Abstract
The aim of the current investigation was to evaluate the effects of administration various levels (400, 800 and 1,200 ppm) of pomposia extracts as natural antioxidant in comparison with BHT as synthetic antioxidant on some biochemical activities and histopathological examination of rats. Some of biochemical tests i.e. Alkaline phosphatase, transaminases]Aspartate transferase (AST) and alanine transferase (ALT) [,bilirubin, urea and uric acid were conducted. Histopathological examinations were carried out on the liver and kidney tissue of rats administrated tested substances. The biochemical results indicated that the administration of polyphenolic compounds present in pomposia juice did not cause any significant (p ≥ 0.05) changes in the biochemical parameters whereas the administration of BHT at 200 ppm caused significant (p ≤ 0.05) increase in the activities of enzymes relevant to the functions of liver and kidney. Microscopically examinations of liver and kidney of rat administered various levels of pomposia juice had the same character as that of control rats (this means that the polyphenolic compounds present in pomposia juice did not cause any adverse affect in liver and kidney), in contrast the administration of 200 ppm of BHT caused marked pathological changes in liver and kidney of rats. The results of the current investigation suggest using pomposia juice as safe food grade substance.
Keywords: Pomposia fruits (Syzygium Cumini), Sunflower oil, Liver and kidney function tests, Microscopic examination
Introduction
Deep fat frying is a traditional and long used method of cooking and is popular throughout the world for preparation and manufacture of many foods. This method is commonly used in traditional catering restaurants, fast food chains and in industrial frying operations such as potato chips, instant noodles Ahmad (1999) During frying, oil is subjected to prolonged periods of heating at high temperatures of about 180°C in the presence of air and water. This leads to a wide range of complex chemical reactions categorized as thermal oxidation, hydrolysis, and polymerization. Undesirable constituents and off-flavors are developed in frying oil due to these chemical reactions Innawong et al. (2004). In this regard, lipid oxidation is a major cause of muscle food deterioration, affecting color, flavor, texture and nutritional value (Innawong et al. 2004; Chan et al. 1993; Kanner et al. 1991; Lee et al. 1986; Rhee et al. 1996 and Yin and Cheng 1997) This oxidative deterioration of muscle involves the oxidation of the unsaturated fatty acids, catalyzed by hemoproteins as well as non-heme iron (Yin and Cheng 1997 and Sato and Hegarty 1971) To avoid or delay the lipid oxidation in food processing, antioxidants have been used for over 50 years (Cuvelier et al. 1994). The antioxidants play an important role in the manufacturing, packaging and storage of fats and fatty foods and have been proven to retard oxidation (Lin et al. 1981). The most commonly used antioxidants are butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT) and tert-butylhydroquinone (TBHQ). They are added to a wide variety of foods in the market (Chang et al. 1977). However, their use is increasingly contested or even banned in certain countries. The recent consumer interest in “natural” products drives the need for natural antioxidative substances to replace the conventional antioxidants (Cuvelier et al. 1994 and Farag et al. 2007).Antioxidant compounds like phenolic acids, polyphenols and flavonoids (such as Anthocyanins) scavenge free radicals such as peroxide, hydroperoxide or lipidperoxyl and thus inhibit the oxidative mechanisms that lead to degenerative diseases (Scartezzini and Speroni 2000 and Timberlake and Bridle 1982). Phenolic compounds are responsible for the antioxidant activity of several vegetables, thus are potential inhibitors of carcinogens, acting in different stages of pathological process of initiation of tumors (Blum 1996) In this respect, anthocyanins are known to possess excellent antioxidant properties (Kong et al. 2003). Anthocyanins from different sources have been reported to inhibit lipid peroxidation, and platelet aggregation (Ghiselli et al. 1998), and possess anti-tumor (Kamei et al. 1998), antimutagenic (Yoshinaga et al. 1999), and hepatoprotective properties (Obi et al. 1998).Jamun (Syzygium cumini) is an evergreen tropical tree in the flowering plant family Myrtaceae, native to India and Indonesia. (Chowdhury and Ray 2007). It produces fruit with high antioxidant activity, and it is an important source of phenolic compounds such as anthocyanins, ellagic acid, quercetin and rutin. (Vizzotto and Pereira 2008) Jamun also known as pompozia fruits, anthocyanins were the main and predomint pigments of pomposia fruits, total anthocyanin content of pomposia fruits was 906.82 mg/100 g of pomposia fruits as dry weight basis (Hamed et al. 2001), and was 216 mg/100 ml of (Syzygium cumini) extract which is equivalent to 230 mg/100 g fruit on a dry weight basis (Veigas et al. 2007). In this regard Liya et al. 2009 reported that Jamun fruit extract (JFE) contained 3.5% anthocyanins (as cyanidin-3-glucoside equivalents) . Pomposia fruits are oblong berries, deep purple or bluish in colour with pinkish pulp, having various medicinal properties and used in Ayurveda as a stomachic, astringent, antiscorbutic, diuretic, antidiabetic, and in chronic diarrhea and enlargement of spleen (Achrekar et al. 1991; Morton 1987 and Nadkarni 1954). The deep purple colour of the fruit is due to anthocyanins, namely delphinidin-3-gentiobioside and malvidin-3-laminaribioside, along with petunidin-3-gentiobioside(Venkateswarlu 1952).; cyanidin diglycoside and glycosides of petunidin and malvidin (Sharma and Sheshadri 1955). petunidin,delphindin,cyaniding,pelargondin and malvidin (Hamed et al. 2001) malvidin-3-glucoside and petunidin-3-glucoside in the Brazilian variety (Lago et al. 2004), delphinidin, petunidin and malvidin-diglucosides (Veigas et al. 2007; Sharma et al. 2008); delphinidin, cyanidin, petunidin, peonidin and malvidin (Liya et al. 2009. The antioxidative effects of different level (200, 400,800 and 1,200 ppm) of pomposia juice and extracts as natural antioxidant compared to BHT (200 ppm) on the stability of fried sunflower oil was investigated by Ali 2010 who found that the phenolic compounds of pomposia extract at levels 800 and 1,200 ppm induced powerful antioxidant effects which was almost equal to the synthetic antioxidant (BHT) at level 200 ppm.
