervals. Comorbidity (none, 1, 2 or ≥3) was assessed by using Klabunde’s adaption of the Charlson comorbidity index based on outpatient, inpatient and carrier claims in 2007.14, 15 Similar to other studies measuring comorbidities using claims data,16 we used a one year look-back period for comorbidities. Race/ethnicity was obtained from the Medicare Part D denominator file and categorized as White, Black, Hispanic or Other/Unknown. We used median household income and percentage of high school graduates in the patient zip code area to measure patient socio-economic status and categorized them by quartiles.
Estimation of life expectancy
The methods used to develop life expectancy estimates were described in our previous publication.13 Briefly, we followed a cohort of women aged 67–90 in 2001 100% Texas Medicare data (n=716,279) through 2009. The 2001 cohort was stratified into 48 strata by age (in two year intervals) and by comorbidity score14, 15 (0, 1, 2 or >3). About 18.8% of the cohort of women aged 67–90 years in 2001 were in age-comorbidity strata with median survivals <7 years. The median survival of those strata ranged from 3.6 to 6.4 years. When those strata were combined, 63.3% of the women died within 7 years and 78.1% within 9 years. When those age-comorbidity strata with median survivals ≥7 years were combined, 75.9% of the women lived for at least 7 years.
Then, the median survival estimates for the 2001 cohort were applied to the corresponding age-comorbidity strata of the 2008 cohort.13 Only women with an identifiable UCP in Texas were included in the study. We identified 125,593 women aged 67–90 with an estimated life expectancy of <7 years and 363,003 women aged 67–84 with an estimated life expectancy of ≥7 years. The analyses were limited to UCPs with at least 10 patients in each of the two life-expectancy groups. This resulted in 90,903 women with <7 years and 222,584 women with ≥7 years estimated life expectancy, cared for by 3,595 UCPs.
Identification of the UCP
A woman’s UCP was identified as the physician who saw that woman on two or more occasions in an outpatient setting for evaluation and management (CPT codes 99201–99205 and 99211–99215) in the year 2007. The physician was identified from the Unique Provider Identification Number (UPIN) in the performing provider field. If a woman had more than one identified physician, the physician who provided most evaluation and management services was assigned as her UCP. In the case of ties, we assigned the most recently visited provider as the UCP.17
UCP characteristics
UCP age, gender, country of medical training, board certification and specialty were obtained from the American Medical Association Masterfile that was linked with Medicare claims via provider UPIN. UCPs were categorized as <50 vs. 50+ years, US vs. foreign trained, and board certified vs. not board certified. We categorized UCP specialty as Family Medicine, General Internal Medicine, OB/GYN, Geriatric or Other. The “Other” category contains specialists, such as Internal Medicine subspecialists and surgeons. The specialists were included because specialists increasingly provide routine follow-up and preventive care.18 UCP patient panel size was based on the total number of female Medicare beneficiaries aged 65 years or older under the care of each UCP during the year 2007 regardless of life expectancy and was categorized into quartiles. UCP geographic location was measured by the rural continuum codes based on the county where the UCP practiced and was categorized as Metro vs. Non-Metro area.
Screening Mammography Utilization
We defined a screening mammogram as a bilateral mammogram (Carrier files with a Current Procedure Terminology [CPT] or Healthcare Common Procedure Coding System [HCPCS] code of 76056, 76057, G0202 or G0204) with no mammogram (CPT or HCPCS codes of 76055, 76056, 76057, G0202, G0204 and G0206) in the previous 11 months and with no diagnosis of breast cancer or breast mass (any claim with ICD-9-CM codes 174xx, 2330 and 61172) in the previous two years.19, 20 We validated the algorithm with mammography record review and estimated that 92% of the algorithm-identified screening mammograms were confirmed screenings.19 Appropriate use was defined as a screening mammogram in a woman with a life expectancy of at least seven years while over-use was defined as screening mammography in women with shorter life expectancy.
Statistical Analysis
Descriptive analysis was used to summarize the patient and UCP characteristic and rates of mammography screening for women with a life expectancy of <7 years and ≥7 years, respectively. We used multilevel logistic regression modeling21 for both groups to (1) evaluate the association between UCP characteristics and screening utilization, adjusting for patient characteristics and (2) generate rates of mammography screening for each UCP, adjusting for within-UCP clustering. The average adjusted screening rates derived from the multilevel model were used as a statistical benchmark.22 The adjusted screening rate of each UCP was compared to these average rates to identify UCPs with statistically significantly higher or lower than average rates at a significance level of 0.05. Pearson correlation and Wilcoxon signed rank test were used to test the association of UCP screening rates for women with a life expectancy of <7 years vs. those for women with a life expectancy of ≥7 years.
