Abstract
AIM: To investigate the performance and diagnostic accuracy of interferon-gamma (IFN-γ) for tuberculous peritonitis (TBP) by meta-analysis.
METHODS: A systematic search of English language studies was performed. We searched the following electronic databases: MEDLINE, EMBASE, Web of Science, BIOSIS, LILACS and the Cochrane Library. The Standards for Reporting Diagnostic Accuracy initiative and Quality Assessment for Studies of Diagnostic Accuracy tool were used to assess the methodological quality of the studies. Sensitivity, specificity, and other measures of the accuracy of IFN-γ concentration in the diagnosis of peritoneal effusion were pooled using random-effects models. Receiver operating characteristic (ROC) curves were applied to summarize overall test performance. Two reviewers independently judged study eligibility while screening the citations.
RESULTS: Six studies met the inclusion criteria. The average inter-rater agreement between the two reviewers for items in the quality checklist was 0.92. Analysis of IFN-γ level for TBP diagnosis yielded a summary estimate: sensitivity, 0.93 (95%CI, 0.87-0.97); specificity, 0.99 (95%CI, 0.97-1.00); positive likelihood ratio (PLR), 41.49 (95%CI, 18.80-91.55); negative likelihood ratio (NLR), 0.11 (95%CI, 0.06-0.19); and diagnostic odds ratio (DOR), 678.02 (95%CI, 209.91-2190.09). χ2 values of the sensitivity, specificity, PLR, NLR and DOR were 5.66 (P = 0.3407), 6.37 (P = 0.2715), 1.38 (P = 0.9265), 5.46 (P = 0.3621) and 1.42 (P = 0.9220), respectively. The summary receiver ROC curve was positioned near the desirable upper left corner and the maximum joint sensitivity and specificity was 0.97. The area under the curve was 0.99. The evaluation of publication bias was not significant (P = 0.922).
CONCLUSION: IFN-γ may be a sensitive and specific marker for the accurate diagnosis of TBP. The level of IFN-γ may contribute to the accurate differentiation of tuberculosis (TB) ascites from non-TB ascites.
Keywords: Tuberculosis, Tuberculous peritonitis, Interferon-gamma, Diagnosis, Meta-analysis
INTRODUCTION
Tuberculous peritonitis (TBP) is a manifestation of tuberculosis (TB), which constitutes about 3% of extrapulmonary tuberculosis (EPTB) cases, and EPTB constitutes about 15%-20% of all cases of TB in immunocompetent patients and accounts for more than 50% of cases in human immunodeficiency virus-positive individuals[1]. TBP is one of the most common forms of EPTB and cases of TBP are expected to increase with the increasing incidence of TB worldwide[2,3]. Early diagnosis of TBP is beneficial for anti-TB treatment, the prevention of complications, and reduction of mortality rate[4]. However, current clinical diagnostic techniques for TBP are time-consuming and inefficient. The definitive diagnosis requires histological confirmation of caseous granulomas. As ascites is one of the clinical signs of TBP, bacteriologic confirmation can be performed using ascitic fluid-derived acid-fast bacilli smears as well as cultures for Mycobacterium tuberculosis (M. tuberculosis). However, four weeks are required for the cultivation of M. tuberculosis, and acid-fast bacilli smears are too insensitive to meet the current diagnostic demand[5]. Laparoscopy-guided biopsy is advantageous for rapid TBP diagnosis, but has complications related to anesthesia and potential injury and bleeding[6]. An evaluation of existing techniques is urgently required as is the development of new methods with high sensitivity and specificity for early and accurate TBP diagnosis.
M. tuberculosis infection initiates an immunologic cascade involving the secretion of various cytokines and recruitment of Th1 lymphocytes. With abundant cell recruitment at the morbid site, the levels of various cytokines are markedly elevated. Interferon-gamma (IFN-γ) is an important cytokine following infection with M. tuberculosis[7,8]. Studies assessing the level of IFN-γ have been reported. Several studies from different parts of the world have demonstrated the efficacy of IFN-γ for the diagnosis of TB pleural and pericardial effusions[9,10], and its diagnostic efficacy has been compared with that of adenosine deaminase (ADA) in terms of cost-effectiveness[11]. Some studies have also evaluated the role of IFN-γ in the diagnosis of TB ascites[12-14]. However, whether IFN-γ detection contributes to accurate TBP diagnosis remains controversial. In the present study, we systematically analyzed and assessed the overall efficacy of IFN-γ in the diagnosis of TBP via meta-analysis techniques.
MATERIALS AND METHODS
Search strategy and study selection
We searched the following electronic databases: MEDLINE (1980-2011); EMBASE (1980-2011); Web of Science (1990-2011); BIOSIS (1993-2011) and LILACS (1980-2011). We also reviewed the Cochrane Library to identified relevant studies. Updated searches were carried out in December 2011. The following search terms were used: “tuberculosis” “Mycobacterium tuberculosis” “peritonitis” “peritoneal effusion/peritoneal fluid/abdominal effusion/ ascitic fluid/ascites” “interferon/IFN” “sensitivity and specificity” and “accuracy”. We contacted experts in the specialty and searched the reference lists of primary and review articles. Although no language restrictions were imposed initially, our resources only permitted the review of articles published in the English language for the full text review and final analysis. Conference abstracts and letters were excluded due to unavailable data.
