Abstract
Effect of soaking time (20, 40, 60, 80, 100, 120, 140, 160, 180, 200, 220, 240, 260, 280, 300, 320, 340, 360, 380, 400, 420, 440, 460, 480 and 500 min) and temperature (20, 30, 40, 50, 60, 70, 87, 92 and 97°C) together with ultrasound application (25 kHz 100 W and 25 kHz 300 W) on texture of chickpea was investigated. Soaking time, temperature, application of ultrasounds and power of ultrasounds had significant effect (P < 0.05) on texture of chickpea. An Asymptotic first order texture model was successfully fitted to correlate texture of chickpea with soaking time and temperature. Texture model rate constant (kF) increased from 1.68 × 10−4 to 19.20 × 10−4 s−1 for a temperature change from 20 to 97°C without and with 25 kHz 100 W, 25 kHz 300 W ultrasound treatments. Average gelatinization temperature of chickpea using the model was found to be 60 ± 1°C. Activation energy (Ea) values of chickpea for below and above gelatinization temperature were found to be 26.49 and 9.73 kJ mol−1, respectively. Time to reach equilibrium texture level (te) of soaked chickpeas decreased from 941 to 82 min with increase of temperature from 20 to 97°C ultrasounds of 25 kHz 100 W and 25 kHz 300 W. In the food industry, chickpea is pre-processed (soaked and cooked) to produce humus, canned products and blended powder products. Ultrasonic treatments can be applied to soften and inturn decrease the cooking time of the chickpeas.
Keywords: Chickpea, Soaking, Texture, Ultrasound, Modeling
Introduction
In general, legumes are sources of complex carbohydrates, protein and dietary fiber, having significant amounts of vitamins and minerals, and high energetic value (Tharanathan and Mahadevamma 2003). Carbohydrate contents vary, but often include long chain carbohydrates that are difficult to digest and lead to flatulence. Most grain legumes contain antinutrients or poisonous substances and need to be thoroughly cooked before eating. Grain legumes are considered to be good for health due to their mutual compatibility for their properties in disease prevention, including cardiovascular diseases, diabetes, obesity and, possibly, colon cancer (Bhathena and Velasquez 2002). Different domestic processing methods (decortication, soaking, sprouting, fermentation, boiling, roasting, parching, frying, steaming) remove anti-nutritional factors and increase the protein digestibility of chickpea seed (Attia et al. 1994). Legumes are usually cooked before being used in the human diet to improve the protein quality by destruction or inactivation of the heat labile anti-nutritional factors (Wang et al. 1997). Recently, there has been increasing demand for research to improve cooking of chickpeas in developed countries where chickpeas are mainly consumed to improve overall nutritional status by replacing animal foods with legumes (Guillon and Champ 1996). The most common process of pre-soaking usually is not sufficient to decrease overall cooking time of chickpea. Soaking may also reduce the firmness of cooked legumes (Gandhi and Bourne 1991). Water absorption causes the chickpea to become softer and uniform in texture (Abu-Ghannam 1998; Deshpande and Bal 2001). Hardness of legumes is the most important factor and sometimes it is problem during processing. Hardness is related with some chemical compositions of legumes. Also, chemical fortifications that are used either texture or nutritional purposes. Storage of legumes at high temperatures and low humidity (≥25°C, RH ≥ 65%) sometimes generates the hardshell condition. The level of water that hard to cook seeds imbibe is approximately the same as that of normal seeds, but the hard to cook legumes do not soften during cooking because the cotyledon cells do not separate. Hardshell legumes have a defective seed coat and fail to soften during cooking because they do not imbibe sufficient water (Hussain et al. 1989; Liu et al. 1992; Reyes-Moreno and Paredes-Lopez 1993).
Ultrasound is a form of energy generated by sound waves of frequencies that are too high to be detected by human ear, i.e. above 16 kHz (Jayasooriya et al. 2004; Knorr et al. 2004). Ultrasound has been used physically or chemically in many aspects of food processing and preservation, for example pasteurization, sterilization, generation of emulsions, disruption of cells, promotion of chemical reactions, inhibition of enzymes, tenderizing meat and modification of crystallization (Chemat and Hoarau 2004; Mason et al. 1996).
It has been shown that for legumes, a soaking operation prior to cooking is necessary to eliminate the toxic factors contained in the raw seed and to decrease the cooking time. However, the only pre-soaking may not be enough for decreasing cooking time of legumes. Ultrasonic waves can cause a rapid series of alternative compressions and expansions, in a similar way to a sponge when it is squeezed and released repeatedly. In addition, ultrasound produces cavitations which may be helpful to moisture uptake strongly. But, in literatures there are no researches for ultrasonic application of legumes to decrease the soaking time of chickpea. So, the objective of this study is to search the effects of ultrasound treatments combination with temperature to soften the chickpea during soaking operation.
Materials and methods
Raw materials
Special pure culture and homogeneous in physical and chemical properties of chickpea samples (Cider arietinum L.) was used in experiments. It was Certified chickpea, Inci- 2003, was obtained from Çukurova Agricultural Research Institute (Adana, Turkey). After removing foreign materials and damaged seeds, it was sieved using screens with opening of 7.50 and 9 mm in order to standardize the sizes. The average diameter was found to be 8.00 (±0.27) mm using digital micrometer.
Soaking operation
Soaking of chickpea was performed at 20, 30, 40, 50, 60, 70, 87, 92 and 97°C without and with 25 kHz 100 W (4 liter capacity ultrasonic tank, acoustic energy density (EAD) of 0.020 W cm−3) and 25 kHz 300 W (18 liter capacity ultrasonic tank, EAD of 0.015 W cm−3) ultrasound treatments. The temperature was kept constant by using a tubular cooler inserted into ultrasound tank. Diamentions of 4 L (25 kHz 100 W) and 18 L (25 kHz 300 W) volume capacity ultrasound tanks were 240 × 140 × 150 and 330 × 300 × 200 mm, respectively. Four and 18 liter capacity ultrasonic tanks had 6, 12 probes (lead titanate) and 3, 6 transuders (piezoelectric crystals), respectively. Each ultrasonic probe was 60 mm in diameter and 75 mm in length. Conventional and ultrasonic soaking was both performed in ultrasonic tanks (Intersonik, Turkey). Seeds splitted into two halves and observed complete color darkening ensured the water to reach the center of chickpea. For texture determination, 400 grams of chickpeas were immersed in 2400 milliliters of deionized water (1:6) in soaking tank. At pre-defined time intervals (20 min) for each temperature, 10 chickpea seeds were removed from soaking ultrasonic tanks, gently wiped with clean paper towel in order to remove excess water. Then, seed coats were removed manually and seeds were lightly pressed between tips of thumb and pointing finger to separate them into two cotyledons. The cotyledons were placed on a Texture Analyzer to determine hardness as maximum compression force (Fmax, N).
