Abstract
Cooking methods have a significant impact on flavour compounds in fish soup. The effects of cooking temperatures (55, 65, 75, 85, 95, and 100 °C) on sensory properties and protein hydrolysates were studied in crucian carp (Carassius auratus) soup. The results showed that the soup prepared at 85 °C had the best sensory quality in color, flavour, amour, and soup pattern. Cooking temperature had significant influence on the hydrolysis of proteins in the soup showed by SDS-PAGE result. The contents of water soluble nitrogen (WSN) and non-protein nitrogen (NPN) increased with the cooking temperature, but the highest contents of total peptides and total free amino acids (FAA) were obtained at the cooking temperature of 85 °C. The highest contents of umami-taste active amino acid and branched-chain amino acids were also observed in the 85 °C sample. In conclusion, a cooking temperature of 85 °C was preferred for more excellent flavor and higher nutritional value of crucian carp soup.
Keywords: Crucian carp, Cooking temperature, Protein, Hydrolysates, Flavour
Introduction
Crucian carp (Carassius auratus), native to China, Russia, and Korea, is one of the most important fresh water fish species (Tang et al. 2008). It is rich in protein, carbohydrates, Vitamin A, B, minerals and so on (Bastić et al. 2002). Crucian carp is usually prepared to make fish soup in many Asian-Pacific countries (Xia and Xu 2005), because the fish has a small figure but delicious taste. It is generally recognized that crucian carp soup is rich in nutrients and also has the function of regulating menstruation, lactogenesis and help the recovery of puerperants.
Flavour is one of the most important characteristics of food quality, particularly for soup/broth products. In general, most flavour components are water-soluble including nucleotides, amino acids, peptides, organic acids, organic bases, and inorganic ions. It is well known that peptides and amino acids are the primary ingredients that contribute to the taste of fish and meat products (Biswas et al. 2005, Shah et al. 2010a). Cambero et al. (2000) analyzed the relationship of compounds with the flavour of beef soup developed at different cooking temperatures. A significant rise (P < 0.05) in the concentration of nitrogen in HPPN (higher than 600 Da peptidic nitrogen), SNAN (small non-amino acid nitrogen compounds), creatinine, IMP (inosine 5'-monophosphate) and AMP (adenosine 5'-monophosphate) occurred with the increase of cooking temperature. The results also suggested the reduction of PN (protein nitrogen) and increase of SNAN were important to the beef soup flavour. Some other studies on fish soup have been reported. de Godoy et al. (2010) evaluated the acceptance of soups prepared with different kinds of smoked fish carcasses (Nile Tilapia, carp, and pacu). Shah et al. (2010b) isolated flavour-enhancing compounds, creatine and creatinine from water-soluble extracts of dried herring fillet.
Fish protein is a very important food protein to human as it provides an excellent balance of amino acids. The hydrolysates of fish protein include flavour peptides, umami-taste amino acids, branched-chain amino acids (BCAA) etc. The BCAAs have been known in reducing fatigue in both anaerobic and endurance sports, which also have been used clinically to aid the recovery of burn victims (Shimomura et al. 2006). Thermal processes for fish products can significantly influence the composition of protein and protein hydrolysates, especially the peptides and free amino acids, ultimately influence the quality of the fish food (Cambero et al. 1998, Pandey et al. 2008).
To our knowledge, there was no literature report on the relationship of the cooking methods and the nutrition and taste components in fresh water fish soup. The objective of this study was to evaluate the effects of cooking temperature on the sensory properties and contents of protein hydrolysates of crucian carp soup, to provide useful information for the preparation of crucian carp soup, both for family cooking and industrialized production.
Materials and methods
Soup preparation
Flesh crucian carp (Carassius auratus) with mean body weight of 300 ± 50 g were obtained from Century Mart Hypermarkets in Hangzhou City. Thirty individual fish were gutted and washed with tap water. After draining, two pieces of whole fish (460 ± 75 g) were cooked in a reflux with a 0.5% NaCl solution in a proportion of 6:1 (v/w) at temperatures of 55, 65, 75, 85, 95 and 100 °C for 2 h, respectively. The NaCl solutions were heated to the desired temperature before cooking. The cooked soup were cooled down to room temperature and clarified by sieving through a 0.2-mm-mesh sieve. The crucian carp soups obtained were then used in the following analysis.
Sensory evaluation
The sensory analysis was carried out following the Morita (Morita et al. 2003) method with some modifications. The samples were randomly distributed to 14 trained panelists (seven females and seven males) and quantitative descriptive analysis was performed. The color, aroma, taste and soup pattern were used as descriptive index and evaluated by using a 15 cm line scale. Each index has its highest score of 30 and the total score was 120. The sample with the best flavour was assigned as the highest score. All samples were evaluated once.
Protein and SDS-PAGE analysis
Protein was extracted according to the methods of Bechtel (Bechtel and Parrish 1983). The protein concentration was measured by the Biuret method (Robinson and Hodgen 1940) and adjusted with deionised water to a final concentration of 4 mg/mL. SDS-PAGE was performed according to the procedure of Laemmli (Laemmli 1970), using broad molecular weight standards, and the gels were stained with Coomassie Brilliant Blue.
