Abstract
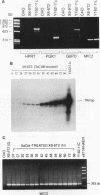
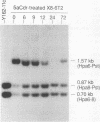
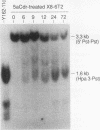
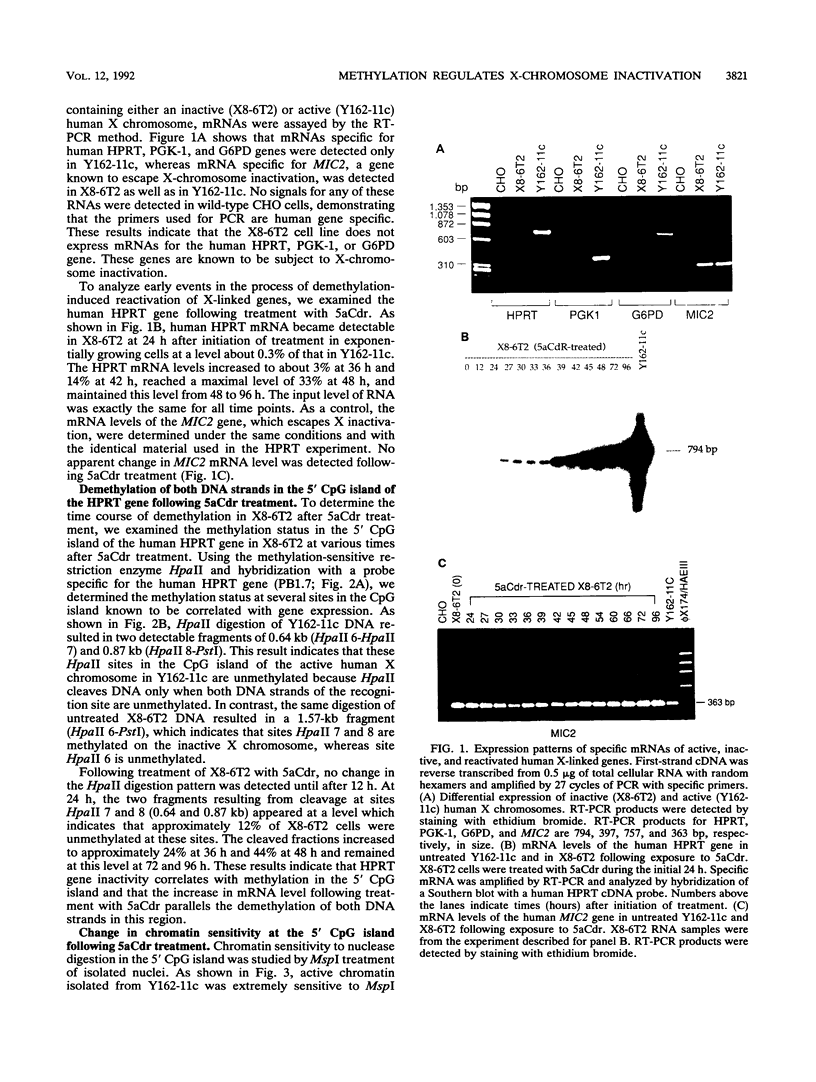
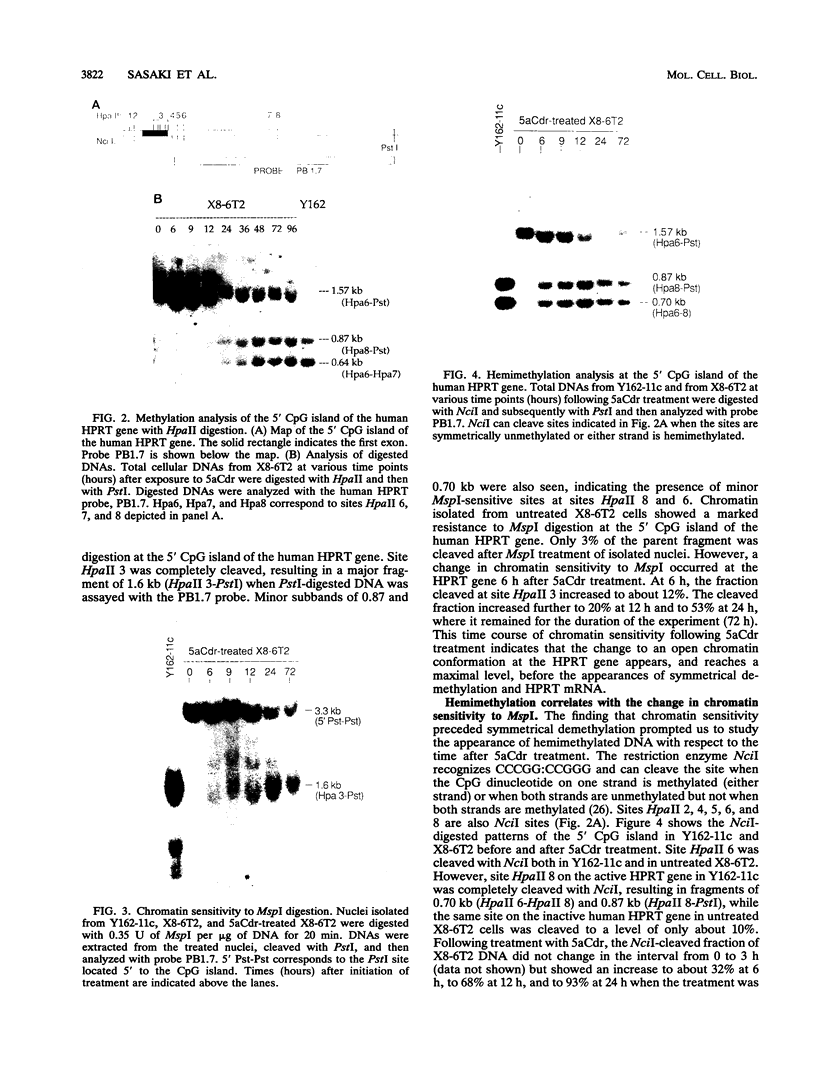
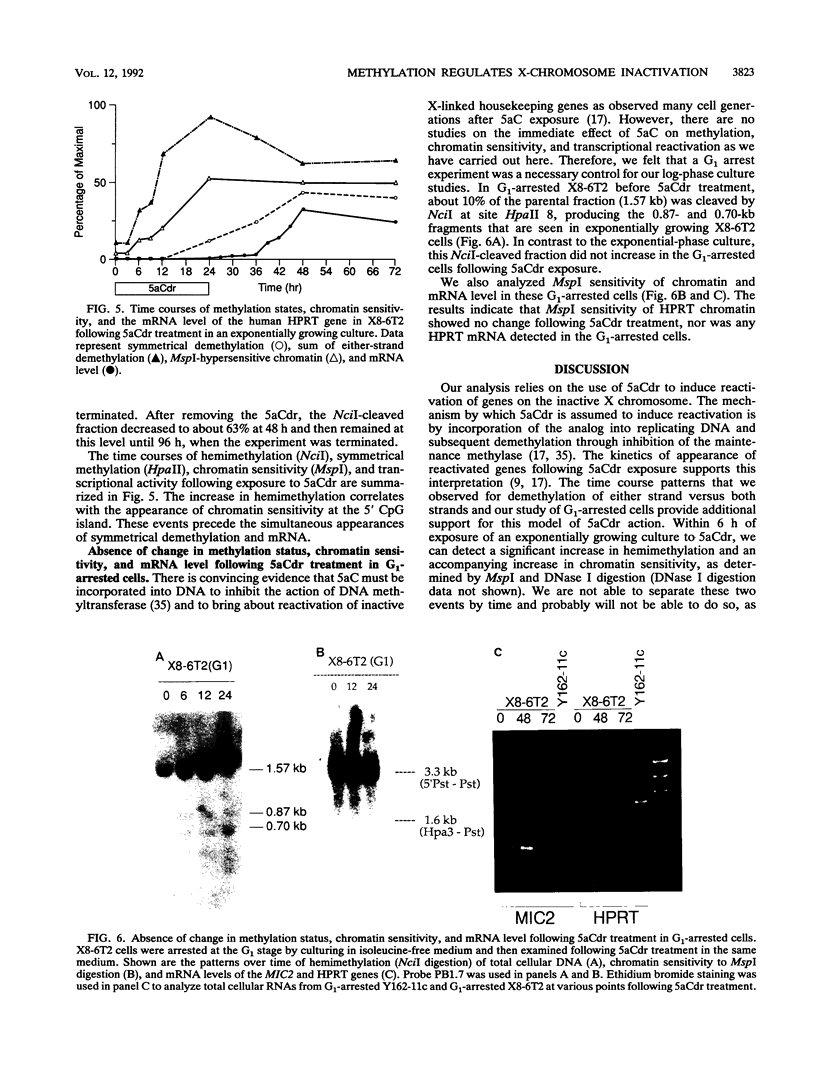
Reactivation of the hypoxanthine phosphoribosyltransferase (HPRT) gene on an inactive human X chromosome in a somatic cell hybrid was analyzed following exposure to 5-aza-2'-deoxycytidine. Hemimethylation and chromatin hypersensitivity in the 5' CpG island appeared by 6 h after exposure and continued to increase for 24 h in an exponentially growing cell culture. These results imply that the conformation of inactive chromatin requires a symmetrically methylated 5' G+C-rich promoter region. In addition, quantitative analysis of the time course patterns suggest that chromatin sensitivity changes may depend on strand-specific demethylation. Symmetrically demethylated DNA was first detected at 24 h and continued to increase until 48 h. HPRT mRNA was first detected at 24 h and increased in a biphasic pattern until 48 h. These results suggest that hemimethylation permits nuclease attack but not transcription factor binding, which requires symmetrically demethylated DNA. We also show that in G1-arrested cells, 5-aza-2'-deoxycytidine has no effect on methylation, chromatin conformation, or transcription. We conclude that reactivation of the HPRT gene present on the inactive X chromosome of a somatic cell hybrid involves the initial events of DNA hemimethylation and chromatin hypersensitivity at the 5' CpG island, followed by symmetrical demethylation and transcriptional reactivation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antequera F., Macleod D., Bird A. P. Specific protection of methylated CpGs in mammalian nuclei. Cell. 1989 Aug 11;58(3):509–517. doi: 10.1016/0092-8674(89)90431-5. [DOI] [PubMed] [Google Scholar]
- Bartlett M. H., Adra C. N., Park J., Chapman V. M., McBurney M. W. DNA methylation of two X chromosome genes in female somatic and embryonal carcinoma cells. Somat Cell Mol Genet. 1991 Jan;17(1):35–47. doi: 10.1007/BF01233203. [DOI] [PubMed] [Google Scholar]
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Boyes J., Bird A. DNA methylation inhibits transcription indirectly via a methyl-CpG binding protein. Cell. 1991 Mar 22;64(6):1123–1134. doi: 10.1016/0092-8674(91)90267-3. [DOI] [PubMed] [Google Scholar]
