Abstract
Baicalin is a flavonoid from the root of huangqin (Scutellaria baicalensis gcorsi, a kind of Traditional Chinese Medicine and food condiment) with two pro phenolic hydroxyls. In this manuscript, high purity of baicalin (95.5 %) was isolated from the root of huangqin and its antioxidant activities were investigated. The antioxidant properties of baicalin were evaluated by scavenging of the diphenylpicrylhydrazyl radical (DPPH), reducing power, and iron-chelating assays, compared to ascorbic acid and BHT. The results of antioxidant activity showed that the activities of baicalin were significantly better than these of ascorbic acid and BHT, and the linear correlations were good in these three assays.
Keywords: Baicalin, Huangqin, Flavonoid, Purification, Antioxidant activity
Introduction
More and more researches have been found that free radicals including singlet oxygen (1O2), superoxide ion (O-2), hydroxyl ion (·OH), peroxyl radical (ROO·), quinones (Q·) and polycyclic aromatic hydrocarbons (PAH·) were highly reactive and toxic molecules generated in cells under normal metabolic activities. They are very dangerous to human health, for example, reactive oxygen species (ROS, including 1O2, O-2, ·OH) can cause oxidative damage to proteins, enzymes, DNA, and lipids. They have also been linked to pathogenesis of oxidative diseases (Halliwell and Gutteridge 1989). Moreover, the factors which produce these harmful ingredients such as tobacco smoke, pollutants, alcohol, synthetic pesticides and solvent are increasing.
People realize that antioxidants can scavenge free radicals, which is beneficial to the body. However, some synthetic antioxidants, such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT) and propyl gallate three of the most common antioxidants as preservative added in juice, biscuit, perfume, and so on, have shown DNA damage induction (Sasaki et al. 2002). Therefore, researchers turned their attention to the natural resources, such as herbs, seeds, leaves, cereals, spices and fruits to find natural antioxidants to replace synthetic additives. Flavonoids play a significant role in natural resources and may be beneficial to human beings as antioxidants and they have been caused the attention of researchers (Loganayaki et al. 2011; Sharma et al. 2011; Kosanić et al. 2011). Baicalin is a flavonoid from the root of huangqin. Huangqin is a kind of Traditional Chinese Medicine with anti-inflammatory, antiallergic, antibacterial, antihypertensive effect (Zheng et al. 2005) and food condiment. Based on some published papers, it may have some other uses. Such as Su (Su et al. 2000) detected the activity of baicalin with the experiments of oxygen consumption test and fatty acid analysis.
It’s worth noting that some authors (Starzynska et al. 2008; Liu et al. 2009) reported there was a good linear relationship between the antioxidant activities and the quantification of total phenols determined, however, some authors did not find linear relationship (Hinneburg et al. 2006; Prakash et al. 2010) or not significant relationship (Tung and Chang 2010; Chen et al. 2011) between them. So, in this manuscript, the relationship between them was also discussed.
The investigation of this paper was that, (1) isolation and purification of baicalin from huangqin, (2) investigation the antioxidant activity of the isolated baicalin by DPPH radical scavenging, reducing power, and iron- chelating assays, (3) the correlation between the amount of baicalin and antioxidant capacity.
Materials and methods
Standard baicalin was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (NICPBP, Beijing, China). HPLC Conditions were: Venusil ODS column (5.0 μm, 150 mm × 4.6 mm), with methanol–water-H3PO4 (60:40:0.2, v/v) as mobile phase at the wavelength of 280 nm. NMR conditions were: Varian INOVA-501 (1 H, 500 MHz; 13C, 125 MHz), in DMSO-d6 with TMS as an internal standard. Ascorbic acid and BHT are two commonly used antioxidants as food additives. They have a polyphenol structure or similar structure. Therefore, both of them were selected as controls in these three antioxidant activities assays. All of the three antioxidant assays were performed three times and the data were analyzed by SPSS 12.0.
