Abstract
We evaluated the phenolic content and antioxidant capacities of pod and seed extracts (in methanol, ethanol, and water) of an underutilized legume, Clitoria fairchildiana (Howard). The antioxidant capacity of the extracts was determined using the ferric reducing antioxidant potential assay, and the free radical-scavenging capacity was evaluated using 2,2-diphenyl-1-picrylhydrazyl radical-scavenging and ABTS assays. In addition, the total flavonoids, flavonols, and tannin contents were also determined. Overall, the methanol extracts of the pod contained high concentration of phenolics and showed high antioxidant capacities compared to seed extracts. In addition, a positive correlation was found between total phenol and tannin versus antioxidant capacity. Results of the present study indicate pods and seeds of C. fairchildiana to possess rich amount of natural antioxidants, and can be further explored for their possible use as a natural additive in food or in pharmaceutical industries.
Keywords: Antioxidant activity, Clitoria fairchildiana, Flavonoids, Polyphenols, Solvent extraction
Introduction
With increased knowledge on the health-promoting and disease-preventive effects of phenolic compounds and antioxidants in plants, diverse plant resources are being researched throughout the world. The discovery of natural and safe sources of antioxidants of plant origin has increased markedly in recent years, and they have been recognized to be a better alternative than synthetic antioxidants such as butylated hydroxytoluene, butylated hydroxyanisole, and others. However, considering the wide range of plant resources available in the world, many of them still remain unexplored. In particular, a wide gap exists regarding the evaluation of antioxidant compounds from underutilized legumes. Exploring underutilized legume resources is of great importance considering their rich nutraceutical value and the presence of bioactive compounds (Bhat and Karim 2009). Moreover, some of the underutilized legume seeds (e.g. Canavalia sp., Mucuna sp., and Sesbania sp.) possess high amounts of bioactive and health-promoting compounds (Bhat and Karim 2009).
Clitoria fairchildiana (family: Leguminosae) is usually grown as a shade tree in tropical regions and as an ornamental plant in temperate regions of the world (Silva et al. 1998a). Various parts of this plant are reported to be used for anti-inflammatory treatments, as a diuretic, expectorant, and as an emenagogue (Silva et al. 1998b; Silva and Parente 2002). However, apart from the isolation of rotenoid glycosides from the seeds of C. fairchildiana (Mathias et al. 2005), no scientific reports are available on the antioxidant or phytochemical constituents of the pods or seeds of this plant. Hence, the main objectives of this study were to (a) individually evaluate the antioxidant capacity of the young pods and dried seed of C. fairchildiana and (b) to comparatively evaluate the effects of various extracting solvents on various antioxidant compounds. The results of this study is expected to provide sufficient baseline information for further exploration of this underutilized legume for nutraceutical purposes as well as for developing new, cheaper, and safe food products.
Materials and methods
Chemicals and reagents
Potassium persulfate, 2,2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ), folin–ciocalteu reagent (FC reagent), vanillin, sodium carbonate, aluminium chloride hexahydrate, ferric chloride, sodium acetate, ascorbic acid, gallic acid, quercetin, and catechin were purchased from Sigma Aldrich (Laborchemikalien GmbH, 30926, Seelze, Germany). All of the other non-specified reagents and solvents used in the study were of analytical grade and procured from Fisher Scientific Ltd., (Shah Alam, Selangor Darul Ehsan, Malaysia).
Plant material
Fresh, young (tender) pods and dried seeds of Clitoria fairchildiana (Howard) were collected from the campus garden of Universiti Sains Malaysia, Penang, Malaysia. Physically damaged pods and seeds were sorted out and discarded; the retained samples were surface cleaned with a fine muslin cloth to remove the adherent dirt prior to use.