Lastly, we evaluated the stability of the UCP profiling by comparing the screening rates among women with a life expectancy of <7 years for each UCP in 2008–09 to their rates in 2006–07, using the Spearman rank correlation and Wilcoxon signed rank test. The 2006–07 cohort was identified using the same inclusion and exclusion criteria described above. The analysis included only the 2743 UCPs with 10 or more women with a life expectancy of <7 years in both 2006–2007 and 2008–2009 cohorts. SAS version 9.2 (SAS Institute, Cary, NC) was used for all statistical analyses.
RESULTS
Table 1 presents the characteristics of the women in the limited life expectancy (<7 years) and longer life expectancy (≥7 years) cohorts, and the mammography screening rates of each. Among women with limited life expectancy, non-Hispanic whites had the highest screening rates (33.0%), followed by non-Hispanic Blacks (31.7%) and Hispanics (25.6%). Screening rates increased with higher zip code income and education. The patterns were similar for women with longer life expectancy, but the screening rates were higher for each category.
Table 1.
Screening Mammography Use in 2008–2009 for Women with an Estimated Life Expectancy of <7 Years and ≥7 Years on 1/1/2008
| Patient Characteristics | N of patients (% receiving screening mammography)
|
|
|---|---|---|
| Life Expectancy <7 years | Life Expectancy ≥ 7 years | |
| All | 90903 (32.5) | 222584 (56.5) |
| Age (years) | ||
| 67–68 | 1347 (42.8) | 19595 (61.8) |
| 69–70 | 1972 (41.3) | 32595 (62.3) |
| 71–72 | 2101 (42.6) | 31579 (60.9) |
| 73–74 | 2411 (42.5) | 31713 (59.3) |
| 75–76 | 2408 (39.0) | 29101 (56.3) |
| 77–78 | 5557 (40.7) | 25753 (54.8) |
| 79–80 | 5317 (36.6) | 24146 (50.4) |
| 81–82 | 12377 (37.5) | 14804 (47.5) |
| 83–84 | 11151 (33.5) | 13298 (42.0) |
| 85–86* | 19982 (32.5) | 0 |
| 87–88* | 15887 (26.3) | 0 |
| 89–90* | 10393 (19.8) | 0 |
| Race | ||
| Non-Hispanic White | 67995 (34.2) | 173883 (58.8) |
| Non-Hispanic Black | 6259 (33.2) | 12970 (54.4) |
| Hispanics | 15503 (25.4) | 31749 (45.7) |
| Other/Unknown | 1094 (23.9) | 3898 (46.9) |
| Comorbidity score | ||
| 0 | 25511 (30.8) | 151933 (58.3) |
| 1 | 25721 (32.9) | 57730 (53.2) |
| 2 | 16319 (33.1) | 12921 (50.2) |
| 3+ | 23352 (33.6) | 0 (n/a) |
| Household income§ | ||
| Q1 (low) | 22022 (28.6) | 46374 (49.9) |
| Q2 | 24118 (32.7) | 58363 (55.4) |
| Q3 | 23079 (35.1) | 59030 (58.9) |
| Q4 (high) | 17352 (34.6) | 47509 (61.8) |
| Education¥ | ||
| Q1 (low) | 18598 (27.9) | 37342 (48.3) |
| Q2 | 22691 (31.3) | 55238 (53.6) |
| Q3 | 24891 (34.0) | 64910 (58.6) |
| Q4 (high) | 20396 (36.9) | 53810 (63.1) |
No women aged >84 were in the strata with ≥7 years estimated life expectancy.13
Household income was measured by the median household income in the patient zip code area from US Census data. It was categorized into quartiles (Q1–Q4) from low to high.
Education was measured by the percentage of high school graduates in the patient zip code area from US Census data. It was categorized into quartiles (Q1–Q4) from low to high.
Table 2 shows the association between UCP characteristics and screening mammography utilization. These are presented as unadjusted rates and also adjusted in a multilevel model controlling for patient characteristics. The number of women with limited life expectancy per UCP ranged from 10 to 182, with a median of 20. The range for women with longer life expectancy was 10 to 382, with a median of 54. The UCP characteristics associated with screening women with limited and longer life expectancy were remarkably similar. Women in both cohorts were more likely to receive screening if their UCP was older, female, a graduate of a non-US medical school, board qualified and practiced in metro areas. Family practice physicians were significantly less likely to screen each group, compared to Internal Medicine UCPs. There were small numbers of OB/GYN and geriatric physicians, leading to wide confidence intervals.
Table 2.