A study was included when it provided both the sensitivity (true-positive rate) and specificity (false-positive rate) of IFN-γ for TBP diagnosis, or provided IFN-γ values in a dot-plot form that allowed results to be extracted for individual study subjects. Patients of any age diagnosed with TBP underwent smear or culture of M. tuberculosis and/or histologic observation of peritoneal tissue, as well as clinical diagnosis, such as response to anti-TB therapy. In addition, we selected studies including at least 10 TBP specimens which were eligible for inclusion in order to reduce selection bias due to a small number of participants. Two reviewers (Su SB and Jiang HX) independently judged study eligibility while screening the citations. Disagreements were resolved by consensus.
Data extraction and quality assessment
Two reviewers (Su SB and Jiang HX) checked and extracted data independently. The reviewers were blinded to publication details, and disagreements were resolved by consensus. Data retrieved from the reports included participant characteristics, assay methods, sensitivity and specificity data, cutoff values, year of publication, and methodological quality. Peritonitis IFN-γ values provided in dot plots were measured by placing scalar grids over the plots, and were analyzed by a receiver operating characteristic (ROC) curve for each study (SPSS; Chicago, IL, United States). A summary of each study, including the numbers of true-positive, false-positive, false-negative and true-negative findings, is displayed in Table 1.
Table 1.
Summary of included studies
| Ref. | Patients | Assay method | Cut off |
Test results |
Quality score |
||||
| TP | FP | FN | TN | STARD | QUADAS | ||||
| Ribera et al[12] | 86 | RIA | 3 U/mL or 9 U/mL | 16 | 0 | 0 | 70 | 11 | 9 |
| Soliman et al[13] | 50 | ELISA | 26 pg/mL | 13 | 0 | 3 | 33 | 15 | 12 |
| Sathar et al[14] | 92 | RIA | 3.2 U/mL | 25 | 1 | 2 | 54 | 13 | 10 |
| Saleh et al[28] | 41 | ELISA | 0.35 IU/mL | 13 | 0 | 1 | 27 | 16 | 11 |
| Sharma et al[29] | 119 | ELISA | 112 pg/mL | 30 | 3 | 1 | 85 | 18 | 13 |
| Sathar et al[30] | 52 | ELISA | 20 pg/mL | 21 | 0 | 2 | 29 | 14 | 12 |
ELISA: Enzyme-linked immunosorbent assay; RIA: Radioimmunoassay; TP: True-positive; FP: False-positive; FN: False-negative; TN: True-negative; STARD: Standards for Reporting Diagnostic Accuracy, maximum score 25, guidelines that aim to improve the quality of reporting in diagnostic studies; QUADAS: Quality Assessment for Studies of Diagnostic Accuracy, appraisal by use of empirical evidence, maximum score 14, expert opinion and formal consensus to assess the quality of primary studies of diagnostic accuracy.
We assessed the methodological quality of studies using guidelines established by the Standards for Reporting Diagnostic Accuracy (STARD)[15] initiative and the Quality Assessment for Studies of Diagnostic Accuracy (QUADAS) tool[16]. In addition, the following study design characteristics were retrieved: (1) cross-sectional design vs case-control design; (2) consecutive or random sampling of patients; (3) blind (single or double) interpretation of determination and reference standard results; and (4) prospective data collection. If primary studies did not show data that met the above criteria, we requested them from the authors. The “unknown” items were then treated as “no” if the authors did not respond.
Statistical analysis
We used standard methods recommended for meta-analyses of diagnostic test evaluations[17]. Analyses were performed using a professional statistical software program (Meta-DiSc for Windows; XI Cochrane Colloquium; Barcelona, Spain). The following measures of test accuracy were analyzed for each study: sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR) and diagnostic odds ratio (DOR).
The analysis was based on a summary ROC (SROC) curve[17]. Sensitivity and specificity as a single test threshold identified for each study were used to plot an SROC curve[18]. A random-effects model was adopted to calculate the average sensitivity, specificity, and other measures across studies[19,20].
The term heterogeneity refers to the degree of variability in results across studies, which was used in relation to meta-analyses. We detected statistically significant heterogeneity with the χ2 test (Fisher exact tests). To assess the effects of STARD and QUADAS scores on the diagnostic ability of IFN-γ, we included them as covariates in univariate meta-regression analysis (inverse variance weighted). We also analyzed the effects of other covariates on DOR, such as cross-sectional design, consecutive or random sampling of patients, single or double interpretation of determination, reference standard results, and prospective data collection. The relative DOR (RDOR) was calculated according to standard methods to analyze the change in diagnostic precision in the study per unit increase in the covariate[21,22]. Since publication bias is of concern for meta-analyses of diagnostic studies, we tested for the potential presence of this bias with funnel plots and the Egger test[23].
