Abstract
High frequency spinal cord stimulation (HF-SCS) is a method of inspiratory muscle activation resulting in phrenic motoneuron activation via stimulation of spinal cord pathways. The specific pathways mediating this response, however, are unknown. The aim of this study was to assess the potential role of upper cervical (C1–C4) pre-phrenic interneurons (UCI) and localize the pathways in the thoracic spinal cord mediating activation of phrenic motoneurons during HF-SCS. In 7 anesthetized, spinalized (C1 level) dogs, HF-SCS was applied at the T2 level. Diaphragm EMG, inspired volume and airway pressure generation were monitored before and following sequential spinal cord sections at the C4 and C8 levels. Section at the C4 level and dorsal columns at C8 resulted in no significant changes. However, lateral funiculi section (C8 level) resulted in significant reductions in each parameter. We conclude that during upper thoracic HF-SCS, the phrenic motoneuron pools are activated via spinal pathways located in the lateral funiculus but UCI are not involved.
Keywords: spinal cord stimulation, inspiratory muscles, spinal cord injury
1. Introduction
Based upon previous animal studies, the application of electrical stimulation in the region of the upper thoracic spinal cord with high stimulus frequencies (~300 Hz) results in activation of spinal cord pathways that synapse with the inspiratory motoneuron pools (DiMarco and Kowalski, 2009, 2010, 2011). This method results in processing of the stimulus within these neurons and consequent physiologic activation of the inspiratory muscles. Physiologic activation is evidenced by the fact that high frequency spinal cord stimulation (HF-SCS) results in an asynchronous pattern of diaphragm and inspiratory intercostal activation and single motor unit firing frequencies similar to those observed during spontaneous breathing. Consequently, HF-SCS may be a useful method of inspiratory muscle pacing in subjects with ventilator-dependent cervical spinal cord injury (SCI).
Optimal activation of the inspiratory muscles occurred via application of HF-SCS with a single electrode positioned at the T2 spinal level on the ventral epidural surface (DiMarco et al., 1987; DiMarco and Kowalski, 2009, 2010, 2011). The specific neuronal networks in the cervical spinal cord mediating phrenic motoneuron activation during HF-SCS, however, are unknown. Potential mechanisms include activation of pre-phrenic interneurons, located in the upper cervical spinal cord (C1–C4), which synapse with the phrenic and upper thoracic inspiratory motoneuron pools and/or activation of neuronal circuits either cephalad to, or in close vicinity to the phrenic motoneurons (Dobbins and Feldman, 1994; Duffin and Iscoe, 1996; Hayashi et al., 2003; Hilaire et al., 1986; Lane et al., 2008b, 2009; Lipski and Duffin, 1986; Lois et al., 2009; Nakazono and Aoki, 1994; Palisses and Viala, 1987). If the mechanism of HF-SCS to activate the phrenic motoneurons is dependent upon functional neuronal structures in the upper cervical spinal cord, this method would not be successful in providing inspiratory muscle pacing in ventilator-dependent subjects with SCI. To investigate the potential role of upper cervical pre-phrenic interneurons on diaphragm activation, the degree of phrenic activation was monitored during HF-SCS, before and after sequential section of the spinal cord at the upper C4 level.
The spinal cord pathways in the vicinity of the stimulating electrode, which mediate activation of the phrenic motoneuron pools via HF-SCS, are also unknown. Previous investigators, however, have described afferent inputs from the lower thoracic intercostal and abdominal muscles that reflexly facilitate phrenic motoneuron discharge via spinal cord pathways (intercostal to phrenic reflex) (Decima et al., 1967, 1969a, 1969b). These pathways have been shown to reside bilaterally in the ventrolateral funiculus of the thoracic spinal cord. We hypothesized that pathways in this region of the spinal cord mediate activation of the phrenic motoneurons during HF-SCS. To test this hypothesis, the degree of phrenic activation was monitored before and after sequential section of the spinal cord at the C8 level. The degree of phrenic activation was monitored by measurements of diaphragm EMG, inspired volume and airway pressure generation during HF-SCS.
