Abstract
The epithelium forms a physical barrier important to the detection of pathogens. P. aeruginosa infections are frequently encountered in Cystic Fibrosis lungs, lead to ERK1/ERK2 activation and contribute to tissue destruction. We report here that in bronchial airway epithelial cells (BEAS-2B), diffusible material from P. aeruginosa and TLR2, TLR3 and TLR5 ligands activates ERK1/ERK2 via the protein kinase TPL2 and not the growth factor receptor EGFR. Activation of TPL2 by these agonists in airway epithelial cells requires the protein kinases TAK1 and IKKβ in accordance with the previously reported model of activation of TPL2 in macrophages. Inhibition of TPL2 activity with a pharmacological inhibitor (Compound 1) not only prevented ERK1/ERK2 activation but also decreased cytokine synthesis in response to pathogen-associated molecular patterns. These results suggest that inhibition of the protein kinase TPL2 is an attractive strategy to decrease inflammation in the lungs when it is not warranted.
Introduction
The Extracellular-signal Regulated Kinase (ERK)1/ERK2, also known as Mitogen activated Protein kinase (MAPK) 3/MAPK1, are well recognized for their roles in cell growth and differentiation occurring downstream of the v-raf-1 murine leukemia viral oncogene homolog (RAF)1 in response to growth factors [1]. However, they are also important transducers of inflammatory signals. This role is well conserved in nature, since ERK1/ERK2 confer protection to rectal infections of the nematode C. elegans [2]. Activation of ERK1/ERK2 in mammalian macrophages exposed to lipopolysaccharides (LPS) occurs downstream of the Tumor Progression Locus (TPL) 2 protein kinase, also known as MAPK Kinase Kinase (MAP3K) 8 [3], instead of RAF1, which is linked to growth factor activation of ERK1/ERK2. Both TPL2 and RAF1 activates ERK1/ERK2 via the phosphorylation of their direct upstream activator, MAPK kinase (MKK) 1/MKK2 (also known as MAP2K1/MAP2K2 [1]. This dichotomy in ERK1/ERK2 activation has led to great interest in pharmacological inhibition of one arm of the pathway while leaving the other unaffected.
The regulation of TPL2 activity is complex and many aspects remain unclear. TPL2 is found in unstimulated cells as a complex with TNFAIP3 interacting protein (TNIP) 2 [4] and nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 (NFκB1 p105) [5]. Interaction with TNIP-2 and NFκB1 p105 stabilizes TPL2, while the interaction with NFκB1 p105 also prevents TPL2 activity towards MKK1/MKK2 [6]. Activation of TPL2 requires phosphorylation and degradation of NFκB1 p105 by the IkB Kinase (IKK) complex [7], releasing TPL2 that will autophosphorylate on Thr290 [8] and auto- or transphosphorylate on Ser400 [9]. Once activated TPL2 phosphorylates MKK1/MKK2 direct upstream activators of ERK1/ERK2. In LPS-stimulated macrophages, the cascade TPL2-MKK1/MKK2-ERK1/ERK2 is essential to the production of tumor necrosis factor (TNF)-α [3] by regulating pre-TNFα expression at the plasma membrane [10]. In hematopoietic cell lineages, TPL2 is a master regulator of ERK1/ERK2-dependent gene expression downstream of Toll-like Receptors (TLRs) [11].
Although the role of TPL2 has been well established in immune cells, its role in mediating ERK1/ERK2 activation in other cell types has been much less studied. The epithelium forms a physical barrier important in the protection and detection of pathogens. In human corneal epithelial cells infected by P. aeruginosa, ERK1/ERK2 activation was reported downstream of the Epidermal Growth Factor Receptor (EGFR) [12]. P. aeruginosa infections are frequently encountered in Cystic Fibrosis lungs and contribute to tissue destruction. TLR-stimulation and P. aeruginosa infection of airway epithelial cells activate ERK1/ERK2 [13], [14], but the upstream activator of this pathway is unknown. As this knowledge would be important from a biological and pharmacological standpoint, in this manuscript we have investigated which signals upstream of the ERK1/ERK2 cascade are responsible for their activation by P. aeruginosa in human airway epithelial cells.
Materials and Methods
Materials
Pam3CSK4, flagellin and Poly I:C were bought from InvivoGen (San Diego, CA, USA). 5Z-7-oxozeaenol, BI605906 and C1 were kindly provided by Professor Sir Philip Cohen (MRC PPU, University of Dundee, UK). PD184352, PD153035 and human Epidermal Growth Factor (EGF) were bought from USBiological (Swampscott, MA, USA). AG1478 was bought from Tocris Biosciences (Minneapolis, MN, USA). Pseudomonas aeruginosa diffusible material (PsaDM) was prepared and used as described previously [14].
Antibodies
Anti-phospho Thr202/Tyr204 ERK1/ERK2 (4370) and anti-ERK1/ERK2 (9107) were used at 1/1000 dilution and purchased from Cell Signalling (Danvers, MA, USA). Anti-Raf-1 (sc-133) and anti-TPL2 (sc-720) were purchased form Santa Cruz Biotechnology (Santa Cruz, CA., USA) and used at 1/1000 and 1/500, respectively. Anti-phospho Tyr1068 EGFR (#Ab40815) was purchased from Abcam (Cambridge, MA., USA). Anti-GAPDH was purchased form Millipore (Billerica, MA. USA) and used at 1/4000 dilution. Goat anti-rabbit IgG DyLightTM800 (35571) and Goat anti-mouse IgG DyLightTM680 (35518) were used at 1/15000 dilution and were bought from Thermo Scientific (Rockford, IL, USA).
Cell Culture
Immortalized human bronchial epithelial cells BEAS-2B cells were purchased from ATCC (Rockville, MD, USA) and cultured as described previously [14]. Cells were grown to confluence and serum starved overnight to prevent serum-dependant ERK1/ERK2 activation, prior to stimulation with agonists and/or inhibitors.
Silencing Experiments
RAF1 siRNA (sc-29462), TPL2 siRNA (sc-35095) and siRNA transection reagent (sc-29528) were bought from Santa Cruz Biotechnology. BEAS-2B AECs were transfected in 12-wells plates according to the manufacturer’s protocols.
