Abstract
We examined daytime salivary cortisol and salivary alpha-amylase (sAA) secretion levels and variability in preschool-aged children with autism (AUT) and typically developing children (TYP). Fifty-two subjects (26 AUT and 26 TYP) were enrolled. Salivary samples were obtained at waking, midday, and bedtime on two consecutive days at three phases (baseline, 3 months later, 6 months later). There were modest increases in waking cortisol and sAA levels in AUT relative to TYP, but the increases were not statistically significant. Important differences were observed in cortisol and sAA variability between AUT and TYP. There was also a graded response among AUT by functional status—cortisol and sAA secretion levels were higher when IQ was lower.
Keywords: Autism, Children, Salivary cortisol, Salivary alpha-amylase, Diurnal, Variability
Introduction
Autism, also referred to as autistic disorder, usually presents in children before 3 years of age and is characterized by: deficits in reciprocal social interaction; deficits in communication; and restricted, repetitive behaviors, interests, and activities (1994). Autism is conceptualized as a spectrum of disorders commonly referred to as the autism spectrum disorders (ASD), which includes the pervasive developmental disorders-not otherwise specified and Asperger syndrome. Intelligence ranges from severe intellectual disability to normal intelligence in autism, strictly defined, but there is a tendency to assume that these other aforementioned ASD reflect decreasing severity (Volkmar et al. 2009).
Although autism is of unknown etiology, there are many symptoms and behaviors that suggest the potential involvement of the limbic-hypothalamic-pituitary-adreno-cortical (L-HPA) axis. Among the most visible and troubling manifestations of autism are the affected children's difficulties with emotion regulation, adaptation to change, and accommodation to everyday stressors and perturbations of routine. The L-HPA axis is involved in homeostatic regulation involving many bodily processes working with each other to maintain temperature, blood pressure, and neuroendocrine secretion. Thus, maintenance of appropriate L-HPA responsiveness is crucial for the adaptive functioning of the human body. Cortisol regulation is a central part of the process to prevent excess demand or allostatic load on the organism (McEwen 1998). Allostatic load is a consequence of chronic exposure to fluctuating or variable neuroendocrine response and may contribute to pathogenesis when adaption or homeostasis is poorly regulated.
Dysregulation of the L-HPA axis in autism may be a result of neurobiological or neuroanatomical defects or deficits. It has been proposed that children with autism with a low intelligence quotient (IQ) may have a serotonin regulation disorder, leading to a less refined ability to suppress cortisol following dexamethasone administration (Hoshino et al. 1987). Other forms of circadian regulatory control—feeding, temperature, and sleep—also have been found to function abnormally in autism (Hill et al. 1977; Richdale and Prior 1992). In addition to a neurointegrative defect as above, the L-HPA axis of individuals with autism may become dysregulated as a result of environmental triggers. These triggers might alter the regulation of the diurnal rhythm over time to achieve a hypocortisolism (Gunnar and Vazquez 2001) or hypercortisolism (Cicchetti and Rogosch 2001). A blunting of the slope (Varadhan et al. 2008) and/or and increase in the slope decline across the day (Steptoe et al. 2000) have been reported. There is also research to suggest that variability in the cortisol rhythm may be the best fitting description of altered levels, at least in depression (Peeters et al. 2004; Yehuda et al. 1996). Children with autism may be more sensitive to zeitgebers or environmental cues which may result in greater variability in cortisol secretion (Corbett et al. 2008).
There is a body of published data on the research question of whether diurnal cortisol levels in children with autism are different from typically developing children. There were six studies (Corbett et al. 2006, 2008; Hoshino et al. 1987; Jansen et al. 2003; Marinovic-Curin et al. 2008; Richdale and Prior 1992) that used collection procedures, comparison populations, and settings that were most relevant to the population and question of interest. For children with autism compared to control children, afternoon and evening cortisol levels were higher in the majority of studies (Corbett et al. 2008; Hoshino et al. 1987; Marinovic-Curin et al. 2008; Richdale and Prior 1992). However, due to lack of precision in study estimates, study design differences, and the lack of an examination of potential confounders, this research question has not been resolved.
In the glucocorticoid cascade hypothesis, the inability to cope with ongoing stress and resulting excess glucocorticoids triggers future degeneration and disease (Sapolsky et al. 1986; Oitzl et al. 2010). For example, human and animal data have shown that cumulative exposure to excess glucocorticoids impairs memory, learning, and contributes to hippocampal atrophy (Sapolsky et al. 1986; Lupien et al. 2005). In addition to the L-HPA, the autonomic nervous system (ANS) is activated during stress and plays a more immediate role in the stress response. Autonomic activation is suggested by the release of alpha-amylase, a salivary enzyme, as an indirect indicator of ANS activity (Nater and Rohleder 2009) Early stress has also been associated with increases in the sensitivity of the noradrenergic system and pathological consequences as above (Bremner and Vermetten 2001). Exposure to chronic stress results in long-term alterations in the locus coeruleus and norepinephrine release (Bremner et al. 1996). Resting levels of cortisol and salivary alpha-amylase are considered reliable indicators of allostatic load (McEwen 1998).
In children with autism, there has been no research on diurnal sAA profiles and it is clearly time to examine other CNS pathways, especially the complementary SNS stress pathway. Individuals with autism have atypical responses to stress with hyperreactivity to novel events or changes to their environment as expressed in behavior. Whether the stress in the face of change, unfamiliarity, and social situations leads to ongoing changes in neurohormone release over long periods of time remains to be seen. However, research on chronic stress in typical individuals indicates that individuals with autism with chronic stress may be especially prone to problems with homeostasis of neuro-hormones and the burden that that carries. Basal levels and variability of cortisol and sAA are important to examine given that dysregulation of basal levels contributes to allostastic load independent of stress-reactive neurohormone release.
