Abstract
Although there are numerous decades-old studies drawing attention to the presence of aflatoxins in spices, and particularly in red pepper spice, the problem has not been eradicated. In the present study, information presented in the literature, about production method of red pepper spice, its contamination with aflatoxin, and the uncertainty about the data are assessed to find out the points where improvement may be achieved. Failure Mode and Effect Analysis (FMEA) are performed to assess the risk. The highest total risk attributable to chemical plus physical plus biological causes is associated with the washing stage (RPN=363), which is followed by the receiving (RPN=342) and the storage (RPN=342) stages. The highest risk attributable to biological causes (RPN=180) is associated with microbial growth and aflatoxin production due to insufficient control of drying conditions. The highest chemical risk (RPN=144) is found for the presence of unintentional food additives, such as pesticides, herbicides, hormones, and heavy metals in fresh red pepper fruits. EWMA (exponentially weighted average) charts are employed to monitor aflatoxin production during storage. They successfully distinguished between the batches, which turned to be unsafe. Risk associated with unintentional additives may be reduced by using certified additives only. Better drying control will definitely reduce the risk associated with the drying process. Codex Alimentarius plan has worldwide acceptance for assessing safety of the nuts. Risk of accepting the batches contaminated with aflatoxin may be eliminated by applying the Codex Alimentarius sampling plan before putting the dry pulverized red pepper into the storage facility.
Keywords: Pulverized red pepper, FMEA analysis, Aflatoxin, Codex Alimentarius sampling plan, EWMA chart, Accelerated spoilage
Introduction
Red Pepper spices are made from ground dried fruits of Capsicum family. These fruits are commonly known as chili peppers and have fiery red or orange pods. Red pepper spices are used to achieve the characteristic hot flavor of Mexican, Creole, Cajun, Thai, and Chinese, Indian, Korean, and Turkish foods. They are used in chili, Spanish rice and barbecue sauce, meats, salads and casseroles as well as Adana and Gaziantep kebabs. Turkey is one of the leading producers of red peppers; 60,000 tons of red peppers were produced for the spice industry in Turkey in 2008; production was estimated to be 94,750 tons in 2009 (TurkStat 2010). Most of the red pepper spices reach to consumers as pulverized dry product in 50 to 200 g packages. Red peppers are subject to fungal growth both during drying or subsequent storage process. There are publications reporting presence of aflatoxins in both red pepper and other spices offered to public (Omurtag et al. 1998; Reddy et al. 2001; Erdogan 2004; Hayoglu et al. 2005; Aydin et al. 2006; O’Riordan and Wilkinson 2009). These findings indicate that there is an urgent need for improvement of the quality control procedures for the production and storage stages of these products.
The quality of the peppers is controlled when they arrive to the spice production facility; then they are washed to remove dust and soil, stalks, and seeds. Peppers that are not good for spice production are discarded. Peppers are sliced; cellular water is drained by keeping the slices in the nets for 24 h, and then dried in tunnel driers at 70 to 75 °C for about 90 min. Control of the drying conditions is crucial to prevent production of aflatoxins at this stage of the process. The pulverized bell pepper is produced after breaking the dry slices in the hammer mill (Hayoglu et al. 2005). Retailers may test the presence of the aflatoxins in bulk before buying them by using the Codex Alimentarius sampling plan (Anon 2008a). Pulverized bell pepper is usually put into small, e.g. 50 to 200 g cellophane packages by the retailers to be sold in the markets. Among all the spices, pulverized red pepper is second to salt in terms of consumers’ preference in Turkey. A major fraction of the red pepper spice goes to the restaurants in 10 g paper packages, which is expected to be subject to more spoilage than the other retail packages, since paper is permeable to moisture and does not provide good barrier against environmental mishandling.
Failure Mode and Effect Analysis (FMEA) is a widely used tool for quality assurance in many manufacturing industries, addressing customer and governmental requirements, quality control and safety. It is a systematic preventive method used to define, identify and eliminate the potential product failures from the process. Highly comprehensive studies have recently appeared in the literature to demonstrate application of the FMEA analysis to a corn curl manufacturing plant (Varzakas and Arvanitoyannis 2007), chocolate-producing industry (Arvanitoyannis and Savelides 2007), smoked trout production process (Arvanitoyannis et al. 2009), industrial processing of common octopus (Arvanitoyannis and Varzakas 2009) and ready to eat vegetables (Varzakas and Arvanitoyannis 2009). In FMEA analysis, potential risks of a process are assessed by defining the value for frequency of occurrence of a failure (O), seriousness of the failure (S) and ability to detect the failure (D) before consumption. Values of three variables are multiplied (OxSxD) to calculate the Risk Priority Number (RPN) for each failure. Corrective actions are suggested based on the results of risk assessment to reduce and eliminate the potential failures from the system (Scipioni et al. 2002; Arvanitoyannis and Varzakas 2007a; Arvanitoyannis and Varzakas 2007b; Arvanitoyannis and Varzakas 2008; Ozilgen 2010). In the present study FMEA analysis of the red pepper spice processing and storage is performed. Critical points where failure of the hazard control may be lost are pointed out and the precautions needed to detect and prevent the hazards are explained.
Building quality control charts are one of the most important techniques in understanding of the type of variation that exists in the process. In EWMA charts, the decision depends on statistic zi, which is an exponentially weighted average of all the prior data, including the most recent measurement.
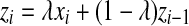 |
1 |
Statistic zi-1 represents the entire history of the measurements since it carries the inherent effect of zi-2 and all of the prior statistics zi-3 …. z0. The weighting factor, λ, determines the rate at which ‘older’ data enter into the calculation of the new statistic zi (Singh 2006). The starting value for the EWMA chart, z0, may be set equal to the population mean; μ. A large value of λ gives more weight to recent data and less weight to older data; a small value of λ gives more weight to the older data and less weight to the recent data. The value of λ is usually set between 0.2 and 0.3 although this choice is somewhat arbitrary (Singh 2006). The UCL, CL, and LCL for the EWMA chart are calculated as (Singh 2006):
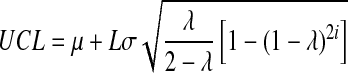 |
2 |
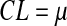 |
3 |
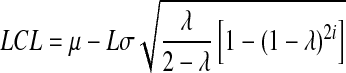 |
4 |
Where LCL and UCL are calculated individually for every zi. Storage is regarded as “absolutely successful” if no aflatoxin is produced. If this goal cannot be achieved confining the growth of aflatoxin below the maximum acceptable limit is considered satisfactory.
Collecting the data for the construction of quality control charts is a long and tedious work. In most cases, these experiments may take unrealistically long periods. Accelerated shelf life studies are performed under such experimental conditions where the chemical, biological, or physical phenomena, which cause the expiration of the shelf life, are accelerated with acceptable accuracy (Lee and Krocta 2002; Achour et al. 2001; Hough et al. 2006; Martins et al. 2005; Jha et al. 2010; Agrahar-Murugkar and Jha 2011). The most common methods of the spoilage acceleration use either elevated temperatures or elevated water activity or both during storage experiments (Gqaleni et al. 1996; Bommakanti and Waliyar 2000; Samapundo et al. 2006; Saad 2009). In this study, experimental data, which are collected with accelerated testing methods, are either obtained in our laboratory or obtained from the literature and EWMA charts are constructed to visualize the quality changes of the solid-pulverized red pepper spice during storage.