Nitrogen fraction determination
Determinations of nitrogen fraction in crucian carp soup were carried out according to the method reported by Careri et al. (1993). Aliquots (10 mL) of crucian carp soup were mixed with the same volume of 15% phosphotungstic acid solutions to obtain non-protein nitrogen (NPN). Mixtures were kept at room temperature for 60 min, and centrifuged at 5000 rpm for 10 min at 4 °C. The supernatants were obtained by filtration through Whatman No.4 paper. The WSN (water-soluble nitrogen) and NPN were determined by the Kjeldahl method using Kjeldahl equipment (Foss 2300, Denmark). The WSN was considered as the total nitrogen in the present work.
Peptides analysis
Peptides were determined following the method described by Liu et al. (2007). A 10 g sample was deproteinized with 0.6 mol/L perchloric acid by homogenizing for 30 s. The homogenates were centrifuged at 15,000 g for 10 min and filtered through Whatman No. 54. After the pH being adjusted to 6 with 30% potassium hydroxide, the filtrates were filtered again. Twenty microlitres of filtrate were used for the HPLC analysis, using a Supelcosil LC-18 containing octadecyldimethylsilyl, 25 cm × 4.6 mm (5 μm particle size) from Supelco (Bellafonte, PA, USA) and a UV detector (214 nm). The eluents used were (A) water (HPLC grade) and (B) acetonitrile containing 0.1% of trifluoroacetic acid. The flow rate was 1 ml/min and the following gradient was employed: initial of 3.2% B; 0.5 min linear change to 4.5% B; 5 min linear change to 8.5% B; 10 min linear change to 11.5% B; 22 min linear change to 99% B and kept for 12 min, then equilibrated at 3.2% for 10 min.
Free amino acids analysis
The amino acids composition was analyzed according to the report of Norziah and Ching (2000), using the Waters Associates AccQ•Tag method, pre-column derivatization of samples with AccQ•fluor reagent and analysis by reverse phase HPLC. Identification of the amino acids in the sample was carried out by comparing their retention times with the standards.
Statistical analysis
Data were analyzed by Duncan’s multiple range tests using statistical package statistica V 5.5 software. A significant level was defined as a probability of 0.05 or less. Determinations were carried out in triplicate.
Results and discussion
Sensory evaluation
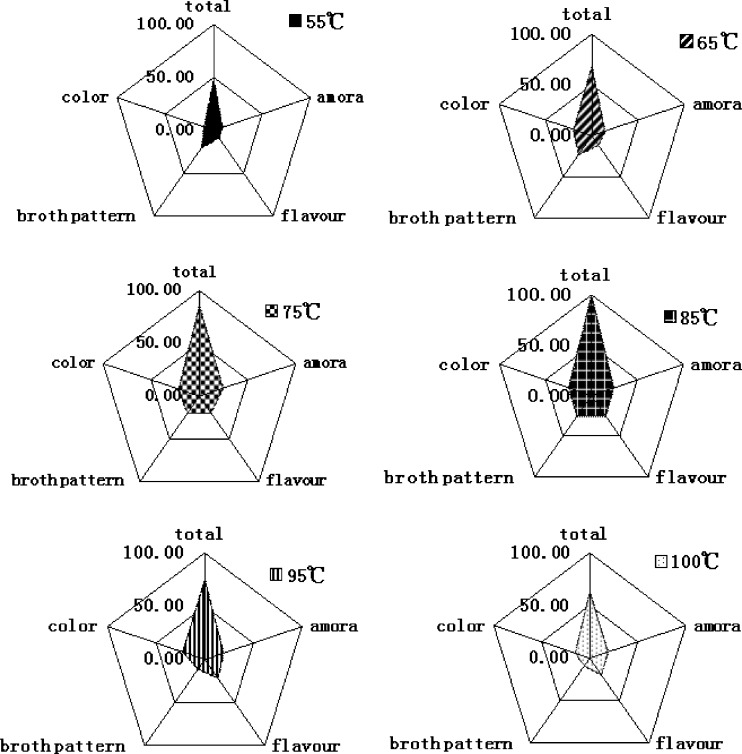
Figure 1 showed the results of sensory evaluation of crucian carp soups obtained at different cooking temperatures. The scores of color, aroma, flavour and pattern of the crucian carp soups had no linear relationship with the cooking temperatures. The crucian carp soup obtained at 85 °C had the most favorable appearance: the soup color was as white as milk. The soup obtained at 75 °C got a similar good appearance, and its score was next to the samples of 85 °C. The 55 °C one showed the lowest scored in the item of color as the soup looked as clear as water. The 75 °C and 85 °C ones got the highest grade of aroma scores (24.43 and 24.36, respectively), especially its specific fish soup aroma. The samples obtained at 95 °C and 100 °C also got relatively high values in aroma evaluation test, with 19.29 and 18.86 scores, respectively. But, the soups under these two temperatures contained many impurity substances, such as suspended meat mince and fishbone. Flavour is another important index for the sensory quality of fish soup. The strongest delicate flavour was obtained in the soup cooked at 85 °C, while the lowest flavour score was for 55 °C. A fishy odour was detected in the 65 °C and 55 °C soups, especially in the 55 °C one (Fig. 1).
Fig. 1.