2. Methods
Studies were performed on 7 mongrel dogs (mean weight 15.6 ± 0.5 kg) with the approval of the Institutional Animal Care and Use Committee of Case Western Reserve University. Animals were anesthetized initially with pentobarbital sodium (PB, 25 mg/kg), administered intravenously. Additional doses of PB (1–2 mg/kg) were provided as needed to maintain an absent corneal reflex and absent response to noxious stimuli.
A size 10 mm ID endotracheal tube was sutured into the trachea in the midcervical region. A catheter was placed in the femoral vein to administer supplemental anesthesia and fluids. Blood pressure and heart rate were monitored from a catheter placed in the femoral artery (Waveline Pro Multi-Function Monitor, DRE Inc, Louisville, KY). Oxygen saturation was monitored from the earlobe and end-tidal PCO2 at the trachea (Waveline Pro). Body temperature was maintained with a heating blanket (Harvard Apparatus, Cambridge, MA) at 38 ± 0.5°C. Airway pressure was measured at functional residual capacity (FRC) following airway occlusion with a pressure transducer (Validyne, MP45, Northridge, CA) which was connected to the airway opening. Tidal volume was measured by electrical integration of the flow signal from a pneumotachograph (Series 3700, Hans Rudolph, Kansas City, MO).
Laminectomies were performed at the C1, C4 and C8 levels to allow spinal cord section at these locations. Based upon previously described techniques, a final laminectomy was performed at the T4–T5 level for placement of an 8 plate stimulation lead with 4 mm contacts (model AD-TEDH Medical Instrument Corp, Racine, WI) at the T2 level on the ventral surface. The lead was positioned under direct vision on the ventral surface of the spinal cord and advanced to the T2 level (as previously described: DiMarco and Kowalski, 2009, 2010, 2011). An indifferent ground electrode was implanted in the back musculature. A grass square-wave pulse stimulator (model S88, Grass Technologies, West Harwick, RI) equipped with a stimulus isolation unit (PSIU6, Grass Technologies) was used to provide monopolar electrical stimulation at 300 Hz over a range of stimulus amplitudes (0 – 6 mA). Stimulus train duration was fixed at 1.2 s since a plateau in pressure and volume generation is generally achieved by this time. Electrical stimulation was provided over a wide range of stimulus amplitudes to evaluate potential stimulus current related effects.
Inspiratory muscle EMG recordings of the parasternal (2nd interspace), and costal diaphragm (via the 7th interspace) were assessed with use of bipolar teflon-coated, stainless steel fine-wire electrodes, uninsulated at their terminal ~5 mm. Inspiratory electrical activity was quantified by measuring the peak amplitudes of the moving average EMG.
In each animal, the dura mater was opened and spinal cord sectioned at the high C1 level using watchmaker forceps under microscopic control. Complete section was verified by lifting a hook across the area of transection. Following C1 section, diaphragm EMG, airway pressure and inspired volume were assessed during HF-SCS (0.75 – 6 mA, 0.2 ms pulse width, 300 Hz) at the T2 level on the ventral surface.
Sequential section of the spinal cord (white) and adjacent gray matter (dorsal columns, lateral funiculi and then ventral funiculi) was performed at the upper C4 level and subsequently at the C8 level (see the schematic diagrams of transverse spinal cord section on the top of each figure). Prior to re-assessment of physiologic parameters during HF-SCS, at least 30 minutes was allowed to elapse after each spinal cord section procedure, at which time the animal was hemodynamically stable.
All recordings were monitored and stored on a computer utilizing a data acquisition and analysis system (Spike 2 with 1401 interface, Cambridge Electronic Design, Cambridge, UK).
