Abstract
AIM: To identify a practical approach for preoperative decision-making in patients with intraductal papillary mucinous neoplasms (IPMNs) of the pancreas.
METHODS: Between March 1999 and November 2006, the clinical characteristics, pathological data and computed tomography/magnetic resonance imaging (CT/MRI) of 54 IPMNs cases were retrieved and analyzed. The relationships between the above data and decision-making for pancreatic resection were analyzed using SPSS 13.0 software. Univariate analysis of risk factors for malignant or invasive IPMNs was performed with regard to the following variables: carcinoembryonic antigen, carbohydrate antigen 19-9 (CA19-9) and the characteristics from CT/MRI images. Receiver operating characteristic (ROC) curve analysis for pancreatic resection was performed using significant factors from the univariate analysis.
RESULTS: CT/MRI images, including main and mixed duct IPMNs, tumor size > 30 mm or a solid component appearance in the lesion, and preoperative serum CA19-9 > 37 U/mL had good predictive value for determining pancreatic resection (P < 0.05), but with limitations. Combining the above factors (CT/MRI images and CA19-9) improved the accuracy and sensitivity for determining pancreatic resection in IPMNs. Using ROC analysis, the area under the curve reached 0.893 (P < 0.01, 95%CI: 0.763-1.023), with a sensitivity, specificity, positive predictive value and negative predictive value of 95.2%, 83.3%, 95.2% and 83.3%, respectively.
CONCLUSION: Combining preoperative CT/MRI images and CA19-9 level may provide useful information for surgical decision-making in IPMNs.
Keywords: Intraductal papillary mucinous neoplasms, Surgical decision-making, Carbohydrate antigen 19-9, Computed tomography/magnetic resonance imaging, Predictor
INTRODUCTION
There is a controversy between pancreatic resection and close follow-up for the treatment of intraductal papillary mucinous neoplasms (IPMNs). The prognosis of noninvasive IPMNs is better than that of invasive IPMNs. The overall prognosis of IPMNs is better than that of pancreatic adenocarcinomas[1,2]. When noninvasive IPMNs transform into invasive IPMNs, the prognosis of this disease is as poor as that of pancreatic ductal adenocarcinomas[3,4]. There is no good way of monitoring this malignant transformation. Considering the nature of potential malignant transformation, some surgeons think that all IPMNs should be resected. However, for patients with benign lesions, close follow-up may be reasonable, as pancreatectomy may have severe adverse consequences due to its high mortality (15% at immature clinics) and high rate of postoperative complications (49%)[5]. It is increasingly clear that not all IPMNs should be resected immediately[6,7]. Wang et al[8] reported that there was no significant difference in the 5-year survival rate between resected IPMNs and non-resected IPMNs. He also suggested that resection is not justified in elderly patients with high surgical risks, especially in asymptomatic and aged patients. A prospective study on the management of 109 asymptomatic patients also indicated that careful non-operative management seemed to be safe and effective[9]. Therefore, it is crucial to establish a protocol to determine which IPMNs should be resected.
According to the Sendai consensus guidelines[10], all main duct IPMNs, symptomatic branch duct IPMNs and IPMNs > 3 cm in size accompanying mural nodules should be resected. The presence of mural nodules is a very reliable malignant predictor in IPMNs. However, there is controversy regarding lesion size > 3 cm as a reliable indicator[11]. The Sendai Criteria also showed that more data are needed to determine whether all branch duct IPMNs > 30 mm in size should be resected immediately[10]. The Sendai criteria have a very high negative predictive value (NPV) and low positive predictive value (PPV)[12], which may miss some malignant cases[12]. Moreover, the management of some asymptomatic branch duct IPMNs < 30 mm in size is not clear using these criteria[10]. Therefore, preoperative guidelines for surgical decision-making in IPMN patients need to be investigated[13].
However, much work have been done to clarify the characteristics of malignant or invasive IPMNs. Both computed tomography (CT) and magnetic resonance imaging (MRI) examinations have good predictive value for invasive IPMNs. They have similar functions in pancreatic diagnosis[14], evaluating the extent of tumor invasion and judging the resectability of pancreatic tumors[15]. In addition, a high serum carbohydrate antigen 19-9 (CA19-9) level is associated with malignant pancreatic disease[16-19]. Because not all lesions predicted to be malignant or invasive are suitable for surgery (e.g., advanced malignancy or those wrongly predicted to be malignant), and some lesions predicted to be benign may require surgery (e.g., borderline tumors or those wrongly predicted to be benign), there are differences between the prediction for malignant or invasive IPMNs and the prediction for surgical decision-making. A pragmatic approach for pancreatic resection decision-making in IPMN patients is lacking. Serum CA19-9 and CT/MRI scans are useful in predicting malignant/invasive IPMNs, but their function in surgical decision-making for IPMNs is still unclear. Moreover, the value of the combination of preoperative serum CA19-9 and dynamic enhanced thin-slice CT/MRI scanning in operative decision-making is also unknown. Therefore, in this study, the above problems were investigated and a simple and pragmatic approach for surgical decision-making in IPMNs was designed.