Materials and methods
The risk priority numbers (RPN) were calculated from three variables, O, S, and D, of failures to identify the risk level of each potential failure. A numerical ranking for variables O, S, and D of failures was established by taking total of the physical, chemical and biological risk into consideration. Biological risk was assessed by considering the epidemiological studies and the best expert opinion about the similar ingredients or processes as a base. Although aflatoxin is a chemical, it is produced by microorganisms; therefore aflatoxin production is considered as a risk attributable to biological causes. Each failure mode was identified whether or not the failure is likely to occur on a scale of 1–10. The highest ranking indicated the greatest probability of the failure to occur. The possibility to detect the failure before occurring and the cost of the failure to the consumer were also rated on a scale of 1–10, where the numbers increase as it becomes more difficult to detect the failure before occurring, or as the potential damage of the failure increases. The risk priority numbers were calculated by multiplying the values of the variables O, S, and D. Possible corrective actions were suggested for each potential failure mode with risk priority numbers higher than 120. At these points, The RPNs after the corrective actions were recalculated to understand the influence of corrective actions on the improvement of the process (Table 1). Pareto diagrams (Fig. 1), was constructed by following the same procedure as Arvanitoyannis and Varzakas (2007a).
Table 1.
Application of failure mode and effect analysis to pulverized red pepper production
| Processing stage: Receiving fresh red peppers | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Failures and Cause | O | S | D | RPN | Corrective actions | O | S | D | RPN after corrective actions |
| Biological Hazards | |||||||||
| Microbial contamination due to the unsanitary conditions of the receiving environment | 6 | 5 | 1 | 30 | – | – | – | – | – |
| Microbial contamination due to improper handling | 6 | 5 | 1 | 30 | – | – | – | – | – |
| Microbial growth due to prolonged storage at room temperature | 5 | 8 | 3 | 120a | Refrigerate perishable foods right after receiving | 2 | 8 | 2 | 32 |
| Chemical Hazards | |||||||||
| Presence of unintentional food additives such as pesticides, herbicides, hormones, heavy metal, etc. | 6 | 8 | 3 | 144a | Use certified ingredients only. Supplier must be reliable. Reject the contaminated lot. | 2 | 9 | 3 | 54 |
| Physical Hazards | |||||||||
| Presence of foreign materials like dust and soil | 6 | 3 | 1 | 18 | – | – | – | – | – |
| Processing stage: Washing to remove dust and soil | |||||||||
| Biological | |||||||||
| Microbial contamination from water- | 5 | 8 | 3 | 120a | Use water from the local authority. Ensure the standards of the pipelines and connectors Sewage and manure control is required. | 2 | 8 | 3 | 48 |
| Chemical | |||||||||
| Uncontrolled chlorine concentration in water | 5 | 8 | 3 | 120a | Use a potable water from the local authority | 2 | 7 | 3 | 42 |
| Metal contamination in water from the worn pipeline connections | 5 | 7 | 3 | 105 | – | – | – | – | – |
| Physical Hazards | |||||||||
| Foreign matter | 6 | 3 | 1 | 18 | – | – | – | – | – |
| Processing stage: Removing the stalks, seeds, green or unsuitable peppers | |||||||||
| Failures and Cause | O | S | D | RPN | Corrective actions | O | S | D | RPN after corrective actions |
| Biological Hazards | |||||||||
| Microbial contamination due to the unsanitary conditions of the processing environment | 6 | 5 | 1 | 30 | – | – | – | – | – |
| Chemical Hazards | not common | ||||||||
| Physical Hazards | |||||||||
| Foreign matter | 6 | 3 | 1 | 18 | – | – | – | – | – |
| Processing stage: Slicing | |||||||||
| Biological Hazards | |||||||||
| Contamination from equipment and improper handling | 6 | 5 | 1 | 30 | – | – | – | – | – |
| Chemical Hazards | not common | ||||||||
| Physical Hazards | |||||||||
| Foreign matter | 6 | 3 | 1 | 18 | – | – | – | – | – |
| Processing stage: Drying | |||||||||
| Biological | |||||||||
| Contamination from the environment | 5 | 2 | 7 | 70 | – | – | – | – | – |
| Microbial growth due to inappropriate control of process parameters-temperature and time control | 6 | 9 | 3 | 162a | Drying process parameters- time and temperature- must be optimized and controlled during the process | 2 | 7 | 2 | 28 |
| Chemical | not common | ||||||||
| Physical | |||||||||
| Foreign materials, i.e., dust coming from the environment | 4 | 3 | 2 | 24 | – | – | – | – | – |
| Processing stage: Grinding | |||||||||
| Failures and Cause | O | S | D | RPN | Corrective actions | O | S | D | RPN after corrective actions |
| Biological | |||||||||
| Contamination from equipment and improper handling | 6 | 5 | 1 | 30 | – | – | – | – | – |
| Chemical | not common | ||||||||
| Physical | |||||||||
| Foreign materials, i.e., dust coming from the environment | 4 | 3 | 2 | 24 | – | – | – | – | – |
| Processing stage: Packaging | |||||||||
| Biological | |||||||||
| Microorganisms from the unclean packaging material | 3 | 6 | 3 | 54 | – | – | – | – | – |
| Chemical | |||||||||
| Migration of chemicals from the non-food grade packaging materials | 4 | 8 | 4 | 128a | Use food grade packaging materials only | 2 | 8 | 2 | 32 |
| Physical Hazards | |||||||||
| Foreign materials | 4 | 3 | 2 | 24 | – | – | – | – | – |
| Processing stage: Storage | |||||||||
| Biological | |||||||||
| Mold growth on dried red pepper due to improper storage conditions—time, temperature and relative humidity abuse | 6 | 9 | 3 | 162a | Drying process parameters- time and temperature- must be optimized. Temperature and humidity of the storage area must be optimized and controlled regularly. Regular sanitation of the storage area is required. | 2 | 7 | 2 | 28 |
| Chemical | |||||||||
| Aflatoxin production in foods by molds | 5 | 9 | 4 | 180a | Drying process parameters- time and temperature- must be optimized. Temperature and humidity of the storage area must be optimized and controlled regularly. Avoid extended storage time | 2 | 9 | 3 | 54 |
| Physical Hazards | not common | ||||||||
acorrective actions are required since RPN is above 120
O: frequency of occurrence for each failure
S: seriousness of the failure to the consumer
D: possibility of detecting the failure
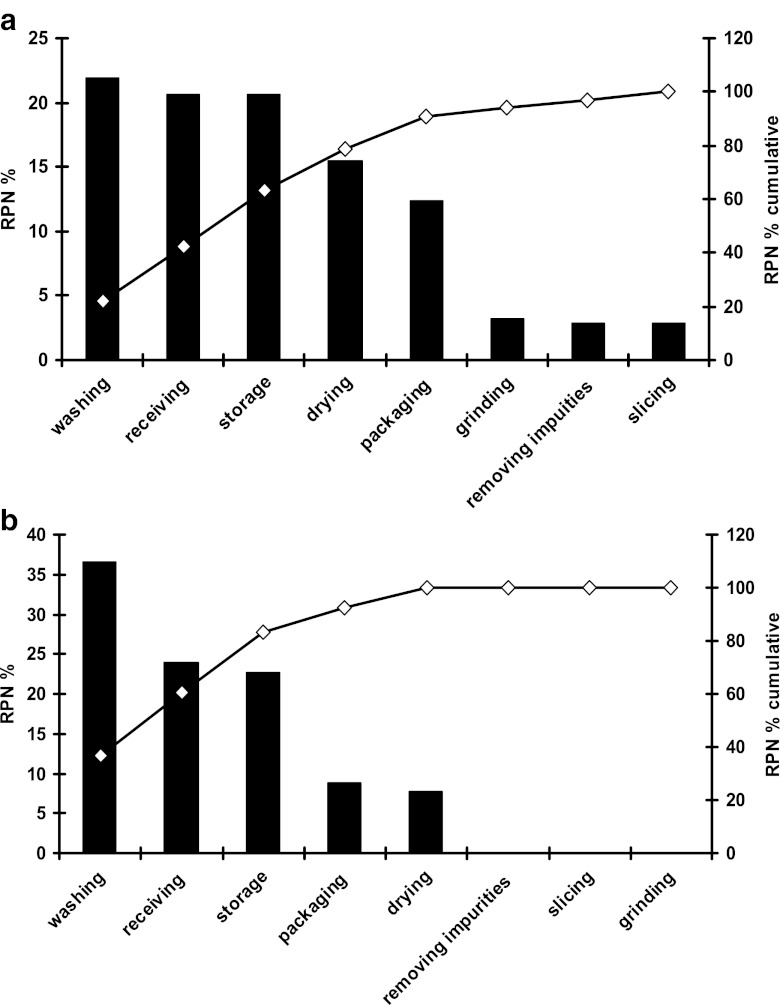
Fig. 1.