Measurement of textural properties of chickpeas during soaking
The texture properties (Fmax, maximum force in N) of chickpeas were measured using a TA-XT2i Texture Analyzer (Texture Technologies Corp, Scarsdale, NY/Stable Micro Systems, Godalming, UK). Penetration test was used to predict the force required to push a stainless needle probe (6 cm in length and 0.2 mm in diameter) into the sample. Fmax was used to explain the hardness of chickpea samples. Texture Analyzer parameters; pre-test speed, test speed, post test speed, distance, load cell and temperature for chickpea were 2 mm.s−1, 1 mm.s−1, 2 mm.s−1, 4 mm, 0.04903 N (5 kg) and 25°C, respectively. The texture values (Fmax) of chickpeas at each temperature (20–97°C) related to soaking times were used for texture modeling.
Modeling of chickpea hardness as a function of soaking time
For symmetry and simplicity, the following primary model (Chemat and Hoarau 2004; Gowen et al. 2007; Cunningham et al. 2008) was applied to the data to describe the decrease of chickpea hardness over time:
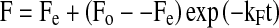 |
1 |
where, F, Fo, Fe and kF are forces at any time (t, in sec), initial and equilibrium, the rate constant of chickpea softening in s−1, respectively. The experimental chickpea texture data at each soaking temperature was fitted by non-linear regression to Eq. 1. Time to reach equilibrium texture level (te) values of chickpeas were also calculated from the Model Eq. 1.
Modeling of chickpea hardness as a function of soaking time and temperature
The rate constant (kF) can be correlated with temperature using the Arrhenius equation (Nourian et al. 2003).
 |
2 |
where Ea–F is the activation energy (kJ mol−1); R is the universal gas constant (8.314 kJ mol−1 K−1); T is the absolute temperature (K), and kFo is the pre-exponential Arrhenius factor (s−1). The regression analysis was performed on the Eq. 2 for a temperature <60°C and >60°C. From the regression of this equation, the activation energy (Ea) and break temperature was predicted. kF values obtained from Eq. 2 were inserted into Eq. 1 to obtain an equation of F as a function of Fe, Fo, T and t.
Statistical analysis