Cell Lysis, RNA Extraction, Real-time PCR, Immunoblotting and ELISA
All these techniques were performed as previously described [14], [15].
NF-κB Promoter-reporter Constructs
The NF-κB consensus response element (ggggactttcc) was synthesized in 4 copies and cloned at the XhoI/BglII sites of the pGL4.28 vector (Promega, Madison, WI, USA). The resulting vector was transformed in DH5α bacterial strain and purified with Invitrogen’s Purelink maxi prep kit (Invitrogen, Burlington, ON, Canada). pGL4.28NF-κB was transfected in BEAS-2B AECs with Fugene HD (Roche Applied Science, Basel, Switzerland) for 48 hrs and then selected for Hygromycin resistance in order to create stable cell lines. Cells were grown and stimulated as mentioned, then lysed with reporter lysis buffer (Promega). Luciferase activity in the cell extract was quantified using a Tecan M1000 microplate reader.
Statistical Analysis
The groups were compared with the non-parametric Wilcoxon-Mann-Withney test using GraphPad Prism software (version 5.0) p-values less then 0.05 were considered to be significant. Indications of significance correspond to p-values associated to untreated cells (*) and to PsaDM-treated cells or EGF-treated cells (#).
Results
TPL2 but not EGFR or RAF1 is Required for MKK1/MKK2-ERK1/ERK2 Activation in BEAS-2B Airway Epithelial Cells Stimulated with P. aeruginosa Diffusible Material
As bacteria present in the lungs of CF patients are found mostly as intra-luminal aggregates distal from airway epithelial cells (AECs) [16], we have exposed these cells to P. aeruginosa diffusible material (PsaDM). Since it is not known whether PsaDM activates ERK1/ERK2 via the EGFR as reported for P. aeruginosa infection of corneal epithelial cells [12], we therefore prevented the activity of the EGFR using two distinct pharmacological inhibitors, AG1478 and PD153035 at 100 nM and 50 nM respectively. These concentrations were found to prevent EGFR receptor phosphorylation at Tyr1068 and ERK1/ERK2 phosphorylation at its activation motif (Thr202/Tyr204) in the immortalized bronchial airway epithelial cell line BEAS-2B stimulated with EGF (Fig. S1A, S1B and S1C). Blocking EGFR activity did not prevent ERK1/ERK2 phosphorylation in BEAS-2B AECs stimulated with PsaDM ( Fig. 1A and 1B ). Moreover, as in the literature AG1478 is often used at concentration 100X higher than those reported here to be required to inhibit EGFR phosphorylation, we also show that even at 10 µM, a dose likely to suppress the activity of protein kinases other than EGFR, no reduction of in ERK1/ERK2 phosphorylation is observed in response to PsaDM ( Fig. 1A and 1B ). Accordingly, although EGF led to phosphorylation of the EGFR at Tyr1068, no phosphorylation at this residue was observed in BEAS-2B exposed to PsaDM ( Fig. 1A ).
Figure 1. PsaDM activates ERK1/ERK2 in an EGFR-independent manner.