Pareto diagram for total risk classification, including chemical, physical, and biological risks, of pulverized red pepper processing a prior to corrective actions, b after corrective actions
Results from the aflatoxin measurements were used to calculate statistic zi as given in Eq. 1. MATLAB (MathWorks, version 2009a, USA) was used for computations and construction of the EWMA charts using Eqs. 1–4. Four sets of data were employed to test the application of the EWMA charts. Data, which are given in Figs. 2 and 3, are obtained in our laboratories. During the accelerated shelf life tests aflatoxin was extracted from10 g of ground red pepper samples as described by O’Riordan and Wilkinson (2009) and measured with HPLC as described by Martins et al. (2005). Data, which are presented in Figs. 4 and 5 were obtained from the literature (Karagöz 2001). Uncertainty of the data has been assessed as described by Augustine and Minvielle (2008) and O’Riordan and Wilkinson (2009). Possible failure modes in the process were identified and the potential hazards for each failure mode were analyzed.
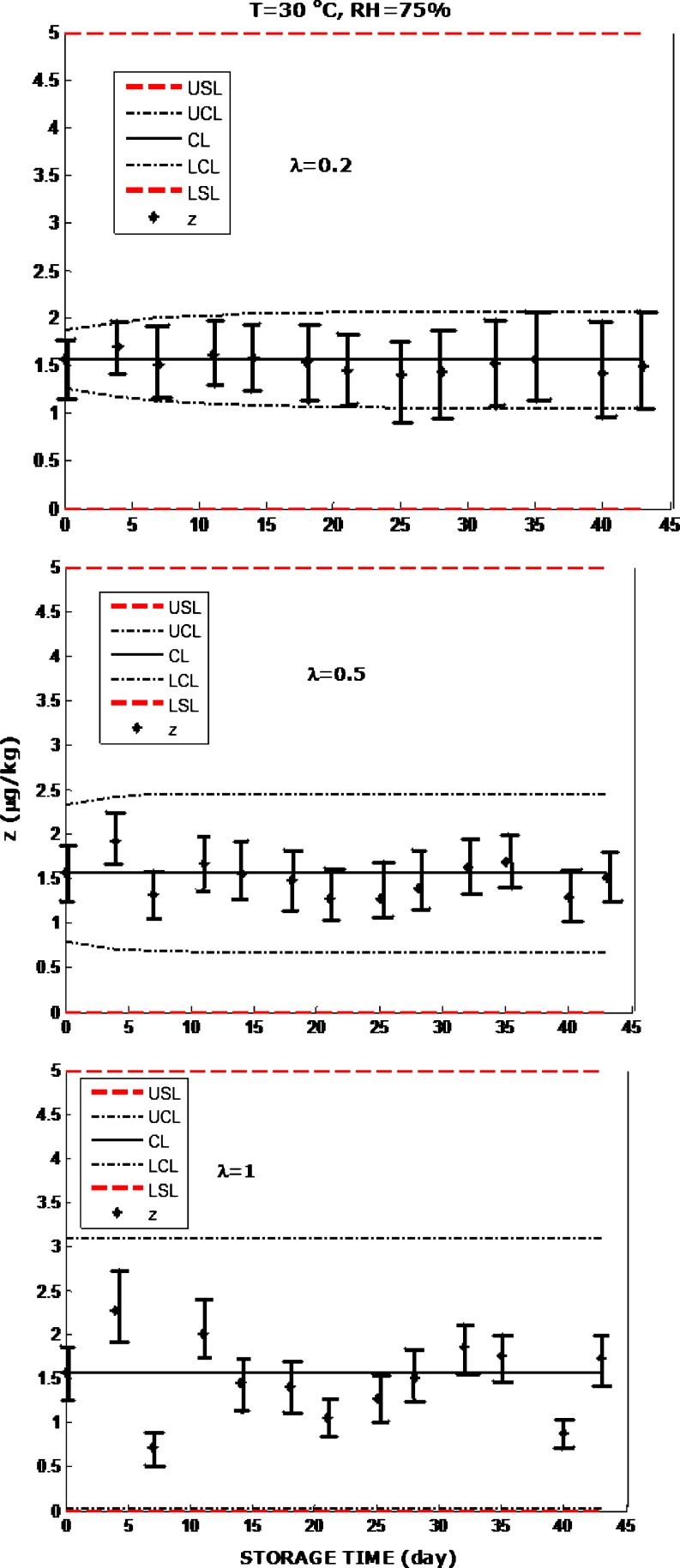
Fig. 2.