MATERIALS AND METHODS
Clinical data
Fifty-four patients with IPMNs of the pancreas observed from March 1999 to November 2006 were retrospectively reviewed. Clinical data and tumor marker levels, including carcinoembryonic antigen and CA19-9, were collected. All patients underwent surgery and had a confirmed pathological diagnosis. Dynamic enhanced thin-slice CT/MRI images were objectively reevaluated by two radiologists and the final decision was made by consensus. The parameters of CT/MRI images included the type of tumor, the morphology of the lesion, the size of the lesion, the diameter of the main pancreatic duct, the diameter of the common bile duct, the septum appearance of the lesion, the solid component appearance in the lesion, and the patulous intraductal papilla.
Premise and hypothesis: Gold standard for pancreatic resection in IPMNs
IPMNs include adenomas, borderline tumors, adenocarcinoma in situ, and invasive IPMNs. Malignant IPMNs include adenocarcinoma in situ and invasive IPMNs. Benign IPMNs include adenomas and borderline tumors. By analyzing the postoperative pathological diagnosis and the clinicopathological stage, we found that some of the 54 patients required surgery, while other patients with benign diseases or more advanced stages were not suitable for pancreatic resection. Because borderline tumors have a high potential to change to malignant tumors, borderline tumors or malignant tumors with stage 0, I and II (UICC 6th edition, 2002) at pathological diagnosis were the gold standard (criterion-G) for surgical resection of IPMNs. According to criterion-G, the 54 cases were divided into two groups: the operative group who required surgical resection and the observational group who required close follow-up. The predictive value of preoperative serum CA19-9 level, CT/MRI scan, or their combination for determining surgical resection were then investigated.
Statistical analysis
Statistical analysis was carried out using SPSS 13.0 for Windows. All continuous data were presented as mean ± SD. Categorical variables were compared by the χ2 test or Fisher’s exact test. The independent-samples t test were used to compare the means of the two groups. Logistic regression analysis was performed to identify independent risk factors; receiver operating characteristic (ROC) curve analysis was used to observe predictive values. P < 0.05 was considered statistically significant.
RESULTS
Clinical and pathological results
There were 36 male and 18 female patients in this study. The average age was 61.43 ± 8.24 years (range, 43-81 years). Conventional pancreatoduodenectomy was performed in 40 patients, total pancreatectomy was performed in 4 cases, distal pancreatectomy with splenectomy was performed in 5 cases, and distal pancreatectomy without splenectomy was performed in 1 case. Biopsy was carried out in 4 cases due to superior mesenteric artery (SMA) invasion, celiac trunk invasion or liver metastasis. The perioperative mortality rate was zero. Postoperative pathological diagnosis of IPMNs showed that there were 8 cases of adenoma, 8 cases of borderline tumors, 2 cases of adenocarcinoma in situ, and 36 cases of invasive IPMNs. There were 6 IPMN cases with a positive margin status, including 5 cases with pancreatic intraepithelial neoplasia 1 (PanIN-1) and 1 case with PanIN-3. According to the stage of pancreatic malignant tumor (UICC, 6th edition), there were 2 Stage 0, 3 Stage I A, 4 Stage I B, 20 StageIIA, 5 Stage IIB, 3 Stage III and 1 Stage IV in 38 malignant IPMNs.