There is no concomitant body of work on chronic stress and basal measures of salivary alpha-amylase (sAA) in young children as there is with cortisol. However, the few studies from the literature on stress and basal sAA in older children are useful to elucidate this question. In a study of the effects of daily stress perception on waking sAA levels in school age children, there was no association between perceived stress and sAA (Maldonado et al. 2008). However, in a study of chronic stress in school-age children with asthma, there were lower levels of sAA across the day in children with high chronic stress (Wolf et al. 2008). In this latter study, the authors suggest that a chronic illness accompanied by autonomic nervous system (ANS) changes may be a necessary factor in order to observe changes in sAA. This hypothesis is also supported by a study that observed higher levels of sAA and increased respiratory problems in children following a stress-reactivity paradigm (Granger et al. 2007).
Bauer et al. (2002) propose that the coordination of the HPA and the sympathetic nervous system (SNS) during stress has important implications for predicting behaviors. Studies that have not examined the HPA and SNS together may be missing information on how each system is moderated by the concurrent activity of the other (Fortunato et al. 2008). The results of the current study should provide data to assist in characterizing the L-HPA and SNS pathways in autism.
The current study provided the opportunity to examine sAA in conjunction with salivary cortisol to comprehensively measure endpoints of the stress pathways. To our knowledge, there have been no studies of sAA in individuals with autism. Our main objectives were to determine whether average levels and variability of daytime cortisol and sAA secretion, based on salivary sampling, differed between preschool-aged children with autism and typically developing children. In addition, we wanted to determine whether the findings might be modified by IQ. Our hypotheses were that we would observe an increased level and variability of cortisol during the day in children with autism, and that levels might be differentiated based on cognitive level (i.e.IQ). We were uncertain whether sAA levels would be different between the diagnostic groups and thus this was an exploratory analysis.
Methods
Study Population
A subgroup of participants from a larger Sleep Study (the Sleep in Children with Autism Study) (Goodlin-Jones et al. 2008) were recruited for this study, the Sleep and Cortisol Study (SACS). Subjects for the SACS included children with autism and typically developing children, 2–5½ years of age, enrolled from 2005 to 2009. The study was reviewed and approved by the Institutional Review Boards of the University of California, Davis and the University of California, Berkeley and written informed consent was obtained from the parent(s) of the subjects. The SACS and the host study used a longitudinal study design with measurements taken at enrollment (baseline), and 3 and 6 months thereafter. Subjects could be enrolled into the SACS from the Sleep Study at any of the three phases of the parent study.
Any children were excluded if they had: Down syndrome, fragile-X, or other genetic or chromosomal disorders; a chronic illness; evidence of significant visual impairment (over 20/200 in better eye); Tourette syndrome, seizure disorder, obsessive compulsive disorder, anxiety, depression, or other psychiatric disorders; parents that sought treatment for a sleep disorder; used oral or inhalant corticosteroids during the study. Typically developing children were excluded if they had any evidence of cognitive impairment or developmental delay.
Measures
Diagnosis of Autism
The Autism Diagnostic Observation Schedule-Generic (ADOS-G) is a semi-structured, standardized assessment in which the researcher observes social interaction, skills in communication, play activities, and imaginative use of materials for children and adults suspected of having an ASD (Lord et al. 2000). The Autism Diagnostic Interview-Revised (ADI-R) is a standardized, semi-structured, investigator-based interview for the primary caregivers of individuals with an ASD (Lord et al. 1993). Both the ADOS-G and ADI-R are scored immediately and are videotaped for additional scoring at a later time. Children with autism were determined to have a diagnosis of strict autism by DSM-IV criteria (American Psychiatric Association 1994). The typically developing children did not receive the ADI-R or the ADOS-G, but were screened clinically to confirm that they did not meet criteria for strict autism or other ASD.
Determination of Salivary Analytes
Cortisol and sAA levels were obtained by salivary sampling at 3 times per day, over 2 consecutive days, and at baseline, 3, and 6 months (18 potential samples). Parents were instructed to collect the samples as follows: (1) morning sample within 30 min of the child waking; (2) mid-afternoon sample at approximately 2:00 pm; and (3) evening sample within 30 min of the child being put to bed. No eating, drinking (except water), or brushing of teeth was allowed in the 30 min before sampling. Parents were asked to pour a pre-measured amount (less than 1/64th of a teaspoon) of cherry flavored Kool-Aid into the child's mouth and then insert a cotton roll to absorb the saliva. Approximately 0.5–1.0 ml of saliva was requested for each sampling. The date and time of saliva collection was recorded by the parent(s) on the tube and on a daily diary. In addition, the TrackCap® system (MEMS 6, Aprex, Inc.) was used for checking compliance. The TrackCap contains a microchip which logs the absolute clock time when opening a plastic bottle containing the sampling kits. The TrackCap time was checked against the sample time that was recorded directly on the sample tube (the “gold” standard).
The cortisol assays were conducted in the Behavioral Endocrinology Laboratory at Pennsylvania State University under the direction of DAG. All samples were assayed for salivary cortisol in duplicate using a commercially available enzyme immunoassay (Salimetrics, LLC). The assay uses 25 microliters of saliva per determination, has a lower limit of detection of 0.003 μg/dl, standard curve range from 0.012 to 3.0 μg/dl, and average intra- and inter-assay coefficients of variation of 3.5 and 5.1 %, respectively.
The samples also were assayed for sAA by a kinetic reaction assay (Salimetrics, LLC). The assay employs a chromagenic substrate, 2-chloro-p-nitrophenol, linked to maltotriose. The enzymatic action of sAA on this substrate yields 2-chloro-p-nitrophenol, which can be measured spectrophotometrically at 405 nm using a standard laboratory plate reader. The amount of sAA activity present in the sample is directly proportional to the increase (over a 2 min period) in absorbance at 405 nm. The intra-assay variation (CV) based on 30 replicate tests was less than 7.5 %. The inter-assay variation based on 16 separate runs was less than 6 %.
Other Data
At baseline, The Hollingshead Four Factor Index of Social Status was used to obtain an overall measure of socioeconomic status (Hollingshead 1975) and the Mullen Scales of Early Learning was used to determine the IQ (Mullen 1997). Inventories regarding child behavior (the Child Behavior Checklist (CBCL) (Achenbach 1992) and stress (child scale of the Parenting Stress Index (PSI) (Loyd and Abidin 1985) were administered at each of the three phases of the study. A two-page questionnaire was self-administered by the parent on each day of the saliva collection. This questionnaire requested information on the use of medications, presence of a cold or fever, and a quantification of events (e.g. errands, enrichment classes) in the last 24 h for the child.