- Brown C. J., Flenniken A. M., Williams B. R., Willard H. F. X chromosome inactivation of the human TIMP gene. Nucleic Acids Res. 1990 Jul 25;18(14):4191–4195. doi: 10.1093/nar/18.14.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Deobagkar D. D., Liebler M., Graessmann M., Graessmann A. Hemimethylation of DNA prevents chromatin expression. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1691–1695. doi: 10.1073/pnas.87.5.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dracopoli N. C., Rettig W. J., Albino A. P., Esposito D., Archidiacono N., Rocchi M., Siniscalco M., Old L. J. Genes controlling gp25/30 cell-surface molecules map to chromosomes X and Y and escape X-inactivation. Am J Hum Genet. 1985 Jan;37(1):199–207. [PMC free article] [PubMed] [Google Scholar]
- Ellis N., Keitges E., Gartler S. M., Rocchi M. High-frequency reactivation of X-linked genes in Chinese hamster X human hybrid cells. Somat Cell Mol Genet. 1987 May;13(3):191–204. doi: 10.1007/BF01535202. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Gartler S. M., Dyer K. A., Graves J. A., Rocchi M. A two step model for mammalian X-chromosome inactivation. Prog Clin Biol Res. 1985;198:223–235. [PubMed] [Google Scholar]
- Graves J. A., Gartler S. M. Mammalian X chromosome inactivation: testing the hypothesis of transcriptional control. Somat Cell Mol Genet. 1986 May;12(3):275–280. doi: 10.1007/BF01570786. [DOI] [PubMed] [Google Scholar]
- Hansen R. S., Ellis N. A., Gartler S. M. Demethylation of specific sites in the 5' region of the inactive X-linked human phosphoglycerate kinase gene correlates with the appearance of nuclease sensitivity and gene expression. Mol Cell Biol. 1988 Nov;8(11):4692–4699. doi: 10.1128/mcb.8.11.4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen R. S., Gartler S. M. 5-Azacytidine-induced reactivation of the human X chromosome-linked PGK1 gene is associated with a large region of cytosine demethylation in the 5' CpG island. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4174–4178. doi: 10.1073/pnas.87.11.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly D. J., Esty A. C., Bernard H. U., Friedmann T. Isolation of a genomic clone partially encoding human hypoxanthine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5038–5041. doi: 10.1073/pnas.79.16.5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly D. J., Okayama H., Berg P., Esty A. C., Filpula D., Bohlen P., Johnson G. G., Shively J. E., Hunkapillar T., Friedmann T. Isolation and characterization of a full-length expressible cDNA for human hypoxanthine phosphoribosyl transferase. Proc Natl Acad Sci U S A. 1983 Jan;80(2):477–481. doi: 10.1073/pnas.80.