Decision-making for pancreatic resection by CT/MRI scans
In 54 patients, 42 patients required surgery according to criterion-G. Following univariate analysis, non-branch duct IPMNs (main-duct and mixed IPMNs), lesions > 30 mm, the cystic and solid appearance of the lesion, the extended main pancreatic duct > 8.25 mm, the dilated common bile duct > 8.7 mm and the solid component appearance in the lesion from CT/MRI images were associated with pathologic findings for invasive IPMNs (Table 1). These features which were considered invasive characteristics from CT/MRI images were then further evaluated to predict surgical decision-making in IPMNs using ROC analysis. This evaluation showed that lesions > 30 mm, non-branch duct IPMNs and the solid component appearance in the lesion were good predictors for determining surgical resection (Figure 1). The patulous intraductal papilla was not analyzed due to a limited number of cases. However, all five cases with patulous intraductal papilla (3 malignant and 2 borderline tumors) harbored malignant potential, and many previous studies have indicated that patulous intraductal papilla is highly associated with invasive IPMNs. Thus, patulous intraductal papilla was also regarded as one of the invasive characteristics in IPMNs in our study. Next, our hypothesis (criterion-CT/MRI) was examined: pancreatic resection would be performed if the patient had any of the following factors on CT/MRI images: lesion size > 30 mm, non-branch duct IPMNs (main-duct and mixed), a solid component appearance in the lesion or patulous intraductal papilla, but no involvement of crucial vessels, such as SMA or celiac trunk, and definitive distant metastasis. According to criterion-CT/MRI, 95% of patients were correctly judged as requiring surgery. Using Fisher’s exact test, it was confirmed that criterion-CT/MRI had good predictive value for determining pancreatic resection in IPMNs (P < 0.05). The sensitivity of criterion-CT/MRI was 95% and the specificity was 50%. The PPV and NPV were 91.3% and 100%, respectively.
Table 1.
Univariate predictors of invasive intraductal papillary mucinous neoplasms
| Factors | Noninvasive | Invasive | t/χ2 | P value |
| Gender | ||||
| Male | 12 | 24 | ||
| Female | 6 | 12 | 0.00 | 1.00 |
| Age (yr) | 60.00 ± 8.48 | 61.94 ± 7.76 | -0.84 | 0.40 |
| Size of lesion (mm)1 | ||||
| ≤ 30 | 16 | 2 | ||
| > 30 | 2 | 20 | 25.47 | < 0.01 |
| Type | ||||
| Non-branch duct | 10 | 36 | ||
| Branch duct | 8 | 0 | 18.78 | < 0.01 |
| Morphology of the lesion1 | ||||
| Unilocular cystic | 4 | 0 | ||
| Multiocular cystic | 14 | 6 | ||
| Cystic and solid | 0 | 16 | 23.03 | < 0.01 |
| Septum | ||||
| Yes | 12 | 6 | ||
| No | 6 | 30 | 20.00 | 0.072 |
| Solid appearance | ||||
| Yes | 1 | 26 | ||
| No | 17 | 10 | 21.33 | < 0.01 |
| Diameter of main pancreatic duct (mm) | ||||
| ≤ 8.25 | 18 | 20 | ||
| > 8.25 | 0 | 16 | 11.36 | < 0.01 |
| Diameter of common bile duct (mm) | ||||
| ≤ 8.7 | 18 | 12 | ||
| > 8.7 | 0 | 24 | 21.60 | < 0.01 |
Not including main-duct intraductal papillary mucinous neoplasms.
Figure 1.
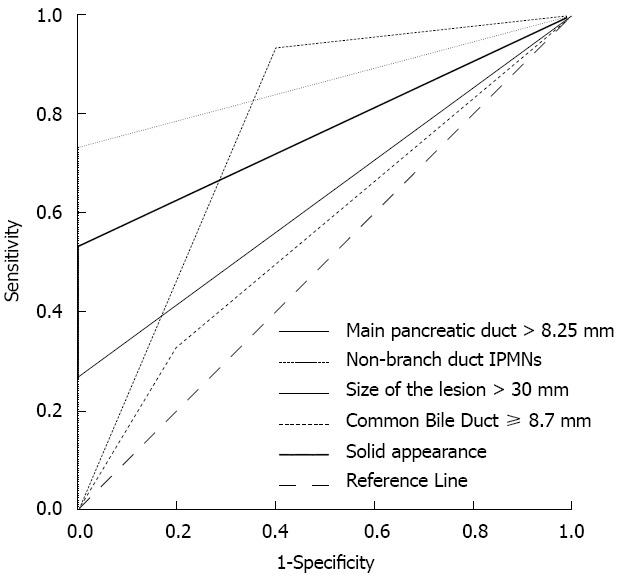
Characteristics of computed tomography/magnetic resonance imaging. Receiver operating characteristic analysis showed that non-branch duct intraductal papillary mucinous neoplasms (IPMNs), lesion size > 30 mm and a solid component appearance in the lesion had great significance for predicting pancreatic resection, and the area under the curve reached 0.76 (P = 0.012, 95%CI: 0.569-0.964), 0.867 (P = 0.001, 95%CI: 0.758-0.976) and 0.76 (P < 0.01, 95%CI: 0.623-0.910), respectively.