A–B. BEAS-2B AECs were left untreated or exposed to 5 µg/ml PsaDM for 15 min or 50 ng/ml EGF for 30 min. Cells were lysed, and the lysates subjected to SDS-PAGE. A. Immunoblotting was performed with an anti-body that recognizes the EGFR phosphorylated at Tyr 1068 (p-EGFR, upper panel), an antibody that recognizes ERK1/ERK2 phosphorylated at Thr202/Tyr204 (p-ERK1/2, middle panel) or an antibody that recognizes all forms of ERK1/ERK2 (ERK1/2, lower panel). B. Quantitative analysis of the signal intensity obtained with an antibody recognizing only the phosphorylated forms of ERK1/ERK2 normalised to the signal obtained with antibody that recognizes all forms of ERK1/ERK2 was performed using Li-Cor infrared Odyssey imaging system.
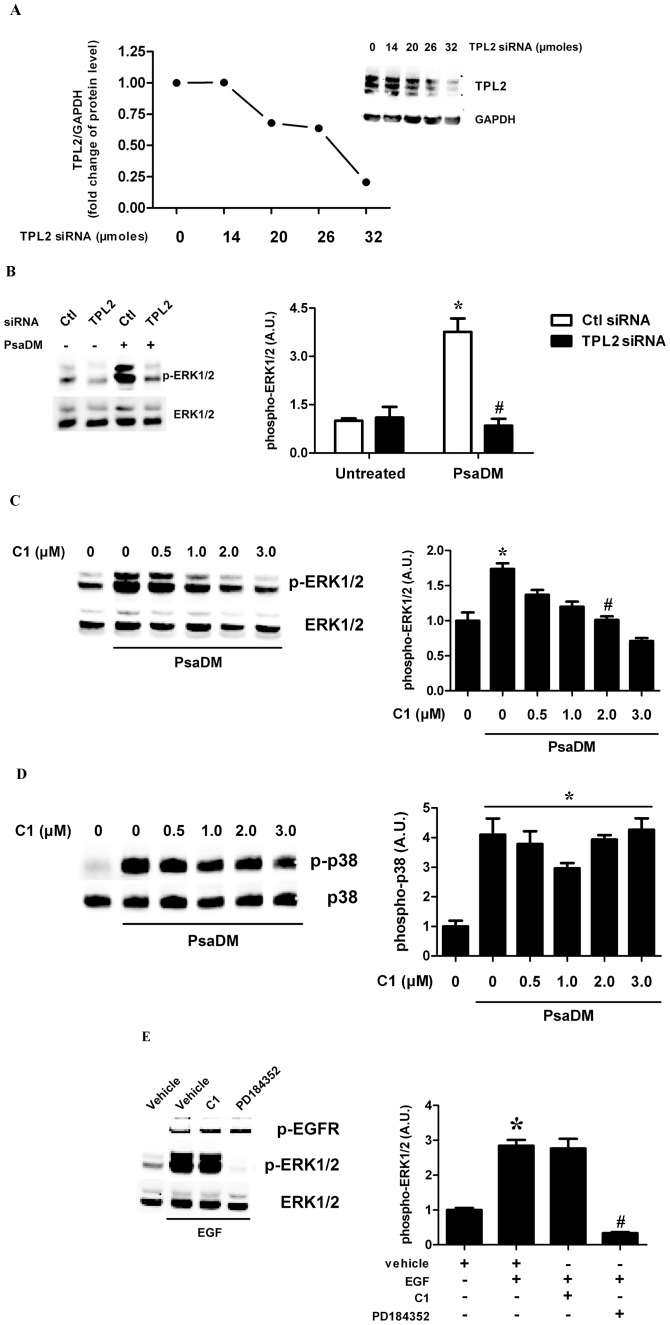
As the MAPK pathway MKK1/MKK2-ERK1/ERK2 is not only activated by RAF1, but also by the protein kinase TPL2, we asked whether this protein kinase contributed to the activation of ERK1/ERK2 in response to PsaDM. In order to determine the contribution of TPL2 to the activation of the ERK1/ERK2, siRNA against the mRNA of this kinase was purchased and transfected in BEAS-2B AECs. By using this siRNA, we were able to knock down TPL2 protein expression in a dose-dependent manner, up to ∼80% at 32 µM ( Fig. 2A ). When BEAS-2B AECs were stimulated with PsaDM for 15 min, ERK1/ERK2 activation was suppressed in cells transfected with the TPL2 siRNA but not the non-targeted siRNA transfected cells ( Fig. 2B ). Accordingly, siRNA against RAF1 did not prevent ERK1/ERK2 phosphorylation in BEAS-2B AECs exposed to PsaDM (Fig. S2A and S2B). To confirm the data obtained with the siRNA, we used the pharmacological inhibitor C1 shown to block TPL2 (MAP3K8) activity [17]. In BEAS-2B AECs stimulated with PsaDM, C1 decreased the phosphorylation of ERK1/ERK2 in a dose-dependent manner, with an IC50 of 1 µM ( Fig. 2C ), whereas p38α (also known as MAPK14) phosphorylation was only marginally affected ( Fig. 2D ). In contrast, EGF-driven ERK1/ERK2 phosphorylation in BEAS-2B AECs was unaffected by inhibition of TPL2 with C1 ( Fig. 2E ). These results show that there exist two parallel pathways that can lead to the activation of the ERK1/ERK2 in airway epithelial cells in response to distinct agonists.
Figure 2. TPL2 is required for PsaDM-dependent ERK1/ERK2 MAPK phosphorylation in AECs. A.
BEAS-2B AECs were transfected with increasing amounts of siRNA (0 to 32 µM) directed against TPL2 as indicated. Following lysis, 30 µg of lysates was submitted to SDS-PAGE followed by immunoblotting with an antibody that recognizes all form of TPL2. B. BEAS-2B AECs were transfected with control or TPL2 siRNA (32 µM) for 72 hours then left untreated or exposed to PsaDM for 15 minutes. ERK1/ERK2 was immunoblotted as in Fig. 1 . C–D. BEAS-2B AECs were pre-treated 1 hour with increasing doses of the TPL2 inhibitor C1 (0–3 µM) and exposed to 5 µg/ml PsaDM for 15 minutes. ERK1/ERK2 phosphorylation was determined as in Fig. 1 (C), while p38 phosphorylation was evaluated by immunoblotting with antibodies recognizing only the phosphorylated forms of p38α or antibodies that recognize all forms of p38α (D). E. BEAS-2B AECs were left untreated or pre-treated for 1 hour with vehicle, 2 µM C1 or 2 µM PD184352 (a MKK1/MKK2 inhibitor) and stimulated with 50 ng/mL EGF for 30 minutes. EGFR and ERK1/ERK2 were immunoblotted as in Fig. 1 . Representative blots from four distinct experiments are shown (left panels). Quantitative analysis of the signals was performed and expressed as graphs (right panels).