Preparation of different extracts
Pods
For extraction with different solvents, fresh pods were blended in a mixer for 3 min followed by extraction (in the absence of light) with a known volume of methanol, ethanol (96%) and distilled water (aqueous) (1:5) with continuous magnetic stirring on a hot plate (magnet 4.5 × 0.5 cm; JENWAY 1000 hot plate and stirrer, JENWAY Ltd., Staffordshire, UK) at 1100 rpm for 3 h at room temperature (25 ± 1°C). The extract obtained was filtered through Whatman filter paper (No.1) followed by centrifugation at 3000 g for 15 min (SIGMA Laborzentrifugen 6–10, Deutschland, Osterode, Germany). Next, the supernatant was concentrated at 50°C using a rotary evaporator (Buchi Rotavapor R-215, Postfach Flawil, Switzerland), and the concentrated solution of respective extracts were lyophilized in a freeze dryer (LABCONCO, Free Zone 6 l, Kansas City, Missouri, USA) to obtain dry form of the extracts. Further, this was stored at 4°C (in dark) until further analysis.
Seeds
Naturally sun dried seeds (moisture 12 ± 1%) were ground to a fine powder to a particle size of <30 mesh size. The same extraction procedure described above for pods was followed.
Determination of total phenolics content
The total phenolic content of the extracts was determined using the Folin–Ciocalteu assay (Singleton and Rossi 1965). A known volume of the aliquot (400 μL) of the extract was mixed with 2.0 mL (10 times pre-diluted) of FC reagent. After standing for 5 min at room temperature, 1.6 mL of (7.5% w/v) sodium carbonate solution was added. The solutions were mixed and allowed to stand for 60 min at room temperature. The absorbance was measured at 765 nm using a UV-visible spectrophotometer (Shimadzu UV-160A, Kyoto, Japan). A calibration curve was prepared using a standard solution of gallic acid. Results were expressed as mg gallic acid equivalents (GAE) per gram of sample.
Determination of total flavonoid content
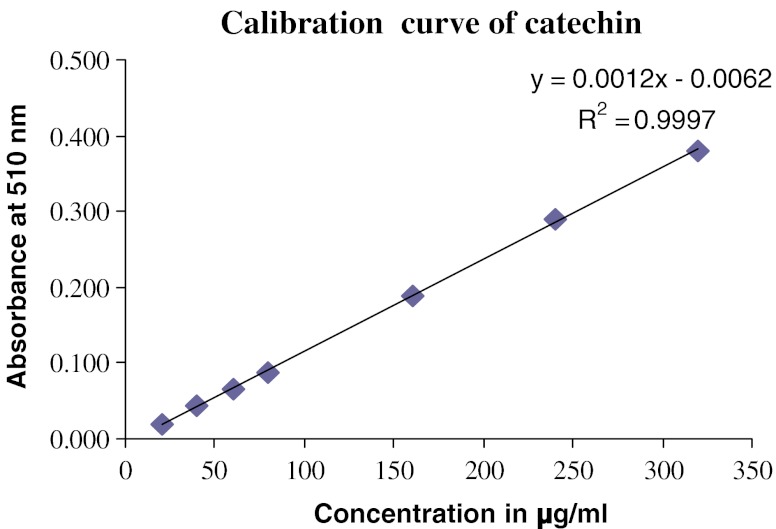
The colorimetric method described by Sakanaka et al. (2005) was employed to determine the total flavonoid content in the extracts. Briefly, 250 μL of the extract or (+)-catechin standard solution was mixed with 1.25 mL of distilled water in a test tube, followed by addition of 75 μL of a 5% sodium nitrite solution. After 6 min, 150 μL of a 10% aluminium chloride solution was added and the mixture was allowed to stand for 5 min before 0.5 mL of 1 M sodium hydroxide was added. The mixture was brought to 2.5 mL with distilled water and mixed well. The absorbance was measured immediately at 510 nm using a UV-visible spectrophotometer (Shimadzu UV-160A). The results were expressed as mg of (+)-catechin equivalent per gram of extract. The calibration curve obtained for catechin is provided in Fig. 1.
Fig. 1.