Samples preparation
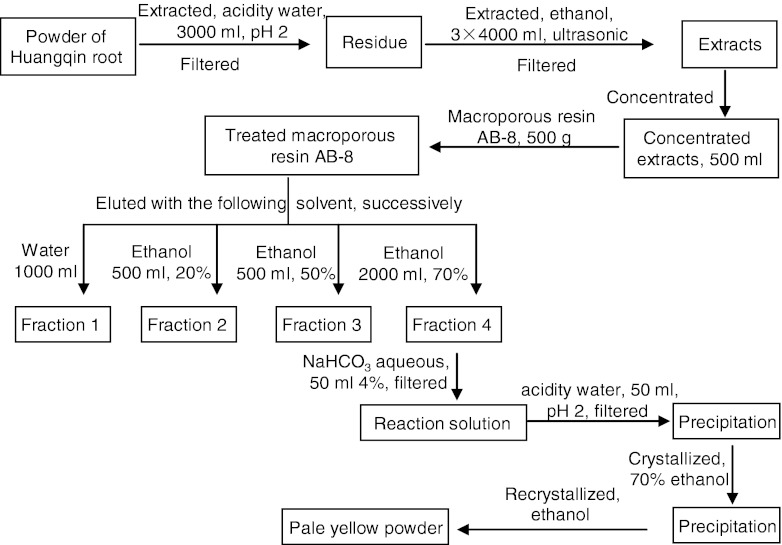
The purification process was shown in Figs. 1 and 2. The pale yellow powder was detected by HPLC and NMR.
Fig. 1.
Purification process of baicalin
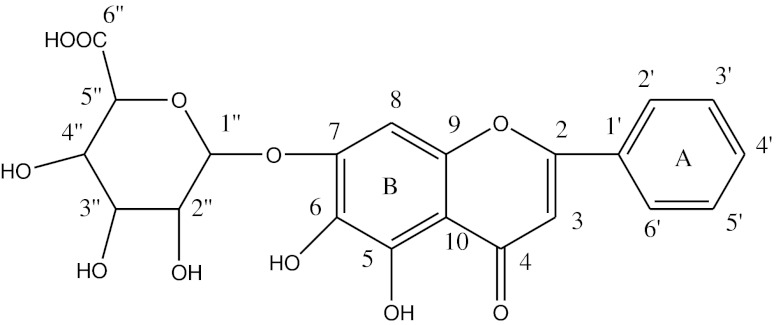
Fig. 2.
Structure of baicalin
DPPH radical- scavenging
The activity of baicalin to scavenge DPPH free radical was determined as previously described (Chen et al. 2011). The process was that, a given volume of baicalin, BHT, or ascorbic acid was put in a test tube, and then added 2.0 ml of DPPH-ethanol solution (0.1260 mg/ml), after that, the content in the test tube was further diluted to 5.0 ml with ethanol. The blank test contained all of the reaction reagents except baicalin or control substance (BHT or ascorbic acid). The reactants were mixed well and kept for 20 min in darkness at room temperature. After that the resultant UV absorbance was recorded at 517 nm. The percentage inhibition values were calculated of the absorbance of the blank test (Ab) and the sample (As) using Eq. 1.
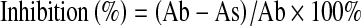 |
1 |
Reducing power activity
The activity of baicalin to reduce iron (III) was assessed with the method of previous paper (Reddy and Urooj 2010; Yogesh and Ali 2012). Briefly, a given volume of baicalin, ascorbic acid, or BHT was mixed with 2.5 ml of phosphate buffer (0.2 M, pH 6.6) and 2.5 ml of 1 % aqueous potassium hexacyanoferrate (K3Fe(CN)6) solution in a 10.0 ml test tube with plug, added ethanol to 8.0 ml, and then mixed well. After 30 min incubation at 50 °C, 2.5 ml of 10 % trichloroacetic acid (Cl3CCOOH) were added to stop the reaction. The mixture was centrifuged (2,500 rmp) for 10 min. Then took 2.5 ml of the upper layer, added 2.5 ml water and 0.5 ml of 0.1 % aqueous FeCl3, well mixed. After 10 min incubation, the resultant UV absorbance was measured at 700 nm.