RESULTS
Selection and summary of studies
Eleven out of 25 publications dealing with peritonitis IFN-γ concentration for TBP diagnosis were considered to be eligible for inclusion in the meta-analysis[10,12-14,24-30]. Among these publications, five studies[10,24-27] were excluded because IFN-γ was detected only in peritoneal dialysis patients[27], there was no detailed data[25] and the number of participants was 10 or less[10,24,26] (Figure 1). Finally, 6 studies[12-14,28-30] including 131 TBP patients and 309 non-TBP patients were available for analysis, and the clinical characteristics of these studies, along with QUADAS scores, are outlined in Table 1.
Figure 1.
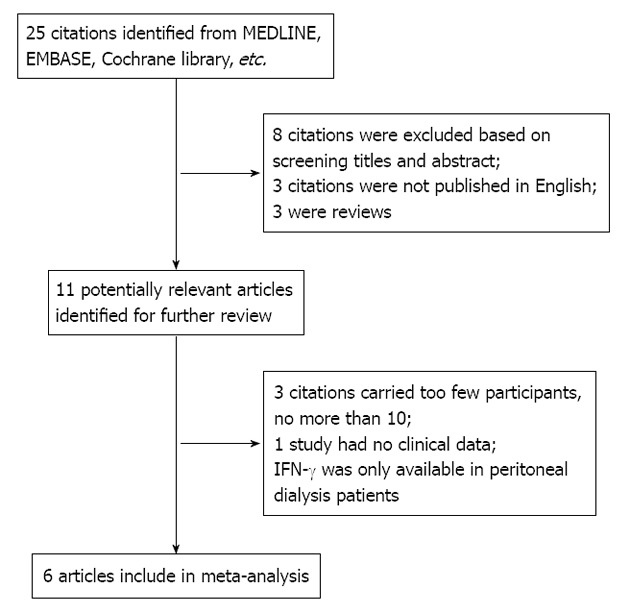
Flowchart of study selection. IFN-γ: Interferon-gamma.
Quality of reporting and study characteristics
The average inter-rater agreement between the two reviewers for items in the quality checklist was 0.92. All studies (100%) were collected from consecutive patients. The average sample size was 69 (range, 41-119) in the included studies. In four studies[12,13,28,30], a small proportion of the patients received the diagnosis according to clinical presentation, peritoneal effusion analysis, radiology findings and responsiveness of the patient to anti-TB chemotherapy. However, the diagnosis of peritoneal TB was confirmed in most of the TBP patients based on the conventional “gold standard” which was a smear or a positive M. tuberculosis culture which was taken from ascitic fluid and/or histology showing a caseating granuloma. In two studies[14,29], all patients were diagnosed with TBP based on a smear or culture that was positive for M. tuberculosis and had been taken from ascitic fluid and/or histology showing a caseating granuloma. All studies (100%) which reported that the study design was prospective could be identified (Table 2). Two studies (33.3%) reported blinded interpretation of the IFN-γ assay independent of the reference standard.
Table 2.
Characteristics of included studies
| Ref. | TB/N-TB patients | Reference standard | Cross-sectional design | Consecutive or random | Blinded design | Prospective |
| Ribera et al[12] | 16/70 | Bac/His or Clin | No | Yes | No | Yes |
| Soliman et al[13] | 17/33 | Bac/His or Clin | No | Yes | Yes | Yes |
| Sathar et al[14] | 30/62 | Bac/His | No | Yes | No | Yes |
| Saleh et al[28] | 14/27 | Bac/His or Clin | No | Yes | No | Yes |
| Sharma et al[29] | 31/88 | Bac/His | Yes | Yes | Yes | Yes |
| Sathar et al[30] | 23/29 | Bac/His or Clin | No | Yes | No | Yes |
TB: Tuberculosis; Bac: Bacteriology; His: Histology; Clin: Clinical course.
Diagnostic accuracy
The sensitivity and specificity of 6 IFN-γ assays for the diagnosis of TBP are shown in the forest plot (Figure 2). Sensitivity of IFN-γ for TBP diagnosis ranged from 0.54 to 1.00 (mean, 0.93; 95%CI, 0.87-0.97), while specificity ranged from 0.87 to 1.00 (mean, 0.99; 95%CI, 0.97-1.00). We also noted that PLR was 41.49 (95%CI, 18.80-91.55), NLR was 0.11 (95%CI, 0.06-0.19) and DOR was 678.02 (95%CI, 209.91 to 2190.09). χ2 values of sensitivity, specificity, PLR, NLR and DOR were 5.66 (P = 0.3407), 6.37 (P = 0.2715), 1.38 (P = 0.9265), 5.46 (P = 0.3621) and 1.42 (P = 0.9220), respectively, indicating no significant heterogeneity for sensitivity, specificity, PLR, NLR and DOR between studies.
Figure 2.