Calibration curve of Catechin
Determination of total flavonol content
The total flavonol content was estimated by adapting the method described by Miliauskas et al. (2004) with slight modifications. The quercetin calibration curve was prepared by mixing 1 mL of 0.150–0.05 mg mL−1 quercetin methanol solution with 1 mL of 2% aluminium trichloride and 3 mL of 5% sodium acetate. The absorption at 440 nm was read after 150 min at 20°C. The same procedure was carried out with 1 mL of plant extract (1 mg/ mL−1) instead of quercetin solution. Total flavonols were expressed as mg quercetin equivalent per gram of extract.
Determination of tannin content
To determine tannins concentration, the vanillin-HCl method with slight modification was used (Bhat et al. 2007). Extracts in methanol (1 mL) were treated with 5 mL of reagent mixture composed of 4% vanillin in methanol and 8% concentrated HCl in methanol (in a 1:1 ratio). After 20 min, the color developed was read at 500 nm using a UV-visible spectrophotometer (Shimadzu UV-160A). A standard calibration curve was prepared using catechin (20–400 μg mL−1) and tannins were expressed as mg catechin equivalent per gram of extract.
Determination of antioxidant capacity (Phosphomolybdenum assay)
The antioxidant capacity of pod and seed extracts were evaluated by the formation of phosphomolybdenum complex, based on the method described by Prieto et al. (1999). Briefly, to a known aliquot (0.4 mL) of the sample solution (250 μg mL−1 in methanol) taken in a vial, 4 mL of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate) was added. Further, the vials were capped and incubated in a water bath at 95°C for 90 min. The blank solution contained 4 mL of reagent solution and the 0.4 mL of methanol. After cooling the samples to room temperature, the absorbance of the mixture was measured at 695 nm against a blank (UV-visible spectrophotometer, Shimadzu UV-160A, Kyoto, Japan). Calibration curve was prepared using a standard solution of ascorbic acid (25–300 μg mL−1) and the antioxidant activity was expressed relative to that of ascorbic acid.
DPPH and ABTS+ radical scavenging activity
The DPPH and ABTS+ radical scavenging activities of the extracts were measured by the method reported by Blois (1958) and Re et al. (1999), respectively. The results were expressed according to the following formula.
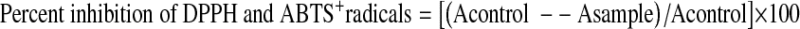 |
Where, Acontrol is the absorbance of the DPPH solution without extract and the Asample is the absorbance of the sample with DPPH solution.
Ferric reducing antioxidant property (FRAP assay)
Ability of the sample extracts to reduce ferric ions was measured according to the modified method described by Benzie and Strain (1996). Ferrous sulphate solution with concentration ranging from 0.1 to 1 μM was used for the preparation of standard calibration curve. The FRAP values were expressed as mM ferrous equivalents per gram of plant material.
Statistical analysis
Data from this study (samples run in triplicates, n = 3) are represented as mean values ± SD. Analysis of variance was performed, and significant differences between mean values were determined by Tukey’s pair-wise comparison test at a level of significance of p < 0.05. Statistical analyses were conducted using SPSS 12.01 (SPSS Inc., Wacker Drive, Chicago, USA).
Results and discussion
Table 1 shows results for the percent yield obtained for the pod and seed extracts after extracting with various solvents. Among the seed extracts, highest yield was obtained for ethanol and methanol (4.5%), while for pod it was methanol followed by water (aqueous) and ethanol (3.9, 3.7 and 3.2, respectively).
Table 1.