Data Analysis
The main effect variables were: diagnosis (children with autism versus typically developing children) and functional status for children with autism (IQ < 55 for extremely low functional status, IQ from 55 to 69 for low functional status, and IQ ≥ 70 for high functional status). The outcome variables were cortisol measured in micrograms per deciliter but transformed and presented in nanomoles per liter (nmol/l) and sAA measured and presented in units per milliliter (U/ml). A natural log transformation was performed on the outcome variables before analysis, but the estimates and the 95 % confidence intervals were back-transformed to the original units. Typically, an estimate is presented with the 95 % confidence interval following in a set of parentheses (lower limit–upper limit). The following covariates were used in the modeling process for choosing the best model by cross-validation: child age in months; phase; day; time; race; whether the mother works; Hollingshead 4-factor social index; count of events during the day; nap on day of saliva collection; hours of television per day; total score on the child scale of the PSI; total score on the CBCL; and hours of school/therapy per day.
The GENMOD procedure in SAS (SAS version 9.1, SAS Inc., Cary, NC) was used to fit a generalized linear model to the data by maximum likelihood estimation. A generalized estimating equations (GEE) approach that considers the repeated measurements was used to obtain variance estimates around the effect estimate. The coefficients and confidence intervals by GEE were given only to get approximate variability around the effect estimates. A linear mixed model approach with the inclusion of a random effect for subject was used to explore between- and within-subject variability (the MIXED procedure in SAS).
In observational studies only one exposure and corresponding outcome is observed for each subject. Thus, we have incomplete or missing data on other potential exposures/outcomes for the subject. One approach to simulate the ideal situation from observational data is based on the concept of counterfactuals. The counterfactual outcome distribution represents the observed exposure and outcome as well as those outcomes that would have occurred if, “counter to fact”, the subject received an exposure other than the one that was actually observed (Maldonado and Greenland 2002). The missing data problem is resolved by creating a pseudopopulation of the full data that would occur if all potential exposures/outcomes occurred (Robins 1999). The use of counterfactuals to solve the missing data problem results in a dataset free of nuisance parameters (i.e. confounding).
G-computation is one type of estimator used in causal inference methods to examine a causal effect of interest (Ahern et al. 2009; Gill and Robins 2001; Snowden et al. 2011). The first step in G-computation is to fit a regression model for the exposure of interest and the outcome adjusted for relevant covariates. The Deletion/Substitution/Addition (DSA) algorithm (the R Project for Statistical Computing, copyright© 2000–2009, R Development Core Team) was used to fit a non-parametric model. The DSA is a data adaptive, machine learning algorithm that selects the best-fitting model over the entire model space of polynomial generalized linear models. In an attempt to avoid model misspecification, the DSA algorithm is used so that model choice is not constrained by human preference for models in decision-making (e.g. stepwise and other traditional regression modeling). A cross-validated loss-function-based (the L2 loss function) estimation procedure is used to select the model with the best fit (Sinisi and van der Laan 2004). A final cross-validation risk is calculated from the empirical average of the loss to obtain the optimal model from among the best models trialed.
This latter model is used to predict the counterfactual outcomes of all possible exposures for each subject (i.e., the outcome when each subject is exposed and unexposed, given covariates). In effect, imputation is used to fill in data for the missing counterfactual observations by prediction. A model of the form E[Y|A = a, W] is fit by maximum likelihood to provide a marginal estimate for cortisol in both exposure states for each subject. For example, E[Y|A = 1, W] is the estimated causal (marginal) effect of A = 1 (having autism) on Ya (cortisol level), after pooling over all strata of confounders, W. The marginal estimates (means) are not constrained by stratification—the results are closer to an unconditional interpretation of an effect rather than an adjusted effect conditional on the strata. For researchers seeking one effect estimate rather than strata specific estimates when there is effect modification or interaction, they can use g-computation to their advantage, given that a single estimate representing the marginal effect can be obtained. Confidence intervals were obtained around the marginal estimate using cluster bootstrapping with 1,000 repetitions.
Results
Descriptive Characteristics
Six families withdrew after enrollment because the child or family could not tolerate or complete the study procedures. There were 52 families that completed at least one phase of the study, 42 that completed 2 phases, and 32 that completed 3 phases. Characteristics of the study population at baseline are shown in Table 1.
Table 1.
Demographic and other characteristics by diagnostic group
| Characteristic | Children with autism N = 26 N (%) | Typically developing children N = 26 N (%) |
|---|---|---|
| Demographic characteristics of children and parents (collected at baseline) | ||
| Child-level | ||
| Male gender | 22 (84.6) | 23 (88.5) |
| Caucasian race | 17 (65.4) | 19 (73.1) |
| Child's age in months—mean (±std; range) | 45.1 (±8.9; 28–64) | 39.4 (±10.5; 24–61) |
| Baseline cognition (IQ*)—mean (±std; range) | 57.5 (±13.5; 49–94) | 99.7 (±18.0; 70–130) |
| Parent-level | ||
| Married parents | 25 (96.1) | 25 (96.1) |
| Parents with at least a bachelors degree | ||
| Mothers | 22 (84.6) | 19 (73.1) |
| Fathers | 16 (61.5) | 17 (65.4) |
| Parent's age in years—mean (±std; range) | ||
| Mothers | 34.0 (±7.0; 25–58) | 34.3 (±5.7; 22–46) |
| Fathers | 35.9 (±8.0; 25–60) | 36.1 (±6.0; 21–47) |
| Parent employed | ||
| Mother | 10 (38.5) | 7 (26.9) |
| Father | 22 (84.6) | 25 (96.1) |
| Hollingshead Four Factor Index of Social Status—mean (±std; range) | 49.4 (±12.0; 27–66) | 49.8 (±9.9; 30–66) |
| Mean (±se) | Mean (±se) | |
|---|---|---|
| Behavioral characteristics (collected at each phase and averaged) | ||
| Child-level | ||
| Child Behavior Checklist (CBCL) total score | 60.9 (0.4) | 46.7 (0.6) |
| Parenting Stress Index (PSI) total score (Child) | 131.8 (1.0) | 97.9 (1.1) |
| Hours of television per day | 1.8 (0.06) | 1.6 (0.05) |
IQ intelligence quotient
A total of 26 children with autism (AUT) and 26 typically developing children (TYP) were included in the analyses. Overall, there was a preponderance of males in the study population (roughly a 4:1 ratio). The children came from families with parents that were generally: Caucasian, married, college-educated, and in their mid 30 s. There were few differences between the two diagnostic groups. By design AUT were older than TYP because younger TYP were recruited selectively by the host study. Baseline IQ was expected to be lower in AUT compared to TYP because of deficits in expressive and receptive language skills among AUT. Total scores on the CBCL and the PSI were higher for AUT versus TYP.