The Protein Kinases TAK1 and IKKβ are Required for MKK1/MKK2-ERK1/ERK2 Activation in BEAS-2B AECs Stimulated with PsaDM
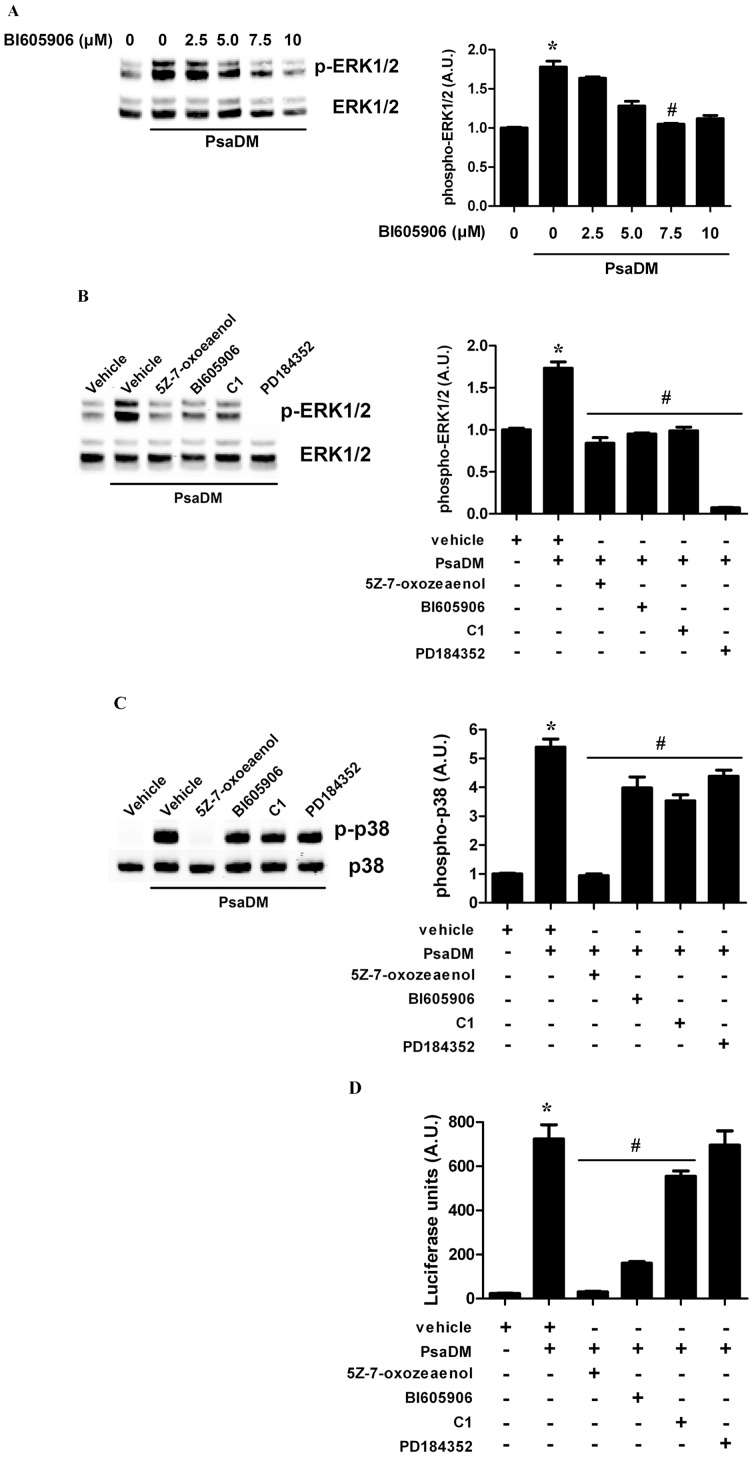
As mentioned in the introduction, the TLR-mediated activation of TPL2 requires the protein kinase TAK1 (also known as MAP3K7) that will in turn phosphorylate and activate the IKK complex. This will lead to the phosphorylation and degradation of NFκB1 p105, releasing TPL2 to become activated. In order to determine whether the same pathway acts in AECs, we prevented TAK1 activity using 5Z-7-oxozeaenol, a resorcylic acid lactone [18] and blocked IKKβ, also known as inhibitor of kappa light polypeptide gene enhancer in B-cells, kinase beta (IKBKB) activity using BI605906 [19]. BI605906 dose-dependently decreased ERK phosphorylation in BEAS-2B stimulated with PsaDM, with an IC50 of 3.75 µM ( Fig. 3A ). Moreover, pre-treating BEAS-2B AECs with 5Z-7-oxozeaenol also decreased ERK1/ERK2 phosphorylation to levels comparable to those obtained in the presence of the IKKβ or TPL2 inhibitor or in the absence of stimulation ( Fig. 3B ). The residual ERK1/ERK2 phosphorylation was suppressed by the MKK1/MKK2 inhibitor PD184352 [20] ( Fig. 3B ). In comparison, p38α phosphorylation was prevented by 5Z-7-oxozeaenol but not BI605906, C1 or PD184352 ( Fig. 3C ), whereas activation of NFκB-driven gene transcription was blocked by both 5Z-7-oxozeaenol and BI605906, but not C1 or PD184352 ( Fig. 3D ). ERK1/ERK2 basal phosphorylation was greatly decreased by PD184352 and mildly affected by 5Z-7-oxozeaenol and C1 (Fig. S3), suggesting that basal activity is mostly driven by the classical growth factor-mediator ERK1/ERK2 MAPK activation, whereas the PsaDM-driven inflammatory response occurs via the TAK1-IKKβ-TPL2-MKK1/MKK2-ERK1/ERK2 pathway.
Figure 3. The protein kinases TAK1 and IKKβ are required for MKK1/MKK2-ERK1/ERK2 activation in BEAS-2B AECs stimulated with PsaDM. A.
BEAS-2B AECs were left untreated or pre-treated 1 hour with increasing doses of the IKKβ inhibitor BI605906 (0–10 µM) and exposed to 5 µg/ml PsaDM for 15 minutes. ERK1/ERK2 phosphorylation was detected as in Fig. 1 . B–C. BEAS-2B AECs were pre-treated for 1 hour with vehicle, TAK1 inhibitor 5Z-7-oxozeaenol (0.25 µM), IKKβ inhibitor BI605906 (7.5 µM), C1 (2 µM) or PD184352 (2 µM) and stimulated with 5 µg/ml PsaDM for 15 minutes. Lysates were immunoblotted for ERK1/ERK2 (B) and p38α (C). D. BEAS-2B AECs stably transfected with pGL4.28-NF-κB were left untreated or pre-treated for 1 hour with vehicle, TAK1 inhibitor 5Z-7-oxozeaenol (0.25 µM), IKKβ inhibitor BI605906 (7.5 µM), C1 (2 µM) or PD184352 (2 µM) and stimulated with 5 µg/ml PsaDM for 2 hours. Cells extracts were subjected to luminescence analysis. Representative blots from four distinct experiments are shown (left panels). Quantitative analysis of the signals was performed and expressed as graphs (right panels).