Percent yield, FRAP, total antioxidant, total phenols, flavonoids, tannin and flavonols values of different extracts of pod and seed of Clitoria fairchildiana
| Seed extracts | Pod extracts | |||||
|---|---|---|---|---|---|---|
| Aqueous | Ethanol | Methanol | Aqueous | Ethanol | Methanol | |
| % yield | 3.4 ± 0.01a | 4.5 ± 0.02b | 4.5 ± 0.01 b | 3.7 ± 0.01 b | 3.2 ± 0.03 a | 3.9 ± 0.03 b |
| Total phenolics1 | 40.5 ± 1.0e | 28.4 ± 0.2f | 58.9 ± 0.3d | 66.8 ± 1.0c | 96.6 ± 0.1b | 141.7 ± 0.4a |
| Antioxidant capacity2 | 64.1 ± 2.0f | 125.6 ± 1.4e | 146.2 ± 2.0d | 152.8 ± 2.2c | 227.8 ± 1.4b | 352.0 ± 2.0a |
| FRAP values3 | 0.31±.01e | 0.28 ± 0.01f | 0.65 ± 0.0d | 1.0 ± 0.01c | 1.4 ± 0.0b | 1.4 ± 0.0a |
| Total flavonoids4 | 27.7 ± 1.2f | 92.7 ± 3.0b | 86.3 ± 2.1c | 41.3 ± 1.0e | 74.3 ± 1.2d | 126.0 ± 2.0a |
| Total flavonols5 | 0.38 ± 0.1e | 9.46 ± 0.2b | 5.9 ± 0.3c | 1.2 ± 0.1d | 6.5 ± 0.3c | 17.1 ± 0.4a |
| Total tannins6 | 40.7 ± 2.0e | 55.7 ± 3.0d | 62.7 ± 1.2c | 62.7 ± 1.0c | 94.7 ± 1.0b | 193.3 ± 3.0a |
1 mg Gallic acid equivalent/gm extract; 2 mg Ascorbic acid equivalent /gm of the extract;
3 mM Fe2+/gm extract; 4 mg Catechin equivalent/gm extract; 5 mg Quercetin equivalent/gm extract; 6mg Catechin equivalent/gm extract.
FRAP: Ferric reducing antioxidant potential
Mean values with different alphabets in the row were significantly different (p < 0.05) from each other. Values were the means of three replicates ± standard deviation (SD).
Total phenolics content
Determining the phenolic concentration in a plant produce is important because this parameter has been shown to directly correspond to the radical scavenging activity and to have a linear correlation with the antioxidant activity (Bagchi et al.2006). Plant phenolics have also been well established in legumes as the most abundant group of secondary metabolites and are known to act as natural antioxidants, which are capable of reducing the oxidative damage associated with many diseases such as arteriosclerosis, cancer and cardiovascular diseases (Alothman et al. 2009a).
In the present study, pod extracts showed higher phenolics content compared to seed extracts. The methanol extract of pods had significantly higher phenols (141.7 mg GAE g−1 extract) compared to ethanol (96.6 mg GAE g−1 extract) and aqueous extracts (66.8 mg GAE/g extract) (p < 0.05). Similarly, in seeds the phenol content of methanol extracts (58.9 mg GAE g−1 extract) was higher than that of the aqueous and ethanol extracts (40.5 and 28.4 mg GAE g−1 extract, respectively) (Table 1). The high concentration of phenolics in pods is an indication that they are the main contributors to antioxidant activity, as reported by earlier researchers (Thirugnanasampandan et al. 2008).
Total flavonoids
Flavonoids occur as natural secondary metabolites of plants, possess rich antioxidant properties, and are able to interact and scavenge the free radicals that can damage cell membranes and biological molecules, including DNA (Paniwnyk et al. 2001). The flavonoids present naturally in plants encompass six sub-classes (flavones, isoflavones, flavonols, flavanols and anthocyanins) that vary in their structural characteristics (Rice-Evans and Miller 1997). As depicted in Table 1, methanol extracts of both pods and seeds exhibited high flavonoid contents (126.0 and 86.3 mg catechin equivalent g−1 extract, respectively). Interestingly, the flavonoid content of the seed ethanol extract was higher than that of the pod ethanol extract (92.7 vs. 74.3 mg catechin equivalent g−1 extract).