Calculated parameters for modeling and plots were compared using SIGMAPLOT 10 (Jandel Scientific, San Francisco, USA) and Excel 2003 (Microsoft, USA). ANOVA, multiple range analysis (Duncan tests) (SPSS version 16, SPSS Inc., USA) at P < 0.05 was performed to predict optimum process conditions such as time, temperature and ultrasound power. The goodness of fit of the equations was evaluated on the basis of the percentage root-mean-square (RMSE (%), R2 and Residual plots.
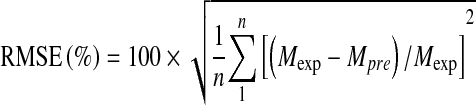 |
3 |
where n is the number of experimental points; Mexp is the experimental texture value (Fmax, N), and Mpre is the predicted texture value (Fmax, N). The lower the value of RMSE (%) the better the ability of the model to fit the data.
Results and discussion
Change in texture of chickpea during soaking
Texture is a quality attribute that is closely related to the structural and mechanical properties of a food. Force/deformation methods are widely used for objective measurement of the textural properties of solid foods (Kilcast 2004). Many earlier researchers employed instrumental texture measurement (also known as the hardness) to quantify product quality. Hardness is often defined as the peak force corresponding to the first compression of the sample (Sila et al. 2005).
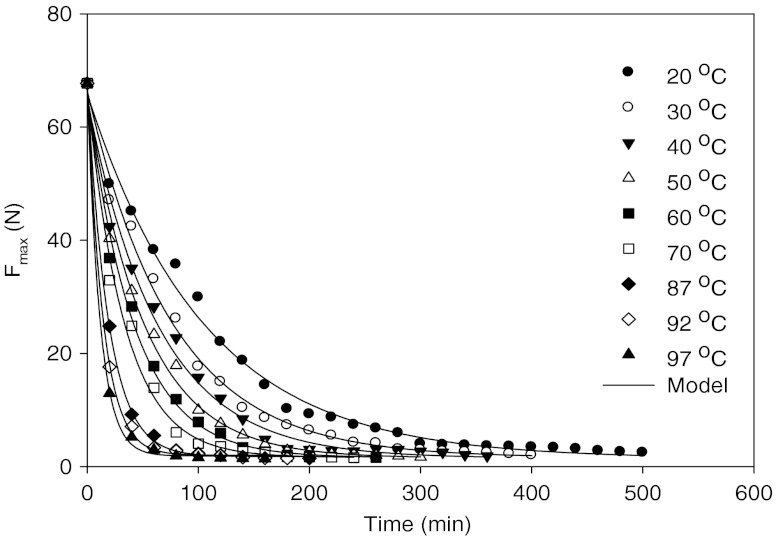
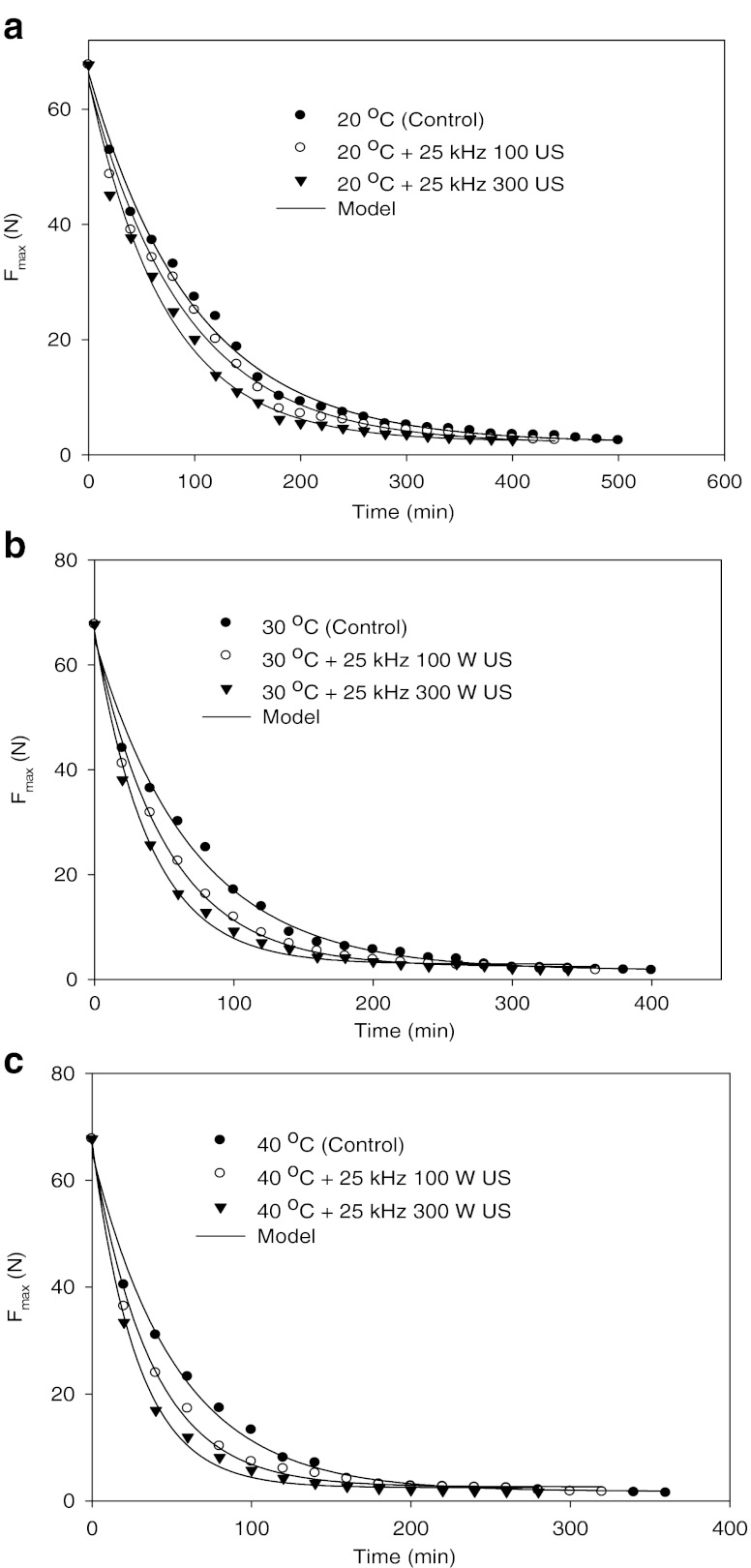
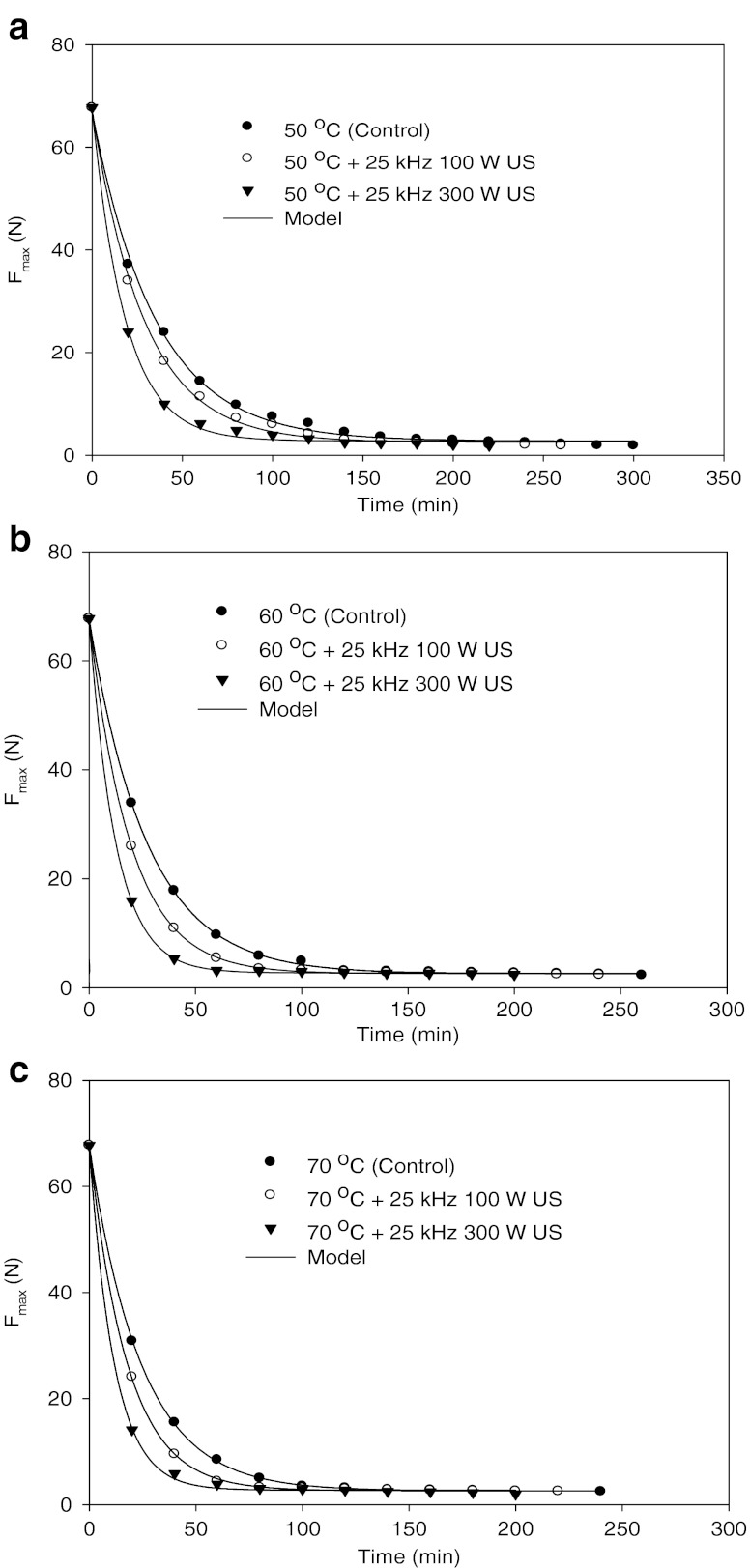
Curves of chickpea hardness (Fmax) versus time, over the set of soak temperatures (20, 30, 40, 50, 60, 70, 87, 92 and 97°C) and ultrasounds (25 kHz 100 and 300 W) studied, were illustrated in Figs. 1, 2, 3 and 4. Summary of multiple range analysis (Duncan tests) on average Fmax (N) of soaked chickpeas at different temperatures, times and ultrasounds were also given in Tables 1, 2, and 3.
Fig. 1.
Means of experimental and predicted texture values (Fmax, N) of chickpeas during soaking at different temperatures
Fig. 2.
Means of experimental and predicted texture values (Fmax, N) of chickpeas during soaking at 20 (a), 30 (b) and 40 (c) °C temperatures without and with ultrasound treatments
Fig. 3.
Means of experimental and predicted texture values (Fmax, N) of chickpeas during soaking at 50 (a), 60 (b) and 70 (c) °C temperatures without and with ultrasound treatments
Fig. 4.
Means of experimental and predicted texture values (Fmax, N) of chickpeas during soaking at 87 (a), 92 (b) and 97 (c) °C temperatures without and with ultrasound treatments
Table 1.