Mean sensory evaluation of the crucian carp (Carassius auratus) soup obtained at different cooking temperatures. (n = 3)
Cooking temperature had a remarkable influence on the quality of crucian carp soup. When the soups were prepared at high temperatures (95 °C and 100 °C), the proteins on the surface of fish flesh coagulated, leading to the decrease of flavour and taste compounds extracted from flesh. As a result, the soup did not taste well. Furthermore, an extended cooking time caused a breakdown of protein structure, which brought to a bad soup pattern and color of the soups. On the other hand, when the cooking temperatures were lower (55 and 65 °C), the denaturation of muscle protein in the fish flesh might not complete and the soluble protein hydrolysates had not been dissolved sufficiently, thus the flavour and taste compounds were also lower in the soups. In contrary, the soup cooked at 85 °C, had the best quality in color, aroma, flavour and soup pattern.
SDS-PAGE
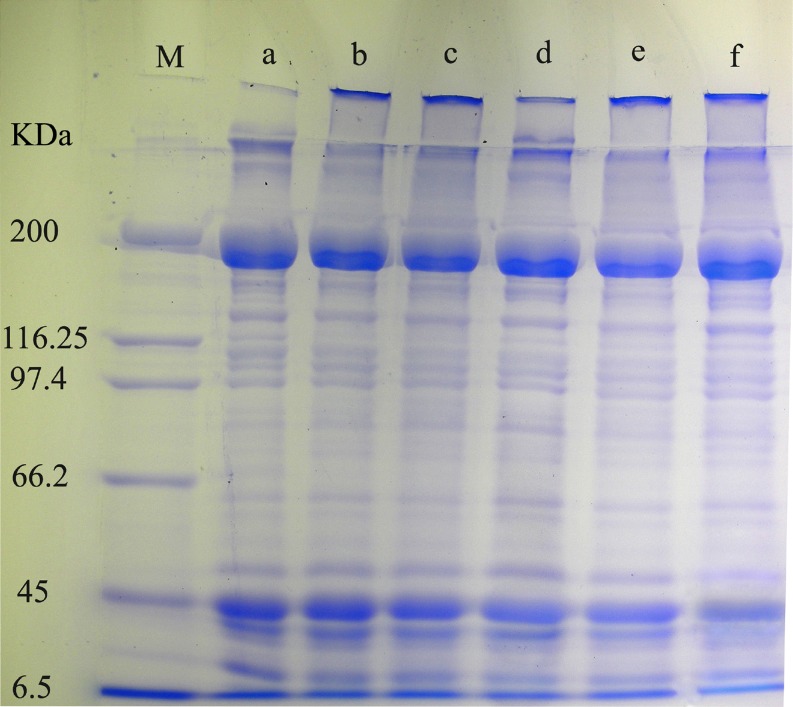
The electrophoretic patterns of proteins in crucian carp soups obtained at different cooking temperatures were shown in Fig. 2. The protein patterns of 55 °C and 65 °C soups were found to be similar with each other, which indicated that the two soup samples have the similar protein fractions. The increase of cooking temperature (75 °C and 85 °C) resulted in the dropping of the intensity of the myosin heavy chain (MHC) and molecular weight of 66–110 kDa bands. This was accompanied by an appearance of low molecular weight bands (14–45 kDa). The degradation of proteins during the cooking process was responsible for the density changes of protein bands. It is well known that high temperature promotes the heat denaturation of proteins. However, the low molecular weight bands did not increase significantly in the higher temperatures. In the present study, it was shown that when the cooking temperatures was 95 °C and 100 °C, the intensity of the heavy molecular weight (HMW) bands (>200 kDa) increased compared to that of 55–85 °C. It could be explained that the formation of protein aggregation occurred in the samples obtained at high temperatures due to protein cross-linking. Furthermore, the content of MHC decreased and some low molecular weights bands occurred as a result of the degradation of proteins in the 95 °C and 100 °C soups. Consequently, the solubility of proteins was reduced, which made contributions to the combination of intermolecular proteins and proteins with other compounds. These resulted in a relative reduction of flavour compounds from fish flesh into the soup. According to the previous research, the color of the soup had a positive correlation with the contents of proteins and lipids, and the milk-white-like soup was caused by high concentration of proteins and lipids (Chidanandaiah et al. 2002, Yamazawa and Koshino 2010). From the above sensory evaluation results, the 85 °C soup had a better milk white color than other samples, which suggested that the concentrations of proteins and lipids in this soup might be higher than those in other ones, and was confirmed by the SDS-PAGE analysis.
Fig. 2.