Total flavonols
The flavonols exhibit diverse chemical structures and characteristics; and are regarded as potent antioxidants with health-promoting activities (Duthie et al. 2000). In the present study, the methanol extract of the pod contained significantly higher (p < 0.05) flavonol concentration (17.1 mg quercetin equivalent g−1 extract) compared to all of the other extracts, followed by the ethanol extract of the seed (9.46 mg quercetin equivalent g−1 extract). Irrespective of pods or seeds, aqueous extracts showed lower flavonol content.
Total tannins
Tannins, which constitute a group of high molecular weight phenolics, have been reported to posses great ability to quench free radicals (ABTS+), the effectiveness of which is depended on the molecular weight, number of aromatic rings, and nature of the hydroxyl group substitution (Cai et al. 2006). The tannin-protein complexes that are formed as a result of conventional food processing have been shown to be potential free radical scavengers and have been suggested to be one of the major contributors for the nutraceutical property. These complexes are able to prevent free radical-mediated diseases occurring in the gastrointestinal tract (Riedl and Hagerman 2001).
In our study, irrespective of the solvent used, pod extracts showed higher tannin content than the seed extracts. The pod extracts contained 193.3 and 94.7 mg catechin equivalent g−1 extract in methanol and ethanol, respectively. In seed extracts, the values were 62.7 and 55.7 mg catechin equivalent g−1 extract in methanol and ethanol, respectively.
Antioxidant capacity
The pods showed higher antioxidant capacity than did the seed extracts. The methanol extract of the pod had a significantly higher antioxidant capacity (352 mg g−1equivalent to ascorbic acid), followed by the ethanol and aqueous extracts (227.8 and 152.8 mg/g equivalent to ascorbic acid, respectively) (p < 0.05). In the seed extract, similar trends were observed, but with a much lower antioxidant capacity compared to the pods (see Table 1).
A strong correlation was observed between the antioxidant capacity and the total phenolics and tannin content in the individual extracts (see Fig. 2a, b). These results are an indication that enhancement of the total phenols and tannin content can correspondingly increase the antioxidant capacity of the plant product. Similar inferences have been drawn by earlier workers who found that the antioxidant activity of different extracts depended on the amount of polyphenols present in the raw produce (Jayaprakasha et al. 2003; Ao et al.2008).
Fig. 2.
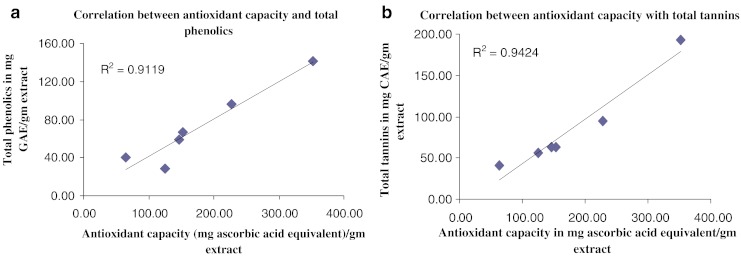
Correlation between antioxidant capacity with a total phenolics and b total tannins
Percentage inhibition for DPPH and ABTS assay
Figure 3a and b show the percentage inhibition of different pod and seed extracts for the DPPH assay. The DPPH assay involves reaction of specific antioxidant with a stable free radical 2, 2-diphenyl-1-picrylhydrazyl (DPPH) wherein a reduction in the DPPH concentration caused by the antioxidant decreases the optical absorbance of DPPH.
Fig. 3.