The current investigation was performed to evaluate the effects of administration various levels of pomposia extracts as natural antioxidant in comparison with BHT as synthetic antioxidant on some liver and kidney functions i.e.(alkaline phosphatase, transaminases (aspartate transferase (AST) and alanine transferase (ALT) [,bilirubin, urea and uric acid and on histopathological status of liver and kidney organs of those rats to ensure the safety use of pomposia extract as food grade.
Materials and methods
Source of pomposia
Pomposia, (Syzygium cumini), Kaatha variety was obtained from the farm of Faculty of Agriculture, Cairo University, Giza, Egypt. (season July 2009). The fruits were ripened and freshly harvested.
Sunflower oil
Freshly refined sunflower oil without antioxidants was obtained from Sila edible oil Co. S.A.E., Kom Osheim, El-Fayoum Governorate Egypt.
Preparation of crude juice of pomposia (Syzygium Cumini)
Pomposia fruit Kaatha variety was cleaned, cut into pieces and then pressed by means of the hydraulic laboratory press model C S/N 37000–156 Freds from Carver (WI, USA). The resultant crude juice was centrifuged at 4,000 rpm for 30 min, the supernatant was lyophilized using a freeze- dryer (Labconco corporation Kansas city, Missouri 64132 USA) .The concentrated juice was kept in a brown bottle at −18°Cuntil further use.
Determination of total polyphenols
Total polyphenols were determined according to the method of (Jayaprakasha et al. 2003). An aliquot from pomposia juice (0.1 mL) was dissolved in a 10 mL mixture of acetone and water (6:4 v/v). Sample (0.2 mL) was mixed with 1.0 mL of ten-fold diluted Folin-Ciocalteu reagent and 0.8 mL of 75 g L-1 sodium carbonate solution. After standing for 30 min at room temperature, the absorbance was measured at 725 nm. Phenolic contents were calculated on the basis of the standard curve for gallic acid (GAL).The results were expressed as mg of gallic acid equivalent per g of dry extract.
Experimental animals
Male Albino rats, with an average weight of 95–100 g were used in the present investigation and were obtained from the Faculty of Veterinary Medicine, Cairo University, Giza, Egypt.
Reagent methodology kits
Alkaline phosphatase, transaminases [Aspartate transferase (AST) and alanine transferase (ALT) [,bilirubin, urea and uric acid kits were obtained from Biodiagnostic CO. (29, Tahrer st., Dokki, Giza, Egypt.). All chemicals used were of analytical reagent grade.
Nutritional experiments
A total of 50 Albino male rats with an average weight of 95–100 g were raised in the animal house of Food Technology Research Institute, Agricultural Research Center, Giza, Egypt. The animals were fed on a basal diet for 15 days as an adaptation period. The basal diet was formulated according to AOAC 2000 and consisted of casein (15%), sunflower oil without antioxidant (10%), cellulose (5%), salt mixture (4%), vitamin mixture (1%) and starch (65%). The rats were divided into 5 groups, the first one presents the control rats and the rats of the second, third and fourth groups were given 400, 800 and 1,200 ppm of pomposia polyphenols, respectively. The last group of rats was given 200 ppm of synthetic antioxidant (BHT). The phenolic compounds were administrated by stomach ingestion tube.
Blood sampling
Blood samples were taken at the start of the experiment and at 1, 2, 3, 4, 5 and 6 weeks from the beginning of the experiment. The blood samples were obtained from the orbital pleux by means of capillary tube (1–1.5 m) from each rat. At the end of the experiment, the animals were killed and the blood of each rat group was collected in tubes. The blood of rats in each group were centrifuged at 1,100 Xg for 20 min to obtain the sera and kept in the deep freezer (−18°C) until analysis.
Serum analysis
Alanine aminotransferase (ALT) (E.C., 2.6.1.2), aspartate aminotransferase (AST) (E.C. 2.6.1.1) and Alkaline phosphatase (AP) (E.C. 3.1.3.1.) activities were measured according to the methods described by Bergmeyer and Harder 1986 and Belfield and Goldberg 1971, respectively.Total Bilirubin was determined according to the method of Walter and Gerade 1970. Urea and uric acid levels in rat sera were determined as outlined by Fawcett and Scott 1960 and Barham and Trinder 1972, respectively.
Histopathological study
At the end of the nutritional experiments, the rats were killed using diethyl ether. The liver and kidney organs were removed, stored in 10% neutral formalin and embedded in paraffin wax. The organs were sectioned (5–6 μ) and stained with hematoxylin and eosin according to Culling 1965. Tissue sections were then examined using ordinary optical microscope (20 magnification) for histological evaluation.
Statistical analysis
Data were statistically analyzed in completely randomized design in factorial arrangement according to the procedures outlined by Gomez and Gomez 1984 and the treatments means were compared by least significant differences (L.S.D) and Duncan multiple range using SPSS program package.
Results and discussion
Polyphenols of pomposia (Kaatha variety)
Pomposia juice and extracts had been shown to be rich in polyphenols (Hamed et al. 2001; Teixeira et al. 1992; Ravi et al. 2004 and Ali 2010). Total polyphenols of crude juice of pomposia was 16.32 mg/g.