Electophoresis pattern of proteins of crucian carp (Carassius auratus) soup obtained at different cooking temperatures.(M: Marker; a 55 °C; b 65 °C; c 75 °C; d 85 °C; e 95 °C; d 100 °C)
Nitrogen fractions analysis
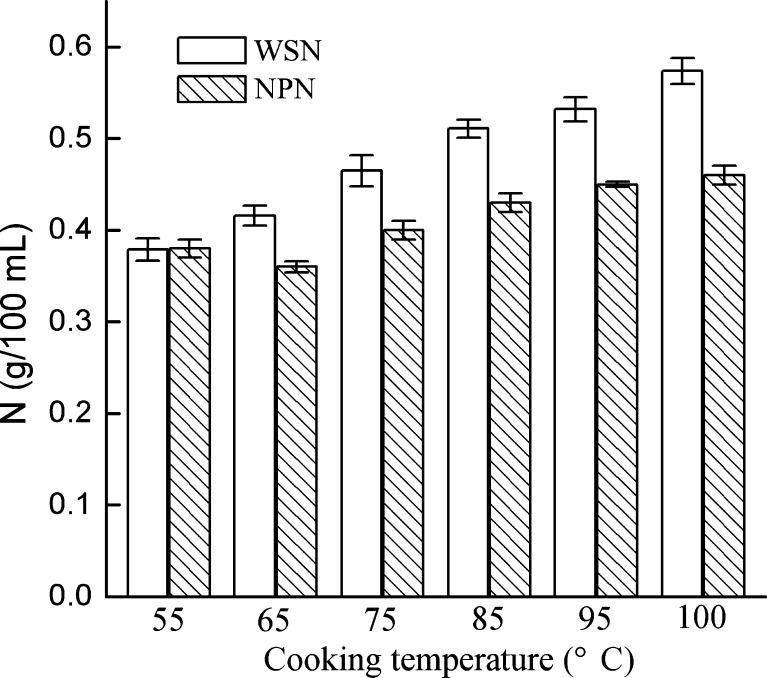
Figure 3 showed the changes of nitrogen fractions in the soups cooked at different temperatures. The content of WSN in all soups ranged from 0.38 to 0.57 g/100 mL. An increase in WSN was observed when the temperature was increased. The composition of NPN in the crucian carp soup was the nitrogen of free amino acids, nucleotides, and peptides, which can directly contribute to the formation of flavour or as flavour precursors (Madruga et al. 2010). In the present study, the content of NPN in soups also increased with the increase of cooking temperature. The behaviour of WSN and NPN coincided with that of the SDS-PAGE pattern, which reflected the protein degradation and the release of low-molecular-weight nitrogen compounds (Sun et al. 2010). Similar results were also reported for shrimp soup (Cambero, et al. 1998), which indicated that cooking temperature played a more important role than cooking time on WSN and NPN concentrations.
Fig. 3.
Concentration (g/100 ml) of water-soluble nitrogen (WSN), non-protein nitrogen (NPN) of crucian carp (Carassius auratus) soup obtained at different cooking temperatures. (n = 3)
Peptides analysis
Table 1 showed the peptides peaks in different soups by using reverse-phase HPLC. An increase in the chromatography area of the total peptides was observed when the cooking temperature was increased up to 85 °C. However, the total peptides tended to slightly reduce over 85 °C. The increases of total peptides in soups might be attributed to two reasons. One might be the flowing out of original peptides from fish meat into the aqueous soup during cooking. On the other hand, an increase of oligopeptides took place because of the degradation of proteins at higher temperature (Liu and Zhao 2010). However, the total peptides did not continue to increase when the cooking temperature was over 85 °C. As a matter of fact, a decrease of the total peptides occurred in the soups when cooked at 95 °C and 100 °C, implying that the rate of degradation of peptides was higher than that of peptide formation at higher temperatures.
Table 1.
Peptide concentrations in crucian carp (Carassius auratus) soup cooked at different temperatures
| 55 °C | 65 °C | 75 °C | 85 °C | 95 °C | 100 °C | |
|---|---|---|---|---|---|---|
| Peak 1 | 10.3 ± 0.23c | 11.7 ± 0.72c | 15.6 ± 0.39b | 17.9 ± 1.03a | 14.5 ± 0.48b | 11.4 ± 0.55c |
| Peak 2 | 3.1 ± 0.03 d | 4.2 ± 0.11 | 6.3 ± 0.17b | 8.4 ± 0.35a | 5.2 ± 0.61c | 3.9 ± 0.08 d |
| Peak 3 | 1.9 ± 0.05a | 1.8 ± 0.02ab | 1. 7 ± 0.35b | 2.1 ± 0.03a | 2.0 ± 0.09a | 1.9 ± 0.16ab |
| Peak 4 | 47.3 ± 1.16e | 65.8 ± 1.03c | 89.3 ± 1.21b | 102.2 ± 3.49a | 69.7 ± 1.46c | 57.1 ± 1.32 d |
| Peak 5 | 5.7 ± 0.22a | 4.6 ± 0.02ab | 4.1 ± 0.23b | 3.2 ± 1.07c | 2.8 ± 0.11c | 2.6 ± 0.04 d |
| Peak 6 | 30.5 ± 1.81c | 38.3 ± 0.31b | 44.7 ± 0.56a | 42.9 ± 2.18ab | 31.4 ± 2.2c | 29.0 ± 1.1 d |
| Peak 7 | 0.9 ± 0.03a | 0.78 ± 0.02a | 0.64 ± 0.01b | 0.57 ± 0.02b | 0.49 ± 0.01b | 0.26 ± 0.01c |
| Peak 8 | 4.2 ± 0.42a | 3.29 ± 0.05c | 3.9 ± 0.32ab | 3.9 ± 0.15a | 3.7 ± 0.11b | 3.2 ± 0.05c |
| Peak 9 | 10.4 ± 0.14a | 9.2 ± 0.01a | 6.5 ± 0.03c | 7.1 ± 1.02b | 6.8 ± 0.23b | 4.9 ± 0.08 d |
| Peak 10 | 7.14 ± 0.92c | 10.7 ± 0.03a | 9.4 ± 0.15ab | 11.5 ± 0.51a | 8.8 ± 0.17b | 6.4 ± 0.12 d |
| Peak 11 | 0.85 ± 0.02 d | 1.5 ± 0.07c | 3.3 ± 0.02a | 2.8 ± 0.06a | 2.2 ± 0.02b | 1.6 ± 0.18c |
| Peak 12 | 0.28 ± 0.01c | 0.72 ± 0.02a | 0.54 ± 0.01b | 0.31 ± 0.01c | 0.49 ± 0.01b | 0.19 ± 0.01 d |
| Peak 13 | 11.3 ± 0.13 d | 15.9 ± 0.02c | 20.7 ± 0.78b | 22.4 ± 1.04a | 19.4 ± 0.49b | 16.2 ± 1.39c |