Decision-making for pancreatic resection by preoperative serum CA19-9 levels
Thirty-eight cases were malignant and 16 cases were non-malignant IPMNs. Following univariate analysis, jaundice, direct bilirubin > 6.0 μmol/L, γ-GT > 50 U/L, alkaline phosphatase > 115 U/L and CA19-9 > 37 U/mL were significantly associated with malignancy (Table 2). Using multivariate analysis, only CA19-9 > 37 U/mL was identified as an independent predictor of malignant IPMNs. The ROC curve also showed that CA19-9 had much better predictive potential for malignant IPMNs than the other factors (Figure 2). The relationship between the high level of serum CA19-9 and surgical decision-making was investigated. The higher the level of serum CA19-9, the higher the possibility of surgery for IPMNs (P = 0.013). The sensitivity of CA19-9 > 37 U/mL for determining surgery was 57% and the specificity was 84%. The PPV and NPV were 100% and 33%, respectively.
Table 2.
Univariate predictors of malignant intraductal papillary mucinous neoplasms
| Factors | Benign | Malignant | t/χ2 | P value |
| (n = 16) | (n = 38) | |||
| Gender | ||||
| Male | 10 | 26 | ||
| Female | 6 | 12 | 0.18 | 0.67 |
| Age (yr) | 61.75 ± 7.22 | 61.29 ± 8.72 | -0.18 | 0.85 |
| Lumbodorsal pain | ||||
| Yes | 0 | 3 | ||
| No | 10 | 31 | 1.50 | 0.22 |
| Jaundice | ||||
| Yes | 0 | 19 | ||
| No | 16 | 19 | 12.34 | < 0.01 |
| Direct bilirubin (μmol/L) | ||||
| ≤ 6.0 | 14 | 12 | ||
| > 6.0 | 2 | 26 | 14.10 | < 0.01 |
| ALP (U/L) | ||||
| ≤ 115 | 13 | 12 | ||
| > 115 | 3 | 26 | 9.26 | 0.02 |
| γ-GT (U/L) | ||||
| ≤ 50 | 13 | 10 | ||
| > 50 | 3 | 28 | 13.80 | < 0.01 |
| CA19-9 (U/mL) | ||||
| ≤ 37 | 14 | 8 | ||
| > 37 | 2 | 30 | 20.59 | < 0.01 |
| CEA (ng/mL) | ||||
| ≤ 5 | 15 | 33 | ||
| > 5 | 1 | 5 | 0.54 | 0.65 |
ALP: Alkaline phosphatase; CEA: Carcinoembryonic antigen; CA19-9: Carbohydrate antigen 19-9.
Figure 2.
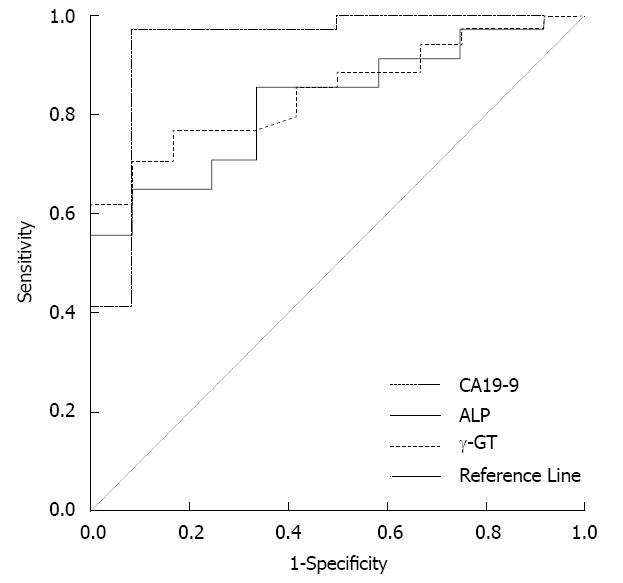
The predictors of malignant intraductal papillary mucinous neoplasms. Receiver operating characteristic analysis showed that γ-GT > 50 U/L, alkaline phosphatase (ALP) > 115 U/L or carbohydrate antigen 19-9 (CA19-9) > 37 U/mL effectively predicted malignant intraductal papillary mucinous neoplasms. However, the area under the curve for CA19-9 > 37 U/mL was the largest among the three indices and reached 0.939 (P < 0.01, 95%CI: 0.843-1.035).
