Abstract
Rice bran water extract (RBWE) and ethanol extract (RBEE) at 1.0 mg/ml markedly inhibited the proliferation of LS174T human colon cancer cells. RBEE but not RBWE induced apoptosis. RBWE promoted the production of intestinal mucin (MUC2). Real-time RT-PCR demonstrated that RBWE up-regulated the expression of MUC2 and sucrase-isomaltase complex (a differentiation marker of colon cancer cells), and attenuated that of proliferating cell nuclear antigen at the mRNA level in a dose-dependent manner. These findings suggested that RBWE suppress the proliferation of colon cancer cells by inducting differentiation not apoptosis.
Keywords: Rice bran extract, Intestinal mucin, Colon cancer differentiation, Proliferating cell nuclear antigen
Introduction
Rice bran generated by the milling and polishing of brown rice is a major agricultural waste in Japan. Rice bran is rich in proteins, fibers, minerals and oils, but its consumption is limited. Recently, the biological properties of rice bran including its anti-oxidant (Minamiyama et al. 1994; Srinivasan et al. 2007), anti-dyslipidemia (Kestin et al. 1990; Gerhardt and Gallo 1998; Most et al. 2005) and anti-tumor (Barmes et al. 1983; Verschoyle et al. 2007) activities have attracted much attention.
Intestinal mucin, MUC2, is a glycoprotein composed of a polypeptide chain with a large number of O-linked glycoside side-branches (Winterfold et al. 1999). MUC2 is mainly produced by goblet cells, and plays an important role in protection against colorectal diseases, and indeed the risk of colorectal cancer and experimental colitis was increased in MUC2-deficient mice (Velcich et al. 2002; Van der Sluis et al. 2006). The human colon cancer cell line, LS174T is known to produce MUC2, and the production was promoted by cytokines and sodium butyrate (Iwashita et al. 2003; Hatayama et al. 2007). In the present study, we investigated the effects of rice bran extracts on cell proliferation and MUC2 production in LS174T cells.
Materials and methods
Cell culture
The human colon adenocarcinoma cell line, LS174T was obtained from American Type Culture Collection, and maintained in Dulbeco’s modified Eagle medium (DMEM) supplemented with 10% FCS and antibiotics.
Rice bran extract
The rice bran from Akitakomachi (1 g) was extracted in 50 ml of distilled water or ethanol, and the extracts were collected by centrifugation. The water and ethanol extracts were dried with lyophilization and evaporation, respectively. The extraction procedure yielded 160.7 mg of rice bran water extract (RBWE) and 145.1 mg of rice bran ethanol extract (RBEE).
Periodic acid siff (PAS) staining
PAS staining is popularly used for detecting sugar chains in glycoproteins such as MUC2, and we evaluated the level of MUC2 on LS174T cells by the assay. LS174T cells (1 × 104) were precultured in 24-well plates (1 ml) for 12 h, and subsequently incubated with various concentrations of rice bran extracts or sodium butyrate for 4 days. The cells were fixed with ethanol-acetic acid (3:1), washed with distilled water twice, and treated with 0.5% periodate, Shiff reagent, and 0.6% sodium metabisulfite containing 0.05 N HCl. The morphology and apoptotic appearance of cells were confirmed with Hoechst33258 (Wako Pure Industry Co. Ltd.) under a fluorescence microscope.
RNA extraction and cDNA synthesis
LS174T cells (2.0 × 104) in 6-well plates were precultured in DMEM containing 10% FCS for 12 h, and incubated with or without various concentrations of rice bran extracts or sodium butyrate for 4 days. Total RNA was isolated using a QuickGene RNA cultured cell kit S (FUJIFILM, Co.). Template cDNA was produced with 5 μg of total RNA using the PrimeScript RT reagent Kit (TAKARA BIO, INC.)