| Total | 133.8 ± 2.71 d | 168.6 ± 1.98c | 206.3 ± 5.82b | 225.3 ± 4.37a | 167.5 ± 3.97c | 138.4 ± 2.64 d |
Contents of peptides were in area 100 mL−1 soup and expressed as means ± standard error (n = 3). Means followed by different lowercase letters in the same row are significantly different (P ≤ 0.05)
The changes of individual peptide areas were different from the changes of total peptides. It was clear that most peptides (Peak 1, 2, 4, 6, 10 and 13) tended to increase as the cooking temperature increased in the range of 55–85 °C (Table 1), yet a decrease was observed above 85 °C. This was in agreement with the results of nitrogen fractions mentioned in section ‘Nitrogen fractions analysis’, which demonstrated that a remarkable increase of formation of these peptides due to the proteolytic breakdown in this temperature range. However, some peptides (Peak 5 and 7) underwent a reducing with the increase of temperatures, suggesting the degradation of these peptides was much more severe. Some peptides (Peak 3, 8, 9 and 12) did not have a significantly correlation with the heating temperatures. The slight changes suggested that a balance between degradation and formation of these peptides was existed simultaneously in the soups under different temperatures.
Peptides and FAA are the main flavour compounds and the precursors of flavour compounds (Sentandeu et al. 2003). The free amino acids are the final products of proteolysis, while the peptides are intermediate products (Hashim et al. 1998). Peptides have the buffer capacity due to the amino-group and carboxyl-group, which can not only contribute to the basic flavors directly, but can also help to improve or change food flavors though the interaction with other flavour compounds (Hansen et al. 2000, Toldrá et al. 1997).
Free amino acids analysis
Like peptides, free amino acids (FAA) are another important flavour component in fish products. The free amino acids concentrations of crucian carp soups cooked at different temperatures were determined. As shown in Table 2, the predominant free amino acids in the crucian carp soup were Arg (27%–40%), Glu (5–17%) and Ala (10–12%), followed by Asp (3–10%), Gly (5–8%), Leu (2–4%) and Lys (2–4%). The remaining FAA of Phe, Thr, Tyr, Val, Ile, Cys2, Met, His and Met accounted to less than 3% of the total FAA.
Table 2.
Concentration (μmol/100 mL) of free amino acids (FAA) in curcial carp (Carassius auratus) soup cooked at different temperatures. (μmol/100 mL)
| AA | Temperatures/°C | |||||
|---|---|---|---|---|---|---|
| 55 | 65 | 75 | 85 | 95 | 100 | |
| Asp | 60.5 ± 1.31 d | 80.3 ± 5.02 cd | 131.6 ± 3.18b | 98.1 ± 4.33c | 197.4 ± 2.69a | 120.4 ± 8.94b |
| Ser | 48.5 ± 2.26c | 66.3 ± 3.15a | 54.7 ± 2.99bc | 59.8 ± 1.86b | 52.6 ± 2.11bc | 28.5 ± 0.08 d |
| Glu | 209.1 ± 7.01a | 189.0 ± 8.43b | 181.7 ± 6.86b | 180.3 ± 5.83b | 141.1 ± 4.26c | 72.7 ± 1.32 d |
| Gly | 107.6 ± 1.04c | 111.3 ± 1.95c | 128.7 ± 2.88b | 138.8 ± 4.09a | 122.5 ± 6.45b | 107.6 ± 2.87c |
| His | 33.4 ± 0.06b | 19.3 ± 0.11 d | 43.3 ± 0.37ab | 48.3 ± 1.02a | 34.4 ± 0.06b | 29.8 ± 0.15c |
| Arg | 523.7 ± 8.93c | 648.33 ± 4.76b | 810.7 ± 7.11a | 884.9 ± 9.93a | 839.9 ± 11.97a | 693.3 ± 4.98b |
| Thr | 30.4 ± 1.34bc | 45.30 ± 1.08a | 35.3 ± 1.00b | 29.3 ± 0.02bc | 21.4 ± 0.01c | 16.9 ± 0.31 d |
| Ala | 402.8 ± 3.55a | 422.7 ± 9.83a | 291.8 ± 7.16c | 336.0 ± 6.81b | 264.1 ± 1.06c | 138.8 ± 3.84 d |
| Pro | 27.8 ± 1.76c | 38.5 ± 1.20ab | 34.3 ± 0.85b | 46.1 ± 0.16a | 44.8 ± 3.76a | 25.9 ± 1.07c |
| Cys2 | 1.7 ± 0.06 cd | 1.3 ± 0.01 cd | 13.5 ± 0.12b | 31.1 ± 0.64a | 5.2 ± 1.07c | 0.89 ± 0.03 d |
| Tyr | 25.0 ± 0.5bc | 35.5 ± 0.02a | 24.5 ± 1.05bc | 30.7 ± 0.67b | 22.4 ± 1.63c | 11.3 ± 4.42 d |