Associations of Usual Care Provider (UCP) Characteristics with Screening Mammography Use
| UCP Characteristics | Number of UCPs | Life Expectancy <7 years
|
Life Expectancy ≥ 7 years
|
||
|---|---|---|---|---|---|
| Number of patients (% receiving mammography screening) | Odds Ratio (95% CI) of receiving mammography*± | Number of patients (% receiving mammography screening) | Odds Ratio (95% CI) of receiving mammography*± | ||
| Overall | 3595 | 90903 (32.5) | n/a | 222584 (56.5) | n/a |
| Age (years) | |||||
| <50 | 1619 | 38840 (32.4) | 0.90 (0.86, 0.95) | 95803 (56.8) | 0.94 (0.90, 0.98) |
| 50+ | 1976 | 52063 (32.6) | ref | 126781 (55.2) | ref |
| Gender | |||||
| Female | 695 | 16394 (38.2) | 1.33 (1.25, 1.42) | 42962 (63.1) | 1.34 (1.28, 1.41) |
| Male | 2900 | 74509 (31.3) | ref | 179622 (54.9) | ref |
| US trained | |||||
| No | 2600 | 64816 (34.0) | 1.30 (1.23, 1.38) | 167580 (58.4) | 1.35 (1.29, 1.41) |
| Yes | 995 | 26087 (28.8) | ref | 55004 (50.7) | ref |
| Board Certification | |||||
| No | 1147 | 26608 (29.9) | ref | 66788 (52.8) | ref |
| Yes | 2448 | 64295 (33.6) | 1.09 (1.03, 1.15) | 155796 (58.1) | 1.13 (1.08, 1.18) |
| Panel Size of women 65+ | |||||
| Q1 (23–77) | 900 | 12186 (31.4) | 0.94 (0.88, 1.01) | 26909 (54.4) | 0.85 (0.80, 0.90) |
| Q2 (78–109) | 903 | 15721 (31.0) | 0.93 (0.87, 1.00) | 41969 (55.5) | 0.90 (0.85, 0.95) |
| Q3 (110–156) | 901 | 22589 (32.6) | 1.00 (0.94, 1.07) | 57961 (56.1) | 0.93 (0.88, 0.98) |
| Q4 (157+) | 891 | 40407 (33.4) | ref | 95745 (57.7) | ref |
| Specialty | |||||
| Family Practice | 1540 | 33433 (28.4) | 0.71 (0.67, 0.75) | 91817 (52.4) | 0.73 (0.70, 0.77) |
| Internal Medicine | 1613 | 49871 (36.2) | ref | 115096 (60.9) | ref |
| OB/GYN | 9 | 123 (43.1) | 1.61 (0.92, 2.80) | 469 (59.1) | 1.04 (0.69, 1.59) |
| Geriatrics | 26 | 727 (31.6) | 0.90 (0.67, 1.21) | 1090 (55.1) | 0.88 (0.68, 1.15) |
| Other | 1540 | 6749 (26.0) | 0.66 (0.60, 0.73) | 14112 (47.0) | 0.62 (0.58, 0.67) |
| Location | |||||
| Metro Area | 3090 | 76662 (33.3) | 1.15 (1.07, 1.24) | 186221 (57.4) | 1.15 (1.08, 1.21) |
| Non-Metro Area | 505 | 14240 (28.5) | ref | 36363 (51.6) | ref |
The odds ratios (OR) and 95% confidence intervals (CI) were from a multilevel model with UCP random intercepts and fixed effects of UCP characteristics and patient characteristics (age, comorbidity, race, median household income and percentage of high school graduate in the zip code area). We repeated these analyses without including patient characteristics and obtained very similar results. We also performed the analysis including all UCPs with at least one patient in either life expectancy category. These results were also very similar.
For women with an estimated life expectancy of <7 years, the intra-class correlation coefficient (ICC) for UCPs was 9.8% for the null model, 8.1% for the model with UCP characteristics and 8.3% with UCP and patient characteristics. For women with an estimated life expectancy ≥7 years, the ICC was 9.8% for the null model, 7.7% after adding UCP characteristics and 7.3% with UCP and patient characteristics.