Real-time RT-PCR
In a fluorescent temperature cycler (Chromo4; Bio-Rad Laboratories, Inc.), 2.5% of each RT reaction solution was amplified in 25 μl of 1 × SYBR Premix Ex Taq (TAKARA BIO, INC.) containing 0.2 μM of each primer. Samples were incubated in the thermal cycler for an initial denaturation at 95 °C for 10 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s. The oligonucleotide primers used in the experiment are indicated in Table 1. To confirm the amplification of specific transcripts, melting curve profiles (cooling to 60 °C and heating slowly to 95 °C with continuous measurements of fluorescence) were produced at the end of each PCR. The relative level of both mRNAs was normalized to the amount of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA.
Table 1.
Gene specific primers used for quantitative real-time PCR
| Gene name | Forward | Reverse |
|---|---|---|
| GAPDH | 5′-GCACCGTCAAGGCTGAGAAC-3′ | 5′-TGGTGAAGACGCCAGTGGA-3′ |
| GUS | 5′-CATTATTCAGAGCGAGTATGGAGCA-3′ | 5′-TCTTCAGTGAACATCAGAGGTGGA-3′ |
| MUC2 | 5′-CAACCAGCACGTCATCCTGAA-3′ | 5′-GATGCAAATGCTGGCATCAAAG-3′ |
| SI | 5′-GACAACTATGCACGATGGGACAAC-3′ | 5′-TTGCATCCAGCGGGTACAGA-3′ |
| PCNA | 5′-GGCCGAAGATAACGCGGATAC-3′ | 5′-GGCATATACGTGCAAATTCACCAG-3′ |
Statistical analysis
Data are expressed as the mean ± standard deviation (SD). The significance of differences was analyzed using a one-way ANOVA with Dunnett’s multiple comparison test. A value of p < 0.05 was considered significant.
Results and discussion
Differentiation- and apoptosis-inducing activity
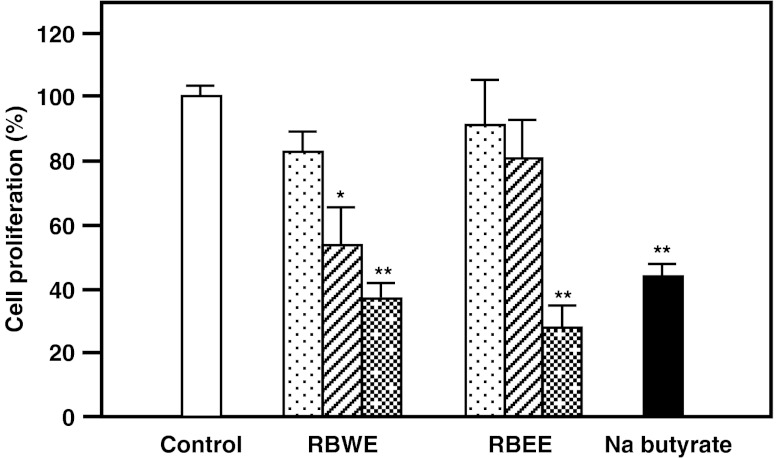
Briefly, we studied the effects of RBWE and RBEE on the proliferation of LS174T cells. 1% (V/V) ethanol used as vehicle control against RBEE, did not affect LS174T cell growth (98.9 ± 5.8%, when compared with those of untreated cells). Sodium butyrate at 1.0 mM (=0.11 mg/ml), known to suppress colon cancer in vitro and in vivo (Dashwooda and Hob 2007), inhibited LS174T cell growth by 43.8%. RBWE suppressed cell growth by 53.4% at 0.1 mg/ml and 37.2% at 1.0 mg/ml, while RBEE at 1.0 mg/ml suppressed it by 28.2% (Fig. 1). We examined whether the anti-proliferative effects of RBWE and RBEE occurred as a result of apoptosis. LS174T cells treated with RBWE or RBEE (final concentration, 1.0 mg/ml) for 48 h were stained with Hoechst 33258. The condensation and fragmentation of nuclei were observed in the RBEE-treated cells (Fig. 2c), but not in untreated cells or the RBWE-treated cells (Fig. 2a and b). These findings show that RBEE inhibited the LS174T cell growth by triggering apoptosis.