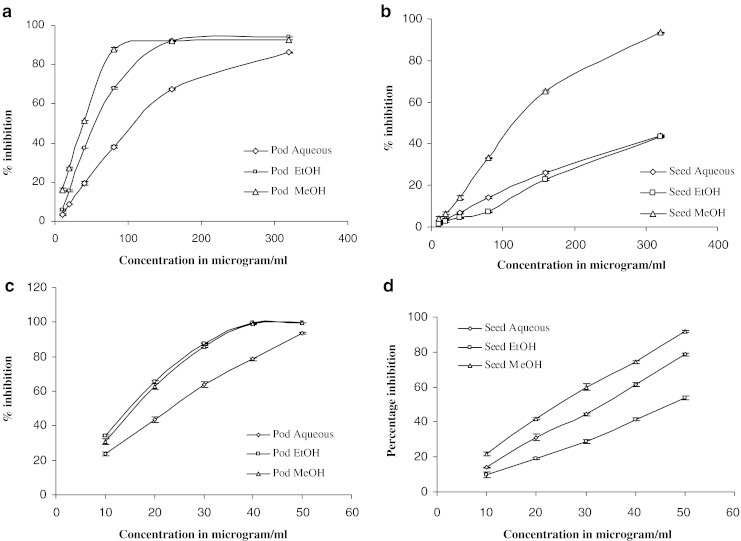
a, b Percentage inhibition of pod and seed extracts after DPPH assay; c, d Percentage inhibition of pod extracts after ABTS assay
In the present study, the methanol extracts of both the pods and seeds showed a significantly higher percent inhibition of DPPH compared to the ethanol or aqueous extracts (p < 0.05). Overall, compared to the seed extracts, the pod extracts displayed higher percent inhibition of DPPH in all of the solvents (upto a concentration range of 160 μg mL−1). In general, methanol extracts showed higher percent inhibition of DPPH in both pod and seed extracts. In pod, the order of inhibition in the extract was methanol > ethanol > water. However, in seed the order of inhibition was methanol > water > ethanol.
Figure 3c and d show the results of percent inhibition of the different seed and pod extracts for the ABTS assay. In pods, both the ethanol and methanol extracts showed better and significantly higher percent inhibition compared to aqueous extracts (p < 0.05). However, at a concentration of 50 μg mL−1, no significant changes were recorded between the various solvent extracts used (p > 0.05). In seeds, only the methanol extract showed significantly higher percent inhibition (91.78% at 50 μg mL−1) (p < 0.05) in the ABTS assay compared with the ethanol or aqueous extracts. Followed by methanol, aqueous extracts showed significantly better percent inhibition than the ethanol extracts at all of the concentrations studied (p < 0.05). These results clearly indicate that for both pods and seeds, all of the solvent extracts had a noticeable effect on the scavenging of free radicals.
Ferric reducing antioxidant potential assay
The FRAP assay is generally employed to measure the antioxidant effect of any substance in the reaction medium, as its reducing ability. Naturally present antioxidants are considered as reductants, and the inactivation of oxidants by reductants is described as a redox-reaction (Siddhuraju and Becker 2007). Table 1 shows the results of the FRAP assay of pod and seed extracts. The methanol extracts of the pod showed significantly (p < 0.05) higher FRAP values compared to all of the other extracts (from both seeds and pods). However, low FRAP values were recorded in the ethanol extract of the seed (0.286 mM Fe2+ per gram of extract).
A general trend found in the present study highlights the fact that the pod extracts have better antioxidant capacities than the seed extracts. A possible reason for this difference is that pods and seeds accumulate different amounts of phytochemicals, which in turn can affect the level of antioxidants present.
Effects of the solvent system
In the present study, we used 3 different extracting solvents (methanol, ethanol, and water), and we found that they differed significantly in their ability to extract antioxidants. Traditionally, solvents such as methanol, ethanol, and acetone have been routinely used to extract phenolic/antioxidant compounds from fresh plants at different concentrations in the presence of water (Alothman et al. 2009b). Because the extraction method used plays a significant role in the accurate quantification of antioxidants, it is very difficult to compare the data available in the literature (Santas et al. 2008; Alothman et al.2009b). However, generally, solvents such as methanol, ethanol, and acetone have different polarities, vapor pressures, and viscosities; solvents with low viscosity have low density and high diffusivity, which can allow them to easily diffuse into the pores of the plant materials to leach out the active constituents (Hemwimol et al. 2006; Naczk and Shahidi 2006). In addition, variations in antioxidant activity and the yield of phenolic compounds have been reported to be influenced by extracting solvents (Sun and Ho 2005).