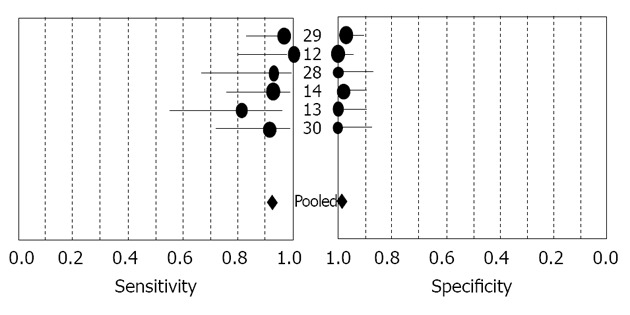
Forest plot showing the sensitivity and specificity of interferon-gamma in the diagnosis of tuberculous peritonitis. Forest plot shows the sensitivity and specificity of interferon-gamma (IFN-γ) for tuberculous peritonitis diagnosis. The point estimates of sensitivity and specificity from each study are shown as solid circles. Error bars indicated 95%CI. Numbers indicate the studies included in the meta-analysis, as cited in the reference list. Pooled estimates for IFN-γ assay were as follows: sensitivity, 0.93 (95%CI, 0.87 to 0.97), specificity, 0.99 (95%CI, 0.97 to 1.00).
The SROC plot is different from the traditional ROC plot and explores the effect of varying thresholds on sensitivity and specificity in a single study. In a SROC plot, any of the data points represent a separate study. The SROC curve represents a global summary of test performance and shows the tradeoff between sensitivity and specificity. A graph of the SROC curve for IFN-γ determination showing true-positive rates and false-positive rates from individual studies is shown in Figure 3. As a global measure of test efficacy, we used the Q-value, the intersection point of the SROC curve with a diagonal line from the left upper corner to the right lower corner of the ROC space, which corresponds to the highest common value of sensitivity and specificity for the test. This point represents an overall measure of the discriminatory power of a test. Our data showed that the SROC curve was positioned near the desirable upper left corner and that the maximum joint sensitivity and specificity was 0.97. The area under the curve (AUC) was 0.99. This indicated a high level of overall accuracy.
Figure 3.
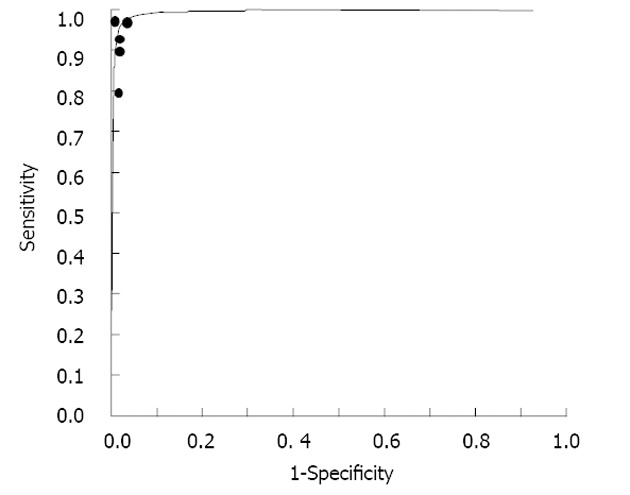
Summary receiver operating characteristic curves for interferon-gamma assays. Solid circles represent each study included in the meta-analysis. The size of each study is indicated by the size of the solid circle. Summary receiver operating characteristic curves summarize the overall diagnostic accuracy.
Multiple regression analysis and publication bias
By using the STARD guidelines[15], a quality score for each study was compiled on the basis of title and introduction, methods, results and discussion (Table 1). Quality scoring was also carried out using QUADAS[16], in which a score of 1 indicated a fulfilled criterion, 0 if an unclear criterion, and -1 if criterion not achieved. These scores were used in the meta-regression analysis to assess the effect of study quality on the RDOR of IFN-γ in the diagnosis of TBP. As shown in Table 3, studies with higher quality (STARD score, ≥ 13; QUADAS score, ≥ 10) produced RDOR values that were not significantly higher than those studies with lower quality. We also noted that differences for studies with or without blinded, cross-sectional, consecutive/random and prospective designs did not reach statistical significance, indicating that the study design did not substantially affect the diagnostic accuracy.
Table 3.
Weighted meta-regression of the effects of study design methods and methodological quality on diagnostic accuracy of interferon-gamma assays
| Covariate | Studies | Coefficient | RDOR (95%CI) | P |
| Consecutive or random | 6 | - | - | - |
| Prospective | 6 | - | - | - |
| Cross-sectional design | 1 | -1.763 | 0.17 (0.00, 2053.55) | 0.593 |
| Blinded design | 2 | -0.649 | 0.52 (0.01, 31.46) | 0.649 |
| Methods | ||||
| RIA | 2 | -0.815 | 0.44 (0.01, 26.58) | 0.571 |
| ELISA | 4 | |||
| QUADAS ≥ 10 | 5 | -2.137 | 0.12 (0.00, 98.54) | 0.387 |
| STARD ≥ 13 | 5 | -2.137 | 0.12 (0.00, 98.55) | 0.387 |
ELISA: Enzyme-linked immunosorbent assay; RIA: Radioimmunoassay; STARD: Standards for Reporting Diagnostic Accuracy, maximum score 25, guidelines that aim to improve the quality of reporting in diagnostic studies; QUADAS: Quality Assessment for Studies of Diagnostic Accuracy, appraisal by use of empirical evidence, maximum score 14, expert opinion and formal consensus to assess the quality of primary studies of diagnostic accuracy; RDOR: Relative diagnostic odds ratio.