Iron- chelating activity
Chelating of iron (II) ions assay was carried out as described by previous work (Babu and Rao 2011; Oboh and Ademosun 2011). Briefly, a given volume of the baicalin, ascorbic acid, or BHT was added in a 5.0 ml test tube, and then added 50 μl of 2.0 mM aqueous FeSO4, then added ethanol to 4.0 ml, well mixed. After 5 min incubation, the reaction was initiated by 1.0 ml of 5.0 mM ferrozine solution. After 10 min of equilibrium, the resultant UV absorbance was recorded at 562 nm. The blank test contained all of the reaction reagents except baicalin or control substance (ascorbic acid or BHT). The iron-chelating activities were calculated from the absorbance of the blank test (Ab) and sample (As) with Eq. 1.
Results and discussion
Sample preparations
The pale yellow powder was baicalin detected by HPLC and NMR (Table 1). Baicalin is one of the main flavonoid in the root of huangqin, and the concentration is 1.5 %. The purity of baicalin was 95.5 % with the yield rate of 5.8 %. Previous published paper reported that, the activity of flavonoid depends on the number of hydroxyl groups in the ring A, B, C and the existence of a C2-C3 double bond. In other words, the greater the number of hydroxyl groups in the two rings especially in ring B, the greater the radical scavenging potency of flavonoids (Carter 1971). Based on the structure of baicalin some results could be inferred that, baicalin is an active flavonoid with two pro phenolic hydroxyls.
Table 1.
NMR data of baicalin
| 13C-NMR(DMSO-d6, 125 MHz) | 1 H-NMR(DMSO-d6, 500 MHz) | ||||
|---|---|---|---|---|---|
| C | δ | C | δ | H | δ |
| C-2 | 163.5 | C-2′, 6′ | 126.4 | 5-OH | 12.545, s |
| C-3 | 104.2 | C-3′, 5′ | 129.2 | 6-OH | 8.664, s |
| C-4 | 182.5 | C-4′ | 130.6 | H-2′, 6′ | 8.070, d |
| C-5 | 149.2 | C-1′′ | 99.9 | H-3′, 4′, 5′ | 7.571-7.534, m |
| C-6 | 132.0 | C-2′′ | 72.8 | H-8 | 7.004, s |
| C-7 | 151.2 | C-3′′ | 75.2 | H-3 | 7.000, s |
| C-8 | 93.7 | C-4′′ | 71.3 | 2′′, 3′′, 4′′-OH | 5.289-5.499, m |
| C-9 | 146.8 | C-5′′ | 75.5 | H-1′′ | 5.240, d |
| C-10 | 106.1 | C-6′′ | 170.0 | H-5′′ | 4.060, d |
| C-1′ | 130.8 | H-2′′, 3′′, 4′′ | 3.330-3.450, m | ||
DPPH radical- scavenging
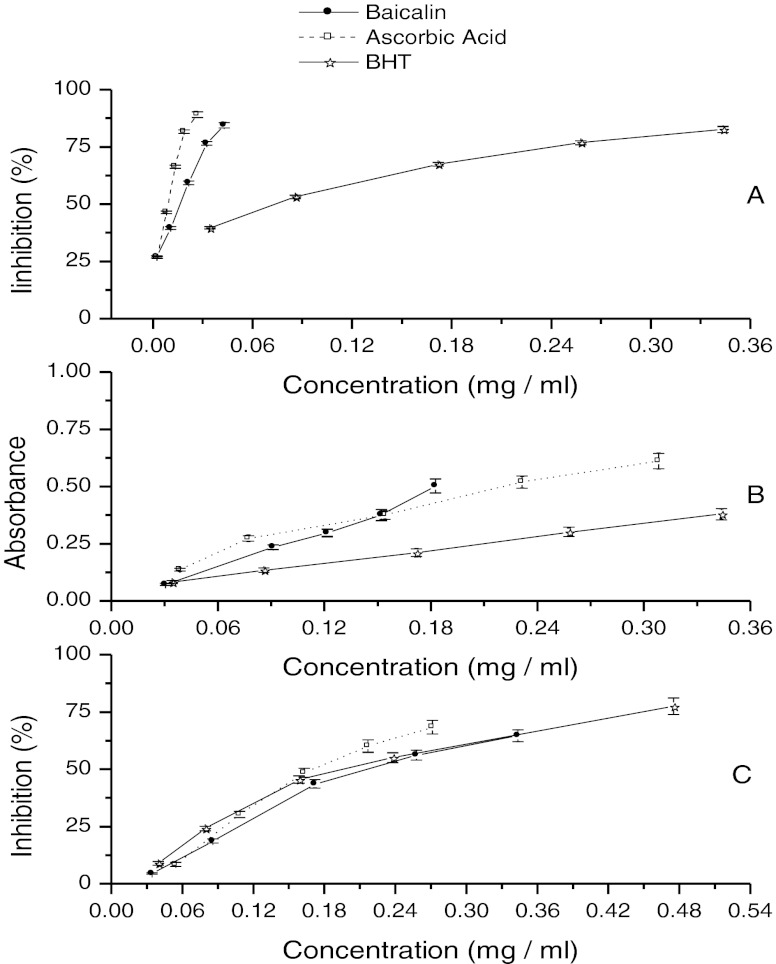