Fig. 1.
Anti-proliferative effects of RBWE and RBEE on LS174T cells. Cells (1 × 104) were precultured in DMEM for 12 h, and subsequently incubated alone ( ) or with 0.01 (
) or with 0.01 ( ), 0.1 (
), 0.1 ( ), or 1.0 mg/ml (
), or 1.0 mg/ml ( ) of RBWE or RBEE, or 1.0 mM sodium butyrate (
) of RBWE or RBEE, or 1.0 mM sodium butyrate ( ) for 4 days. Viable cell numbers were measured using the trypan-blue dye. The data are expressed relative to values for untreated control cells and represent the mean ± SD (n = 4, *p < 0.05, **p < 0.01)
) for 4 days. Viable cell numbers were measured using the trypan-blue dye. The data are expressed relative to values for untreated control cells and represent the mean ± SD (n = 4, *p < 0.05, **p < 0.01)
Fig. 2.
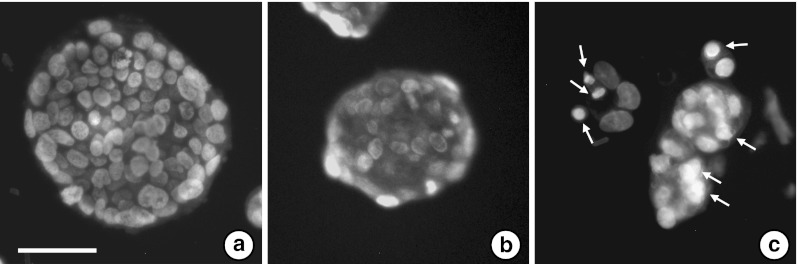
Apoptosis induced by RBEE. Cells (1 × 104) were precultured in DMEM for 12 h, and incubated alone (a) or with 1.0 mg/ml of RBWE (b) or RBEE (c) for 2 days, and the nucleus was stained with Hoechst 33125, and the arrows indicate apoptotic cells. (bar; 40 μm)
Furthermore, we studied the mechanisms involved in the anti-proliferative effect of RBWE on LS174T cells. As our previous study revealed that sodium butyrate promoted production of MUC2 in LS174T cells (Hatayama et al. 2007), we examined the effects of RBWE and RBEE on MUC2 glycoproteins using PAS staining (Fig. 3). MUC2 levels in LS174T cells under normal culture conditions were very low (Fig. 3a), but the proportion of PAS-positive cells increased on incubation with RBWE at 0.1 mg/ml and sodium butyrate at 1.0 mM for 4 days (Fig. 3b and d). However, 0.1 mg/ml of RBEE did not affect MUC2 production in LS174T cells (Fig. 3c).