Operative decision-making by the combination of serum CA19-9 and CT/MRI scans
Serum CA19-9 alone had a low sensitivity and NPV for predicting surgical decision-making for pancreatic resections. CT/MRI scans alone had a low specificity. High sensitivity, specificity, PPV and NPV are necessary for pancreatic resection decision-making. Therefore, the predictive value of combining CA19-9 level and CT/MRI scan images was evaluated. Our final approach (criterion-F) was as follows: patients with negative criterion-CT/MRI and serum CA19-9 ≤ 37 U/mL would be watched, and the remaining patients would undergo pancreatic resection. The value of criterion-F was analyzed by ROC curve analysis (Figure 3). The area under the curve was 0.893 (95%CI: 0.763-1.023), and its sensitivity was 95.2%, specificity was 83.3%, PPV was 95.2% and NPV was 83.3%.
Figure 3.
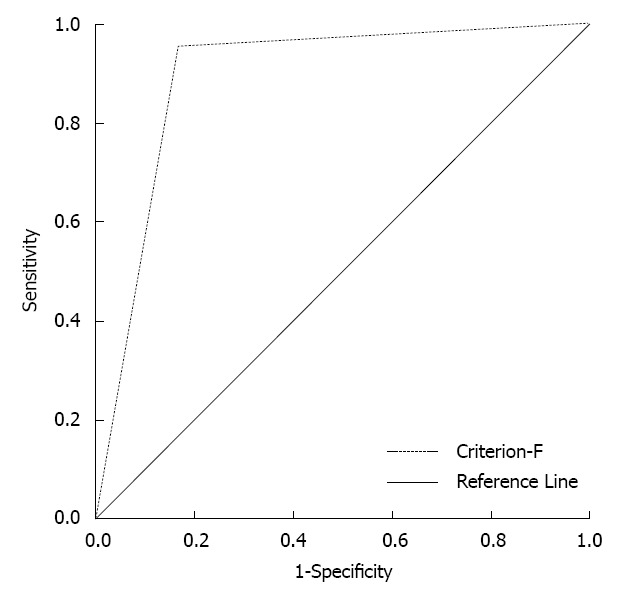
The predictive value of the combination of carbohydrate antigen 19-9 and computed tomography/magnetic resonance imaging (criterion-F). The area under the curve by receiver operating characteristic analysis reached 0.893 (P < 0.01, 95%CI: 0.763-1.023).
DISCUSSION
Pancreatic resection is necessary for invasive IPMNs, while close follow-up is also feasible for definitive benign IPMNs[20,21]. Until now, it has not been possible to effectively identify the nature of IPMNs preoperatively, therefore, it is difficult to accurately judge which IPMNs should be resected. Cytological biopsy from ERCP or fine needle aspiration has a high specificity for differentiation between benign and malignant IPMNs, however, their sensitivity is very low[22] and are less feasible and convenient than serum CA19-9 or CT/MRI scans. The value of CT/MRI scans in pancreatic diseases has been demonstrated[23]. Moreover, many predictive factors of malignant or invasive IPMNs were reported, including serum CA19-9[19,24], jaundice[25], tumor size > 3 cm[21], dilatation of the main pancreatic duct[26], and the presence of patulous papilla[27,28]. Predictors of malignant or invasive IPMNs may help surgical decision-making. However, their value in pancreatic resection decision-making for IPMNs, especially the value of combining preoperative serum CA19-9 and CT/MRI scans, have not been evaluated.
According to criterion-G, 54 patients were divided into the operative group (42 cases) and the watched group (13 cases). In the univariate analysis, main and mixed duct IPMNs, tumor size > 30 mm, an extended main pancreatic duct > 8.25 mm, common bile duct > 8.7 mm and the solid component appearance in the tumor from CT/MRI images were associated with pathologic findings of invasive IPMNs. However, only three parameters had good predictive value for surgical decision-making: main and mixed duct IPMNs, tumor size > 30 mm and the solid component appearance in the lesion. The patulous intraductal papilla has been reported to be a malignant sign in IPMNs by many researchers[7,29] and our all IPMN patients with patulous intraductal papilla required pancreatic resections, thus we combined it with the above three parameters to form criterion-CT/MRI.