EWMA charts constructed with the data obtained during storage of the red pepper spice at 30 °C with 75% RH. Parameter λ=0.2 (top), λ=0.5 (middle), λ=1 (bottom). The spice was produced by a prominent Turkish spice company, marketed in 250 g cellophane packages, and expected to be subject to appropriate sampling and aflatoxin detection tests before reaching the consumer. Error bars were constructed as described by Augustine and Minvielle (2008) and O’Riordan and Wilkinson (2009)
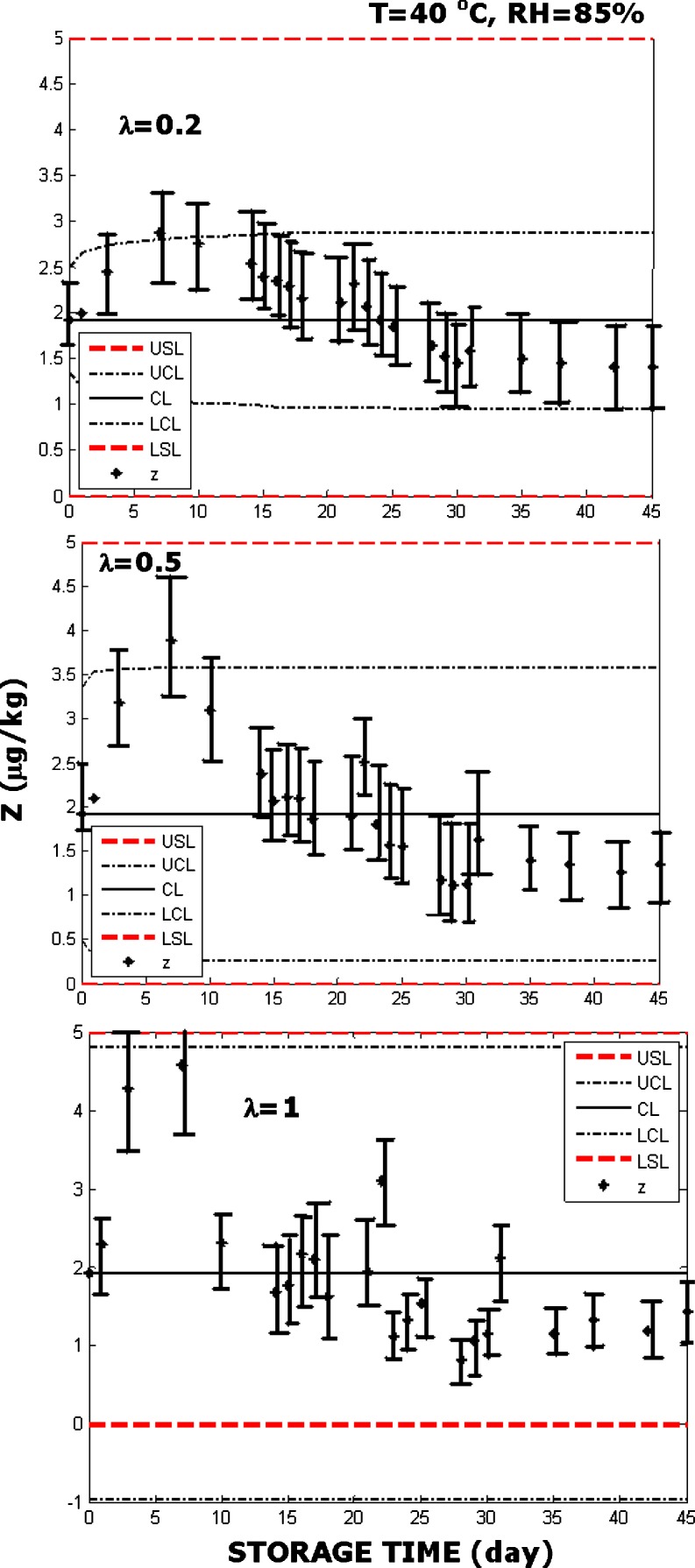
Fig. 3.
EWMA charts constructed with the data obtained during storage of the red pepper spice at 40 °C with 85% RH. Parameter λ = 0.2 (top), λ = 0.5 (middle), λ = 1 (bottom). Parameter λ = 0.2 (top), λ = 0.5 (middle), λ = 1 (bottom). The spice was purchased in a Turkish franchise of an international company, marketed in bulk, and retailed packaged in amounts requested by the consumers. The spice is expected to be subject to appropriate sampling and aflatoxin detection tests before reaching the consumer. Error bars were constructed as described by Augustine and Minvielle (2008) and O’Riordan and Wilkinson (2009)
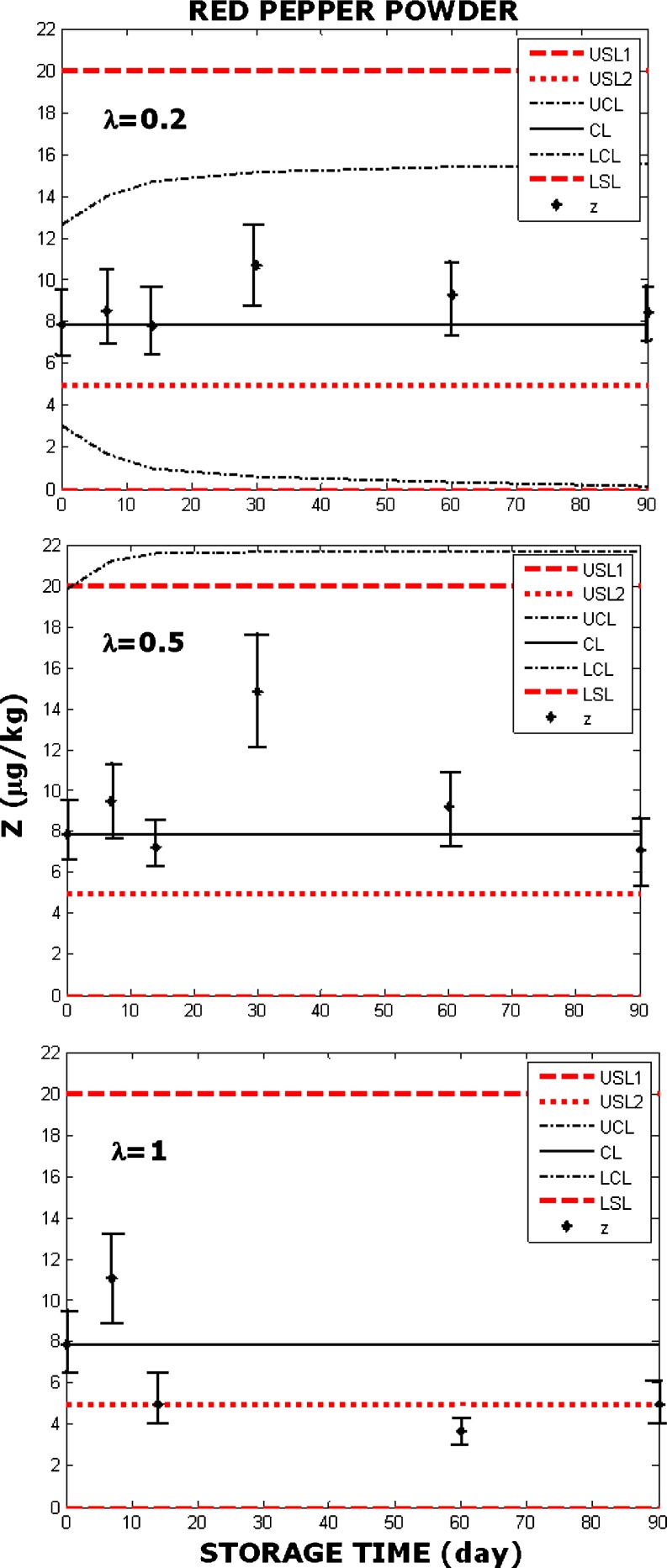
Fig. 4.