There was a very high degree of agreement (intraclass correlation, r = 0.98) between TrackCap time and sample time for both AUT and TYP. The average difference in minutes between the TrackCap and sample times was <2 min and <3 min for AUT and TYP, respectively. The SACS was noteworthy because we were able to examine the collection times related to sleep (waking and bedtime) more accurately given the use of sleep actigraphy.
Association between diagnosis and the salivary biomarkers
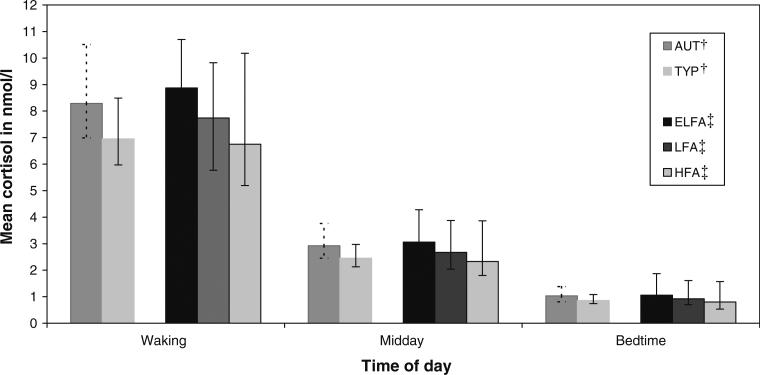
The coefficient from the GEE for the effect of diagnosis on cortisol was 0.17 (–0.08 to 0.49). This value and the confidence interval suggest that the level of cortisol across the day for AUT compared to TYP was similar (p > 0.23). Figure 1 displays the marginal means by diagnostic group at waking, midday, and bedtime.
Fig. 1.
Mean (95 % confidence intervals) salivary cortisol levels in children by diagnosis and by functional status*. *Marginal estimate obtained from causal modeling with g-computation estimation; 95 % confidence interval obtained from cluster bootstrapping. †Diagnosis: AUT = children with autism (N = 26); TYP = typically developing children (N = 26); adjusted for time (ordinal), hours per day of television squared (continuous), nap on day of saliva collection (dichotomous), CBCL total score (continuous), and hours of school/therapy per day (dichotomous). ‡Functional status: ELFA = extremely low functioning children with autism (N = 21); LFA = low functioning children with autism (N = 4); HFA = high functioning children with autism (N = 5); adjusted for time (ordinal), hours per day of television squared (continuous), count of events (dichotomous), Hollingshead index squared (continuous)
An average of 2.92 and 2.45 nmol per liter of cortisol were secreted from waking to bedtime (i.e. the midday value is the daytime average) for AUT and TYP, respectively. The waking values, while 1.34 units higher for AUT versus TYP, had wide confidence intervals suggesting some uncertainty. As specified in Methods, functional status in children with autism was defined as extremely low (ELFA), low (LFA), and high (HFA). The coefficient from the final model indicated that there was a modest 0.14 (0.003–0.31) increase—a 14 % higher level of cortisol over the day for a change to a lower functional status. Figure 1 also displays the data for functional status by time of day. We observed the largest differences of the day at waking for ELFA, LFA and HFA, although they were not statistically significant (p > 0.05 for all differences). Although there was a higher cortisol secretion level for ELFA and LFA compared to HFA during the day, the small sample and lack of precision in the estimates precluded any definitive conclusion.
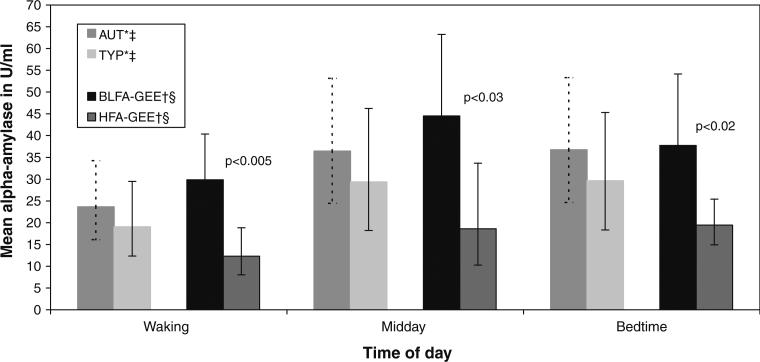
The typical diurnal rhythm of sAA begins with a sharp decline approximately 30 min after waking and then secretion increases over the day achieving a plateau in the afternoon through evening hours (Nater et al. 2007). The coefficient for the association between diagnosis and sAA represented an approximately 26 % (–0.18 to 0.94) increase in sAA levels across the day for AUT compared to TYP, although the confidence interval was wide. In Fig. 2 the mean values of salivary sAA for the AUT were higher than for the TYP at all times of the day, but again there was large variability for both groups suggesting some uncertainty in the estimates.
Fig. 2.