Summary of multiple range analysis (Duncan test) on average Fmax (N) of soaked chickpeas at different temperatures and times
| Time (min) | Fmax (N)exp | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 20°C | 30°C | 40°C | 50°C | 60°C | 70°C | 87°C | 92°C | 97°C | |
| 0 | 67.7j,8 | 67.7i,8 | 67.7h,8 | 67.7h,8 | 67.7i,8 | 67.7g,8 | 67.7g,8 | 67.7h,8 | 67.7g,8 |
| ±3.29 | ±3.29 | ±3.29 | ±3.29 | ±3.29 | ±3.29 | ±3.29 | ±3.29 | ±3.29 | |
| 20 | 52.9i,7 | 44.1h,6 | 40.4g,5 | 33.8g,4 | 29.2h,3 | 28.1f,3 | 24.0f,2 | 23.0g,2,1 | 20.0f,8,1 |
| ±3.16 | ±2.23 | ±2.29 | ±2.26 | ±1.22 | ±1.16 | ±2.15 | ±1.79 | ±1.94 | |
| 40 | 42.1h,7 | 36.4g,6 | 31.0f,5 | 24.6f,4 | 18.1g,3 | 15.6e,2 | 15.5e,2 | 14.0f,2,1 | 13.8e,1 |
| ±2.86 | ±2.58 | ±2.24 | ±2.03 | ±1.76 | ±1.18 | ±0.98 | ±1.10 | ±0.97 | |
| 60 | 37.3g,7 | 30.1f,6 | 23.2e,5 | 15.1e,4 | 12.6f,3 | 10.4d,2 | 8.9d,1 | 9.8e,2,1 | 9.0d,2,1 |
| ±2.36 | ±2.46 | ±1.71 | ±1.47 | ±1.59 | ±1.27 | ±1.40 | ±1.36 | ±0.26 | |
| 80 | 33.1f,6 | 25.2e,5 | 17.4d,4 | 10.9d,3 | 9.5e,2 | 7.0c,1 | 6.6c,1 | 6.4d,1 | 6.1c,1 |
| ±1.87 | ±1.45 | ±1.06 | ±1.08 | ±1.03 | ±0.97 | ±0.42 | ±0.71 | ±0.30 | |
| 100 | 27.4e,7 | 17.1d,6 | 13.3c,5 | 8.0c,4 | 5.9d,3 | 4.7b,2 | 3.1b,1 | 3.6c,1 | 3.2b,1 |
| ±1.13 | ±1.17 | ±1.15 | ±1.43 | ±0.67 | ±1.16 | ±0.39 | ±0.56 | ±3.27 | |
| 120 | 24.1d,7 | 13.9c,6 | 8.1b,5 | 6.5b,4 | 4.2c,3 | 3.7b,3 | 3.1b,3,2 | 2.9b,c,2,1 | 2.2a,1 |
| ±1.44 | ±1.27 | ±1.40 | ±0.87 | ±0.73 | ±0.52 | ±0.42 | ±0.48 | ±0.29 | |
| 140 | 18.7c,7 | 9.0b,6 | 7.1b,5 | 5.9b,4 | 3.4b,c,3 | 2.4a,2 | 2.2a,b,2,1 | 1.8a,b,2,1 | 1.7a,1 |
| ±1.35 | ±0.70 | ±1.29 | ±0.78 | ±0.63 | ±0.44 | ±0.35 | ±0.23 | ±0,25 | |
| 160 | 13.4b,6 | 7.1a,5 | 4.2a,4 | 3.6a,3 | 2.7 a,b,2 | 2.0a,1 | 1.6a,1 | 1.6a,1 | 1.5a,1 |
| ±1.30 | ±0.78 | ±0.84 | ±0.46 | ±0.81 | ±0.31 | ±0.19 | ±0.24 | ±0.08 | |
| 180 | 10.2a,5 | 6.3a,4 | 3.2a,3 | 2.8a,3 | 2.1a,2 | 1.8a,2,1 | 1.5a,1 | 1.5a,1 | 1.4a,1 |
| ±0.96 | ±1.06 | ±0.66 | ±0.18 | ±0.34 | ±0.13 | ±0.16 | ±0.26 | ±0.06 | |
| 200 | 9.3a,5 | 5.7a,4 | 2.8a,3 | 2.6a,3 | 1.8a,2 | 1.6a,2,1 | 1.4a,1 | 1.4a,1 | 1.3a,1 |
| ±0.61 | ±0.85 | ±0.59 | ±0.21 | ±0.09 | ±0.19 | ±0.11 | ±0.07 | ±0.08 | |
a–jIndicate statistical differences between each row (time) at constant temperature, α = 0,05,
1–8Indicate statistical differences between each column (temperature) at constant time, α = 0,05,
Second values are standard deviations
Table 2.
Summary of multiple range analysis (Duncan test) on average Fmax (N) of soaked chickpeas at 20, 30 and 40°C temperatures and different ultrasound treatments
| Time (min) | Fmax (N)exp | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 20°C | 20°C + 25 kHz 100 W | 20°C + 25 kHz 300 W | 30°C | 30°C + 25 kHz 100 W | 30°C + 25 kHz 300 W | 40°C | 40°C + 25 kHz 100 W | 40°C + 25 kHz 300 W | |
| 0 | 67.7a | 67.7a | 67.7a | 67.7a | 67.7a | 67.7a | 67.7a | 67.7a | 67.7a |
| ±3.29 | ±3.29 | ±3.29 | ±3.29 | ±3.29 | ±3.29 | ±3.29 | ±3.29 | ±3.29 | |
| 20 | 52.9c | 46.3b | 42.9a | 44.1b | 42.3a | 40.8a | 40.4c | 36.42b | 34.3a |
| ±3.16 | ±2.04 | ±2.87 | ±2.33 | ±1.29 | ±1.50 | ±2.29 | ±2.82 | ±1.09 | |
| 40 | 42.1b | 41.1b | 38.0a | 36.4b | 34.1a | 32.71a | 31.0b | 29.1a | 27.4a |
| ±2.86 | ±2.02 | ±1.70 | ±2.58 | ±0.71 | ±0.86 | ±2.24 | ±1.82 | ±1.89 | |
| 60 | 37.3b | 34.3a | 34.0a | 30.1c | 27.1b | 24.89a | 23.2c | 20.7b | 18.3a |
| ±2.36 | ±1.63 | ±2.08 | ±2.46 | ±1.71 | ±1.20 | ±1.71 | ±2.20 | ±1.12 | |
| 80 | 33.1c | 30.8b | 28.9a | 25.2c | 22.3b | 20.4a | 17.4c | 15.6b | 11.6a |
| ±1.87 | ±1.52 | ±1.67 | ±1.45 | ±1.60 | ±1.31 | ±1.06 | ±1.97 | ±1.16 | |
| 100 | 27.4b | 26.4b | 24.0a | 17.1c | 14.1b | 12.8a | 13.3c | 11.2b | 7.0a |
| ±1.13 | ±1.63 | ±1.42 | ±1.17 | ±1.37 | ±0.94 | ±1.15 | ±1.18 | ±0.60 | |
| 120 | 24.1c | 20.5b | 17.8a | 13.9c | 9.9b | 7.4a | 8.1c | 6.4b | 4.9a |
| ±1.44 | ±1.59 | ±1.27 | ±1.27 | ±0.48 | ±1.00 | ±1.40 | ±0.88 | ±0.71 | |
| 140 | 18.7c | 15.7b | 13.7a | 9.0c | 7.4b | 6.1a | 7.1c | 5.9b | 4.1a |
| ±1.35 | ±1.39 | ±1.07 | ±0.70 | ±0.85 | ±0.68 | ±1.29 | ±0.57 | ±0.53 | |
| 160 | 13.4c | 11.7b | 9.1a | 7.1c | 5.6b | 4.2a | 4.2b | 3.8b | 3.1a |
| ±1.30 | ±1.32 | ±1.23 | ±0.78 | ±0.70 | ±0.68 | ±0.84 | ±0.64 | ±0.59 | |
| 180 | 10.2b | 7.9a | 7.2 a | 6.3c | 5.2b | 4.0a | 3.2b | 2.9b,a | 2.6a |