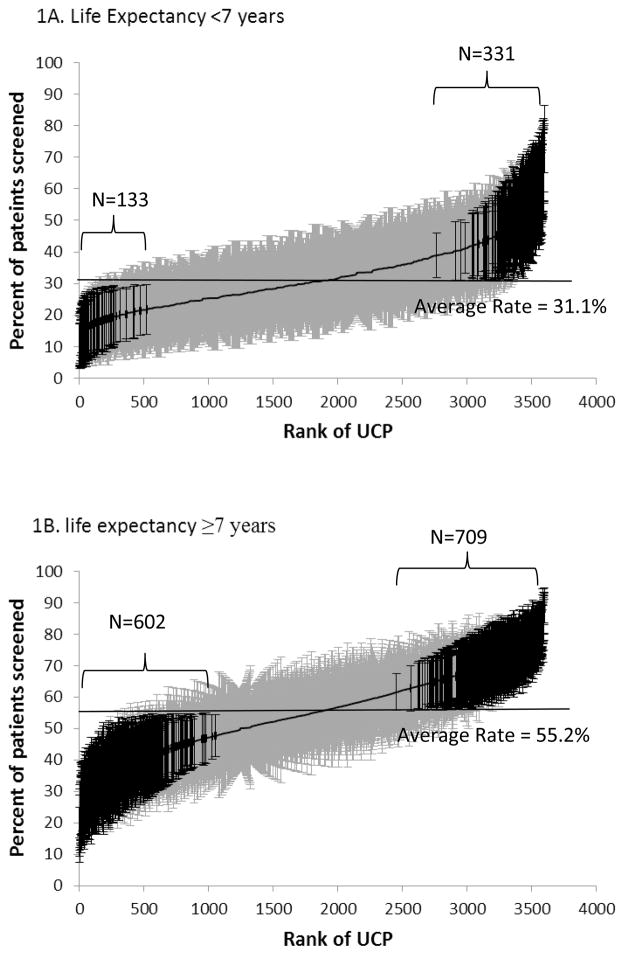
Figure 1 presents screening mammography utilization among the 3,595 UCPs with 10 or more female patients with estimated life expectancies of <7 years, and ≥7 years, derived from a multilevel model with random intercepts for UCP effect. UCPs were ranked by their adjusted screening rates, from low to high. The screening rates of UCPs for women with limited life expectancy ranged from 10.6% to 75.7%, with a mean of 31.1%. For women with longer life expectancy, the range of the screening rates of the UCPs was 17.1% to 89.2%, with a mean of 55.2%. For women with limited life expectancy, 3.7% of UCPs had significantly lower and 9.2% had significantly higher rates than the average adjusted screening rate for all UCPs. For women with longer life expectancy, 16.7% and 19.7% had significantly lower and higher than average rates, respectively. The analyses that adjusted for patient characteristics in the multilevel models had similar results.
Figure 1.
Cumulative distribution of 3595 usual care providers (UCPs) by the rates of screening mammography of their patients in 2008–09. Figure 1A includes the rates for women with an estimated life expectancy of <7 years and Figure 1B includes women with an estimated life expectancy of ≥7 years. Adjusted screening rates and 95% confidence intervals for each UCP are shown, derived from a multilevel null model. UCPs were ranked from 1 to 3595 (horizontal axis) by their screening rates (vertical axis). The UCP with a rank of one had the lowest screening rate, while the UCP with a rank of 3595 had the highest screening rate. UCPs with rates significantly different from the average are indicated with dark ink.
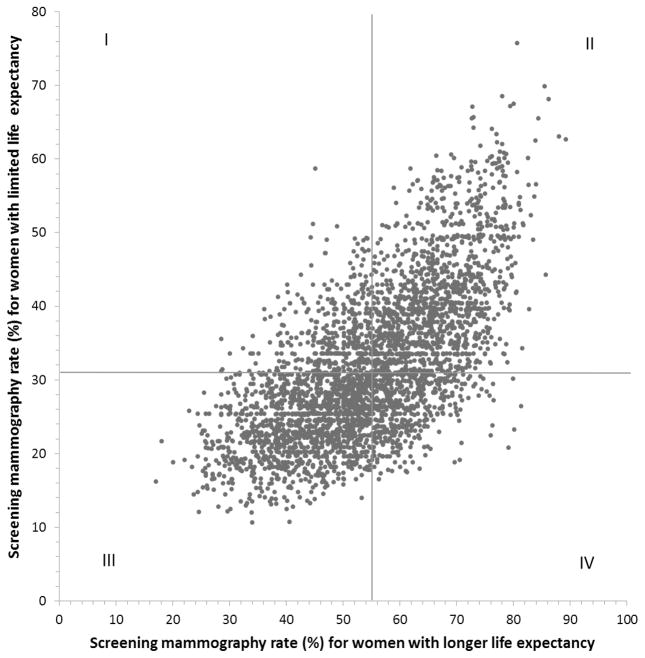
Figure 2 shows a scatterplot showing the association at the UCP level between rates of screening mammography in women with limited life expectancy and rates in women with longer life expectancy, generated from the multilevel models. Each UCP is represented by a point on the plot, indicating the UCP’s rate for screening women with limited life expectancy (vertical axis) and rate for screening women with longer life expectancy (horizontal axis) in 2008–09. Most UCPs tended to have high rates of screening mammography for both groups (Quadrant II) or low rates of screening mammography for both groups (Quadrant III). The Pearson correlation coefficient between the two rates was 0.67 (P<0.001). Wilcoxon signed rank test showed no significant difference between the UCP rankings in screening rates in the two groups (P=0.98). We repeated the analysis using the actual UCP screening rates (i.e., not derived from a multilevel model) and the pattern was similar. Among the 331 UCPs in Figure 1 who had significantly higher than average screening rates in women with limited life expectancy, 255 (77%) also had significantly higher than average, and none had significantly lower than average, rates for women with longer life expectancy. Among the 133 UCPs with significantly lower than average screening mammography rates in women with limited life expectancy, 92 (69.1%) also had significantly lower than average, and only one had significantly higher than average, screening rates in women with longer life expectancy.