Mean (95 % confidence intervals) salivary alpha-amylase levels in children by diagnosis*‡ and by functional status†§. *Marginal estimate obtained from causal modeling with g-computation estimation; 95 % confidence interval obtained from bootstrapping with repeated sampling †Least squares mean and 95 % confidence interval obtained from generalized estimating equation (GEE). We could not obtain CIs around the g-computation estimates from bootstrapping. The CIs around the g-computation estimates would be more conservative and preferred because they are the correct estimates. ‡Diagnosis: AUT = children with autism (N = 26); TYP = typically developing children (N = 26); adjusted for time (dummy), child's age (continuous), CBCL total score2 (continuous), phase (ordinal), and mother works (dichotomous) §Functional status: BLFA = both low functioning children with autism (N = 21); HFA = high functioning children with autism (N = 5); adjusted for time (dummy), child's age (continuous), CBCL total score squared (continuous), and phase (ordinal)
The analysis for functional status combined the ELFA and LFA groups (renamed “both low functioning autism” or BLFA) because the salivary sAA values were very similar across the day in these two groups. The coefficient for the effect of functional status on sAA was 1.43 (0.38–3.27) or close to a 1.5-fold higher level of sAA for the lower functional status group. The results of g-computation estimation suggested large differences between the predicted means of the BLFA and HFA groups and were very similar to those obtained from GEE. The results of GEE were presented in Fig. 2 because we could not obtain confidence intervals around the g-computation estimates from bootstrapping given that there were not enough subjects in clusters to be re-sampled. At waking the estimate for BLFA was 17.6 U/ml higher, and at midday the estimate was 25.9 U/ml higher, than for HFA. The large differences were statistically significantly different even with wide confidence intervals from GEE, but the 95 % CI around the g-computation estimates would be wider and thus more conservative.
Random Effects Analysis (Between- and within-Subject Variability)
Children with autism may not have a fine-tuned pattern of secretion across the day, regardless of the mean level, so that secretion might be more variable compared to typically developing children. Table 2 displays the findings from the linear mixed model for cortisol first followed by the model for sAA.
Table 2.
Linear mixed models using random effects for the comparison of the variability of salivary biomarkers between- and within-subjects
| Variance estimate | (Standard error) | |
|---|---|---|
| CORTISOL | ||
| Covariance parameter | ||
| Model 1: Overall* | ||
| Between-subject variability—ALL | 0.0544 | (0.0165) |
| Within-subject variability—ALL | 0.4248 | (0.0221) |
| Model 2: By diagnostic group† | ||
| Within-subject variability—AUT | 0.4229 | (0.0313) |
| Within-subject variability—TYP | 0.4270 | (0.0313) |
| Model 3: By diagnostic group† | ||
| Between-subject within-group variability—AUT | 0.0663 | (0.0263) |
| Between-subject within-group variability—TYP | 0.0388 | (0.0187) |
| ALPHA-AMYLASE | ||
| Covariance parameter | ||
| Model 1: Overall* | ||
| Between-subject variability—ALL | 0.7603 | (0.1601) |
| Within-subject variability—ALL | 0.7227 | (0.0377) |
| Model 2: By diagnostic group† | ||
| Within-subject variability—AUT | 0.8628 | (0.0638) |
| Within-subject variability—TYP | 0.5824 | (0.0431) |
| Model 3: By diagnostic group† | ||
| Between-subject within-group variability—AUT | 0.5115 | (0.1596) |
| Between-subject within-group variability—TYP | 0.9890 | (0.2884) |
Model 1 includes: random intercept for salivary biomarker; time and day as fixed effects
Models 2 and 3 include: random intercept for salivary biomarker and diagnostic group: time and day as fixed effects
Model 1 (all Models are in Table 2) examines all of the subjects without regard to diagnosis. The between-subject variance suggests that there is some variability in mean cortisol secretion between the study children, but that most of the variability can be explained by the within-subject variability. In Model 2 diagnostic group is added to examine within-subject variability within each diagnostic group. This examines whether the child's own variability between repeated sample collections is different for children with autism versus typically developing children. The within-subject variance within each diagnostic group is not very different from the overall within-subject variance regardless of diagnostic group (Model 1), suggesting that diagnosis does not help to explain the within-subject variability in cortisol. Model 3 examined the between-subject variability within each diagnostic group. Here, the research question is whether there is more variability between children with autism and each other versus between typically developing children and each other. The results suggested that both children with autism and typically developing children had low variability. The 95 % confidence limits overlap and the variance estimates for AUT of 0.0663 (0.0148–0.1178) compared to 0.0388 for TYP (0.0021–0.0756) were not statistically significantly different from each other.
For the sAA analysis, there were striking differences in contrast to the cortisol analysis (Table 2). Both the between-subject and within-subject estimates of variability for all children regardless of diagnosis were large and similar to each other (Model 1). When diagnostic group was entered large differences in within-subject variability between AUT and TYP could be seen (Model 2). There was 28 % (0.13–0.43) more within-subject variability in AUT compared to TYP (p < 0.001). In terms of between-subject within-group variability (Model 3), the 95 % CI for the 48 % difference (–0.1686 to 1.1236) for TYP compared to AUT suggests that there is little precision in the estimate.
Discussion
Although there were higher levels of cortisol secretion at waking for AUT, cortisol secretions at midday and bedtime were not very different between the diagnostic groups on average. Similar findings for AUT and TYP were observed for sAA. There were no statistically significant differences between the diagnostic groups at any time of the day for the salivary biomarkers. In a study of children with ASD there was an equivalent cortisol awakening response, measured approximately 30 min after waking, compared to similar-aged typically developing controls (Zinke et al. 2010). The review of previous studies of diurnal secretion suggested higher levels of cortisol during the day, particularly at late afternoon or bedtime. Corbett, et al. in a study with a similar design observed an increase of 0.15 across the day for AUT compared to TYP (Corbett et al. 2006). This effect size was similar to the SACS results (0.17), however, the largest difference in cortisol in the Corbett study was observed at evening.