Fig. 3.
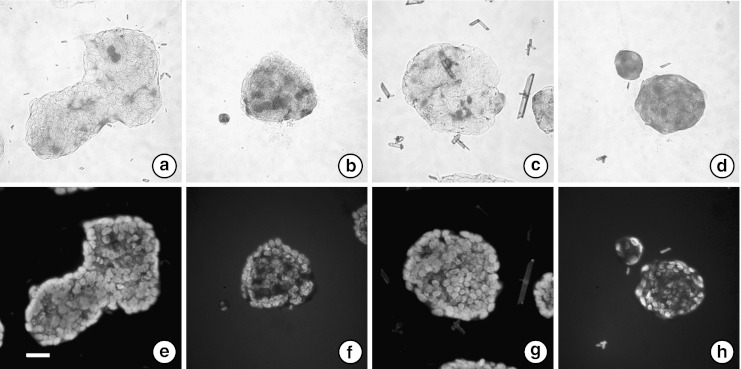
PAS-staining of LS174T cells treated with REWE. Following no treatment (a and e), or treatment with 0.1 mg/ml of RBWE (b and f), 0.1 mg/ml of RBEE (c and g) or 1.0 mM sodium butyrate (d and h) for 4 days, the glycoproteins (MUC2) on cells were visualized by PAS staining, and the cells were counterstained with Hoechst33258 (bar; 40 μm)
Real-time RT-PCR
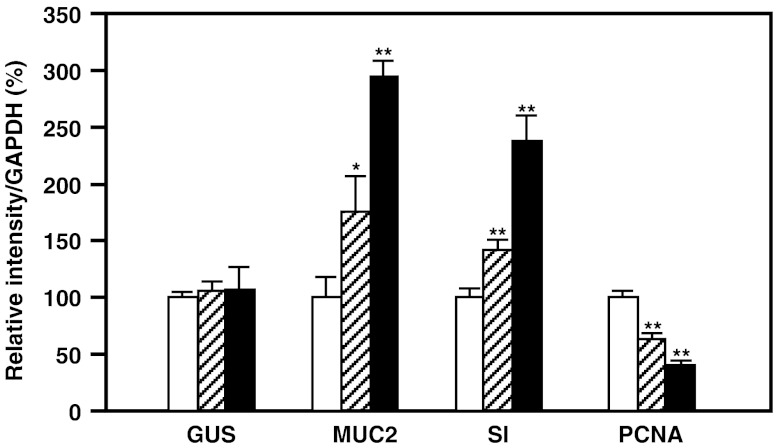
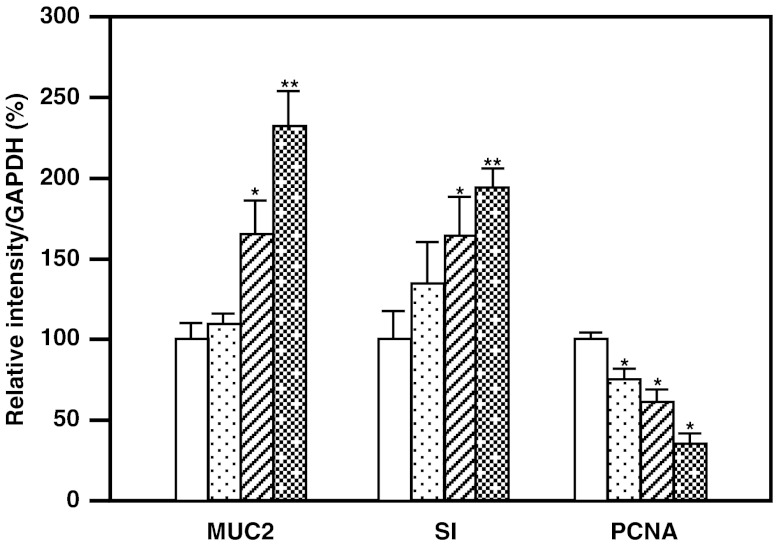
We examined the effects of RBWE on the gene expression of MUC2, sucrase-isomaltase complex (SI), a differentiation marker of colon cancer cells (Celano et al. 1993) and proliferating cell nuclear antigen (PCNA, Fig. 4). Real-time RT-PCR demonstrated that no effect on mRNA expression of β-glucuronidase (GUS). RBWE at 1.0 mg/ml and sodium butyrate at 1.0 mM enhanced MUC2 production in LS174T cells 1.7-fold and 2.9-fold, respectively. SI expression in the cells treated with RBWE and sodium butyrate increased 1.4- and 2.4-fold, respectively. PCNA is a cell-cycle regulator expressed in the nucleus of proliferating cells. In some tumors, the degree of PCNA expression correlates with tumor progression and grade (Zeng and Davis 2003; Hall et al. 1990; Malham et al. 2010). RBWE and sodium butyrate attenuated the PCNA mRNA expression by 63.3% and 40.1%, respectively. Furthermore, we examined the dose-dependent effects of RBWE on MUC2 production and differentiation in LS174T cells (Fig. 5). RBWE at 0.1–1.0 mg/ml enhanced the gene expression of MUC2 and SI, and attenuated PCNA expression at the mRNA level in a dose-dependent manner.
Fig. 4.