Figure 2.
Scatterplot of rates for each usual care provider (UCP) of screening mammography for their patients with limited life expectancy (<7 years) vs. longer life expectancy (≥7 years). Each of the 3595 UCPs is represented by one point, indicating the mammography rates of their patients with limited (vertical axis) and longer life expectancies (horizontal axis) in 2008–2009. The plot is divided into four quadrants based on the average UCP screening rates for patients with longer and limited life expectancy. For example, UCPs in quadrant I have screening rates above the mean for women with limited life expectancy and below the mean for women with longer life expectancy. The rates were generated from multilevel null models. The Pearson correlation coefficient between the two rates is 0.67 (p<0.001).
Finally, we assessed the stability of screening rates among UCPs over time. We studied the 2743 UCPs who provided care for at least 10 women with life expectancy of <7 years in 2006 and also in 2008. We ranked UCPs by the adjusted screening mammography rates in each period (2006–2007 and 2008–2009). The Spearman correlation coefficient of the two sets of rankings was 0.65, P<0.001. Wilcoxon signed rank test showed no significant difference between the two sets of rankings (P=0.67). We also categorized the two sets of rankings into quintiles (Table 3). Of the UCPs in the highest quintile of mammography use in 2006–2007, 75.2% were in the first or second quintile in 2008–2009. Of the UCPs in the lowest quintile in 2006–2007, 86.6% were in the lowest two quintiles in 2008–2009.
Table 3.
Rank Stability, by Quintile, of Usual Care Providers (UCPs) in 2006–07 vs. 2008–09, in Screening Mammography of Women with an Estimated Life Expectancy of <7 Years*
| 2006–2007 Cohort† | 2008–2009 Cohort†
|
||||
|---|---|---|---|---|---|
| Quintile 1 (13.6–27.6%) | Quintile 2 (27.7–32.9%) | Quintile 3 (33.0–38.4%) | Quintile 4 (38.5–45.9%) | Quintile 5 (46.0–74.1%) | |
| Quintile 1 (11.4–23.7%) | 47.5% | 27.7% | 17.9% | 5.7% | 1.3% |
| Quintile 2 (23.7–28.9%) | 29.3% | 27.9% | 25.7% | 13.0% | 4.1% |
| Quintile 3 (29.0–34.3%) | 14.3% | 23.9% | 23.8% | 27.9% | 10.2% |
| Quintile 4 (34.4–42.5%) | 7.7% | 15.9% | 19.1% | 32.3% | 25.0% |
| Quintile 5 (42.6–78.7%) | 1.3% | 4.6% | 13.6% | 21.1% | 59.5% |
The ranges of the percent of women receiving screening mammography in each UCP quintile are given in parentheses. The percent of UCPs in each quintile of mammography screening in 2006–07 are given for each quintile in 2008–09. The Spearman rank correlation coefficient between the two sets of ranking is 0.67 (p<0.001).
The analysis included the 2743 UCPs who had 10 or more female patients with a life expectancy <7 years in both 2006–2007 and 2008–2009 cohorts.
DISCUSSION
As noted by Welch,2 screening mammography rates are widely used to indicate quality in primary care services and to rate primary care physicians. There have been no attempts in those ratings to separate appropriate screening from overscreening.2, 8 Our study suggests that such an approach is possible. We found considerable variation in over-screening at the level of the UCP. Interestingly, the UCP characteristics associated with overutilization (i.e., screening women with an estimated life expectancy of <7 years) were also associated with appropriate utilization. Most of these characteristics have been shown in other studies to be associated with greater utilization of mammography in all women.17, 23–25 Ideally, one might wish a UCP to have a high rate of screening in women with longer life expectancy and a very low rate in women with limited life expectancy. In reality, UCPs with higher rates of screening mammography use in women with longer life expectancy also tended to have higher rates in women with limited life expectancy. UCPs who avoided screening mammography in women with limited life expectancy also tended to have low screening rates in women with longer life expectancy. Very few UCPs in Figure 2 had high rates of mammography screening in women with longer life expectancy and low rates in those with limited life expectancy.