2.1 Data Analysis
Mean peak integrated diaphragm and parasternal EMG, inspired volumes and airway pressures under control conditions were compared with those obtained following sequential sectioning of the spinal cord at the C4 and C8 levels, in separate trials. Statistical analysis was performed by using a one-way analysis of variance and the Newman-Keuls test. A p value of < 0.05 was accepted as reflective of statistical significance. Results are presented as means ± SE.
3. Results
The effects of sequential section of the spinal cord at the C4 and C8 spinal levels on diaphragm and parasternal EMG activation and inspired volume generation during HF-SCS (2 mA, 300 Hz, 0.2 ms pulse width) is shown for one animal in Figure 1. Following sequential section of the dorsal columns, lateral funiculi and complete spinal cord at the C4 level, there were no apparent changes in the degree of inspiratory muscle EMG activity or inspired volume compared to control values. Similarly, following section of the dorsal columns at the C8 level, there were no changes in inspired volume or EMG activity. However, following section of the lateral funiculi at this level, there was a marked reduction in the degree of diaphragm activation and associated marked reduction in inspired volume generation. There were no further changes in these parameters following complete section. In contrast, there were no apparent changes in the degree of parasternal activation during spinal cord section at the C8 level.
Figure 1.
The effects of sequential section of the spinal cord at the C4 and C8 spinal levels on parasternal and diaphragm EMG activation and inspired volume generation during HF-SCS (2 mA, 300 Hz, 200 μs pulse width) in a representative animal.
The mean changes, expressed as % of control, in peak integrated parasternal and diaphragm EMG activity during HF-SCS are provided in Table 1 (2 mA, 300 Hz, 0.2 ms pulse width). Compared to control values, there were no significant changes in parasternal and diaphragm EMG activity during sequential spinal cord section at the C4 level. Following section of the dorsal columns at the C8 level, these parameters were also not significantly different than control values. However, section of the lateral funiculi at the C8 level resulted in a significant decrease in diaphragm EMG to 8% of control values (p < 0.01).
Table 1.
Mean changes in peak integrated EMG activity during HF-SCS
| Control | C4 spinal level | C8 spinal level | |||||
|---|---|---|---|---|---|---|---|
| Dorsal Columns Section | Lateral Funiculi Section | Total Section | Dorsal Columns Section | Lateral Funiculi Section | Total Section | ||
| Parasternal (% of control) | 100 ± 0 | 100 ± 4 | 89 ± 4 | 94 ± 3 | 93 ± 12 | 71 ± 12 | 80 ± 23 |
| Diaphragm (% of control) | 100 ± 0 | 107 ± 7 | 105 ± 12 | 116 ± 7 | 118 ± 9 | 8 ± 7* | 11 ± 8* |
p < 0.01 compared to control
Mean inspired volume generation during HF-SCS at different levels of stimulation under control conditions and following sequential section of the spinal cord is shown in Figure 2A. Mean inspired volumes during stimulation with 0.75, 2 and 6 mA were 260 ± 71, 703 ± 37 and 682 ± 16 ml under control conditions. There were no significant changes in mean inspired volume generation following sequential section at the C4 level or subsequent section of the dorsal columns at the C8 level. However, following section of the lateral funiculi at the C8 level, inspired volume fell to 184 ± 24, 238 ± 40 and 262 ± 41 ml (p < 0.05 for each) during HF-SCS with 0.75, 2 and 6 mA, respectively. There were no further significant changes in inspired volume following complete spinal cord section at this level.
Figure 2.
Mean inspired volume (Panel A) and airway pressure generation (Panel B) during HF-SCS at different levels of stimulation under control conditions and following sequential section of the spinal cord.
The effects of sequential section of the spinal cord at the C4 and C8 spinal levels on airway pressure generation under conditions of airway occlusion at FRC are shown for one animal in Figure 3 (same animal as Figure 1). Following sequential section of the spinal cord at the C4 level and also section of the dorsal columns at the C8 level, airway pressure generation remained unchanged. However, following section of the lateral funiculi at the C8 level, there was a marked reduction in airway pressure to 27% of control values. There were no further significant changes in airway pressure following complete spinal cord section at this level.