In addition to investigation of AUT as a whole, the AUT were divided into high, low, and extremely low functioning groups. The results suggested that there was an increase in cortisol levels, and particularly sAA levels, when comparing diagnostic groups stratified by IQ. The response was graded—cortisol and sAA levels were higher when IQ was lower. Previous research has observed that children with autism with an IQ < 60 (termed poorly developed) had higher plasma cortisol levels before and after the dexamethasone suppression test (Hoshino et al. 1984). In a later study, four of five poorly developed AUT were non-suppressors and tended to have higher afternoon cortisol values compared to suppressors (Hoshino et al. 1987). Studies of individuals with autism by IQ have shown more sensory symptoms in low IQ groups compared to higher IQ groups (Kern et al. 2007; Leekam et al. 2007). This has been hypothesized as ineffective inhibitory control of sensory processing that may reflect an imbalance of neuronal excitation/inhibition in intellectually disabled children with autism (Orekhova et al. 2008). Similarly, in a study of young children, abnormal sensory processing was correlated with a higher autism severity score (a marker for lower functioning and lower IQ) (Kern et al. 2007). Alternatively, given our knowledge regarding the influence of excess output from the L-HPA and SNS systems on learning and memory, it is plausible that the stress exhibited in autism may be associated with deficits in intelligence and/or cognitive performance. In a study of cognitive performance and morning levels of salivary cortisol in typical children with high perceived stress, the authors suggest that stress may diminish cognitive performance because of its modulating effects on the L-HPA (Maldonado et al. 2008).
In the linear mixed models approach the between-subject within-group cortisol variability across the day was greater for AUT compared to TYP, although the variance estimates were small. Corbett et al. observed that the between-subject variance in cortisol for school-aged children with autism was greater than for typically developing children (Corbett et al. 2006, 2008). It is of note that the variability findings in the SACS were not consistent between cortisol and sAA. Unfortunately, we did not have the statistical power to examine whether functional status was associated with cortisol and/or sAA variability. Dysregulation with variability as a consequence may be a more likely characteristic of the L-HPA system in children with autism than hypercortisolism, although either or both may be occurring.
Some researchers posit that irregularities in more than one pathway of the CNS are consistent with a neurointegrative defect in autism (Hill et al. 1977; Nir et al. 1995). It is of note that there was some imbalance between the L-HPA and ANS in the SACS findings. There were differences in the type of variability (between- and within-subject) observed and the magnitude of secretion of cortisol and sAA in the children with autism. These findings of imbalance or asymmetry give support to the interactive model of adaptation from Bauer et al. (2002). In their interactive model optimal adaptation results when there is balance across the systems. In the only study of diurnal secretion in children, Wolf et al. (2008) examined both salivary analytes in school-age children with asthma and observed imbalances in the findings. Among the children with asthma there was lower diurnal secretion of sAA with higher chronic stress compared to healthy children, but no differences in cortisol were seen in the equivalent analysis.
There were some limitations in the study that should be addressed. Waking, midday, and bedtime samples were chosen to reflect a peak (waking), an interim point (midday), and nadir (bedtime) of secretion across the day. While it would be advantageous to collect samples over many times of the day to obtain an estimate of the area under the curve, this undertaking would be prohibitive for families with very young children. A minimal amount of Kool-Aid was deemed necessary to make the saliva collection procedure acceptable to very young children, although it has been suggested to avoid the use of oral stimulants (Schwartz et al. 1998). Recently, it has been reported that sAA is influenced by saliva flow rate (Beltzer et al. 2010). We did not have measurement of the amount of time it took to collect the saliva samples and thus could not incorporate flow rate into the analysis.
Almost all of the missing data were due to whole phases missing prior to SACS enrollment, rather than lapses in collection during any given phase. The missing data in the SACS was considered “missing at random” (MAR). In MAR, the probability that responses are missing depends on the set of observed responses but is not related to specific missing values. Fitzmaurice et al. (2004), consider the MAR assumption to be the default assumption for the analysis of partially missing longitudinal data, but we do not know how well our data truly reflected MAR.
G-computation estimation was used to obtain marginal (unconditional or population-level) estimates of average cortisol secretion levels over a day. While not unique to G-computation estimation, the marginal estimates derived by this method are superior to observational techniques, such as standard regression. However, important features of g-computation estimation are that it depends on the proper specification of the model to avoid a bias in estimation and consistent estimation—both of which can be difficult to achieve with a small sample size.
Conclusions
The SACS is one of a very few studies of cortisol and the first study of sAA secretion in preschool-aged children with autism. We established average salivary cortisol and sAA secretion values at waking, midday, and bedtime in both children with autism and typically developing children. There were two primary findings that are worthy of further exploration. First, the stark differences between cortisol and sAA in the type of variability that was prominent in the groups was unexpected. Second, differences in cortisol and sAA levels between extremely low, low, and high functioning children with autism, as defined by IQ, was a novel finding.
It will be important to replicate these results in larger studies of very young children with autism to determine whether these results are real. In addition, enrolling subgroups of children with differing functional status, differing adaptability, and/or sensory sensitivities will be valuable to parse out what environmental triggers or underlying brain mechanisms may be responsible for any differences in diurnal secretion.
Using a multisystem approach, such as an integrative assessment of how the L-HPA and ANS are working together to differentiate autism phenotypes or subgroups, should prove a fruitful avenue of research. There may be imbalances in these systems that may be an important descriptor of children with autism. This has ramifications regarding the amount of allostatic load these children may be experiencing. Given that proper regulation is a key feature of the stress-response pathways, imbalances from higher diurnal secretion or increased diurnal variability over the long term may be adverse to children's health.
Acknowledgments
The most heartfelt thank you goes to the families and their children who generously participated in the study. We also would like to thank the research staff on the Sleep in Children with Autism Study (Karen Tang, Anny Wu, Stephanie Sitnick, Sara Waters, and Shacunda Burton) who graciously assisted with the families. Melissa Amacher volunteered her time on data entry and other tasks. We are grateful for the management of samples by Mary Curran at Salimetrics, LLC and Alan Hubbard for statistical advice on causal methodology. Some of the infrastructure support for the study was provided by NIMH RO1 MH068232 (PI: Thomas Anders) and the M.I.N.D. Institute. This paper was prepared in part from the dissertation of the corresponding author.