TLR-mediated ERK1/ERK2 Activation Occurs via TAK1-IKKβ-TPL2-pathway in Airway Epithelial Cells
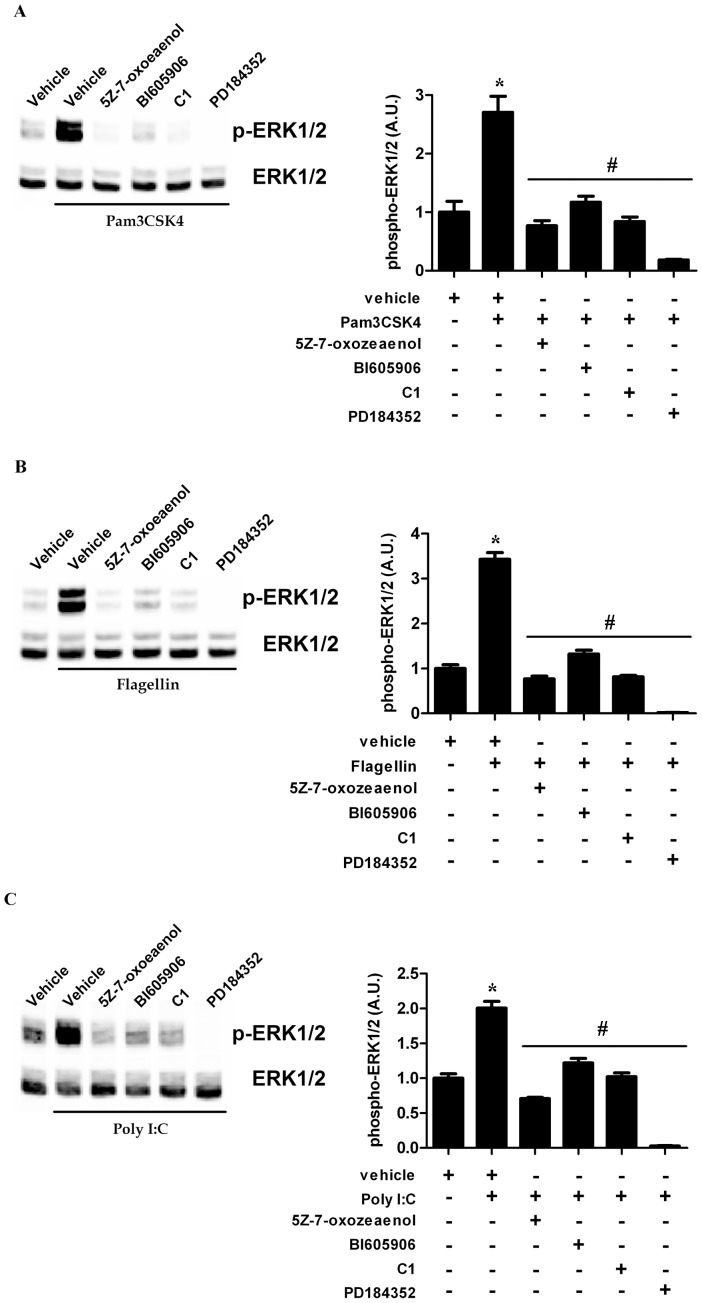
PsaDM activate p38α in a TLR-dependent fashion, acting mainly through TLR5 and TLR2 [15], [21], [22]. Therefore we checked whether activation of ERK1/ERK2 in BEAS-2B AECs occurs via the same mechanism when they are stimulated with isolated TLR ligands. Pam3CSK4 is a triacylated synthetic lipoprotein that binds the heterodimer TLR1/TLR2. Flagellin is a protein constituent of the flagella of motile bacteria that activates TLR5 at the cells surface. Both Pam3CSK4 and Flagellin activates downstream signaling via the MyD88 adaptor [23]. Polyinosine-polycytidylic acid (Poly I:C) is a synthetic analog of dsRNA that binds TLR3 and activates downstream signaling via the TRIF adaptor [23]. Stimulation of BEAS-2B AECs with Pam3CSK4 ( Fig. 4A ), Flagellin ( Fig. 4B ) or Poly I:C ( Fig. 4C ) led to the phosphorylation of the ERK1/ERK2 MAPK in all cases via the TAK1-IKKβ-TPL2-MKK1/MKK2 pathway ( Fig. 4 ).
Figure 4. TLR-mediated ERK1/ERK2 occurs via TAK1-IKKβ-TPL2 pathway in airway epithelial cells.
BEAS-2B AECs were left untreated or pre-treated for 1 hour with vehicle, TAK1 inhibitor 5Z-7-oxozeaenol (0.25 µM), IKKβ inhibitor BI605906 (7.5 µM), C1 (2 µM) or PD184352 (2 µM) and stimulated for 45 min with 1 µg/ml Pam3CSK4 (A), for 30 min with 200 ng/ml Flagellin (B) or for 75 min with 10 µg/ml Poly I:C (C). Following lysis, ERK1/ERK2 phosphorylation was determined as in Fig. 1 . Representative blots from four distinct experiments are shown (left panels). Quantitative analysis of the signals was performed and expressed as graphs (right panels).
Blocking TPL2 Activity Decreases Cytokine Synthesis Driven by PsaDM Stimulation of BEAS-2B AECs
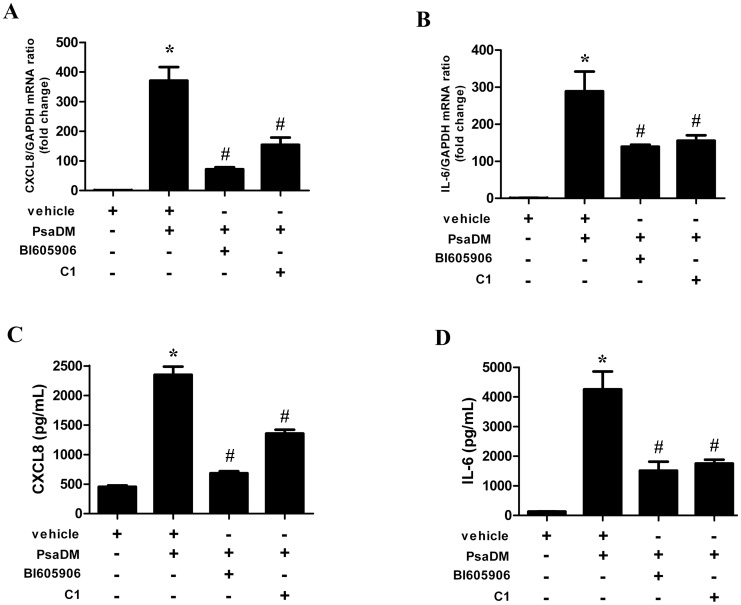
A major attraction of the dichotomy of ERK1/ERK2 activation between growth factors and PAMP is the possibility of pharmacological intervention on one arm of the pathway while leaving the other intact. For example, preventing TPL2 activity could decrease inflammation in chronic inflammatory disorders. Therefore we checked whether blocking TPL2 activity with C1 in BEAS-2B AECs could decrease cytokine synthesis. In accordance with our previous reports that blocking ERK1/ERK2 activation by PD184352 decreased the mRNA levels (Fig. S4) and cytokine release of CXCL8 (IL-8) and IL-6 induced by PsaDM [14], [15], blocking TPL2 with C1 also decreased by 60% and 50% the transcription of both CXCL8 and IL-6 ( Fig. 5A and 5B ). Similarly, pharmacological inhibition of TPL2 impairs cytokines secretion, reducing CXCL8 and IL-6 release into the culture media by 40% and 60% respectively in response to PsaDM ( Fig. 5C and 5D ). Blocking IKKβ with the inhibitor BI605906 also decreased expression and secretion of both cytokines ( Fig. 5 ).