Biochemical evaluation of Pomposia (Syzygium cumini) fruit juice
ALT, AST, AP activities
The assessment of the level of AST, ALT and ALP provides a good and simple tool to measure the protective activity of the target drug against the hepatic damage of the target compounds (Hewawasam et al. 2004). Alkaline phosphatase is the marker enzyme for plasma and endoplasmic reticulum. (Wright and Plummer 1974 and Shahjahan et al. 2004). Tables 1, 2 and 3 show the effect of different levels of pomposia polyphenolic compounds (400,800 and 1,200 ppm) on the activities of ALT, AST, and AP. The administration of polyphenols present in pomposia fruits at levels 400, 800 and 1,200 ppm did not cause any significant changes in the activities of these enzymes and were similar to the control group However, the administration of 200 ppm of synthetic antioxidant (BHT) caused significant (P ≤ 0.05) increase in these enzyme activities after three weeks of the beginning of the experiment and towards the end of the experiment. These results indicate that the phenolic compounds of pomposia fruit juice up to 1,200 ppm are safe, since there is no significant effects were observed on the activities of above-mentioned enzymes, whilst, 200 ppm of BHT caused harmful effect on liver functions. Significant increase in the activity of liver enzymes was showed in rats fed on diets containing 200 mg of BHT/Kg of body weight (Farag et al. 2006 and Abd El Mageid et al. 2009). The rise in the activities of ALT, AST, and AP in rat serum is a sign of hepatocelluar damage (Baron 1987). Hepatic cells participate in a variety of metabolic activities and contain a number of enzymes. In the stage of liver injury, the transport function of hepatocytes is disturbed, which can result in the leakage of plasma membrane, thereby causing an increase in sGPT and sGOT levels. In addition, a defective excretion of bile by the liver was reflected by an increase in the level of sALP. (Rajesh and Latha 2004 and Yanardag and Tunali 2006).This case has been found in rats administered only BHT and not with Pomposia fruit juice.
Table 1.
Influence of various levels of Pomposia fruit juice (PFJ) and BHT on the activity of serum Alanine aminotransferase (ALT) (IU/L) of rat
| Blood withdrawal Period (week) | Concentration of phenolic compound | ||||
|---|---|---|---|---|---|
| Control rats | PFJ(400 ppm) | PFJ(800 ppm) | PFJ(1,200 ppm) | BHT(200 ppm) | |
| 0 | 28.7 d ± 0.55 | 28.8 d ± 0.73 | 29.5 d ± 0.71 | 29.4 d ± 0.47 | 28.9 d ± 0.29 |
| 1 | 28.7 d ± 0.53 | 28.0 d ± 0.11 | 28.4 d ± 0.66 | 28.4 d ± 0.27 | 30.3 d ± 0.60 |
| 2 | 29.1 d ± 1.26 | 29.2 d ± 0.98 | 28.3 d ± 0.64 | 28.7 d ± 1.06 | 32.4 cd ± 0.63 |
| 3 | 30.1 d ± 0.74 | 30.0 d ± 0.76 | 29.2 d ± 0.77 | 29.4 d ± 0.52 | 40.8 bc ± 3.04 |
| 4 | 28.7 d ± 0.98 | 29.2 ± 1.36 | 29.5 d ± 0.54 | 28.8 d ± 0.10 | 46.7 b ± 1.24 |
| 5 | 29.9 d ± 1.76 | 29.7 d ± 0.58 | 28.4 d ± 0.53 | 28.2 d ± 0.21 | 56.9 a ± 0.81 |
| 6 | 29.6 d ± 1.06 | 29.3d ± 1.47 | 28.8 d ± 0.75 | 27.8 d ± 0.45 | 60.8 a ± 1.54 |
| LSD at 0.05 = 8.554 | |||||
Data are expressed as mean ± SD
Values given represent means of five determinations.
Values followed by the same letter are not significantly different (p < 0.05).
Table 2.
Influence of various levels of Pomposia fruit juice (PFJ) and BHT on the activity of serum aspartate aminotransferase (AST IU/L) of rat
| Blood withdrawal Period (week) | Concentration of phenolic compounds | ||||
|---|---|---|---|---|---|
| Control rats | PFJ(400 ppm) | PFJ(800 ppm) | PFJ(1,200 ppm) | BHT(200 ppm) | |
| 0 | 45.5 efgh ± 1.04 | 44.0 egh ± 1.05 | 43.3fgh ± 1.15 | 43.1 gh ± 0.94 | 43.0 h ± 0.94 |
| 1 | 44.5 efgh ± 1.56 | 43.0 h ± 1.05 | 44.1fgh ± 2.12 | 44.3 efgh ± 0.57 | 44.7e fgh ± 0.55 |
| 2 | 45.3 efgh ± 1.21 | 44.4efgh ± 0.50 | 44.5 efgh ± 1.60 | 43.3 fgh ± 1.48 | 46.1 ef ± 1.65 |
| 3 | 43.4 fgh ± 1.59 | 43.9 fgh ± 0.25 | 45.7efgh ± 1.46 | 45.4 efgh ± 0.61 | 53.4 d ± 2.99 |
| 4 | 44.0 fgh ± 2.00 | 44.0 fgh ± 1.00 | 45.6 efgh ± 0.74 | 45.3 efgh ± 1.08 | 56.7 c ± 2.11 |
| 5 | 44.5 efgh ± 0.55 | 44.6efgh ± 0.60 | 45.9 efg ± 0.53 | 44.8 efgh ± 1.56 | 63.0 b ± 2.95 |
| 6 | 45.1efgh ± 0.84 | 44.1 fgh ± 0.33 | 45.2 efgh ± 1.14 | 43.5 fgh ± 0.37 | 70.4 a ± 1.73 |
| LSD at 0.05 = 2.290 | |||||
Data are expressed as mean ± SD
Values given represent means of five determinations.
Values followed by the same letter are not significantly different (p < 0.05).
Table 3.