Figure 3.

The effects of sequential section of the spinal cord at the C4 and C8 spinal levels on airway pressure generation under conditions of airway occlusion at FRC in a representative animal.
The mean changes in airway pressure generation during HF-SCS under control conditions and following sequential section of the spinal cord are provided in Figure 2B. Mean airway pressure generation during stimulation with 0.75, 2 and 6 mA were 20 ± 4, 64 ± 4, 61 ± 4 cm H2O, respectively under control conditions. There were no significant changes in mean airway pressure generation following sequential section at the C4 level or subsequent section of the dorsal columns at the C8 level. However, following section of the lateral funiculi at the C8 level, airway pressure generation fell to 12 ± 2, 16 ± 3 and 17 ± 3cm H2O (p < 0.05 for each). There were no further significant changes in airway pressure generation following complete spinal cord section at this level.
4. Discussion
HF-SCS is a unique method of inspiratory muscle activation resulting in an asynchronous pattern of both diaphragm and inspiratory intercostal muscle activation, which resembles that observed during spontaneous breathing (DiMarco and Kowalski, 2009, 2010, 2011). This pattern of activation stands in marked contrast to the synchronous activation of all axons as occurs with direct phrenic nerve stimulation or ventral root stimulation of the intercostal muscles. Moreover, HF-SCS with a single electrode positioned on the ventral surface of the upper thoracic spinal cord at low stimulus currents (< 3 mA) results in near-maximum diaphragm and intercostal activation, as evidenced by the magnitude of inspired volume production, which approximates the inspiratory capacity (DiMarco and Kowalski, 2009).
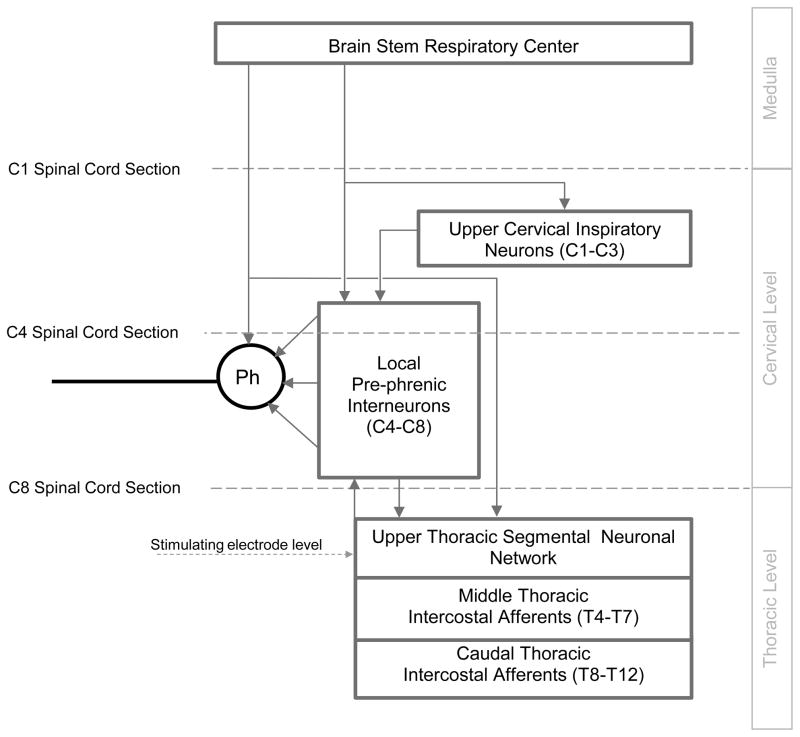
The results of this study shed some light on the spinal cord tracts and synaptic pathways resulting in phrenic motoneuron activation. A simplified diagram of the spinal tracts of interest in this discussion is shown in Figure 4. It is important to note that in our prior studies, HF-SCS with a single electrode was evaluated at multiple sites on the dorsal and ventral epidural surface between the T1 and T5 area. HF-SCS applied at the T2 level on the ventral surface resulted in maximum inspired volume and airway pressure generation (DiMarco and Kowalski, 2009). This finding suggests that tracts in the immediate vicinity of this area were the most likely candidates mediating the observed responses. Indeed, the results of sequential sectioning at the C8 level in the present study indicate that activation of the phrenic motoneuron pools via HF-SCS occurs via stimulation of spinal cord tracts located within the lateral funiculi.