Figure 5. Blocking TPL2 activity decreases cytokine synthesis driven by PsaDM stimulation of BEAS-2B AECs.
BEAS-2B AECs were left untreated or pre-treated for 1 hour with vehicle, 7.5 µM BI605906 or 2 µM C1 and stimulated with 5 µg/ml PsaDM for 1 hour (A–B) or 24 h (C–D). A–B. Total RNA was extracted and subjected to QRT-PCR for CXCL8 (A) and IL-6 (B). C–D. Following stimulation, the medium was collected and the amount of CXCL8 (C) and IL-6 (D) was determined by ELISA. Results from four independent experiments are shown.
Discussion
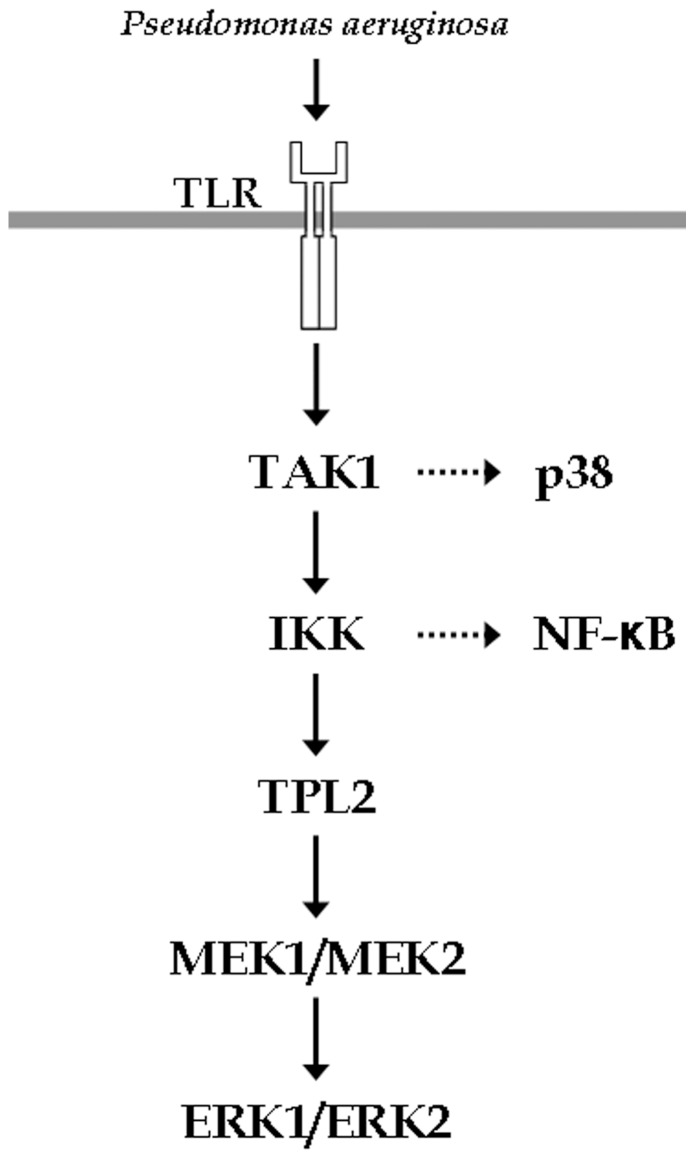
In this manuscript we have shown that the MKK1/MKK2-ERK1/ERK2 cascade is activated by the protein kinase TPL2 in airway epithelial cells exposed to TLR agonists and material derived from a clinical isolate of P. aeruginosa. In accordance with the pathway shown in macrophages [7], TPL2 is activated downstream of the protein kinases TAK1 and IKKβ ( Fig. 6 ). As expected, p38α is also activated downstream of TAK1 but independently from IKKβ and TPL2, whereas NFκB activation depends on TAK1, IKKβ but not TPL2. Therefore, following TLR activation signaling diverges downstream of TAK1, with one arm that leads to p38α activation and another arm downstream of the IKK complex required for both TPL2 and NFκB activation.
Figure 6. ERK1/ERK2 activation in airway epithelial cells exposed to TLR agonist occurs via the protein kinase TPL2.
TLR-dependent stimulation of AECs sequentially induces the activation of TAK1, IKKβ and TPL-2, leading to the phosphorylation of ERK1/ERK2.
In a previous report, it was shown that the MKK1/MKK2-ERK1/ERK2 MAPK cascade was activated downstream of the EGFR in corneal epithelial cells infected with P. aeruginosa [12]. This is in contrast with the data reported herein, where PsaDM did not lead to EGFR phosphorylation and acted via TAK1-IKKβ-TPL2. The major difference between the two models, beside the source of epithelial cells, is the live P. aeruginosa infection (mimicking an acute infection) versus our P. aeruginosa diffusible material (mimicking the chronic infection encountered in CF), which may be the reason for the differences observed.
Activation of the ERK1/ERK2 contributes to innate immunity, a function conserved in evolution [2]. Therefore, this pathway is an attractive target to decrease inflammation when it is not wanted. As the pathway regulates multiple functions, the ability to prevent or decrease its activation in response to bacterial ligands without compromising growth factor signaling is a valuable asset. We have shown that preventing ERK1/ERK2 activation using a TPL2 inhibitor, prevented ERK1/ERK2 activation in response to TLR ligands without compromising their activation in response to EGF. This supports the above notion that inflammatory and growth factor signaling can be separated in regards to ERK1/ERK2 activation. Moreover, we have shown that C1 also decreased the mRNA expression as well as the released into the media of the cytokine IL-6 and the neutrophil chemoattractant CXCL8. Therefore, these results support the idea of targeting TPL2 to decrease inflammation in chronic inflammatory lung diseases such as Cystic Fibrosis.