| ±0.96 | ±1.33 | ±1.10 | ±1.06 | ±0.51 | ±0.60 | ±0.66 | ±0.45 | ±0.36 | |
| 200 | 9.3c | 6.8b | 5.6a | 5.7c | 4.1b | 3.5a | 2.8b | 2.8b | 2.4a |
| ±0.61 | ±0.97 | ±1.22 | ±0.85 | ±0.54 | ±0.43 | ±0.59 | ±0.32 | ±0.42 | |
| 220 | 8.3c | 6.6b | 5.2a | 5.2b | 3.5a | 3.2a | 2.7b | 2.6b | 2.1a |
| ±0.39 | ±0.52 | ±0.85 | ±0.71 | ±0.42 | ±0.36 | ±0.50 | ±0.24 | ±0.20 | |
| 240 | 7.4c | 6.1b | 4.6a | 4.2b | 3.1a | 2.9a | 2.6b | 2.4b,a | 2.1a |
| ±0.56 | ±0.48 | ±0.27 | ±0.51 | ±0.35 | ±0.52 | ±0.26 | ±0.25 | ±0.59 | |
| 260 | 6.6c | 5.3b | 4.1a | 4.0b | 2.6a | 2.5a | 2.5c | 2.2b | 1.9a |
| ±0.65 | ±0.56 | ±0.87 | ±0.52 | ±0.32 | ±0.62 | ±0.24 | ±0.28 | ±0.34 | |
| 280 | 5.4b | 4.2a | 3.8a | 3.0b | 2.1a | 2.1a | 2.1b | 1.9b,a | 1.7a |
| ±0.53 | ±0.38 | ±0.31 | ±0.45 | ±0.32 | ±0.49 | ±0.28 | ±0.42 | ±0.34 | |
| 300 | 5.2b | 3.8a | 3.5a | 2.4b | 1.9a | 1.8a | 1.8a | 1.7a | 1.6a |
| ±0.56 | ±0.47 | ±0.42 | ±0.21 | ±0.16 | ±0.43 | ±0.34 | ±0.62 | ±0.31 | |
a–c Indicate statistical differences between each column (different US powers) at constant temperatures, α = 0,05,
Second values are standard deviations
Table 3.
Summary of multiple range analysis (Duncan test) on average Fmax (N) of soaked chickpeas at 50, 60, 70, 87, 92 and 97°C temperatures and different ultrasound treatments
| Time (min) | Fmax (N)exp | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 50°C | 50°C + 25 kHz 100 W | 50°C + 25 kHz 300 W | 60°C | 60°C + 25 kHz 100 W | 60°C + 25 kHz 300 W | 70°C | 70°C + 25 kHz 100 W | 70°C + 25 kHz 300 W | |
| 0 | 67.7a | 67.7a | 67.7a | 67.7a | 67.7a | 67.7a | 67.7a | 67.7a | 67.7a |
| ±3.29 | ±3.29 | ±3.29 | ±3.29 | ±3.29 | ±3.29 | ±3.29 | ±3.29 | ±3.29 | |
| 20 | 33.8c | 26.0b | 18.7a | 29.2c | 22.9b | 14.1a | 28.1c | 22.4b | 13.3a |
| ±2.26 | ±1.81 | ±1.09 | ±1.22 | ±1.41 | ±1.56 | ±1.16 | ±1.93 | ±1.37 | |
| 40 | 24.6c | 18.3b | 13.0a | 18.1c | 13.0b | 9.1a | 15.6c | 11.9b | 6.3a |
| ±2.03 | ±1.98 | ±1.01 | ±1.76 | ±1.01 | ±1.22 | ±1.18 | ±1.82 | ±0.76 | |
| 60 | 15.1c | 11.4b | 8.7a | 12.6c | 8.0b | 6.1a | 10.4c | 6.3b | 5.1a |
| ±1.47 | ±0.54 | ±0.95 | ±1.59 | ±1.39 | ±0.88 | ±1.27 | ±0.72 | ±0.57 | |
| 80 | 10.9c | 7.2b | 6.3a | 9.5c | 6.1b | 4.2a | 7.0c | 4.3b | 3.1a |
| ±1.08 | ±0.91 | ±0.60 | ±1.03 | ±1.20 | ±0.86 | ±0.97 | ±0.89 | ±0.45 | |
| 100 | 8.0c | 6.0b | 4.2a | 5.9c | 4.1b | 3.0a | 4.7b | 3.1a | 2.7a |
| ±1.43 | ±0.82 | ±0.80 | ±0.67 | ±0.94 | ±1.05 | ±1.16 | ±0.64 | ±0.47 | |
| 120 | 6.5c | 4.2b | 3.2a | 4.2c | 3.4b | 2.7a | 3.7c | 2.7b | 2.3a |
| ±0.87 | ±0.76 | ±0.24 | ±0.73 | ±0.54 | ±0.82 | ±0.52 | ±0.28 | ±0.39 | |
| 140 | 5.9c | 3.0b | 2.4a | 3.4c | 2.3b | 1.7a | 2.4b | 2.0b,a | 1.8a |
| ±0.78 | ±0.51 | ±0.34 | ±0.63 | ±0.29 | ±0.17 | ±0.44 | ±0.57 | ±0.46 | |
| 160 | 3.6b | 2.6a | 2.3a | 2.7b | 1.6a | 1.6a | 2.0a | 1.9a | 1.8a |
| ±0.46 | ±0.34 | ±0.35 | ±0.81 | ±0.16 | ±0.18 | ±0.31 | ±0.27 | ±0.35 | |
| 180 | 2.8b | 2.5b,a | 2.3a | 2.1b | 1.6a | 1.5a | 1.8a | 1.7a | 1.6a |
| ±0.18 | ±0.46 | ±0.29 | ±0.34 | ±0.22 | ±0.15 | ±0.13 | ±0.40 | ±0.26 | |
| 200 | 2.6c | 2.2b | 2.0a | 1.8b | 1.4a | 1.6a | 1.6a | 1.5a | 1.4a |
| ±0.21 | ±0.27 | ±0.21 | ±0.09 | ±0.14 | ±0.26 | ±0.19 | ±0.27 | ±0.10 | |
| 87°C | 87°C + 25 kHz 100 W | 87°C + 25 kHz 300 W | 92°C | 92°C + 25 kHz 100 W | 92°C + 25 kHz 300 W | 97°C | 97°C + 25 kHz 100 W | 97°C + 25 kHz 300 W | |
| 0 | 67.7a | 67.7a | 67.7a | 67.7a | 67.7a | 67.7a | 67.7a | 67.7a | 67.7a |
| ±3.29 | ±3.29 | ±3.29 | ±3.29 | ±3.29 | ±3.29 | ±3.29 | ±3.29 | ±3.29 | |
| 20 | 24.0c | 19.4b | 11.4a | 23.0c | 15.2b | 9.6a | 22.0c | 11.0b | 8.3a |
| ±2.15 | ±1.85 | ±1.37 | ±1.79 | ±0.69 | ±1.74 | ±1.94 | ±1.08 | ±0.97 | |
| 40 | 15.5c | 7.1b | 5.3a | 14.0c | 9.4b | 6.5a | 13.8c | 5.4b | 4.3a |
| ±0.98 | ±1.18 | ±0.68 | ±1.10 | ±0.93 | ±0.85 | ±0.97 | ±0.75 | ±0.98 | |
| 60 | 8.9c | 3.9b | 2.8a | 9.8c | 6.9b | 4.5a | 9.0c | 4.0b | 2.9a |
| ±1.40 | ±0.78 | ±0.47 | ±1.36 | ±0.81 | ±0.53 | ±0.26 | ±0.40 | ±0.38 | |
| 80 | 6.6c | 2.8b | 2.1a | 6.4b | 3.0a | 2.9a | 6.1c | 2.9b | 1.8a |
| ±0.42 | ±0.43 | ±0.29 | ±0.71 | ±0.30 | ±0.37 | ±0.30 | ±0.34 | ±0.24 | |
| 100 | 3.1b | 1.8a | 1.7a | 3.6c | 2.8b | 2.3a | 3.2c | 2.2b | 1.6a |
| ±0.39 | ±0.37 | ±0.10 | ±0.56 | ±0.45 | ±0.18 | ±0.27 | ±0.20 | ±0.18 | |