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M., Mohandas T., Shapiro L. J. Cell cycle-specific reactivation of an inactive X-chromosome locus by 5-azadeoxycytidine. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1215–1219. doi: 10.1073/pnas.79.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith D. H., Singer-Sam J., Riggs A. D. Active X chromosome DNA is unmethylated at eight CCGG sites clustered in a guanine-plus-cytosine-rich island at the 5' end of the gene for phosphoglycerate kinase. Mol Cell Biol. 1986 Nov;6(11):4122–4125. doi: 10.1128/mcb.6.11.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet I., Lieman-Hurwitz J., Cedar H. DNA methylation affects the formation of active chromatin. Cell. 1986 Feb 28;44(4):535–543. doi: 10.1016/0092-8674(86)90263-1. [DOI] [PubMed] [Google Scholar]
- LYON M. F. Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature. 1961 Apr 22;190:372–373. doi: 10.1038/190372a0. [DOI] [PubMed] [Google Scholar]
- Lester S. C., Korn N. J., DeMars R. Derepression of genes on the human inactive X chromosome: evidence for differences in locus-specific rates of derepression and rates of transfer of active and inactive genes after DNA-mediated transformation. Somatic Cell Genet. 1982 Mar;8(2):265–284. doi: 10.1007/BF01538681. [DOI] [PubMed] [Google Scholar]
- Lin D., Chinault A. C. Comparative study of DNase I sensitivity at the X-linked human HPRT locus. Somat Cell Mol Genet. 1988 May;14(3):261–272. doi: 10.1007/BF01534587. [DOI] [PubMed] [Google Scholar]
- Lock L. F., Takagi N., Martin G. R. Methylation of the Hprt gene on the inactive X occurs after chromosome inactivation. Cell. 1987 Jan 16;48(1):39–46. doi: 10.1016/0092-8674(87)90353-9. [DOI] [PubMed] [Google Scholar]
- Meehan R. R., Lewis J. D., McKay S., Kleiner E. L., Bird A. P. Identification of a mammalian protein that binds specifically to DNA containing methylated CpGs. Cell. 1989 Aug 11;58(3):499–507. doi: 10.1016/0092-8674(89)90430-3. [DOI] [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Shapiro L. J. Reactivation of an inactive human X chromosome: evidence for X inactivation by DNA methylation. Science. 1981 Jan 23;211(4480):393–396. doi: 10.1126/science.6164095. [DOI] [PubMed] [Google Scholar]