Physician recommendation is a major driver of cancer screening.23 Physician-level quality assessment and interventions are crucial for quality improvement of screening services. However, concern has arisen about the instability of quality measures, with top ranked providers in one year often appearing as low ranked providers the next year.26 Our quality measures (UCP rates of appropriate- and over-screening) were stable over time. The majority of the top and low performers in one period were identified as the top and low performers in the subsequent period. Such stable quality assessment is a critical first-step in implementing a systematic accountability program in health care.
The current mammography quality measures by HEDIS27 and AHRQ28 use upper age limits because age is used as a proxy for life expectancy. For example, both quality measures exclude women aged 75 years or older (ages 40–69 by HEDIS27 and ages 50–75 by AHRQ28). Our study substituted age-comorbidity based life expectancy estimates for age-alone in defining appropriate patient populations to generate quality measures. If an estimated life expectancy of >7 years were used to define the population appropriate for screening, then approximately three quarters of women aged 75–85 would be included. Conversely, approximately 6.4% of women aged 67–75 had an estimated life expectancy of <7 years and would not be included in the population appropriate for screening. Further, including an indicator of overuse, in addition to appropriate uses, might influence physicians to reduce such practices over time.29, 30
One potential barrier to reducing overuse of screening is that physicians are not necessarily skilled in estimating life expectancy. Gill has recently noted the importance of applying prognostic information in medical decision making, and has called for more attention given to that subject in medical education.31 A recent systematic review described the quality and limitations of current prognostic indices.32 Most of them focus on relatively short term survival, and only one was validated for survival of 5 years or more.33 We have validated our method in comparisons against age alone in predicting 1, 5, 7 and 10 year survival for older women. The C statistics for our method range from 0.79 to 0.81, compared to 0.69 to 0.74 for age alone (Tan A., Kuo Y-F and Goodwin JS, manuscript in preparation).
All prognostic instruments, including ours, lack precision at the level of the individual.31,32 However, the rationale and evidence underlying virtually all screening programs, including mammography, is at the population level, not the individual. The same is true of physician quality measures. Our method is developed for studies using administrative data. We are not proposing that administrative data be used to generate prognostic estimates in clinical practice. Clinicians have access to much richer information, such as functional status, nutritional status, self-rated health and severity of the comorbid conditions, which should allow for more accurate estimates of life expectancy.34 What we are proposing is that provider quality indicators for cancer screening should be refined to more accurately define appropriate and inappropriate target populations based on patient life expectancy.
We chose a cutoff of seven years’ life expectancy based on evidence from randomized mammography trials.5 Other life expectancy cutoffs could be used. For example, one might define the population not appropriate for screening as <7 years estimated life expectancy and the population appropriate for screening as >10 years estimated life expectancy, with those with 7–10 years estimated life expectancy not included in the measurements. However, various cutoffs are unlikely to affect the overall physician practice patterns found in our study.
The study has several limitations. First, information on patient preference, another major driver of screening mammography utilization,35 is not available in administrative claims data. We could only identify receipt of mammography, not what the provider recommended. Stefanek36 has suggested that continuing efforts to define populations appropriate for screening will not be effective without comparable efforts to educate individual screening candidates about potential harms and benefits of screening in a shared decision model.
Limited by the use of claims data, we were unable to include in life expectancy measures information on risk of breast cancer, nor information on self-rated health and functional status.37,38 Nevertheless, the age-comorbidity based algorithm we used provides improved life expectancy estimates compared to the age-alone method. Our algorithm for identifying UCPs included surgical and medical specialists, who might not all be functioning as the patient’s primary care physician. However, the results were similar after dropping specialists from the analyses. Another limitation is that we studied the Texas Medicare population, which is slightly younger and poorer, and contains more Hispanics than the US Medicare population.39 The study findings may be not generalizable to regions outside Texas. Lastly, we did not assess the frequency of doctor visits which may be associated with screening use.40
In conclusion, we found that within physicians, there is a strong correlation between mammography screening of women with limited and longer life expectancies. Quality assessment of physician screening practices can be improved by distinguishing appropriate use from overuse based on patient life expectancy.
Acknowledgments
We thank Sarah Toombs Smith, PhD, Science Editor and Assistant Professor in the Sealy Center on Aging, University of Texas Medical Branch at Galveston, for her editorial assistance.
The study was supported by the Cancer Prevention Research Institute of Texas (RP101201) and the National Institutes of Health (K05CA134923, 8UL1TR000071 and 5P30AG024832-08).
Sponsor’s Role: The funding agencies had no role in the study’s design, conduct, analysis, or interpretation of data or in the preparation, review, or approval of the manuscript.
Footnotes
Conflict of Interest: All authors have no financial or other kind of personal conflicts with this paper.