Considering the variable results which can be obtained during extracting of antioxidants using various solvents, it is quite difficult to develop a standard extraction protocol that can provide better or more consistent results. From the toxicological point of view, ethanol and water are considered to be safer than methanol and other organic solvents (Oktay et al.2003), particularly with regard to applications or use in the food industries. However, in general, the least polar solvents are considered to be most suitable for the extraction of antioxidant compounds, unless very high pressure is employed.
Conclusion
The results of this study indicate that both the pods and seeds of Clitoria fairchildiana are potent antioxidant sources, with pod extracts exhibiting better antioxidant capacities than seed extracts. Overall, methanol proved to be a better solvent for the extraction of antioxidant compounds compared to ethanol and water by providing high extraction yields with strong antioxidant capacities. A good correlation existed between total phenolics and tannin content versus antioxidant capacity of the pod and seed extracts. Further studies are warranted to identify and characterize the phenolic compounds present in various extracts. Clitoria fairchildiana may prove to be a cheap source of natural polyphenolic phytochemicals, thus the pods and seeds of this species should be explored further for other health-promoting properties. Additionally, research work needs to be initiated on evaluating the nutritional qualities and safety issues of this underutilized legume for commercialization and for edible purposes.
References
- Alothman M, Bhat R, Karim AA. Effects of radiation processing on phytochemicals and antioxidants in plant produce. Trends Food Sci Technol. 2009;20:201–212. doi: 10.1016/j.tifs.2009.02.003. [DOI] [Google Scholar]
- Alothman M, Bhat R, Karim AA. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem. 2009;115:785–788. doi: 10.1016/j.foodchem.2008.12.005. [DOI] [Google Scholar]
- Ao C, Li A, Elzaawely AA, Xuan TD, Tawata S. Evaluation of antioxidant and antibacterial activities of Ficus microcarpa L. extract. Food Cont. 2008;19:940–948. doi: 10.1016/j.foodcont.2007.09.007. [DOI] [Google Scholar]
- Bagchi D, Roy S, Patel V, He G, Khanna S, Ojha N, Phillips C, Ghosh S, Bagchi M, Sen CK. Safety and whole-body antioxidant potential of a novel anthocyanin rich formulation of edible berries. Mol Cell Biochem. 2006;281:197–209. doi: 10.1007/s11010-006-1030-6. [DOI] [PubMed] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power, The FRAP Assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bhat R, Karim AA. Exploring the nutritional potential of wild and underutilized legumes. Compr Rev Food Sci Food Saft. 2009;8:305–331. doi: 10.1111/j.1541-4337.2009.00084.x. [DOI] [Google Scholar]
- Bhat R, Sridhar KR, Yokotani KT. Effect of ionising radiation on antinutritional features of velvet bean seeds (Mucuan pruriens) Food Chem. 2007;103:860–866. doi: 10.1016/j.foodchem.2006.09.037. [DOI] [Google Scholar]
- Blois MS. Antioxidant determination by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Cai YZ, Sun M, Xing J, Luo Q, Corke H. Structure–radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006;78:2872–2888. doi: 10.1016/j.lfs.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Duthie G, Duthie SJ, Kyle JAM. Plant polyphenols in cancer and heart disease: implications as nutritional antioxidants. Nutr Res Rev. 2000;13:79–106. doi: 10.1079/095442200108729016. [DOI] [PubMed] [Google Scholar]