Effects of rice bran extracts on the MUC2 gene in LS174T cells. Cells (2 × 104) were precultured in DMEM for 12 h, and subsequently incubated alone ( ), or with 0.1 mg/ml of RBWE (
), or with 0.1 mg/ml of RBWE ( ) or 1.0 mM sodium butyrate (
) or 1.0 mM sodium butyrate ( ) for 4 days. Total RNA was isolated, and the mRNA expression was analyzed with real-time RT-PCR. The data are expressed relative to values for untreated control cells and represent the mean ± SD (n = 3, *p < 0.05, **p < 0.01)
) for 4 days. Total RNA was isolated, and the mRNA expression was analyzed with real-time RT-PCR. The data are expressed relative to values for untreated control cells and represent the mean ± SD (n = 3, *p < 0.05, **p < 0.01)
Fig. 5.
MUC2 gene expression in RBWE-treated LS174T cells. Cells (2 × 104) were precultured in DMEM for 12 h, and subsequently incubated alone ( ), or with 0.01 (
), or with 0.01 ( ), 0.1 (
), 0.1 ( ) or 1.0 mg/ml (
) or 1.0 mg/ml ( ) of RBWE for 4 days. Total RNA was isolated, and the mRNA expression was analyzed with real-time RT-PCR. The data are expressed relative to values for untreated control cells and represent the mean ± SD (n = 3, *p < 0.05, **p < 0.01)
) of RBWE for 4 days. Total RNA was isolated, and the mRNA expression was analyzed with real-time RT-PCR. The data are expressed relative to values for untreated control cells and represent the mean ± SD (n = 3, *p < 0.05, **p < 0.01)
In the present study, we demonstrated that two rice bran extracts suppressed the proliferation of LS174T human colon cancer cells via separate mechanisms. The alcoholic extract induced apoptosis, while the water extract showed cytostatic effects, for example promoting of MUC2 production and cell differentiation. Some phenolic compounds in rice bran such as vitamin E derivatives (Xu et al. 2009) and tricin (Hudson et al. 2000), were found to exhibit apoptotic and/or cytotoxic effects on colon cancer cells in vitro, and these compounds might contribute to apoptotic effects in RBEE. However, the information concerning differentiation inducers of colon cancer is very limited as compared with those that induce apoptosis. We try to isolate the active compounds from rice bran, and investigate signaling mechanisms involved in their differentiation- and apoptosis-inducing activities.
References
- Barmes DS, Clapp NK, Scott DA, Oberst DL, Berry SG. Effects of wheat, rice, corn, and soybean bran on 1,2-dimethylhydrazine-induced large bowel tumorigenesis in F344 rats. Nutr Cancer. 1983;5:1–9. doi: 10.1080/01635588309513772. [DOI] [PubMed] [Google Scholar]
- Celano P, Berchtold CM, Mabry M, Carrol M, Sidransky D, Casero RA, Jr, Lupu R. Induction of markers of normal differentiation in human colon carcinoma cells by the V-rasH oncogene. Cell Growth Differ. 1993;4:341–347. [PubMed] [Google Scholar]
- Dashwooda RH, Hob E. Dietary histone deacetylase inhibitors: from cells to mice to man. Semin Cancer Biol. 2007;17:363–369. doi: 10.1016/j.semcancer.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt AL, Gallo NB. Full-fat rice bran and oat bran similarly reduce hypercholesterolemia in humans. J Nutr. 1998;128:865–869. doi: 10.1093/jn/128.5.865. [DOI] [PubMed] [Google Scholar]
- Hall PA, Levision DA, Woods LA. Proliferating cell nuclear antigen (PCNA) immunolocalization in paraffin sections: an index of cell proliferation with evidence of deregulated expression in some neoplasms. J Pathol. 1990;162:285–294. doi: 10.1002/path.1711620403. [DOI] [PubMed] [Google Scholar]