Author Contributions: Tan: Study concept and design, analysis and interpretation of data, drafting the manuscript. Kuo: Study concept and design, interpretation of data and critical review of the manuscript. Elting: Interpretation of data and critical review of the manuscript. Goodwin: Study concept and design, interpretation of data, drafting and critical review of the manuscript.
References
- 1.Carrier ER, Schneider E, Pham HH, et al. Association between quality of care and the sociodemographic composition of physicians’ patient panels: a repeat cross-sectional analysis. J Gen Intern Med. 2011;26(9):987–994. doi: 10.1007/s11606-011-1740-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Welch HG. Screening mammography--a long run for a short slide? N Engl J Med. 2010;363(13):1276–1278. doi: 10.1056/NEJMe1008369. [DOI] [PubMed] [Google Scholar]
- 3.Satariano WA, Ragland DR. The effect of comorbidity on 3-year survival of women with primary breast cancer. Ann Intern Med. 1994;120(2):104–110. doi: 10.7326/0003-4819-120-2-199401150-00002. [DOI] [PubMed] [Google Scholar]
- 4.Paci E, Miccinesi G, Puliti D, et al. Estimate of overdiagnosis of breast cancer due to mammography after adjustment for lead time. A service screening study in Italy. Breast Cancer Res. 2006;8(6):R68. doi: 10.1186/bcr1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nyström L, Andersson I, Bjurstam N, et al. Long-term effects of mammography screening: updated overview of the Swedish randomised trials. Lancet. 2002;359(9310):909–919. doi: 10.1016/S0140-6736(02)08020-0. [DOI] [PubMed] [Google Scholar]
- 6.Walter LC, Eng C, Covinsky KE. Screening mammography for frail older women: what are the burdens? J Gen Intern Med. 2001;16(11):779–784. doi: 10.1111/j.1525-1497.2001.10113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walter LC. What is the right cancer screening rate for older adults?: comment on “prevalence of cancer screening in older, racially diverse adults”. Arch Intern Med. 2011;171(22):2037–2038. doi: 10.1001/archinternmed.2011.556. [DOI] [PubMed] [Google Scholar]
- 8.Lee SJ, Walter LC. Quality indicators for older adults: preventing unintended harms. JAMA. 2011;306(13):1481–1482. doi: 10.1001/jama.2011.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta KM, Fung KZ, Kistler CE, et al. Impact of cognitive impairment on screening mammography use in older US women. Am J Public Health. 2010;100(10):1917–1923. doi: 10.2105/AJPH.2008.158485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sima CS, Panageas KS, Schrag D. Cancer screening among patients with advanced cancer. JAMA. 2010;304(14):1584–1591. doi: 10.1001/jama.2010.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellizzi KM, Breslau ES, Burness A, et al. Prevalence of cancer screening in older, racially diverse adults: still screening after all these years. Arch Intern Med. 2011;171(22):2031–2037. doi: 10.1001/archinternmed.2011.570. [DOI] [PubMed] [Google Scholar]
- 12.Schonberg MA, McCarthy EP, Davis RB, et al. Breast cancer screening in women aged 80 and older: results from a national survey. J Am Geriatr Soc. 2004;52(10):1688–1695. doi: 10.1111/j.1532-5415.2004.52462.x. [DOI] [PubMed] [Google Scholar]
- 13.Tan A, Kuo Y-F, Goodwin JS. Integrating age and comorbidity to assess screening mammography utilization. American Journal of Preventive Medicine. 2012;42(3):229–234. doi: 10.1016/j.amepre.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 16.Klabunde CN, Warren JL, Legler JM. Assessing comorbidity using claims data: an overview. Med Care. 2002;40(8 Suppl):IV-26–35. doi: 10.1097/00005650-200208001-00004. [DOI] [PubMed] [Google Scholar]
- 17.Finison KS, Wellins CA, Wennberg DE, et al. Screening mammography rates by specialty of the usual care physician. Eff Clin Pract. 1999;2(3):120–125. [PubMed] [Google Scholar]
- 18.Valderas JM, Starfield B, Forrest CB, et al. Ambulatory care provided by office-based specialists in the United States. Ann Fam Med. 2009;7(2):104–111. doi: 10.1370/afm.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freeman JL, Goodwin JS, Zhang D, et al. Measuring the performance of screening mammography in community practice with Medicare claims data. Women Health. 2003;37(2):1–15. doi: 10.1300/J013v37n02_01. [DOI] [PubMed] [Google Scholar]