The evaluation of publication bias showed that the results from the Egger test were not significant (P = 0.922). These results indicated little potential for publication bias.
DISCUSSION
The diagnosis of extrapulmonary mycobacterial infection is often difficult to establish since it has a nonspecific clinical presentation. Conventional diagnostic tests such as microscopic examination of peritonitis fluid by Ziehl-Neelsen staining, culture of mycobacteria from peritoneal effusion and peritonitis pathological examinations, are not always helpful in making the diagnosis because of their limitations. Invasive procedures, such as peritoneoscopy, laparotomy and peritoneal biopsy, which require appropriate and adequate clinical specimens are complex and sometimes risky[6,14]. A negative smear for acid-fast bacilli, a lack of granulomas on histopathology, and failure to culture M. tuberculosis do not exclude the diagnosis[5,31-33]. Furthermore, the culture of M. tuberculosis takes 4 wk, and acid-fast bacilli smears are too insensitive to meet current needs[5,34].
The most popular biomarkers which have been proposed for TBP diagnosis are ADA and INF-γ. The levels of both were significantly higher in TBP patients than in non-TBP patients. Both showed relatively high sensitivity and specificity in diagnosing TBP[12,14,24,26,28,29]. However, there are also other methods for TBP diagnosis, such as the molecular rapid amplification-based tests [for example polymerase chain reaction (PCR)] which detect specific DNA or RNA fragments of M. tuberculosis. Many reports suggest that various PCR tests have good performance with sensitivity reaching up to 95% in smear-positive patients. However, Ziehl-Neelsen staining in patients with TB peritonitis is positive in only 3% of cases, and PCR sensitivity would be similarly very low[35]. At present, the ADA assay has been recommended not only in the TB peritonitis diagnostic test[36], but also as a tool for the differential diagnosis of different forms of EPTB, such as pleuritis, synovitis, and meningitis, infections of the female genital system and peripheral lymph nodes, and uveitis[37]. Liang et al[38] and his colleagues have completed a meta-analysis (including 63 studies) to estimate ADA in the diagnosis of tuberculous pleurisy. The meta-analysis showed that the mean sensitivity of the ADA assays was 0.92, while the mean specificity was 0.90, the maximum joint sensitivity and specificity was 0.91, while AUC was 0.96, indicating a relatively high level of overall accuracy. However, the present meta-analysis showed that the mean sensitivity of the IFN-γ assay was 0.93, while the mean specificity was 0.99, and that the maximum joint sensitivity and specificity was 0.97, while the AUC was 0.99, indicating a higher level of overall accuracy.
The DOR is a single indicator of test accuracy[39] that combines the data from sensitivity and specificity into a single number. The DOR of a test is the ratio of the odds of positive test results in the patient with disease relative to the odds of positive test results in the patient without disease. The value of DOR ranges from 0 to infinity, the higher values indicate better discriminatory test performance (higher accuracy). A DOR of 1.0 indicates that a test does not discriminate between patients with and those without disease. In the present meta-analysis, we found that the mean DOR was 678.02, also indicating a high level of overall accuracy.
Since the SROC curve and the DOR are not easy to interpret or use in clinical practice[40], and likelihood ratios are considered to be more clinically meaningful[40], we also presented both PLR and NLR as our measures of diagnostic accuracy. Likelihood ratios of > 10 or < 0.1 generate large and often conclusive shifts from pretest to posttest probability (indicating high accuracy). A PLR value of 41.49 suggests that patients with TBP have an approximately 41-fold higher chance of being IFN-γ assay-positive compared with patients without TBP. This high probability would be considered high enough to begin or to continue anti-TB treatment of TBP patients, especially in the absence of any evidence of malignancy. On the other hand, NLR was found to be 0.11 in the present meta-analysis. If the IFN-γ assay result was negative, the probability that this patient has TBP is approximately 10%, which is not low enough to rule out TBP. These data suggest that a negative IFN-γ assay result should not be used alone as a justification to deny or to discontinue anti-TB therapy. The choice of therapeutic strategy should be based on the results of microscopic examination of smear or culture of M. tuberculosis and/or histologic observation of peritoneal tissue, as well as other clinical data, such as response to anti-TB therapy.
An exploration of the reasons for heterogeneity rather than computation of a single summary measure is an important goal of meta-analysis[41]. In our meta-analysis, both STARD and QUADAS scores were used in the meta-regression analysis to assess the effect of study quality on RDOR. Most of the studies were of high quality (STARD score of ≥ 13 or QUADAS score of ≥ 10), with the exception of one study[12] which was assessed to be of low quality (STARD score of 11 and QUADAS score of 9). We found that there was no statistical heterogeneity for sensitivity, specificity, PLR, NLR, and DOR among the studies, which indicated that the differences for studies with or without a blinded, cross-sectional, consecutive/random and prospective design did not reach statistical significance, and the study design did not substantially affect diagnostic accuracy.