- Paroush Z., Keshet I., Yisraeli J., Cedar H. Dynamics of demethylation and activation of the alpha-actin gene in myoblasts. Cell. 1990 Dec 21;63(6):1229–1237. doi: 10.1016/0092-8674(90)90418-e. [DOI] [PubMed] [Google Scholar]
- Pfeifer G. P., Riggs A. D. Chromatin differences between active and inactive X chromosomes revealed by genomic footprinting of permeabilized cells using DNase I and ligation-mediated PCR. Genes Dev. 1991 Jun;5(6):1102–1113. doi: 10.1101/gad.5.6.1102. [DOI] [PubMed] [Google Scholar]
- Pfeifer G. P., Steigerwald S. D., Hansen R. S., Gartler S. M., Riggs A. D. Polymerase chain reaction-aided genomic sequencing of an X chromosome-linked CpG island: methylation patterns suggest clonal inheritance, CpG site autonomy, and an explanation of activity state stability. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8252–8256. doi: 10.1073/pnas.87.21.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Riley D. E., Canfield T. K., Gartler S. M. Chromatin structure of active and inactive human X chromosomes. Nucleic Acids Res. 1984 Feb 24;12(4):1829–1845. doi: 10.1093/nar/12.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstraus M., Chasin L. A. Isolation of mammalian cell mutants deficient in glucose-6-phosphate dehydrogenase activity: linkage to hypoxanthine phosphoribosyl transferase. Proc Natl Acad Sci U S A. 1975 Feb;72(2):493–497. doi: 10.1073/pnas.72.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saluz H. P., Feavers I. M., Jiricny J., Jost J. P. Genomic sequencing and in vivo footprinting of an expression-specific DNase I-hypersensitive site of avian vitellogenin II promoter reveal a demethylation of a mCpG and a change in specific interactions of proteins with DNA. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6697–6700. doi: 10.1073/pnas.85.18.6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saluz H. P., Jiricny J., Jost J. P. Genomic sequencing reveals a positive correlation between the kinetics of strand-specific DNA demethylation of the overlapping estradiol/glucocorticoid-receptor binding sites and the rate of avian vitellogenin mRNA synthesis. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7167–7171. doi: 10.1073/pnas.83.19.7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamame M., Antequera F., Villanueva J. R., Santos T. High-frequency conversion to a "fluffy" developmental phenotype in Aspergillus spp. by 5-azacytidine treatment: evidence for involvement of a single nuclear gene. Mol Cell Biol. 1983 Dec;3(12):2287–2297. doi: 10.1128/mcb.3.12.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. M., Jones P. A. Mechanism of action of eukaryotic DNA methyltransferase. Use of 5-azacytosine-containing DNA. J Mol Biol. 1982 Dec 15;162(3):679–692. doi: 10.1016/0022-2836(82)90395-3. [DOI] [PubMed] [Google Scholar]
- Tobey R. A. Production and characterization of mammalian cells reversibly arrested in G1 by growth in isoleucine-deficient medium. Methods Cell Biol. 1973;6:67–112. doi: 10.1016/s0091-679x(08)60048-5. [DOI] [PubMed] [Google Scholar]
- Toniolo D., Martini G., Migeon B. R., Dono R. Expression of the G6PD locus on the human X chromosome is associated with demethylation of three CpG islands within 100 kb of DNA. EMBO J. 1988 Feb;7(2):401–406. doi: 10.1002/j.1460-2075.1988.tb02827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venolia L., Gartler S. M., Wassman E. R., Yen P., Mohandas T., Shapiro L. J. Transformation with DNA from 5-azacytidine-reactivated X chromosomes. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2352–2354. doi: 10.1073/pnas.79.7.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H. Assembly of an active chromatin structure during replication. Nucleic Acids Res. 1979 Oct 10;7(3):781–792. doi: 10.1093/nar/7.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Wolf S. F., Jolly D. J., Lunnen K. D., Friedmann T., Migeon B. R. Methylation of the hypoxanthine phosphoribosyltransferase locus on the human X chromosome: implications for X-chromosome inactivation. Proc Natl Acad Sci U S A. 1984 May;81(9):2806–2810. doi: 10.1073/pnas.81.9.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S. F., Migeon B. R. Clusters of CpG dinucleotides implicated by nuclease hypersensitivity as control elements of housekeeping genes. Nature. 1985 Apr 4;314(6010):467–469. doi: 10.1038/314467a0. [DOI] [PubMed] [Google Scholar]
- Yen P. H., Patel P., Chinault A. C., Mohandas T., Shapiro L. J. Differential methylation of hypoxanthine phosphoribosyltransferase genes on active and inactive human X chromosomes. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1759–1763. doi: 10.1073/pnas.81.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]