DPPH is a stable free radical and accepts an electron or hydrogen radical to form a stable diamagnetic molecule. It is widely used to detect the antioxidant of active components. The reduction capability on DPPH radicals was determined by the decrease of its absorbance at 517 nm, because it was visually noticeable a discoloration from violet to yellow. Violet color could be absorbed maximum but with tiny absorbance of yellow color at 517 nm by UV spectrophotometer. In this assay, ascorbic acid and BHT were tested as comparison. The results of the DPPH method are shown in Fig. 3. In this assay, all of the samples could discolor DPPH, which implied that all of them had antioxidant activities. Free radical scavenging potentials of baicalin and controls were found in the order of ascorbic acid > baicalin > BHT, approximately. The median inhibitory concentration (IC50) values of ascorbic acid, baicalin, and BHT were 8.1 μg/ml, 16.4 μg/ml, and 75.3 μg/ml, respectively. The results could prove that a flavonoid with more phenolic hydroxyl (there is only one phenolic hydroxyl in BHT) had stronger antioxidant activity. The linear correlation coefficient (R2) between the concentrations and inhibitions of DPPH was 98.2 %, which meant there was a good linear of them.
Fig. 3.
DPPH radical scavenging activity a, Reducing power activity b and Iron-chelating activity c of baicalin, ascorbic acid, and BHT (n = 3)
Reducing power activity
Fe (III) reduction is a method to detect antioxidant activity which is widely used as an indicator of electron-donating activity process, and is an important mechanism of phenolic antioxidant action adopted by some researchers (Reddy and Urooj 2010; Yogesh and Ali 2012). In this assay, K3Fe(CN)6 reduced to objects and Fe3+ (excessive) added can react to generate a blue color complex. The blue complex can be detected by UV spectrophotometer at 700 nm with a max absorbance. So the higher absorbance of the reaction mixture indicates the greater reducing power. As shown in Fig. 3, the absorbances of the three sample are in the order of baicalin > ascorbic acid > BHT. Which meant the reducing power of baicalin was better than these of ascorbic acid and BHT. BHT performed worst in this assay. It was slightly different from the results of DPPH assays. The consequences implied that baicalin, ascorbic acid and BHT had noticeable potency to donate electrons to reactive free radicals, to convert them into more stable non-reactive species and to terminate the free radical chain reaction. The linear correlation coefficient (R2) in this assay was 97.5 %.