It should be emphasized that a definite TBP diagnosis is achieved when M. tuberculosis is demonstrated in peritonitis specimens, or when caseating granulomas are found in peritonitis biopsy specimens. As mentioned above, M. tuberculosis requires 4 wk of culture, and acid-fast bacilli smears are too insensitive to meet current needs[5,34]. Where diagnostic difficulty exists, measuring the levels of several biomarkers, such as ADA and IFN-γ, in ascitic fluid is useful, and clinicians can embark on empirical anti-TB therapy while awaiting culture results, especially in young patients from areas with a high prevalence of TB. One criticism of the use of biomarkers rather than cultures for TBP diagnosis is that culture results are not available to guide anti-TB therapy. In short, none of the biomarkers, including IFN-γ, provide culture and sensitivity data. Culture results are particularly useful if drug resistant TB is prevalent[42].
Our meta-analysis had several limitations. Firstly, the exclusion of conference abstracts, letters to the editor, and non-English-language studies might have led to publication bias, which was not found in the present review. However, a review of these abstracts and letters suggested that the overall results were similar to the results in the English language studies included. Secondly, misclassification bias may occur. TBP is not always diagnosed by either histologic or microbiological examination. Actually, some patients were diagnosed with TBP infection based just on the clinical course. This issue regarding accuracy of diagnosis could cause nonrandom misclassification, leading to biased results. Finally, the number of studies that met the inclusion criteria was not large enough. Multi-center and large blinded randomized controlled trials with IFN-γ assays using peritoneal effusion for TBP diagnosis should be conducted.
Based on this study, IFN-γ may play a potential role in accurate TBP diagnosis. This may be helpful in clinical findings and conventional tests including microbiological examination and peritoneal biopsy. Numerous studies are required to further establish the role of IFN-γ for early and accurate TBP diagnosis.
ACKNOWLEDGMENTS
We are grateful to Professor Shi HZ for his great assistance in the statistical analysis and translating foreign language articles.
COMMENTS
Background
Tuberculous peritonitis (TBP) is a manifestation of tuberculosis. Its diagnosis is still challenging and of great importance. Early and accurate diagnosis contributes to effective therapy and good survival rates. However, current clinical diagnostic techniques for TBP are time-consuming and inefficient. Interferon-gamma (IFN-γ) in peritoneal effusion has been shown to be a marker for the diagnosis of TBP.
Research frontiers
How does IFN-γ in ascitic fluid testing relate to blood interferon-γ release assay (IGRA) tests? The blood IGRA test is an established test for latent tuberculous (TB) infection, however, there is little information on the difference between IFN-γ and IGRA tests in clinical diagnosis. Multi-center and large blinded randomized controlled trials using both these tests for TB diagnosis should be conducted in the future.
Innovations and breakthroughs
Authors assessed IFN-γ for TBP diagnosis by meta-analysis, and the clinical findings may greatly facilitate the diagnosis and differential diagnosis of TBP.
Applications
IFN-γ may play a potential role in the accurate diagnosis of TBP. This may be helpful in clinical findings and conventional tests including microbiological examination and peritoneal biopsy. The level of IFN-γ may contribute to the accurate differentiation of tuberculosis ascites from non-tuberculosis ascites.
Peer review
This systematic review and meta-analysis of IFN-γ testing for TBP was generally of good quality, and adhered to relevant guidelines for systematic review and quality assessment.
