Abstract
Choroidal or cutaneous metastasis of gastric cancer is rare. Gastrointestinal cancer was found in only 4% in patients with uveal metastasis. Choroidal metastasis from gastric cancer was reported in two cases in earlier literature. The frequency of gastric cancer as a primary lesion was 6% in cutaneous metastasis of men, and cutaneous metastasis occurs in 0.8% of all gastric cancers. We report a patient with gastric adenocarcinoma who presented with visual disorder in his left eye and skin pain on his head as his initial symptoms. These symptoms were diagnosed to be caused by choroidal and cutaneous metastasis of gastric adenocarcinoma. Two cycles of chemotherapy consisted of oral S-1 and intravenous cisplatin (SPIRITS regimen); this was markedly effective to reduce the primary gastric lesion and almost all the metastatic lesions.
Keywords: Stomach neoplasms, Neoplasm metastasis, Choroid neoplasms, Skin neoplasms
INTRODUCTION
Gastric adenocarcinoma is one of the most common causes of death from cancer worldwide and more than half of the world’s gastric adenocarcinoma cases arise in Asia[1]. Gastric adenocarcinoma usually develops slowly but eventually grows to show multiple sites of metastasis. Choroidal metastasis of gastric cancer is, however, extremely rare. The uvea consists of the iris, ciliary body, and choroid. Uveal metastasis was reported in 4% of the patients who had a primary cancer in the gastrointestinal tract. Metastasis was found in the iris in 90 (9%), ciliary body in 22 (2%), and choroid in 838 (88%) of the 950 metastatic foci in the uvea[2]. Cutaneous metastasis of gastric cancer is also relatively rare[3,4]. We report a patient with gastric adenocarcinoma who presented with a visual disorder in his left eye and skin pain on his head as his initial symptoms.
CASE REPORT
A 75-year-old man visited our hospital in February 2011 because of a visual disorder in his left eye and skin pain on his head. He had two cutaneous nodules on his head. One of the cutaneous nodules grew rapidly one month after the first visit (Figure 1A and B). To evaluate the cause of the visual disorder, fundoscopic examination (Figure 2A), optical coherence tomographic examination (Figure 2B), and magnetic resonance imaging (MRI) (Figure 2C and D) were performed and an elevated choroidal neoplasm was detected. Uveal melanoma is known as the most common primary intraocular malignant tumor[5]. The diagnostic efficacy of N-isopropyl-p-(123I) iodoamphetamine single photon emission computed tomography (123I-IMP SPECT) for uveal melanoma has been established[6]. We performed 123I-IMP SPECT to evaluate the elevated choroidal neoplasm. It, however, showed no accumulation in bilateral eyes that is not specific for melanoma (Figure 3). We diagnosed his choroidal tumor as metastatic tumor, not melanoma.
Figure 1.
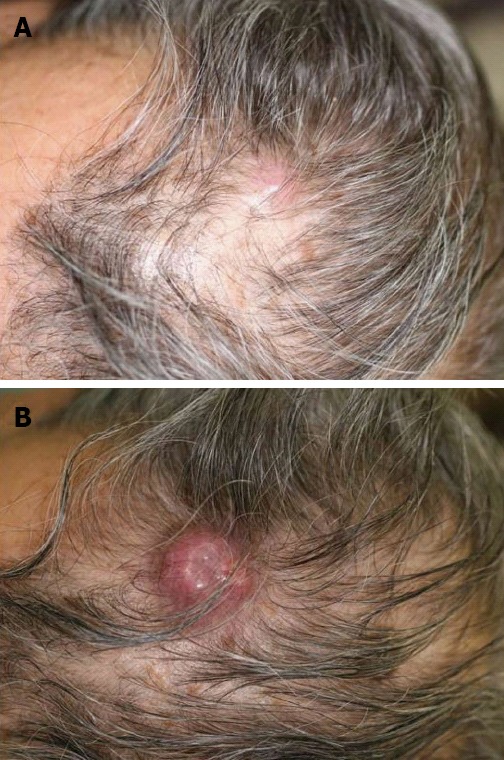
Cutaneous metastasis lesion in the head. A: One of the two cutaneous nodules on his head at first visit; B: The size of the cutaneous tumor was rapidly enlarged at 1 mo after the first visit.
Figure 2.