Our data showed that criterion-CT/MRI or CA19-9 > 37 U/mL alone showed good predictive value for determining pancreatic resection in IPMNs (P < 0.05), however, they both had limitations. The specificity of criterion-CT/MRI was only 50%. The sensitivity of CA19-9 > 37 U/mL was just 57% and the NPV of CA19-9 > 37 U/mL was just 33%. Thus, CA19-9 level and criterion-CT/MRI were combined to improve the accuracy and sensitivity. This led to our final approach (criterion-F): patients with negative criterion-CT/MRI and normal CA19-9 level (≤ 37 U/mL) would be observed; while the remaining patients would undergo pancreatic resection. Criterion-F had higher accuracy for pancreatic resection decision-making in IPMNs, with acceptable sensitivity, specificity, PPV and NPV (95.2%, 83.3%, 95.2% and 83.3%, respectively).
The Sendai criteria[10] have a high NPV, but PPV is low (14%-22%)[30]. Moreover, the management of some IPMNs, such as lesions > 3 cm without main pancreatic duct dilation or mural nodules, is not very clear using the Sendai criteria. The management of some asymptomatic branch duct IPMNs < 30 mm in size is also not clear using the Sendai criteria[10]. Our criterion-F can be applied in all patients, including the patients mentioned above. According to our criterion-F, a very small number of patients would be misjudged. This small number may be less than 3% of all patients, which is acceptable considering the high risk of mortality due to pancreatic resection (3%-15%)[5]. For elderly patients, there is controversy over operative therapy. The implementation of our criterion-F should be appropriately adjusted, especially for noninvasive IPMNs, because the time required for the noninvasive type to develop into the invasive type might be longer than an elderly patient’s life expectancy[31].
In conclusion, a combination of serum CA19-9 and CT/MRI findings may be necessary for surgical decision-making in IPMNs. Criterion-F is effective for making the correct surgical decision, and can be used in all IPMNs. Of course, this study has limitations due to its retrospective design and relatively small sample size. Our findings should be further audited and improved by prospective large-scale clinical trials.
COMMENTS
Background
Due to the potential of malignant transformation from benign intraductal papillary mucinous neoplasms (IPMNs), all patients with IPMNs were previously recommended to undergo pancreatic surgery. There is currently more data available to support non-operative management of some benign IPMNs which is reasonable and feasible. However, it is difficult for surgeons to distinguish between benign and malignant IPMNs preoperatively and make decisions regarding pancreatic resection for IPMNs.
Research frontiers
Although there have been several articles on the malignant or invasive predictive factors for IPMNs, accurate preoperative criteria have not been available to determine pancreatic resection in IPMNs. Surgical decision-making for individual patients with IPMNs is still difficult. It is important to identify a practical approach for preoperative decision-making in IPMNs.
Innovations and breakthroughs
The combined strategy of serum carbohydrate antigen 19-9 level and computed tomography/magnetic resonance imaging provided useful information for preoperative surgical decision-making in IPMNs, with acceptable sensitivity, specificity, positive predictive value and negative predictive value of 95.2%, 83.3%, 95.2% and 83.3%, respectively.
Applications
It would be helpful for surgeons to determine which IPMNs should be resected.
Terminology
IPMN: This is a type of neoplasm which grows within the pancreatic ducts (intraductal) and is characterized by the production of thick fluid by the tumor cells.
Peer review
The paper provides a practical approach for preoperative surgical decision-making for IPMNs. The topic is very interesting, the results are applicable and the conclusions are valuable.
Footnotes
Supported by The National Natural Science Foundation of China, No. 81001007; the Program for Young Excellent Talents in Tongji University, No. 2008KJ060; and Youth Fund of the Shanghai Tenth People’s Hospital, No. 10RQ105
P- Reviewer Fu DL S- Editor Wen LL L- Editor A E- Editor Yan JL