Figure 4.
Potential spinal cord tracts mediating activation of the phrenic motoneuron pools during HF-SCS. See text for further explanation.
Spinal cord tracts with facilitatory effects on phrenic motoneuron activity, have been described by Decima et al (1967, 1969a, 1969b). In a set of elegant experiments, these investigators demonstrated that electrical stimulation with single shocks applied to the lower thoracic external or internal intercostal nerves (T8–T12) resulted in activation of the diaphragm as demonstrated by bursts of activity recorded from both the unilateral and contralateral phrenic nerve (intercostal to phrenic reflex). The upper thoracic intercostal nerves did not evoke any significant reflex responses. By performing successive lesioning of the spinal cord, the ascending pathways mediating these responses were found to run bilaterally in the ventrolateral funiculi. We speculate, therefore, that these same tracts mediate phrenic activation during HF-SCS.
It is important to mention that there is an extensive distribution of pre-phrenic interneurons throughout the cervical spinal cord with projections to both the phrenic and intercostal motoneuron pools (Billing et al., 2000; Lane et al., 2008b). Moreover, recent work raises the possibility of a network of interneurons comprising a central pattern generator at the level of the phrenic motoneuron pools (Alilain et al., 2008). It is also possible therefore that stimulation of axons of these interneurons may mediate the observed responses.
The mechanism by which stimulation of these tracts results in excitation of the phrenic motoneurons is more complex. During normal breathing, the primary input to the phrenic motoneurons arises from monosynaptic bulbospinal projections (Bianchi et al., 1995; de Castro et al., 1994; Davies et al., 1985; Dobbins and Feldman, 1994; Ellenberger et al., 1990; Tian and Duffin, 1998). However, a population of pre-phrenic upper cervical (C1–C4) interneurons with projections to the phrenic motoneuron pools has also been identified (Bellingham 1999; Dobbins and Feldman, 1994; Duffin and Iscoe, 1996; Hayashi et al., 2003; Lane et al., 2008b, 2009; Lipski and Duffin 1986; Lois et al., 2009; Nakazono and Aoki, 1994; Palisses and Viala, 1987). Studies by several previous investigators have shown that these neurons display inspiratory-related phasic bursting and have direct input from the rostral ventral respiratory group (Duffin and Iscoe, 1996; Hayashi et al., 2003; Hilaire et al., 1986; Lane et al., 2009; Palisses and Viala, 1987). Their functional role, if any, during spontaneous breathing, however, is unknown. Recent studies suggest that the pre-phrenic cervical interneurons may serve as a synaptic relay between the brainstem and the phrenic and intercostal motoneurons (Bellingham 1999; Decima et al., 1967; Lane et al., 2008a, 2008b, 2009; Lee and Fuller, 2011; Sandhu et al., 2009). They may take on greater importance in certain pathological conditions such as cervical spinal cord injury (Sandhu et al., 2009). Since the degree of diaphragm electrical activation was not affected by C4 spinal section over a wide range of applied stimulation in this acute preparation, the presence of pre-phrenic upper cervical interneuronal networks are not required in the generation of the observed responses. Rather, stimulated neuronal tracts in the lateral funiculi synapse with interneurons in the vicinity of, or directly with the phrenic motoneurons.