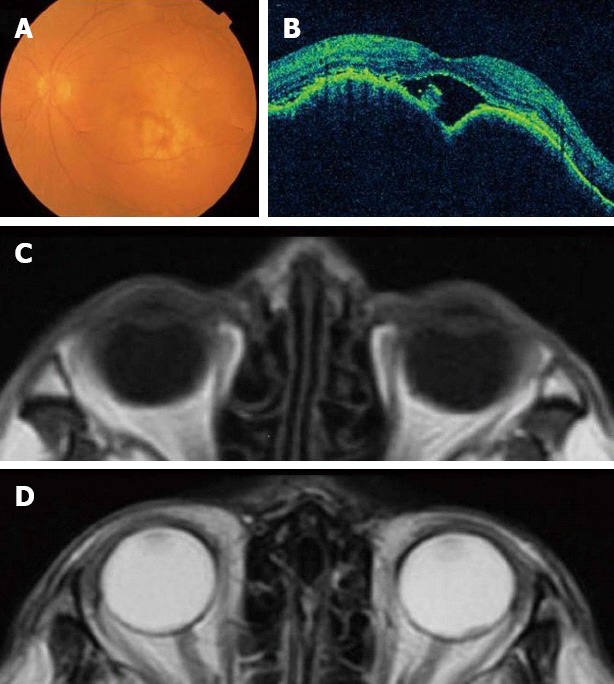
An elevated choroidal neoplasm. A: Fundoscopic examination; B: Optical coherence tomographic examination; C: Magnetic resonance imaging (MRI) T1-weighted image; D: MRI T2-weighted image.
Figure 3.
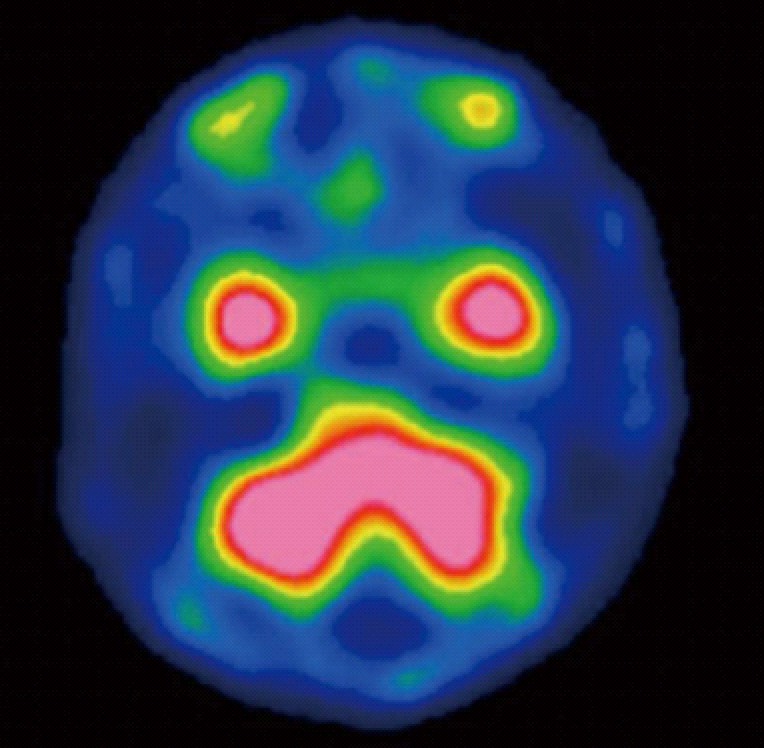
N-isopropyl-p-(123I) iodoamphetamine single photon emission computed tomography findings. 123 Iodoamphetamine single photon emission computed tomography showed no accumulation in bilateral eyes.
To detect the primary malignancy, we performed 18F-fluorodeoxyglucose-positron emission tomography/computed tomography (18-FDG-PET/CT) and it showed multiple uptake in the stomach, the bilateral adrenal glands, the abdominal lymph nodes, the right pubis, the femur, and one of the head nodules, but there was no abnormal uptake in his left eye. He underwent esophagogastroduodenoscopy because of the gastric hot spot in the 18-FDG-PET/CT. Endoscopic finding revealed an advanced gastric cancer on the lesser curvature of the stomach (Figure 4A). Gastric biopsy specimens showed moderately differentiated adenocarcinoma (Figure 4B). Pathologic examination of the biopsy specimens of the cutaneous nodule also revealed poorly differentiated adenocarcinoma. Carcinoembryonic antigen (CEA) and alpha fetoprotein (AFP) were elevated to 82 ng/mL and 21 ng/mL, respectively. Abdominal contrast-enhanced CT showed multiple liver metastases, in addition to the known lesions. Thus, we made a final diagnosis of stage IV gastric cancer with multiple metastases; cutaneous, choroid, bilateral adrenal glands, lymph nodes, and liver, right pubis, femur (T4N2M1).
Figure 4.