| 120 | 3.1b | 1.6a | 1.5a | 2.9c | 2.5b | 2.2a | 2.2b | 1.5a | 1.4a |
| ±0.42 | ±0.30 | ±0.21 | ±0.48 | ±0.19 | ±0.15 | ±0.29 | ±0.20 | ±0.12 | |
| 140 | 2.2b | 1.6a | 1.5a | 1.8b | 1.7b,a | 1.5a | 1.7b | 1.4a | 1.3a |
| ±0.35 | ±0.21 | ±0.10 | ±0.23 | ±0.22 | ±0.14 | ±0.25 | ±0.08 | ±0.07 | |
| 160 | 1.6b | 1.5b,a | 1.4a | 1.6b | 1.5b,a | 1.4a | 1.5c | 1.3b | 1.2a |
| ±0.19 | ±0.23 | ±0.13 | ±0.24 | ±0.19 | ±0.08 | ±0.08 | ±0.10 | ±0.08 | |
a–cIndicate statistical differences between each column (different US powers) at constant temperatures, α = 0,05,
Second values are standard deviations
As the soaking temperature increased time needed for water to reach the center of chickpea decreased from 500 min at 20°C to 200 min at 97°C soaking (Fig. 1). Chickpea hardness decreased from an initial dry value (Fo-exp, 67.7 N) towards an average predicted equilibrium value 2.1 ± 0.43 N for all temperatures (20, 30, 40, 50, 60, 70, 87, 92 and 97°C) after a certain amount of soaking time. Increasing soaking time significantly (P < 0.05) decreased Fmax values (Tables 1, 2 and 3). Also, increase in soaking temperature and ultrasound application decreased Fmax values. The texture graphs show two phases, a rapid softening phase, which may be associated with the high rate of water absorption followed by a saturation phase where texture degradation rate slows down until an equilibrium texture property is achieved. As soaking proceeds, water was absorbed, resulting in a more uniform texture, and intra-bean variability consequently decreased.
Modeling of chickpea hardness as a function of soaking time
In the powder and granular food materials, researchers have paid attention on granular compression stress (Peleg 1977). Chickpea hardness decreased towards an asymptotic equilibrium state during soaking, and the general shape of the textural degradation curves (Figs. 1, 2, 3 and 4) resembled the inverse shape of the water absorption curves. Furthermore, Chemat and Hoarau (2004); Gowen et al. (2007); Cunningham et al. (2008) showed that the change in hardness of foods during processing such as soaking has been followed as an asymptotic first order model.
With the aim of building a general model to describe chickpea hardness as a function of time, the estimated model parameters (Fo, Fe and kF) were investigated (Table 4). Fitted curves for different soaking temperatures (20, 30, 40, 50, 60, 70, 87, 92 and 97°C) were illustrated in Fig. 1. The goodness of fits was predicted by root mean square error (RMSE %) which was changed from 2.86 to 24.83 and R2 (0.9914–0.9999) for a temperature range of 20–97°C without and with ultrasound treatments. The residual plots (fitted texture values versus residuals) for the general model regressed on the data are displayed in Fig. 5 and the residual points seem to be randomly distributed, with most residuals lying within two standard deviations, indicating good model fit. The predicted values for both the initial hardness, Fo, and the equilibrium hardness, Fe. were changed from 64.83 to 67.71 N and from 1.61 to 2.73 N for chickpeas over the range of 20–97°C temperatures studied, respectively. The experimental Fmax value of dry chickpea samples was measured as 67.73 N which is close to the predicted value (66.84 N). Increase in temperature from 20 to 97°C increased kF value from 1.68 × 10−4 to 8.78 × 10−4 s−1 (Table 4 and Fig. 1). Softening time of chickpea decreased as the soaking temperature was increased due to increase in kF resulting more softening. Predicted time (te) values of chickpea taken to reach equilibrium texture level was decreased from 941 to 180 min when the soaking temperature was increased from 20 to 97°C. Increase in soaking temperature of chickpea from 20 to 60°C decreased in te value from 941 to 260 min (681 min decrease). On the other hand, when the soaking temperature was increased from 60 to 97°C, te value decreased from 260 to 180 min (80 min decrease). So, soaking temperatures below gelatinization affected texture more than that of above gelatinization temperature. The Asymptotic first order texture model was successfully fitted to correlate texture of chickpea with soaking time for a temperature range of 20–97°C without and with ultrasound treatments.
Table 4.