Supporting Information
AG1478 and PD153035 prevent ERK1/ERK2 activation by EGF. BEAS-2B cells were pre-treated for 1 hour with increasing dose of EGFR inhibitor AG1478 or PD153035 and stimulated for 30 minutes with 50 ng/mL of EGF. Cells extracts were then subjected to EGFR and ERK1/ERK2 phosphorylation analysis. Representative blots are shown in A. Quantitative analysis of the signals was performed and expressed as graphs for EGFR phosphorylation (B) and ERK1/ERK2 phosphorylation (C).
(TIF)
RNA interference of RAF1 does not prevent ERK1/ERK2 activation by PsaDM. A. BEAS-2B AECs were transfected with increasing amounts of siRNA directed against RAF1 (0 to 32 µM). 75% of protein knock-down was achieves with 32 µmoles of siRNA. B. BEAS-2B AECs were transfected with control or RAF1 siRNA for 72 hours then left untreated or exposed to 5 µg/ml PsaDM for 15 minutes. Cells extracts were then subjected to ERK1/ERK2 phosphorylation analysis. Compared to control siRNA, targeting RAF1 had no impact on ERK1/ERK2 activation by PsaDM.
(TIF)
Basal ERK1/ERK2 activation is mostly occurring independently of TAK1-IKKβ-TPL2. BEAS-2B AECs were pre-treated for 1 hour with vehicle, TAK1 inhibitor 5Z-7-oxozeaenol (0.25 µM), IKKβ inhibitor BI605906 (7.5 µM), C1 (2 µM) or PD184352 (2 µM) and exposed to 5 µg/ml PsaDM for 15 minutes. Cells extracts were then subjected to ERK1/ERK2 phosphorylation analysis. Except for PD184352, all the others inhibitors had minor impacts on basal ERK1/ERK2 phosphorylation levels. Representative blots from four distinct experiments are shown (left panel). Quantitative analysis of the signals was performed and expressed as graphs (right panel).
(TIF)
ERK1/ERK2 activation by PsaDM contributes to CXCL8 and IL-6 gene expression. BEAS-2B AECs were left untreated or pre-treated for 1 hour with 2 µM PD184352 and stimulated with 5 µg/ml PsaDM for 1 hour. A–B. Total RNA was extracted and subjected to QRT-PCR for CXCL8 (A) and IL-6 (B). Results from four independent experiments are shown.
(TIF)
Acknowledgments
We would like to thank Sir Philip Cohen (MRC PPU, University of Dundee, UK) for the kind gift of 5Z-7-oxozeaenol, BI605906 and C1. We thank Suzanne Cossette for her advice on luciferase assays.
Funding Statement
The authors acknowledge the financial support of Cystic Fibrosis Canada. The Meakins-Christie Laboratories - MUHC-RI, are supported by a Centre grant from Les Fonds de la Recherche en Santé du Québec (FRSQ). GM was supported by the CIHR Québec Respiratory Health Network fellowship. SR would like to acknowledge a salary award from the FRSQ. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kyriakis JM, App H, Zhang XF, Banerjee P, Brautigan DL, et al. (1992) Raf-1 activates MAP kinase-kinase. Nature 358: 417–421. [DOI] [PubMed] [Google Scholar]
- 2. Nicholas HR, Hodgkin J (2004) The ERK MAP kinase cascade mediates tail swelling and a protective response to rectal infection in C. elegans. Curr Biol 14: 1256–1261. [DOI] [PubMed] [Google Scholar]
- 3. Dumitru CD, Ceci JD, Tsatsanis C, Kontoyiannis D, Stamatakis K, et al. (2000) TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell 103: 1071–1083. [DOI] [PubMed] [Google Scholar]
- 4. Lang V, Symons A, Watton SJ, Janzen J, Soneji Y, et al. (2004) ABIN-2 forms a ternary complex with TPL-2 and NF-kappa B1 p105 and is essential for TPL-2 protein stability. Mol Cell Biol 24: 5235–5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Belich MP, Salmeron A, Johnston LH, Ley SC (1999) TPL-2 kinase regulates the proteolysis of the NF-kappaB-inhibitory protein NF-kappaB1 p105. Nature 397: 363–368. [DOI] [PubMed] [Google Scholar]
- 6. Beinke S, Deka J, Lang V, Belich MP, Walker PA, et al. (2003) NF-kappaB1 p105 negatively regulates TPL-2 MEK kinase activity. Mol Cell Biol 23: 4739–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beinke S, Robinson MJ, Hugunin M, Ley SC (2004) Lipopolysaccharide activation of the TPL-2/MEK/extracellular signal-regulated kinase mitogen-activated protein kinase cascade is regulated by IkappaB kinase-induced proteolysis of NF-kappaB1 p105. Mol Cell Biol 24: 9658–9667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Handoyo H, Stafford MJ, McManus E, Baltzis D, Peggie M, et al. (2009) IRAK1-independent pathways required for the interleukin-1-stimulated activation of the Tpl2 catalytic subunit and its dissociation from ABIN2. Biochem J 424: 109–118. [DOI] [PubMed] [Google Scholar]
- 9. Robinson MJ, Beinke S, Kouroumalis A, Tsichlis PN, Ley SC (2007) Phosphorylation of TPL-2 on serine 400 is essential for lipopolysaccharide activation of extracellular signal-regulated kinase in macrophages. Mol Cell Biol 27: 7355–7364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rousseau S, Papoutsopoulou M, Symons A, Cook D, Lucocq JM, et al. (2008) TPL2-mediated activation of ERK1 and ERK2 regulates the processing of pre-TNF alpha in LPS-stimulated macrophages. J Cell Sci 121: 149–154. [DOI] [PubMed] [Google Scholar]