References
- 1.Sadakari Y, Ohuchida K, Nakata K, Ohtsuka T, Aishima S, Takahata S, Nakamura M, Mizumoto K, Tanaka M. Invasive carcinoma derived from the nonintestinal type intraductal papillary mucinous neoplasm of the pancreas has a poorer prognosis than that derived from the intestinal type. Surgery. 2010;147:812–817. doi: 10.1016/j.surg.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Wente MN, Schmied BM, Schmidt J, Büchler MW. [Differentiated therapy for intraductal papillary mucinous neoplasms] Chirurg. 2009;80:7–13. doi: 10.1007/s00104-008-1579-6. [DOI] [PubMed] [Google Scholar]
- 3.Poultsides GA, Reddy S, Cameron JL, Hruban RH, Pawlik TM, Ahuja N, Jain A, Edil BH, Iacobuzio-Donahue CA, Schulick RD, et al. Histopathologic basis for the favorable survival after resection of intraductal papillary mucinous neoplasm-associated invasive adenocarcinoma of the pancreas. Ann Surg. 2010;251:470–476. doi: 10.1097/SLA.0b013e3181cf8a19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wasif N, Bentrem DJ, Farrell JJ, Ko CY, Hines OJ, Reber HA, Tomlinson JS. Invasive intraductal papillary mucinous neoplasm versus sporadic pancreatic adenocarcinoma: a stage-matched comparison of outcomes. Cancer. 2010;116:3369–3377. doi: 10.1002/cncr.25070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin SC, Shan YS, Lin PW. Adequate preoperative biliary drainage is determinative to decrease postoperative infectious complications after pancreaticoduodenectomy. Hepatogastroenterology. 2010;57:698–705. [PubMed] [Google Scholar]
- 6.Ikeuchi N, Itoi T, Sofuni A, Itokawa F, Tsuchiya T, Kurihara T, Ishii K, Tsuji S, Umeda J, Moriyasu F, et al. Prognosis of cancer with branch duct type IPMN of the pancreas. World J Gastroenterol. 2010;16:1890–1895. doi: 10.3748/wjg.v16.i15.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pitman MB, Lewandrowski K, Shen J, Sahani D, Brugge W, Fernandez-del Castillo C. Pancreatic cysts: preoperative diagnosis and clinical management. Cancer Cytopathol. 2010;118:1–13. doi: 10.1002/cncy.20059. [DOI] [PubMed] [Google Scholar]
- 8.Wang SE, Shyr YM, Chen TH, Su CH, Hwang TL, Jeng KS, Chen JH, Wu CW, Lui WY. Comparison of resected and non-resected intraductal papillary mucinous neoplasms of the pancreas. World J Surg. 2005;29:1650–1657. doi: 10.1007/s00268-005-0035-8. [DOI] [PubMed] [Google Scholar]
- 9.Salvia R, Crippa S, Falconi M, Bassi C, Guarise A, Scarpa A, Pederzoli P. Branch-duct intraductal papillary mucinous neoplasms of the pancreas: to operate or not to operate? Gut. 2007;56:1086–1090. doi: 10.1136/gut.2006.100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka M. Controversies in the management of pancreatic IPMN. Nat Rev Gastroenterol Hepatol. 2011;8:56–60. doi: 10.1038/nrgastro.2010.193. [DOI] [PubMed] [Google Scholar]
- 12.Sawhney MS, Al-Bashir S, Cury MS, Brown A, Chuttani R, Pleskow DK, Callery MP, Vollmer CM. International consensus guidelines for surgical resection of mucinous neoplasms cannot be applied to all cystic lesions of the pancreas. Clin Gastroenterol Hepatol. 2009;7:1373–1376. doi: 10.1016/j.cgh.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 13.Correa-Gallego C, Ferrone CR, Thayer SP, Wargo JA, Warshaw AL, Fernández-Del Castillo C. Incidental pancreatic cysts: do we really know what we are watching? Pancreatology. 2010;10:144–150. doi: 10.1159/000243733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahani DV, Kadavigere R, Blake M, Fernandez-Del Castillo C, Lauwers GY, Hahn PF. Intraductal papillary mucinous neoplasm of pancreas: multi-detector row CT with 2D curved reformations--correlation with MRCP. Radiology. 2006;238:560–569. doi: 10.1148/radiol.2382041463. [DOI] [PubMed] [Google Scholar]
- 15.Kinney TP, Punjabi G, Freeman M. Technology insight: applications of MRI for the evaluation of benign disease of the pancreas. Nat Clin Pract Gastroenterol Hepatol. 2007;4:148–159. doi: 10.1038/ncpgasthep0760. [DOI] [PubMed] [Google Scholar]