4.1 Study Limitations
Peak integrated EMG was used in this study as an index of the magnitude of inspiratory muscle activation. Previous studies have established that this measurement has significant limitations. Changes in muscle length result in substantial alterations in EMG activity independent of the level of neural drive. Consequently, the magnitude of EMG will not be solely reflective of changes in motor drive to the muscle. In the present study, this was not an issue with the diaphragm since the EMG was either unchanged or virtually disappeared following the sequential sectioning. With parasternal EMG, this was also not a significant concern so long as the level of diaphragm activation was maintained. Lack of diaphragm contraction would have resulted in greater parasternal muscle shortening resulting in a greater EMG signal for a given level of stimulation. We cannot discount, therefore, the possibility that some of the parasternal EMG changes following section of the lateral funiculi at the C8 level were related to this phenomenon.
It is possible that cut axons from phrenic interneurons and even bulbospinal neurons may remain functional under acute experimental conditions (McDonald, 1972). Electrical stimulation of these severed tracts, therefore, remains a potential mechanism by which the inspiratory motoneuronal pools were activated under the experimental conditions of this study. A more complex experimental design involving prolonged study duration of several days to allow degeneration of these axons (in a C1 preparation) would be necessary to evaluate this possibility.
4.2 Clinical Implications
Analysis of single motor unit activity during HF-SCS indicates that the firing frequency of diaphragm motor units is similar to that occurring during spontaneous breathing (DiMarco and Kowalski, 2009, 2010, 2011). With adjustment of stimulus amplitude to achieve inspired volumes comparable to spontaneous breathing, peak firing frequencies were comparable to that reported in spontaneously breathing dogs and spontaneously breathing humans (Saboisky et al., 2007). Since this method allows processing of the stimulus within the phrenic motoneurons pools, phrenic motoneuron recruitment presumably occurs in an orderly pattern in accordance with Henneman’s principle (Berger, 1979; Dick et al., 1987; Donnelly et al., 1985; Henneman et al., 1965; Hilaire et al., 1987; Mantilla and Sieck, 2003). Since long-term ventilation can be maintained with HF-SCS, this method has the potential to provide a more natural and effective method of inspiratory muscle pacing in patients with ventilator-dependent tetraplegia (DiMarco and Kowalski, 2009, 2010).
Most patients with ventilator-dependent tetraplegia and who are candidates for diaphragm pacing (i.e. the phrenic motoneuron pools are largely intact) have suffered damage to the spinal cord cephalad to the phrenic motoneuron pools. The fact that activation of the inspiratory motoneurons via HF-SCS does not involve cervical pre-motor neurons and interneuronal networks in the upper portion of the cervical spinal cord indicates that this technique may be a useful method of diaphragm pacing in patients with damage to this portion of the spinal cord.
Highlights.
HF-SCS results in physiologic activation of the diaphragm
HF-SCS of the phrenic motoneurons does not involve cervical pre-phrenic interneurons
HF-SCS is mediated by spinal pathways located in the lateral funiculi
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Anthony F. DiMarco, Email: afd3@case.edu.
Krzysztof E. Kowalski, Email: kek5@case.edu.