| Val | 25.6 ± 2.89c | 47.4 ± 6.07b | 47.1 ± 5.32b | 71.7 ± 3.88a | 48.5 ± 1.46b | 26.4 ± 5.00c |
| Met | 40.3 ± 0.73c | 82.3 ± 2.89b | 102.3 ± 4.93a | 108.5 ± 5.91a | 31.6 ± 6.11 d | 10.3 ± 0.22e |
| Lys | 37.8 ± 0.79 d | 49.6 ± 3.30 cd | 71.7 ± 2.58b | 75.4 ± 1.16ab | 86.6 ± 5.20a | 58.3 ± 2.04c |
| Ile | 27.6 ± 0.12 cd | 22.8 ± 2.06 d | 33.9 ± 2.21c | 51.6 ± 3.04a | 43.7 ± 4.11b | 18.5 ± 0.97 d |
| Leu | 50.1 ± 2.07b | 63.2 ± 1.99a | 53.8 ± 3.75b | 66.8 ± 2.00a | 55.3 ± 2.98b | 26.9 ± 0.01c |
| Phe | 36.5 ± 3.95c | 26.1 ± 4.00 d | 52.8 ± 0.91b | 67.0 ± 1.57a | 23.7 ± 3.97 d | 15.8 ± 0.01e |
| ∑UTAA | 816.6 ± 4.09a | 829.3 ± 2.01a | 786.5 ± 3.01b | 831.0 ± 1.25a | 748.7 ± 3.08c | 455.3 ± 2.15 d |
| ∑BCAAs | 103.4 ± 1.05c | 133.4 ± 2.81bc | 134.8 ± 1.32bc | 190.0 ± 1.18a | 147.6 ± 0.97b | 71.9 ± 0.54 d |
| ∑FAA | 1688.7 ± 9.07 d | 1949.1 ± 7.12c | 2111.6 ± 3.99b | 2335.1 ± 5.80a | 2035.1 ± 9.67bc | 1402.4 ± 5.96 d |
Means followed by different letters in a row are significantly different (P ≤ 0.05) (n = 3). FAA free amino acids, UTAA Unami-Taste amino acids, BCAAs Branched-Chain Amino Acids
The Glu, Asp, Phe, Ala and Gly, presented in relatively large quantities in soups, belonged to the umami-taste active amino acids, and were very important to the flavour of food (Itou et al. 2006). These amino acids have been considered as the main contributors to the flavour of a soup (Randhawa and Ranote 2004, Shiau et al. 1997). Furthermore, their sodium salts have a synergistic effect on the flavour with nucleotides (Kayim et al. 2011). Table 2 showed the total tasty amino acids in crucian carp soup accounted for more than 50% of the total FAA. The decreases of some amino acids (Glu and Ala) could be explained by the reaction between these amino acids and other compounds. On the other hand, Gly and Phe concentrations increased stably until the cooking temperature increased up to 85 °C; followed by a slight reduction above 85 °C. The highest total tasty amino acids concentration took place in the soup obtained at 85 °C, suggesting that the strongest flavour was obtained in the 85 °C soup. This result was in agreement with that of the sensory evaluation. The Leu, Ile and Val compose the Branched-Chain Amino Acids (BCAAs) which has special physiological functions, such as strengthen the immunity (Wakshlag et al. 2006). The concentration of BCAA accounted about 6–8% of the total FAA (Table 2). The content of BCAA increased when the cooking temperatures increased up to 85 °C. Thereafter, it showed a decreasing tendency in the soups cooked at a higher than 85 °C. It can be concluded that the highest content of BCAA was obtained in the 85 °C soup.
Although the effect of heating temperature on the contents of each type of the amino acids was quite different, the total FAA concentration had a clear correlation with cooking temperatures, the concentration of total FAA increased continuously until the temperature reached 85 °C, thereafter, the concentration decreased. There were two possible ways to constitute source of FAA in the soup, one was the original FAA flew into soup from the fish flesh and the other the degradation of proteins and peptides due to the aminopeptidase (Buscailhon et al. 1994). Therefore, the concentration of total FAA was determined by the ratio of formation and decomposition of FAA in the food. Previous researchers observed that some amino acids involved in the Maillard reaction with reducing sugars to produce hexaldehydes, heptaldehydes and so on (Chawla and Sahu 2007). Also, a small part of degradation of amino acids generated esters, ketonesvia through the Strecker degradation pathway. These reactions provided flavour compounds to food through the pathway of interaction of amino acids with compounds from lipid oxidation (Ardö, 2006).