Influence of various levels of Pomposia fruit juice (PFJ) and BHT on the activity of serum Alkaline phosphatase (AP) (IU/L) of rat
| Blood withdrawal Period (week) | Concentration of phenolic compounds | ||||
|---|---|---|---|---|---|
| Control rats | PFJ(400 ppm) | PFJ(800 ppm) | PFJ(1,200 ppm) | BHT(200 ppm) | |
| 0 | 64.2 e ± 0.13 | 62.7 efgh ± 1.07 | 63.6 ef ± 1.15 | 63.2ef ± 0.99 | 61.9 efgh ± 0.31 |
| 1 | 63.5 ef ± 0.51 | 62.2 efgh ± 0.65 | 62.8 efgh ± 0.62 | 62.4efgh ± 0.61 | 62.0 efgh ± 1.10 |
| 2 | 63.1 efg ± 0.02 | 62.1 efgh ± 0.07 | 61.4 fgh ± 2.33 | 62.4efgh ± 0.81 | 62.1 efgh ± 0.89 |
| 3 | 62.1 efgh ± 1.98 | 63.0 efgh ± 0.07 | 63.1 ef ± 0.04 | 62.8 efgh ± 1.14 | 71.3 d ± 0.06 |
| 4 | 61.7 fgh ± 1.47 | 62.2 efgh ± 1.00 | 62.7 efgh ± 0.53 | 63.7 ef ± 0.87 | 74.7 c ± 1.12 |
| 5 | 62.3 efgh ± 2.09 | 61.4 fgh ± 0.51 | 62.6 efgh ± 0.69 | 63.1 ef ± 0.90 | 79.5 b ± 0.67 |
| 6 | 61.3 fgh ± 2.21 | 60.6 h ± 0.57 | 60.7 gh ± 3.84 | 63.7 ef ± 0.48 | 85.1 a ± 0.84 |
| LSD at 0.05 = 1.972 | |||||
Data are expressed as mean ± SD
Values given represent means of five determinations.
Values followed by the same letter are not significantly different (p < 0.05).
Total bilirubin content
Bilirubin concentration has been used to evaluate chemically induced hepatic injury. Besides various normal functions, liver excretes the breakdown product of hemoglobin namely bilirubin into bile (Plaa and Hewitt 1982).Table 4 show the influence of various levels of polyphenolic compounds present in pomposia juice at levels (400, 600 and 1,200 ppm) and 200 ppm of synthetic antioxidant (BHT) on the level of total bilirubin. No significant (p ≥ 0.05) changes in the level of total bilirubin were shown for those rats administrated various levels of pomposia juice and for control group, however the administration of 200 ppm of synthetic antioxidant (BHT) caused significant (P ≤ 0.05) and gradual increase in total bilirubin starting from the third week of the experiment and towards the end of the experiment. This increase indicates that BHT may produce sufficient injury to hepatic parenchyma to cause large increases in bilirubin content. The abovementioned results consider as a good indicator for proving the safe of pomposia juice
Table 4.
Influence of various levels of Pomposia fruit juice(PFJ) and BHT on the activity of serum total bilirubin of rat
| Blood withdrawal Period (week) | Concentration of phenolic compounds | ||||
|---|---|---|---|---|---|
| Control rats | PFJ(400 ppm) | PFJ(800 ppm) | PFJ(1,200 ppm) | BHT(200 ppm) | |
| 0 | 1.013 e ± 0.005 | 1.013 e ± 0.005 | 1.007 e ± 0.015 | 1.023 e ± 0.011 | 1.007 e ± 0.015 |
| 1 | 1.020 e ± 0.010 | 1.023 e ± 0.005 | 1.023 e ± 0.011 | 1.010 e ± 0.000 | 1.013 e ± 0.005 |
| 2 | 0.990 e ± 0.034 | 1.023 e ± 0.011 | 0.996 e ± 0.083 | 1.010 e ± 0.020 | 1.040 e ± 0.010 |
| 3 | 1.027 e ± 0.005 | 1.030e ± 0.000 | 1.007 e ± 0.015 | 1.003 e ± 0.020 | 1.240 d ± 0.078 |
| 4 | 1.027 e ± 0.005 | 1.020 e ± 0.000 | 1.017 e ± 0.011 | 1.007 e ± 0.011 | 1.317 c ± 0.030 |
| 5 | 1.013 e ± 0.005 | 1.007e ± 0.015 | 1.017 e ± 0.011 | 1.020 e ± 0.020 | 1.380 b ± 0.026 |
| 6 | 1.013 e ± 0.005 | 1.003e ± 0.011 | 1.027 e ± 0.005 | 1.013 e ± 0.028 | 1.477 a ± 0.037 |
| LSD at 0.05 = 0.05150 | |||||
Data are expressed as mean ± SD
Values given represent means of five determinations.
Values followed by the same letter are not significantly different (p < 0.05).
Serum uric acid
Uric acid is the main end- product of nucleic acid and purine catabolism in human liver. Hyperuricemia or elevated serum ureate levels results from high production (metabolic) or decreased exrection (renal) (Baron 1987)Table 5 illustrate the changes in the levels of serum uric acid for control rats and those rats administrated various level of pomposia juice and 200 ppm of BHT . The results showed that there were no significant (p ≥ 0.05) changes were noted in the levels of uric acid for control rats and for rats administrated different level of phenolic compounds of pomposia fruit juice up to 1,200 ppm. On the other hand rats administrated 200 ppm of BHT had significant (p ≤ 0.05) and gradual increase in uric acid level starting from the fourth week of the experiment and towards the end of the experiment.
Table 5.