References
- Alilain WJ, Li X, Horn KP, Dhingra R, Dick TE, Herlitze S, Silver J. Light-induced rescue of breathing after spinal cord injury. J Neurosci. 2008;28:11862–11870. doi: 10.1523/JNEUROSCI.3378-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham MC. Synaptic inhibition of cat phrenic motoneurons by internal intercostal nerve stimulation. J Neurophysiol. 1999;82:1224–1232. doi: 10.1152/jn.1999.82.3.1224. [DOI] [PubMed] [Google Scholar]
- Berger AJ. Phrenic motoneurons in the cat: subpopulations and nature of respiratory drive potentials. J Neurophysiol. 1979;42:76–90. doi: 10.1152/jn.1979.42.1.76. [DOI] [PubMed] [Google Scholar]
- Bianchi AL, Denavit-Saubié M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Billing I, Foris JM, Enquist LW, Card JP, Yates BJ. Definition of neuronal circuitry controlling the activity of phrenic and abdominal motoneurons in the ferret using recombinant strains fo pseudorabies virus. J Neuroscience. 2000;20:7446–7454. doi: 10.1523/JNEUROSCI.20-19-07446.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JG, Kirkwood PA, Sears TA. The detection of monosynaptic connexions from inspiratory bulbospinal neurones to inspiratory motoneurones in the cat. J Physiol. 1985;368:33–62. doi: 10.1113/jphysiol.1985.sp015845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro D, Lipski J, Kanjhan R. Electrophysiological study of dorsal respiratory neurons in the medulla oblongata of the rat. Brain Res. 1994;639:49–56. doi: 10.1016/0006-8993(94)91763-9. [DOI] [PubMed] [Google Scholar]
- Decima EE, von Euler C, Thoden U. Spinal intercostal-phrenic reflexes. Nature. 1967;214:312–313. doi: 10.1038/214312a0. [DOI] [PubMed] [Google Scholar]
- Decima EE, von Euler C. Excitability of phrenic motoneurones to afferent input from lower intercostal nerves in the spinal cat. Acta Physiol Scand. 1969a;75:580–591. doi: 10.1111/j.1748-1716.1969.tb04413.x. [DOI] [PubMed] [Google Scholar]
- Decima EE, von Euler C, Thoden U. Intercostal-to-phrenic reflexes in the spinal cat. Acta Physiol Scand. 1969b;75:568–579. [PubMed] [Google Scholar]
- Dick TE, Kong FJ, Berger AJ. Recruitment order of diaphragmatic motor units obeys Henneman’s size principle. In: Sieck GC, Gandevia SC, Cameron WE, editors. Respiratory Muscles and Their Neuromotor Control. Alan R Liss Inc; New York: 1987. pp. 239–247. [Google Scholar]
- DiMarco AF, Altose MD, Cropp A, Durand D. Activation of the inspiratory intercostal muscles by electrical stimulation of the spinal cord. Am Rev Respir Dis. 1987;136:1385–1390. doi: 10.1164/ajrccm/136.6.1385. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE. High frequency spinal cord stimulation of inspiratory muscles in dogs: a new method of inspiratory muscle pacing. J Appl Physiol. 2009;107:662–669. doi: 10.1152/japplphysiol.00252.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE. Intercostal muscle pacing with high frequency spinal cord stimulation in dogs. Respir Physiol Neurobiol. 2010;171:218–224. doi: 10.1016/j.resp.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE. Distribution of electrical activation to the external intercostal muscles during high frequency spinal cord stimulation in dogs. J Physiol. 2011;589:1383–1395. doi: 10.1113/jphysiol.2010.199679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- Donnelly DF, Cohen MI, Sica AL, Zhang H. Responses of early and late onset phrenic motoneurons to lung inflation. Respir Physiol. 1985;61:69–83. doi: 10.1016/0034-5687(85)90029-5. [DOI] [PubMed] [Google Scholar]