EWMA chart constructed with powdered red pepper according to the USL defined in Chinese or American standards (USL1) and Turkish standards (USL2). Parameter λ=0.2 (top), λ=0.5 (middle), λ=1 (bottom). Original data was adapted from Karagöz (2001). Karagöz (2001) obtained crushed red pepper and pulverized red pepper samples from the local market in Ankara, Turkey. Autoclaved them for 15 min at 121 °C, than contaminated them with 105 spores/g of Aspergilus flavus spores and measured the amounts of the aflatoxins at 7th, 14th, 30th, 60th and 90th days. The samples employed in this study were 50 g each, stored at room temperature (25 °C) and at 50% RH. Aflatoxin measurements were average of values obtained with four replicas. Karagöz (2001) reported no correlation between aflatoxin measurements and Aspergilus flavus colony counts. Error bars were constructed as described by Augustine and Minvielle (2008) and O’Riordan & Wilkinson (2009)
Fig. 5.
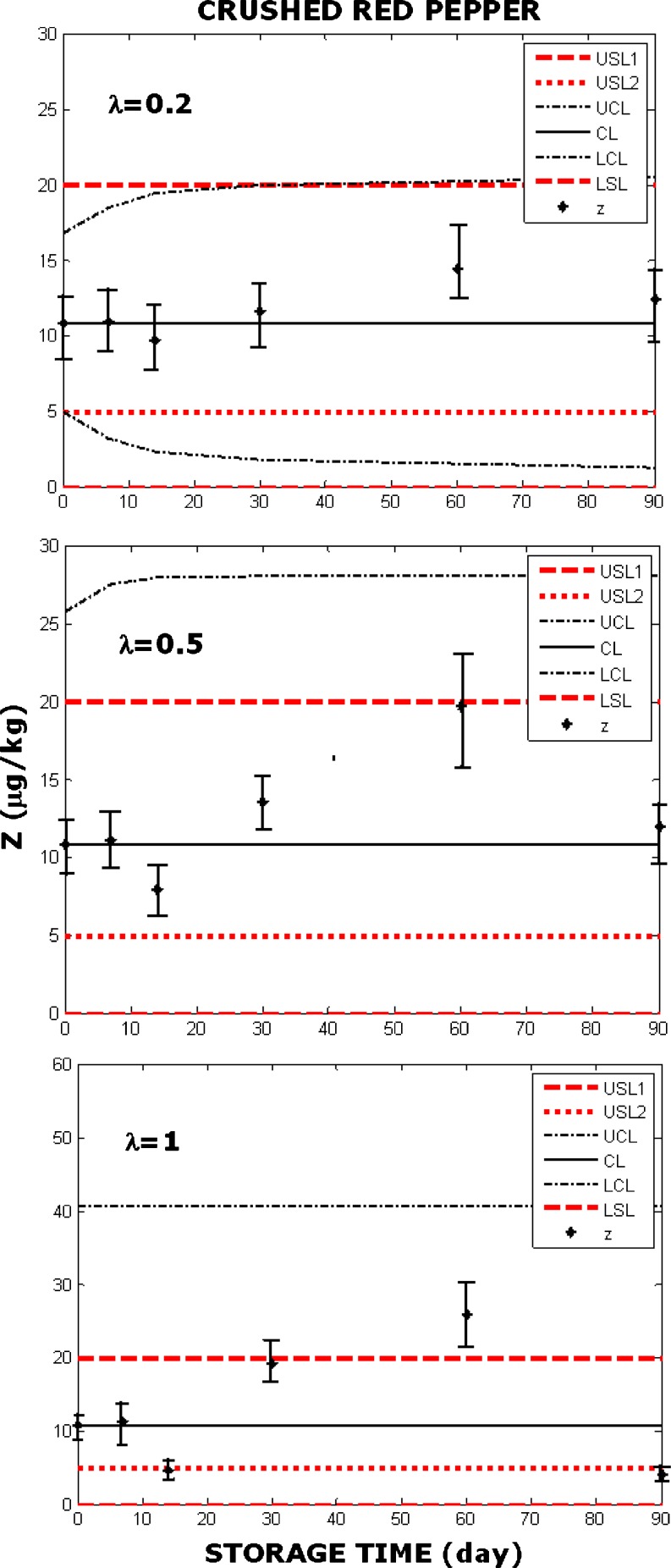
EWMA chart constructed with crushed red pepper according to the USL defined in Chinese or American standards (USL1) and Turkish standards (USL2). Parameter λ=0.2 (top), λ=0.5 (middle), λ=1 (bottom). Original data was adapted from Karagöz (2001) and obtained under the conditions as explained in Fig. 4. Error bars were constructed as described by Augustine and Minvielle (2008) and O’Riordan and Wilkinson (2009)
Results and discussion
Percentage of the risk priority numbers (RPNs) associated with the total (chemical plus physical plus biological) risk, associated with each stage of the process are detailed in Table 1. The highest total risk (RPN=363) was associated with the washing stage, which is followed by the receiving (RPN=342), storage (RPN=342), drying (RPN=256), packaging (RPN=206), grinding (RPN=54), removing stalks etc. (RPN=48) and slicing (PRN=48) stages. Percent contribution of the RPN of each stage to that of the total process is depicted with a Pareto diagram (Fig. 1a). After taking the corrective actions as described in Table 1, both the magnitude and the order of the RPNs of each stage have changed (Fig. 1b). The highest total risk (RPN=90) was associated with washing, which was followed by receiving (RPN=86), storage (RPN=82), packaging (RPN=32) drying (RPN=28) stages.
The highest RPN attributable to biological causes before the corrective actions was associated with aflatoxin production (RPN=180) and yeast and mold growth (RPN=162) in storage due to inappropriate storage conditions. The second highest RPN associated with microbial growth and aflatoxin growth is due to insufficient control of the drying conditions (RPN=162) during production of the bell pepper spice. The highest RPN number attributable to chemical causes before taking the corrective actions is found to be associated with the presence of unintentional food additives, such as pesticides, herbicides, hormones and heavy metals in the fresh red pepper fruit (RPN=144). Unintentional addition of heavy metals, pesticides, and herbicides into foods and the migration of chemicals from the packaging materials are the possible chemical contamination risks at different stages of processing. Potential sources of these contaminants in the process need to be detected and removed from the process since prolonged exposure to these chemicals might have severe effects on human health (Table 1). For example, neurological disorders, Alzheimer disease, Parkinson's disease, cancer, low-birth-weight are some of the reported effects of heavy metal poisoning on human health (Muhammad et al. 2009). The most significant sources of toxic metals to the food chain are the soil, water, environmental pollution, and equipment used for processing. Although the use of pesticides is controlled by legislation, pesticide contamination is widespread especially in developing countries due to indiscriminate usage and lack of control. Prolonged exposure to pesticides and herbicides can result in soft tissue, brain, lung, liver, digestive system and urinary tract cancers as well as birth defects and damage to the nervous systems. Exclusively using certified raw ingredients, supplying water from the local authority, ensuring the standards of water pipelines and connectors, and using only food-grade packaging materials will minimize the chemical contamination risks at many stages of the process.