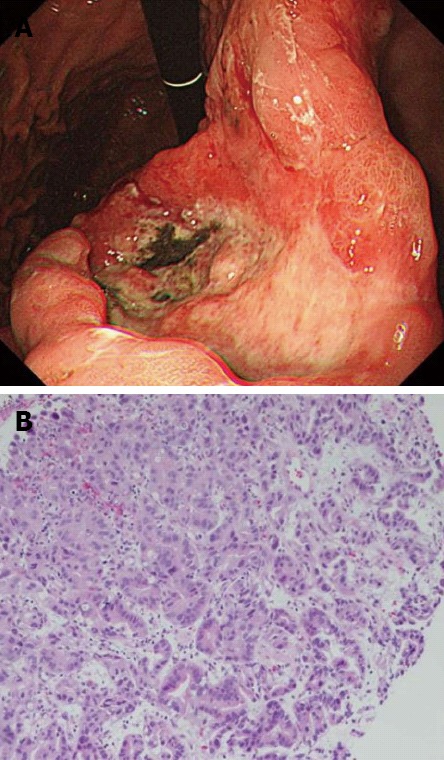
Esophagogastroduodenoscopy and pathological findings of gastric cancer. A: Esophagogastroduodenoscopy was performed and revealed an advanced gastric cancer; B: The gastric biopsy specimens revealed moderately differentiated adenocarcinoma (hematoxylin and eosin stain, ×400).
We started chemotherapy with oral S-1 (40 mg/m2, twice a day, on days 1-21) and intravenous cisplatin (60 mg/m2, on day 8), every 5 wk (SPIRITS regimen)[7]. Chemotherapy was effective at shrinking a primary gastric lesion (Figure 5A), an elevated choroidal lesion (Figure 5B and C), and other metastatic tumors, and improving skin pain relief on his head after 2 cycles, but was not effective in treating the larger cutaneous lesion of the head. The larger cutaneous nodule of the head increased from 25 mm to 32 mm (Figure 5D). Serum levels of CEA and AFP decreased to 8 ng/mL and 8 ng/mL, respectively.
Figure 5.
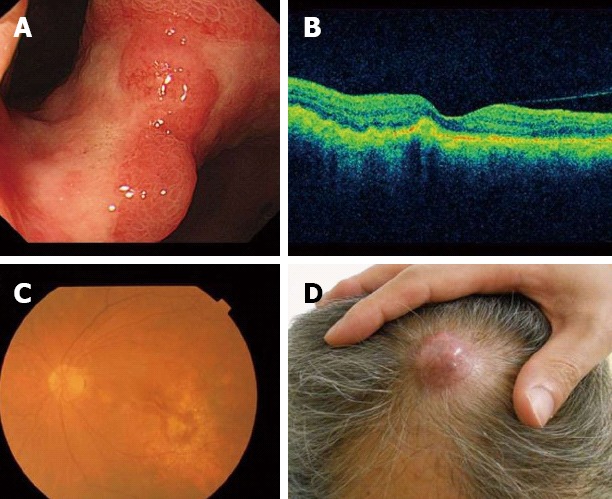
Response to chemotherapy. A: The primary gastric lesion was markedly reduced after 2 cycles of chemotherapy; B, C: The choroidal metastatic tumor was also markedly reduced; D: The larger cutaneous nodule of head enlarged from 25 mm to 32 mm, and headache vanished.
DISCUSSION
Gastric adenocarcinoma shows multiple sites of metastasis. The most common metastatic sites from gastric cancer are liver, peritoneum, and non-regional or distant lymph nodes. Choroidal metastasis is extremely rare, and cutaneous metastasis is also relatively rare. In our case, the initial symptoms related to an advanced gastric cancer showed a visual disorder in his left eye and skin pain on his head. Therefore we reported this case as a remarkably rare case because of both metastases occurring concurrently.
Choroidal metastasis has been increasing due to the improvement of ophthalmic diagnosis techniques. A previous report showed choroidal metastasis mostly comes from breast and lung cancers. In a survey, gastrointestinal cancer was found in only 4% of 420 patients with uveal metastasis[2]. Choroidal metastasis from gastric cancer was reported in two cases in earlier literature[8,9], and these cases were found to be recurrent cases of gastric cancer. To the best of our knowledge, this is the first case in which a choroidal tumor was found because of a visual disorder as the first symptom before gastric cancer was diagnosed.