- Duffin J, Iscoe S. The possible role of C5 segment inspiratory interneurons investigated by cross-correlation with phrenic motoneurons in decerebrate cats. Exp Brain Res. 1996;112:35–40. doi: 10.1007/BF00227175. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Vera PL, Haselton JR, Haselton CL, Schneiderman N. Brainstem projections to the phrenic nucleus: an anterograde and retrograde HRP study in the rabbit. Brain Res Bull. 1990;24:163–174. doi: 10.1016/0361-9230(90)90201-a. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Kirkwood PA. Spinal breathing: stimulation and surprises. J Physiol. 2011;589:2661–2662. doi: 10.1113/jphysiol.2011.210476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F, Hinrichsen CF, McCrimmon DR. Short-term plasticity of descending synaptic input to phrenic motoneurons in rats. J Appl Physiol. 2003;94:1421–1430. doi: 10.1152/japplphysiol.00599.2002. [DOI] [PubMed] [Google Scholar]
- Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Khatib M, Monteau R. Central drive on Renshaw cells coupled with phrenic motoneurons. Brain Res. 1986;376:133–139. doi: 10.1016/0006-8993(86)90907-8. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Monteau R, Khatib M. Determination of recruitment order for phrenic motoneurons. In: Sieck GC, Gandevia SC, Cameron WE, editors. Respiratory Muscles and Their Neuromotor Control. Alan R Liss Inc; New York: 1987. pp. 249–261. [Google Scholar]
- Lane MA, Fuller DD, White TE, Reier PJ. Respiratory neuroplasticity and cervical spinal cord injury: translational perspectives. Trends Neurosci. 2008a;31:538–547. doi: 10.1016/j.tins.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, White TE, Coutts MA, Jones AL, Sandhu MS, Bloom DC, Bolser DC, Yates BJ, Fuller DD, Reier PJ. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J Comp Neurol. 2008b;511:692–709. doi: 10.1002/cne.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, Lee KZ, Fuller DD, Reier PJ. Spinal circuitry and respiratory recovery following spinal cord injury. Respir Physiol Neurobiol. 2009;169:123–132. doi: 10.1016/j.resp.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KZ, Fuller DD. Neural control of phrenic motoneuron discharge. Respir Physiol Neurobiol. 2011;179:71–79. doi: 10.1016/j.resp.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J, Duffin J. An electrophysiological investigation of propriospinal inspiratory neurons in the upper cervical cord of the cat. Exp Brain Res. 1986;61:625–637. doi: 10.1007/BF00237589. [DOI] [PubMed] [Google Scholar]
- Lois JH, Rice CD, Yates BJ. Neural circuits controlling diaphragm function in the cat revealed by transneuronal tracing. J Appl Physiol. 2009;106:138–152. doi: 10.1152/japplphysiol.91125.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Sieck GC. Mechanisms underlying motor unit plasticity in the respiratory system. J Appl Physiol. 2003;94:1230–1241. doi: 10.1152/japplphysiol.01120.2002. [DOI] [PubMed] [Google Scholar]
- McDonald WI. The time course of conduction failure during degeneration of a central tract. Exp Brain Res. 1972;14:550–556. doi: 10.1007/BF00236596. [DOI] [PubMed] [Google Scholar]
- Nakazono Y, Aoki M. Excitatory connections between upper cervical inspiratory neurons and phrenic motoneurons in cats. J Appl Physiol. 1994;77:679–683. doi: 10.1152/jappl.1994.77.2.679. [DOI] [PubMed] [Google Scholar]
- Palisses R, Viala D. Existence of respiratory interneurons in the cervical spinal cord of the rabbit. C R Acad Sci III. 1987;305:321–324. [PubMed] [Google Scholar]
- Saboisky JP, Gorman RB, De Troyer A, Gandevia SC, Butler JE. Differential activation among five human inspiratory motoneuron pools during tidal breathing. J Appl Physiol. 2007;102:772–780. doi: 10.1152/japplphysiol.00683.2006. [DOI] [PubMed] [Google Scholar]
- Sandhu MS, Dougherty BJ, Lane MA, Bolser DC, Kirkwood PA, Reier PJ, Fuller DD. Respiratory recovery following high cervical hemisection. Respir Physiol Neurobiol. 2009;169:94–101. doi: 10.1016/j.resp.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian GF, Duffin J. The role of dorsal respiratory group neurons studied with cross-correlation in the decerebrate rat. Exp Brain Res. 1998;121:29–34. doi: 10.1007/s002210050433. [DOI] [PubMed] [Google Scholar]