- Hemwimol S, Pavasant P, Shotipruk A. Ultrasound-assisted extraction of anthraquinones from roots of Morinda citrifolia. Ultrason Sonochem. 2006;13:543–548. doi: 10.1016/j.ultsonch.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Jayaprakasha GK, Selvi T, Sakariah KK. Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extracts. Food Res Int. 2003;36:117–122. doi: 10.1016/S0963-9969(02)00116-3. [DOI] [Google Scholar]
- Mathias L, Silva BP, Mors WB, Parentene JP. Isolation and structural elucidation of a novel rotenoids from the seeds of Clitoria fairchildiana. Nat Prod Res. 2005;19:325–329. doi: 10.1080/14786410412331272022. [DOI] [PubMed] [Google Scholar]
- Miliauskas G, Venskutonis PR, Van Beek TA. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004;85:231–237. doi: 10.1016/j.foodchem.2003.05.007. [DOI] [Google Scholar]
- Naczk M, Shahidi F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J Pharm Biomed Anal. 2006;41:1523–1542. doi: 10.1016/j.jpba.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Oktay M, Gulcin I, Kufrevioglu OI. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. LWT Food Sci Technol. 2003;36:263–271. doi: 10.1016/S0023-6438(02)00226-8. [DOI] [Google Scholar]
- Paniwnyk L, Beaufoy E, Lorimer JP, Mason TJ. The extraction of rutin from flower buds of Sophora japonica. Ultrason Sonochem. 2001;8:299–301. doi: 10.1016/S1350-4177(00)00075-4. [DOI] [PubMed] [Google Scholar]
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a Phosphomolybdenum Complex: Specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorizing assay. Free Rad Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Rice-Evans CA, Miller NJ. Structure-antioxidant activity relationships of flavonoids and isoflavonoids. In: Rice-Evans CA, Packer L, editors. Flavonoids in health and disease. New York: Marcel Dekker; 1997. pp. 199–219. [Google Scholar]
- Riedl K, Hagerman AE. Tannin–protein complexes as radical scavengers and radical sinks. J Agric Food Chem. 2001;49:4917–4923. doi: 10.1021/jf010683h. [DOI] [PubMed] [Google Scholar]
- Sakanaka S, Tachibana Y, Okada Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha) Food Chem. 2005;89:569–575. doi: 10.1016/j.foodchem.2004.03.013. [DOI] [Google Scholar]
- Santas J, Carbó R, Gordon MH, Almajano MP. Comparison of the antioxidant activity of two Spanish onion varieties. Food Chem. 2008;107:1210–1216. doi: 10.1016/j.foodchem.2007.09.056. [DOI] [Google Scholar]
- Siddhuraju P, Becker K. The antioxidant and free radical scavenging activities of processed cowpea (Vigna unguiculata (L.) Walp.) seed extracts. Food Chem. 2007;101:10–19. doi: 10.1016/j.foodchem.2006.01.004. [DOI] [Google Scholar]
- Silva BP, Bernardo RR, Parentene JP. Clitoriacetal 11-O-β-D-Glucopyranoside from roots of Clitoria fairchildiana. Phytochemistry. 1998;47:121–124. doi: 10.1016/S0031-9422(97)00537-2. [DOI] [PubMed] [Google Scholar]
- Silva BP, Bernardo RR, Parentene JP. Rotenoids from roots of Clitoria fairchildiana. Phytochemistry. 1998;49:1787–1789. doi: 10.1016/S0031-9422(98)00235-0. [DOI] [PubMed] [Google Scholar]
- Silva BP, Parente JP. Antiinflammatory activity of rotenoids from Clitoria fairchildiana. Phytother Res. 2002;16:S87–S88. doi: 10.1002/ptr.807. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am J Enol Viticul. 1965;16:144–158. [Google Scholar]
- Sun T, Ho CT. Antioxidant activities of buckwheat extracts. Food Chem. 2005;90:743–749. doi: 10.1016/j.foodchem.2004.04.035. [DOI] [Google Scholar]
- Thirugnanasampandan R, Mahendran G, Bai VN. Antioxidant properties of some medicinal Aristolochiaceae species. Afr J Biotechnol. 2008;7:357–361. [Google Scholar]