Drying and storage stages were the most critical stages in the process. Mycotoxins are extremely toxic by-products of mold growth. Aflatoxins have received greater attention than any other mycotoxins since it has severe carcinogenic and mutagenic affects on human health. Erdogan (2004), while reviewing the literature, reported that aflatoxin production in red peppers is associated with presence of Aspergillus species, e.g., A.fumigatus, A. flavus, A. niger, A. ochraceus in most pepper samples. Although it is almost not possible to achieve absolute safety, many countries limit exposure to mycotoxins by imposing regulatory limits on commodities to minimize the potential risk from the ingestion of food products. Relative humidity, environmental temperature, moisture content of foods, and the number of insects in the environment need to be managed during production, processing, transportation, and storage of foods to avoid mold growth and mycotoxin production (van Egmond 2004; Ozyaral et al. 2007; Bircan et al. 2008; Chulze 2010). Drying process parameters, time and temperature, needs to be optimized and controlled during the process. Application of hygiene rules and regulations at place, staff education about food safety, and necessary temperature controls throughout the process will decrease the microbial load on final food product (Table 1).
Ahn et al. (2010) while working on the occurrence of ochratoxin A in Korean red paprika pointed to the fact that the toxin production may occur at any stage of transportation, production or storage, and recommended that all the possible measures should be taken at all stages to prevent the production of the toxin. The entire process from farm to fork is considered in our analysis and it is found that the risk of aflatoxin production was associated mainly with storage, where RPN was calculated to be 180 before and 54 after corrective actions. The second highest risk attributable to biological reasons before corrective actions, as indicated with RPN=162 in Table 1, is associated with the drying process, therefore better drying control may reduce the risk. The dried and pulverized red pepper spices are traded in bulk. Codex Alimentarius (Anon 2008a) includes an internationally accepted sampling plan for aflatoxin detection in almonds, Brazil nuts, hazelnuts and pistachios. It is the most widely used sampling plan employed for testing the safety of treenuts. This plan is designed to make decisions about acceptance of large masses of commodities. It determines the number and weights of the increments to bring together 20 kg of sample from tons of treenuts. The sample is ground, well mixed and its aflatoxin content is analytically determined. If the treenuts are destined for further processing, they are required to have less than 15 μg/kg of aflatoxin, in case of consumption without further processing the limit becomes 8 μg/kg. Codex Alimentarius sampling plan is also used with other nut-like foods, including spices, when a better alternative is not available. In the Turkish Food Codex upper aflatoxin specification limit (USL) for spices including dried, whole or pulverized red peppers is 5.0 μg/kg for aflatoxins B1 and 10 μg/kg for the sum of B1, B2, G1 and G2 (Anon 2008b). The USL is 20 μg/kg in the US and China, and 2 μg/kg in EU. Other countries adapt USLs ranging between those of the US and the EU (Liu et al. 2006). In red pepper spice production process, if the pulverized bell pepper is purchased from another producer, then its safety may be assessed at after grinding. Since Codex Alimentarius has gained international acceptance and is being used with other foods there is no need to question its reliability at this point.
A product, which is accepted as safe and put into storage facility, may turn to be unsafe during storage under unfavorable conditions. Millions of small dried, pulverized bell pepper packages may spend some time in storage facilities before reaching the consumer. Therefore, there is a need for reassurance of safety of the stored products. Aflatoxin determination in packed red pepper spice is done with destructive techniques, where the packages are taken randomly from the shelves, opened; the red peppers are ground, extracted and discarded after chemical analysis. Due to the very large number of the packages dealt with and destructive nature of the techniques employed in quality control, it is not possible to test every package for the presence of aflatoxin during this period; therefore, the tests are needed to be carried out on the samples. The sample size is usually much smaller than the total number of the packages on the shelves in order to reduce the cost of the analysis. In liquid foods, the probability of detecting the microbial contamination with random sampling is large, since it is easy for the microorganisms to travel around and grow homogeneously. On the other hand, the movement of the contaminated solid particles or the toxin-producing microorganisms is restricted in solid foods; which increases the repeatability variance in aflatoxin measurements and reduces the possibility of detecting contamination with randomly taken samples. In the present study, all the replicate aflatoxin measurements with the extracts from the same sample usually gave the same results. Therefore, it may be concluded that the fluctuations between different samples were caused by either a real increase or decrease in the aflatoxin content of the entire stored product, or because of the inhomogeneous nature of the simulated packages.
The most common methods of the spoilage acceleration are to use either elevated temperatures or elevated water activity or both during storage experiments. In this study, EWMA charts and accelerated shelf life testing are applied in quality control of solid-pulverized red pepper during storage. Since there were no previously established EWMA charts available in the literature for pulverized red pepper storage, the CL, UCL, and LCL of the control charts were calculated both from our own data and from Karagöz’s data (Karagöz 2001) by using Eq. 2–4. The USL was established by the Turkish Food Codex or Chinese and US standards (Anon 2008b; Liu et al. 2006).
Weighting factor, λ, of Eqs. 1–4 allows adjusting the sensitivity of the EWMA charts to sudden shifts in measurements. Three different values of λ (0.2, 0.5, and 1) were tested in the present study. Setting λ=1 employs the most recent data only and yields a Shewhart control chart. A small value of λ gives more weight to older data and less weight to recent data and results in a smoother plot. Usually, it is recommended to set the value of λ to 0.2. In the present study, since the amount of aflatoxin changes daily depending on the most recent measurement, the influence of older data is also important. If zi fall within the control limits, the process is under control with respect to the chosen LCL and the UCL. Parameter zi was calculated by using Eq. 1, where xi refers to the measurement of the aflatoxin content in the ith sample. Uncertainty associated with xi is therefore needs to be considered while reporting the values of the parameter z and discussing Figs. 2, 3, 4, and 5. The variance σ2of the aflatoxin measurements (combined uncertainty) may be stated as (Augustine and Minvielle 2008):
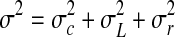 |
5 |
Where 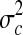 the variance of the aflatoxin levels between the samples is,
the variance of the aflatoxin levels between the samples is, 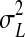 is the variance of the aflatoxin levels when the same measurement is done at different laboratories (method recovery) and
is the variance of the aflatoxin levels when the same measurement is done at different laboratories (method recovery) and 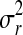 is the repeatability variance (sample recovery). O’Riordan and Wilkinson (2009) while working with the level of aflatoxin contamination in a range of imported spice preparations on the Irish retail market reported the combined uncertainty as 7.8%. In a normally distributed population, 95.46% of the individual measurements from the population fall between±2* of the population mean (Ozilgen 2011); therefore, the error bars given in Figs. 2, 3, 4, and 5 were chosen to represent the upper and the lower values of the parameter zi as zi±2*0.078 zi after considering the uncertainty.
is the repeatability variance (sample recovery). O’Riordan and Wilkinson (2009) while working with the level of aflatoxin contamination in a range of imported spice preparations on the Irish retail market reported the combined uncertainty as 7.8%. In a normally distributed population, 95.46% of the individual measurements from the population fall between±2* of the population mean (Ozilgen 2011); therefore, the error bars given in Figs. 2, 3, 4, and 5 were chosen to represent the upper and the lower values of the parameter zi as zi±2*0.078 zi after considering the uncertainty.