Footnotes
Present Address: National Fragile X Foundation, 1615 Bonanza Street, Suite 202, Walnut Creek, CA 94596, USA
Present Address: Vanderbilt Kennedy Center, PMB 40, 230 Appleton Place, Nashville, TN 37203, USA
Present Address: Center for Interdisciplinary Salivary Bioscience Research, School of Nursing and Bloomberg School of Public Health, The Johns Hopkins University, 525 N. Wolfe Street, Baltimore, MD 21205, USA
Contributor Information
Sharon A. Kidd, School of Public Health, University of California, 101 Haviland Hall, Berkeley, CA 94720, USA
Blythe A. Corbett, M.I.N.D. Institute, University of California at Davis Medical Center, 2825 50th Street, Sacramento, CA 95817, USA
Douglas A. Granger, Department of Biobehavioral Health, Pennsylvania State University, University Park, PA 16802, USA
W. Thomas Boyce, College for Interdisciplinary Studies and Faculty of Medicine, University of British Columbia, Vancouver, BC V6T-123, Canada.
Thomas F. Anders, M.I.N.D. Institute, University of California at Davis Medical Center, 2825 50th Street, Sacramento, CA 95817, USA
Ira B. Tager, School of Public Health, University of California, 101 Haviland Hall, Berkeley, CA 94720, USA
References
- Achenbach T. Manual for the Child Behavior Checklist/2–3 and 1992 profile. University of Vermont Department of Psychiatry; Burlington, VT: 1992. [Google Scholar]
- Ahern J, Hubbard A, Galea S. Estimating the effects of potential public health interventions on population disease burden: A step-by-step illustration of causal inference methods. American Journal of Epidemiology. 2009;169(9):1140–1147. doi: 10.1093/aje/kwp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . The diagnostic and statistical manual of mental disorders. 4th ed. American Psychiatric Publishing, Inc; Washington, D.C.: 1994. [Google Scholar]
- Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children's behavior: Advantages of a multisystem approach. Journal of Developmental and Behavioral Pediatrics. 2002;23(2):102–113. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Beltzer EK, Fortunato CK, Guaderrama MM, Peckins MK, Garramone BM, Granger DA. Salivary flow and alpha-amylase: Collection technique, duration, and oral fluid type. Physiology & Behavior. 2010;101(2):289–296. doi: 10.1016/j.physbeh.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse (New York, N. Y.) 1996;23(1):28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E. Stress and development: Behavioral and biological consequences. Development and Psychopathology. 2001;13(3):473–489. doi: 10.1017/s0954579401003042. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. The impact of child maltreatment and psychopathology on neuroendocrine functioning. Development and Psychopathology. 2001;13(4):783–804. [PubMed] [Google Scholar]
- Corbett BA, Mendoza S, Abdullah M, Wegelin JA, Levine S. Cortisol circadian rhythms and response to stress in children with autism. Psychoneuroendocrinology. 2006;31(1):59–68. doi: 10.1016/j.psyneuen.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Corbett BA, Mendoza S, Wegelin JA, Carmean V, Levine S. Variable cortisol circadian rhythms in children with autism and anticipatory stress. Journal of Psychiatry and Neuroscience. 2008;33(3):227–234. [PMC free article] [PubMed] [Google Scholar]
- Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. 1st ed. Wiley; Hoboken, NJ: 2004. [Google Scholar]
- Fortunato CK, Dribin AE, Granger DA, Buss KA. Salivary alpha-amylase and cortisol in toddlers: Differential relations to affective behavior. Developmental Psychobiology. 2008;50(8):807–818. doi: 10.1002/dev.20326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill RD, Robins JM. Causal inference for complex longitudinal data: The continuous case. The Annals of Statistics. 2001;29(6):1785–1811. [Google Scholar]
- Goodlin-Jones BL, Tang K, Liu J, Anders TF. Sleep patterns in preschool-age children with autism, developmental delay, and typical development. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(8):930–938. doi: 10.1097/CHI.ObO13e3181799f7c. [DOI] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, el-Sheikh M, Gordis EB, Stroud LR. Salivary alpha-amylase in biobehavioral research: Recent developments and applications. Annals of the New York Academy of Sciences. 2007;1098:122–144. doi: 10.1196/annals.1384.008. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology. 2001;13(3):515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Hill SD, Wagner EA, Shedlarski JG, Jr, Sears SP. Diurnal cortisol and temperature variation of normal and autistic children. Developmental Psychobiology. 1977;10(6):579–583. doi: 10.1002/dev.420100612. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. Working Paper. Yale University; New Haven: 1975. [Google Scholar]
- Hoshino Y, Ohno Y, Murata S, Yokoyama F, Kaneko M, Kumashiro H. Dexamethasone suppression test in autistic children. Folia Psychiatrica et Neurologica Japonica. 1984;38(4):445–449. doi: 10.1111/j.1440-1819.1984.tb00793.x. [DOI] [PubMed] [Google Scholar]
- Hoshino Y, Yokoyama F, Watanabe M, Murata S, Kaneko M, Kumashiro H. The diurnal variation and response to dexamethasone suppression test of saliva cortisol level in autistic children. The Japanese Journal of Psychiatry and Neurology. 1987;41(2):227–235. doi: 10.1111/j.1440-1819.1987.tb00406.x. [DOI] [PubMed] [Google Scholar]
- Jansen LM, Gispen-de Wied CC, van der Gaag RJ, van Engeland H. Differentiation between autism and multiple complex developmental disorder in response to psychosocial stress. Neuropsychopharmacology. 2003;28(3):582–590. doi: 10.1038/sj.npp.1300046. [DOI] [PubMed] [Google Scholar]
- Kern JK, Trivedi MH, Grannemann BD, Garver CR, Johnson DG, Andrews AA, Schroeder JL. Sensory correlations in autism. Autism. 2007;11(2):123–134. doi: 10.1177/1362361307075702. [DOI] [PubMed] [Google Scholar]