Influence of various levels of Pomposia fruit juice(PFJ) and BHT on the activity of serum uric acid of rat
| Blood withdrawal Period (week) | Concentration of phenolic compounds | ||||
|---|---|---|---|---|---|
| Control rats | PFJ(400 ppm) | PFJ(80 0 ppm) | PFJ(1,200 ppm) | BHT(200 ppm) | |
| 0 | 4.507 fg ± 0.005 | 4.530 fg ± 0.010 | 4.553 fg ± 0.020 | 4.490 g ± 0.036 | 4.503 fg ± 0.005 |
| 1 | 4.517 fg ± 0.011 | 4.533 fg ± 0.049 | 4.523 fg ± 0.041 | 4.510 fg ± 0.020 | 4.727 ef ± 0.015 |
| 2 | 4.520 fg ± 0.034 | 4.513 fg ± 0.011 | 4.617 efg ± 0.167 | 4.610 efg ± 0.164 | 4.820 de ± 0.010 |
| 3 | 4.507 fg ± 0.049 | 4.547 fg ± 0.101 | 4.480 g ± 0.043 | 4.520 fg ± 0.010 | 4.963 d ± 0.032 |
| 4 | 4.510 fg ± 0.010 | 4.473 g ± 0.064 | 4.607 fg ± 0.176 | 4.647 efg ± 0.228 | 5.930 c ± 0.228 |
| 5 | 4.520 fg ± 0.034 | 4.470 g ± 0.040 | 4.507 fg ± 0.056 | 4.560 fg ± 0.010 | 7.447 b ± 0.412 |
| 6 | 4.533 fg ± 0.028 | 4.523fg ± 0.015 | 4.603 fg ± 0.020 | 4.653 efg ± 0.159 | 8.133 a ± 0.205 |
| LSD at 0.05 = 0.1857 | |||||
Data are expressed as mean ± SD
Values given represent means of five determinations.
Values followed by the same letter are not significantly different (p < 0.05).
Serum urea
Urea is the major end-product of nitrogen catabolism in human. Urea is synthesized in the liver released to the blood and cleared (excreted) by the kidney. The change in plasma urea leval are due to alteration of renal function (diseases kidney) (Baron 1987).Table 6 show the influence of various levels of polyphenolic compounds present in pomposia juice at levels (400, 600 and 1,200 ppm) and 200 ppm of synthetic antioxidant (BHT) on the level of serum urea. Data showed that there were no significant(p ≥ 0.05) changes were observed in levels of urea during whole the experiment for control rats group and for rats administrated different level of phenolic compounds of pomposia fruit juice. On the contrary, administration of 200 ppm of synthetic antioxidant (BHT) caused significant (P ≤ 0.05) increase in urea levels, this increase was observed starting from the 4 th week of the experiment and towards the end of the experiment . Blood Urea Nitrogen (BUN) only begins to rise after a marked renal parenchyma injury occurs, the increase in the level of BUN indicated that BHT could induce renal dysfunction Lin et al. 2007 Such findings were coincided with that found by Ali 2010 who showed that administration of BHT at level 200 ppm caused severe damage to the tissues of liver and kidney and in the mean time caused an increases in the levels of creatinine, uric acid, urea, total bilirubin and rise in the activities of aspartate aminotransferase, alanine aminotransferase,and alkaline phosphatase . The abovementioned results of the current study suggest using pomposia juice as safe food grade substance.
Table 6.
Influence of various levels of Pomposia fruit juice(PFJ) and BHT on the activity of serum urea of rat
| Blood withdrawal Period (week) | Concentration of phenolic compounds | ||||
|---|---|---|---|---|---|
| Control rats | PFJ(400 ppm) | PFJ(80 0 ppm) | PFJ(1,200 ppm) | BHT(200 ppm) | |
| 0 | 30.0 d ± 0.05 | 30.0 d ± 0.06 | 30.0 d ± 0.04 | 30.5 d ± 0.50 | 30.1 d ± 0.04 |
| 1 | 30.1 d ± 0.04 | 30.1 d ± 0.03 | 31.0 d ± 0.04 | 29.3 d ± 0.54 | 30.7 d ± 0.51 |
| 2 | 30.1 d ± 0.02 | 30.2 d ± 0.01 | 30.4 d ± 0.54 | 29.6 d ± 0.54 | 30.4 d ± 0.60 |
| 3 | 30.1 d ± 0.10 | 30.2 d ± 0.07 | 30.1 d ± 0.10 | 29.5 d ± 0.45 | 31.0 d ± 0.64 |
| 4 | 31.0d ± 0.06 | 30.1 d ± 0.02 | 30.0 d ± 0.06 | 30.0 d ± 0.06 | 41.0 c ± 0.90 |
| 5 | 30.7 d ± 0.58 | 30.4 d ± 0.57 | 30.0 d ± 0.11 | 30.0 d ± 0.02 | 51.8 b ± 2.00 |
| 6 | 30.3 d ± 0.04 | 30.1 d ± 0.01 | 30.1 d ± 0.07 | 29.7 d ± 0.51 | 56.0 a ± 0.00 |
| LSD at 0.05 = 3.286 | |||||
Data are expressed as mean ± SD
Values given represent means of five determinations.
Values followed by the same letter are not significantly different (p < 0.05).