Iron- chelating activity
H2O2 can be formed in human’s body and is harmful. Fe2+ is indispensable to the human body and exists in the human body widely. But Fenton Reaction can be occurred between H2O2 and Free State Fe2+. In Fenton Reaction, H2O2 can be reduced to HO· which is more harmful to human body. So, to reduce the concentration of free state Fe2+ can hinder the formation of HO· to protect human body. In this assay, ferrozine was usually used to detect the Fe2+ which was not involved in the previous redox reaction. That’s because, ferrozine and Fe2+ can generate a red color complex which can be detected with UV spectrophotometer at 562 nm. So the lower absorbance of the reaction mixture indicates the greater chelating capacity of the antioxidant samples. The chelating capacities were calculated from the absorbance of the blank control (Ab) and sample (As) using Eq. 1. As shown in Fig. 3, the efficiencies of Fe2+-ferrozine complex increase with the increasing concentration of the three antioxidants. In this assay, baicalin performed best and the other two samples did nearly the same but worse than that of baicalin. The median inhibitory concentration (IC50) values of the baicalin, ascorbic acid and BHT were 17.0 μg/ml, 19.6 μg/ml and 21.7 μg/ml, respectively. When the inhibition was under 40 %, ascorbic acid was the best, but, when the inhibition was above 40 %, baicalin was the best. The linear correlation coefficient (R2) of baicalin was 96.5 %.
Correlation between the amount of antioxidants and antioxidant activity
The correlation between the antioxidant activities and the quantity of the flavonoid is still under discussion, good linear relationships were observed in some published papers (Starzynska et al. 2008; Liu et al. 2009). However, a research found linear relationships between them were not very significant (Prakash et al. 2010); a research found linear relationships between them in reducing power assay was good but in other assays were not very significant (Tung and Chang 2010); a research found linear relationships between them in DPPH assay was good but in other assays were not very significant (Chen et al. 2011); a research found there were no linear relationships between them (Hinneburg et al. 2006). The controversy might be contributed to the complexity of plant materials used. There were massive components in the extract and these components might produce various results. Some of them might not perform antioxidant activities, some others might exhibit opposite activities, and moreover, some might have a strong activity on DPPH radical scavenging but a weak activity on iron-chelating capacity. The target products in most researches were determined by Folin-Ciocalteu method, which was used to determine the total phenols in the extract, but not of certain components. That might result in inevitable deviation when the extract was considered as single component. In order to eliminate the interferences of the impure materials, a certain component, baicalin, was used in this manuscript. Baicalin’s antioxidant activities increased with the increasing concentration and good linear correlation coefficients were observed.
Conclusions
High purity baicalin (95.5 %, detected by HPLC) was obtained with the yield rate of 5.8 %, in the sample preparation experiment. The process to obtain baicalin from huangqin root was feasible. Three assays of experiment were carried out to detect the antioxidant of baicalin. As a flavonoid with two pro hydroxyl groups, baicalin showed strong antioxidant activities in all of the three assays experiment based on the results. Other more, good linear correlation coefficients (R2) between the amount of substance and antioxidant activity were found in these assays. All of the results showed that, baicalin might have potential as a natural antioxidant to add in some fields.