Footnotes
P- Reviewer Turner AM S- Editor Gou SX L- Editor A E- Editor Xiong L
References
- 1.Sharma SK, Mohan A. Extrapulmonary tuberculosis. Indian J Med Res. 2004;120:316–353. [PubMed] [Google Scholar]
- 2.Sharma MP, Bhatia V. Abdominal tuberculosis. Indian J Med Res. 2004;120:305–315. [PubMed] [Google Scholar]
- 3.Riquelme A, Calvo M, Salech F, Valderrama S, Pattillo A, Arellano M, Arrese M, Soza A, Viviani P, Letelier LM. Value of adenosine deaminase (ADA) in ascitic fluid for the diagnosis of tuberculous peritonitis: a meta-analysis. J Clin Gastroenterol. 2006;40:705–710. doi: 10.1097/00004836-200609000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Lingenfelser T, Zak J, Marks IN, Steyn E, Halkett J, Price SK. Abdominal tuberculosis: still a potentially lethal disease. Am J Gastroenterol. 1993;88:744–750. [PubMed] [Google Scholar]
- 5.Inadomi JM, Kapur S, Kinkhabwala M, Cello JP. The laparoscopic evaluation of ascites. Gastrointest Endosc Clin N Am. 2001;11:79–91. [PubMed] [Google Scholar]
- 6.Vogel Y, Bous JC, Winnekendonk G, Henning BF. Tuberculous peritonitis in a German patient with primary biliary cirrhosis: a case report. J Med Case Rep. 2008;2:32. doi: 10.1186/1752-1947-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes PF, Fong SJ, Brennan PJ, Twomey PE, Mazumder A, Modlin RL. Local production of tumor necrosis factor and IFN-gamma in tuberculous pleuritis. J Immunol. 1990;145:149–154. [PubMed] [Google Scholar]
- 8.Jalapathy KV, Prabha C, Das SD. Correlates of protective immune response in tuberculous pleuritis. FEMS Immunol Med Microbiol. 2004;40:139–145. doi: 10.1016/S0928-8244(03)00303-1. [DOI] [PubMed] [Google Scholar]
- 9.Poyraz B, Kaya A, Ciledağ A, Oktem A, Gönüllü U. Diagnostic significance of gamma-interferon in tuberculous pleurisy. Tuberk Toraks. 2004;52:211–217. [PubMed] [Google Scholar]
- 10.Burgess LJ, Reuter H, Carstens ME, Taljaard JJ, Doubell AF. The use of adenosine deaminase and interferon-gamma as diagnostic tools for tuberculous pericarditis. Chest. 2002;122:900–905. doi: 10.1378/chest.122.3.900. [DOI] [PubMed] [Google Scholar]
- 11.Sharma SK, Banga A. Pleural fluid interferon-gamma and adenosine deaminase levels in tuberculosis pleural effusion: a cost-effectiveness analysis. J Clin Lab Anal. 2005;19:40–46. doi: 10.1002/jcla.20054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ribera E, Martínez Vásquez JM, Ocaña I, Ruiz I, Jimínez JG, Encabo G, Segura RM, Pascual C. Diagnostic value of ascites gamma interferon levels in tuberculous peritonitis. Comparison with adenosine deaminase activity. Tubercle. 1991;72:193–197. doi: 10.1016/0041-3879(91)90007-f. [DOI] [PubMed] [Google Scholar]
- 13.Soliman AA, el-Aggan HA, el-Hefnawy AM, Mahmoud SA, Abo Deya SH. The value of ascites adenosine deaminase activity and interferon gamma level in discriminating tuberculous from non-tuberculous ascites. J Egypt Soc Parasitol. 1994;24:93–105. [PubMed] [Google Scholar]
- 14.Sathar MA, Simjee AE, Coovadia YM, Soni PN, Moola SA, Insam B, Makumbi F. Ascitic fluid gamma interferon concentrations and adenosine deaminase activity in tuberculous peritonitis. Gut. 1995;36:419–421. doi: 10.1136/gut.36.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Lijmer JG, Moher D, Rennie D, de Vet HC. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. The Standards for Reporting of Diagnostic Accuracy Group. Croat Med J. 2003;44:635–638. [PubMed] [Google Scholar]
- 16.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devillé WL, Buntinx F, Bouter LM, Montori VM, de Vet HC, van der Windt DA, Bezemer PD. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol. 2002;2:9. doi: 10.1186/1471-2288-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau J, Ioannidis JP, Balk EM, Milch C, Terrin N, Chew PW, Salem D. Diagnosing acute cardiac ischemia in the emergency department: a systematic review of the accuracy and clinical effect of current technologies. Ann Emerg Med. 2001;37:453–460. doi: 10.1067/mem.2001.114903. [DOI] [PubMed] [Google Scholar]
- 19.Irwig L, Tosteson AN, Gatsonis C, Lau J, Colditz G, Chalmers TC, Mosteller F. Guidelines for meta-analyses evaluating diagnostic tests. Ann Intern Med. 1994;120:667–676. doi: 10.7326/0003-4819-120-8-199404150-00008. [DOI] [PubMed] [Google Scholar]
- 20.Vamvakas EC. Meta-analyses of studies of the diagnostic accuracy of laboratory tests: a review of the concepts and methods. Arch Pathol Lab Med. 1998;122:675–686. [PubMed] [Google Scholar]