Predicted parameters of texture model during soaking of chickpeas at different temperatures without and with ultrasound application
| Process | Fo (N) | Fe (N) | kF × 104 (s−1) | te (min) | R2 | RMSE* (%) |
|---|---|---|---|---|---|---|
| 20 | 66.25 | 2.11f,y | 1.68a,x | 941 | 0.9945 | 9.10 |
| 20°C + 25 kHz 100 W | 64.90 | 2.07x | 1.89y | 836 | 0.9924 | 9.38 |
| 20°C + 25 kHz 300 W | 65.19 | 2.37z | 2.36z | 670 | 0.9935 | 7.14 |
| 30 | 64.83 | 1.78c,x | 2.37b,x | 667 | 0.9914 | 9.81 |
| 30°C + 25 kHz 100 W | 65.82 | 2.51y | 3.29y | 480 | 0.9957 | 16.64 |
| 30°C + 25 kHz 300 W | 66.32 | 2.92z | 4.26z | 371 | 0.9956 | 24.35 |
| 40 | 65.22 | 1.79d,x | 3.16c,x | 500 | 0.9928 | 12.54 |
| 40°C + 25 kHz 100 W | 66.35 | 2.64z | 4.53y | 349 | 0.9953 | 23.19 |
| 40°C + 25 kHz 300 W | 67.17 | 2.48y | 5.85z | 270 | 0.9968 | 24.83 |
| 50 | 66.94 | 2.73i,y | 4.72d,x | 335 | 0.9979 | 19.19 |
| 50°C + 25 kHz 100 W | 66.33 | 2.52x | 5.77y | 274 | 0.9986 | 17.16 |
| 50°C + 25 kHz 300 W | 67.61 | 2.75z | 9.10z | 174 | 0.9986 | 23.14 |
| 60 | 67.72 | 2.57g,x | 6.09e,x | 260 | 0.9998 | 5.88 |
| 60°C + 25 kHz 100 W | 67.74 | 2.59y | 8.56y | 185 | 0.9999 | 4.76 |
| 60°C + 25 kHz 300 W | 67.73 | 2.70z | 13.30z | 119 | 0.9999 | 5.78 |
| 70 | 67.64 | 2.61h,x | 6.84f,x | 231 | 0.9999 | 2.86 |
| 70°C + 25 kHz 100 W | 67.77 | 2.62y | 9.33y | 169 | 0.9999 | 5.26 |
| 70°C + 25 kHz 300 W | 67.69 | 2.73z | 14.20z | 111 | 0.9993 | 16.13 |
| 87 | 67.57 | 1.91e,y | 7.82g,x | 202 | 0.9995 | 10.59 |
| 87°C + 25 kHz 100 W | 67.67 | 1.92z | 10.90y | 145 | 0.9996 | 16.15 |
| 87°C + 25 kHz 300 W | 67.72 | 1.82x | 15.10z | 105 | 0.9999 | 21.66 |
| 92 | 67.65 | 1.72b,x | 8.28h,x | 191 | 0.9999 | 4.33 |
| 92°C + 25 kHz 100 W | 67.70 | 1.89z | 11.70y | 135 | 0.9996 | 11.62 |
| 92°C + 25 kHz 300 W | 67.72 | 1.80y | 17.50z | 90 | 0.9999 | 7.89 |
| 97 | 67.71 | 1.61a,x | 8.78i,x | 180 | 0.9999 | 5.82 |
| 97°C + 25 kHz 100 W | 67.76 | 1.62y | 12.60y | 125 | 0.9998 | 9.99 |
| 97°C + 25 kHz 300 W | 67.72 | 1.75z | 19.20z | 82 | 0.9998 | 12.52 |
x–zIndicate statistical differences between each column (different US powers) at constant temperature and a–i Indicate statistical differences between each row, α = 0,05
*: 
Fig. 5.

Normal residual plots for primary model (Eq. (1)) regressed on texture data at different temperatures without and with ultrasounds (US)
Modeling of chickpea hardness as a function of soaking time and temperature
As the soaking temperature was increased, the shape of curves was changed (Fig. 1). Arrhenius plots of the natural logarithm of the estimated value of kF versus 1/T for chickpeas (Eq. 2) were shown in Fig. 6. The slope of curve is related to the activation energy, Ea, for the process of chickpea softening. For chickpeas, a break in the Arrhenius curve was apparent, after which the slope or activation energy changed. In order to find where the break occurred, the natural log of kF was fitted to a linear model with break point (Muggeo 2003), and the break temperature (gelatinization temperature) was estimated to be 60 ± 1°C (R2 ≥ 0.9938). This is in agreement with the break temperature estimated (61 ± 0.75) from the water absorption model in the study of Yildirim et al. (2011), and other literature results (Sayar et al. 2001; Gowen et al. 2007).
Fig. 6.
Arrhenius plot of Texture model of texture degradation rate constant, kF, over the temperature range 20–97°C for chickpea soaking
The regression analysis was performed and the Eq. 4 (R2 = 0.9963) and 5 (R2 = 0.9938) were predicted for temperatures range of 20–60°C and 60–97°C, respectively.
 |
4 |
 |
5 |
From the regression of these equations, the activation energies (Ea) of soaked chickpeas for 20–60 and 60–97°C temperature ranges were calculated as 26.49 and 9.73 kJ/mol, respectively. These values were comperable to the results reported by Gowen et al. (2007) for the texture kinetics of chickpeas during soaking in the temperature range of 25–60°C (46 kJ mol−1) and Cunningham et al. (2008) for the thermal degradation of potatoes soaked in water in the temperature range 20–80°C (41.12 kJ mol−1). The lower activation energy for the rate of water transfer above the gelatinization temperature implies that water travels faster in gelatinized chickpea than in ungelatinized chickpea.
Incorporating the temperature break at 60°C for the texture model, time and temperature dependence of texture (F) value for soaked chickpeas, and dependence of initial (Fo) and equilibrium (Fe) texture values, the following general models were derived to describe the texture kinetics of chickpeas:
For 20–60°C temperature range;
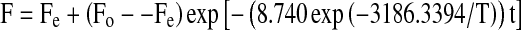 |
6 |
and for 60–97°C temperature range;
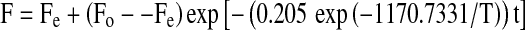 |
7 |
Equations 6 and 7 can be used to find the texture of chickpea during soaking at any time (seconds) and temperature (K) providing that Fo and Fe are known.
Effect of ultrasounds on texture of chickpeas during soaking
From the Figs. 2, 3 and 4, the ultrasound treatments of chickpea during soaking significantly (P < 0.05) decreased texture values (Fmax, N) at all studied temperatures (20–97°C). Fmax (in N) value at 20°C and 120 min soaking of chickpea was found as 24.05 N. Its value decreased to 20.49 and 17.81 N with 25 kHz 100 W and 25 kHz 300 W ultrasounds at the same temperature and time. A similar decrease in Fmax (N) was observed for other temperatures and times. Fo, Fe, kF and te values of chickpeas were also affected from ultrasound application (Table 4). kF value of chickpea increased from 1.68 × 10−4 to 1.89 × 10−4 (s−1) with the application of 25 kHz 100 W ultrasounds from 1.68 × 10−4 to 2.36 × 10−4 (s−1) with 25 kHz 300 W ultrasound at 20°C (Table 4).
The benefit of ultrasounds is evident from Table 4 and Figs. 2, 3 and 4. For example, when 25 kHz 100 W ultrasound was applied to chickpea at 20°C, the equilibrium texture time (te) value was 836 min, compared to 941 min for without ultrasound. This represents 105 min benefit for 25 kHz 100 W ultrasound application to chickpea soaking. At the same temperature (20°C), when 25 kHz 300 W ultrasound was applied for soaking of chickpea, time to reach equilibrium texture level (te) value decreased from 941 to 670 min which represents 271 min benefit during soaking. Time to reach equilibrium texture level (te) of chickpea also decreased for other temperatures when ultrasound applied during soaking. 25 kHz 100 W and 25 kHz 300 W ultrasound applied chickpea during soaking at 30°C represented 187 and 296 min benefits, respectively. te value at 87, 92 and 97°C without ultrasound was found as 202, 191 and 180 min, respectively. Equilibrium texture time (te) value for 25 kHz 100 W applied chickpea during soaking at 87, 92 and 97°C decreased from 202 to 105 min, 191 to 90 min and 180 to 82 min. As a result, application of ultrasounds increased in kF values and decreased in Fmax and te values. The effect of ultrasound at low temperatures was higher than that of high temperatures. Furthermore, high power ultrasound such as 300 W affected texture more than low power (100 W) ultrasound.