- Leekam SR, Nieto C, Libby SJ, Wing L, Gould J. Describing the sensory abnormalities of children and adults with autism. Journal of Autism and Developmental Disorders. 2007;37(5):894–910. doi: 10.1007/s10803-006-0218-7. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr., Leventhal BL, DiLavore PC, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Storoschuk S, Rutter M, Pickles A. Using the ADI-R to diagnose autism in preschool children. Infant Mental Health Journal. 1993;14(3):234–252. [Google Scholar]
- Loyd BH, Abidin RR. Revision of the Parenting Stress Index. Journal of Pediatric Psychology. 1985;10(2):169–177. doi: 10.1093/jpepsy/10.2.169. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Fiocco A, Wan N, Maheu F, Lord C, Schramek T, et al. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology. 2005;30(3):225–242. doi: 10.1016/j.psyneuen.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Maldonado EF, Fernandez FJ, Trianes MV, Wesnes K, Petrini O, Zangara A, Ambrosetti L. Cognitive performance and morning levels of salivary cortisol and alpha-amylase in children reporting high versus. low daily stress perception. The Spanish Journal of Psychology. 2008;11(1):3–15. doi: 10.1017/s1138741600004066. [DOI] [PubMed] [Google Scholar]
- Maldonado G, Greenland S. Estimating causal effects. International Journal of Epidemiology. 2002;31(2):422–429. [PubMed] [Google Scholar]
- Marinovic-Curin J, Marinovic-Terzic I, Bujas-Petkovic Z, Zekan L, Skrabic V, Dogas Z, et al. Slower cortisol response during ACTH stimulation test in autistic children. European Child and Adolescent Psychiatry. 2008;17(1):39–43. doi: 10.1007/s00787-007-0632-1. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338(3):171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Mullen E. Mullen scales of early learning. Western Psychological Services; Los Angeles: 1997. [Google Scholar]
- Nater UM, Rohleder N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology. 2009;34(4):486–496. doi: 10.1016/j.psyneuen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Nater UM, Rohleder N, Schlotz W, Ehlert U, Kirschbaum C. Determinants of the diurnal course of salivary alpha-amylase. Psychoneuroendocrinology. 2007;32(4):392–401. doi: 10.1016/j.psyneuen.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Nir I, Meir D, Zilber N, Knobler H, Hadjez J, Lerner Y. Brief report: circadian melatonin, thyroid-stimulating hormone, prolactin, and cortisol levels in serum of young adults with autism. Journal of Autism and Developmental Disorders. 1995;25(6):641–654. doi: 10.1007/BF02178193. [DOI] [PubMed] [Google Scholar]
- Oitzl MS, Champagne DL, van der Veen R, de Kloet ER. Brain development under stress: Hypotheses of glucocorticoid actions revisited. Neuroscience and Biobehavioral Reviews. 2010;34(6):853–866. doi: 10.1016/j.neubiorev.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Prokofyev AO, Nygren G, Gillberg C, Elam M. Sensory gating in young children with autism: Relation to age, IQ, and EEG gamma oscillations. Neuroscience Letters. 2008;434(2):218–223. doi: 10.1016/j.neulet.2008.01.066. [DOI] [PubMed] [Google Scholar]
- Peeters F, Nicolson NA, Berkhof J. Levels and variability of daily life cortisol secretion in major depression. Psychiatry Research. 2004;126(1):1–13. doi: 10.1016/j.psychres.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Richdale AL, Prior MR. Urinary cortisol circadian rhythm in a group of high-functioning children with autism. Journal of Autism and Developmental Disorders. 1992;22(3):433–447. doi: 10.1007/BF01048245. [DOI] [PubMed] [Google Scholar]
- Robins JM. Association, causation, and marginal structural models. Synthese. 1999;121:151–179. [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: The glucocorticoid cascade hypothesis. Endocrine Reviews. 1986;7(3):284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Schwartz EB, Granger DA, Susman EJ, Gunnar MR, Laird B. Assessing salivary cortisol in studies of child development. Child Development. 1998;69(6):1503–1513. [PubMed] [Google Scholar]
- Sinisi SE, van der Laan MJ. Deletion/substitution/addition algorithm in learning with applications in genomics. Statistical Applications in Genetics and Molecular Biology. 2004;3 doi: 10.2202/1544-6115.1069. Article 18. [DOI] [PubMed] [Google Scholar]
- Snowden JM, Rose S, Mortimer KM. Implementation of G-computation on a simulated data set: Demonstration of a causal inference technique. American Journal of Epidemiology. 2011;173(7):731–738. doi: 10.1093/aje/kwq472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Cropley M, Griffith J, Kirschbaum C. Job strain and anger expression predict early morning elevations in salivary cortisol. Psychosomatic Medicine. 2000;62(2):286–292. doi: 10.1097/00006842-200003000-00022. [DOI] [PubMed] [Google Scholar]
- Varadhan R, Walston J, Cappola AR, Carlson MC, Wand GS, Fried LP. Higher levels and blunted diurnal variation of cortisol in frail older women. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2008;63(2):190–195. doi: 10.1093/gerona/63.2.190. [DOI] [PubMed] [Google Scholar]
- Volkmar FR, State M, Klin A. Autism and autism spectrum disorders: diagnostic issues for the coming decade. Journal of Child Psychology and Psychiatry. 2009;50(1–2):108–115. doi: 10.1111/j.1469-7610.2008.02010.x. [DOI] [PubMed] [Google Scholar]
- Wolf JM, Nicholls E, Chen E. Chronic stress, salivary cortisol, and alpha-amylase in children with asthma and healthy children. Biological Psychology. 2008;78(1):20–28. doi: 10.1016/j.biopsycho.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ. Cortisol regulation in posttraumatic stress disorder and major depression: a chronobiological analysis. Biological Psychiatry. 1996;40(2):79–88. doi: 10.1016/0006-3223(95)00451-3. [DOI] [PubMed] [Google Scholar]
- Zinke K, Fries E, Kliegel M, Kirschbaum C, Dettenborn L. Children with high-functioning autism show a normal cortisol awakening response (CAR). Psychoneuroendocrinology. 2010;35(10):1578–1582. doi: 10.1016/j.psyneuen.2010.03.009. [DOI] [PubMed] [Google Scholar]