References
- Babu DR, Rao GN (2011) Antioxidant properties and electrochemical behavior of cultivated commercial Indian edible mushrooms. J Food Sci Technol. doi:10.1007/s13197-011-0338-8 [DOI] [PMC free article] [PubMed]
- Carter P. Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine) Anal Biochem. 1971;40:450–458. doi: 10.1016/0003-2697(71)90405-2. [DOI] [PubMed] [Google Scholar]
- Chen HX, Zhang Y, Lu XM, Qu ZS (2011) Comparative studies on the physicochemical and antioxidant properties of different tea extracts. J Food Sci Technol. doi:10.1007/s13197-011-0291-6 [DOI] [PMC free article] [PubMed]
- Halliwell B, Gutteridge J. Free radicals in biology and medicine. 2. Oxford: Clarendon; 1989. pp. 22–65. [Google Scholar]
- Hinneburg I, Dorman HJD, Hiltunen R. Antioxidant activities of extracts from selected culinary herbs and spices. Food Chem. 2006;97:122–129. doi: 10.1016/j.foodchem.2005.03.028. [DOI] [Google Scholar]
- Kosanić M, Ranković B, Vukojević J. Antioxidant properties of some lichen species. J Food Sci Technol. 2011;48(5):584–590. doi: 10.1007/s13197-010-0174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PF, Deng TS, Hou XL, Wang JG. Antioxidant properties of isolated isorhamnetin from the sea buckthorn marc. Plant Foods Human Nutr. 2009;64:141–145. doi: 10.1007/s11130-009-0116-1. [DOI] [PubMed] [Google Scholar]
- Loganayaki N, Siddhuraju P, Manian S (2011) Antioxidant activity and free radical scavenging capacity of phenolic extracts from Helicteres isora L. and Ceiba pentandra L. J Food Sci Technol. doi:10.1007/s13197-011-0389-x [DOI] [PMC free article] [PubMed]
- Oboh G, Ademosun AO (2011) Characterization of the antioxidant properties of phenolic extracts from some citrus peels. J Food Sci Technol. doi:10.1007/s13197-010-0222-y [DOI] [PMC free article] [PubMed]
- Prakash B, Shukla R, Singh P, Kumar A, Mishra PK, Dubey NK. Efficacy of chemically characterized Piper betle L. essential oil against fungal and aflatoxin contamination of some edible commodities and its antioxidant activity. Inter J of Food Microbio. 2010;142(1):114–119. doi: 10.1016/j.ijfoodmicro.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Reddy VP, Urooj A (2010) Antioxidant properties and stability of aegle marmelos leaves extracts. J Food Sci Technol. doi:10.1007/s13197-010-0221-z [DOI] [PMC free article] [PubMed]
- Sasaki YF, Kawaguchi S, Kamaya A, Ohshita M, Kabasawa K, Iwana K, Taniguchi K, Tsuda S. The comet assay with 8 mouse organs: results with 39 currently used food additives. Mutation Res. 2002;519:103–119. doi: 10.1016/S1383-5718(02)00128-6. [DOI] [PubMed] [Google Scholar]
- Sharma KD, Stähler K, Smith B, Melton L. Antioxidant capacity, polyphenolics and pigments of broccoli-cheese powder blends. J Food Sci Technol. 2011;48(4):510–514. doi: 10.1007/s13197-010-0211-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzynska AJ, Stodolak BZ, Jamroz M. Antioxidant properties of extracts from fermented and cooked seeds of Polish cultivars of Lathyrus sativus. Food Chem. 2008;109:285–292. doi: 10.1016/j.foodchem.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Su YL, Leunga LK, Bi YR, Huang Y, Chen ZY. Antioxidant activity of flavonoids isolated from scutellaria rehderiana. J Am oil chem soc. 2000;77(8):807–813. doi: 10.1007/s11746-000-0129-y. [DOI] [Google Scholar]
- Tung YT, Chang ST. Variation in antioxidant activity of extracts of Acacia confusa of different ages. Nat Prod Comm. 2010;5(1):73–76. [PubMed] [Google Scholar]
- Yogesh K, Ali J (2012) Antioxidant potential of thuja (Thuja occidentalis) cones and peach (Prunus persia) seeds in raw chicken ground meat during refrigerated (4 ± 1 °C) storage. J Food Sci Technol. doi:10.1007/s13197-012-0672-5 [DOI] [PMC free article] [PubMed]
- Zheng XY, et al. Chinese pharmacopoeia. Beijing: Chemical Industry; 2005. pp. 141–142. [Google Scholar]