Histological examination
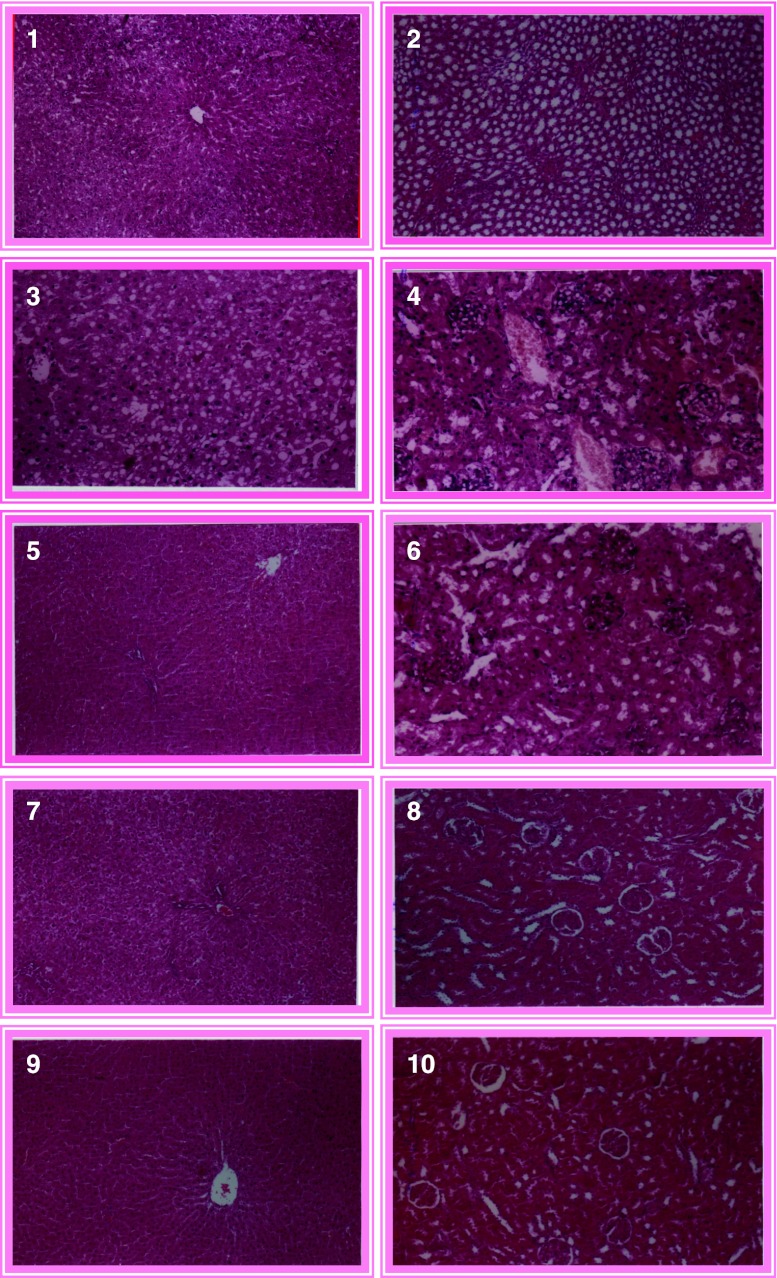
To ensure the safety of pomposia juice histopathological examinations were conducted. In General, The results of histopathological examinations of rat liver and kidney tissues administrated BHT(200 ppm) and pomposia juice at levels (400, 600 and 1,200 ppm)were in accordance with the results of biochemical measurements of serum rat liver and kidney. Figures 1 and 2 show the microscopically examination of liver and kidney tissues of rats given only the basal diet (control). The microscopically examination of liver tissue revealed the presence of normal hepatic parenchyma (Fig. 1). The microscopically examination of kidney tissues demonstrated of normal cortical and medullary structures including renal tubules and glumeruli (Fig. 2).
Figs. 1–10.
1 Microscopical examination of liver tissues of rats given only the basal diet (control) (H.E X 200). 2 Microscopical examination of kidney tissues of rats given only the basal diet (control) (H.E X 200). 3 Histological examination of liver tissues of rats administered the basal diet and BHT (200 ppm.) (H.E X 200). 4 Histological examination of kidney tissues of rats administered the basal diet and BHT (200 ppm.) (H.E X 200). 5 Cross section of liver tissues of rats administered the basal diet and Pomposia fruit juice (200 ppm.) (H.E X 200). 6 Cross section of kidney tissues of rats administered the basal diet and Pomposia fruit juice (200 ppm.) (H.E X 200). 7 Cross section of liver tissues of rats administered the basal diet and Pomposia fruit juice (400 ppm.) (H.E X 200). 8 Cross section of kidney tissues of rats administered the basal diet and Pomposia fruit juice (400 ppm.) (H.E X 200). 9 Cross section of liver tissues of rats administered the basal diet and Pomposia) fruit juice (1,200 ppm.) (H.E X 200). 10 Cross section of kidney tissues of rats administered the basal diet and Pomposia fruit juice (1,200 ppm.) (H.E X 200)
Figure 3 show the microscopically examination of liver tissues of rat administered BHT (200 ppm) at the end of the experiment. The results showed marked pathological changes, i.e. a wide extension at hepatocelluar degeneration including cloudy swelling and vacuolar degeneration. Several pathogenic changes were observed in kidney sections of rats administrated BHT at level 200 ppm i.e. modularly blood vessels together with degenerative change in the renal tubules especially the proximal ones (Fig. 4).
Figures 5, 6, 7, 8, 9 and 10 show the microscopically examination of liver and kidney of rat administered pomposia juice at levels (400, 600 and 1,200 ppm). In general, liver and kidney tissue of rat administered various levels of pomposia juice had the same character as that of control rats (this means that the polyphenolic compounds present in pomposia juice did not cause any adverse affect in liver and kidney).
Conclusion
The biochemical results indicated that the administration of polyphenolic compounds present in pomposia juice did not cause any changes in the biochemical parameters whereas the administration of BHT at 200 ppm caused significant (p ≤ 0.05) and gradual increase in the activities of enzymes relevant to the functions of liver and kidney.
The histopathological results were as quality assurance indices to ensure that the administration of polyphenolic compounds present in pomposia juice did not cause any harmful effects on the liver and kidney of rats. The abovementioned results of the current investigation suggest using pomposia juice as safe food grade substance.