- 21.Suzuki S, Moro-oka T, Choudhry NK. The conditional relative odds ratio provided less biased results for comparing diagnostic test accuracy in meta-analyses. J Clin Epidemiol. 2004;57:461–469. doi: 10.1016/j.jclinepi.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 22.Westwood ME, Whiting PF, Kleijnen J. How does study quality affect the results of a diagnostic meta-analysis? BMC Med Res Methodol. 2005;5:20. doi: 10.1186/1471-2288-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ariga H, Kawabe Y, Nagai H, Kurashima A, Masuda K, Matsui H, Tamura A, Nagayama N, Akagawa S, Machida K, et al. Diagnosis of active tuberculous serositis by antigen-specific interferon-gamma response of cavity fluid cells. Clin Infect Dis. 2007;45:1559–1567. doi: 10.1086/523591. [DOI] [PubMed] [Google Scholar]
- 25.Kim SH, Cho OH, Park SJ, Ye BD, Sung H, Kim MN, Lee SO, Choi SH, Woo JH, Kim YS. Diagnosis of abdominal tuberculosis by T-cell-based assays on peripheral blood and peritoneal fluid mononuclear cells. J Infect. 2009;59:409–415. doi: 10.1016/j.jinf.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Liao CH, Chou CH, Lai CC, Huang YT, Tan CK, Hsu HL, Hsueh PR. Diagnostic performance of an enzyme-linked immunospot assay for interferon-gamma in extrapulmonary tuberculosis varies between different sites of disease. J Infect. 2009;59:402–408. doi: 10.1016/j.jinf.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Palomar R, Arias Guillén M, Robledo C, Agüero R, Agüero J, Rodríguez C, Molinos L, Rodrigo E, Ortega F, Arias M. [Detection of latent tuberculosis infection in peritoneal dialysis patients: new methods] Nefrologia. 2011;31:169–173. doi: 10.3265/Nefrologia.pre2011.Jan.10765. [DOI] [PubMed] [Google Scholar]
- 28.Saleh MA, Hammad E, Ramadan MM, Abd El-Rahman A, Enein AF. Use of adenosine deaminase measurements and QuantiFERON in the rapid diagnosis of tuberculous peritonitis. J Med Microbiol. 2012;61:514–519. doi: 10.1099/jmm.0.035121-0. [DOI] [PubMed] [Google Scholar]
- 29.Sharma SK, Tahir M, Mohan A, Smith-Rohrberg D, Mishra HK, Pandey RM. Diagnostic accuracy of ascitic fluid IFN-gamma and adenosine deaminase assays in the diagnosis of tuberculous ascites. J Interferon Cytokine Res. 2006;26:484–488. doi: 10.1089/jir.2006.26.484. [DOI] [PubMed] [Google Scholar]
- 30.Sathar MA, Ungerer JP, Lockhat F, Simjee AE, Gouws E. Elevated adenosine deaminase activity in patients with HIV and tuberculous peritonitis. Eur J Gastroenterol Hepatol. 1999;11:337–341. doi: 10.1097/00042737-199903000-00020. [DOI] [PubMed] [Google Scholar]
- 31.Geake TM, Spitaels JM, Moshal MG, Simjee AE. Peritoneoscopy in the diagnosis of tuberculous peritonitis. Gastrointest Endosc. 1981;27:66–68. doi: 10.1016/s0016-5107(81)73152-3. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Vazquez JM, Ocaña I, Ribera E, Segura RM, Pascual C. Adenosine deaminase activity in the diagnosis of tuberculous peritonitis. Gut. 1986;27:1049–1053. doi: 10.1136/gut.27.9.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menzies RI, Fitzgerald JM, Mulpeter K. Laparoscopic diagnosis of ascites in Lesotho. Br Med J (Clin Res Ed) 1985;291:473–475. doi: 10.1136/bmj.291.6493.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Portillo-Gómez L, Morris SL, Panduro A. Rapid and efficient detection of extra-pulmonary Mycobacterium tuberculosis by PCR analysis. Int J Tuberc Lung Dis. 2000;4:361–370. [PubMed] [Google Scholar]
- 35.Sanai FM, Bzeizi KI. Systematic review: tuberculous peritonitis--presenting features, diagnostic strategies and treatment. Aliment Pharmacol Ther. 2005;22:685–700. doi: 10.1111/j.1365-2036.2005.02645.x. [DOI] [PubMed] [Google Scholar]
- 36.Ruiz-Manzano J, Blanquer R, Calpe JL, Caminero JA, Caylà J, Domínguez JA, García JM, Vidal R. [Diagnosis and treatment of tuberculosis] Arch Bronconeumol. 2008;44:551–566. [PubMed] [Google Scholar]
- 37.Titarenko OT, Potapenko EI, Kokkanen BM, Prokhorovich NA, Oleĭnik AN, Nakonechnyĭ GD, Molodykh AZ. [Adenosine deaminase in the complex diagnosis of different forms of extrapulmonary tuberculosis] Probl Tuberk Bolezn Legk. 2006:14–18. [PubMed] [Google Scholar]
- 38.Liang QL, Shi HZ, Wang K, Qin SM, Qin XJ. Diagnostic accuracy of adenosine deaminase in tuberculous pleurisy: a meta-analysis. Respir Med. 2008;102:744–754. doi: 10.1016/j.rmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. 2003;56:1129–1135. doi: 10.1016/s0895-4356(03)00177-x. [DOI] [PubMed] [Google Scholar]
- 40.Deeks JJ. Systematic reviews in health care: Systematic reviews of evaluations of diagnostic and screening tests. BMJ. 2001;323:157–162. doi: 10.1136/bmj.323.7305.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petitti DB. Approaches to heterogeneity in meta-analysis. Stat Med. 2001;20:3625–3633. doi: 10.1002/sim.1091. [DOI] [PubMed] [Google Scholar]
- 42.Light RW. Establishing the diagnosis of tuberculous pleuritis. Arch Intern Med. 1998;158:1967–1968. doi: 10.1001/archinte.158.18.1967. [DOI] [PubMed] [Google Scholar]