Conclusion
The data obtained suggested that the best quality soup (in color, aroma, flavour and soup pattern) was obtained by heating crucian carp in a 0.5% NaCl solution (1:6, w/v) at 85 °C for 2 h. From the analysis of variance of the sensory data, temperature plays a more important role than cooking time in the generation of the sensory properties in crucian carp soup. The heating temperature played a crucial role on the proteolysis and solubility of protein in the fish soup, the highest contents of total peptides, total FAA, tasty amino acids and BCAA in crucian carp soup were obtained at the cooking temperature of 85 °C. In conclusion, a cooking temperature of 85 °C was preferred for more excellent flavor and higher nutritional value of crucian carp soup.
Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities (KYJD09033), NSFC (30972282) and the national program of ‘985’ (project number 707034). The authors also acknowledge the Science and Technology Department of Zhejiang Province for partial financial support (2008 C12019, 2010 C12012).
References
- Ardö Y. Flavour formation by amino acid catabolism. Biotechnol Adv. 2006;24:238–242. doi: 10.1016/j.biotechadv.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Bastić L, Kočovski T, Antonović D, Vidarić D. The meat quality of some freshwater fish—Nutritive and technological aspects. Acta Vet Beograd. 2002;52:259–266. doi: 10.2298/AVB0204259B. [DOI] [Google Scholar]
- Bechtel PJ, Parrish FC., Jr Effects of postmortem storage and temperature on muscle protein degradation: analysis by SDS gel electrophoresis. J Food Sci. 1983;48:294–295. doi: 10.1111/j.1365-2621.1983.tb14857.x. [DOI] [Google Scholar]
- Biswas AK, Keshri RC, Chidanandaiah Effect of different cooking methods on quality of enrobed pork patties. J Food Sci Technol. 2005;42:173–175. [Google Scholar]
- Buscailhon S, Monin G, Cornet M, Bousset J. Time-related changes in nitrogen fractions and free amino acids of lean tissue of frech dry-cured ham. Meat Sci. 1994;37:449–456. doi: 10.1016/0309-1740(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Cambero MI, Jaramillo CJ, Ordoñez JA, Cobos A, Pereira-Lima CI, García De Fernando GD. Effect of cooking conditions on the flavour compounds and composition of shrimp (Parapenaeus longirostris) soup. Eur Food Res Technol. 1998;206:311–322. [Google Scholar]
- Cambero MI, Pereira-Lima CI, Ordoñez JA, García De Fernando GD. Beef broth flavour: relation of components with the flavour developed at different cooking temperatures. J Sci Food Agr. 2000;80:1519–1528. doi: 10.1002/1097-0010(200008)80:10<1519::AID-JSFA674>3.0.CO;2-R. [DOI] [Google Scholar]
- Careri M, Mangia A, Barbieri G, Bouoni L, Virgili R, Parolari G. Sensory property relationships to chemical data of Italian-type dry-cured ham. J Food Sci. 1993;58:968–972. doi: 10.1111/j.1365-2621.1993.tb06090.x. [DOI] [Google Scholar]
- Chawla HM, Sahu SN. Effect of spice essential oils on Maillard browning model reaction of glucose and glycine: an UV-visible and reverse phase HPLC analysis. J Food Sci Technol. 2007;44:602–606. [Google Scholar]
- Chidanandaiah, Sanyal MK, Keshri RC. Changes in quality of chicken soup and chicken-whey soup in laminated pouches during refrigerated storage. J Food Sci Technol. 2002;39:288–291. [Google Scholar]
- de Godoy LC, Franco MLRS, Franco NP, da Silva AF, de Assis MF, de Souza NE, Matsushita M, Visentainer JV. Sensorial analysis of soups and broths made by smoked fish carcasses meal: its utilization to supplement school meals. Cienc Tecnol Aliment. 2010;30:86–89. doi: 10.1590/S0101-20612010000500014. [DOI] [Google Scholar]
- Hansen CE, Mañez A, Burri C, Bousbaine A. Comparison of enzyme activities involved in flavour precursor formation in unfermented beans of different cocoa genotypes. J Sci Food Agr. 2000;80:1193–1198. doi: 10.1002/1097-0010(200006)80:8<1193::AID-JSFA619>3.0.CO;2-7. [DOI] [Google Scholar]
- Hashim P, Selamat J, Muhammad SKS, Ali A. Changes in free amino acid, peptide-N, sugar and pyrazine concentration during cocoa fermentation. J Sci Food Agr. 1998;78:535–542. doi: 10.1002/(SICI)1097-0010(199812)78:4<535::AID-JSFA151>3.0.CO;2-6. [DOI] [Google Scholar]
- Itou K, Kobayashi S, Ooizumi T, Akahane Y. Changes in proximate composition and extractive components of rice-bran-fermented mackerel Heshiko during processing. Fish Sci. 2006;72:1269–1276. doi: 10.1111/j.1444-2906.2006.01285.x. [DOI] [Google Scholar]