- 16.Tessler DA, Catanzaro A, Velanovich V, Havstad S, Goel S. Predictors of cancer in patients with suspected pancreatic malignancy without a tissue diagnosis. Am J Surg. 2006;191:191–197. doi: 10.1016/j.amjsurg.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 17.Hwang DW, Jang JY, Lee SE, Lim CS, Lee KU, Kim SW. Clinicopathologic analysis of surgically proven intraductal papillary mucinous neoplasms of the pancreas in SNUH: a 15-year experience at a single academic institution. Langenbecks Arch Surg. 2012;397:93–102. doi: 10.1007/s00423-010-0674-6. [DOI] [PubMed] [Google Scholar]
- 18.Hammad N, Heilbrun LK, Philip PA, Shields AF, Zalupski MM, Venkatramanamoorthy R, El-Rayes BF. CA19-9 as a predictor of tumor response and survival in patients with advanced pancreatic cancer treated with gemcitabine based chemotherapy. Asia Pac J Clin Oncol. 2010;6:98–105. doi: 10.1111/j.1743-7563.2010.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu B, Zheng WY, Jin DY, Ding WX, Lou WH, Ramsohok L. Predictive value of serum carbohydrate antigen 19-9 in malignant intraductal papillary mucinous neoplasms. World J Surg. 2011;35:1103–1109. doi: 10.1007/s00268-011-1003-0. [DOI] [PubMed] [Google Scholar]
- 20.Pausawasdi N, Scheiman JM. Pancreatic cystic lesions. Curr Opin Gastroenterol. 2010;26:506–512. doi: 10.1097/MOG.0b013e32833d115a. [DOI] [PubMed] [Google Scholar]
- 21.Grützmann R, Saeger HD. [Cystic tumors of the pancreas] Chirurg. 2010;81:755–768; quiz 769. doi: 10.1007/s00104-009-1861-2. [DOI] [PubMed] [Google Scholar]
- 22.Iglesias-Garcia J, Dominguez-Munoz E, Lozano-Leon A, Abdulkader I, Larino-Noia J, Antunez J, Forteza J. Impact of endoscopic ultrasound-guided fine needle biopsy for diagnosis of pancreatic masses. World J Gastroenterol. 2007;13:289–293. doi: 10.3748/wjg.v13.i2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Wang PJ, Yuan XD. Correlation between CT patterns and pathological classification of intraductal papillary mucinous neoplasm. Eur J Radiol. 2010;73:96–101. doi: 10.1016/j.ejrad.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 24.Ingkakul T, Sadakari Y, Ienaga J, Satoh N, Takahata S, Tanaka M. Predictors of the presence of concomitant invasive ductal carcinoma in intraductal papillary mucinous neoplasm of the pancreas. Ann Surg. 2010;251:70–75. doi: 10.1097/SLA.0b013e3181c5ddc3. [DOI] [PubMed] [Google Scholar]
- 25.Wiesenauer CA, Schmidt CM, Cummings OW, Yiannoutsos CT, Howard TJ, Wiebke EA, Goulet RJ, McHenry L, Sherman S, Lehman GA, et al. Preoperative predictors of malignancy in pancreatic intraductal papillary mucinous neoplasms. Arch Surg. 2003;138:610–617; discussion 617-618. doi: 10.1001/archsurg.138.6.610. [DOI] [PubMed] [Google Scholar]
- 26.Al-Refaie WB, Choi EA, Tseng JF, Tamm EP, Lee JH, Lee JE, Evans DB, Pisters PW. Intraductal papillary mucinous neoplasms of the pancreas. Med Princ Pract. 2006;15:245–252. doi: 10.1159/000092985. [DOI] [PubMed] [Google Scholar]
- 27.Jang JY, Kim SW, Ahn YJ, Yoon YS, Choi MG, Lee KU, Han JK, Kim WH, Lee YJ, Kim SC, et al. Multicenter analysis of clinicopathologic features of intraductal papillary mucinous tumor of the pancreas: is it possible to predict the malignancy before surgery? Ann Surg Oncol. 2005;12:124–132. doi: 10.1245/ASO.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 28.Gourgiotis S, Ridolfini MP, Germanos S. Intraductal papillary mucinous neoplasms of the pancreas. Eur J Surg Oncol. 2007;33:678–684. doi: 10.1016/j.ejso.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 29.Ferrone CR, Correa-Gallego C, Warshaw AL, Brugge WR, Forcione DG, Thayer SP, Fernández-del Castillo C. Current trends in pancreatic cystic neoplasms. Arch Surg. 2009;144:448–454. doi: 10.1001/archsurg.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang RS, Weinberg B, Dawson DW, Reber H, Hines OJ, Tomlinson JS, Chaudhari V, Raman S, Farrell JJ. Evaluation of the guidelines for management of pancreatic branch-duct intraductal papillary mucinous neoplasm. Clin Gastroenterol Hepatol. 2008;6:815–819; quiz 719. doi: 10.1016/j.cgh.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Terris B, Ponsot P, Paye F, Hammel P, Sauvanet A, Molas G, Bernades P, Belghiti J, Ruszniewski P, Fléjou JF. Intraductal papillary mucinous tumors of the pancreas confined to secondary ducts show less aggressive pathologic features as compared with those involving the main pancreatic duct. Am J Surg Pathol. 2000;24:1372–1377. doi: 10.1097/00000478-200010000-00006. [DOI] [PubMed] [Google Scholar]