Bell pepper spice RP3, which was purchased from the farmers market, had more aflatoxin than what is permitted by the standards, therefore not considered for further analysis. EWMA charts were used to test whether RP1 and RP2 remained safe in storage under accelerated spoilage conditions. Although large number of storage experiments was performed and the use of the EWMA charts was assessed with all of them, only a few examples are presented here due to the limitation of the space. When red pepper spice was stored at 30 °C with 75% RH and with λ=0.2, λ=0.5 and λ=1 did not indicate violation of the control limits with all the values of λ (Fig. 2). This result may imply that the product remained safe during the storage process. The use of EWMA charts are depicted with the red pepper spice stored at 40 °C with 85% RH in Fig. 3, where the product was determined to be turned unsafe.
In Fig. 4 EWMA charts indicate that the product was safe when assessed with the US or Chinese standards, but unsafe according to the Turkish standard. In Fig. 5 the product seems like safe according to the US and Chinese standards when λ=0.2, but employing λ=0.5 or λ=1 shows that the previous conclusion with only one value of λ was misleading. Figure 5 shows that the product is not safe according to the Turkish standards, regardless of the value of λ.
Conclusion
Red peppers are subject to fungal growth both during drying or subsequent storage processes. As red pepper is the second spice to salt in Turkey, and one of the most popular spices all around the world, an urgent need for improvement of the quality control procedures for the production and storage stages of these products has been addressed.
FMEA analysis indicated that the highest total risk including chemical plus physical plus biological was associated with the washing stage (RPN=363), which is followed by the receiving (RPN=342) and the storage (RPN=342) stages. The highest biological risk (RPN=180) was associated with microbial growth and aflatoxin production due to insufficient control of the drying conditions. The highest chemical risk (RPN=144) was found for the presence of unintentional food additives, such as pesticides, herbicides, hormones, and heavy metals in the fresh red pepper fruit. Taking corrective actions causes substantial decrease in the RPN numbers associated with all the risks. Based on the FMEA analysis, improvements for all stages of red pepper spice form field to table are suggested. Better drying control will definitely reduce the risk associated with the drying process. Risk associated with unintentional additives may be reduced by using certified additives only. Although the acceptable toxin limits may vary from one region to the other, Codex Alimentarius plan has worldwide acceptance for assessing safety of the nuts. Risk of accepting the batches contaminated with aflatoxin may be eliminated by applying the Codex Alimentarius sampling plan before putting the dry pulverized bell pepper spice into the storage facility.
EWMA charts are applied in quality control of solid-pulverized red pepper during storage. Using accelerated shelf-life testing methods, aflatoxin growth was either determined or data from the literature was used to construct these charts. EWMA charts are found to be successful to distinguish between the batches, which turned to be unsafe in storage. It is recommended to construct the EWMA charts with different values of weighting factor, λ in order to reach a better conclusion considering the variability involved in the measurements.
Abbreviations
- zi
Weighted sum (EWMA statistic)
- xi
ith measurement in a sample
- λ
Weighting factor
- σ
Population standard deviation of the historical data
- μ
Population mean
- CL
Center line
- D
Ability to detect the failure
- EWMA
Exponentially weighted moving average
- L
Width of the control limits (L=3 in Shewhart charts)
- LCL
Lower control limit
- LSL
Lower specification limit
- O
Occurrence
- RPN
Risk priority number
- RH
Relative humidity
- S
Seriousness of the failure
- UCL
Upper control limit
- USL
Upper specification limit
References
- Achour M, Mtimet N, Cornelius C, Zgouli S, Mahjoub A, Thonart P, Hamdi M. Application of the accelerated shelf life testing method (ASLT) to study the survival rates of freeze-dried Lactococcus starter cultures. J Chem Tech Biotechnol. 2001;76(6):624–628. doi: 10.1002/jctb.427. [DOI] [Google Scholar]
- Agrahar-Murugkar D, Jha K. Influence of storage and packaging conditions on the quality of soy flour from sprouted soybean. J Food Sci Technol. 2011;48(3):325–328. doi: 10.1007/s13197-011-0242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn J, Kim D, Jang H, Kim Y, Shim W, Chung D. Occurance of ochoratoxin A in Korean red paprika and factors to be considered in prevention strategy. Mycotox Res. 2010;26(279):286. doi: 10.1007/s12550-010-0067-2. [DOI] [PubMed] [Google Scholar]
- Anon (2008a) Codex Alimentarius comission joint FAO/WHO food standards programme, Proposed draft sampling plans for aflatoxin contamination in almonds, Brasilnuts, hazelnuts and pistachios (N07-2004)
- Anon (2008b) Gida Maddelerindeki Bulasanlarin Maksimum Limitleri Hakkinda Teblig, Resmi Gazete: 17.05.2008- 26879
- Arvanitoyannis IS, Savelides SC. Application of failure mode and effect analysis and cause and effect analysis and Pareto diagram in conjunction with HACCP to a chocolate- producing industry: A case study of tentative GMO detection at pilot plant scale. Int J Food Sci Tech. 2007;42(11):1265–1289. doi: 10.1111/j.1365-2621.2006.01304.x. [DOI] [Google Scholar]
- Arvanitoyannis SI, Varzakas TH. Application of failure mode and effect analysis (FMEA), cause and effect analysis and Pareto diagram in conjunction with HACCP to a potato chips manufacturing plant. Int J Food Sci Tech. 2007;42(12):1424–1442. doi: 10.1111/j.1365-2621.2006.01361.x. [DOI] [PubMed] [Google Scholar]
- Arvanitoyannis SI, Varzakas TH. A Conjoint study of quantitative and semi- quantitative assessment of failure in a strudel manufacturing plant by means of FMEA and HACCP, Cause and Effect and Pareto diagram. Int J Food Sci Tech. 2007;42(10):1156–1176. doi: 10.1111/j.1365-2621.2006.01301.x. [DOI] [Google Scholar]
- Arvanitoyannis SI, Varzakas TH. Application of ISO 22000 and Failure Mode and Effect Analysis (FMEA) for industrial processing of salmon: Case study. Crit rev in Food Sci Nutr. 2008;48(5):411–429. doi: 10.1080/10408390701424410. [DOI] [PubMed] [Google Scholar]
- Arvanitoyannis I, Palaiokostas C, Panagiotaki P. A Comparative Presentation of Implementation of ISO 22000 versus HACCP and FMEA in a Small Size Greek Factory Producing Smoked Trout: A Case Study. Crit rev in Food Sci Nutr. 2009;49(2):176–201. doi: 10.1080/10408390701856058. [DOI] [PubMed] [Google Scholar]