- 20.Randolph WM, Mahnken JD, Goodwin JS, et al. Using Medicare data to estimate the prevalence of breast cancer screening in older women: Comparison of different methods to identify screening mammograms. Health Serv Res. 2002;37(6):1643–1657. doi: 10.1111/1475-6773.10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leyland A, Goldstein H, editors. Multilevel modelling of health statistics. New York, NY: John Wiley & Sons, Inc; 2001. [Google Scholar]
- 22.Normand S-LT, Glickman ME, Gatsonis CA. Statistical methods for profiling providers of medical care: Issues and applications. J Am Stat Assoc. 1997;92(439):803–814. [Google Scholar]
- 23.Bhosle M, Samuel S, Vosuri V, et al. Physician and patient characteristics associated with outpatient breast cancer screening recommendations in the United States: analysis of the National Ambulatory Medical Care Survey Data 1996–2004. Breast Cancer Res Treat. 2007;103(1):53–59. doi: 10.1007/s10549-006-9344-3. [DOI] [PubMed] [Google Scholar]
- 24.Henderson JT, Weisman CS. Physician gender effects on preventive screening and counseling: An analysis of male and female patients’ health care experiences. Med Care. 2001;39(12):1281–1292. doi: 10.1097/00005650-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Andersen MR, Urban N. Physician gender and screening: Do patient differences account for differences in mammography use? Women Health. 1997;26(1):29–39. doi: 10.1300/J013v26n01_03. [DOI] [PubMed] [Google Scholar]
- 26.Parry GJ, Gould CR, McCabe CJ, et al. Annual league tables of mortality in neonatal intensive care units: longitudinal study. International Neonatal Network and the Scottish Neonatal Consultants and Nurses Collaborative Study Group. BMJ. 1998;316(7149):1931–1935. doi: 10.1136/bmj.316.7149.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Committee for Quality Assurance. [Accessed August 9, 2012];Continous improvement and the expansion of quality measurement. 2011 Available at: http://www.ncqa.org/LinkClick.aspx?fileticket=FpMqqpADPo8%3D.
- 28.Agency for Healthcare Research and Quality. [Accessed August 9, 2012];National health care quality report. 2011 Available at: http://www.ahrq.gov/qual/nhqr11/nhqr11.pdf.
- 29.Feasby TE, Barnett HJ. Improving the appropriateness of carotid endarterectomy. Neurology. 2007;68(3):172–173. doi: 10.1212/01.wnl.0000254507.52005.7d. [DOI] [PubMed] [Google Scholar]
- 30.Halm EA, Tuhrim S, Wang JJ, et al. Has evidence changed practice?: Appropriateness of carotid endarterectomy after the clinical trials. Neurology. 2007;68(3):187–194. doi: 10.1212/01.wnl.0000251197.98197.e9. [DOI] [PubMed] [Google Scholar]
- 31.Gill T. The central role of prognosis in clinical decision making. JAMA. 2012;307(2):199–200. doi: 10.1001/jama.2011.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yourman LC, Lee SJ, Schonberg MA, et al. Prognostic indices for older adults: A systematic review. JAMA. 2012;307(2):182–192. doi: 10.1001/jama.2011.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schonberg MA, Davis RB, McCarthy EP, et al. Index to predict 5-year mortality of community-dwelling adults aged 65 and older using data from the National Health Interview Survey. J Gen Intern Med. 2009;24(10):1115–1122. doi: 10.1007/s11606-009-1073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fried LP, Kronmal RA, Newman AB, et al. Risk factors for 5-year mortality in older adults: The Cardiovascular Health Study. JAMA. 1998;279(8):585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 35.Charles C, Gafni A, Whelan T. Decision-making in the physician-patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med. 1999;49(5):651–661. doi: 10.1016/s0277-9536(99)00145-8. [DOI] [PubMed] [Google Scholar]
- 36.Stefanek ME. Uninformed compliance or informed choice? A needed shift in our approach to cancer screening. J Natl Cancer Inst. 2011;103(24):1821–1826. doi: 10.1093/jnci/djr474. [DOI] [PubMed] [Google Scholar]
- 37.Lee SJ, Lindquist K, Segal MR, et al. Development and validation of a prognostic index for 4-year mortality in older adults. JAMA. 2006;295(7):801–808. doi: 10.1001/jama.295.7.801. [DOI] [PubMed] [Google Scholar]
- 38.Lesser GT. Social and productive activities in elderly people. Self rated health is important predictor of mortality. BMJ. 2000;320(7228):185. [PubMed] [Google Scholar]
- 39.The Kaiser Family Foundation. [Accessed September 10, 2012];Texas: Medicare population demographics. Available at: http://www.statehealthfacts.org/profileind.jsp?cat=6&sub=75&rgn=45.
- 40.Burns RB, McCarthy EP, Freund KM, et al. Variability in mammography use among older women. J Am Geriatr Soc. 1996;44(8):922–926. doi: 10.1111/j.1532-5415.1996.tb01861.x. [DOI] [PubMed] [Google Scholar]