- Kayim M, Cimen M, Can E, Kocabas M. Biochemical taste parameters in meat and sea products. Asian J Anim Vet Adv. 2011;6:233–237. doi: 10.3923/ajava.2011.233.237. [DOI] [Google Scholar]
- Laemmli UK. Cleavage and structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu TX, Zhao MM. Thermal pretreatment and chemical modifications as a means to alter hydrolytic characteristics and prevent bitterness in hydrolysates of fishery by-catch (Decapterus maruadsi) protein. Int J Food Sci Technol. 2010;45:1852–1861. doi: 10.1111/j.1365-2621.2010.02344.x. [DOI] [Google Scholar]
- Liu Y, Xu XL, Zhou GH. Changes in taste compounds of duck during processing. Food Chem. 2007;102:22–26. doi: 10.1016/j.foodchem.2006.03.034. [DOI] [Google Scholar]
- Madruga MS, Elmore JS, Oruna-Concha MJ, Balagiannis D, Mottram DS. Determination of some water-soluble aroma precusors in goat meat and their enrolment on flavour profile of goat meat. Food Chem. 2010;123:513–520. doi: 10.1016/j.foodchem.2010.04.004. [DOI] [Google Scholar]
- Morita K, Kubota K, Aishima T. Comparison of aroma characteristics of 16 fish species by sensory evaluation and gas chromatographic analysis. J Sci Food Agr. 2003;83:289–297. doi: 10.1002/jsfa.1311. [DOI] [Google Scholar]
- Norziah MH, Ching CY. Nutritional composition of edible seaweed Gracilaria changgi. Food Chem. 2000;68:69–76. doi: 10.1016/S0308-8146(99)00161-2. [DOI] [Google Scholar]
- Pandey MC, Jayathilakan K, Manral M, Radhakrishna K, Bawa AS. Heat and mass transfer kinetics of Catla catla fish during frying. J Food Sci Technol. 2008;45:323–327. [Google Scholar]
- Randhawa GK, Ranote PS. Storage stability of processed oyster mushroom (Pleurotus spp.) into soup powder. J Food Sci Technol. 2004;41:525–529. [Google Scholar]
- Robinson HW, Hodgen CG. The Biuret reaction in the determination of serum protein. I. A study of the condition necessary for the production of the stablele color which bears a quantitative relationship to the protein concentration. J Biol Chem. 1940;135:707–725. [Google Scholar]
- Sentandeu MA, Stoeva S, Aristory MC, Laib K, Voelter W, Toldrá F. Idetification of small peptides generated in Spanish dry-cured ham. J Food Sci. 2003;68:64–69. doi: 10.1111/j.1365-2621.2003.tb14115.x. [DOI] [Google Scholar]
- Shah AKMA, Ishihara T, Ogasawara M, Kurihara H, Baba N, Takahashi K. Mechanism involved in the formation of characteristic taste and flavor during the production of dried herring (clupea pallasii) fillet. Food Sci Technol Res. 2010;16:201–208. doi: 10.3136/fstr.16.201. [DOI] [Google Scholar]
- Shah AKMA, Ogasawara M, Egi M, Kurihara H, Takahashi K. Identification and sensory evaluation of flavour enhancers in Japanese traditional dried herring (Clupea pallasii) fillet. Food Chem. 2010;122:249–253. doi: 10.1016/j.foodchem.2010.02.072. [DOI] [Google Scholar]
- Shiau CY, Tseng IT, Chiou TK, Cheng CC. Changes in extractive nitrogenous components and quality of canned milkfish during thermal sterilization. J Chin Nutr Soc. 1997;22:349–359. [Google Scholar]
- Shimomura Y, Yamamoto Y, Bajotto G, Sato J, Murakami T, Shimomura N, Kobayashi H, Mawatari K. Nutraceutical effects of branched-chain amino acids on skeletal muscle. J Nutr. 2006;136:529S–532S. doi: 10.1093/jn/136.2.529S. [DOI] [PubMed] [Google Scholar]
- Sun W, Zhao Q, Zhao H, Zhao M, Yang B. Volatile compounds of Cantonese sausage released at different stages of processing and storage. Food Chem. 2010;121:319–325. doi: 10.1016/j.foodchem.2009.12.031. [DOI] [Google Scholar]
- Tang XY, Chen J, Li GG, Zhu LJ, Dai YF. Effect of processing conditions on the nutrition value of fish soup. Sci Technol Food Ind. 2008;10:248–255. [Google Scholar]
- Toldrá F, Flores M, Sanz Y. Dry-cured ham flavour: enzymatic generation and process influence. Food Chem. 1997;59:523–530. doi: 10.1016/S0308-8146(97)00013-7. [DOI] [Google Scholar]
- Wakshlag JJ, Kallfelz FA, Wakshlag RR, Davenport GM. The effects of brancked-chain amino acids on canine neoplastic cell proliferation and death. J Nutr. 2006;136:1633S–1635S. doi: 10.1093/jn/136.7.2007S. [DOI] [PubMed] [Google Scholar]
- Xia QQ, Xu HQ. Cooking technology of thick crucian carp soup and its nutritional analysis. Cul Sci J Yangzhou Univ. 2005;3:24–26. [Google Scholar]
- Yamazawa M, Koshino S. Relationship between the turbidity of dashi (soup stock) made from fushi (boiled, smoke-dried fish fillet) and the chemical components present, and the component mainly responsible for the turbidity. Nippon Shokuhin Kagaku Kogaku Kaishi. 2010;57:251–256. doi: 10.3136/nskkk.57.251. [DOI] [Google Scholar]