- Hatayama H, Iwashita J, Kuwajima A, Abe T. The short chain fatty acid, butyrate, stimulates MUC2 mucin production in the human colon cancer cell line LS174T. Biochem Biophys Res Commun. 2007;356:599–603. doi: 10.1016/j.bbrc.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Hudson EA, Dinh PA, Kokubun T, Simmonds MS, Gescher A. Cancer epidemiology. Biomark Prev. 2000;9:1163–1170. [PubMed] [Google Scholar]
- Iwashita J, SatoY SH, Takahashi N, Sasaki H, Abe T. mRNA of MUC2 is stimulated by IL-4, IL-13 or TNF-a through a mitogen-activated protein kinase pathway in human colon cancer cells. Immunol Cell Biol. 2003;81:275–282. doi: 10.1046/j.1440-1711.2003.t01-1-01163.x. [DOI] [PubMed] [Google Scholar]
- Kestin M, Moss R, Clifton PM, Nestel PJ. Comparative effects of three cereal brans on plasma lipids, blood pressure, and glucose metabolism in mildly hypercholesterolemic men. Am J Clin Nutr. 1990;52:661–666. doi: 10.1093/ajcn/52.4.661. [DOI] [PubMed] [Google Scholar]
- Malham P, Husain N, Bhalla S, Gupta RK, Husain M. Proliferating cell nuclear antigen, p53 and micro vessel density: Grade II vs. Grade III astrocytoma. Indian J Pathol Microbiol. 2010;53:20–23. doi: 10.4103/0377-4929.59177. [DOI] [PubMed] [Google Scholar]
- Minamiyama Y, Yoshikawa T, Tanigawa T, Takahashi S, Naito Y, Ichikawa H, Kondo M. Antioxidative effects of a processed grain food. J Nutr Sci Vitaminol (Tokyo) 1994;40:467–477. doi: 10.3177/jnsv.40.467. [DOI] [PubMed] [Google Scholar]
- Most MM, Tulley R, Morales S, Lefevre M. Rice bran oil, not fiber, lowers cholesterol in humans. Am J Clin Nutr. 2005;81:64–68. doi: 10.1093/ajcn/81.1.64. [DOI] [PubMed] [Google Scholar]
- Srinivasan M, Sudheer AR, Meon VP. Ferulic acid: therapeutic potential through its antioxidant property. J Clin Biochem Nutr. 2007;40:92–100. doi: 10.3164/jcbn.40.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Sluis M, De Koning BAE, De Bruijn ACJM, Velcich A, Meijerink JPP, Van Goudoever JB, Buller HA, Dekker J, Van Seuningen I, Renes IB, Einerhand AWC. Muc2-deficient mice spontaneously develop colitis, indicating that Muc2 is critical for colon protection. Gastroentelogy. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Velcich A, Yang W, Hayer J, Fragale A, Nicholas C, Viani R, Kucharpati R, Lipkin M, Yang A, Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin. MUC2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- Verschoyle RD, Greaves P, Cai H, Edwards RE, Steward WP, Gescher AJ. Evaluation of the cancer chemopreventive efficacy of rice bran in genetic mouse models of breast, prostate and intestinal carcinogenesis. Br J Cancer. 2007;96:248–254. doi: 10.1038/sj.bjc.6603539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterfold CM, Walsh MD, Leggett BA, Jass JR. Ultrastructural localization of epithelial mucin core proteins in colorectal tissues. J Hisotochem Cytochem. 1999;47:1063–1074. doi: 10.1177/002215549904700811. [DOI] [PubMed] [Google Scholar]
- Xu WL, Liu JR, Liu HK, Qi GY, Sun XR, Sun WG, Chen BQ. Inhibition of proliferation and induction of apoptosis by gamma-tocotrienol in human colon carcinoma HT-29 cells. Nutrition. 2009;25:555–566. doi: 10.1016/j.nut.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Zeng H, Davis CD. Down-regulation of proliferating cell nuclear antigen gene expression occurs during cell cycle arrest induced by human fecal water in colonic HT-29 cells. J Nutr. 2003;133:2682–2687. doi: 10.1093/jn/133.8.2682. [DOI] [PubMed] [Google Scholar]