- 11. Banerjee A, Gugasyan R, McMahon M, Gerondakis S (2006) Diverse Toll-like receptors utilize Tpl2 to activate extracellular signal-regulated kinase (ERK) in hemopoietic cells. Proc Natl Acad Sci U S A 103: 3274–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang J, Li H, Wang J, Dong Z, Mian S, et al. (2004) Role of EGFR transactivation in preventing apoptosis in Pseudomonas aeruginosa-infected human corneal epithelial cells. Invest Ophthalmol Vis Sci 45: 2569–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berube J, Bourdon C, Yao Y, Rousseau S (2009) Distinct intracellular signaling pathways control the synthesis of IL-8 and RANTES in TLR1/TLR2, TLR3 or NOD1 activated human airway epithelial cells. Cell Signal 21: 448–456. [DOI] [PubMed] [Google Scholar]
- 14. Berube J, Roussel L, Nattagh L, Rousseau S (2010) Loss of cystic fibrosis transmembrane conductance regulator function enhances activation of p38 and ERK MAPKs, increasing interleukin-6 synthesis in airway epithelial cells exposed to Pseudomonas aeruginosa. J Biol Chem 285: 22299–22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roussel L, Martel G, Berube J, Rousseau S (2011) P. aeruginosa drives CXCL8 synthesis via redundant toll-like receptors and NADPH oxidase in CFTRF508 airway epithelial cells. J Cyst Fibros 10: 107–113. [DOI] [PubMed] [Google Scholar]
- 16. Baltimore RS, Christie CD, Smith GJ (1989) Immunohistopathologic localization of Pseudomonas aeruginosa in lungs from patients with cystic fibrosis. Implications for the pathogenesis of progressive lung deterioration. Am Rev Respir Dis 140: 1650–1661. [DOI] [PubMed] [Google Scholar]
- 17. Hall JP, Kurdi Y, Hsu S, Cuozzo J, Liu J, et al. (2007) Pharmacologic inhibition of tpl2 blocks inflammatory responses in primary human monocytes, synoviocytes, and blood. J Biol Chem 282: 33295–33304. [DOI] [PubMed] [Google Scholar]
- 18. Ninomiya-Tsuji J, Kajino T, Ono K, Ohtomo T, Matsumoto M, et al. (2003) A resorcylic acid lactone, 5Z-7-oxozeaenol, prevents inflammation by inhibiting the catalytic activity of TAK1 MAPK kinase kinase. J Biol Chem 278: 18485–18490. [DOI] [PubMed] [Google Scholar]
- 19. Clark K, Peggie M, Plater L, Sorcek RJ, Young ER, et al. (2011) Novel cross-talk within the IKK family controls innate immunity. Biochem J 434: 93–104. [DOI] [PubMed] [Google Scholar]
- 20. Sebolt-Leopold JS, Dudley DT, Herrera R, Van Becelaere K, Wiland A, et al. (1999) Blockade of the MAP kinase pathway suppresses growth of colon tumors in vivo. Nat Med 5: 810–816. [DOI] [PubMed] [Google Scholar]
- 21. Beaudoin T, Lafayette S, Nguyen D, Rousseau S (2012) Mucoid Pseudomonas aeruginosa caused by mucA mutations result in activation of TLR2 in addition to TLR5 in airway epithelial cells. Biochem Biophys Res Commun. 428: 150–154. [DOI] [PubMed] [Google Scholar]
- 22. Zhang Z, Reenstra W, Weiner DJ, Louboutin JP, Wilson JM (2007) The p38 mitogen-activated protein kinase signaling pathway is coupled to Toll-like receptor 5 to mediate gene regulation in response to Pseudomonas aeruginosa infection in human airway epithelial cells. Infect Immun 75: 5985–5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA (1999) Phylogenetic perspectives in innate immunity. Science 284: 1313–1318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AG1478 and PD153035 prevent ERK1/ERK2 activation by EGF. BEAS-2B cells were pre-treated for 1 hour with increasing dose of EGFR inhibitor AG1478 or PD153035 and stimulated for 30 minutes with 50 ng/mL of EGF. Cells extracts were then subjected to EGFR and ERK1/ERK2 phosphorylation analysis. Representative blots are shown in A. Quantitative analysis of the signals was performed and expressed as graphs for EGFR phosphorylation (B) and ERK1/ERK2 phosphorylation (C).
(TIF)
RNA interference of RAF1 does not prevent ERK1/ERK2 activation by PsaDM. A. BEAS-2B AECs were transfected with increasing amounts of siRNA directed against RAF1 (0 to 32 µM). 75% of protein knock-down was achieves with 32 µmoles of siRNA. B. BEAS-2B AECs were transfected with control or RAF1 siRNA for 72 hours then left untreated or exposed to 5 µg/ml PsaDM for 15 minutes. Cells extracts were then subjected to ERK1/ERK2 phosphorylation analysis. Compared to control siRNA, targeting RAF1 had no impact on ERK1/ERK2 activation by PsaDM.
(TIF)
Basal ERK1/ERK2 activation is mostly occurring independently of TAK1-IKKβ-TPL2. BEAS-2B AECs were pre-treated for 1 hour with vehicle, TAK1 inhibitor 5Z-7-oxozeaenol (0.25 µM), IKKβ inhibitor BI605906 (7.5 µM), C1 (2 µM) or PD184352 (2 µM) and exposed to 5 µg/ml PsaDM for 15 minutes. Cells extracts were then subjected to ERK1/ERK2 phosphorylation analysis. Except for PD184352, all the others inhibitors had minor impacts on basal ERK1/ERK2 phosphorylation levels. Representative blots from four distinct experiments are shown (left panel). Quantitative analysis of the signals was performed and expressed as graphs (right panel).
(TIF)
ERK1/ERK2 activation by PsaDM contributes to CXCL8 and IL-6 gene expression. BEAS-2B AECs were left untreated or pre-treated for 1 hour with 2 µM PD184352 and stimulated with 5 µg/ml PsaDM for 1 hour. A–B. Total RNA was extracted and subjected to QRT-PCR for CXCL8 (A) and IL-6 (B). Results from four independent experiments are shown.
(TIF)