Conclusion
Texture (Fmax) of chickpea increased (P < 0.05) during soaking. Soaking time, temperature, ultrasound treatments (25 kHz 100 W and 25 kHz 300 W) and power of ultrasounds (100–300 W) had significant effect (P < 0.05) on texture (Fmax) of chickpea. An Asymptotic first order texture model was successfully fitted to correlate texture of chickpea with soaking time and temperature. Texture model rate constant (kF) increased from 1.68 × 10−4 to 19.20 × 10−4 s−1 for a temperature range of 20–97°C and 25 kHz 100 W, 25 kHz 300 W ultrasound applications. Average gelatinization temperature of chickpea from the texture models was found to be 60 ± 1°C during soaking of chickpea. Time to reach equilibrium texture level (te) of soaked chickpeas decreased from 941 to 82 min when temperature increased from 20 to 97°C without and with ultrasounds (25 kHz 100 W and 25 kHz 300 W). Ultrasounds decreased soaking and cooking times of chickpea, however, complete and detailed energy analysis should be performed to see if the process lowers the energy requirement.
Acknowledgement
Thanks to Intersonik Co., Turkey for the support.
References
- Abu-Ghannam N. Modeling textural changes during the hydration process of red beans. J Food Eng. 1998;28:341–351. doi: 10.1016/S0260-8774(98)00127-7. [DOI] [Google Scholar]
- Attia RS, El-Tabey Shehata AM, Aman ME, Hamza MA. Effect of cooking and decortication on the physical properties, the chemical composition and the nutritive value of chickpea (C. arietinum L.) Food Chem. 1994;50:125–131. doi: 10.1016/0308-8146(94)90108-2. [DOI] [Google Scholar]
- Bhathena SJ, Velasquez MT. Beneficial role of dietary phytoestrogens in obesity and diabetes. J Clin Nutr. 2002;76:1191–1201. doi: 10.1093/ajcn/76.6.1191. [DOI] [PubMed] [Google Scholar]
- Chemat F, Hoarau N. Hazard analysis and critical control point (HACCP) for an ultrasound food processing operation. Ultrason Sonochem. 2004;11:257–260. doi: 10.1016/j.ultsonch.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Cunningham SE, Mcminn WAM, Magee TRA, Richardson PS. Effect of processing conditions on the water absorption and texture kinetics of potato. J Food Eng. 2008;84:214–223. doi: 10.1016/j.jfoodeng.2007.05.007. [DOI] [Google Scholar]
- Deshpande SD, Bal S. Effect of soaking time and temperature on textural properties of soybean. J Texture Stud. 2001;32:343. doi: 10.1111/j.1745-4603.2001.tb01241.x. [DOI] [Google Scholar]
- Gandhi AP, Bourne MC. Short communication: effect of pre-soaking on the rate of thermal softening of soybeans. Int J Food Sci Tech. 1991;26:117–121. doi: 10.1111/j.1365-2621.1991.tb01147.x. [DOI] [Google Scholar]
- Gowen A, Abu-Ghannam N, Frias J, Oliveira J. Modelling the water absorption process in chickpeas (Cicer arietinum L.)-The effect of blanching pre-treatment on water intake and texture kinetics. J Food Eng. 2007;78:810–819. doi: 10.1016/j.jfoodeng.2005.11.022. [DOI] [Google Scholar]
- Guillon F, Champ M (1996) Grain legumes and transit in humans. In: Grain Legumes, AEP edn, pp 11–18
- Hussain A, Watts BM, Bushuk W. Hard to cook phenomenon in beans: changes in protein electrophoretic patterns during storage. J Food Sci. 1989;54:1367–1390. doi: 10.1111/j.1365-2621.1989.tb05996.x. [DOI] [Google Scholar]
- Jayasooriya SD, Bhandari BR, Torley P, D’arcy BR. Effect of high power ultrasound waves on properties of meat: a review. Int J Food Prop. 2004;7:301–319. doi: 10.1081/JFP-120030039. [DOI] [Google Scholar]
- Kilcast D. Solid foods: texture in foods. Washington, DC: CRC Press; 2004. [Google Scholar]
- Knorr D, Zenker M, Heinz V, Lee DU. Applications and potential of ultrasonics in food processing. Trends Food Sci Tech. 2004;15:261–266. doi: 10.1016/j.tifs.2003.12.001. [DOI] [Google Scholar]
- Liu KS, McWatters KH, Philipps RD. Protein insolubilization and thermal destabilization during storage as related to hard-to-cook defect in cowpeas. J Agri Food Chem. 1992;40:2483–2487. doi: 10.1021/jf00024a028. [DOI] [Google Scholar]
- Mason TJ, Paniwnyk L, Lorimer JP. The uses of ultrasound in food technology. Ultrason Sonochem. 1996;3:253–260. doi: 10.1016/S1350-4177(96)00034-X. [DOI] [Google Scholar]
- Muggeo VM. Estimating regression models with unknown breakpoints. Stat Med. 2003;22:3055–3071. doi: 10.1002/sim.1545. [DOI] [PubMed] [Google Scholar]
- Nourian F, Ramaswamy HS, Kushalappa AC. Kinetic changes in cooking quality of potatoes stored at different temperatures. J Food Eng. 2003;60:257–266. doi: 10.1016/S0260-8774(03)00046-3. [DOI] [Google Scholar]
- Peleg M. Flowability of food powders and methods for its evaluation—A review. J Food Process Eng. 1977;1:303–328. doi: 10.1111/j.1745-4530.1977.tb00188.x. [DOI] [Google Scholar]
- Reyes-Moreno C, Paredes-Lopez O. Hard-to-cook phenomenon in common beans. A review. Crit Rev Food Sci. 1993;33:227–286. doi: 10.1080/10408399309527621. [DOI] [PubMed] [Google Scholar]
- Sayar S, Turhan M, Gunasekaran S. Analysis of chickpea soaking by simultaneous water transfer and water–starch reaction. J Food Eng. 2001;50:91–98. doi: 10.1016/S0260-8774(00)00196-5. [DOI] [Google Scholar]
- Sila DN, Smout C, Vu TS, Van-Loey AM, Hendrickx M. Influence of pretreatment conditions on texture and cell wall components of carrot during thermal processing. Effect of preheating on thermal degradation kinetics of carrot texture. J Food Sci. 2005;70:85–91. doi: 10.1111/j.1365-2621.2005.tb07095.x. [DOI] [Google Scholar]
- Tharanathan RN, Mahadevamma S. Grain legumes—a boon to human nutrition. Trends Food Sci Tech. 2003;14:507–518. doi: 10.1016/j.tifs.2003.07.002. [DOI] [Google Scholar]
- Wang N, Lewis MJ, Brennan JG, Westby A. Effect of processing methods on nutrients and anti-nutritional factors in cowpea. Food Chem. 1997;58:59–68. doi: 10.1016/S0308-8146(96)00212-9. [DOI] [Google Scholar]
- Yildirim A, Öner MD, Bayram M. Fitting Fick’s model to analyze water diffusion into chickpeas during soaking with ultrasound treatment. J Food Eng. 2011;104:134–142. doi: 10.1016/j.jfoodeng.2010.12.005. [DOI] [Google Scholar]