In this case, we could not pathologically diagnose the choroidal tumor of this patient as a metastatic tumor. Generally, melanoma and metastatic tumors are the most common malignant choroid tumors[5]. Therefore we needed to differentiate melanoma and metastatic tumor. Normally, 123I-IMP SPECT is clinically useful for the differential diagnosis of these conditions[6]. In this case 123I-IMP SPECT showed no accumulation in bilateral eyes. In addition, MRI of the orbits demonstrated a well-circumscribed subretinal mass, where the T1-weighted image was isointense and the T2-weighted image was hypointense. MRI findings were also compatible with a metastatic tumor, not a melanoma[10]. Chemotherapy for the gastric adenocarcinoma was effective in treating the choroidal tumor. In general, malignant melanoma is resistant to chemotherapy and its treatment regimen is quite different from gastric adenocarcinoma[11,12]. In our case, the choroidal tumor was markedly reduced in size after chemotherapy for gastric adenocarcinoma (consisting of S-1 and cisplatin). Based on these reasons, it would be appropriate to diagnose this choroidal tumor as a metastatic tumor, not a melanoma.
The frequency of cutaneous metastasis is relatively low, and varies with gender according to the type of underlying malignancies. The most common origins of cutaneous metastasis were reported in lung cancer, colon cancer, melanoma, squamous cell carcinoma of the oral cavity, and renal cell carcinoma in men[3]. On the other hand, the most common origins in women were breast cancer, colon cancer, melanoma, ovarian cancer, and lung cancer[3]. The frequency of gastric cancer as a primary lesion was 6% in cutaneous metastasis of men[3], and cutaneous metastasis occurs in 0.8% of all gastric cancers[13]. In addition, the cutaneous metastasis was reported to be close to the site of the primary tumor, therefore the abdomen was the most frequent site in gastric cancer[14]. Our patient, however, had cutaneous metastasis only on his head and we could make a pathological diagnosis based on pathological findings.
In summary, choroidal or cutaneous metastasis of gastric cancer is rare. We report a patient with gastric adenocarcinoma who presented with both choroidal and cutaneous metastasis of the gastric cancer as initial symptoms.
Footnotes
P- Reviewer Fujisaki J S- Editor Song XX L- Editor O’Neill M E- Editor Li JY
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Shields CL, Shields JA, Gross NE, Schwartz GP, Lally SE. Survey of 520 eyes with uveal metastases. Ophthalmology. 1997;104:1265–1276. doi: 10.1016/s0161-6420(97)30148-1. [DOI] [PubMed] [Google Scholar]
- 3.Brownstein MH, Helwig EB. Metastatic tumors of the skin. Cancer. 1972;29:1298–1307. doi: 10.1002/1097-0142(197205)29:5<1298::aid-cncr2820290526>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33:161–182; quiz 183-186. doi: 10.1016/0190-9622(95)90231-7. [DOI] [PubMed] [Google Scholar]
- 5.Tong KA, Osborn AG, Mamalis N, Harrie RP, Call NB. Ocular melanoma. AJNR Am J Neuroradiol. 1993;14:1359–1366. [PMC free article] [PubMed] [Google Scholar]
- 6.Goto H. Clinical efficacy of 123I-IMP SPECT for the diagnosis of malignant uveal melanoma. Int J Clin Oncol. 2004;9:74–78. doi: 10.1007/s10147-003-0380-2. [DOI] [PubMed] [Google Scholar]
- 7.Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 8.Melo DH, Pierre Filho Pde T, Gomes PR, Holanda AG, Amadei LP. Choroidal metastasis of gastric adenocarcinoma as a first sign of systemic disease recurrence: case report. Arq Bras Oftalmol. 2010;73:467–468. doi: 10.1590/s0004-27492010000500017. [DOI] [PubMed] [Google Scholar]
- 9.Sahin A, Kiratli H. Choroidal metastasis as a first sign of recurrence in a patient with gastric adenocarcinoma. Can J Ophthalmol. 2007;42:331–332. [PubMed] [Google Scholar]
- 10.Peyster RG, Augsburger JJ, Shields JA, Hershey BL, Eagle R, Haskin ME. Intraocular tumors: evaluation with MR imaging. Radiology. 1988;168:773–779. doi: 10.1148/radiology.168.3.3406407. [DOI] [PubMed] [Google Scholar]
- 11.Sasse AD, Sasse EC, Clark LG, Ulloa L, Clark OA. Chemoimmunotherapy versus chemotherapy for metastatic malignant melanoma. Cochrane Database Syst Rev. 2007;(1):CD005413. doi: 10.1002/14651858.CD005413.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Olver IN, Bishop JF, Green M, Zimet A, Laidlaw C. A phase II study of cisplatinum and continuous infusion 5-fluorouracil for metastatic melanoma. Am J Clin Oncol. 1992;15:503–505. doi: 10.1097/00000421-199212000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Hu SC, Chen GS, Wu CS, Chai CY, Chen WT, Lan CC. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60:379–387. doi: 10.1016/j.jaad.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Brownstein MH, Helwig EB. Patterns of cutaneous metastasis. Arch Dermatol. 1972;105:862–868. [PubMed] [Google Scholar]