References
- Abd El Mageid MM, Salama NA, Saleh MAM, Abo Taleb HM. Antioxidant and antimicrobial characteristics of red and brown algae extracts 4th Conference on Recent Technologies in Agriculture. Faculty of Agriculture: Cairo Univerisity; 2009. pp. 818–828. [Google Scholar]
- Achrekar S, Kaklij GS, Pote MS, Kelkar SM. Hypoglycemic activity of Eugenia jambolana and Ficus bengalensis: mechanism of action. In Vivo. 1991;5:133–148. [PubMed] [Google Scholar]
- Ahmad I. Chemistry and technology of deep-frying oils Pak. J Food Sci. 1999;9:24–29. [Google Scholar]
- Ali RFM (2004) Biochemical Studies on some heated vegetable oils. Master of Science thesis, Biochemistry dept., Faculty of Agriculture, Cairo University, Egypt
- Ali RFM. Effect of Pomposia (Syzygium cumini) fruit juice on the stability of fried sunflower oil. J Food Technol. 2010;8(2):30–38. doi: 10.3923/jftech.2010.30.38. [DOI] [Google Scholar]
- AOAC (2000) Official methods of analysis of the Association of Official Analytical Chemists, 17th edn.In Horwitz W (ed) Washington, DC
- Barham D, Trinder P. Enzymatic, colorimatric method for determination uric acid in serum plasma and urine. Analyst. 1972;97:142–146. doi: 10.1039/an9729700142. [DOI] [PubMed] [Google Scholar]
- Baron DN (1987) A short textbook of chemical pathology, 4th edition- English Language Book Society/Hodder and Stoughton Ltd, Mill Road, Dunton Green, Sevenoaks, Kent, Great Britain, pp 188–228
- Belfield A, Goldberg DM. A colorimetric method for determination of alkaline phosphatase in serum. Enzyme. 1971;12:561–565. doi: 10.1159/000459586. [DOI] [PubMed] [Google Scholar]
- Bergmeyer HU, Harder M. A colorimetric method of determination of serum glutamic oxaloacetic and pyruvic tranaminase. Clin Biochem. 1986;24:28–34. [Google Scholar]
- Blum M. Designing food for better health. Int Food Ingredients. 1996;3:25–29. [Google Scholar]
- Chan KM, Decker EA, Means WJ. Extraction and activity of carnosine, a naturally occurring antioxidant in beef muscle. J Food Sci. 1993;58:1–4. doi: 10.1111/j.1365-2621.1993.tb03199.x. [DOI] [Google Scholar]
- Chang SS, Ostric-Matijasevic B, Hsieh OAL, Cheng LH. Natural antioxidants from rosemary and sage. J Food Sci. 1977;42(4):1102–1106. doi: 10.1111/j.1365-2621.1977.tb12676.x. [DOI] [Google Scholar]
- Chowdhury P, Ray RC. Fermentation of Jamun (Syzgium cumini L.) fruits to form red wine. Asean Food J. 2007;14(1):15–23. [Google Scholar]
- Culling CFA. Hand book of histopathological techniques. 2. London: Butterworth; 1965. [Google Scholar]
- Cuvelier ME, Berset C, Richard H. Antioxidant constituents in sage (Salvia officinalis) J Agric Food Chem. 1994;42:655–669. doi: 10.1021/jf00039a012. [DOI] [Google Scholar]
- Farag RS, Mohamoud EA, Basuny AM, Ali RFM. Influence of crude olive leaf juice on rat liver and kidney functions. Int J Food Sci Tech. 2006;41:1–10. doi: 10.1111/j.1365-2621.2006.01093.x. [DOI] [Google Scholar]
- Farag RS, Mohamoud EA, Basuny AM. Use crude olive leaf juice as a natural antioxidant for the stability of sunflower oil during heating. Int J Food Technol. 2007;42:107–111. doi: 10.1111/j.1365-2621.2006.01374.x. [DOI] [Google Scholar]
- Fawcett JK, Scott JE. Enzymatic, colorimetric method for determination urea in serum, plasma and urine. J Clin Pathol. 1960;13:156–162. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiselli A, Nardini M, Baldi A, Scaccini C. Antioxidant activity of different phenolic fractions separated from an Italian red wine. J Agric Food Chem. 1998;46:361–367. doi: 10.1021/jf970486b. [DOI] [PubMed] [Google Scholar]
- Gomez KA, Gomez AA. Statistical procedures for agricultural research. New York: Wiley; 1984. [Google Scholar]
- Hamed HS Mattuke, Hemmat I, Attia EA. Production of anthocyanins from pompozia fruitsEgyptian. J Agric Res. 2001;79(1):233–243. [Google Scholar]
- Hewawasam RP, Jayatilaka KAPW, Pathirana C, Mudduwa LKB. Hepatoprotective effect of Epaltes divaricata extract on carbon tetrachloride induced hepatotoxicity in mice. Ind J Med Res. 2004;120:30–34. [PubMed] [Google Scholar]
- Innawong B, Mallikarjunan P, Irundayaraj J, Marcy JE. The determination of frying oil quality using Fourier transform infrared attenuated total reflectance. Lebensm Wiss U Technol. 2004;37:23–28. [Google Scholar]
- Jayaprakasha G, Selvi T, Sakariah K. Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extracts. Food Res Int. 2003;36:117–122. doi: 10.1016/S0963-9969(02)00116-3. [DOI] [Google Scholar]
- Kamei H, Hashimoto Y, Koide T, Kojima T, Hasegawa M. Anti-tumor effect of methanol extracts from red and white wines. Cancer Biother Radiopharm. 1998;13:447–452. doi: 10.1089/cbr.1998.13.447. [DOI] [PubMed] [Google Scholar]
- Kanner J, Harel S, Jaffe R. Lipid peroxidation of muscle food as affected by NaCl. J Agric Food Chem. 1991;39:1017–1021. doi: 10.1021/jf00006a002. [DOI] [Google Scholar]
- Kong J-M, Chia L-S, Goh N-K, Chia TF, Brouillard R. Analysis and biological activities of anthocyanins. Phytochemistry. 2003;64:923–933. doi: 10.1016/S0031-9422(03)00438-2. [DOI] [PubMed] [Google Scholar]
- Lago ES, Gomes E, da Silva R (2004) Extraction and anthocyanic pigment quantification of the Jamun fruit (Syzygium cumini Lamark). http://www.iqsc.usp.br/outros/eventos/2004/bmcfb/trabalhos/docs/trab-76.pdf.