- Arvanitoyannis IS, Varzakas TH. Application of failure mode and effect analysis (FMEA) and cause and effect analysis for industrial processing of common octopus (Octopus vulgaris)—Part II. Int J Food Sci Tech. 2009;44(1):79–92. doi: 10.1111/j.1365-2621.2007.01640.x. [DOI] [Google Scholar]
- Augustine JC, Minvielle B. Design of control charts to monitor the microbiological contamination of pork meat cuts. Food Control. 2008;19(1):82–97. doi: 10.1016/j.foodcont.2007.02.007. [DOI] [Google Scholar]
- Aydin A, Erkan ME, Başkaya R, Ciftcioglu G. Determination of aflatoxin B1 levels in powdered red pepper. Food Control. 2006;18(9):1015–1018. doi: 10.1016/j.foodcont.2006.03.013. [DOI] [Google Scholar]
- Bircan C, Barringer SA, Ulken U, Pehlivan R. Aflatoxin levels in dried figs, nuts and paprika for export from Turkey. Int J Food Sci Tech. 2008;43(8):1492–1498. doi: 10.1111/j.1365-2621.2008.01726.x. [DOI] [Google Scholar]
- Bommakanti AS, Waliyar F (2000) The importance of Aflatoxins in Human and Livestock Health. International Crops Research Institute for the Semi-Arid Tropics. http://www.aflatoxin.info/health.asp. Accessed June 18 2009
- Chulze SN. Strategies to reduce mycotoxin levels in maize during storage: a review. Food Addit Contam. 2010;27(5):651–657. doi: 10.1080/19440040903573032. [DOI] [PubMed] [Google Scholar]
- van Egmond HP. Natural toxins: risks, regulations and analytical situations in Europe. Anal Bioanal Chem. 2004;378(1152):1160. doi: 10.1007/s00216-003-2373-4. [DOI] [PubMed] [Google Scholar]
- Erdogan A. The aflatoxin contamination of some pepper types sold in Turkey. Chemosphere. 2004;56(4):321–325. doi: 10.1016/j.chemosphere.2004.02.020. [DOI] [PubMed] [Google Scholar]
- Gqaleni N, Smith JE, Lacey J, Gettinby G. Effects of temperature, water activity, and ıncubation time on production of aflatoxins and cyclopiazonic acid by an ısolate of aspergillus flavus in surface agar culture. Appl Environ Microbiol. 1996;63(3):1048–1053. doi: 10.1128/aem.63.3.1048-1053.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayoglu I, Didin M, Turkoglu H, Fenercioglu H. The effects of processing methods on some properties of hot red and red-blackish ground peppers. Pak J Biol Sci. 2005;8(10):1420–1423. doi: 10.3923/pjbs.2005.1420.1423. [DOI] [Google Scholar]
- Hough G, Garitta L, Gomez G. Sensory-shelf-life predictions by survival analysis accelerated storage models. Food Qual Pref. 2006;17(6):468–473. doi: 10.1016/j.foodqual.2005.05.009. [DOI] [Google Scholar]
- Jha SN, Narsaiah K, Sharma AD, Singh M, Bansal S, Kumar R. Quality parameters of mango and potential of non-destructive techniques for their measurement—a review. J Food Sci Technol. 2010;47(1):1–14. doi: 10.1007/s13197-010-0004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagöz S. Growth and aflatoxin production of Aspergillus flavus on some spices marketed in Turkey. Mycotox Res. 2001;17(1):2–8. doi: 10.1007/BF02946111. [DOI] [PubMed] [Google Scholar]
- Lee SY, Krocta JM. Accelerated shelf life testing of whey-protein-coated peanuts analyzed by static headspace gas chromatography. J Agr Food Chem. 2002;50(7):2022–2028. doi: 10.1021/jf010501j. [DOI] [PubMed] [Google Scholar]
- Liu Z, Gao J, Yu J. Aflatoxins in stored maize and rice grains in Lianing Province, China. J Stored Prods Res. 2006;42(4):468–479. doi: 10.1016/j.jspr.2005.09.003. [DOI] [Google Scholar]
- Martins RC, Lopez IC, Silva CLM. Accelerated shelf life testing of fozen green beans (Phaseoulus vulgaris, L.) quality loss kinetics: color and starch. J Food Eng. 2005;67(3):339–346. doi: 10.1016/j.jfoodeng.2004.04.037. [DOI] [Google Scholar]
- Muhammad F, Akhtar M, Javed I, Zu-Rahman JI, Anwar MI, Hayat S. Quantitative structure activity relationship and risk analysis of some heavy metal residues in the milk of cattle and goat. Toxicol Indust Health. 2009;25(3):177–181. doi: 10.1177/0748233709105592. [DOI] [PubMed] [Google Scholar]
- Omurtag GZ, Atak G, Yurdun T, Ersoy O. Aflatoxin Analysis in Dried Red Pepper Samples by TLC and HPL. Acta Pharm Turcica. 1998;40(3):125–130. [Google Scholar]
- O’Riordan MJ, Wilkinson MG. Comparison of analytical methods for aflatoxin determination in commercial chili spice preparations and subsequent development of an ımproved method. Food Control. 2009;20(8):700–705. doi: 10.1016/j.foodcont.2008.09.009. [DOI] [Google Scholar]
- Ozilgen S (2010) Application of failure mode and effect analysis model to foodservice systems operated by chefs in practice and by chefs from a culinary school in Turkey. J Consumer Prot Food Safety doi:10.1007/s00003-010-0626-7 (in press)
- Ozilgen M. Handbook of food process modeling and statistical quality control. 2. USA: CRC; 2011. [Google Scholar]
- Ozyaral O, Keskin Y, Başkaya R, Luleci E, Gulen D. Şeker ve şeker katkılı besin maddelerinde kserofilik-kserotoleran küfler Xerophilic and xerotolerant molds in candies and candied products. J Turkish Microbiol Soc. 2007;37(1):43–50. [Google Scholar]
- Reddy SV, Mayi DK, Reddy MU, Thirumala-Devi K, Reddy DVR. Aflatoxins B1 in different grades of chilies in India as determined by ındirect competitive ELISA. Food Addit and Contam. 2001;18(6):553–558. doi: 10.1080/02652030119491. [DOI] [PubMed] [Google Scholar]
- Saad N (2009) Aflatoxins: Occurrence and Health Risks (2004) Cornell University http://www.ansci.cornell.edu/plants/toxicagents/aflatoxin/aflatoxin.html#Home. Accessed June 18 2009
- Samapundo S, Devlieghere F, Geeraerd AH, De Meulenaer B, Van Impe JF, Debevere J. Modelling of the ındividual and combined effects of water activity and temperature on the radial growth of aspergillus flavus and a parasiticus on corn. Food Microb. 2006;24(5):517–529. doi: 10.1016/j.fm.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Scipioni A, Saccarola G, Centazzo A, Arena F. FMEA methodology design, implementation and integration with HACCP system in food company. Food Control. 2002;13(8):495–501. doi: 10.1016/S0956-7135(02)00029-4. [DOI] [Google Scholar]
- Singh NSS (2006) EWMA control chart in detecting and diagnosing a persistent shift in a process mean, Proceedings of the 2nd IMT-GT Regional Conference on Mathematics, Statistics and Applications, Universiti Sains Malaysia, Penang, June 13–15
- TurkStat (2010) Turkish Statistics Institute Crop Production Statistics http://www.Tuik.gov.tr; Acessed July 15 2010
- Varzakas TH, Arvanitoyannis IS. Application of failure mode and effect analysis (FMEA), cause and effect analysis, and Pareto diagram in conjunction with HACCP to a corn curl manufacturing plant. Crit rev in Food Sci Nutr. 2007;47(4):363–387. doi: 10.1080/10408390600781316. [DOI] [PubMed] [Google Scholar]
- Varzakas TH, Arvanitoyannis IS. Application of failure mode and effect analysis and cause and effect analysis on processing of ready to eat vegetables—Part II. Int J Food Sci Tech. 2009;44(5):932–939. doi: 10.1111/j.1365-2621.2007.01682.x. [DOI] [Google Scholar]