- Lee YB, Kim YS, Ashmore CR. Antioxidant property in ginger rhizome and its application to meat products. J Food Sci. 1986;51:20–23. doi: 10.1111/j.1365-2621.1986.tb10826.x. [DOI] [Google Scholar]
- Lin FS, Warner CR, Fazio T. Alteration of phenolic antioxidants in heated vegetable oil. J Am Chem Soc. 1981;65:789–792. [Google Scholar]
- Lin HM, Yen FL, Ng LT, Lin CC. Protective effects of Ligustrum lucidum fruit extract on acute butylated hydroxytoluene-induced oxidative stress in rats. J Ethnopharmacol. 2007;111:129–136. doi: 10.1016/j.jep.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Liya L, Lynn SA, Shiuan C, Caroline K, Aftab A, Navindra PS. Eugenia jambolana Lam. Berry extract inhibits growth and induces apoptosis of human breast cancer but not non-tumorigenic breast cells. J Agric Food Chem. 2009;11; 57(3):826–831. doi: 10.1021/jf803407q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton J. Fruits of warm climates. FL: Miami; 1987. [Google Scholar]
- Nadkarni AK. Indian Materia Medica, vol. 1. Bombay: Popular Prakashan; 1954. p. 1331. [Google Scholar]
- Obi FO, Usenu IA, Osayande JO. Prevention of CCl4-induced hepatotoxicity in the rat by H. rosasinensis anthocyanin extracts administered in ethanol. Toxicology. 1998;131:93–98. doi: 10.1016/S0300-483X(98)00119-X. [DOI] [PubMed] [Google Scholar]
- Plaa GL, Hewitt WR. Detection and evaluation of chemical induced liver injury. In: Hayes AW, editor. Principles and methods of toxicology. New York: Raven; 1982. p. 407. [Google Scholar]
- Rajesh MG, Latha MS. Preliminary evaluation of the antihepatotoxic activity of Kamilari, a polyherbal formulation. J Ethnopharmacol. 2004;91:99–104. doi: 10.1016/j.jep.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Ravi K, Ramachandran B, Subramanian S. Effect of Eugenia Jambolana seed kernel on antioxidant defense system in streptozotocin-induced diabetes in rats. Life Sci. 2004;75:2717–2731. doi: 10.1016/j.lfs.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Rhee KS, Anderson LM, Sams AR. Lipid oxidation potential of beef, chicken and pork. J Food Sci. 1996;61:8–12. doi: 10.1111/j.1365-2621.1996.tb14714.x. [DOI] [Google Scholar]
- Sato K, Hegarty GR. Warmed-over flavour in cooked meat. J Food Sci. 1971;36:1098–1102. doi: 10.1111/j.1365-2621.1971.tb03355.x. [DOI] [Google Scholar]
- Scartezzini P, Speroni E. Review on some plants of Indian traditional medicine with antioxidant activity. J Ethnopharmacol. 2000;71(1–2):23–43. doi: 10.1016/S0378-8741(00)00213-0. [DOI] [PubMed] [Google Scholar]
- Shahjahan MK, Sabitha JM, Shyamala-Devi CS. Effect of Solanum trilobatum against carbon tetrachloride induced hepatic damage in albino rats. Indian J Med Res. 2004;120:194–198. [PubMed] [Google Scholar]
- Sharma JN, Sheshadri TR. Survey of anthocyanins from Indian sources: Part II. J Sci Ind Res. 1955;14:211–214. [Google Scholar]
- Sharma B, Viswanath G, Salunke R, Roy P. Effects of flavonoid-rich extract from seeds of Eugenia jambolana (L.) on carbohydrate and lipid metabolism in diabetic mice. Food Chem. 2008;110:697–705. doi: 10.1016/j.foodchem.2008.02.068. [DOI] [Google Scholar]
- Teixeira CC, Blotta RM, Costa AP, Mussnich DG, Ranquetat GG, Fuchs FD. Plants employed in the treatment of diabetes mellitus results of an ethnopharmacological survey in Porto Alegre. Brazil Fitoterapia LXIII. 1992;4:320–322. [Google Scholar]
- Timberlake CF, Bridle P. Distribution of anthocyanins in food plant. In: Markakis P, editor. Anthocyanins as food colors. New York: Academic; 1982. p. 137. [Google Scholar]
- Veigas JM, Narayan MS, Laxman PM, Neelwarne B. Chemical nature, stability and bioefficacies of anthocyanins from fruit peel of Syzygium cumini skeels. Food Chem. 2007;105:619–627. doi: 10.1016/j.foodchem.2007.04.022. [DOI] [Google Scholar]
- Venkateswarlu G. On the nature of the colouring matter of the jambul fruit (Eugenia jambolana) J Indian Chem Soc. 1952;29(6):434–437. [Google Scholar]
- Vizzotto M, Pereira MC. Characterization of functional properties of Jambolan (Caracterização das Propriedades Funcionais do Jambolão) Bol Pesqui Desenvolvimento. 2008;79:1–27. [Google Scholar]
- Walter M, Gerade H. A colorimetric method for determination bilirubin in serum and plasma. Micro Chem J. 1970;15:231–236. doi: 10.1016/0026-265X(70)90045-7. [DOI] [Google Scholar]
- Wright PJ, Plummer DT. The use of urinary enzyme measurement to detect renal damage caused by nephrotoxic compounds. Biochem Pharmacol. 1974;23(1):65–73. doi: 10.1016/0006-2952(74)90314-1. [DOI] [PubMed] [Google Scholar]
- Yanardag R, Tunali S. Vanadyl sulfate administration protects the streptozotocin-induced oxidative damage to brain tissue in rats. Mol Cell Biochem. 2006;286:153–159. doi: 10.1007/s11010-005-9107-1. [DOI] [PubMed] [Google Scholar]
- Yin MC, Cheng WS. Oxymyoglobin and lipid oxidation in phosphatidylcholine liposomes retarded by α- tocopherol and b-carotene. J Food Sci. 1997;62:1095–1097. doi: 10.1111/j.1365-2621.1997.tb12220.x. [DOI] [Google Scholar]
- Yoshinaga M, Yamakawa O, Nakatani M. Genotypic diversity of anthocyanin content and composition in purple-fleshed sweet potato (Ipomoea batatas (L.) Lam) Breed Sci. 1999;49:43–47. doi: 10.1270/jsbbs.49.43. [DOI] [Google Scholar]